Introduction
Hepatocellular carcinoma (HCC) is the commonest
primary liver malignancy and its incidence is increasing worldwide
(1). According to the GLOBOCAN
database, in 2020, including intrahepatic bile ducts, there were
905,677 new cases of liver cancer around the world, accompanied by
830,180 new deaths (2). Liver
cirrhosis is a major risk factor for HCC (3). Although liver transplantation is the
primary long term curative treatment, HCC still has a recurrence
rate of 10–15% in transplant patients (4). Since biopsy is not mandatory for HCC
diagnosis (5), the availability of
tumor specimens for analysis is limited, which in turn has limited
our understanding of the molecular mechanisms involved in
carcinogenesis.
The calcium ion (Ca2+) is an
intracellular second messenger involved in energy metabolism, cell
cycle control, gene expression, cell proliferation, cell migration,
necrosis and apoptosis in nearly every cell type, including
hepatocytes (6). The inositol
1,4,5-trisphosphate receptor (ITPR) is the only intracellular
Ca2+ release channel expressed in the liver, with all
three isoforms described in humans and rodents (7,8). The
expression of each ITPR varies among cell type, having distinct
subcellular distribution and biophysical properties (9). For instance, under physiological
conditions type 1 (ITPR1) and 2 (ITPR2) are the predominant
isoforms in hepatocytes (10,11),
while isoform 3 (ITPR3) is absent or minimally expressed (12). However, ITPR3 becomes significantly
expressed in hepatocytes in acute (13,14)
and chronic liver diseases, as well as in HCC and in liver cancer
cell lines (15,16). ITPR3 expression is also increased
in other malignancies, including cholangiocarcinoma (17), colon cancer (18), melanoma (19), mesothelioma and prostate cancer
(20).
Epigenetic events have been associated with the
expression of ITPR3 in HCC, with hypomethylation of the promoter
region of the ITPR3 gene upregulating its expression (16). In contrast, the promoter regions of
ITPR1 and ITPR2 are already demethylated in normal hepatocytes
(16), consistent with the
observation that ITPR1 and ITPR2 are constitutively expressed in
this cell type (10).
To understand the biological role of ITPR3 in HCC,
the present study compared ITPR3 expression in HCC and adjacent
cirrhotic parenchyma in patients with different types of underlying
chronic liver disease. It also correlated ITPR3 expression with
clinical and morphological parameters in order to investigate
whether ITPR3 could serve as a prognostic marker.
Materials and methods
Sample characterization
A total of 53 liver explants from patients who
underwent orthotopic liver transplantation (OLT) in Hospital das
Clínicas, Universidade Federal de Minas Gerais (UFMG; Federal
University of Minas Gerais), Brazil, between January 2002 and
December 2017, were retrospectively reviewed after the study was
approval by the local Ethics Committee, COEP-UFMG, in the city of
Belo Horizonte, state of Minas Gerais, Brazil (CAAE
71206617.8.0000.5149) and written informed consent was obtained
from the patients or their relatives. Histologically normal tissues
were obtained from liver resections of patients with metastatic
colon cancer between 2010 and 2017 at Hospital das Clínicas (UFMG),
after rigorous examination by an experienced pathologist.
A representative slide of each case was selected and
analyzed after staining with conventional hematoxylin and eosin
(H&E) and immunohistochemistry. Formalin-fixed (concentration
10%; duration 12 h at room temperature) and paraffin-embedded
samples were deparaffinized and antigen retrieval was performed in
citrate buffer (10 mM) containing 0.6% peroxide of hydrogen
(heating temperature, 65°C for 8 h in the thermal oven), followed
by four immersions in xylene (the first for 20 min and the others
with 1 min duration) and three immersions in absolute alcohol (the
first for 5 min and the others with 1 min duration). Afterwards,
the slides were immersed in running water for 3 min. The Novolink
Polymer Detection System (cat. no. RE7200-CE; Leica Microsystems,
Inc.) was used in the subsequent steps as described previously
(21). Primary antibodies against
ITPR3 (anti-ITPR3; Sigma-Aldrich; Merck KGaA; cat. no. HPA003915)
and ssDNA labeling (anti-Single Stranded DNA; cat. no. 18731;
Immuno-Biological Laboratories, Co., Ltd.) were incubated overnight
at room temperature in a 1:100 dilution, followed by incubation
with detection polymer for 40 min at room temperature. DAB was used
for signal detection.
Clinical pre-OLT data and histopathological
parameters of the explanted liver were retrospectively collected
from the medical records, including sex, age, etiology of the
underlying chronic liver disease (CLD), model for end-stage liver
disease (MELD) and CHILD-Pugh scores (CHILD); prognostic models
that estimate the severity of underlying liver condition, pre-OLT
serum levels of α fetoprotein (AFP), number of nodules, size of the
greatest nodules, microvascular invasion and histological grade.
The medians of MELD, AFP, total bilirubin, international normalized
ratio, creatinine, alanine aminotransferase, aspartate
aminotransferase and serum albumin were obtained from the sum of
the values of each of these clinical data for each patient.
Therefore, the medians reflect the mean of the total sum of the 53
patients in this study, between the minimum and maximum values.
Outcomes, such as death from any cause or tumor
recurrence since OLT and event-free survival (EFS), defined as the
time interval between the OLT date and the occurrence of the event
or ending of the follow-up period (December 20, 2017), were
analyzed.
Analysis of ITPR3 expression intensity
and ssDNA labeling index
To evaluate ITPR3 expression, images of
immunohistochemistry slides stained for ITPR3 from 10 different
fields (magnification, ×40) were captured in tumor and adjacent
cirrhotic parenchyma through an optical microscope (Zeiss GmbH).
The intensity of ITPR3 staining was evaluated by delimiting 10
cytoplasmic regions, excluding the nucleus, using ImageJ 1.50i
software (National Institutes of Health). The software performed
this analysis through a histogram, which expressed the intensity of
the pixels in each selected region on a scale between 0–255. ITPR3
expression intensity was expressed as the mean of 10 analyzed cells
in each of the 10 imaged fields.
Mitotic index evaluation
A total of two experienced liver pathologists
identified areas of interest and counted the number of mitotic
cells in 10 high-power fields (HPF) of H&E stained slides
(magnification, ×20) in order to identify areas containing mitotic
figures, in metaphase or anaphase. Following Baak (22), the mitotic cells were counted only
with a complete agreement between the two pathologists through a
multi-head microscope. The delineation between low mitotic index in
samples with >5 mitotic figures at 10 HPF and high mitotic index
in samples with ≥5 mitotic figures at 10 HPF was based on previous
studies (23–27).
Pre-processing of raw RNA sequencing
(RNA-seq) data
Raw read sequences were pre-processed by filtering
out read sequences with less than 36 bp, removing low quality or N
bases from the read ends, trimming Illumina adapters, scanning the
read with a 4-base wide sliding window and cutting when the average
quality per base dropped below 15 (LEADING:3 TRAILING:3
SLIDINGWINDOW:4:15 MINLEN:36) by Trimmomatic v0.39 (28).
The raw RNA-seq data were obtained in our previous
publication, through experiments with HepG2 (16), considered a cell line of liver
cancer. A commercially available CRISPR/Cas9 system was used to
eliminate ITPR3 in HepG2 liver cancer cells (Santa Cruz
Biotechnology, Inc.). Cells were grown in Dulbecco's Modified
Eagles Medium (cat. no. 11320033) supplemented with L-glutamine (1
mM; cat. no. 25030149), fetal bovine serum (10% v/v; cat. no.
A4766801) and penicillin/streptomycin (100 units/ml and 100 mg/ml;
cat. no. 15140122) at 37°C in a humid environment with an
artificial atmosphere containing 5% CO2.
Quantification and differential gene
expression analysis
Quantification of the gene expression (−g) was
performed by Salmon v1.2.1 (29).
