Introduction
Lung cancer is one of the most common and lethal
cancer types with a high rate of mortality worldwide (1,2).
Lung cancer accounts for 14% of new cancer cases and has a 26%
mortality rate in males; for females, it accounts for 13% of new
cancer cases and has a 25% mortality rate (3). Non-small-cell lung cancer (NSCLC) is
the primary subtype of lung cancer and is composed of two major
histologic groups: Lung squamous cell carcinoma (LUSC) and lung
adenocarcinoma. Treatment strategies, including surgical resection,
radiotherapy and chemotherapy, have developed rapidly in recent
years, but the overall survival of patients with lung cancer
remains poor (4,5). Biomarkers that may be used to detect
or diagnose LUSC, improve the survival rate and reduce recurrence
are lacking (6). Therefore, it is
necessary to develop novel therapeutic strategies for LUSC.
Long non-coding RNAs (lncRNAs) range from 200
nucleotides to 100 kb in length and are a class of
non-protein-coding RNAs (7,8).
lncRNAs are known to have various roles in disease development,
regulation of metabolism, epigenetic gene control and
transcriptional regulation (9,10).
Over the past decade, lncRNAs have been determined to be widely
involved in the proliferation, apoptosis, invasion and metastasis
of malignancies (11,12). Increasing evidence in lung cancer
has indicated that dysregulated lncRNAs may affect the development
and occurrence of lung cancer by modifying the biological functions
and self-renewal abilities of cancer cells (12–14).
Furthermore, it was reported that dysregulated lncRNAs have a role
in the drug resistance of lung cancer (15,16).
lncRNA γ-butyrobetaine hydroxylase 1
(BBOX1)-antisense 1 (AS1) [National Center of Biotechnology
Information (NCBI) Gene ID: 103695435] is located on chromosome
11p14.2-p14.1. As previously reported, BBOX1-AS1 has a role in the
development of various diseases, including NSCLC, colorectal
cancer, cervical cancer and gastric cancer (17–20).
BBOX1-AS1 was overexpressed in NSCLC and in colorectal, cervical
and gastric cancers. BBOX1-AS1 may also facilitate the
proliferation, invasion and migration of these cancer cells
(17–20). However, the functions of BBOX1-AS1
in LUSC have yet to be studied independently.
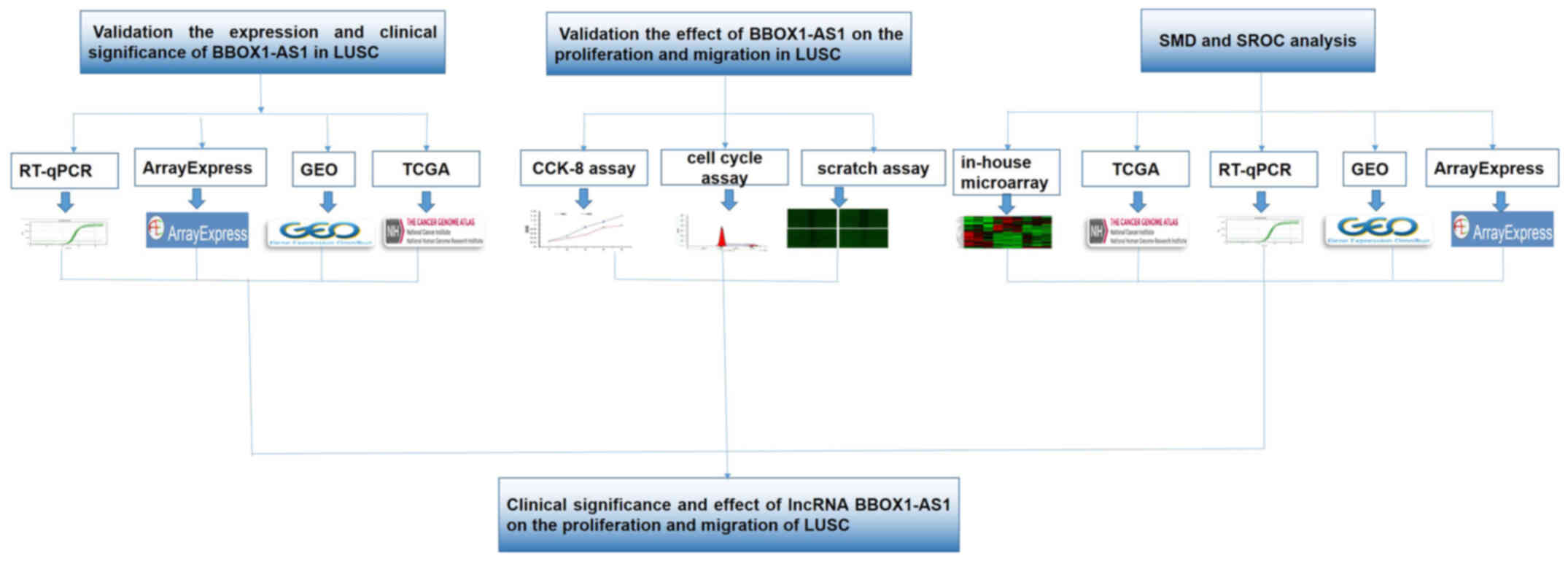
In the present study, the clinical significance and
effect of the differential expression of BBOX1-AS1 in LUSC was
investigated. In vitro assays and in silico analyses
were performed to examine the biological functions and probable
mechanisms of BBOX1-AS1 in LUSC. A flow diagram of the present
study is presented in Fig. 1.
Materials and methods
Patient tissue samples
A total of three pairs of LUSC tissues and normal
lung tissues obtained >5 cm from the edge of the LUSC lesion
were collected between August 2017 and October 2017 from the
Department of Pathology of the First Affiliated Hospital of the
Guangxi Medical University (Nanning, China). The patients' age
ranged from 51 to 58 years. All three patients were definitively
diagnosed with LUSC and underwent surgical resection without any
further treatment. The pathological diagnoses were independently
performed by at least two blinded pathologists. All methods used
adhered to the relevant guidelines and were approved by the Ethical
Committee of the First Affiliated Hospital of Guangxi Medical
University (Nanning, China). Consent forms for the use of tissues
were signed by the doctors and patients involved in the study.
In-house microarray analysis
An in-house microarray analysis was performed to
detect differentially expressed lncRNAs between LUSC and normal
lung tissues. Microarray hybridization and sample analysis were
performed by Kangchen Biotech. The detailed methods were performed
as previously described by Zhang et al (21). The significantly differentially
expressed lncRNAs between LUSC and normal lung tissues were
selected using the criteria of |fold change| ≥2 and P≤0.05.
Similarly, the differentially expressed mRNAs were identified using
microarray analysis.
Validation of the expression and
clinical role of BBOX1-AS1
Expression analysis in clinical samples was
performed using the original RNA sequencing (RNA-seq) data from The
Cancer Genome Atlas (TCGA, cancergenome.nih.gov) database to
determine BBOX1-AS1 expression between LUSC and noncancerous lung
tissues. The LUSC cohort comprised 502 LUSC cases and 49
corresponding noncancerous lung samples. The original expression
data were normalized using the R package DESeq. Differences in the
expression of BBOX1-AS1 between LUSC tissues and normal lung
tissues were identified from the TCGA dataset (22,23).
The relationships of BBOX-AS1 expression and clinicopathological
parameters (age, sex, ethnicity, tumor stage, regional lymph nodes,
distant metastasis and stage) were determined from the TCGA data. A
receiver operating characteristic (ROC) curve was created to
determine the clinical value of BBOX1-AS1 expression in
distinguishing normal and LUAD tissue. Gene Expression Omnibus
(GEO, http://www.ncbi.nlm.nih.gov/geo/) data and the
ArrayExpress (ebi.ac.uk/arrayexpress/) database were used to verify
BBOX1-AS1 expression in LUSC. Kaplan-Meier curve analysis was used
to assess BBOX1-AS1 expression and the 5-year survival rates of
patients with LUSC with an endpoint of death or the end of the
study period.
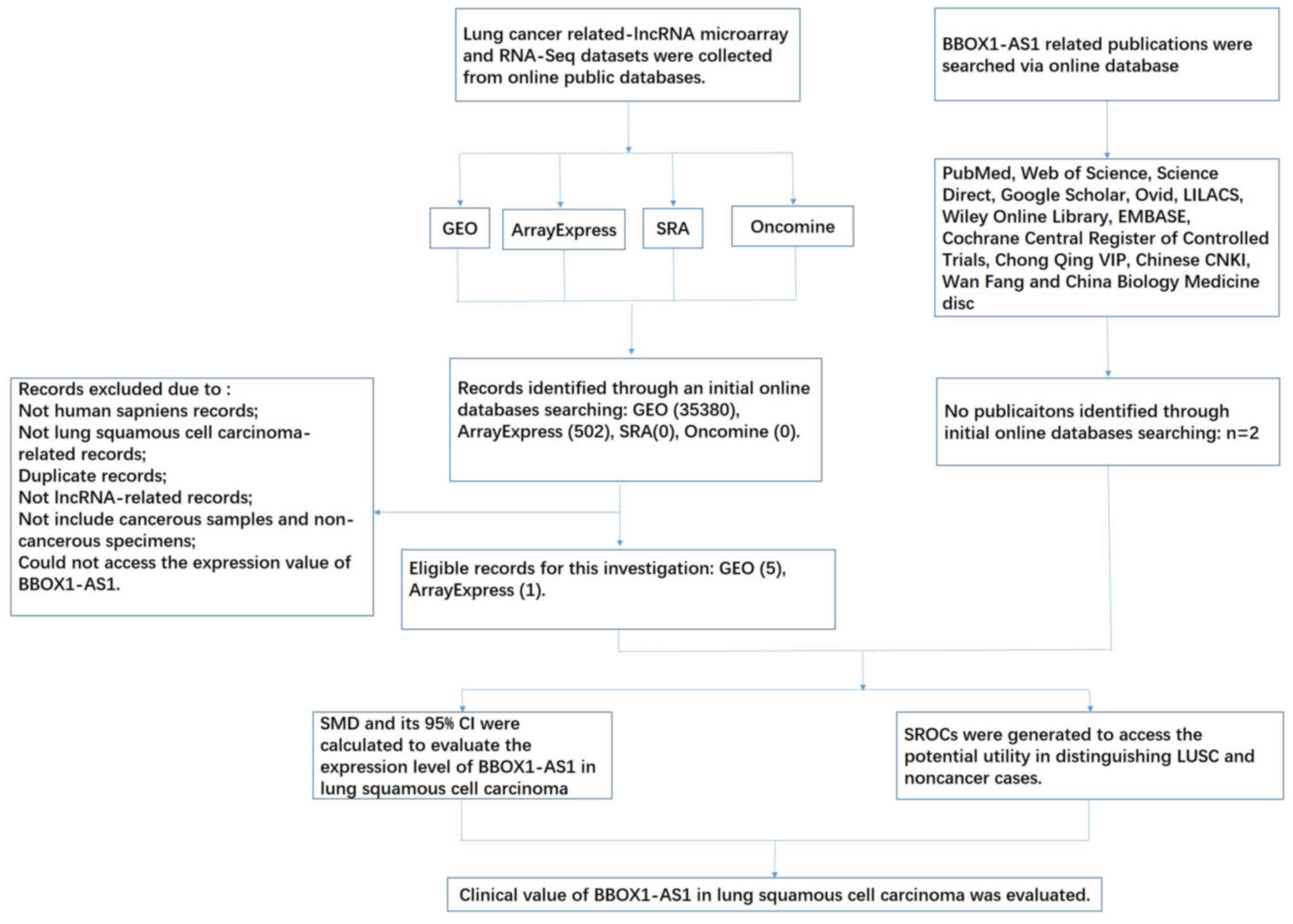
The LUSC-related BBOX1-AS1 microarray and RNA-seq
datasets were downloaded from GEO, ArrayExpress, the TCGA, Sequence
Read Archive (SRA, http://trace.ncbi.nlm.nih.gov/Traces/sra/) and
Oncomine (https://www.oncomine.org/).
