Introduction
Breast cancer (BC) is the most frequently occurring
cancer in women worldwide, with >2 million new cases diagnosed
in 2018 (1). ~70% of BC cases are
hormone receptor-positive (HR+) and human epidermal
growth factor receptor 2-negative (HER2−). Endocrine
therapy (ET) is considered the mainstay of treatment for both pre-
and postmenopausal women with HR+/HER2−
metastatic BC (mBC) (2). Regarding
mBC, the most relevant therapeutic improvement of the last few
years has been the introduction of cyclin-dependent kinase (CDK) 4
and 6 inhibitors (CDK4/6i) (palbociclib, ribociclib and
abemaciclib) combined with ET. Pivotal trials, namely PALOMA 2 and
3, MONALEESA 2, 3 and 7, and MONARCH 2 and 3, showed an improvement
in progression free survival (PFS) of 5 to 10 months (3–8). A
recent meta-analysis reported that CDK4/6-i + ET combinations,
compared with ET alone, improved overall survival (OS) independent
of age, menopausal status, endocrine sensitivity and visceral
involvement (9). Except for
patients with extensive visceral involvement, CDK4/6i + ET
combinations remain the treatment of choice for
HR+/HER2− mBC (2).
Despite the significant improvements in survival
determined by CDK4/6i, resistance represents a major clinical
challenge. Resistance might be present immediately at treatment
initiation. Primary or de novo resistance occurs in ~15% of
patients receiving CDK4/6i with anti-aromatase inhibitors, and ~30%
of those receiving CDK4/6i with fulvestrant (10). Currently, there are no valid
prognostic factors for response to CDK4/6i. Baseline lymphopenia
has been reported in several publications as a prognostic factor in
different types of cancer (11–17). Thus, lymphopenia is an
independent predictive factor of survival in metastatic colorectal
cancer patient with shorter PFS (median 4 vs. 7 months; P=0.033)
and OS (median 16 vs. 24 months, P=0.024)(11).
The present study aimed to assess the impact of
baseline absolute lymphocyte count (ALC) on response to
CDK4/6i.
Materials and methods
Design
Between April 2016 and February 2019, a descriptive
retrospective single center study was performed at the François
Baclesse Comprehensive Cancer Center, Caen, Calvados, Normandy.
Eligible patients were women aged >18 years with
HR+/HER2− mBC treated with palbociclib in
combination with ET (an aromatase inhibitor or fulvestrant).
Premenopausal women also received luteinizing hormone-releasing
hormone agonists. A total of 114 patients were included; there were
no predefined exclusion criteria. The primary end-point, PFS, was
evaluated from palbociclib initiation to radiological progression,
death or last follow-up. Secondary end points were OS (time from
palbociclib initiation to death), best radiological response and
safety. Tumor assessment was performed every 2–3 cycles and disease
response was categorized as complete response (CR), partial
response (PR), stable disease (SD) or progressive disease (PD),
according to the response evaluation criteria in solid tumors
(version 1.1) (18). Objective
response rate (ORR) was defined as the percentage of patients in
whom either CR or PR was observed. Disease control rate (DCR) was
defined as the proportion of patients with either CR, PR or SD as
best overall response. All patients underwent baseline routine
blood tests, including white blood cell and ALC. Lymphopenia was
defined as ALC <1.5 g/l; lymphopenia and other adverse events
(AEs) were graded according to National Cancer Institute Common
Terminology Criteria for AEs (version 5.0) (19). Initial dose of palbociclib and dose
reductions were reported and analyzed for their impact on PFS and
OS.
In accordance with regulations regarding research
involving human subjects, the present study was registered in the
corresponding data protection document. As an observational
retrospective study, institutional review board approval was not
required. Patients' non-opposition to the use of their data was
sought after checking whether the patient was still living; as a
result, no data exclusion due to death was necessary. All data were
anonymized for statistical analysis.
Statistical analysis
Qualitative variables are presented as the number
and frequency; quantitative variables are presented as the mean ±
standard deviation or median and extreme values. The
characteristics of lymphopenic and non-lymphopenic patients were
compared by χ2 test (or Fisher's exact test, in case of
observed values per category <5) for the qualitative variables,
and by the unpaired Student's t-test for the quantitative variables
(or Wilcoxon non-parametric test if data were not normally
distributed). P<0.05 was considered to indicate a statistically
significant difference. PFS and OS were calculated according to the
Kaplan-Meier method and comparison of survival between different
patient populations was performed by the log-rank or supremum
log-rank test (Renyi-type test) in case of crossing curves. The
impact of known prognostic factors (age, number of previous lines
of treatment, palbociclib dose reduction and occurrence of AE) was
assessed by univariate and multivariate Cox models. All incident
cases were assessed (no calculation of the number of subjects
needed). Analyses were conducted using R software, version 4.0.2
(https://cran.r-project.org/bin/windows/base/).