The transcriptome index was built using the cDNA sequences of the
Homo sapiens assembly version GRCh38 downloaded from Ensembl
release 104 (30). The estimated
number of reads were used for the differential gene expression
analysis. Genes with low expression levels were removed; genes with
counts scaled by total mapped reads per million >1 in at ≥9
samples were retained for differential gene expression analysis.
DESeq2 (31) was applied to
evaluate the differential gene expression using DEBrowser v1.16.1
(32). Genes were considered
differentially expressed when they had an adjusted P-value
<0.05; fold-change ≤-1.5 (downregulated or more enriched in
control condition).
Identification of differentially
expressed genes associated with apoptosis
Genes participating in the human apoptosis route
were extracted from the Kyoto Encyclopedia of Genes and Genomes
(KEGG) Pathways (33) Apoptosis
map (hsa04210) was obtained using the rest application programming
interface of KEGG. Genes on the apoptosis pathway were mapped to
their corresponding UniProt identifiers by UniProt's idmapping (The
Uniprot Consortium 2021) (34).
The same was done for the ensemble gene IDs of differentially
expressed genes. An in-house custom python script was used to
compare both lists and identify differentially expressed genes
participating on the apoptosis pathway. A custom colorized version
of the apoptosis pathway, containing upregulated genes in green,
downregulated genes in red and non-differentially expressed genes
in white was generated using KEGG Mapper (35).
Heatmap
Heatmaps of the top 50 differentially expressed
genes sorted by the average Reads per Kilobase per Million of
mapped reads were generated for each condition by Heatmapper
(36) using average linkage
clustering and Euclidean measurement methods.
Statistical analysis
Descriptive statistics summarized the data, which
were expressed as numbers and percentages (categorical) and as
median and interquartile range (continuous). A normality test
(Shapiro-Wilk) was performed for each continuous variable. For
comparison between the etiological groups, χ2 or the
Fisher's exact test was applied in categorical variables and Mann
Whitney or Kruskal-Wallis test in continuous data. In multiple
comparisons, Dunn's test was applied after the Kruskal-Wallis test.
Multivariate COX regression model with covariance structure was
performed to determine the model for EFS. Moreover, to evaluate the
correlation between the clinicopathological parameter and ITPR3
expression, Spearman's coefficient was used. SPSS software, v20
(IBM Corp.), was used for statistical analysis. Three technical
replicates were used per sample in the analyses. Final results were
expressed as the mean of the three values. P<0.05 was considered
to indicate a statistically significant difference.
Results
The etiologies of the underlying chronic liver
diseases among the explanted livers were distributed as follows:
Hepatitis C virus (HCV) infection (n=31; 58.5%), alcoholic liver
disease (ALD; n=16; 30.2%) and cryptogenic cirrhosis (CC; n=6;
11.3%). Multiple etiologies or coexistence of other liver
pathologies were considered exclusion criteria.
Among all etiological groups, the median age of the
patients was 57.09 years (range, 41.11-74.03). The median MELD
score was 15.0 (range, 6.0-32.0) and the median AFP was 123.78
(range, 1.00-2883.00). The median total bilirubin was 2.65 (range,
0.50-8.70), the median international normalized ratio was 1.045
(range, 0.990-2.690) and the median creatinine was 1.02 (range,
0.14-3.00). The median alanine aminotransferase was 91.3 (range,
24.0-325.0), the median aspartate aminotransferase was 85.54
(range, 25.00-267.00) and the median serum albumin was 3.20 (range,
2.00-4.80). All clinical and laboratory data are summarized in
Table I.
 | Table I.Clinical and laboratory data of
patients with hepatocellular carcinoma. |
Table I.
Clinical and laboratory data of
patients with hepatocellular carcinoma.
| Parameter | Minimum | Median | Maximum |
|---|
| Age (years) | 41.11 | 57.09 | 74.03 |
| MELD | 6.0 | 15.0 | 32.0 |
| AFP (ng/ml) | 1.00 | 123.78 | 2883.00 |
| TBIL (mg/dl) | 0.50 | 2.65 | 8.70 |
| INR | 0.990 | 1.045 | 2.690 |
| Creatinine
(mg/dl) | 0.14 | 1.02 | 3.00 |
| ALT (U/l) | 24.0 | 91.3 | 325.0 |
| AST (U/l) | 25.00 | 85.54 | 267.00 |
| ALB (g/dl) | 2.00 | 3.20 | 4.80 |
Morphological descriptions of the livers from HCC
patients are presented in Table
II. The number of nodules was categorized into two groups: ≤3
nodules and >3 nodules. Vascular invasion was categorized as
present or absent. Differentiation grade, according to Edmondson
and Steiner (37), was classified
into I and II (Low grade; well differentiated) and III and IV (high
grade; poorly differentiated). The non-categorical data (mitosis
and nodule size) are expressed as median values.
 | Table II.Anatomopathological characteristics
of liver from patients with hepatocellular carcinoma. |
Table II.
Anatomopathological characteristics
of liver from patients with hepatocellular carcinoma.
| Clinical
parameters | Value |
|---|
| Nodules
numbera |
|
| ≤3 | 26 (55.3%) |
|
>3 | 21 (44.7%) |
| Vascular
invasiona |
|
|
Present | 23 (54.8%) |
|
Absent | 19 (45.2%) |
| Differentiation
degreea |
|
| Low
degree (I–II) | 35 (72.9%) |
| High
degree (III–IV) | 13 (27.1%) |
| Histological
typea |
|
|
Pseudo-acinar | 6 (12.8%) |
|
Solid | 3 (6.4%) |
|
Trabecular | 22 (46.8%) |
|
Pseudo-acinar/trabecular | 16 (34.0%) |
| Mitosis (10
HPF)b | 5.0 (0-22.0) |
| Nodule size
(cm)b | 3.0 (1-10.5) |
As ITPR3 expression is a common event in HCC
development (16), the presence
and distribution of this Ca2+ channel was evaluated in
HCC samples from different etiologies. ITPR3 staining of
cholangiocytes was used as an internal positive control, as this
liver cell type constitutively expresses ITPR3 (29). There was greater ITPR3 labeling,
both in the non-tumor regions of the explants (Fig. 1A) and the tumor regions (Fig. 1B), relative to hepatocytes from
healthy control livers. ITPR3 labeling in the tumor region of
patients with HCC caused by HCV (Fig.
1C) or ALD (Fig. 1D) were
higher in comparison with the adjacent non-tumor region, while no
difference was observed between these two regions in CC patients
(Fig. 1E). Although the level of
ITPR3 staining was similar among the etiologic groups in both the
cirrhotic parenchyma and the tumor regions (Fig. 1F), the pattern of labelling varied
among groups. In the tumor regions and in the underlying cirrhotic
parenchyma, homogeneous staining of ITPR3 was observed, with ITPR3
dispersed throughout the hepatocyte cytoplasm (Fig. 1A and B; blue arrows). In some
cases, as observed in CC, a predominant perinuclear region of ITPR3
staining (Fig. 1A and B; black
arrows) was present.
