Introduction
Ovarian carcinoma is a major gynecological malignant
tumor with high incidence and mortality (1). In China, ovarian carcinoma has
increased significantly by nearly 30% in recent years (1). Although the mortality of ovarian cancer
generally decreases with early diagnosis and treatment, patients
with advanced or late stage of ovarian squamous cell carcinoma
(OSCC) still have a poor prognosis, with a 5-year survival rate of
below 20% in China (1,2).
Epithelial ovarian cancer is the leading
pathological type of ovarian malignant tumors and accounts for
nearly 50% in all types of ovarian malignant tumor worldwide
(3,4). The tumor microenvironment plays an
important role in invasion and metastasis, and is composed of
cancer-associated fibroblasts (CAFs) and other types of stromal
cells, including endothelial cells and inflammatory cells (5). CAFs can regulate tumor biological
processes and contribute to cancer progression by various
mechanisms, such as affecting extracellular matrix (ECM) remodeling
(6). CAFs also secrete inflammatory
cytokines, which could play an important role in the regulation of
immune cells (6). TGF-β is a
cytokine that is mainly secreted by CAFs, which can promote the
epithelial-mesenchymal transition (EMT), contributing to the
invasion and metastasis in colorectal and breast cancer (7). In addition, CAFs play a vital role in
ECM remodeling through the regulation of production and degradation
of metalloproteinases via Rho/Rho-associated protein kinase
signaling (8). The physical
remodeling of ECM enhances tumor growth and facilitates metastatic
invasion in various cancer types, such as breast cancer (9), pancreatic cancer (10), colorectal cancer (11). In addition, CAFs also can synthesize
soluble inflammatory tumor-promoting factors involved in cancerous
biological processes, such as stromal cell-derived factor 1, which
enhances invasiveness via activation of integrin β1-related
signaling pathways (9). In addition,
the co-culture of CAFs with melanoma cells results in the
production of various pro-inflammatory cytokines, such as
interleukin (IL)-6, IL-8 and IL-1β and concurrently represses the
expression of inflammatory mediators that inhibit the invasiveness
of cancer cells (12). These results
indicated that CAFs serve a key role in the regulation of immune
responses by secreting several inflammatory mediators (12–14).
Cancer-associated fibroblasts can also secrete
exosomes that transport mediators of cell-cell communication in a
paracrine manner, including non-coding RNA molecules or some
inflammatory mediators, such as IL-33, IL-1β and tumor necrosis
factor (TNF)-α (15). A previous
report showed that the components in exosomes produced by CAFs
could promote breast cancer cell motility and metastasis via
activation of Wnt-planar cell polarity (Wnt-PCP) cell signaling
(16). Exosome-stimulated breast
cancer cells display excessive activation of Wnt-planar cell
polarity signaling (15). In breast
cancer mouse models, administration of breast cancer cells (BCCs)
combined with fibroblasts promotes metastasis dependent on
cell-cell communication mediators contained in exosomes, Cd81 and
Wnt-PCP signaling in BCCs (15,16).
The present study aimed to demonstrate the role of
IL-33 transported by exosomes secreted by CAFs in ovarian
epithelial carcinoma by regulation of the differentiation and
function of cancer-associated macrophages (CAMs).
Materials and methods
Animals
Wild-type (WT) and ST2-deficient female nude BALB/c
mice were purchased from the Experimental Animal Center of Nanjing
University. The protocols for animal experiments were approved by
the Baoji People's Hospital Ethics Committee on the Use of Live
Animals in Teaching and Research (Baoji, China). A total of 30
female mice (12 weeks old) with a weight of ~30 g were housed under
12 h of light and 12 h dark cycle with free access to food and
water at the Laboratory Animal Unit.
