Introduction
Cancer is a major health issue across the globe
(1,2). Chemotherapy is an important step in
the systemic therapy for cancer, especially for metastatic cancer
(3,4). Oxaliplatin is one of the
platinum-based anticancer chemotherapy drugs approved by the Food
and Drug Administration for the treatment of digestive cancer,
including gastric and colon cancer (5). Its anticancer effect is attributed to
the formation of intra- or inter-strand crosslinks with nuclear
DNA, which breaks the double bond of DNA strands, leading to the
failure in DNA translation and transcription (6). Oxaliplatin has widely been applied
for the treatment of various types of tumors for many years
(7). However, one of the
limitations associated with platinum-based chemotherapy is the
development of dose-limiting toxicities that prevent continuation
of the treatment (8). Therefore,
there is a need to make improvements in its use in clinical
practice. At present, strategies to limit the chemotherapy of
toxicity, such as neurotoxicity, include the co-administration of
antioxidants, such as thiols, particularly glutathione (GSH), or
vitamin E, together with the platinum agent (9). Thus, we were interested to explore
whether herbal medicine might be a feasible add-on to oxaliplatin
cancer treatment.
The combined herbal medicine and Western medical
treatment has been used in many patients for a long time. Owing to
the poor prognosis of some cancer types when treated with Western
medicine, many patients prefer the option of herbal medicine as the
adjuvant therapy, expecting to enhance therapeutic efficiency,
reduce adverse effects, and improve quality of life (10,11).
Based on the patient-tailored diagnosis and treatment, such a
combined therapy has attracted more attention in the clinical
setting. The physical functions were significantly improved in
participants with non-small-cell lung cancer treated with the
combined herbal-Western medicine than in those treated with Western
medicine only (12). In addition,
nature-derived products have recently attained a lot of interest
due to their potentiation of anticancer effects by modulating the
signaling pathways involved in cancer proliferation, and owing to
their protective potential in radiotherapy and chemotherapy
(13). For example, some in
vitro and in vivo tests confirmed that alteronol, a
herbal medicine-extracted ingredient, could potentiate the
therapeutic effects of Adriamycin on breast cancer cells and reduce
toxicities to major organs in mice (14). Thus, introducing novel bioactive
components with natural origins could be considered to treat
different types of human cancer on the basis of their selective
molecular targets (1).
Luteolin (3′,4′,5,7-tetrahydroxyflavone) is a
natural flavonoid, widely existing in medical plants, such as
Lonicera japonica Thunb and Ajuga nipponensis Makino
(1). Luteolin possesses various
pharmacological effects, including anticancer, antioxidant,
anti-inflammatory, immunoregulatory, and cardioprotective ones
(15). Luteolin is compatible with
various drugs and enhances their therapeutic effects in the
treatment of many diseases, such as Alzheimer disease, diabetic
cystopathy, and sciatic nerve ligation-induced neuropathy (16–18).
Moreover, luteolin can be utilized as an agent that both improves
the therapeutic effects on various types of cancer and decreases
toxicity in the host. Furthermore, luteolin is known to effectively
act in combination with silibinin (19), sorafenib (20), 5-flurouracil (21), and lycopene (22) against hepatocellular carcinoma
cells, glioblastoma cells, ovarian cancer cells, and Solid Ehrlich
Carcinoma (SEC). Research on the use of the combined chemotherapy
in clinical setting is of even higher interest. Normally,
chemotherapeutic drugs exhibit antitumor activities along with
deleterious effects, which has been the challenge in the cancer
treatment. The dose reduction of these drugs may alleviate their
side effects, and it can also limit their efficiency of inhibiting
tumor growth (23). Therefore,
further studies on potential chemotherapeutic properties of
luteolin in cancer treatment are needed, including studies of the
underlying mechanism.
In the present study, mouse forestomach carcinoma
(MFC) cells were used as the cell model to identify the anticancer
effects of the combined treatment with luteolin and oxaliplatin.
The MFC cell line with high metastasis is prone to blood-born
metastasis to lung. The aim of the present study was to investigate
the enhancing effects of luteolin on low-dose oxaliplatin-induced
proliferation inhibition in MFC cells. The difference between the
combined and monotherapy were demonstrated, supporting the
potential of luteolin to enhance the therapeutic effects of
oxaliplatin. And this work was also conducted to confirm the key
signaling pathway involved in the enhanced effects of the combined
treatment with luteolin and oxaliplatin. Therefore, findings of the
present study may provide a theoretical foundation for further
clinical chemotherapy research on the use of luteolin.
Materials and methods
Reagents
Luteolin (purity, ≥98%) was purchased from
Sigma-Aldrich (cat. no. L9283). DMSO (cat. no. D8371; Beijing
Solarbio Science & Technology Co., Ltd.) was used to dissolve
with luteolin as the mother solution. Oxaliplatin was purchased
from Tai-Tianqing Pharmaceutical Co., Ltd. (cat. no. H20143263).
Oxaliplatin was dissolved with PBS (P1020; Beijing Solarbio Science
& Technology Co., Ltd.). Luteolin and/or oxaliplatin were
diluted to the required concentration (luteolin: 10, 20, 30, 40,
50, 60, 70 and 80 µM; and oxaliplatin: 5, 10, 20, 30, 40, 50, 60,
70 and 80 µM) with RPMI-1640 complete medium. RPMI-1640 complete
medium (cat. no. 31800; Beijing Solarbio Science & Technology
Co., Ltd.) containing 0.1% DMSO was used as a control.
Cell culture
The MFC cell line was purchased from the National
Infrastructure of Cell Line Resource (cat. no. 1101MOU-PUMC000143).
The cells were cultured with RPMI-1640, containing 10% fetal bovine
serum (cat. no. REF10091-48; Gibco; Thermo Fisher Scientific,
Inc.), and incubated in an incubator (HF90/HF240; Heal Force) at 5%
CO2 at 37°C. The cells were treated with different
concentrations of luteolin and/or oxaliplatin for 24 h in the
subsequent experiments.
CCK-8 cell viability assay and
Chou-Talalay combination index (CI) method analysis
The effect of luteolin and/or oxaliplatin on MFC
cell viability was determined by CCK-8 assay (24). Briefly, MFC cell suspension (100
µl/well) was seeded at a density of 6,000-7,000 cells/well in a
96-well plate. After incubation for 24 h, the supernatant was
discarded, and the cells were treated with luteolin (10, 20, 30,
40, 50, 60, 70, and 80 µM) and oxaliplatin (5, 10, 20, 30, 40, 50,
60, 70, and 80 µM) (total volume 200 µl/well) for 24 h. Based on
the CCK-8 cell proliferation and cytotoxicity assay kit (cat. no.
CA1210; Beijing Solarbio Science & Technology Co., Ltd.)
protocol, 100 µl testing solution (CCK-8: Complete RPMI-1640, 1:10)
was added to each well. After incubation at 37°C in 5%
CO2 in the dark for 1 h, the absorbance at a wavelength
of 450 nm was detected via a Thermo 3001 multi-function microplate
reader (Infinite 200 PRO; Tecan Austria GmbH). IC50
indicated the drug concentration resulted in 50% reduction in cell
survival. The Chou-Talalay CI method and Compusyn 2.0 software were
used to calculate the CI and the dose reduction index (DRI). The CI
was calculated as (D)1/(Dx)1 +
(D)2/(Dx)2, where (Dx)1 and
(Dx)2 were the doses of drug 1 and 2 alone that inhibit
x%, while (D)1 and (D)2 were the portions of
drug 1 and 2 in combination to achieve x%. This equation was used
to quantitatively depict the synergism (CI <1), additive effect
(CI=1), and antagonism (CI >1) (25).
DNA contents analysis
DNA contents analysis was conducted by PI staining
and flow cytometry (26). MFC
cells were seeded in a 6-well plate at 2.5×105
cells/well and incubated at 37°C in a 5% CO2 incubator
for 24 h. After treatment with luteolin (20 µM) and/or oxaliplatin
(5 µM) (total volume 3 ml/well) for one day, the cells were
collected and fixed in 75% ethanol at 4°C for 4–5 h. In accordance
with the manufacturer's instructions of the DNA content
quantitation assay kit (cat. no. CA1510; Beijing Solarbio Science
& Technology Co., Ltd.), the fixed cells were stained by PI
staining solution (50 ng/ml) and RNase A (0.1 mg/ml) at 37°C in the
dark for 30 min. FACSCanto II flow cytometer (BD Biosciences)
recorded the DNA content in MFC cells. The data were analyzed by
FACSDiva software (version 6.1.3; BD Biosciences).