Results
Clinicopathological data of
patients
Between April 2016 and February 2019, a total of 114
patients were recruited. The median age at palbociclib initiation
was 51 years. Most patients had a good Eastern Cooperative Oncology
Group performance status (PS; PS0, 41.2%; PS1, 44.7%). The median
number of previous lines of treatment was four and 85.1% of
patients received fulvestrant in combination with palbociclib. Only
16.7% of patients exhibited de novo mBC (Table I). Median baseline ALC was 1.4 g/l
(range, 0.2-4.3 g/l). A total of 65 (57%) and 49 (43%) patients had
baseline ALC<1.5 and ≥1.5 g/l, respectively. PS, number of
previous lines of treatment and palbociclib dose reduction were not
significantly different in these two groups (Table II).
 | Table I.Characteristics of patients. |
Table I.
Characteristics of patients.
| Characteristic | Number (n=114) | % |
|---|
| Median age, years
(range) | 51 (30–75) |
|
| PS |
|
|
| 0 | 47 | 41.2 |
| 1 | 51 | 44.7 |
| 2 | 13 | 11.4 |
| 3 | 3 | 2.6 |
| Histological
diagnosis |
|
|
|
Invasive lobular
carcinoma | 21 | 19.6 |
|
Invasive ductal carcinoma | 81 | 75.7 |
|
Other | 5 | 4.7 |
|
Missing | 7 | 6.1 |
| Initial stage |
|
|
|
I–III | 95 | 83.3 |
| IV
(de novo) | 19 | 16.7 |
| Hormone receptor
status |
|
|
|
ER+/PR+ | 94 | 82.5 |
|
ER+/PR− | 19 | 16.6 |
|
ER−/PR+ | 1 | 0.9 |
| Endocrine
therapy |
|
|
|
Fulvestrant | 97 | 85.1 |
|
Letrozole | 17 | 14.9 |
| ALC, g/l |
|
|
|
≥1.50 | 49 | 43.0 |
|
1.49-0.80 | 47 | 41.0 |
|
0.79-0.50 | 13 | 12.0 |
|
0.49-0.20 | 5 | 4.0 |
 | Table II.Characteristics of patients according
to pretreatment absolute lymphocyte count. |
Table II.
Characteristics of patients according
to pretreatment absolute lymphocyte count.
|
| Number |
|
|---|
|
|
|
|
|---|
| Characteristic | ALC >1.5 g/l
(n=49) | ALC <1.5 g/l
(n=65) | P-value |
|---|
| PS |
|
| 0.170 |
|
0/1 | 45.0 (91.8%) | 53.0 (81.5%) |
|
|
2/3 | 4.0 (8.2%) | 12.0 (18.5%) |
|
| Previous lines of
treatment, median (range) | 3.0 (2.0-4.0) | 4.0 (2.0-6.0) | 0.170 |