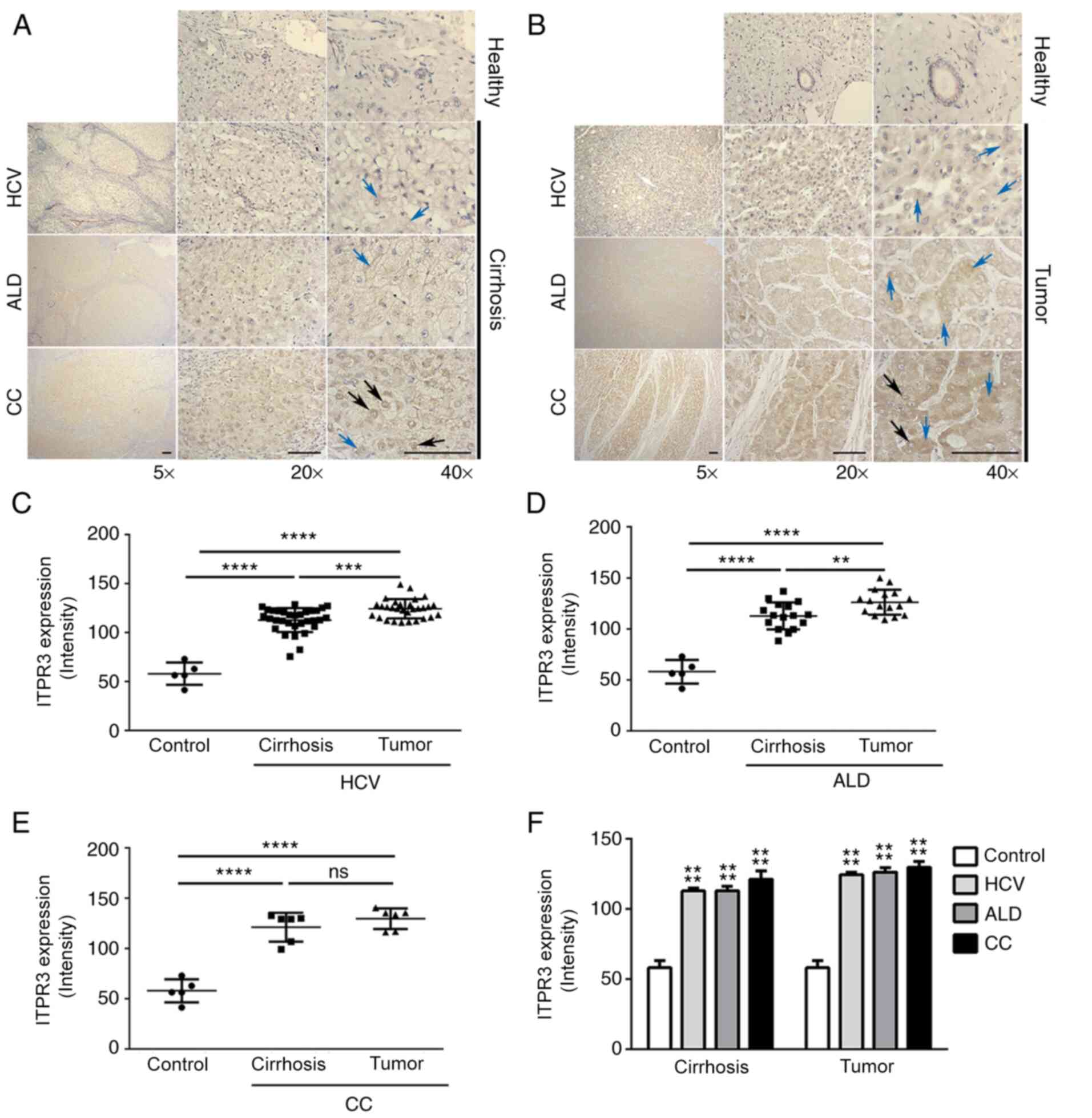 | Figure 1.Representative images of ITPR3
labeling in HCC, underlying cirrhotic parenchyma and non-tumor
livers (controls). (A) ITPR3 labeling in cirrhosis in patients
diagnosed with HCV (n=31), ALD (n=16), CC (n=6) and healthy
(control; n=2). Scale bar=200 µm (magnification, ×5), 50 µm
(magnification, × 20) and 25 µm (magnification, ×40). Black arrows
indicate ITPR3 perinuclear localization in hepatocytes. Blue arrows
indicate ITPR3 cytoplasmatic localization in hepatocytes. (B) ITPR3
labeling in tumor (HCC) region of patients diagnosed with HCV
(n=31), ALD (n=16), CC (n=6) and healthy (control; n=3). Black
arrows indicate ITPR3 perinuclear localization in hepatocytes. Blue
arrows indicate ITPR3 cytoplasmatic localization in hepatocytes.
(C) Representative graph of the intensity of ITPR3 marking in HCC
regions and cirrhotic parenchyma of patients with HCV compared with
non-tumor livers (control; n=31; ****P<0.0001; ***P<0.0001).
(D) Representative graph the intensity of ITPR3 marking in HCC
regions and cirrhotic parenchyma of patients with ALD compared with
non-tumor livers (control; n=16; **P<0.0057; ***P<0.0001).
(E) Representative graph the intensity of ITPR3 marking in HCC
regions and cirrhotic parenchyma of patients with CC compared with
non-tumor livers (control; n=06; ****P<0.0001). (F)
Representative graph the ITPR3 labeling intensity in tumor samples
and cirrhotic parenchyma comparing the three etiologies
(****P<0.0001). ITPR3, inositol 1,4,5-trisphosphate type 3
receptor; HCC, hepatocellular carcinoma; HCV, hepatitis C virus;
ALD, alcoholic liver disease; CC, cryptogenic cirrhosis; ALD,
alcoholic liver disease. |
As in other malignancies, ITPR3 expression is an
early event in HCC carcinogenesis (16,18)
which may then relate to morphological aspects as well as clinical
outcomes of patients affected by the disease. Thus, the correlation
between ITPR3 expression and clinicopathological parameters was
investigated. Table III showed
that serum AST levels positively correlated with the intensity of
the tumor ITPR3 labeling profile (P<0.02), a correlation that
was not observed in the adjacent parenchyma (P>0.4; using
Spearman's statistical test).
 | Table III.Correlation between ITPR3 labeling
intensity with anatomopathological and clinical data
parameters. |
Table III.
Correlation between ITPR3 labeling
intensity with anatomopathological and clinical data
parameters.
| Clinical
parameters | Cirrhosis
P-value | Tumor P-value |
|---|
| Number nodules | 0.120 | 0.296 |
| Vascular
invasion | 0.637 | 0.746 |
| Necrosis | 0.114 | 0.461 |
| Fibrosis | 0.752 | 0.456 |
| Tumor
inflammation | 0.383 | 0.552 |
| Intracellular
characteristics | 0.927 | 0.825 |
| Differentiation
degree | 0.677 | 0.668 |
| Histological
pattern | 0.601 | 0.941 |
| MELD | 0.395 | 0.393 |
| AFP (ng/ml) | 0.703 | 0.781 |
| TBIL (mg/dl) | 0.496 | 0.694 |
| INR | 0.403 | 0.344 |
| Creatinine
(mg/dl) | 0.216 | 0.248 |
| ALT (U/l) | 0.410 | 0.281 |
| AST (U/l) | 0.497 | 0.018a |
| Mitosis (10
HPF) | - | 0.0098b |
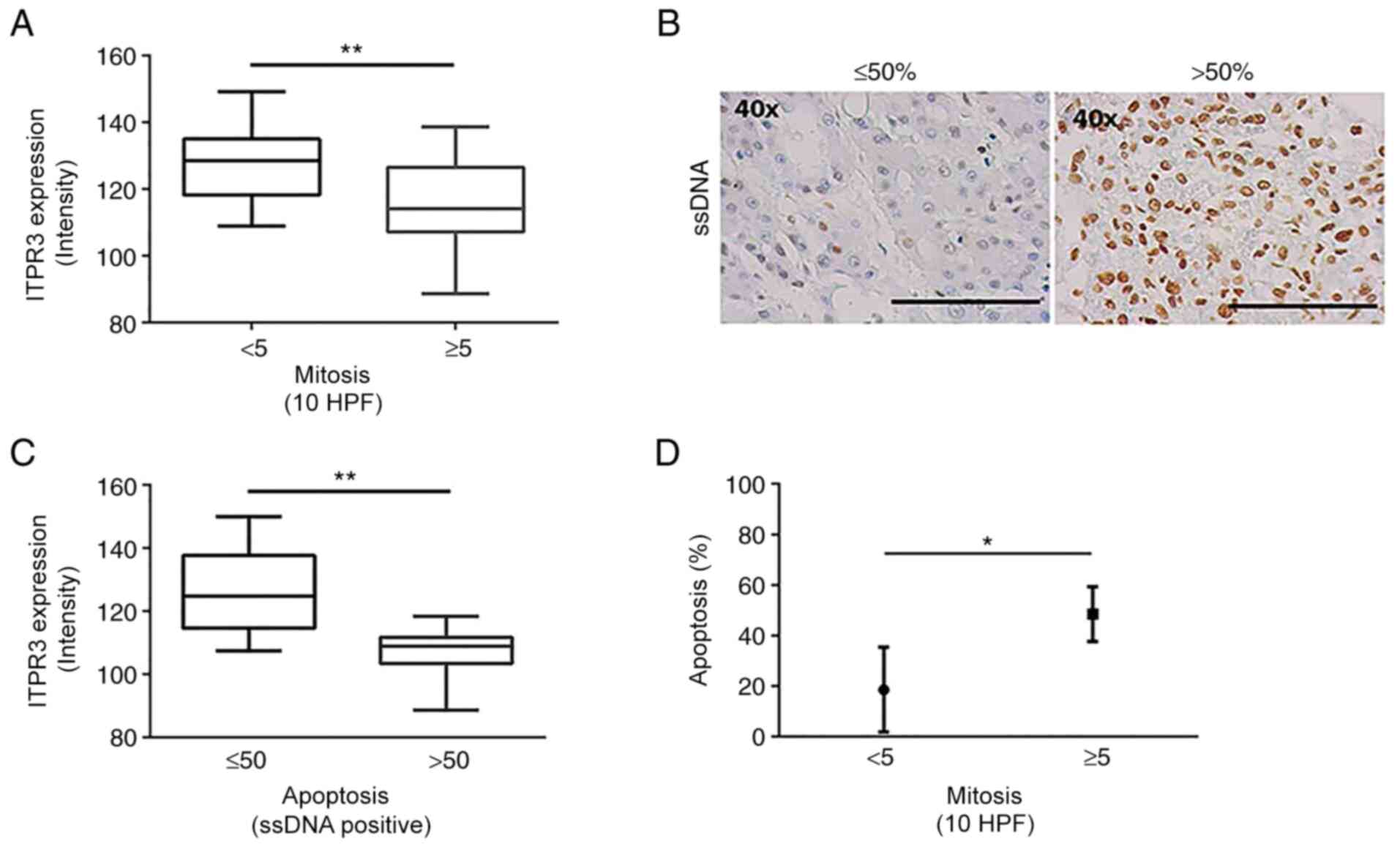
A high mitotic index (≥5/10 HPF) is a poor
prognostic factor in HCC, as well as with intrahepatic metastasis,