Hoechst-33258 staining
MFC cells were seeded in a 6-well plate at
2.0×105 cells/well. The cells were treated with luteolin
(20 µM) and/or oxaliplatin (5 µM) (total volume 3 ml/well) for 24
h. Based on Hoechst-33258 stain solution (cat. no. C0021; Beijing
Solarbio Science & Technology Co., Ltd.) instructions (14), the cells were incubated with 500 µl
staining working solution at room temperature in the dark for 5 min
and soaked in PBS three times for 5 min. Inverted fluorescence
microscopy (DMI3000; Leica Microsystems GmbH) was used to detect
blue nuclei.
Annexin V-FITC/PI double staining
assay
Apoptosis induced by luteolin was examined by
fluorescein isothiocyanate (Annexin V-FITC) and propidium iodide
(PI) double-staining assay (27).
MFC cells were seeded in a 6-well plate at 2.5×105
cells/well and incubated at 37°C in 5% CO2 for 24 h. The
cells were treated with luteolin (20 µM) and/or oxaliplatin (5 µM)
(total volume 3 ml/well) for 24 h. After the treatment, the
supernatant was discarded. In line with the instructions of the
Annexin V-FITC/PI apoptosis detection kit (cat. no. CA1020; Beijing
Solarbio Science & Technology Co., Ltd.), the cells were
pre-processed and stained using Annexin V-FITC/PI in the dark for
30 min. The fluorescence intensity was measured using FACSCanto II
flow cytometer (BD Biosciences), and the apoptotic rates were
analyzed using FACSDiva software (version 6.1.3, BD
Biosciences).
Reactive oxygen species (ROS) levels
testing
Subsequent to the indicated drug treatment for 24 h,
the supernatant was discarded. The cells were rinsed with PBS, and
stained with 10 µM DCFH-DA solution in the dark for 30 min in
accordance with the reactive oxygen species assay kit (cat. no.
CA1410; Beijing Solarbio Science & Technology Co., Ltd.)
protocol (28). Inverted
fluorescence microscopy (DMI3000, Leica) was used to record the
morphological changes. The stained cells were analyzed by FACSCanto
II flow cytometer (BD Biosciences). The fluorescence mean value in
P2 from the flow cytometer was collected using FACSDiva software
(version 6.1.3, BD Biosciences), which was used for quantitative
analysis. The data were presented as fold increase normalized to
the control group.
Measurement of mitochondrial membrane
potential (MMP)
MMP was measured using a mitochondrial membrane
potential assay kit with JC-1 (cat. no. M8650; Beijing Solarbio
Science & Technology Co., Ltd.) (29). The treated MFC cells were stained
with JC-1 fluorescence working solution in accordance with the
protocol. MMP of stained cells was measured by FACSCanto II flow
cytometer (BD Biosciences) and analyzed by FACSDiva software
(version 6.1.3, BD Biosciences). The relative ratio of P2 (red
fluorescence) to P3 (green fluorescence) was used for quantitative
analysis of MMP change in MFC cells.
Protein extraction and
quantification
A total of 3×106 MFC cells were
inoculated into 100-mm culture dishes. After overnight culture, the
cells were incubated with luteolin (20 µM) and/or oxaliplatin (5
µM) (total volume 7 ml/well) for 24 h. Then, the cells were rinsed
with PBS and lysed on ice for 30 min with RIPA buffer (cat. no.
R0010; Beijing Solarbio Science & Technology Co., Ltd.)
containing 0.1 M PMSF (cat. no. P0100; Beijing Solarbio Science
& Technology Co., Ltd.) and protease phosphatase inhibitor
(cat. no. P1261; Beijing Solarbio Science & Technology Co.,
Ltd.). The cell lysate was centrifuged at 12,000 × g (Thermo
Scientific™ Sorvall™ Legend™ Micro 21R Microcentrifuge; Thermo
Fisher Scientific, Inc.) for 15 min at 4°C, and the supernatant was
collected for the subsequent tests. The protein quantification was
performed using the BCA protein assay kit (cat. no. PC0020; Beijing
Solarbio Science & Technology Co., Ltd.) (30). The data were obtained at the
absorbance of 562 nm by a microplate reader (Infinite 200 PRO,
Tecan, Tecan Austria GmbH). The amount of protein was calculated in
accordance with the prescribed computational formula of the kit's
protocol.
Western blot assay
In total, 50 µg cell lysates from each group were
loaded per lane and separated by SDS-PAGE (6% spacer gel and 10%
separating gel). Next, cell lysates in the gel were transferred to
PVDF membranes (cat. no. ISEQ00010; MilliporeSigma). The
transferred membranes were blocked with Tris-buffered saline (10 mM
Tris-Cl; pH 7.4), containing 0.5% Tween-20 and 5% skimmed dry milk,
at room temperature for 2 h, and then incubated with primary
antibodies at 4°C overnight (31),
respectively. The primary antibodies used were: β-actin mouse
monoclonal antibody (cat. no. TA-09; 1:2,000; OriGene Technologies,
Inc.), Bcl-2 rabbit polyclonal antibody (cat. no. ab196495,
1:1,000; Abcam), BCL-2-associated X protein (Bax) rabbit monoclonal
antibody (cat. no. ab182734; 1:1,000; Abcam), cyclin A2 rabbit
monoclonal antibody (cat. no. ab181591; 1:2,000; Abcam), cyclin B1
rabbit monoclonal antibody (cat. no. ab32053; 1:1,000; Abcam),
cyclin-dependent kinase-1 (CDK1) rabbit monoclonal antibody (cat.
no. ab133327; 1:20,000; Abcam), tumor necrosis factor
receptor-associated protein 1 (TRAP1) rabbit polyclonal antibody
(cat. no. 10325-1-AP; 1:2,000; ProteinTech Group, Inc.), cell
division cycle 25 homolog C (CDC25C) mouse monoclonal antibody
(cat. no. 66912-1-lg; 1:2,000; ProteinTech Group, Inc.),
extracellular-regulated protein kinases1/2 (ERK1/2) rabbit
polyclonal antibody (cat. no. 9102s; 1:1,000; Cell Signaling
Technology, Inc.) and p-ERK1/2 mouse monoclonal antibody (cat. no.
9106s; 1:1,000; Cell Signaling Technology, Inc.). The membranes
were rinsed with TBST and then probed with appropriate secondary
antibodies (peroxidase-conjugated goat anti-mouse IgG (H+L); cat.
no. ZB-2305; 1:50,000; OriGene Technologies, Inc.; and goat
anti-rabbit IgG H&L; ab6721; cat. no. 1:20,000; Abcam). The
immunoreactive protein bands were visualized with the gel imaging
analysis system (BioSpectrum 510 Imaging System Motorized
Platform). Scanning gray analysis was analyzed by Photoshop CC 2019
software (Adobe Systems Inc.). The grayscale value of each band was
used to plot histograms.
Statistical analysis
The experiments were performed at least 3 times.
Data were analyzed using the SPSS 21.0 software package (version
21.0, SPSS Inc.), and presented as mean ± standard deviation (SD).