| Best response |
|
| 0.016a |
| CR | 1.0 (2.0%) | 0.0 (0.0%) |
|
| PR | 11.0 (22.4%) | 9.0 (13.8%) |
|
| SD | 27.0 (55.1%) | 26.0 (40.0%) |
|
| PD | 10.0 (20.4%) | 30.0 (46.2%) |
|
| PFS, months
(range) | 10.0
(7.0-16.0) | 6.0 (4.0-8.0) | 0.004a |
| OS, months
(range) | 33.0 (27.0-NA) | 20.0
(17.0-27.0) | 0.020a |
| Adverse events |
|
| 0.900 |
| Grade
1/2 | 11.0 (26.2%) | 14.0 (26.4%) |
|
| Grade
3/4 | 31.0 (73.8%) | 39.0 (73.6%) |
|
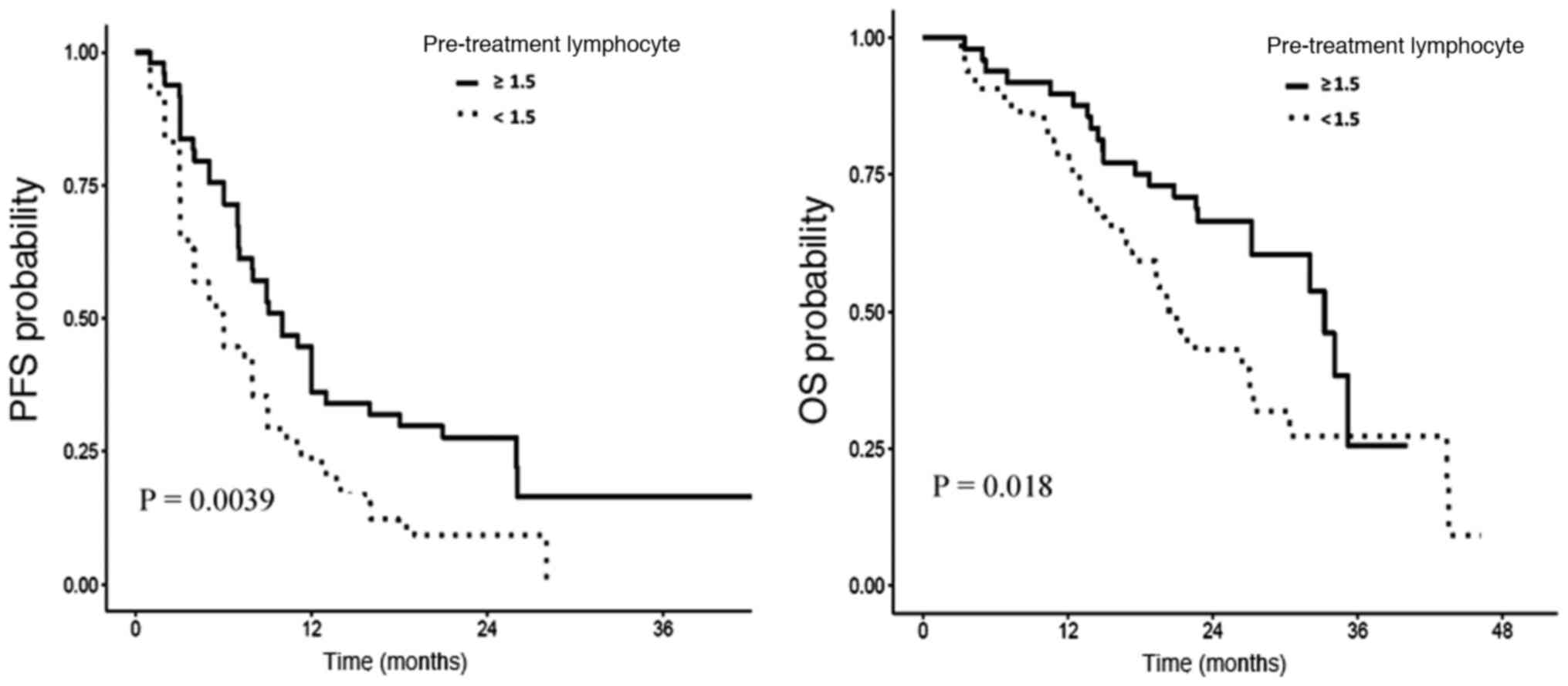
PFS was shorter in patients with
lymphopenia
Median PFS in the whole population was 7.9 months.
Patients with baseline lymphopenia had significantly shorter PFS (6
vs. 10 months; log-rank P=0.004; Fig.
1). Univariate analysis demonstrated that age did not influence
PFS. Patients who received <5 previous lines of treatment had a
significantly longer PFS (9 vs. 5 months; P<0.0001). Palbociclib
dose reduction and the absence of AE were associated with worse PFS
(6 vs. 8 months; P<0.0002 and 4 vs. 8 months; P=0.0088,
respectively) (Table III). In
multivariate analysis, age did not influence PFS. Lymphopenia and
palbociclib dose reduction were associated with worse PFS [hazard
ratio (HR)=1.71 (1.13-2.60); P=0.01 and HR=2.38 (1.45-3.89);
P<0.001, respectively]. Presence of AE and <5 previous lines
of treatment were significantly associated with better PFS [HR=0.39
(0.23-0.68) and 0.41 (0.26-0.64), respectively; P<0.001)
(Table IV).
 | Table III.Univariate analysis of factors
associated with progression-free survival. |
Table III.
Univariate analysis of factors
associated with progression-free survival.
| Variable | HR | 95% CI | P-value |
|---|
| Age <51
years | 1.12 | 0.74-1.68 | 0.6000 |
| Dose reduction of
palbociclib | 2.38 | 1.45-3.89 |
<0.0002a |
| Baseline ALC
<1.5 g/l | 1.71 | 1.13-2.60 | 0.0115a |
| Occurrence of
adverse events | 0.39 | 0.23-0.68 | 0.0088a |
| <5 treatment
lines | 0.41 | 0.26-0.63 |
<0.0001a |
 | Table IV.Multivariate analysis of factors
associated with overall survival. |
Table IV.