larger tumor size, higher AFP levels, advanced tumor stage
(24,28), vascular invasion, poor histological
differentiation and recurrence rate (26). Therefore, the mitotic index was
correlated with the intensity of ITPR3 labeling. Mitotic index was
categorized as either low (<5) or high (>5). The majority of
cases (62.8%) had a low mitotic index (n=22), while 37.2% (n=13)
exhibited high mitotic index. In addition, patients with greater
ITPR3 staining exhibited a lower mitotic index, while patients with
less ITPR3 labeling intensity exhibited a higher mitotic index
(Fig. 2A). The mitotic index was
inversely associated with ITPR3 expression in the tumor region
(P<0.01; using unpaired Student's t-test). These results suggest
that cell proliferation is not the process by which ITPR3 regulates
liver tumor maintenance. Therefore it was investigated whether
Ca2+ may instead modulate apoptosis.
Apoptosis is involved in both cancer growth
(19) and liver regeneration
(30). Our group has previously
demonstrated that ITPR3 expression can induce resistance to cell
death in HCC (16). Thus, the
correlation between the apoptotic marker ssDNA labelling and ITPR3
expression was investigated. In the HCC samples, 69.7% (n=23) had a
low apoptotic index (≤50%), whereas 30.3% (n=10) exhibited a high
apoptotic percentage (>50%) (Fig.
2B). Patients with higher intensity of ITPR3 staining exhibited
a low apoptotic percentage, while patients with lower intensity of
ITPR3 displayed a higher apoptotic percentage (P<0.0005;
Fig. 2C). These findings suggested
that upregulation of ITPR3 correlates with higher apoptosis
resistance, which could contribute to tumor maintenance.
As the mitotic index and apoptotic percentage were
both inversely associated with the intensity of tumor ITPR3
staining, whether these two parameters correlated with each other
in HCC was evaluated. In the specimens of the present study, the
degree of apoptosis and mitosis were directly related (P<0.05 by
unpaired Student's t-test; Fig.
2D), indicating that these events were closely related in the
context of the role of ITPR3 in the modulation of the cell cycle
and tumoral maintenance in HCC.
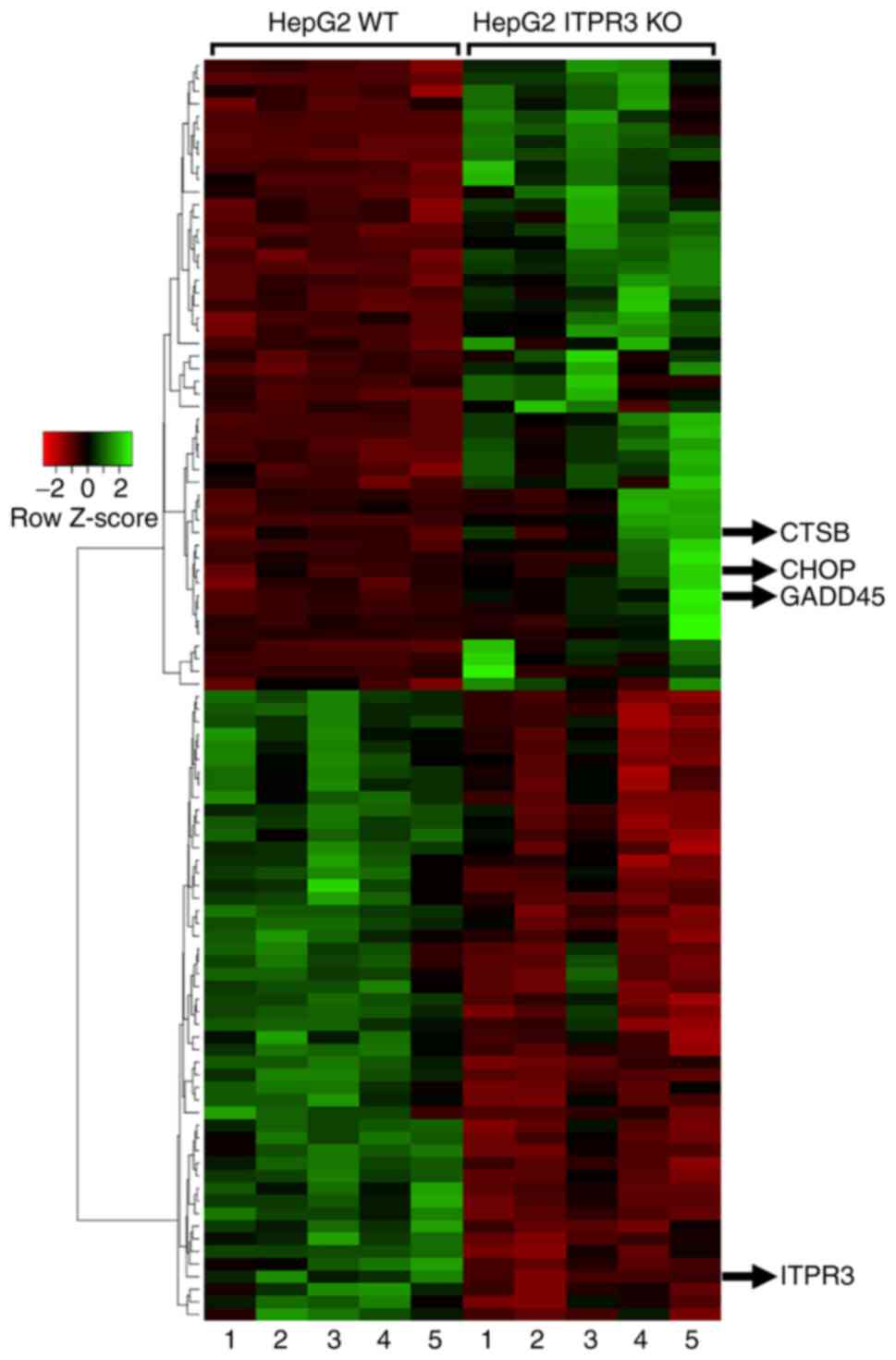
To improved understanding of the mechanism by which
ITPR3 expression in hepatocytes is protective, rather than
permissive, of cell death by apoptosis, RNA-seq was analyzed
comparing global gene expression patterns between wild-type and
ITPR3 knockout HepG2 cells. The apoptotic genes CTSB, CHOP and
GADD45 were found to be upregulated in the ITPR3 KO cells (Figs. 3 and S1). CTSB is a protease whose
overexpression indicates poor prognosis of patients with HCC,
facilitating tumor migration and invasion (38). CHOP is a potential pro-apoptotic
oncogene in HCC, participating in the promotion of hepatic
carcinogenesis through pathways involved in crosstalk with ATF6
(39). The GADD45 family of
proteins responds to physiological and environmental cellular
stress (40) through apoptosis
(41), with a specific
under-expression in HCC that correlates with worse prognosis
(41). These results suggest that
chronic ITPR3 expression may promote an anti-apoptotic response
pathway in liver cancer through modulation of gene expression and
proteasome activity.