Scanning gray analysis was calculated by Photoshop CC software
(Adobe Systems Inc.) Statistical differences were calculated using
ANOVA followed by Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Inhibitory effects of luteolin and/or
oxaliplatin on MFC cell viability
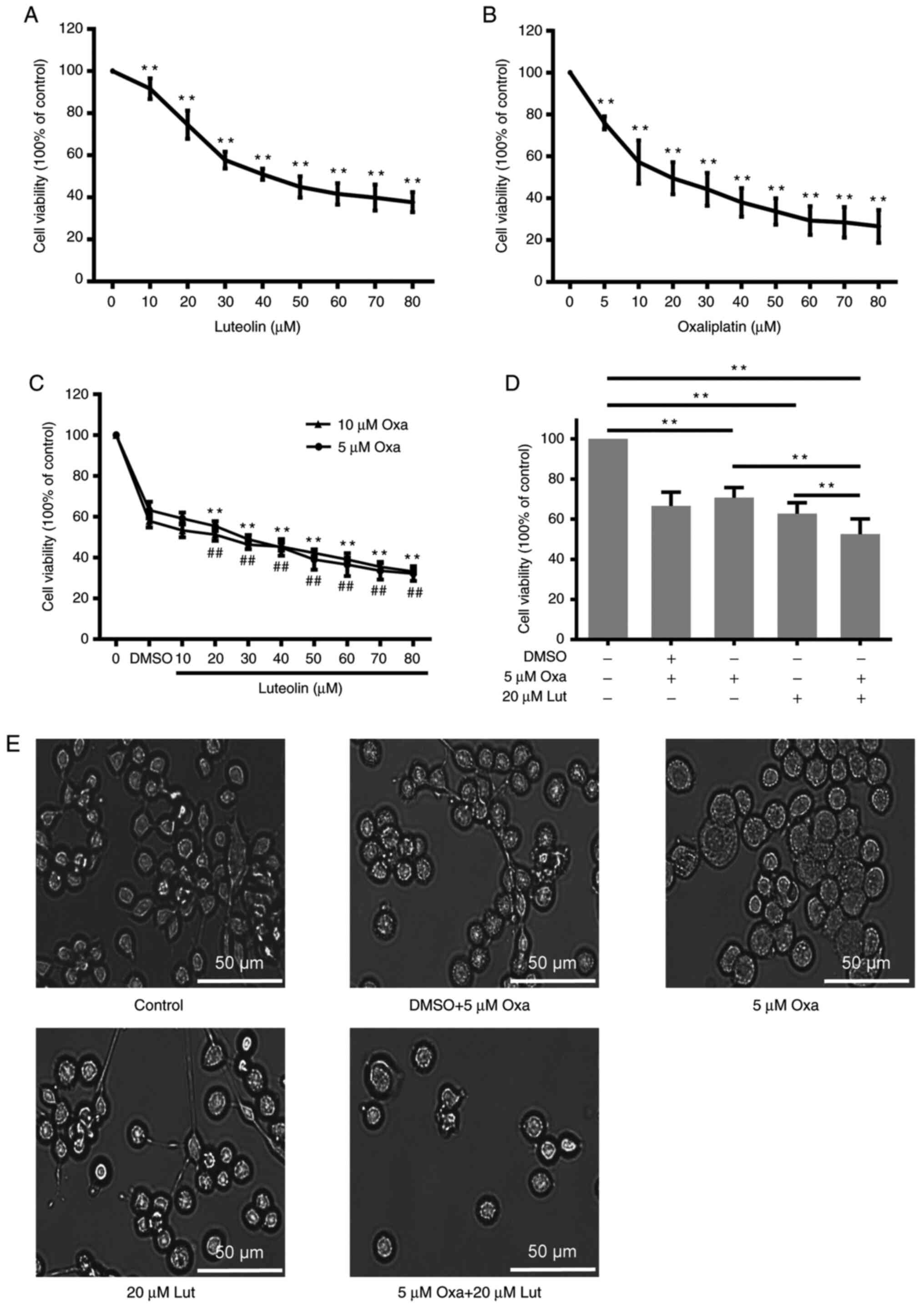
To investigate the inhibitory effects of luteolin
and/or oxaliplatin on mouse forestomach carcinoma MFC cells, CCK-8
assay was performed on MFC cells exposed to a series of luteolin
(10, 20, 30, 40, 50, 60, 70, and 80 µM) and oxaliplatin
concentrations (5, 10, 20, 30, 40, 50, 60, 70, and 80 µM) for 24 h.
Luteolin and oxaliplatin effectively exerted inhibitory effects on
MFC cells proliferation in a dose-dependent manner (Fig. 1A and B). Based on the cell
viability curve, 50 and 20 µM were calculated as the
IC50 values of luteolin and oxaliplatin to MFC cells,
respectively. The CI was used to quantitatively depict the
synergism (CI <1), additive effect (CI=1), and antagonism (CI
>1) in the combined treatment (25). DRI is a measure of how many folds
the dose of each drug in a synergistic combination may be reduced
at a given effect level when compared with the doses of each drug
alone. A greater DRI value indicates a greater dose reduction of
the single drug to create the same effect (14). According to the Compusyn analysis
in Table I, the CI of luteolin (20
µM) and oxaliplatin (5 µM) was 0.801, suggesting that luteolin and
oxaliplatin exerted synergic effects on inhibiting MFC cell
proliferation. DRIs for luteolin (20 µM) and oxaliplatin (5 µM) was
>1, indicating that the dose reduction led to toxicity reduction
in the therapeutic applications (25). The data from CCK-8 assays showed
that the combined treatment with luteolin (20 µM) and oxaliplatin
(5 µM) exerted a much stronger anticancer effect than the
monotherapies (Fig. 1C).
Oxaliplatin at 10 µM did not exhibit much stronger inhibitory
effects on cell proliferation compared with 5 µM oxaliplatin
(Fig. 1D). The morphological
change was observed under a light microscope, showing that the cell
number was more markedly reduced after the combined treatment than
after the monotherapy (Fig.
1E).
 | Table I.Analysis of luteolin and
oxaliplatina. |
Table I.
Analysis of luteolin and
oxaliplatina.
|
|
| Combined
treatment | Drug alone | DRI |
|---|
| Fa | CI | Luteolin (µM) | Oxaliplatin
(µM) | Luteolin (µM) | Oxaliplatin
(µM) | Luteolin | Oxaliplatin |
|---|
| 0.403 | 0.703 | 10 | 5 | 35.080 | 11.956 | 3.508 | 2.391 |
| 0.456 | 0.801 | 20 | 5 | 41.048 | 15.927 | 2.052 | 3.185 |
| 0.523 | 0.821 | 30 | 5 | 49.878 | 22.729 | 1.663 | 4.546 |
| 0.580 | 0.840 | 40 | 5 | 58.967 | 30.853 | 1.474 | 6.171 |
| 0.645 | 0.808 | 50 | 5 | 71.955 | 44.373 | 1.439 | 8.875 |
| 0.675 | 0.851 | 60 | 5 | 79.288 | 52.973 | 1.321 | 10.595 |
| 0.697 | 0.902 | 70 | 5 | 85.389 | 60.649 | 1.220 | 12.130 |
| 0.706 | 0.986 | 80 | 5 | 88.094 | 64.202 | 1.101 | 12.840 |
G2/M phase arrest induced
by luteolin and/or oxaliplatin in MFC cells
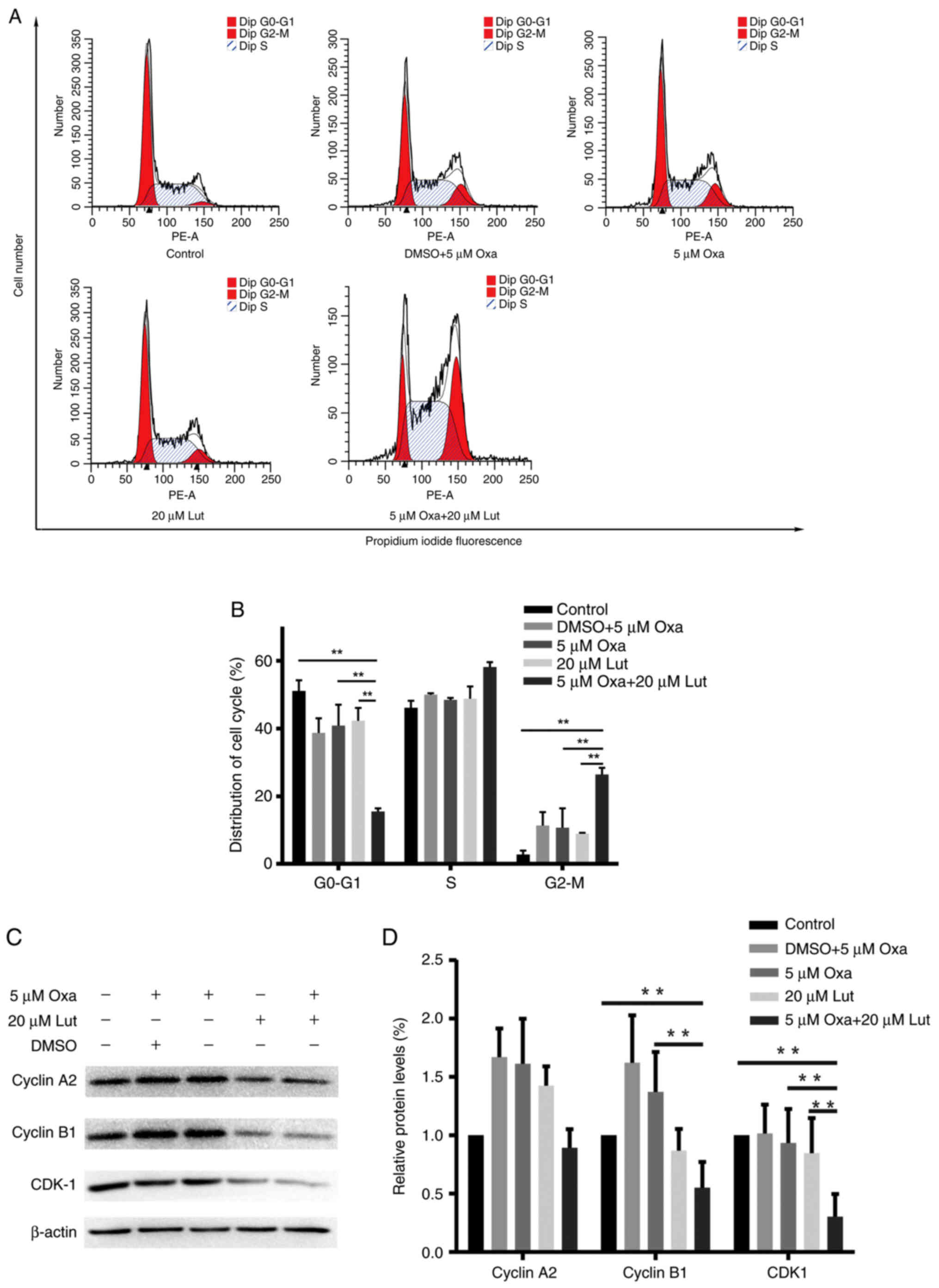
Cell cycle arrest is one of the main causes of the
inhibition of cell proliferation (1,32).