Publications related to BBOX1-AS1 in LUSC were also selected from
12 online databases: PubMed, EMBASE, Web of Science, Science
Direct, Cochrane Central Register of Controlled Trials, Google
Scholar, Ovid, Chinese CNKI, LILACS, Wiley Online Library, Wan
Fang, Chong Qing VIP and China Biology Medicine disc. The retrieval
date was set to September 15, 2020, with the following Boolean
search terms: (lung OR pulmonary OR ‘NSCLC’ OR ‘LC’) AND (‘lncRNA’
OR ‘noncoding RNA’ OR ‘noncoding RNA’ OR ‘noncoding RNA’ OR gene).
Two different investigators (YZ and XW) cross-checked the
literature. A group discussion was used to resolve any
disagreement. The number of false positives, true positives, false
negatives and true negatives was extracted as previously reported
(24,25). The standard mean deviation (SMD)
and summary receiver operating characteristic (SROC) curve of
BBOX1-AS1 in LUSC were presented in Stata 14.0 (Stata Corp.). A
fixed-effects model was used to analyze the pooled SMD to determine
the expression of BBOX1-AS1 in LUSC compared to that in the normal
group. The funnel plot used to evaluate the publication bias. The
asymmetry of the funnel plot could display the validity of
conclusions.
Cell culture and transfection with
small interfering (si)RNA
Three human LUSC cell lines (NCI-H1703, NCI-H226 and
SK-MES-1) and human bronchial epithelial cells (BEAS-2B) were
obtained from the American Type Culture Collection. These four cell
lines were cultured in DMEM with 10% heat-inactivated fetal bovine
serum (Invitrogen; Thermo Fisher Scientific, Inc.). All of the
cells were incubated in a humidified atmosphere with 5%
CO2 at 37°C.
RNA interference (RNAi) was performed to determine
the underlying effect of BBOX1-AS1 on biological processes in LUSC.
The lentiviral vector containing siRNA targeting BBOX1-AS1 was
synthesized by GeneChem (sense, 5′-CTGCTTTGCTCTTCAGACTTATT-3′;
antisense, 5′-TAAGTCTGAAGAGCAAAGCAG-3′). The siRNA vectors used to
knock down the BBOX1-AS1 gene (KD) and the negative siRNA controls
(NC; cat. no. CON077, GeneChem) were transfected into the NCI-H1703
and SK-MES-1 cell lines using Lipofectamine 2000®
(Thermo Fisher Scientific, Inc.) when the cells reached 80%
confluency. Puromycin (2 µg/ml; Corning, Inc.; cat. no. 21-031-CVR)
was applied for filtering the stable cell lines at 48 h after
transfection. A fluorescence microscope was used to observe cell
growth after transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from NCI-H1703, NCI-H266, SK-MES-1 and
BEAS-2B cells was isolated using TRIzol reagent (Shanghai Pufei
Biotech Co., Ltd.) and reverse-transcribed using a Promega M-MLV
Kit (Shanghai Pufei Biotech Co., Ltd.). qPCR was performed using
the LightCycler 480 Real-time PCR System (Roche). The specific
primers were synthesized as follows: BBOX1-AS1 forward,
5′-GATGGGCACATTTGGAAGTT-3′ and reverse, 5′-CAGCGTTAGGTTTGGAGTTG-3′;
and GAPDH (internal control) forward, 5′-TGACTTCAACAGCGACACCCA-3′
and reverse, 5′-CACCCTGTTGCTGTAGCCAAA-3′. All experiments were
performed in triplicate. The levels of BBOX1-AS1 expression were
standardized to those of GAPDH and calculated using the delta Cq
method (26). Furthermore, the
transfection efficiency of the lentiviral vector containing
BBOX1-AS1 siRNA was detected via RT-qPCR.
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 assay was performed to examine the
proliferation of LUSC cells in two different groups (KD group and
NC group). Cell viability was determined with a CCK-8 (Beyotime
Institute of Biotechnology) following the manufacturer's
instructions. The detailed methods were performed as previously
described by Zhang et al (27). The viability of NCI-H1703 and
SK-MES-1 cells at days one, two, three, four and five was assessed
and the absorbance at 450 nm was read using a microplate reader
(Infinite M200 Micro Plate Reader; Tecan Group). The fold change in
the optical density at 450 nm was considered to represent changes
in cell viability. Experiments were performed in triplicate.
Flow cytometric cell cycle
analysis
Flow cytometry was used to measure the effect of
BBOX1-AS1 on the cell cycle distribution. The detailed method of
the flow cytometric cell cycle assay was previously described by
Zhang et al (27). Cells in
the different phases of the cell cycle (G1, S and G2) were
identified using FACSCalibur (EMD Millipore) and data analysis was
performed using ModFit 3.2.1 (Verity Software House, Inc.). Each
test was performed in triplicate.
Scratch assay
A scratch assay was performed to observe the
potential effect of BBOX1-AS1-siRNA on the migration of LUSC cells.
The detailed method of the scratch assay was performed as
previously described by Zhang et al (27,28).
Several random fields of the monolayer were captured directly under
a microscope at 0 and 24 h after scratching. All experiments were
performed in triplicate.
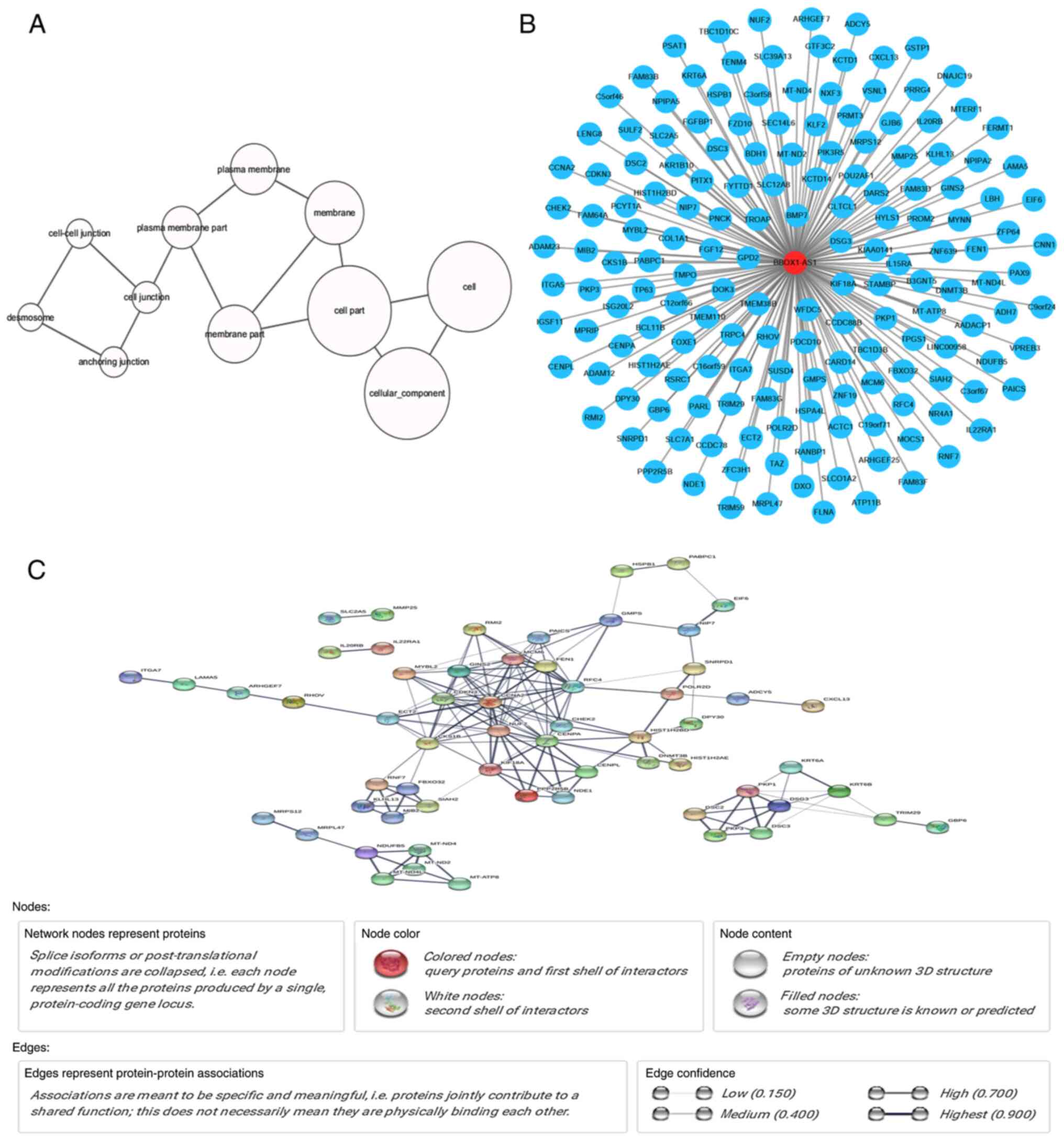
Potential pathways and functions
associated with BBOX1-AS1
Genes associated with BBOX1-AS1 in LUSC were
subjected to Gene Ontology (GO) and Kyoto Encyclopedia of Genes and
Genomes (KEGG) analyses to determine fundamental biological
functions and downstream pathways (29–31).