Multivariate analysis of factors
associated with overall survival.
| Variable | HR | 95% CI | P-value |
|---|
| Age <51
years | 0.79 | 0.48-1.30 | 0.3485 |
| Dose reduction of
palbociclib | 1.42 | 0.80-2.51 | 0.2319 |
| Baseline ALC
<1.5 g/l | 1.76 | 1.03-3.02 | 0.0399a |
| Occurrence of
adverse events | 1.14 | 0.54-2.40 | 0.7245 |
| <5 treatment
lines | 0.72 | 0.42-1.26 | 0.2517 |
OS was shorter in patients with
lymphopenia..
Median OS in the whole population was 27 months.
Patients with baseline lymphopenia had significantly shorter OS (20
vs. 33 months; log-rank P=0.018; supremum log-rank P=0.013;
Fig. 1). In multivariate analysis,
lymphopenia was independently associated with worse OS [HR=1.76
(1.02-3.02); P=0.04]. Palbociclib dose reduction, occurrence of AE,
age and number of lines of treatment did not have any impact on OS
(Table III).
Response rate was lower in patients
with lymphopenia
In the whole group, the ORR was 18.4% (21 patients),
with CR achieved for one patient (1.7%). A total of 53 patients
(46.5%) had SD. The PD rate was 35.1%, resulting in a DCR of 64.9%
(74 patients). There was significantly less partial response (13.8
vs. 22.4%; P=0.016) and more disease progression at first disease
evaluation (46.2 vs. 20.4%; P=0.016) in patients with baseline
ALC<1.5 g/l compared with those with ALC≥1.5 g/l (Table II).
Security data are compatible with
those already known and reported in published phase 3 trials
The majority of patients experienced hematological
toxicity, as expected. A total of 96 patients (84.2%) experienced
AE. The most common AE was neutropenia (82.5%). More than 50% of
patients had grade 3 or higher neutropenia and only one patient had
febrile neutropenia. A total of eight patients (7%) experienced
thrombocytopenia.
Discussion
CDK4/6i and ET combinations are effective for most
patients with HR+/HER2− mBC (9), but certain patients fail to respond
and no biomarker is currently available to predict response to
treatment (20). To the best of
our knowledge, the present study is the first that demonstrates an
association between baseline lymphopenia and worse survival and
response rate in this population.
The host immune system serves a key role in cancer
control (21). Lymphocytes,
whether in peripheral blood or as tumor-infiltrating lymphocytes,
are key factors contributing to the body's immune response (22–24).
Baseline lymphopenia has been shown to be a poor prognostic factor
for various types of cancer (11–17). For example, in a study by
Ray-Coquard et al (17),
lymphopenia was found to be an independent prognostic factor for OS
and PFS in mBC [relative risk (RR), 1.8; 95% CI, 1.3-2.4], in
advanced soft tissue sarcoma (RR, 1.46; 95% CI, 1.0-2.1) and in
non-Hodgkin's lymphoma (RR, 1.48; 95% CI, 1.03-2.1). Lymphopenia is
a powerful predictor of chemotherapy-induced toxicity and is also a
predictive factor of the efficacy of chemotherapy in colorectal,
breast and lung cancer (11,25–27).
Several studies have reported a worse ORR in
patients with baseline lymphopenia compared with patients with
normal ALC (11,25–27),
as seen in the present study. Here, disease progression was
observed at the first evaluation in 46.2% of patients with baseline
lymphopenia vs. 20.4% with normal ALC (P=0.016). For
HR+/HER2− BC, fewer data are available
concerning the impact of lymphopenia on survival, although it is
known that higher ALC is associated with better response to ET
(28). Here, the majority of
patients received fulvestrant (85%); to the best of our knowledge,
the type of ET does not influence ALC. More recently, it was
reported that neutrophil-to-lymphocyte ratio is a predictive marker
for response to ET in mBC (29).
In the Ray-Coquard et al study (17) the ALC threshold was set at 1 g/l to
predict OS in different types of tumor. In the present study, OS
and PFS were impacted regardless of ALC.
Tumor cells can elude immune surveillance. One of
the mechanisms of this escape is the recruitment of
immunosuppressive regulatory T lymphocytes (TLs) (30). The previous success of
immunotherapy based on anti-cytotoxic TL-associated antigen 4 or
anti-programmed death-1/programmed death ligand-1 antibodies
confirms the relevance of TL-based anti-tumor immunity and suggests
that restoration of the immune system could promote tumor control
(31). Several animal and in
vitro models have demonstrated the immune actions of CDK4/6i
(32–34). CDK4/6i increase the immunogenicity of tumor cells
(32) and enhance tumor
infiltration via TL activation (35,36).