We examined whether cell cycle arrest had an impact on the combined
treatment. MFC cells were treated with luteolin (20 µM) and/or
oxaliplatin (5 µM) for 24 h and stained with PI prior to flow
cytometry analysis. As shown in Fig.
2A, the combined treatment significantly induced
G2/M phase arrest and exhibited much stronger effects on
G2/M phase arrest than any other single drug did
(Fig. 2B). Next, western blotting
was used to test the expression levels of the key proteins related
to G2/M phase arrest in the treated MFC cells. The
combined therapy of MFC cells markedly reduced the expression
levels of cyclin B1, and CDK1 compared with controls and any other
monotherapy (Fig. 2C and D). These
data indicated that luteolin and oxaliplatin effectively induced
MFC cell cycle arrest by downregulating the expression levels of
certain key proteins, such as cyclin B1 and CDK1. The combined
treatment had significant effects on triggering G2/M
phase arrest (P<0.01). Thus, the combined treatment with
luteolin and oxaliplatin possibly suppressed cell proliferation via
inducing G2/M phase arrest.
Key signaling protein affected by
luteolin and/or oxaliplatin in MFC cells
TRAP1, ERK1/2, and CDC25C are closely related to
G2/M cell cycle arrest and apoptosis (33,34).
Thus, the changes in expression levels of TRAP1, P-ERK1/2/ERK1/2,
and CDC25C proteins in MFC cells treated with luteolin and/or
oxaliplatin as mentioned before were examined by western blot
analysis. As shown in Fig. 3, the
combined treatment reduced the expression levels of TRAP 1
(Fig. 3A), P-ERK1/2 (Fig. 3B) and CDC25C (Fig. 3C) protein more effectively than the
monotherapies. Compared with the control and oxaliplatin group, the
combined treatment significantly reduced the TRAP1 and CDC25C
proteins expressions (*P<0.05). The combined treatment also
significantly downregulated ERK1/2 phosphorylation, thereby
suppressing ERK1/2 activation. Thus, the combined treatment with
luteolin and oxaliplatin induced G2/M cell cycle arrest
through suppression of TRAP 1/P-ERK1/2/CDC25C in MFC cells.
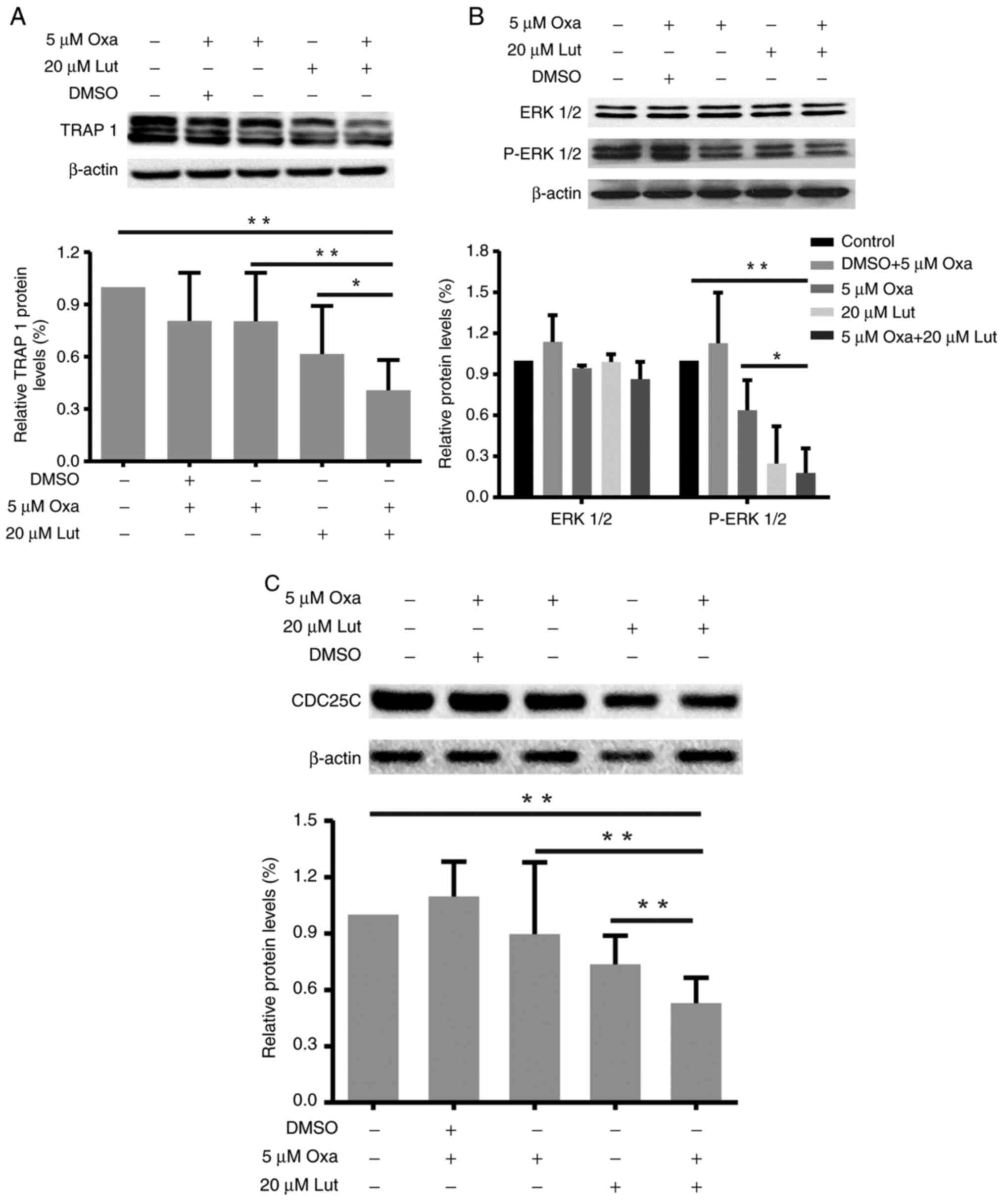 | Figure 3.Changes in TRAP1, P-ERK1/2/ERK1/2 and
CDC25C protein expression levels in mouse forestomach carcinoma
cells induced by luteolin (20 µM) and/or oxaliplatin (5 µM). (A-C)
TRAP1, P-ERK1/2/ERK1/2, CDC25C and β-actin protein expression
levels were assessed by western blot analysis, and quantitative
analysis of protein expression levels is shown in the histogram.
*P<0.05, **P<0.01. Experiments were repeated at least in
triplicate. Lut, luteolin; Oxa, oxaliplatin; TRAP1, tumor necrosis
factor receptor-associated protein 1; ERK1/2,
extracellular-regulated protein kinases1/2; CDC25C, cell division
cycle 25 homolog C. |
Apoptosis induced by luteolin and/or
oxaliplatin in MFC cells
The artificial regulation of apoptosis remains a
considerable focus of attention in cancer treatment (35). Thus, whether apoptosis was involved
in anticancer activities of luteolin and/or oxaliplatin was
examined. MFC cells were exposed to luteolin (20 µM) and/or
oxaliplatin (5 µM), and stained by Hoechst-33258 staining. Images
captured from a fluorescence inversion microscope system showed
that the combined treatment group manifested a more apparent
apoptotic morphology, such as karyopyknosis and chromosome
condensation, than other groups (Fig.
4A). By contrast, in the control group, the cells were still in
the process of mitosis. The quantitative analysis was used to
measure the apoptotic rate by Annexin-V FITC/PI double staining and
flow cytometry. The percentage of apoptotic cells in the combined
therapy group was much higher than that in the control group
(Fig. 4B and C), but for 20 µM
luteolin and 5 µM oxaliplatin as monotherapies, the result was not
significant compared with the control. Usually, the balance between
proapoptotic and antiapoptotic protein regulators is a critical
point to determine whether a cell undergoes apoptosis (36). Thus, the protein expression levels
of Bcl-2 and Bax were assessed by western blot analysis (Fig. 4D). In accordance with the results
of flow cytometry, the ratio of Bcl-2/Bax was markedly lower in the
combined group (Fig. 4E). Thus,
the combined treatment exerted stronger effects on inducing
apoptosis in MFC cells.