Cell culture
The human ovarian cancer cell line COC1 was
purchased from the American Type Culture Collection. COC1 cells
were cultured in DMEM with 1,000 mg/l D-Glucose (Stemcell
Technologies, Inc.) supplemented with 10% FBS (Thermo Fisher
Scientific, Inc.) and 100 U/ml of penicillin/streptomycin (Thermo
Fisher Scientific, Inc.) in a humidified incubator. Primary normal
ovarian fibroblasts (NOFs) and CAFs were isolated from human benign
ovarian biopsies from five patients with ovarian epithelial cancer
and five patients with benign ovarian cysts in the Yulin First
Hospital (Yulin, China) from February 2018 to September 2019. The
mean ± SD age of these subjects was 56.5±8.2 years. Verbal informed
consent was provided by all patients. Briefly, the stroma was
separated from the ovarian epithelial tissues after incubation
overnight at 4°C in HEPES buffer with 500 µg/ml thermolysin
(Sigma-Aldrich; Merck KGaA). The fibroblasts were enzymatically
dissociated from the ECM by treating the stroma with 0.125 U/ml
collagenase H (Roche Diagnostics) for 30 min at 37°C under gentle
agitation. Then, fibroblasts were cultured in DMEM supplemented
with 10% FBS (Invitrogen; Thermo Fisher Scientific, Inc.) and
antibiotics (100 U/ml penicillin and 25 µg/ml gentamicin;
Sigma-Aldrich; Merck KGaA). All cells were cultured for fewer than
three passages after purchasing or receiving them for all the
experiments and tested for mycoplasma contamination by microscopic
examination. The CAFs and NOFs were centrifuged at 1,000 × g at
room temperature and the supernatants were collected and stored at
−80°C until further experiments. The venous blood-derived monocytes
from the aforementioned patients mentioned were also isolated. The
CD14high or CD16high blood-derived monocytes
were sorted by flow cytometry sorting technology from peripheral
blood mononuclear cells (PBMCs) isolated by Ficoll centrifugation
at 1,000 × g at 25°C according to the markers of CD14 and CD16.
Blood monocyte cells were cultured in RPMI-1640 medium supplemented
with 10% FBS (Thermo Fisher Scientific, Inc.) and 100 U/ml of
penicillin/streptomycin (Thermo Fisher Scientific, Inc.) in a
humidified incubator.
Isolation of exosomes derived from
NOFs and CAFs
CAFs and NOFs cellular supernatant was treated with
the FBS Exosome Depletion kit (Norgen Biotek Corp.) to remove any
residual bovine exosomes in FBS, according to the manufacturer's
instructions. CAFs and NOFs isolated from normal ovarian tissues
and cancerous tissues were cultured in DMEM containing 10%
exosome-depleted FBS for 48 h. Then, the conditioned medium was
centrifuged at 2,000 × g for 30 min to remove cells and debris, and
the supernatant was mixed with 0.5 ml volume of the Total Exosome
Isolation Reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Samples were mixed by vortexing and incubated at 4°C overnight.
Then, they were centrifuged at 10,000 × g for 60 min at 4°C.
Exosomes, contained in the pellet, were resuspended in PBS. The
protein concentration was measured using a BCA kit (Pierce; Thermo
Fisher Scientific, Inc.). Transmission electron microscopy (Hitachi
HT7700; Hitachi Ltd.) was used for the visualization of
extracellular vesicles (exosomes). Briefly, the isolated exosomes
were fixed with 2% paraformaldehyde for 15 min at 37°C and spotted
onto a glow-discharged copper grid on filter paper. Then, the
copper grids were dried for 15 min at room temperature to remove
the excessive liquid. The samples were stained by 2%
phosphotungstic acid (PTA) for 5 min at room temperature.
Subsequently, the samples were examined at 100 keV. The mean
diameter and concentration per milliliter of exosomes were
calculated by the qNano (Izon Science Ltd.).
Pathological examination by
immunofluorescence staining
The ovarian epithelial cancerous tissues were fixed
with 4% (v/v) paraformaldehyde for 24 h at 25°C and cut into 5-µm
slices. For immunocytochemistry, permeabilization was performed
using 0.25% (v/v) Triton X-100/PBS (Sigma-Aldrich; Merck KGaA) for
30 min followed by blocking using 5% BSA in PBS for 30 min at 25°C.
Incubation with primary antibodies against α-SMA (1:1,000; cat. no.
ab7817; Abcam), IL-33 (1:1,000; cat. no. ab187060; Abcam), PAX8
(1:1,000; cat. no. 406421; BioLegend, Inc.) and CD45 (1:2,000; cat.
no. ab40763; Abcam) was performed at room temperature for 1 h in
PBS containing 5% BSA. The tissue slices were rinsed in PBS before
incubation with anti-rabbit Alexa Fluor 488-conjugated secondary
antibody and anti-mouse Alexa Fluor 647-conjugated secondary
antibody (both 1:1,000; cat. nos. R37116 and A32731; Invitrogen;
Thermo Fisher Scientific, Inc.) for 1 h. Nucleus staining was
performed with DAPI for 5 min at 25°C (Vector Laboratories, Inc.;
Maravai LifeSciences). The co-localization of IL-33-positive cells
with other markers staining positive cells was visualized using a
fluorescence microscope (Olympus Corporation).