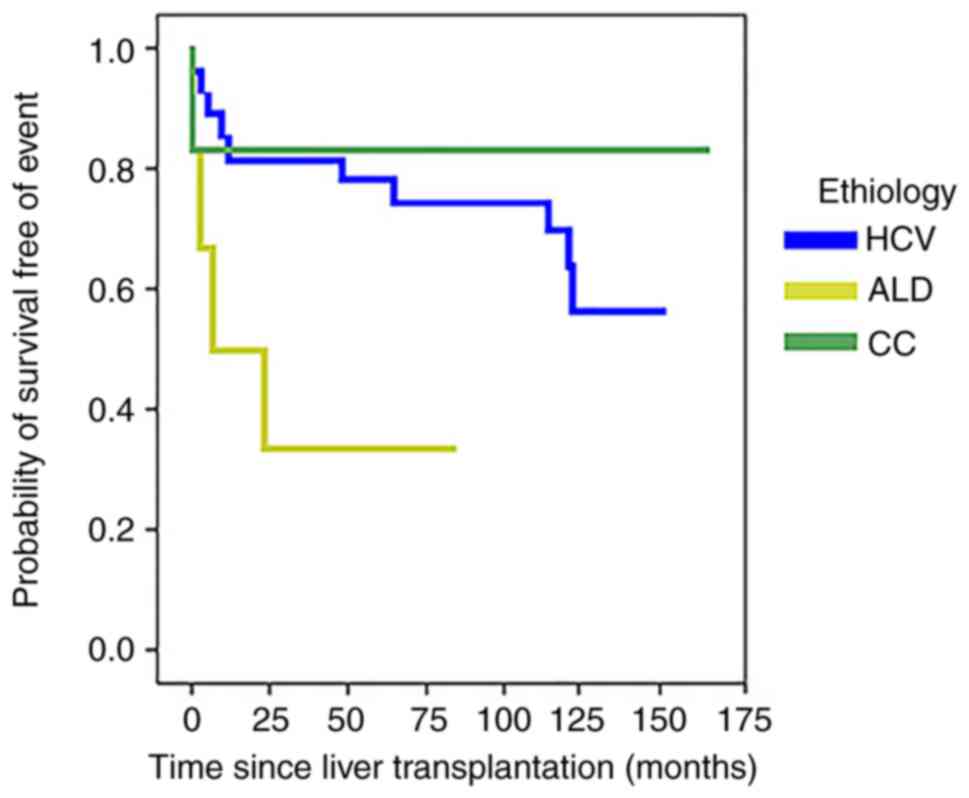
In the survival analysis, the median event-free
survival (EFS) was 113.2 months (range, 93.1-133.2), considering
all etiologies together. The Kaplan-Meier curve showed a
significant difference comparing the EFS among etiologic groups
(Renyi test=0.015; Fig. 4). This
difference was identified between CC and HCV etiologies (P=0.001).
However, neither ITPR3 expression in the tumor nor in the cirrhotic
region was associated with EFS outcome. Together, the results
suggested that ITPR3 expression in transformed hepatocytes is
implicated in evasion of apoptosis, which contributes to the
maintenance of HCC.
Discussion
Hepatocarcinogenesis is a process that involves
multiple steps and in which several pathways can interact (42). Among them, regulators of cell
proliferation and survival are prominent (43). Although different types of
underlying CLD can lead to tumorigenesis through different
mechanisms (44,45), de novo expression of the
ITPR3 Ca2+ channel was noted as a common molecular
signaling pathway that occurs in all HCC-related etiologies
(16). The present study confirmed
the involvement of ITPR3 in HCC of patients with HCV, ALD and
CC.
Together, two main molecular classes of HCC can be
identified (46). The
proliferation class, characterized by a higher availability of
signals involved in cell cycle proliferation and cell cycle
progression, as well as progenitor cell markers, is associated with
a more aggressive clinical course. Such tumors have higher AFP
levels, less cell differentiation and more frequent vascular
invasion (47,48). These characteristics are in turn
associated with greater chances of recurrence after initial
treatment and lower survival rates (48,49).
HCC from hepatitis B virus infection is mostly in this class
(50). The non-proliferative class
of HCC possesses at least two central molecular characteristics: A
predominance of Wnt signaling in ≤25% of cases and an immune
response in the remainder of cases; in the physiological context,
the tumor transcriptome of this class is similar to normal liver
physiology (50). From a clinical
perspective, tumors in this class exhibit less aggressive
characteristics, such as higher cell differentiation index and
lower AFP levels. Regarding the underlying etiology, HCV and
ALD-related HCC are more frequent in this class (48).
In the sample of the present study, the main CLD
etiologies were HCV and ALD, with average AFP serum levels of 123.8
ng/ml, which is relatively low, as only levels above 400 ng/ml are
considered as a threshold in the diagnosis of HCC (51). Regarding morphology, the tumor was
well differentiated (grade I or II of Edmondson and Steiner score)
in 72.9% of patients, the median nodule size was <3.0 cm and
62.8% of tumors had a low mitotic index. Taken together, these data
indicated that the majority of the tumors in the present study
would be classified as non-proliferative. Moreover, the tumor stage
was not as advanced, which is compatible with some methods of
treatment received; OLT is considered for patients fulfilling the
Milan Criteria, as in our hospital: Hospital das Clínicas of the
Federal University of Minas Gerais.
The prognostic importance of the mitotic index and
tumor stage is appreciated in a number of malignancies (52,53).
In addition, its value is similar to that of techniques such as
nuclear antigen of cell proliferation (54) or DNA analysis (55,56).
In the context of HCC, there is evidence that a high mitotic index
is an unfavorable prognostic indicator (24,25,57,58),
as well as an independent predictor of short-term survival in
patients who undergo hepatectomy with curative intent (23). In addition, a high mitotic index
(≥5/10 HPF is associated with intrahepatic metastasis, larger tumor
size, higher AFP levels and advanced tumor stage (23,27),
as well as vascular invasion (26), poor histological differentiation
and high recurrence rate (23). In
the samples in the present study, most patients (62.8%) had a low
mitotic index (<5/10 HPF). This corroborated in vivo and
in vitro studies which demonstrated that both HCV and ALD
negatively affect cell cycle, promoting a G2/M phase arrest in HCC
cell lines (59,60).
Demethylation of the promoter of the ITPR3 gene is
necessary for its expression in hepatocytes and has been associated
with cell proliferation in mouse livers after partial hepatectomy
(PH) (16). The data of the
present study showed that the higher the expression of ITPR3, the
lower the mitotic index. These apparent discrepancies can be
explained, in part, by the methodologies used in these studies
(PCNA vs. mitotic index), as well as the type of samples analyzed
(animal PH model vs. human HCC specimen). PCNA, used in the
previous study, is known to serve an important role in the
metabolism of nucleic acids and its main function is associated
with DNA replication (61).