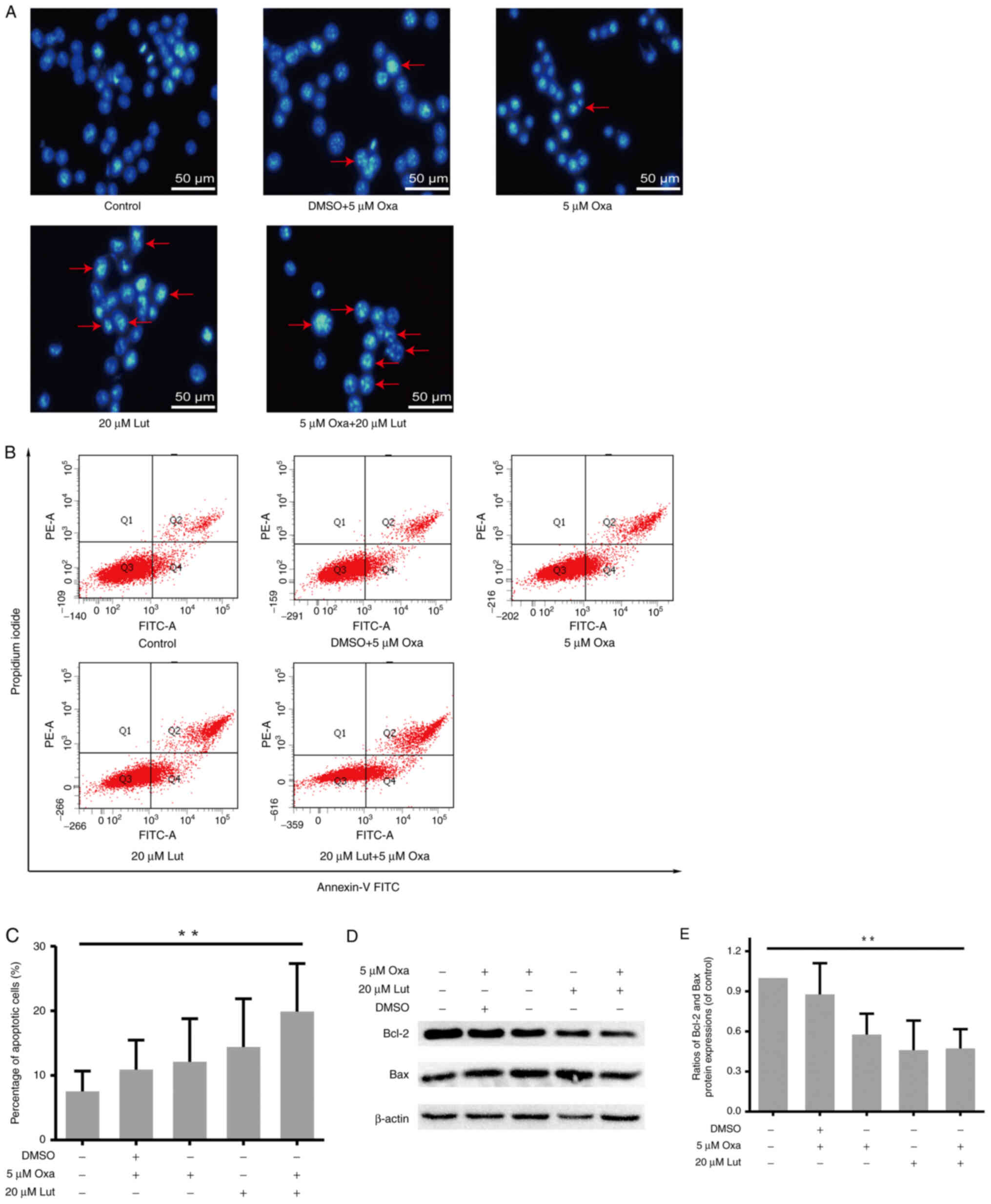 | Figure 4.MFC cell apoptosis induced by
luteolin (20 µM) and/or oxaliplatin (5 µM). (A) The morphological
changes indicative of MFC cell apoptosis were assessed by
fluorescence inverted microscopy (magnification, ×200). (B and C)
After double-staining with Annexin-V FITC and PI, flow cytometry
was used for the quantitative analysis of MFC cell apoptosis. (D)
Protein expression levels (Bcl-2, Bax, and β-actin) were assessed
by western blot analysis. (E) Scanning gray analysis was calculated
by Photoshop CC software (Adobe Systems Inc.). Data are presented
as mean ± SD, **P<0.01. Experiments were repeated at least 3
times. MFC, mouse forestomach carcinoma; Lut, luteolin; Oxa,
oxaliplatin; Bax, BCL-2-associated X protein; Bcl-2, B-cell
lymphoma 2. |
ROS and mitochondrial membrane
potential influenced by luteolin and/or oxaliplatin in MFC
cells
ROS are the primary secondary messengers, and are
involved in many biological processes, including apoptosis and cell
cycle (37). Therefore, the role
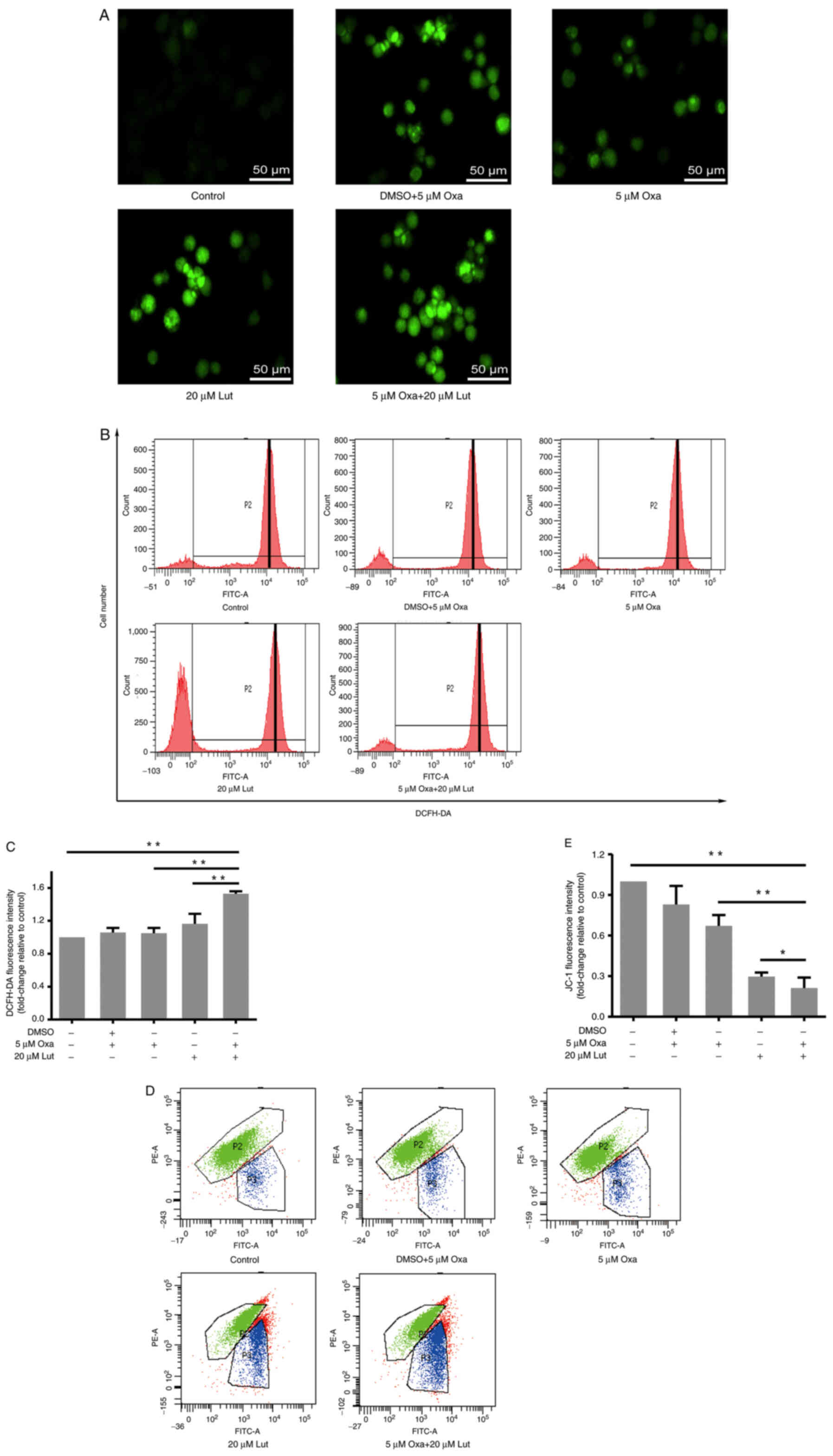
of ROS in these processes was examined. DCFH-DA staining and flow
cytometry were performed to examine ROS accumulation in all of the
groups. Based on the fluorescence inversion microscope system,
stronger green fluorescence was evident in the combined group than
in the other groups (Fig. 5A). The
results from the flow cytometry showed that P2 peaks of the drug
groups moved to the right compared with the control group, and
quantitative analysis verified that the combined treatment was able
to more effectively induce ROS accumulation in MFC cells than any
monotherapy (Fig. 5B and C). Thus,
the combined treatment exerted stronger effects on inducing ROS
accumulation in MFC cells. Mitochondria are the main cell
organelles where ROS are generated. MMP becomes abnormal when ROS
generation exceeds the threshold value, and mitochondrial function
is damaged by ROS over-capacity (37). JC-1 fluorescence staining and flow
cytometry were applied to examine the MMP change of MFC cells
exposed to drug treatments. The results showed that the combined
treatment significantly induced MMP reduction, compared with any
monotherapy (Fig. 5D and E). Thus,
it was suggested that luteolin and oxaliplatin jointly exerted
destructive effects on MMP and destroyed mitochondrial function in
MFC cells.