CDK4/6i enable reactivation of nuclear factor of activated T cell
proteins and their target genes, including the gene encoding IL-2,
a major cytokine that activates TL effectors (36). Finally, CDK4/6i may decrease the
proliferation of regulatory TL (32,36),
reversing the balance of TL effectors and TL regulators in favor of
tumor control. CDK4/6i also increase expression of genes involved
in antigen processing and presentation in vivo in mouse and
patient-derived xenograft models and suppress the proliferation of
immune-suppressive regulatory TL, thus promoting cytotoxic
TL-mediated tumor cell destruction (35). Lymphopenia may reflect T cell
dysfunction with limited ability to perform antitumor functions and
immune actions during palbociclib therapy (37).
Both host characteristics and a high tumor burden
can result in lymphopenia (38).
In a pooled series, lymphopenia was associated with patient age
(39). Inflammation-induced cell
death and decreased thymic function have been suggested as
potential mechanisms of peripheral lymphopenia observed in patients
with metastasis (40). In our
study, median age and PS were similar in patients regardless of
ALC. A possible explanation is that our population was young
(median age was only 51 years).
A large number of the patients in the present study
were from the French Compassionate Access Program, which provides
temporary authorization for use of unlicensed drugs outside of
clinical trials to treat serious or rare diseases when no
appropriate treatment exists. This program was implemented to
improve early access to promising drugs (41). Thus patients were heavily
pretreated, as in the Battisti et al study (42). This may explain the shorter PFS and
OS in the present study compared with those reported in published
registration studies (3–8). All patients received palbociclib
because it was the first drug to have obtained marketing
authorization in France. Lymphopenia was independent of the number
of previous lines of treatment. Lymphopenia has been reported as a
risk factor for the occurrence of chemotherapy-induced
hematotoxicity, especially neutropenia, severe thrombocytopenia and
anemia requiring transfusion and early death following chemotherapy
(17,25,26,43,44).
ALC of 0.7 g/l was previously identified as the most discriminative
predictive value for hematological AE (45). Here, there was no association
between baseline ALC and the probability of AE. Regarding dose
reduction, the present results are consistent with previously
published studies showing that reduce the dose of CDK4/6i has a
negative impact on treatment efficacy (46,47).
A recent publication assessed the role of certain
genomic markers in circulating tumor DNA to identify patients at
higher risk of early progression following fulvestrant therapy in
the presence or absence of palbociclib (48). The aforementioned study found that
high-circulating tumor fraction, TP53 mutation and fibroblast
growth factor receptor 1 amplification were associated with worse
PFS even following the addition of CDK4/6i. Despite the interest in
these genomic markers in prognostic estimation, they remain
expensive and difficult to monitor in daily practice. The present
study suggested that assessment of ALC, a routine and less
expensive test, may serve as a significant prognostic factor for
patients with HR+/HER2− mBC.
The present study had certain limitations, including
the small sample size and retrospective single-center design. Due
to lack of data, lactate dehydrogenase LDH dosage, an indicator of
high tumor burden as suggested in another study (49), was not assessed. The present study
did not have a long follow-up, however it was sufficient to obtain
fairly discriminative survival information. Due to the limited
sample size, retrospective design and heterogeneity of the
population, other elements, such as markers of inflammation and
CD4/8 TL count ratio could not be evaluated. The present results
need to be confirmed by large-scale studies with extensive
follow-up and assessment of other inflammation markers.
To the best of our knowledge, the present study is
the first to demonstrate the impact of baseline lymphopenia as a
strong and easy-to-use prognostic factor for patients with
HR+/HER2− mBC treated with palbociclib in
combination with ET. Lymphopenia may also be a predictive factor of
early progression. A larger study is needed to confirm these
results.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GE was responsible for the conception and design of
the study. GE, SP and ADS collected the data and wrote the
manuscript. GE and ADS confirm the authenticity of all the raw
data. JL performed statistical analysis, participated in data
analysis, interpreted the data and wrote the manuscript. GE, SP,
ADS, CL, AJ, DA, IH, CS, AM, KG, AF and FC interpreted the data and
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
In accordance with the regulations regarding
research involving human subjects, the present study was registered
with corresponding data protection. Patients' non-opposition to the
use of their data was sought after verification of their vital
status.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALC
|
absolute lymphocyte count
|
|
BC
|
breast cancer
|
|
mBC
|
metastatic breast cancer
|
|
ET
|
endocrine therapy
|
|
CDK4/6i
|
cyclin dependent kinase 4–6
inhibitor
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
AE
|
adverse event
|
|
HR+
|
hormone receptor-positive
|
|
HER2−
|
human epidermal growth factor receptor
2-negative
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
ORR
|
objective response rate
|
|
DCR
|
disease control rate
|
|
PS
|
performance status
|
|
HR
|
hazard ratio
|
|
TL
|
T lymphocytes
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cardoso F, Paluch-Shimon S, Senkus E,
Curigliano G, Aapro MS, André F, Barrios CH, Bergh J, Bhattacharyya
GS, Biganzoli L, et al: 5th ESO-ESMO international consensus
guidelines for advanced breast cancer (ABC 5). Ann Oncol.