However, PCNA may also be present in repair by DNA excision, cell
cycle control, chromatin assembly and RNA transcription (61). Therefore, PCNA staining might also
reflect cells in phases preceding mitosis. Another important factor
is the type of sample used, either the PH model, in which
hepatocytes are prompted to proliferate as a physiological process
of the liver (62), or the liver
with already established HCC. So, while previous work showed that
ITPR3 stimulates hepatocyte proliferation and that its sustained
expression in hepatocytes might lead to liver cancer (16), the findings of the current study
supported a role for ITPR3 in the maintenance of the liver tumor by
preventing cell death, rather than through enhanced hepatocyte
proliferation.
It is known that liver growth involves apoptosis,
both in physiological conditions, such as in regeneration (29) and in pathological situations, such
as in HCC (16). Additionally,
Ca2+ signaling through ITPR3 is part of apoptotic cell
death (30) and the role of ITPR3
in carcinogenesis has been recently reviewed (63). ITPR3 can participate in both
inhibiting and promoting apoptosis, depending on the type of tumor
(16). The present study
demonstrated an inverse correlation between apoptosis and liver
tumor ITPR3 expression. This is consistent with a previous finding
that ITPR3 triggers anti-apoptotic gene expression in HCC cell
lines (16). Similarities occur in
colon cancer cells, in which ITPR3 overexpression is associated
with decreased apoptosis (18) and
in hepatocytes infected with yellow fever virus, in which higher
ITPR3 hepatocyte expression prevents cell death through apoptosis
(14).
ITPR3 expression can induce resistance to cell death
in liver cancer by increasing the expression of POU4F1 and
decreasing SIAH2 in WT cells compared with HepG2 ITPR3KO (16). The present study extended these
previous observations using RNA-seq and observed that more genes
have their expression profile altered in relation to ITPR3 and,
also, it is suggested that the signaling pathways associated with
apoptosis resistance were altered in HepG2 ITPR3 KO cells (Fig. S1). In particular, a significant
upregulation of CTSB, CHOP and GADD45 was observed, genes
associated with either cell survival or apoptosis.
In the context of HCC, CHOP is pro-apoptotic
(39), performing a
pro-tumorigenic function, being an oncogene in hepatic
carcinogenesis (39). CHOP is
ubiquitously expressed at very low levels normally, but is strongly
expressed in cells subjected to severe stress (64). However, activation of this gene is
not universally observed in human HCC, which leads to the
suggestion that there are other oncogenic pathways that promote
hepatocarcinogenesis independently of CHOP. Thus, this gene may
participate more in the progression of HCC rather than in its
induction (39). The data from the
present study suggested that the action of ITPR3 on CHOP expression
was associated with the apoptotic signaling pathway by promoting
maintenance of liver cancer development.
Growth arrest and damage to DNA 45 (GADD45) is a
family composed of three homologous acidic proteins (α, β and γ)
(65), which respond to cellular
stresses of environmental and physiological origin (41). GADD45 proteins are also involved in
hepatic tumorigenesis, in both carcinogenesis and established HCC
(65). In the context of HCC,
GADD45β has a pro-apoptotic action and occurs at a low or absent
expression level in neoplastic cells, a specific characteristic of
this type of tumor (41). This is
critically correlated with fundamental clinical-pathological
characteristics of tumor development (41). The synergistic effect of aspirin
and sorafenib treatment induces an increase in GADD45β in HCC,
promoting apoptosis and control of tumor growth (66), an opposite effect of growth in the
non-neoplastic liver (65),
reflecting the opposing effects of this gene depending on the
biological context. GADD45β responses reflect its dynamics in rapid
adaptations (65), being a
possible diagnostic and selection biomarker for the treatment of
HCC (66). These data suggest that
the appearance of ITPR3 in liver cancer may promote tumor
maintenance through the suppressive effect of GADD45 expression,
especially of the β isoform.
The present study demonstrated the upregulation of
important genes involved in the apoptotic pathway in liver cancer,
such as GADD45 and CHOP. However, high-throughput technology would
add stronger evidence. Thus, the absence of microarray experiments
is a limitation of the present study.
In other types of neoplasms or liver diseases, ITPR3
can assume several roles. In prostate cancer, the mechanisms that
promote the degradation of ITPR3 and prevent apoptosis have been
elucidated (19). In
ischemia-reperfusion injury, ITPR3 serves a protective role in
which expression of the protein serves to change the mechanism of
hepatocyte death from necrosis to apoptosis (15). This demonstrates the versatility
that ITPR3 assumes within the varied contexts of different diseases
and organs, but all associated with apoptotic events. It has been
demonstrated that ITPR3 serves a role in proliferative stimulation
and apoptosis block in liver cell lines and normal mouse liver
(16). The current study provided
evidence in human HCC samples that ITPR3 serves a role in
hepatocarcinogenesis by modulating apoptosis. Additional work will
be needed to better understand the mechanism by which de
novo expression of ITPR3 correlates with mitosis and apoptosis
in the pathogenesis of HCC, as well as whether this represents a
potential target for therapy.
In conclusion, ITPR3 was highly expressed in HCC
tumor cells relative to its expression level in the underlying CLD
and healthy livers. ITPR3 expression was inversely correlated with
apoptotic and mitotic indices in HCC, suggesting that ITPR3
contributed to the maintenance of HCC, promoting resistance to
apoptosis. This resistance to apoptosis and alteration of tumor
cell survival occurred, at least in part, through an intracellular
signaling pathway in which ITPR3 demonstrated a negative effect on
the expression of CHOP and GADD45, providing a reduction in
apoptotic cell death. The expression of ITPR3 in the liver may be a
promising prognostic marker of HCC and other types of liver
cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from CNPq, FAPEMIG and
NIH (grant nos. P01-DK57751, P30-DK34989, R01-DK114041 and
R01-DK112797).
Availability of data and materials
The datasets analyzed during the current study are
available in the NCBI Sequence Read Archive under the Accession
code PRJNA758563 (https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA758563).
All data generated or analyzed during this study are included in
this published article.
Authors' contributions
The entire project was conducted, written and
analyzed by MLDS, including carrying out the experiments. AF
contributed in all aspects, mainly in the analysis and
interpretation of data. RMF contributed with the assistance in
carrying out the experiments, analysis and discussion of data and
with the preparation of graphics and images. ACMLF contributed with
the assistance in carrying out the experiments, analysis and
discussion of data and with the preparation of graphics and images.
PHD contributed to the statistical analysis and in the organization
of the survival data. CAXG and VLC elaborated all the
bioinformatics data, analysis and RNA-seq results. PVTV performed
the mitotic cell count on the HCC samples in the tumor mitotic
index experiment. In addition, PVTV helped to analyse and interpret
the data in conjunction with the literature already published and
was involved in the writing of the manuscript and in the critical
review of important intellectual content. PVTV also helped with the
construction of the data discussion. MFL participated in carrying
out the experiments and articulating the data obtained from the
works previously published by the group. FOL was involved in the
writing of the manuscript and critical review of important
intellectual content, and also contributed to the analysis of some
of the data presented. MHN contributed to the analysis and
discussion of the data in this study, in addition to approving the
final version submitted for publication. GF participated in the
orientation and design of the study, particularly in the
contribution of immunohistochemical staining image analysis. CXL
participated in the acquisition of clinical data from patients and
in the interpretation of these data in conjunction with the
results. MLDS and PVTV confirm the authenticity of all the raw
data. All authors reviewed and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Hospital das Clínicas, Federal University of Minas
Gerais (approval number CAAE 71206617.8.0000.5149) and written
informed consent was obtained from the patients or their relatives,
including for use of their tissue in the research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AFP
|
α fetoprotein
|
|
ALD
|
alcoholic liver disease
|
|
CAAE
|
Certificado de Apresentação de
Apreciação Ética (Certificate of Presentation of Ethical
Appreciation)
|
|
CC
|
cryptogenic cirrhosis
|
|
CHILD
|
CHILD-Pugh scores
|
|
CHOP
|
C/EBP homologous protein
|
|
CLD
|
chronic liver disease
|
|
CTSB
|
cathepsin B
|
|
EFS
|
event-free survival
|
|
H&E
|
hematoxylin and eosin
|
|
HCC
|
hepatocellular carcinoma
|
|
HCV
|
hepatitis C virus
|
|
HepG2
|
immortalized liver cancer cell
line
|
|
HPF
|
high-power fields
|
|
INR
|
international normalized ratio
|
|
ITPR
|
inositol 1,4,5-trisphosphate
receptor
|
|
ITPR1
|
inositol 1,4,5-trisphosphate type 1
receptor
|
|
ITPR2
|
inositol 1,4,5-trisphosphate type 2
receptor
|
|
ITPR3
|
inositol 1,4,5-trisphosphate type 3
receptor
|
|
MELD
|
model for end-stage liver disease
|
|
OLT
|
orthotopic liver transplantation
|
|
PH
|
partial hepactomy
|
|
RNA-seq
|
RNA sequencing
|
|
TBIL
|
total bilirubin
|
References
|
1
|
Agni RM: Diagnostic histopathology of
hepatocellular carcinoma: A case-based review. Semin Diagn Pathol.