The genes associated with BBOX1-AS1 in the GEO datasets were
determined using the Psych R programming package. The co-expressed
genes in the TCGA database overlapping with the upregulated mRNAs
in the in-house microarray for bioinformatics analysis were
selected. The Database for Annotation, Visualization and Integrated
Discovery (DAVID; http://david.abcc.ncifcrf.gov/) website was used for
GO and KEGG analyses. The functional terms in the categories
biological process (BP), cellular component (CC) and molecular
function (MF) were obtained from the GO analysis and the GO
functional network was drawn using Cytoscape (v3.5.1, http://cytoscape.org).
The interaction pairs of the co-expressed genes were
determined using the Search Tool for the Retrieval of Interacting
Genes (STRING; version 9.0; http://string-db.org) (32). STRING provides a global database
for numerous organisms and the predicted and known interactions
were scored. The interactional pairs in the protein-protein
interaction (PPI) network were nominated based on a combined score
of >0.4.
Fluorescence in situ hybridization
(FISH)
The FISH assay was performed to determine the
localization of BBOX1-AS1 in the nucleus or cytoplasm of LUSC
tissues. Cy3-labeled BBOX1-AS1 probes were designed and synthesized
by General Biol. Hybridization was performed overnight with
BBOX1-AS1 probes according to the manufacturer's instructions.
Images were collected using a Leica DM 4000B laser scanning
confocal microscope (Leica Instrument, Inc.). The sequence of the
BBOX1-AS1 probe for FISH was CAGGGTAACCGTAG
CATGACCTAGAAATAGTCCTCTCA. All experiments were performed at least
three times.
Statistical analysis
Statistical analysis was performed using SPSS v22.0
(IBM Corporation). Student's t-test was used to compare the
differences between the two groups and the Kruskal-Wallis H-test
was employed to evaluate differences among >2 groups. Spearman's
rank correlation was utilized to evaluate the correlation between
BBOX1-AS1 expression and clinicopathological features. A ROC curve
was plotted to distinguish LUSC from normal lung tissues by gene
expression. A two-sided P<0.05 was considered to indicate
statistical significance.
Results
Differentially expressed lncRNAs via
in-house microarray analysis
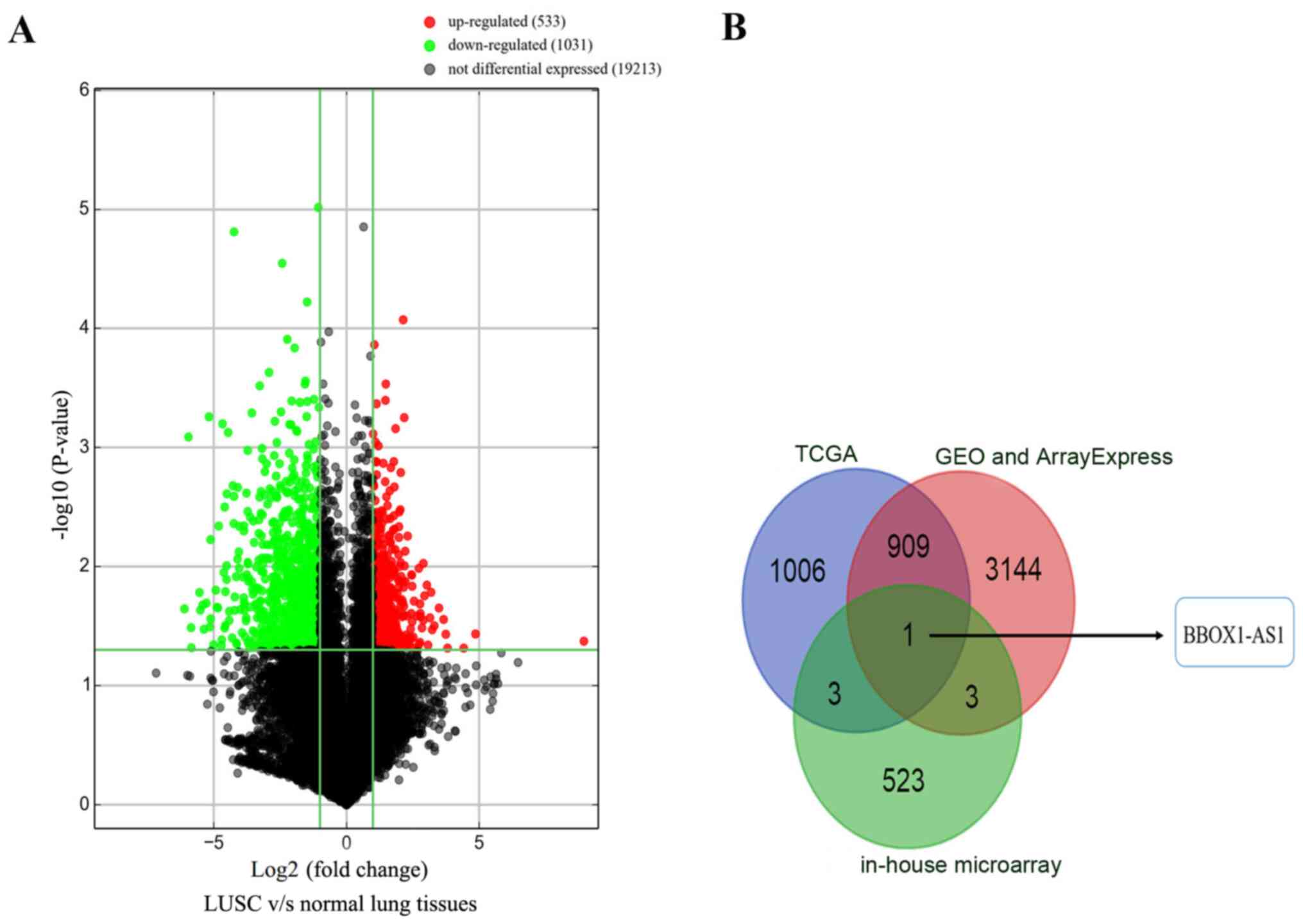
Based on the in-house microarray analysis, a total
of 20,777 lncRNAs were identified. Only 533 upregulated lncRNAs and
1,031 downregulated lncRNAs were significantly differentially
expressed between LUSC and normal lung tissues (|fold change| ≥2,
P≤0.05; Fig. 2A). The upregulated
lncRNAs were extracted from the TCGA, GEO and ArrayExpress
databases. Only one upregulated lncRNA, BBOX1-AS1, overlapped among
the TCGA, GEO, ArrayExpress and in-house microarray data (Fig. 2B).
Clinical significance of BBOX1-AS1 in
LUSC
Increased relative expression of BBOX1-AS1 was
observed in LUSC tissues (5.358±0.174) compared with that in
noncancerous tissues based on TCGA (0.042±0.006, P<0.0001;
Fig. 3A). High BBOX1-AS1
expression was related to the clinicopathological characteristic of
lymphatic metastasis (P<0.05; Fig.
3B; Table I). No statistical
significance was obtained for any of the other clinicopathological
parameters in the TCGA database. Furthermore, the AUC of BBOX1-AS1
was 0.983 (95% CI: 0.973-0.993, P<0.0001; Fig. 3C), which indicated a high
possibility that BBOX1-AS1 was able to discriminate LUSC from
normal lung tissues. A trend that patients with low BBOX1-AS1
expression survived longer than patients with high BBOX1-AS1
expression was determined via Kaplan-Meier curve analysis (P=0.505;
Fig. 3D).
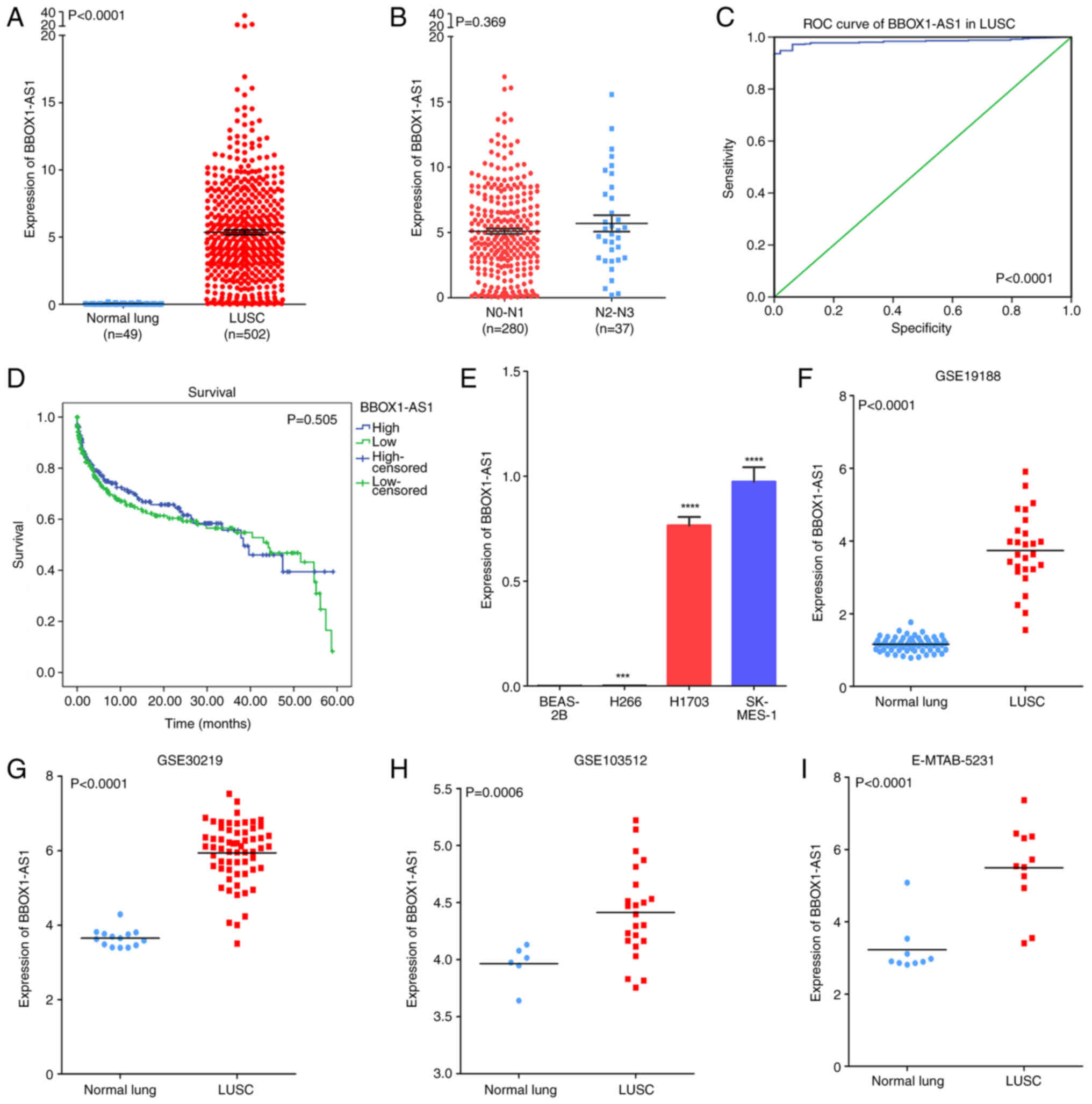 | Figure 3.Clinical significance of BBOX1-AS1 in
LUSC based on RT-qPCR, as well as TCGA, GEO and ArrayExpress
datasets. (A) Differential expression of BBOX1-AS1 between LUSC and
normal lung tissue based on the TCGA dataset. (B) Differential
expression of BBOX1-AS1 in the lymphatic metastasis group vs. no
lymphatic metastasis group based on the TCGA dataset. (C) ROC curve
of BBOX1-AS1 to discriminate LUSC from normal tissues. (D)
Kaplan-Meier survival curves for BBOX1-AS1 expression in LUSC. (E)
Differential expression of BBOX1-AS1 in LUSC cell lines based on
RT-qPCR; ***P<0.001, ****P<0.0001 vs. BEAS-2B. (F-I)
Differential expression of BBOX1-AS1 between LUSC and normal lung
tissue in GEO and ArrayExpress datasets, including the (F) GSE19188
profile, (G) GSE30219 profile, (H) GSE103512 profile, and (I)
E-MTAB-5231 profile. LUSC, lung squamous cell carcinoma; BBOX1-AS1,
γ-butyrobetaine hydroxylase 1 antisense 1; ROC, receiver operating
characteristic; TCGA, The Cancer Genome Atlas; GEO Gene Expression
Omnibus; RT-qPCR, reverse transcription-quantitative PCR. |
 | Table I.Correlation between BBOX1-AS1
expression and clinicopathological features based on TCGA
database. |
Table I.