31:1623–1649. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Finn RS, Crown JP, Lang I, Boer K,
Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, et
al: The cyclin-dependent kinase 4/6 inhibitor palbociclib in
combination with letrozole versus letrozole alone as first-line
treatment of oestrogen receptor-positive, HER2-negative, advanced
breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study.
Lancet Oncol. 16:25–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cristofanilli M, Turner NC, Bondarenko I,
Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, et
al: Fulvestrant plus palbociclib versus fulvestrant plus placebo
for treatment of hormone-receptor-positive, HER2-negative
metastatic breast cancer that progressed on previous endocrine
therapy (PALOMA-3): Final analysis of the multicentre,
double-blind, phase 3 randomised controlled trial. Lancet Oncol.
17:425–439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hortobagyi GN, Stemmer SM, Burris HA, Yap
YS, Sonke GS, Paluch-Shimon S, Campone M, Petrakova K, Blackwell
KL, Winer EP, et al: Updated results from MONALEESA-2, a phase III
trial of first-line ribociclib plus letrozole versus placebo plus
letrozole in hormone receptor-positive, HER2-negative advanced
breast cancer. Ann Oncol. 29:1541–1547. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slamon DJ, Neven P, Chia S, Fasching PA,
De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martín
M, et al: Phase III randomized study of ribociclib and fulvestrant
in hormone receptor-positive, human epidermal growth factor
receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin
Oncol. 36:2465–2472. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goetz MP, Toi M, Campone M, Sohn J,
Paluch-Shimon S, Huober J, Park IH, Trédan O, Chen SC, Manso L, et
al: MONARCH 3: Abemaciclib as initial therapy for advanced breast
cancer. J Clin Oncol. 35:3638–3646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sledge GW Jr, Toi M, Neven P, Sohn J,
Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, et al:
The effect of abemaciclib plus fulvestrant on overall survival in
hormone receptor-positive, ERBB2-negative breast cancer that
progressed on endocrine therapy-MONARCH 2: A Randomized Clinical
Trial. JAMA Oncol. 6:116–124. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schettini F, Giudici F, Giuliano M,
Cristofanilli M, Arpino G, Del Mastro L, Puglisi F, De Placido S,
Paris I, De Placido P, et al: Overall survival of
CDK4/6-inhibitor-based treatments in clinically relevant subgroups
of metastatic breast cancer: Systematic review and meta-analysis. J
Natl Cancer Inst. 112:1089–1097. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Migliaccio I, Leo A, Galardi F, Guarducci
C, Fusco GM, Benelli M, Di Leo A, Biganzoli L and Malorni L:
Circulating biomarkers of CDK4/6 inhibitors response in hormone
receptor positive and HER2 negative breast cancer. Cancers (Basel).