Western blotting
Total protein of blood-derived monocytes and ovarian
cancerous tissues were extracted by RIPA lysis and extraction
buffer (Thermo Fisher Scientific, Inc.) and T-PER™
tissue protein extraction reagent (cat. no. 78510; Thermo Fisher
Scientific, Inc.). Equal amounts of protein (10 µg/lane) were
loaded onto 12% polyacrylamide gels, resolved by SDS-PAGE and
transferred to polyvinylidene difluoride membranes. The membranes
were blocked for 30 min with 5% non-fat milk and 0.05% Tween-20 in
PBS at 4°C. The membranes were incubated with primary antibodies
overnight at 4°C followed by 45 min at room temperature with
HRP-conjugated secondary antibodies (Jackson Immunoresearch
Laboratories, Inc.). Protein expression was detected using Amersham
ECL Western Blotting Detection reagent (GE Healthcare). Bands were
visualized using a Fusion Fx7 imager (Vilber Lourmat) and analyzed
using ImageJ software v.6.0 (National Institutes of Health). The
following antibodies were used: TGF-β (1:1,000; cat. no. 846802;
BioLegend, Inc.), NF-κB p65 (1:2,000; cat. no. ab207297; Abcam),
IκB (1:1,000; cat. no. AI096-1; BioLegend, Inc.), IκK (1:1000; cat.
no. ab32518; Abcam), cleaved caspase-3 (1:1,000; cat. no.
25546-1-AP; ProteinTech, Inc.), cleaved caspase-8 (1:1,000; cat.
no. ab32397; Abcam), α-SMA (1:2,000; cat. no. ab5931; Abcam),
vimentin (1:1,000; cat. no. ab92547; Abcam), GAPDH (1:5,000; cat.
no. ab8245; Abcam), ZEB1 (1:1,000; cat. no. ab203829; Abcam), Snail
(1:1,000; cat. no. ab216347; Abcam), ST2 (1:1,000; cat. no.
ab194113; Abcam), IL-33 (1:1,000; cat. no. H00090865-B01; Novus
Biologicals), p-Smad2 (1:100; cat. no. ab280888; Abcam), p-Smad3
(1:1,000; cat. no. ab52903; Abcam) and tubulin (1:1,000; cat. no.
NA100-1639; Novus Biologicals, Ltd.).
Reverse transcription-quantitative
PCR
Total RNA of ovarian cancerous tissues was extracted
using TRIzol® reagent according to the manufacturer's
instructions (Invitrogen; Thermo Fisher Scientific, Inc.). RNA
concentration and purity were confirmed using a Nanodrop 2000
(Thermo Fisher Scientific, Inc.). Samples with a relative
absorbance ratio of 260/280 between 1.8 and 2.0 were used. All RNA
samples were reverse-transcribed using the High-capacity cDNA
reverse transcription kit (cat. no. 4368813; Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to manufacturer's
protocol. Quantification of specific mRNAs was performed using an
ABI PRISM 7300 Sequence Detection system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) with the SYBR Green Real-time PCR
kit (Takara Bio, Inc.). The thermocycling conditions used were as
follows: 35 cycles of 94°C for 10 min, 55°C for 1 min and 70°C for
10 min. The primer sequences used in the study are shown in
Table I. Relative mRNA amounts were
normalized to GAPDH and calculated using the standard curve method
(17). In brief, the pre-PCR product
of each gene was used as the standard. The standard curve was
established with a 10-fold serial dilution of the product and was
included in all PCR runs. The ratio of target housekeeping gene was
used to determine the expression level of each gene. A control
consisting of ddH2O was negative in all runs.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| CD209 |
GATGCAGGGTTGGACAA |
GCCATCCATTGGACCCATC |
| MRC1 |
ATGCGATGCCCTACAGC |
TCGACTACGTTGGACCAA |
| SLC40A1 |
CTGACTGTTTCACGGGAT |
CTGACTGCATTCCAGCCT |
| FOLR2 |
TCAGTCAGACCTTACCAT |
TCGAGGCTGCTTGCACTC |
| GAPDH |
CAGGCCTGCAGCCTGCT |
TGTCCAACTTAGCCACCC |
ELISA
The cell supernatant was collected, and the
concentration of TGF-β and IL-10 were measured using a commercially
available ELISA kit (cat. no. D1000B; R&D Systems, Inc.)
according to the protocol described by the manufacturer.