Correlation between BBOX1-AS1
expression and clinicopathological features based on TCGA
database.
|
|
| BBOX1-AS1
expression |
|---|
|
|
|
|
|---|
| Clinicopathological
feature | N | Mean ± SD | R | P-value |
|---|
| Age, years |
|
| 0.05 | 0.272 |
|
<60 | 91 | 5.752±3.939 |
|
|
|
≥60 | 401 | 5.260±3.838 |
|
|
| Sex |
|
| 0.054 | 0.230 |
|
Male | 373 | 5.243±3.951 |
|
|
|
Female | 128 | 5.723±3.736 |
|
|
| Ethnicity |
|
| 0.077 | 0.004 |
|
White | 348 | 5.219±3.533 |
|
|
|
Black | 31 | 5.777±4.309 |
|
|
|
Asian | 9 | 9.209±4.086 |
|
|
| T stage |
|
| 0.019 | 0.674 |
|
T1+T2 | 406 | 5.330±3.637 |
|
|
|
T3+T4 | 95 | 5.518±4.883 |
|
|
| N stage |
|
| 0.135 | 0.140 |
|
N0-N1 | 280 | 5.096±3.376 |
|
|
|
N2-N3 | 37 | 6.721±6.450 |
|
|
| M stage |
|
| 0.006 | 0.919 |
| M0 | 321 | 5.134±3.567 |
|
|
| M1 | 6 | 5.286±3.639 |
|
|
| Stage |
|
| 0.045 | 0.318 |
|
I+II | 408 | 5.310±3.641 |
|
|
|
III+IV | 89 | 5.766±4.924 |
|
|
The RT-qPCR results suggested that BBOX1-AS1 was
more highly expressed in LUSC cells (NCI-H1703, NCI-H266, SK-MES-1)
than in BEAS-2B cells, particularly in NCI-H1703 and SK-MES-1 cells
(Fig. 3E). NCI-H1703 and SK-MES-1
cells were selected for further research. The original data from
six datasets (E-MTAB-5231, GSE4824-GPL97, GSE19188, GSE27489,
GSE30219 and GSE103512) from GEO and the ArrayExpress database were
used to verify the expression of BBOX1-AS1 in LUSC. Of these, four
datasets (E-MTAB-5231, GSE19188, GSE30219 and GSE103512) confirmed
high expression of BBOX1-AS1 (Fig.
3F-I).
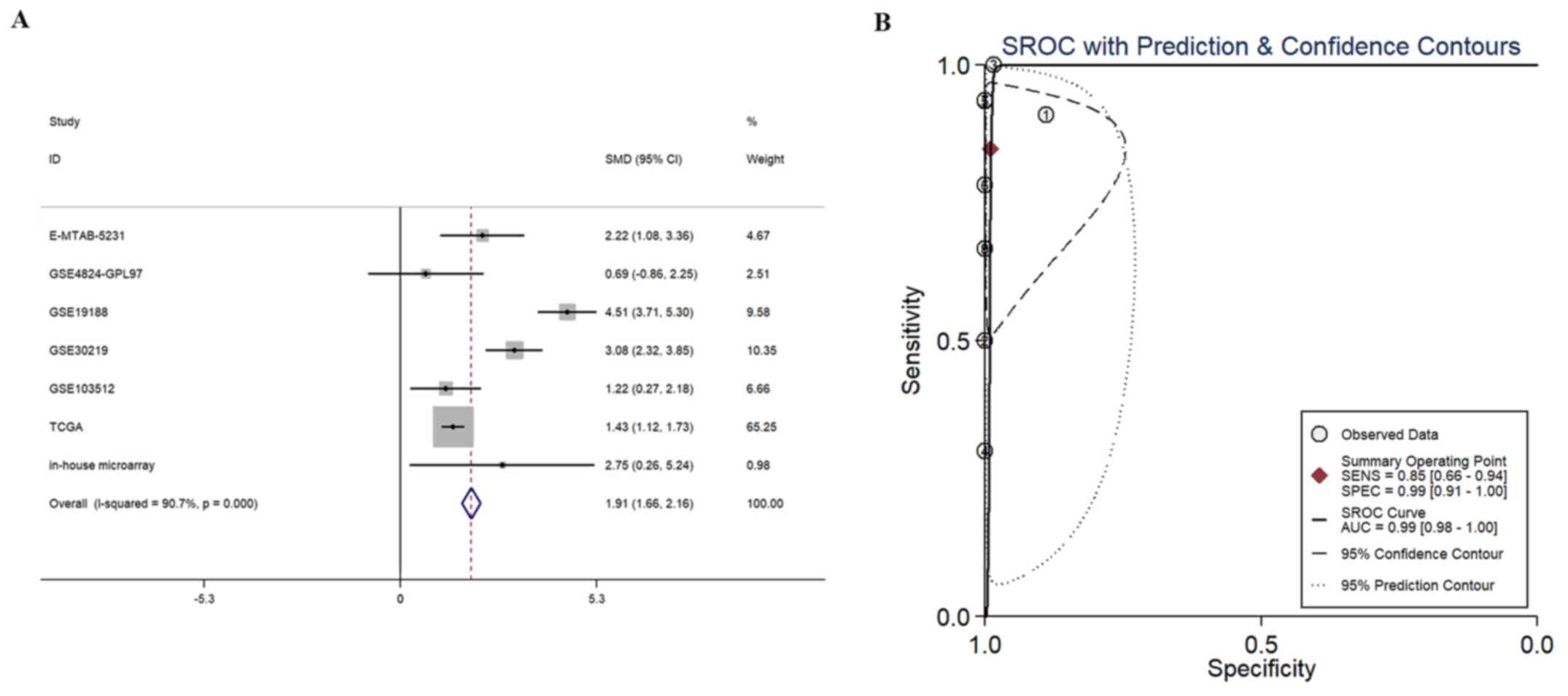
The results of the SMD analysis (including 641 cases
of LUSC and 159 controls) indicated that the combined SMD and 95%
CI reached 1.91 (1.66, 2.16), suggesting significantly higher
BBOX1-AS1 expression in the LUSC group than in the control group
(P<0.001; Fig. 4A). Of note,
publication bias was detected in the present study (P<0.05;
Fig. S1A and B). The pooled
sensitivity and specificity of BBOX1-AS1 were 0.85 (0.66-0.94) and
0.99 (0.91-1.00), respectively (Fig.
S1C). The positive diagnostic likelihood ratio (DLR-positive)
and negative diagnostic likelihood ratio (DLR-negative) scores were
83.24 (9.10-761.45) and 0.15 (0.06-0.38), respectively (Fig. S1D). LR<0.1 or >10.0 was
indicative of high accuracy. The diagnostic score and odds ratio
were 6.30 (3.82-8.77) and 542.07 (45.73-6425.35), respectively
(Fig. S1E). The AUC of the SROC
was 0.99 (0.98-1.00; Fig. 4B),
which indicated a high potential utility of BBOX-AS1 for
distinguishing between LUSC and noncancer cases. Publication bias
occurred in the SROC analysis (P<0.05; Fig. S1B). A flow chart of the SMD and
SROC analyses is provided in Fig.
5.