13:26402021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cézé N, Thibault G, Goujon G, Viguier J,
Watier H, Dorval E and Lecomte T: Pre-treatment lymphopenia as a
prognostic biomarker in colorectal cancer patients receiving
chemotherapy. Cancer Chemother Pharmacol. 68:1305–1313. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grossman SA, Ye X, Lesser G, Sloan A,
Carraway H, Desideri S and Piantadosi S; NABTT CNS Consortium, :
Immunosuppression in patients with high-grade gliomas treated with
radiation and temozolomide. Clin Cancer Res. 17:5473–5480. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balmanoukian A, Ye X, Herman J, Laheru D
and Grossman SA: The association between treatment-related
lymphopenia and survival in newly diagnosed patients with resected
adenocarcinoma of the pancreas. Cancer Invest. 30:571–576. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He JR, Shen GP, Ren ZF, Qin H, Cui C,
Zhang Y, Zeng YX and Jia WH: Pretreatment levels of peripheral
neutrophils and lymphocytes as independent prognostic factors in
patients with nasopharyngeal carcinoma. Head Neck. 34:1769–1776.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kobayashi N, Usui S, Kikuchi S, Goto Y,
Sakai M, Onizuka M and Sato Y: Preoperative lymphocyte count is an
independent prognostic factor in node-negative non-small cell lung
cancer. Lung Cancer. 75:223–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng JF, Liu JS and Huang Y: Lymphopenia
predicts poor prognosis in patients with esophageal squamous cell
carcinoma. Medicine (Baltimore). 93:e2572014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ray-Coquard I, Cropet C, Van Glabbeke M,
Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P,
Labidi I, et al: European Organization for Research and Treatment
of Cancer Soft Tissue and Bone Sarcoma Group: Lymphopenia as a
prognostic factor for overall survival in advanced carcinomas,
sarcomas, and lymphomas. Cancer Res. 69:5383–5391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwartz LH, Seymour L, Litière S, Ford R,
Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, et
al: RECIST 1.1 - Standardisation and disease-specific adaptations:
Perspectives from the RECIST Working Group. Eur J Cancer.
62:138–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
US Department of Health Human Services:
Common Terminology Criteria for Adverse Events (CTCAE).v.5.0. Natl
Institutes Heal Natl Cancer Institute. p1552017.PubMed/NCBI
|
|
20
|
Garrido-Castro AC and Goel S: CDK4/6
inhibition in breast cancer: Mechanisms of response and treatment
failure. Curr Breast Cancer Rep. 9:26–33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: From immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rosenberg SA: The immunotherapy of solid
cancers based on cloning the genes encoding tumor-rejection
antigens. Annu Rev Med. 47:481–491. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bates GJ, Fox SB, Han C, Leek RD, Garcia
JF, Harris AL and Banham AH: Quantification of regulatory T cells
enables the identification of high-risk breast cancer patients and
those at risk of late relapse. J Clin Oncol. 24:5373–5380. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schreiber RD, Old L and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–7150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ray-Coquard I, Ghesquière H, Bachelot T,
Borg C, Biron P, Sebban C, LeCesne A, Chauvin F and Blay JY; ELYPSE
Study Group, : Identification of patients at risk for early death
after conventional chemotherapy in solid tumours and lymphomas. Br
J Cancer. 85:816–822. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ray-Coquard I, Borg C, Bachelot T, Sebban
C, Philip I, Clapisson G, Le Cesne A, Biron P, Chauvin F and Blay
JY; ELYPSE study group, : Baseline and early lymphopenia predict
for the risk of febrile neutropenia after chemotherapy. Br J
Cancer. 88:181–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lissoni P, Brivio F, Fumagalli L, Messina
G, Ghezzi V, Frontini L, Giani L, Vaghi M, Ardizzoia A and Gardani
GS: Efficacy of cancer chemotherapy in relation to the pretreatment
number of lymphocytes in patients with metastatic solid tumors. Int
J Biol Markers. 19:135–140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Franks CR and Williams Y: Prognostic value
of peripheral lymphocyte count in hormone therapy of advanced
breast cancer. Br J Cancer. 34:641–644. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Limori N, Shinichiro K, Yuka A, Wataru G,
Koji T, Katsuyuki T and Takaharu H: Clinical significance of the
neutrophil-to-lymphocyte ratio in endocrine therapy for stage IV
breast cancer. In Vivo. 32:669–675. 2018.PubMed/NCBI
|
|
30
|
Gobert M, Treilleux I, Bendriss-Vermare N,
Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I,
Olive D, et al: Regulatory T cells recruited through CCL22/CCR4 are
selectively activated in lymphoid infiltrates surrounding primary
breast tumors and lead to an adverse clinical outcome. Cancer Res.
69:2000–2009. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang Y: Cancer immunotherapy: Harnessing
the immune system to battle cancer. J Clin Invest. 125:3335–3337.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goel S, DeCristo MJ, Watt AC, BrinJones H,
Sceneay J, Li BB, Khan N, Ubellacker JM, Xie S, Metzger-Filho O, et
al: CDK4/6 inhibition triggers anti-tumour immunity. Nature.
548:471–475. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lelliott EJ, Kong IY, Zethoven M,
Ramsbottom KM, Martelotto LG, Meyran D, Zhu JJ, Costacurta M, Kirby
L, Sandow JJ, et al: CDK4/6 inhibition promotes antitumor immunity
through the induction of T-cell memory. Cancer Discov. May
14–2021.doi: 10.1158/2159-8290.CD-20-1554. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang QF, Li J, Jiang K, Wang R, Ge JL,
Yang H, Liu SJ, Jia LT, Wang L and Chen BL: CDK4/6 inhibition
promotes immune infiltration in ovarian cancer and synergizes with
PD-1 blockade in a B cell-dependent manner. Theranostics.