Flow cytometry
After stimulation, PBMCs and blood-derived monocytes
were cultured in RPMI-1640 medium with 10% FBS and resuspended at a
density of 0.5×105 cells per ml. The cells were then
stained with directly conjugated antibodies for 30 min at 4°C in
flow tubes, and then fixed with PBS containing 4% paraformaldehyde.
The antibodies used for flow cytometry included anti-human CD16-APC
(cat. no. 561304), anti-human CD14-PeCy7 (cat. no. 557154),
anti-human CD206-APC-Cy7 (cat. no. 551136) and anti-human 163-Alexa
Fluor 488 (cat. no. 560459) (all BD Biosciences). For the flow
cytometry analysis, the cells were collected according to the
multi-color flow cytometer (BD Biosciences), and the FACS data were
analyzed using FlowJo 10.0 software (BD Biosciences). For monocyte
sorting, CD14high or CD16high monocytes were
isolated from PBMCs according to standard protocols.
Transwell assay
Cells were suspended in serum-free medium at a
density of 1.0×105/ml. Transwell chambers with or
without Matrigel pre-coating were inserted into 24-well plates
containing 200 µl of the cell suspension in the apical chamber and
500 µl of medium with 10% FBS in the basolateral chamber at 37°C.
At 48 h, the chambers were removed and the penetrating cells were
fixed with 5% paraformaldehyde for 20 min 25°C and dyed with 0.1%
crystal violet for 20 min at 25°C. Images of penetrating cells in
four randomly selected fields of each sample were captured for
counting using a light microscope (magnification, ×20; Olympus
Corporation). Transwell chambers for the invasion assay were
pre-coated with Matrigel at room temperature for 30 min.
Cell viability analysis
COC1 cells were maintained in RPMI-1640 medium
(Sigma-Aldrich; Merck KGaA), supplemented with 10% FBS at 37°C in a
humidified atmosphere with 5% CO2. Different
concentrations of cisplatin (at concentrations of 2, 4, 8 and 16
µg/ml) were used to treat the cells. After 48 h incubation at 37°C,
cells were subjected to MTT reagent dissolved in DMSO for 4 h, and
the absorbance at 570 nm was recorded using a Spectra Max i3
microplate reader (Molecular Devices, LLC).
Xenograft models in mice
Female 4-week old BALB/c mice (weight, ~15 g) with
or without ST2-deficiency were bred and purchased from the Model
Animal Research Center of Nanjing University. The mice were housed
with a 12 h of light and 12 h dark cycle with free access to food
and water in a pathogen-free environment. A xenograft humanized
ovarian carcinoma mouse model was established by injecting
1×107 COC1 cells subcutaneously in the flank region of
each female nude BALB/c mice with or without ST2-deficiency. After
4 days, the mice were euthanized by intraperitoneal injection of
phenobarbital at a dose of 0.1 mg/g of body weight. The loss of
breath (disappearance of thorax movement) and adiaphoria to
clamping of the toes were used to confirm death. The engrafting
tumors were harvested, weighed and then used for protein isolation
and immunoblot assays.
Immunohistochemical staining
A total of 156 patients with ovarian cancer (median
age 61 years; age range, 35–69 years) were included in the present
retrospective study. The carcinoma tissues from these patients were
fixed in 5% formalin at room temperature for 30 min and then
embedded in paraffin blocks after surgical resection. Before
immunohistochemical staining, antigens were retrieved according to
the thermal remediation method (18), and the slides were incubated at 25°C
with 3% goat serum (cat. no. C0265; Beyotime Institute of
Biotechnology) for 30 min. The paraffin block was cut into 5-µm
sections and then incubated with primary antibodies against rat
anti-human IL-33 (cat. no. ab187060) and ST2 (cat. no. ab194113)
(both Abcam) in 5% rabbit serum (cat. no. BMS0090; Abbkine
Scientific Co., Ltd.) overnight at 4°C followed by washing and
incubation with avidin-linked biotin complex rabbit anti-rat Ig
(cat. no. 5597; Cell Signaling Technology Inc.). The signals were
detected using a DAB substrate and hematoxylin was used for
counterstaining at room temperature for 5 min, respectively. The
slides were dehydrated by ethanol, cover slips were added, and they
were observed under a light microscope (magnification, ×20) (Zeiss
GmbH). A negative control in the absence of primary antibodies and
incubated with isotype-matched immunoglobulins was also used for
immunostaining. All captured images were analyzed by ImageJ to
quantify the expression levels of IL-33 and ST2, according to the
ratio of fluorescent intensity of primary antibody-stained sections
to the negative control. The mean expression level was used to
divide the subjects into two groups.