Effect of BBOX1-AS1 on the
proliferation and migration of LUSC cells in vitro
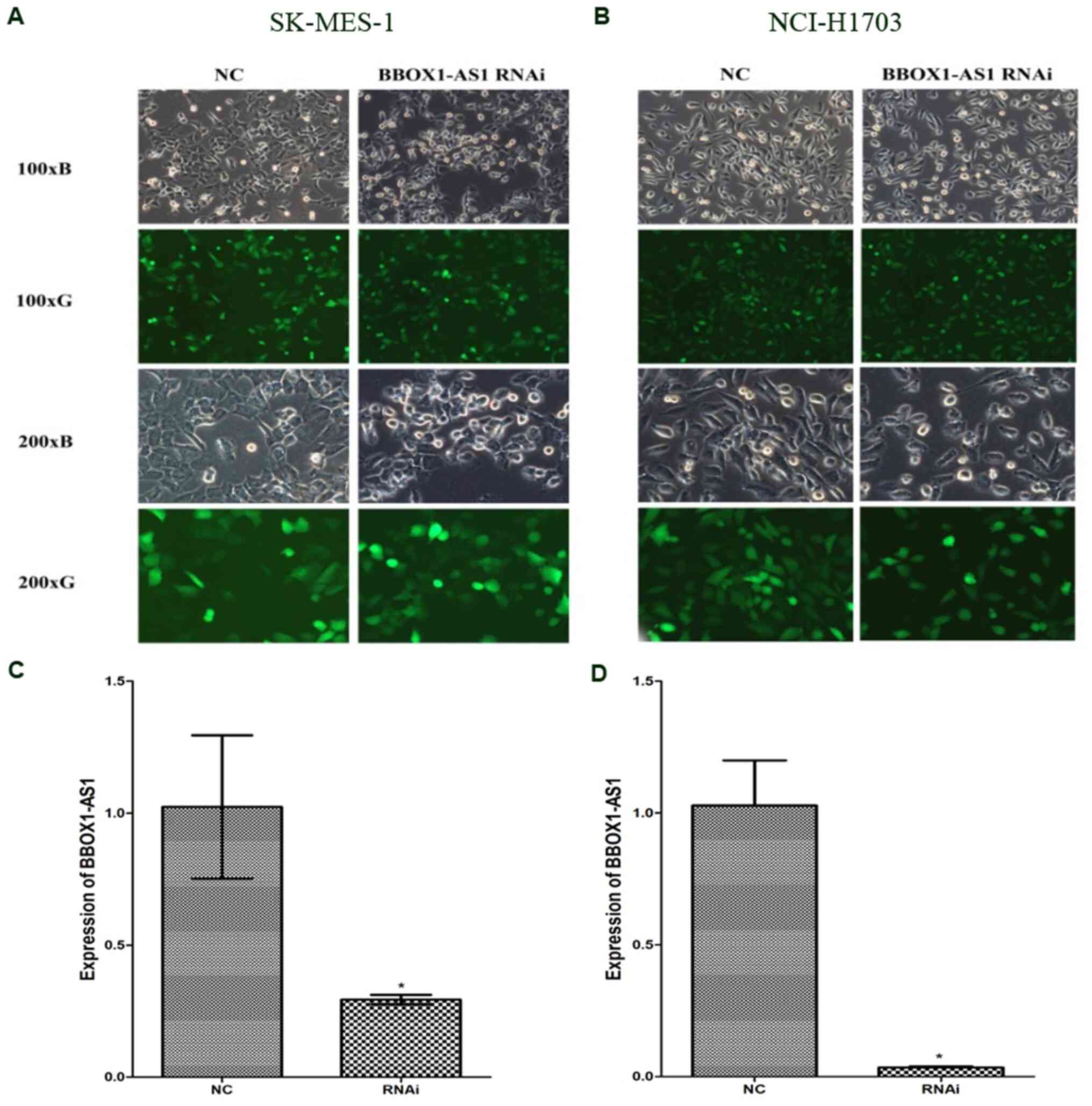
The transfection efficiency of BBOX1-AS1 RNAi
plasmid was observed using a light microscope and fluorescence
microscope. The transfection efficiency in the BBOX1-AS1 RNAi group
in LUSC cell lines was>80% and the knockdown efficiency of
BBOX1-AS1 was >70%. Images of cells after transfection with
BBOX1-AS1 RNAi and control lentivirus are provided in Fig. 6A and B. RT-qPCR indicated that
BBOX1-AS1 expression was significantly lower in the BBOX1-AS1 RNAi
groups than in the control groups in both NCI-H1703 and SK-MES-1
cells (P<0.05; Fig. 6C and
D).
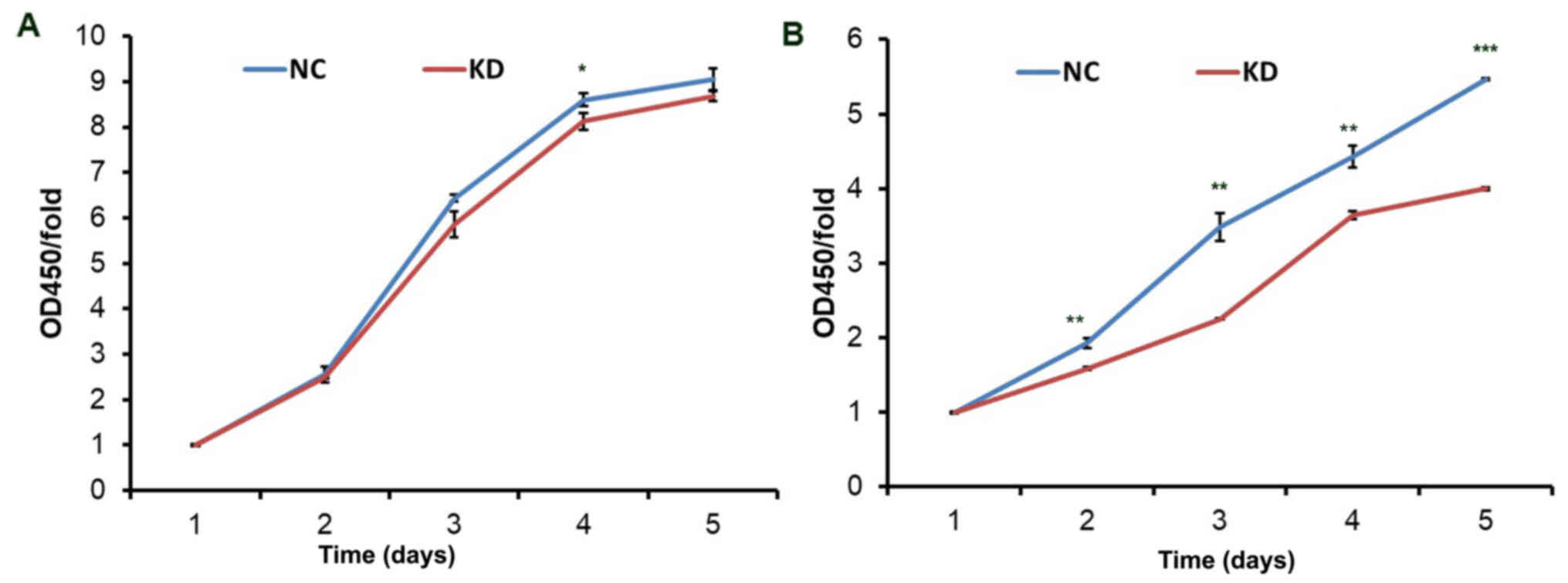
According to the CCK-8 assay, proliferation was
inhibited in the KD group in two cell lines (NCI-H1703 and
SK-MES-1) compared to the NC groups (Fig. 7). In NCI-H1703 and SK-MES-1 cells,
no obvious difference was observed between the KD group and the NC
group on the first day. The proliferation of SK-MES-1 cells in the
KD group was markedly reduced on days 2–5 (P<0.05) and most
significantly reduced on day five. There was no clear difference
observed in NCI-H1703 cells on the second and third days, whereas a
significant decrease was determined on day four (P<0.05). Based
on these results, it may be concluded that silencing of BBOX1-AS1
inhibited the proliferation of LUSC cells.
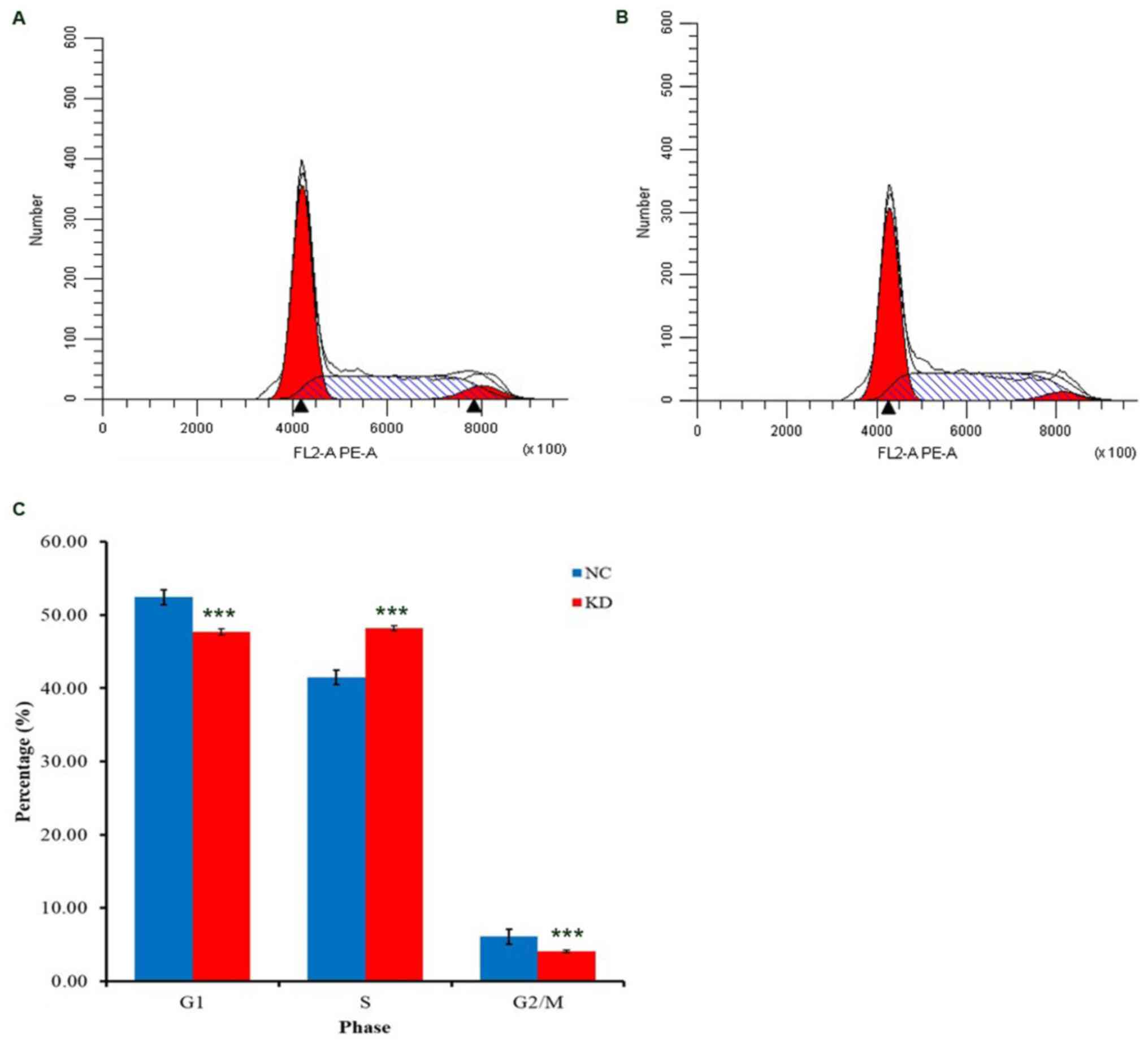
In NCI-H1703 cells, the results of the cell cycle
analysis revealed that BBOX1-AS1 RNAi caused cell cycle arrest in S
phase (P<0.05; Fig. 8). Thus,
BBOX1-AS1 RNAi may inhibit the proliferation of LUSC cells partly
because silencing of BBOX1-AS1 arrested cell cycle progression.