34:126–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bodzin AS, Lunsford KE, Markovic D,
Harlander-Locke MP, Busuttil RW and Agopian VG: Predicting
mortality in patients developing recurrent hepatocellular carcinoma
after liver transplantation: Impact of treatment modality and
recurrence characteristics. Ann Surg. 266:118–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amaya MJ and Nathanson MH: Calcium
signaling in the liver. Compr Physiol. 3:515–539. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patel S, Joseph SK and Thomas AP:
Molecular properties of inositol 1,4,5-trisphosphate receptors.
Cell Calcium. 25:247–264. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wojcikiewicz RJ: Type I, II, and III
inositol 1,4,5-trisphosphate receptors are unequally susceptible to
down-regulation and are expressed in markedly different proportions
in different cell types. J Biol Chem. 270:11678–11683. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yule DI, Ernst SA, Ohnishi H and
Wojcikiewicz RJ: Evidence that zymogen granules are not a
physiologically relevant calcium pool. Defining the distribution of
inositol 1,4,5-trisphosphate receptors in pancreatic acinar cells.
J Biol Chem. 272:9093–9098. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cruz LN, Guerra MT, Kruglov E, Mennone A,
Garcia CR, Chen J and Nathanson MH: Regulation of multidrug
resistance-associated protein 2 by calcium signaling in mouse
liver. Hepatology. 52:327–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirata K, Pusl T, O'Neill AF, Dranoff JA
and Nathanson MH: The type II inositol 1,4,5-trisphosphate receptor
can trigger Ca2+ waves in rat hepatocytes.
Gastroenterology. 122:1088–1100. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dufour JF, Lüthi M, Forestier M and
Magnino F: Expression of inositol 1,4,5-trisphosphate receptor
isoforms in rat cirrhosis. Hepatology. 30:1018–1026. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lemos FO, França A, Lima Filho ACM,
Florentino RM, Santos ML, Missiaggia DG, Rodrigues GOL, Dias FF,
Souza Passos IB, Teixeira MM, et al: Molecular mechanism for
protection against liver failure in human yellow fever infection.
Hepatol Commun. 4:657–669. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lima Filho ACM, França A, Florentino RM,
Dos Santos ML, de Oliveira Lemos F, Missiaggia DG, Fonseca RC,
Gustavo Oliveira A, Ananthanarayanan M, Guerra MT, et al: Inositol
1,4,5-trisphosphate receptor type 3 plays a protective role in
hepatocytes during hepatic ischemia-reperfusion injury. Cell
Calcium. 91:1022642020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leite MF, Thrower EC, Echevarria W, Koulen
P, Hirata K, Bennett AM, Ehrlich BE and Nathanson MH: Nuclear and
cytosolic calcium are regulated independently. Proc Natl Acad Sci
USA. 100:2975–2980. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guerra MT, Florentino RM, França A, Lima
Filho AC, Dos Santos ML, Fonseca RC, Lemos FO, Fonseca MC, Kruglov
E, Mennone A, et al: Expression of the type 3 InsP3
receptor is a final common event in the development of
hepatocellular carcinoma. Gut. 68:1676–1687. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ueasilamongkol P, Khamphaya T, Guerra MT,
Rodrigues MA, Gomes DA, Kong Y, Wei W, Jain D, Trampert DC,
Ananthanarayanan M, et al: Type 3 inositol 1,4,5-trisphosphate
receptor is increased and enhances malignant properties in
cholangiocarcinoma. Hepatology. 71:583–599. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shibao K, Fiedler MJ, Nagata J, Minagawa
N, Hirata K, Nakayama Y, Iwakiri Y, Nathanson MH and Yamaguchi K:
The type III inositol 1,4,5-trisphosphate receptor is associated
with aggressiveness of colorectal carcinoma. Cell Calcium.
48:315–323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuchay S, Giorgi C, Simoneschi D, Pagan J,
Missiroli S, Saraf A, Florens L, Washburn MP, Collazo-Lorduy A,
Castillo-Martin M, et al: PTEN counteracts FBXL2 to promote IP3R3-
and Ca2+-mediated apoptosis limiting tumour growth.
Nature. 546:554–558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bononi A, Giorgi C, Patergnani S, Larson
D, Verbruggen K, Tanji M, Pellegrini L, Signorato V, Olivetto F,
Pastorino S, et al: BAP1 regulates IP3R3-mediated Ca2+
flux to mitochondria suppressing cell transformation. Nature.
546:549–553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fonseca MC, França A, Florentino RM,
Fonseca RC, Lima Filho AC, Vidigal PT, Oliveira AG, Dubuquoy L,
Nathanson MH and Leite MF: Cholesterol-enriched membrane
microdomains are needed for insulin signaling and proliferation in
hepatic cells. Am J Physiol Gastrointest Liver Physiol.
315:G80–G94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baak JP: Mitosis counting in tumors. Hum
Pathol. 21:683–685. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ha SY, Choi M, Lee T and Park CK: The
prognostic role of mitotic index in hepatocellular carcinoma
patients after curative hepatectomy. Cancer Res Treat. 48:180–189.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haratake J, Takeda S, Kasai T, Nakano S
and Tokui N: Predictable factors for estimating prognosis of
patients after resection of hepatocellular carcinoma. Cancer.
72:1178–1183. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nanashima A, Tanaka K, Yamaguchi H,
Shibasaki S, Morino S, Yoshinaga M, Sawai T, Nakagoe T and Ayabe H:
Fibrosis and inflammatory activity in noncancerous tissue and
mitotic index of cancer tissue in patients with hepatocellular
carcinoma: Relationship to clinicopathological factors and
prognosis after hepatic resection. Dig Dis Sci. 48:1517–1522. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Osório FM, Vidigal PV, Ferrari TC, Lima
AS, Lauar GM and Couto CA: Histologic grade and mitotic index as
predictors of microvascular invasion in hepatocellular carcinoma.
Exp Clin Transplant. 13:421–425. 2015.PubMed/NCBI
|
|
27
|
Ouchi K, Sugawara T, Ono H, Fujiya T,
Kamiyama Y, Kakugawa Y, Mikuni J, Yamanami H, Komatsu S and
Horikoshi A: Mitotic index is the best predictive factor for
survival of patients with resected hepatocellular carcinoma. Dig
Surg. 17:42–48. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bolger AM, Lohse M and Usadel B:
Trimmomatic: A flexible trimmer for illumina sequence data.