10:10619–10633. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Teh JL and Aplin AE: Arrested
developments: CDK4/6 inhibitor resistance and alterations in the
tumor immune microenvironment. Clin Cancer Res. 25:921–927. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Deng J, Wang ES, Jenkins RW, Li S, Dries
R, Yates K, Chhabra S, Huang W, Liu H, Aref AR, et al: CDK4/6
inhibition augments antitumor immunity by enhancing T-cell
activation. Cancer Discov. 8:216–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Williams KM, Hakim FT and Gress RE: T cell
immune reconstitution following lymphodepletion. Semin Immunol.
19:318–330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ménétrier-Caux C, Ray-Coquard I, Blay JY
and Caux C: Lymphopenia in cancer patients and its effects on
response to immunotherapy: An opportunity for combination with
Cytokines? J Immunother Cancer. 7:852019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ferrando-Martínez S, Franco JM, Hernandez
A, Ordoñez A, Gutierrez E, Abad A and Leal M: Thymopoiesis in
elderly human is associated with systemic inflammatory status. Age
(Dordr). 31:87–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Manuel M, Tredan O, Bachelot T, Clapisson
G, Courtier A, Parmentier G, Rabeony T, Grives A, Perez S, Mouret
JF, et al: Lymphopenia combined with low TCR diversity (divpenia)
predicts poor overall survival in metastatic breast cancer
patients. OncoImmunology. 1:432–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Degrassat-Théas A, Paubel P, Parent de
Curzon O, Le Pen C and Sinègre M: Temporary authorization for use:
Does the French patient access programme for unlicensed medicines
impact market access after formal licensing? PharmacoEconomics.
31:335–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Battisti NM, Kingston B, King J, Denton A,
Waters S, Sita-Lumsden A, Rehman F, Stavraka C, Kristeleit H,
Sawyer E, et al: Palbociclib and endocrine therapy in heavily
pretreated hormone receptor-positive HER2-negative advanced breast
cancer: The UK Compassionate Access Programme experience. Breast
Cancer Res Treat. 174:731–740. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Blay J, Ray-Coquard I and Mermet C: A
multicentric prospective study of prognostic factors for febrile
neutropenia after chemotherapy in general and cancer hospitals. J
Clin Oncol. 16:56a1997.
|
|
44
|
Blay JY, Le Cesne A, Mermet C, Maugard C,
Ravaud A, Chevreau C, Sebban C, Guastalla J, Biron P and
Ray-Coquard I: A risk model for thrombocytopenia requiring platelet
transfusion after cytotoxic chemotherapy. Blood. 92:405–410. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Borg C, Ray-Coquard I, Philip I, Clapisson
G, Bendriss-Vermare N, Menetrier-Caux C, Sebban C, Biron P and Blay
JY: CD4 lymphopenia as a risk factor for febrile neutropenia and
early death after cytotoxic chemotherapy in adult patients with
cancer. Cancer. 101:2675–2680. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Moftakhar B, Lekkala M, Strawderman M,
Smith TC, Meacham P, Fitzgerald B, Falkson CI and Dhakal A: Impact
of early dose intensity reduction of Palbociclib on clinical
outcomes in patients with hormone-receptor-positive metastatic
breast cancer. Breast Cancer Res Treat. 183:411–418. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Groenland SL, Martínez-Chávez A, van
Dongen MG, Beijnen JH, Schinkel AH, Huitema AD and Steeghs N:
Clinical Pharmacokinetics and Pharmacodynamics of the
Cyclin-Dependent Kinase 4 and 6 Inhibitors Palbociclib, Ribociclib,
and Abemaciclib. Clin Pharmacokinet. 59:1501–1520. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
O'Leary B, Cutts RJ, Huang X, Hrebien S,
Liu Y, André F, Loibl S, Loi S, Garcia-Murillas I, Cristofanilli M,
et al: Circulating tumor DNA markers for early progression on
fulvestrant with or without palbociclib in ER+ advanced
breast cancer. J Natl Cancer Inst. 113:309–317. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Che YQ, Zhang Y, Wang D, Liu H-Y, Shen D
and Luo Y: Baseline lymphopenia: A predictor of poor outcomes in
HER2 positive metastatic breast cancer treated with trastuzumab.
Drug Des Devel Ther. 13:3727–3734. 2019. View Article : Google Scholar : PubMed/NCBI
|