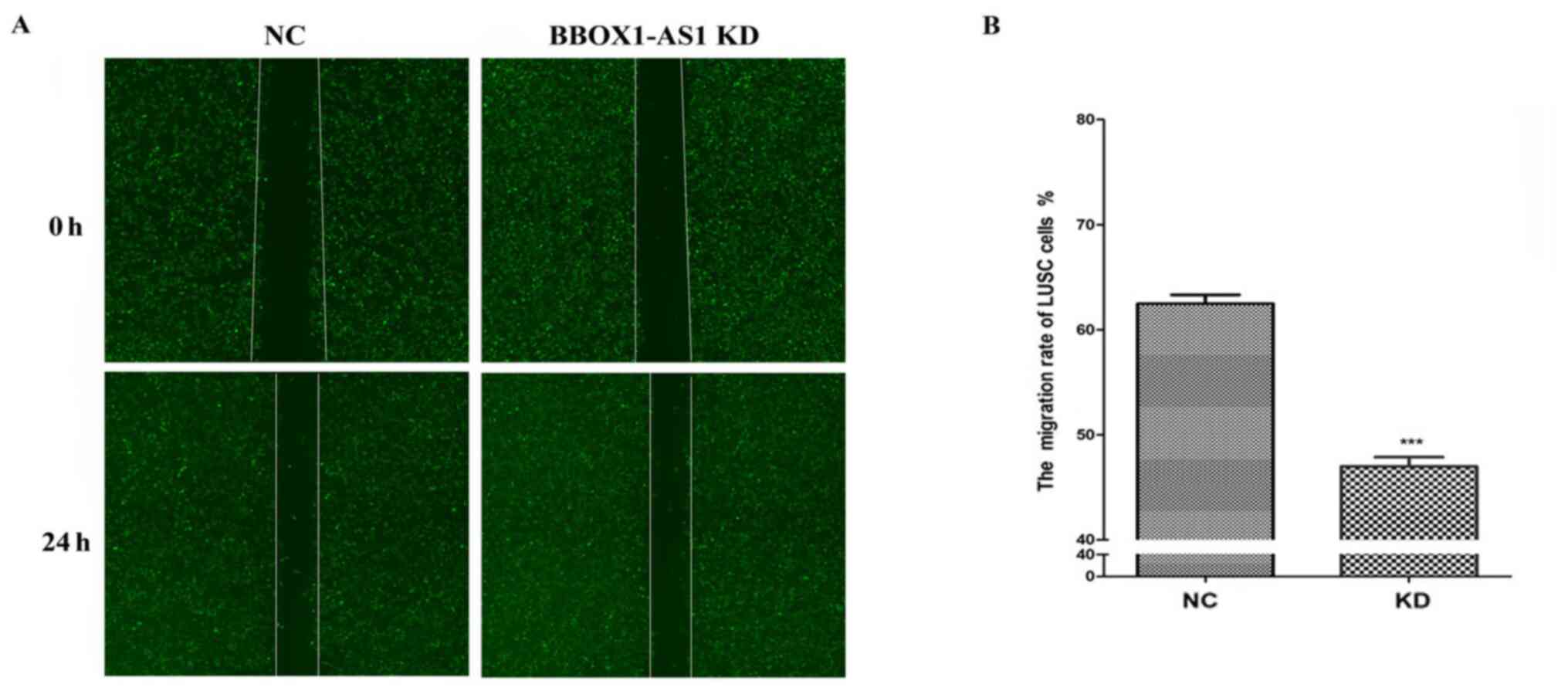
Furthermore, the results of the Celigo scratch assay
suggested that migration was inhibited after knockdown of BBOX1-AS1
in SK-MES-1 cells (P<0.01; Fig. 9A
and B).
Potential pathways associated with
BBOX1-AS1
The in-house microarray, TCGA and GEO datasets were
used to explore the genes co-expressed with BBOX1-AS1. A total of
167 genes were selected for downstream analysis. The possible GO
and KEGG pathways were examined based on these 167 genes. The GO
and KEGG analyses suggested that these genes may participate in
various important biological processes, including cell-cell
junctions, protein complex binding, tissue morphogenesis, focal
adhesion, ECM-receptor interaction and DNA replication (Fig. 10A; Tables II and III).
 | Table II.Top enrichment GO terms in the
categories BP, CC and MF by the co-expressed genes of
γ-butyrobetaine hydroxylase 1 antisense 1. |
Table II.
Top enrichment GO terms in the
categories BP, CC and MF by the co-expressed genes of
γ-butyrobetaine hydroxylase 1 antisense 1.
| GO ID | Term | Ontology | Count | P-value |
|---|
| GO:0034621 | Cellular
macromolecular complex subunit organization | BP | 10 | 0.004493 |
| GO:0034622 | Cellular
macromolecular complex assembly | BP | 9 | 0.007375 |
| GO:0042476 | Odontogenesis | BP | 4 | 0.012155 |
| GO:0048730 | Epidermis
morphogenesis | BP | 3 | 0.020492 |
| GO:0007155 | Cell adhesion | BP | 13 | 0.021424 |
| GO:0022610 | Biological
adhesion | BP | 13 | 0.021636 |
| GO:0048729 | Tissue
morphogenesis | BP | 6 | 0.022049 |
| GO:0007229 | Integrin-mediated
signaling pathway | BP | 4 | 0.024231 |
| GO:0048732 | Gland
development | BP | 5 | 0.032087 |
| GO:0007398 | Ectoderm
development | BP | 6 | 0.032126 |
| GO:0030057 | Desmosome | CC | 5 | 1.88E-05 |
| GO:0070161 | Anchoring
junction | CC | 8 | 5.32E-04 |
| GO:0005911 | Cell-cell
junction | CC | 7 | 0.004754 |
| GO:0043296 | Apical junction
complex | CC | 5 | 0.008914 |
| GO:0016327 | Apicolateral plasma
membrane | CC | 5 | 0.009878 |
| GO:0044427 | Chromosomal
part | CC | 9 | 0.0141 |
| GO:0031965 | Nuclear
membrane | CC | 4 | 0.022122 |
| GO:0005694 | Chromosome | CC | 9 | 0.035488 |
| GO:0019866 | Organelle inner
membrane | CC | 7 | 0.05378 |
| GO:0030054 | Cell junction | CC | 9 | 0.062978 |
| GO:0032403 | Protein complex
binding | MF | 5 | 0.094009 |
| GO:0005178 | Integrin
binding | MF | 3 | 0.094724 |
 | Table III.Enriched KEGG pathway terms by the
co-expressed genes of γ-butyrobetaine hydroxylase 1 antisense
1. |
Table III.
Enriched KEGG pathway terms by the
co-expressed genes of γ-butyrobetaine hydroxylase 1 antisense
1.
| KEGG ID | KEGG term | Count | P-value | Gene symbols |
|---|
| hsa04510 | Focal adhesion | 6 | 0.03381 | ITGA5, LAMA5,
ITGA7, PIK3R5, COL1A1, FLNA |
| hsa04512 | ECM-receptor
interaction | 4 | 0.039674 | ITGA5, LAMA5,
ITGA7, COL1A1 |
| hsa03030 | DNA
replication | 3 | 0.041514 | RFC4, FEN1,
MCM6 |
| hsa05414 | Dilated
cardiomyopathy | 4 | 0.049758 | ACTC1, ITGA5,
ADCY5, ITGA7 |
A gene network of the co-expressed genes of
BBOX1-AS1 was also constructed (Fig.
10B), from which it was possible to detect relationships
between BBOX1-AS1 and these co-expressed genes.
The PPI network was constructed using STRING online
and 253 PPI pairs with a combined score >0.4 were selected
(Fig. 10C). The interactions
between BBOX1-AS1 and coexpressed genes were obtained through the
nodes and strings in the network. Centromere protein A (CENPA) had
the highest degree of connectivity (degree=15) according to the PPI
network, which indicates that CENPA may be the key gene coexpressed
with BBOX1-AS1 in LUSC. In addition, CENPA was downregulated in
LUSC based on the TCGA and GEPIA datasets (Fig. S2A and B). Furthermore, the
correlation between BBOX1-AS1 and CENPA was determined with the
TCGA and GEPIA datasets, indicating that BBOX1-AS1 expression was
positively correlated with CENPA in LUSC (R=0.1512; Fig. S2C and D).