Bioinformatics. 30:2114–2120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patro R, Duggal G, Love MI, Irizarry RA
and Kingsford C: Salmon provides fast and bias-aware quantification
of transcript expression. Nat Methods. 14:417–419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Howe KL, Achuthan P, Allen J, Allen J,
Alvarez-Jarreta J, Amode MR, Armean IM, Azov AG, Bennett R, Bhai J,
et al: Ensembl 2021. Nucleic Acids Res. 49D:D884–D891. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kucukural A, Yukselen O, Ozata DM, Moore
MJ and Garber M: DEBrowser: Interactive differential expression
analysis and visualization tool for count data. BMC Genomics.
20:62019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
UniProt Consortium: UniProt: The universal
protein knowledgebase in 2021. Nucleic Acids Resy. 49D:D480–D489.
2021.PubMed/NCBI
|
|
35
|
Kanehisa M and Sato Y: KEGG Mapper for
inferring cellular functions from protein sequences. Protein Sci.
29:28–35. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Babicki S, Arndt D, Marcu A, Liang Y,
Grant JR, Maciejewski A and Wishart DS: Heatmapper: Web-enabled
heat mapping for all. Nucleic Acids Res. 44W:W147–W153. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: A study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ruan J, Zheng H, Rong X, Rong X, Zhang J,
Fang W, Zhao P and Luo R: Over-expression of cathepsin B in
hepatocellular carcinomas predicts poor prognosis of HCC patients.
Mol Cancer. 15:172016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Scaiewicz V, Nahmias A, Chung RT, Mueller
T, Tirosh B and Shibolet O: CCAAT/enhancer-binding protein
homologous (CHOP) protein promotes carcinogenesis in the
DEN-induced Hepatocellular carcinoma model. PLoS One. 8:e810652013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liebermann DA and Hoffman B: Gadd45 in
stress signaling. J Mol Signal. 3:152008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qiu W, David D, Zhou B, Chu PG, Zhang B,
Wu M, Xiao J, Han T, Zhu Z, Wang T, et al: Down-regulation of
growth arrest DNA damage-inducible gene 45beta expression is
associated with human hepatocellular carcinoma. Am J Pathol.
162:1961–1974. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Llovet JM, Peña CE, Lathia CD, Shan M,
Meinhardt G and Bruix J; SHARP Investigators Study Group, : Plasma
biomarkers as predictors of outcome in patients with advanced
hepatocellular carcinoma. Clin Cancer Res. 18:2290–2300. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Alves RC, Alves D, Guz B, Matos C, Viana
M, Harriz M, Terrabuio D, Kondo M, Gampel O and Polletti P:
Advanced hepatocellular carcinoma. Review of targeted molecular
drugs. Ann Hepatol. 10:21–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ho DW, Lo RC, Chan LK and Ng IO: Molecular
pathogenesis of hepatocellular carcinoma. Liver Cancer. 5:290–302.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lu LC, Hsu CH, Hsu C and Cheng AL: Tumor
heterogeneity in hepatocellular carcinoma: Facing the challenges.
Liver Cancer. 5:128–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hoshida Y, Nijman SM, Kobayashi M, Chan
JA, Brunet JP, Chiang DY, Villanueva A, Newell P, Ikeda K,
Hashimoto M, et al: Integrative transcriptome analysis reveals
common molecular subclasses of human hepatocellular carcinoma.
Cancer Res. 69:7385–7392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chiang DY, Villanueva A, Hoshida Y, Peix
J, Newell P, Minguez B, LeBlanc AC, Donovan DJ, Thung SN, Solé M,
et al: Focal gains of VEGFA and molecular classification of
hepatocellular carcinoma. Cancer Res. 68:6779–6788. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Villanueva A, Hoshida Y, Battiston C,
Tovar V, Sia D, Alsinet C, Cornella H, Liberzon A, Kobayashi M,
Kumada H, et al: Combining clinical, pathology, and gene expression
data to predict recurrence of hepatocellular carcinoma.
Gastroenterology. 140:1501–1512.e2. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z,
Roskams T, Durnez A, Demetris AJ and Thorgeirsson SS:
Classification and prediction of survival in hepatocellular
carcinoma by gene expression profiling. Hepatology. 40:667–676.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zucman-Rossi J, Villanueva A, Nault JC and
Llovet JM: Genetic landscape and biomarkers of hepatocellular
carcinoma. Gastroenterology. 149:1226–1239.e4. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Masuzaki R and Omata M: Screening program
in high-risk populations. In: Hepatocellular Carcinoma. (2nd
edition). Mcmasters KM and Vauthey JN: Springer. (New York, NY).
55–68. 2011.
|
|
52
|
Bloom HJ and Richardson WW: Histological
grading and prognosis in breast cancer; a study of 1409 cases of
which 359 have been followed for 15 years. Br J Cancer. 11:359–377.
1957. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vang R, Shih IM and Kurman RJ: Ovarian
low-grade and high-grade serous carcinoma: Pathogenesis,
clinicopathologic and molecular biologic features, and diagnostic
problems. Adv Anat Patht. 16:267–282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Suehiro T, Matsumata T, Itasaka H,
Yamamoto K, Kawahara N and Sugimachi K: Clinicopathologic features
and prognosis of resected hepatocellular carcinomas of varied sizes
with special reference to proliferating cell nuclear antigen.
Cancer. 76:399–405. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Okada S, Shimada K, Yamamoto J, Takayama
T, Kosuge T, Yamasaki S, Sakamoto M and Hirohashi S: Predictive
factors for postoperative recurrence of hepatocellular carcinoma.
Gastroenterology. 106:1618–1624. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ruà S, Comino A, Fruttero A, Torchio P,
Bouzari H, Taraglio S, Torchio B and Capussotti L: Flow cytometric
DNA analysis of cirrhotic liver cells in patients with
hepatocellular carcinoma can provide a new prognostic factor.
Cancer. 78:1195–1202. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Soini Y, Virkajärvi N, Lehto VP and Pääkkö
P: Hepatocellular carcinomas with a high proliferation index and a
low degree of apoptosis and necrosis are associated with a
shortened survival. Br J Cancer. 73:1025–1030. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tannapfel A, Geissler F, Köckerling F,
Katalinic A, Hauss J and Wittekind C: Apoptosis and proliferation
in relation to histopathological variables and prognosis in
hepatocellular carcinoma. J Pathol. 187:439–445. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Alisi A, Mele R, Spaziani A, Tavolaro S,
Palescandolo E and Balsano C: Thr 446 phosphorylation of PKR by HCV
core protein deregulates G2/M phase in HCC cells. J Cell Physiol.
205:25–31. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Clemens DL, Calisto LE, Sorrell MF and
Tuma DJ: Ethanol metabolism results in a G2/M cell-cycle arrest in
recombinant Hep G2 cells. Hepatology. 38:385–393. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Juríková M, Danihel L, Polák Š and Varga
I: Ki67, PCNA, and MCM proteins: Markers of proliferation in the
diagnosis of breast cancer. Acta Histochem. 118:544–552. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Michalopoulos GK: Liver regeneration after
partial hepatectomy: Critical analysis of mechanistic dilemmas. Am
J Pathol. 176:2–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mangla A, Guerra MT and Nathanson MH: Type
3 inositol 1,4,5-trisphosphate receptor: A calcium channel for all
seasons. Cell Calcium. 85:1021322020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chikka MR, McCabe DD, Tyra HM and
Rutkowski DT: C/EBP homologous protein (CHOP) contributes to
suppression of metabolic genes during endoplasmic reticulum stress
in the liver. J Biol Chem. 288:4405–4415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tian J and Locker J: Gadd45 in the liver:
Signal transduction and transcriptional mechanisms. In: Gadd45
Stress Sensor Genes. Liebermann DA and Hoffman B: Springer. (New
York, NY). 69–80. 2013.
|
|
66
|
Xia H, Lee KW, Chen J, Kong SN, Sekar K,
Deivasigamani A, Seshachalam VP, Goh BKP, Ooi LL and Hui KM:
Simultaneous silencing of ACSL4 and induction of GADD45B in
hepatocellular carcinoma cells amplifies the synergistic
therapeutic effect of aspirin and sorafenib. Cell Death Discov.
3:170582017. View Article : Google Scholar : PubMed/NCBI
|