Discussion
To verify the enhanced effects of luteolin on
efficiency of low-dose oxaliplatin for inhibiting tumor growth, we
examined the combined effects of luteolin and oxaliplatin in MFC
cells and revealed the underlying mechanism. First of all, the
results showed that the combination of luteolin and oxaliplatin
significantly inhibited MFC cell proliferation, which was even more
effective than any monotherapy. According to the Chou-Talalay
method, the CI was much lower than 1, indicating that the combined
treatment of luteolin and oxaliplatin exerted synergistic effects
on reducing MFC cell viability. Throughout all of the experiments,
luteolin did not separate from oxaliplatin solution, suggesting
that it had excellent stability and compatibility with
oxaliplatin.
The next step involved the manner of inhibiting the
proliferation. Oxaliplatin, a kind of metal compound, is an
alkylating agent extensively applied in the treatment of
gastrointestinal and gynecological cancers. The key of its
anticancer activity lies in the formation of
intrastrand/interstrand DNA cross-links and the impairment of DNA
base pairing, replication, and gene transcription (38). Moreover, luteolin is capable of
blocking cell cycle development and suppressing cancer cell
proliferation by regulating extrinsic and intrinsic signaling
pathways (1,39,40).
Therefore, it is essential to study the effects of the combined
treatment on cell cycle progression in MFC cells. Results of the
present study showed that the percentage of cells at
G2/M cell cycle arrest in the combined treatment group
was much higher than that in any other treatment group. Binding
CDK1 to cyclin B1 is required for the integration of mitochondrial
fission with the onset of G2/M transition (41). Cyclin B1 and CDK1 expression levels
were reduced by luteolin and oxaliplatin, suggesting that
G2/M cell cycle arrest played a significant role in
inhibiting cancer cell proliferation by luteolin and oxaliplatin.
Cell cycle is a highly ordered biological event, which is
stringently controlled by cyclins and cyclin-dependent kinases
(CDKs). By translocating between cytoplasm and nucleus, cyclin
B1/CDK1 is involved in the regulation of the entry into mitosis,
nuclear envelope breakdown, and centrosome separation (42). Therefore, the combined treatment
with luteolin and oxaliplatin is more likely to suppress the cyclin
B1/CDK1 complex expression and to interfere with mitochondrial
function, leading to G2/M cell cycle arrest and
apoptosis. The combined treatment induced G2/M cell
cycle arrest by reducing cyclin B1/CDK1, rather by interfering with
cyclin A2. In mitotic cell cycles, cyclin A2 begins to accumulate
in the S phase and continues to increase from the S phase to late
G2 phase before peaking in early prometaphase (43). The combined treatment with luteolin
and oxaliplatin acted on the transition from G2-M phase,
which did not depend on cyclin A2. This suggests that cyclin A2 is
not the target of the combined treatment with luteolin and
oxaliplatin in MFC cells.
TRAP1, P-ERK1/2, and CDC25C protein expression
levels were subsequently assessed. The results showed that they
were inhibited by the combined treatment with luteolin and
oxaliplatin much more effectively than in any single drug group.
Luteolin and oxaliplatin inhibited TRAP1 expression. TRAP1 is a
molecular chaperone and belongs to the heat shock protein 90
(Hsp90) family. It is closely related to the occurrence and
development of various tumors and is involved in anti-apoptosis and
drug resistance, cell cycle progression, cell metabolism, and
quality control of specific client proteins (33,44).
TRAP1 silencing induces the attenuation of ERK phosphorylation,
inhibition of cell cycle progression with cell accumulation in
G0-G1 and G2-M transitions,
extensive reprogramming of genes involved in cell cycle regulation,
and loss of the stem-like signature (2,33).
ERK1/2 is involved in the regulation of various cellular processes,
including cell proliferation, migration, growth, differentiation,
and tumor progression (45). In
addition, inactivation of p-ERK1/2 is involved in cell apoptosis
induced by pharmacological intervention (46). CDC25C is a novel MAPK ERK1/2
target. ERK1/2 promotes CDC25C ubiquitination and proteasomal
degradation, and CDC25C proteolysis is required for sustained
G2 phase arrest (47).
It has also been reported that p38MAPK-ERK-JNK signal transduction
participates in cell cycle arrest, which has been attributed to
nuclear inactivation and degradation of CDC25C (48). The dual-specificity phosphatase
CDC25C plays an important role in the regulation of cell cycle
progression. CDC25C, as a pivotal upstream regulator of cyclin
B1/CDK1, is responsible for the promotion of G2/M phase
transition by triggering CDK1 dephosphorylation to activate the
cyclin B1/CDK1 complex (47).
CDC25C mainly localizes in the cytoplasm and enters the nucleus to
activate the cyclin B1/CDK1 complex before mitosis (48). Results of the western blot analysis
revealed that luteolin and oxaliplatin in MFC cells reduced the
expression of CDC25C and prevented it from activating the cyclin
B1/CDK1 complex, thereby limiting the G2/M transition
and delaying the mitotic process. In addition, it has also been
reported that TRAP1 regulates cell cycle arrest by modulating the
expression and/or the ubiquitination of key cell cycle regulators,
such as CDK1 and cyclin B1. The dual mechanism involves the
transcriptional regulation of key proteins and post-transcriptional
quality control by inducing the ubiquitination of key proteins
enhanced upon TRAP1 downregulation (49). Thus, luteolin and oxaliplatin are
more likely to induce TRAP1/ERK1/2/CDC25C downregulation, leading
to cyclin B1/CDK1 inhibition, and ultimately triggering
G2/M cell cycle arrest.
Generally, the uncontrolled proliferation of cancer
cells is not only related to the disorder of cell cycle
progression, but also to the abnormality of apoptosis (50). Therefore, increasing apoptosis is
considered as a promising method of cancer treatment. In the
present study, the combination of luteolin and oxaliplatin
effectively induced apoptosis, much more markedly than the single
drug treatments. Therefore, apoptosis may be indispensable to the
impact of luteolin and oxaliplatin on inhibiting MFC cell
proliferation. Moreover, ROS participates in many biological
events, including apoptosis (37).
Owing to the structure of phenolic hydroxyl, luteolin may
effectively regulate the oxidation-reduction state by interfering
with cellular ROS levels (1).
Under the combined treatment, ROS significantly increased, and
mitochondrial potential significantly decreased, suggesting that
the combined treatment damaged mitochondria. ROS levels in cancer
cells are normally much higher than those in normal cells; luteolin
and oxaliplatin treatment further increased ROS above the
steady-state level associated with homeostatic function, which is a
lethal threshold for cancer cells (51). More interestingly, oxaliplatin did
not effectively exert destructive effects on ROS and the
mitochondrial potential; instead, adding luteolin markedly enhanced
them, suggesting that the combined treatment with luteolin and
oxaliplatin has a different mechanism from the single drug.