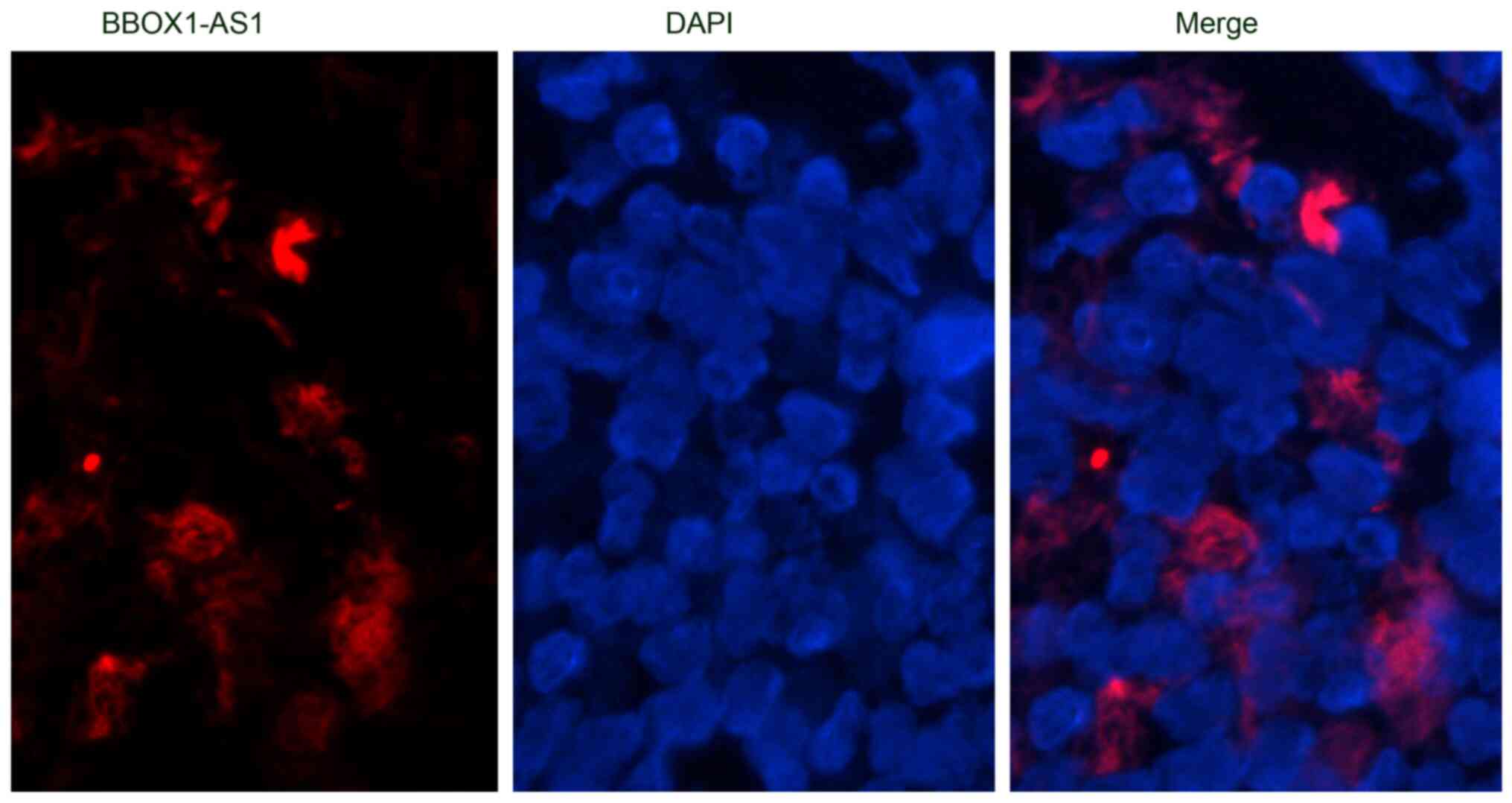
Cellular localization of
BBOX1-AS1
FISH analysis was performed to observe the cellular
location of BBOX1-AS1 in LUSC cells. The majority of BBOX1-AS1
(indicated in red) was located in the cytoplasm (magnification,
×400), indicating that BBOX1-AS1 is able to act as a competing
endogenous (ce)RNA in LUSC (Fig.
11).
Discussion
Previous studies have revealed the pivotal role of
BBOX1-AS1 in NSCLC, as well as colorectal, cervical and gastric
cancers (17–20). Liu et al (17) indicated that BBOX1-AS1 was
overexpressed in colorectal cancer cell lines and that BBOX1-AS1
knockdown enhanced cell proliferation, invasion and migration while
decreasing apoptosis. In cervical cancer, Xu et al (19) determined that BBOX1-AS1 was
upregulated. Increased expression of BBOX1-AS1 had stimulating
effects on cervical cancer cell growth and migration. Yang et
al (18) determined that
BBOX1-AS1 was significantly upregulated in gastric cancer tissues
and cells and that overexpression of BBOX1-AS1 promoted gastric
cancer cell proliferation and inhibited gastric cancer cell
apoptosis. Shi et al (20)
revealed that KLF5-induced BBOX1-AS1 expression exerted a
tumor-promoting role in NSCLC by sponging microRNA
(miRNA/miR)-27a-5p. Several obvious differences were observed
between the present study and that by Shi et al (20). First, the present study used an
in-house microarray analysis to detect differentially expressed
lncRNAs between LUSC and normal lung tissues. BBOX1-AS1 was the
only overlapping upregulated lncRNA based on the datasets from the
TCGA, GEO, ArrayExpress and the in-house microarray. Next,
BBOX1-AS1 was indicated to act as a competing ‘sponge’ of miRNA,
but this requires BBOX1-AS1 to be located in the cytoplasm. FISH
analysis was performed in the present study to determine the
location of BBOX1-AS1 in the nucleus or cytoplasm of LUSC tissues.
Furthermore, SMD and SROC analyses were included in the present
study. SMD analysis confirmed upregulated expression of BBOX1-AS1
in LUSC. SROC analysis indicated the high potential utility of
BBOX-AS1 in distinguishing between LUSC and noncancer cases.
In the present study, RT-qPCR and in vitro
verification were used to further determine the effect of BBOX1-AS1
in LUSC. LUSC cell proliferation and migration were reduced
following BBOX1-AS1 RNAi. These discoveries confirmed the oncogenic
role of BBOX1-AS1 in LUSC. NCI-H1703, NCI-H226 and SK-MES-1 cells
are three well-known human LUSC cell lines. According to the
RT-qPCR analysis, BBOX1-AS1 expression was higher in NCI-H1703 and
SK-MES-1 cells than in NCI-H226 cells. Therefore, the NCI-H1703 and
SK-MES-1 cell lines were selected for further transfection with
BBOX1-AS1 RNAi, and it was indicated that the tumor cell clones
were heterogeneous with respect to both morphology and function.
Lung cancer is highly heterogeneous in cell origin, histology,
clinical features, molecular biology and other aspects, resulting
in a lack of effective and specific therapy strategies (33). Future therapies will undoubtedly
focus on precision medicine and personalized treatments as the
study of nosogenesis continues.
In the present study, it was discovered that high
BBOX1-AS1 expression was associated with lymphatic metastasis,
which is an important clinical finding in LUSC. Furthermore, the
high AUC value indicated that BBOX1-AS1 expression is able to
discriminate between LUSC and noncancerous tissues. First, lncRNA
high-throughput chips and original RNA-seq data were collected from
multiple databases to calculate the SMD and SROC curve of BBOX1-AS1
for LUSC. The combined values of sensitivity (0.85) and specificity
(0.99) demonstrated the high accuracy of BBOX1-AS1 for the
detection of LUSC. The present results verified that the SROC curve
was located near the upper left corner. PLR and NLR were determined
to predict the diagnostic accuracy of BBOX1-AS1. LR<0.1 or
>10.0 was indicative of high accuracy. A PLR value of 83.24
suggested that patients with LUSC had an ~83.24-fold higher chance
of expressing high levels of BBOX1-AS1. In addition, the NLR (0.15)
suggested that if the BBOX1-AS1 result was negative, there was an
~15% chance that the patient had LUSC. SMD analysis confirmed
higher expression of BBOX1-AS1 in LUSC, in accordance with the
results of the RT-qPCR and in-house microarray analyses.
The GO and KEGG analyses indicated that the target
genes may have various biological roles. Previous studies suggested
that these enriched terms may have essential functions in various
cancer types, including cervical, lung and liver cancer (34,35).
These enriched functions may be associated with tumor occurrence,
and the progression and development of lung cancer (34,36).
CENPA had the highest degree of involvement based on the PPI
network. CENPA was reported to be significantly involved in the
prognosis and predicted outcome of targeted therapy of LUSC
(37,38). It was hypothesized that CENPA is
the key gene downstream of BBOX1-AS1 in LUSC. BBOX1-AS1 may affect
the progression and development of LUSC by regulating various
biological functions. However, the precise molecular mechanisms
should be determined in future functional experiments.
lncRNAs may be located in the cytoplasm and function
as ceRNAs in cancer (39,40). Kong et al (39) reported that lncRNA-CDC6 may act as
a ceRNA to target CDC6 by sponging miR-215 to promote the
progression of breast cancer. Tang and Yang (40) indicated that lncRNA SNHG14 may
function as a ceRNA in hepatocellular carcinoma and the
SNHG14/miR-656-3p/SIRT5 axis was able to aggravate the invasion and
migration of hepatocellular carcinoma. The results of the FISH
assay suggested that BBOX1-AS1 was located in the cytoplasm. Thus,
BBOX1-AS1 may act as a ceRNA to affect the biological functions of
LUSC. BBOX1-AS1/miR-361-3p/AEG-1 may have significant roles in the
proliferation, invasion and metastasis of LUSC based on the
literature and bioinformatic prediction software, but functional
experiments should be performed for verification (17,19,41).
There were certain limitations to the SMD and SROC
analyses. Publication bias was detected and heterogeneity (high
I-square values) was unavoidable due to the nonsignificant results
in the two GEO datasets. Furthermore, the use of blinding was
inconsistent among the five different data sources (GEO,
ArrayExpress, TCGA, RT-qPCR and in-house microarray), which
contributed to the heterogeneity. To the best of our knowledge, the
present study was the first to explore the relationship between
BBOX1-AS1 expression and the biological activity of LUSC in
vitro. It was determined that BBOX1-AS1 was able to promote the
proliferation and migration of LUSC cells. Additional functional
experiments should be performed to verify the potential molecular
mechanism of BBOX1-AS1 in LUSC.
In conclusion, the present study indicated that
BBOX1-AS1 may have an oncogenic effect in LUSC by regulating
various biological functions. However, additional functional
experiments should be designed to verify the exact mechanism.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Shandong, China (grant no. ZR202102240355).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YZ and XW made a significant contribution to the
study design, execution and acquisition of data. XKC, YYZ and RQH
contributed to data analysis and interpretation. GC and YJQ
analyzed the experimental data and wrote and revised the
manuscript. YZ and GC confirm the authenticity of the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
All methods used adhered to the relevant guidelines
and were approved by the Ethical Committee of the First Affiliated
Hospital of Guangxi Medical University (Nanning, China). Consent
forms for the use of tissues were signed by the doctors and
patients involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
lncRNA
|
long noncoding RNA
|
|
NSCLC
|
non-small cell lung cancer
|
|
LUSC
|
lung squamous cell carcinoma
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
GEO
|
Gene Expression Omnibus
|
|
TCGA
|
The Cancer Genome Atlas
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
ROC
|
receiver operating characteristic
|
|
BP
|
biological process
|
|
CC
|
cellular component
|
|
MF
|
molecular function
|
|
DAVID
|
Database for Annotation, Visualization
and Integrated Discovery
|
|
STRING
|
Search Tool for the Retrieval of
Interacting Genes
|
|
NCBI
|
National Center of Biotechnology
Information
|
|
SROC
|
summary receiver operating
characteristic
|
|
SRA
|
Sequence Read Archive
|
|
siRNA
|
small interfering RNA
|
|
SMD
|
standard mean deviation
|
References
|
1
|
Zhao Y, Wang XX, Wu W, Long H, Huang J,
Wang Z, Li T, Tang S, Zhu B and Chen D: EZH2 regulates PD-L1
expression via HIF-1α in non-small cell lung cancer cells. Biochem
Biophys Res Commun. 517:201–209. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laurans M, Botticella A, Moukasse Y, Lévy
A and Le Péchoux C: Lung cancer and elective nodal irradiation: A
solved issue? Cancer Radiother. 23:701–707. 2019.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buentzel J, Heinz J, Bleckmann A, Bauer C,
Röver C, Bohnenberger H, Saha S, Hinterthaner M, Baraki H, Kutschka
I, et al: Sarcopenia as Prognostic Factor in Lung Cancer Patients:
A Systematic Review and Meta-analysis. Anticancer Res.