Previous reports have suggested that the mechanism of anticancer
effects of luteolin involves ROS-mediated mitochondrial targeting
(52,53). Therefore, a mitochondria-related
pathway may participate in the anticancer effects of luteolin and
oxaliplatin in MFC cells. Interactions between pro- and
anti-apoptotic members of the Bcl-2 protein family highly control
mitochondrial outer membrane integrity (54). The expression of Bcl-2 and Bax
proteins were interfered by the combined treatment. On intrinsic
apoptotic stimuli, such as DNA damage and oxidative stress, Bcl-2
homology 3 (BH3)-only proteins were activated, leading to Bax and
Bcl-2 antagonist or killer (BAK) activation and mitochondrial outer
membrane permeabilization (MOMP) (55,56).
However, the anti-apoptotic protein Bcl-2 could prevent MOMP by
binding BH3-only proteins and reversing BAX or BAK activation
(55). Therefore, we inferred that
the combination treatment may cause MOMP via regulating Bcl-2 and
Bax proteins and destroying the integrity of the outer
mitochondrial membrane, thereby inducing apoptosis by impairing
mitochondrial membrane potential and ROS balance. Notably, cyclin
B1/CDK1 is pivotal in regulating mitochondrial metabolism in
tumors. Among all the subunits of mitochondrial respiration chain
(complex I–V), 12 subunits, including eight complex I subunits
(NADH ubiquinone oxidoreductase), can be potentially phosphorylated
by cyclin B1/CDK1, indicating that cyclin B1/CDK1 is involved in
controlling ATP output and triggering ROS generation (41,57).
In conclusion, luteolin and oxaliplatin exerted
synergistic effects in inhibiting MFC cell proliferation by
inducing G2/M cell cycle arrest and apoptosis.
Inhibition of the TRAP1/P-ERK1/2/CDC25C/CDK1/cyclin B1 pathway was
indispensable to the induction of G2/M cell cycle
arrest. In addition, the combined therapy significantly interfered
with oxidative balance and MMP, and regulated Bax and Bcl-2 protein
expression levels, thereby leading to apoptosis. Therefore, our
findings indicated that luteolin potentiated low-dose
oxaliplatin-induced inhibitory effects on proliferation in MFC
cells. Findings of the present study may therefore provide the
theoretical basis for the use of luteolin in clinical practice in
the future.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science
Foundation of China (grant no. 31471338), the Key Research and
Development Program of Shandong Province of China (grant no.
2019GSF108214) and the Natural Science Foundation of Shandong
Province (grant no. ZR2016HB51).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JM conceived the experiments and was responsible for
writing the manuscript. JM and XC conducted and completed the
experiments. XZ and ZP interpreted and analyzed the data. DL and WH
were responsible for data interpretation and revised the final
version of the manuscript. QZ and XT participated into study design
and provided funding. JM and XC confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TRAP1
|
tumor necrosis factor
receptor-associated protein 1
|
|
ERK1/2
|
extracellular-regulated protein
kinases1/2
|
|
CDC25C
|
cell division cycle 25 homolog C
|
|
CDK1
|
cyclin-dependent kinase-1
|
|
Bax
|
BCL-2-associated X protein
|
|
Bcl-2
|
B-cell lymphoma 2
|
|
MFC
|
mouse forestomach carcinoma
|
References
|
1
|
Imran M, Rauf A, Abu-Izneid T, Nadeem M,
Shariati MA, Khan IA, Imran A, Orhan IE, Rizwan M, Atif M, et al:
Luteolin, a flavonoid, as an anticancer agent: A review. Biomed
Pharmacother. 112:1086122019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lettini G, Maddalena F, Sisinni L,
Condelli V, Matassa DS, Costi MP, Simoni D, Esposito F and
Landriscina M: TRAP1: A viable therapeutic target for future cancer
treatments? Expert Opin Ther Targets. 21:805–815. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Limagne E, Thibaudin M, Nuttin L, Spill A,
Derangère V, Fumet JD, Amellal N, Peranzoni E, Cattan V and
Ghiringhelli F: Trifluridine/tipiracil plus oxaliplatin improves
PD-1 blockade in colorectal cancer by inducing immunogenic cell
death and depleting macrophages. Cancer Immunol Res. 7:1958–1969.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patel TH and Cecchini M: Targeted
therapies in advanced gastric cancer. Curr Treat Options Oncol.
21:702020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei TT, Lin YT, Tang SP, Luo CK, Tsai CT,
Shun CT and Chen CC: Metabolic targeting of HIF-1α potentiates the
therapeutic efficacy of oxaliplatin in colorectal cancer. Oncogene.
39:414–427. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujita S, Hirota T, Sakiyama R, Baba M and
Ieiri I: Identification of drug transporters contributing to
oxaliplatin-induced peripheral neuropathy. J Neurochem.
148:373–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao P, Xia Y, He W, Zhang T, Hong L, Zheng
P, Shen X, Liang G, Cui R and Zou P: Enhancement of
oxaliplatin-induced colon cancer cell apoptosis by alantolactone, a
natural product inducer of ROS. Int J Biol Sci. 15:1676–1684. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riddell IA: Cisplatin and oxaliplatin: Our
current understanding of their actions. Met Ions Life Sci.
18:2018.PubMed/NCBI
|
|
9
|
Stankovic JSK, Selakovic D, Mihailovic V
and Rosic G: Antioxidant supplementation in the treatment of
neurotoxicity induced by platinum-based chemotherapeutics - a
review. Int J Mol Sci. 21:77532020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao LX, Chen ZQ, Jiang Z, Chen QC, Fan XH,
Xia SJ, Lin JX, Gan HC, Wang T and Huang YX: Rapid rehabilitation
technique with integrated traditional Chinese and Western medicine
promotes postoperative gastrointestinal function recovery. World J
Gastroenterol. 26:3271–3282. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee YC, Chen YH, Huang YC, Lee YF and Tsai
MY: Effectiveness of combined treatment with traditional Chinese
medicine and Western medicine on the prognosis of patients with
breast cancer. J Altern Complement Med. 26:833–840. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang WR, Yang SH, Yu CT, Wang CC, Huang
ST, Huang TH, Chiang MC and Chang YC: Long-term effectiveness of
combined treatment with traditional Chinese medicine and Western
medicine on the prognosis of patients with lung cancer. J Altern
Complement Med. 22:212–222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martinez-Torres AC, Reyes-Ruiz A,
Benítez-Londoño M, Franco-Molina MA and Rodriguez-Padilla C:
IMMUNEPOTENT CRP induces cell cycle arrest and caspase-independent
regulated cell death in HeLa cells through reactive oxygen species
production. BMC Cancer. 18:132018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren B, Ye L, Gong J, Ren H, Ding Y, Chen
X, Liu X, Lu P, Wei F, Xu W, et al: Alteronol enhances the
anti-tumor activity and reduces the toxicity of high-dose
adriamycin in breast cancer. Front Pharmacol. 10:2852019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pu Y, Zhang T, Wang J, Mao Z, Duan B, Long
Y, Xue F, Liu D, Liu S and Gao Z: Luteolin exerts an anticancer
effect on gastric cancer cells through multiple signaling pathways
and regulating miRNAs. J Cancer. 9:3669–3675. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu J, Xu H, Yu Y, He Y, Liu Q and Yang B:
Combination of luteolin and solifenacin improves urinary
dysfunction induced by diabetic cystopathy in rats. Med Sci
Monitor. 24:1441–1448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park S, Kim DS, Kang S and Kim HJ: The
combination of luteolin and l-theanine improved Alzheimer
disease-like symptoms by potentiating hippocampal insulin signaling
and decreasing neuroinflammation and norepinephrine degradation in
amyloid-β-infused rats. Nutr Res. 60:116–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hashemzaei M, Abdollahzadeh M, Iranshahi
M, Golmakani E, Rezaee R and Tabrizian K: Effects of luteolin and
luteolin-morphine co-administration on acute and chronic pain and
sciatic nerve ligated-induced neuropathy in mice. J Complement
Integr Med. 14:2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chakrabarti M and Ray SK: Anti-tumor
activities of luteolin and silibinin in glioblastoma cells:
Overexpression of miR-7-1-3p augmented luteolin and silibinin to
inhibit autophagy and induce apoptosis in glioblastoma in vivo.
Apoptosis. 21:312–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng XQ, Rong LW, Wang RX, Zheng XL, Zhang
L, Zhang L, Lin Y, Wang X and Li ZP: Luteolin and sorafenib
combination kills human hepatocellular carcinoma cells through
apoptosis potentiation and JNK activation. Oncol Lett. 16:648–653.
2018.PubMed/NCBI
|
|
21
|
Soliman NA, Abd-Ellatif RN, ELSaadany AA,
Shalaby SM and Bedeer AE: Luteolin and 5-flurouracil act
synergistically to induce cellular weapons in experimentally
induced solid ehrlich carcinoma: Realistic role of P53; a guardian
fights in a cellular battle. Chem Biol Interact. 310:1087402019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu Y, Liu R, Shen Z and Cai G:
Combination of luteolin and lycopene effectively protect against
the ‘two-hit’ in NAFLD through Sirt1/AMPK signal pathway. Life Sci.
256:1179902020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang HH and Guo XL: Combinational
strategies of metformin and chemotherapy in cancers. Cancer
Chemother Pharmacol. 78:13–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao XQ, Tang H, Yang J, Gu XY, Wang SM
and Ding Y: MicroRNA-15a-5p down-regulation inhibits cervical
cancer by targeting TP53INP1 in vitro. Eur Rev Med Pharmacol Sci.
23:8219–8229. 2019.PubMed/NCBI
|
|
25
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bressy C, Droby GN, Maldonado BD,
Steuerwald N and Grdzelishvili VZ: Cell cycle arrest in
G2/M phase enhances replication of interferon-sensitive
cytoplasmic RNA viruses via inhibition of antiviral gene
expression. J Virol. 93:e01885–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gong Y, Luo S, Fan P, Zhu H, Li Y and
Huang W: Growth hormone activates PI3K/Akt signaling and inhibits
ROS accumulation and apoptosis in granulosa cells of patients with
polycystic ovary syndrome. Reprod Biol Endocrinol. 18:1212020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang X, Qin Y, Pan Z, Li M, Liu X, Chen
X, Qu G, Zhou L, Xu M, Zheng Q and Li D: Cannabidiol induces cell
cycle arrest and cell apoptosis in human gastric cancer SGC-7901
cells. Biomolecules. 9:3022019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Si L, Yan X, Wang Y, Ren B, Ren H, Ding Y,
Zheng Q, Li D and Liu Y: Chamaejasmin B decreases malignant
characteristics of mouse melanoma B16F0 and B16F10 cells. Front
Oncol. 10:4152020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu X, Yang Z, Liu W, Pan Z, Zhang X, Li M,
Liu X, Zheng Q and Li D: The anti-tumor effects of p-coumaric acid
on melanoma A375 and B16 cells. Front Oncol. 10:5584142020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choi YH: Isorhamnetin induces
ROS-dependent cycle arrest at G2/M phase and apoptosis in human
hepatocarcinoma Hep3B cells. Gen Physiol Biophys. 38:473–484. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iida K, Naiki T, Naiki-Ito A, Suzuki S,
Kato H, Nozaki S, Nagai T, Etani T, Nagayasu Y, Ando R, et al:
Luteolin suppresses bladder cancer growth via regulation of
mechanistic target of rapamycin pathway. Cancer Sci. 111:1165–1179.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Palladino G, Notarangelo T, Pannone G,
Piscazzi A, Lamacchia O, Sisinni L, Spagnoletti G, Toti P, Santoro
A, Storto G, et al: TRAP1 regulates cell cycle and apoptosis in
thyroid carcinoma cells. Endocr Relat Cancer. 23:699–709. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie S, Wang X, Gan S, Tang X, Kang X and
Zhu S: The mitochondrial chaperone TRAP1 as a candidate target of
oncotherapy. Front Oncol. 10:5850472020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Obeng E: Apoptosis (programmed cell death)
and its signals-a review. Braz J Biol. 81:1133–1143. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: Function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging (Albany NY). 8:603–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Y, Karakhanova S, Hartwig W, D'Haese
JG, Philippov PP, Werner J and Bazhin AV: Mitochondria and
mitochondrial ROS in cancer: Novel targets for anticancer therapy.
J Cell Physiol. 231:2570–2581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rogers BB, Cuddahy T, Briscella C, Ross N,
Olszanski AJ and Denlinger CS: Oxaliplatin: Detection and
management of hypersensitivity reactions. Clin J Oncol Nurs.
23:68–75. 2019.PubMed/NCBI
|
|
39
|
Chen ZC, Zhang B, Gao F and Shi RJ:
Modulation of G2/M cell cycle arrest and apoptosis by
luteolin in human colon cancer cells and xenografts. Oncol Lett.
15:1559–1565. 2018.PubMed/NCBI
|
|
40
|
Anson DM, Wilcox RM, Huseman ED, Stump TA,
Paris RL, Darkwah BO, Lin S, Adegoke AO, Gryka RJ, Jean-Louis DS
and Amos S: Luteolin decreases epidermal growth factor
receptor-mediated cell proliferation and induces apoptosis in
glioblastoma cell lines. Basic Clin Pharmacol. 123:678–686. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie BW, Wang SY, Jiang N and Li JJ: Cyclin
B1/CDK1-regulated mitochondrial bioenergetics in cell cycle
progression and tumor resistance. Cancer Lett. 443:56–66. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang ZQ, Fan M, Candas D, Zhang TQ, Qin L,
Eldridge A, Wachsmann-Hogiu S, Ahmed KM, Chromy BA, Nantajit D, et
al: Cyclin B1/Cdk1 coordinates mitochondrial respiration for
cell-cycle G2/M progression. Dev Cell. 29:217–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Silva Cascales H, Burdova K, Middleton A,
Kuzin V, Müllers E, Stoy H, Baranello L, Macurek L and Lindqvist A:
Cyclin A2 localises in the cytoplasm at the S/G2 transition to
activate PLK1. Life Sci Alliance. 4:e2020009802021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li XT, Li YS, Shi ZY and Guo XL: New
insights into molecular chaperone TRAP1 as a feasible target for
future cancer treatments. Life Sci. 254:1177372020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shingyochi Y, Kanazawa S, Tajima S, Tanaka
R, Mizuno H and Tobita M: A low-level carbon dioxide laser promotes
fibroblast proliferation and migration through activation of Akt,
ERK, and JNK. PLoS One. 12:e01689372017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu L, Xing YH, Cao MY, Xu JL and Chen JJ:
Exogenous NO induces apoptosis of hepatocellular carcinoma cells
via positive p38/JNK signaling pathway and negative ERK signaling
pathways. Mol Cell Biochem. 476:1651–1661. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu K, Zheng M, Lu R, Du J, Zhao Q, Li Z,
Li Y and Zhang S: The role of CDC25C in cell cycle regulation and
clinical cancer therapy: A systematic review. Cancer Cell Int.
20:2132020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu K, Lu R, Zhao Q, Du J, Li Y, Zheng M
and Zhang S: Association and clinicopathologic significance of
p38MAPK-ERK-JNK-CDC25C with polyploid giant cancer cell formation.
Med Oncol. 37:62020. View Article : Google Scholar
|
|
49
|
Sisinni L, Maddalena F, Condelli V,
Pannone G, Simeon V, Li Bergolis V, Lopes E, Piscazzi A, Matassa
DS, Mazzoccoli C, et al: TRAP1 controls cell cycle G2-M transition
through the regulation of CDK1 and MAD2 expression/ubiquitination.
J Pathol. 243:123–134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kinnaird A and Michelakis ED: Metabolic
modulation of cancer: A new frontier with great translational
potential. J Mol Med (Berl). 93:127–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hirata T, Cho YM, Suzuki I, Toyoda T,
Akagi JI, Nakamura Y, Numazawa S and Ogawa K:
4-Methylthio-3-butenyl isothiocyanate (MTBITC) induced apoptotic
cell death and G2/M cell cycle arrest via ROS production in human
esophageal epithelial cancer cells. J Toxicol Sci. 44:73–81. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Seydi E, Salimi A, Rasekh HR, Mohsenifar Z
and Pourahmad J: Selective cytotoxicity of luteolin and kaempferol
on cancerous hepatocytes obtained from rat model of hepatocellular
carcinoma: Involvement of ROS-mediated mitochondrial targeting.
Nutr Cancer. 70:594–604. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang Q, Wang H, Jia Y, Pan H and Ding H:
Luteolin induces apoptosis by ROS/ER stress and mitochondrial
dysfunction in gliomablastoma. Cancer Chemother Pharmacol.
79:1031–1041. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Peña-Blanco A and García-Sáez AJ: Bax, Bak
and beyond-mitochondrial performance in apoptosis. FEBS J.
285:416–431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Flores-Romero H, Ros U and Garcia-Saez AJ:
Pore formation in regulated cell death. FEBS J.
39:e1057532020.PubMed/NCBI
|
|
57
|
Shendge AK, Chaudhuri D and Mandal N: The
natural flavones, acacetin and apigenin, induce Cdk-Cyclin mediated
G2/M phase arrest and trigger ROS-mediated apoptosis in
glioblastoma cells. Mol Biol Rep. 48:539–549. 2021. View Article : Google Scholar : PubMed/NCBI
|