39:4603–4612. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang N, Lin W, Shi X and Tao T: STK24
expression is modulated by DNA copy number/methylation in lung
adenocarcinoma and predicts poor survival. Future Oncol.
14:2253–2263. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoang LT, Domingo-Sabugo C, Starren ES,
Willis-Owen SAG, Morris-Rosendahl DJ, Nicholson AG, Cookson WOCM
and Moffatt MF: Metabolomic, transcriptomic and genetic integrative
analysis reveals important roles of adenosine diphosphate in
haemostasis and platelet activation in non-small-cell lung cancer.
Mol Oncol. 13:2406–2421. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu L, Wang H, Wei D, Wang B, Zhang C, Zhu
T, Ma Z, Li Z, Wu Y and Yu G: The value of CEP55 gene as a
diagnostic biomarker and independent prognostic factor in LUAD and
LUSC. PLoS One. 15:e02332832020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li K, Tian Y, Yuan Y, Fan X, Yang M, He Z
and Yang D: Insights into the Functions of lncRNAs in
Drosophila. Int J Mol Sci. 20:202019.
|
|
8
|
Murillo-Maldonado JM and Riesgo-Escovar
JR: The various and shared roles of lncRNAs during development. Dev
Dyn. 248:1059–1069. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu J, Yu W, Wang Y, Xia K, Huang Y, Xu A,
Chen Q, Liu B, Tao H, Li F, et al: lncRNAs: Function and mechanism
in cartilage development, degeneration, and regeneration. Stem Cell
Res Ther. 10:3442019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S, Jin J, Xu Z and Zuo B: Functions
and Regulatory Mechanisms of lncRNAs in Skeletal Myogenesis, Muscle
Disease and Meat Production. Cells. 8:82019. View Article : Google Scholar
|
|
11
|
Lim LJ, Wong SYS, Huang F, Lim S, Chong
SS, Ooi LL, Kon OL and Lee CG: Roles and Regulation of Long
Noncoding RNAs in Hepatocellular Carcinoma. Cancer Res.
79:5131–5139. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chi Y, Wang D, Wang J, Yu W and Yang J:
Long Non-Coding RNA in the Pathogenesis of Cancers. Cells. 8:82019.
View Article : Google Scholar
|
|
13
|
Yang J, Qiu Q, Qian X, Yi J, Jiao Y, Yu M,
Li X, Li J, Mi C, Zhang J, et al: Long noncoding RNA LCAT1
functions as a ceRNA to regulate RAC1 function by sponging
miR-4715-5p in lung cancer. Mol Cancer. 18:1712019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Feng C, Li Y, Ma Y and Cai R:
lncRNA H19 promotes lung cancer proliferation and metastasis by
inhibiting miR-200a function. Mol Cell Biochem. 460:1–8. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang N, Guo W, Ren K, Li W, Jiang Y, Sun
J, Dai W and Zhao W: lncRNA AFAP1-AS1 Supresses miR-139-5p and
Promotes Cell Proliferation and Chemotherapy Resistance of
Non-small Cell Lung Cancer by Competitively Upregulating RRM2.
Front Oncol. 9:11032019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin D, Guo J, Wu Y, Du J, Yang L, Wang X,
Di W, Hu B, An J, Kong L, et al: m6A mRNA methylation initiated by
METTL3 directly promotes YAP translation and increases YAP activity
by regulating the MALAT1-miR-1914-3p-YAP axis to induce NSCLC drug
resistance and metastasis. J Hematol Oncol. 12:1352019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Zhu J, Xiao Z, Wang X and Luo J:
BBOX1-AS1 contributes to colorectal cancer progression by sponging
hsa-miR-361-3p and targeting SH2B1. FEBS Open Bio. 2211–5463,
12802. 2020.
|
|
18
|
Yang Y, Yu Q, Li B, Guan R, Huang C and
Yang X: BBOX1-AS1 Accelerates Gastric Cancer Proliferation by
Sponging miR-3940-3p to Upregulate BIRC5 Expression. Dig Dis Sci.
66:1054–1062. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J, Yang B, Wang L, Zhu Y, Zhu X, Xia Z,
Zhao Z and Xu L: lncRNA BBOX1-AS1 upregulates HOXC6 expression
through miR-361-3p and HuR to drive cervical cancer progression.
Cell Prolif. 53:e128232020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi J, Yang C, An J, Hao D, Liu C, Liu J,
Sun J and Jiang J: KLF5-induced BBOX1-AS1 contributes to cell
malignant phenotypes in non-small cell lung cancer via sponging
miR-27a-5p to up-regulate MELK and activate FAK signaling pathway.
J Exp Clin Cancer Res. 40:1482021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, He RQ, Dang YW, Zhang XL, Wang X,
Huang SN, Huang WT, Jiang MT, Gan XN, Xie Y, et al: Comprehensive
analysis of the long noncoding RNA HOXA11-AS gene interaction
regulatory network in NSCLC cells. Cancer Cell Int. 16:892016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Linehan WM and Ricketts CJ: The Cancer
Genome Atlas of renal cell carcinoma: Findings and clinical
implications. Nat Rev Urol. 16:539–552. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xia L, Zhang W and Gao L: Clinical and
prognostic effects of CDKN2A, CDKN2B and CDH13 promoter methylation
in ovarian cancer: A study using meta-analysis and TCGA data.
Biomarkers. 24:700–711. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai KT, Liu AG, Wang ZF, Jiang HW, Zeng
JJ, He RQ, Ma J, Chen G and Zhong JC: Expression and potential
molecular mechanisms of miR 204 5p in breast cancer, based on
bioinformatics and a meta analysis of 2,306 cases. Mol Med Rep.
19:1168–1184. 2019.PubMed/NCBI
|
|
25
|
Gan BL, Zhang LJ, Gao L, Ma FC, He RQ,
Chen G, Ma J, Zhong JC and Hu XH: Downregulation of miR 224 5p in
prostate cancer and its relevant molecular mechanism via TCGA, GEO
database and in silico analyses. Oncol Rep. 40:3171–3188.
2018.PubMed/NCBI
|
|
26
|
Rao X, Huang X, Zhou Z and Lin X: An
improvement of the 2ˆ(−delta delta CT) method for quantitative
real-time polymerase chain reaction data analysis. Biostat
Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI
|
|
27
|
Zhang Y, Chen WJ, Gan TQ, Zhang XL, Xie
ZC, Ye ZH, Deng Y, Wang ZF, Cai KT, Li SK, et al: Clinical
Significance and Effect of lncRNA HOXA11-AS in NSCLC: A Study Based
on Bioinformatics, In Vitro and In Vivo Verification. Sci Rep.
7:55672017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Li ZY, Hou XX, Wang X, Luo YH,
Ying YP and Chen G: Clinical significance and effect of AEG-1 on
the proliferation, invasion, and migration of NSCLC: A study based
on immunohistochemistry, TCGA, bioinformatics, in vitro and in vivo
verification. Oncotarget. 8:16531–16552. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng Q, Huang C, Cao H, Lin J, Gong X, Li
J, Chen Y, Tian Z, Fang Z and Huang J: A Novel Prognostic Signature
of Transcription Factors for the Prediction in Patients With GBM.
Front Genet. 10:9062019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ding J and Zhang Y: Analysis of key GO
terms and KEGG pathways associated with carcinogenic chemicals.
Comb Chem High Throughput Screen. Dec 18–2017.(Epub ahead of
print). doi: 10.2174/1386207321666171218120133. PubMed/NCBI
|
|
31
|
Zhou Z, Li Y, Hao H, Wang Y, Zhou Z, Wang
Z and Chu X: Screening Hub Genes as Prognostic Biomarkers of
Hepatocellular Carcinoma by Bioinformatics Analysis. Cell
Transplant. 28 (Suppl 1):76S–86S. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang Y, Zhang C, Ma MH and Dai DQ:
Identification and prediction of novel non-coding and coding
RNA-associated competing endogenous RNA networks in colorectal
cancer. World J Gastroenterol. 24:5259–5270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Sousa VML and Carvalho L: Heterogeneity
in Lung Cancer. Pathobiology. 85:96–107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aboubakar Nana F, Vanderputten M and Ocak
S: Role of Focal Adhesion Kinase in Small-Cell Lung Cancer and Its
Potential as a Therapeutic Target. Cancers (Basel). 11:112019.
View Article : Google Scholar
|
|
35
|
Mughal MJ, Mahadevappa R and Kwok HF: DNA
replication licensing proteins: Saints and sinners in cancer. Semin
Cancer Biol. 58:11–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yenerall P, Das AK, Wang S, Kollipara RK,
Li LS, Villalobos P, Flaming J, Lin YF, Huffman K, Timmons BC, et
al: RUVBL1/RUVBL2 ATPase Activity Drives PAQosome Maturation, DNA
Replication and Radioresistance in Lung Cancer. Cell Chem Biol.
27:105–121.e14. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang L and Zhao L, Bi J, Guan Q, Qi A,
Wei Q, He M, Wei M and Zhao L: Glycolysis gene expression
profilings screen for prognostic risk signature of hepatocellular
carcinoma. Aging (Albany NY). 11:10861–10882. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qi L, Gao C, Feng F, Zhang T, Yao Y, Wang
X, Liu C, Li J, Li J and Sun C: MicroRNAs associated with lung
squamous cell carcinoma: New prognostic biomarkers and therapeutic
targets. J Cell Biochem. 120:18956–18966. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kong X, Duan Y, Sang Y, Li Y, Zhang H,
Liang Y, Liu Y, Zhang N and Yang Q: lncRNA-CDC6 promotes breast
cancer progression and function as ceRNA to target CDC6 by sponging
microRNA-215. J Cell Physiol. 234:9105–9117. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tang SJ and Yang JB: lncRNA SNHG14
aggravates invasion and migration as ceRNA via regulating
miR-656-3p/SIRT5 pathway in hepatocellular carcinoma. Mol Cell
Biochem. 473:143–153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yao H, Chen R, Yang Y and Jiang J: lncRNA
BBOX1-AS1 Aggravates the Development of Ovarian Cancer by
Sequestering miR-361-3p to Augment PODXL Expression. Reprod Sci.
28:736–744. 2021. View Article : Google Scholar : PubMed/NCBI
|