Statistical analysis
All data were presented as mean ± SD. The
experiments were repeated 3 times. Unpaired Student's t-tests or
Mann-Whitney tests were applied to analyze the difference between
two groups. One-way ANVOA was used to analyze the difference among
three groups. Survival curves were depicted by Kaplan-Meier methods
and the difference between two groups was analyzed using the
log-rank test. P<0.05 was considered to indicate a statistically
significant difference. P-values were two-tailed. All statistical
tests were performed using SPSS statistical software, version 24.0
(IBM Corp.).
Results
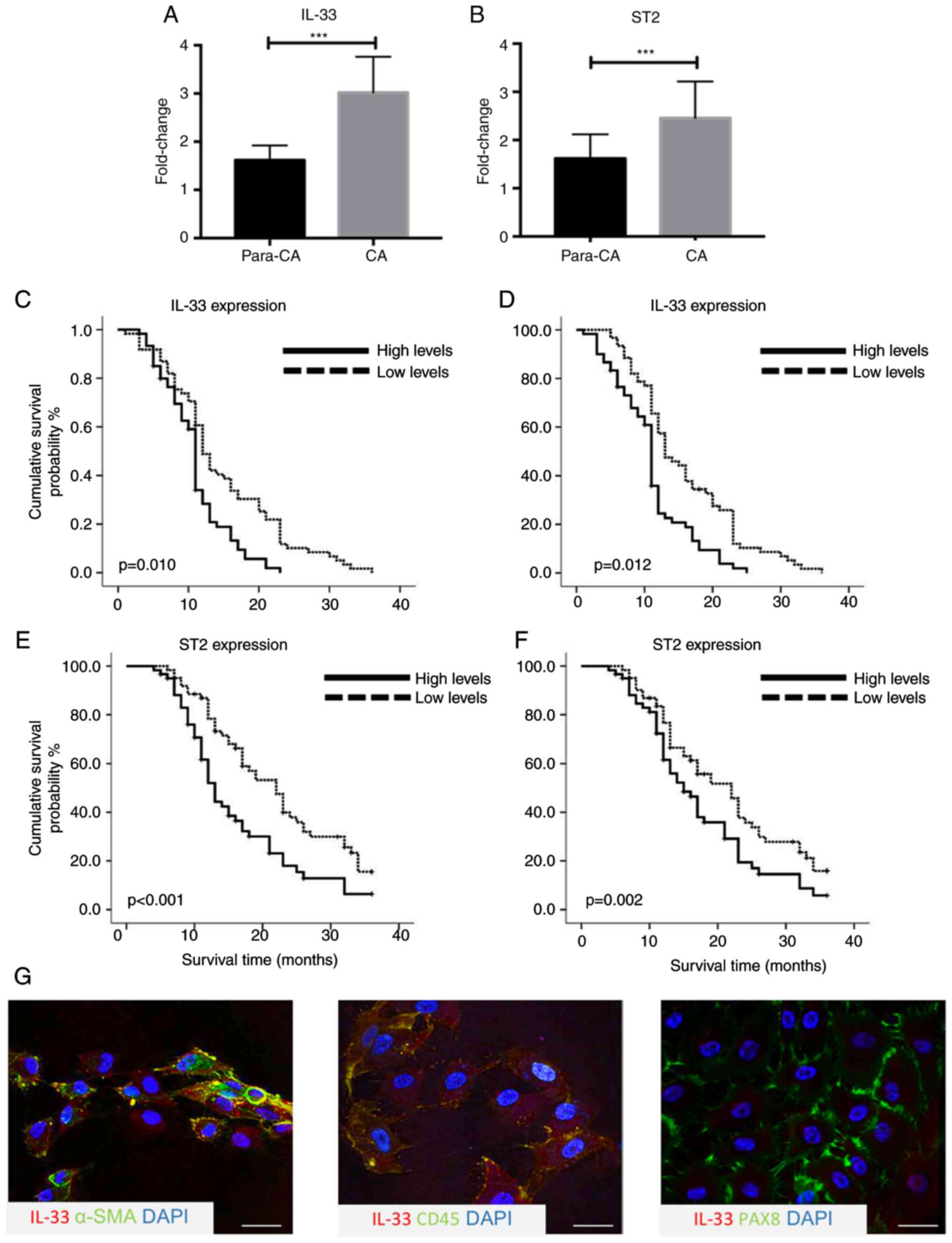
A higher expression of IL-33 in
cancerous tissues correlates with poor prognosis
To explore the potential role of the IL-33/ST2 axis
in ovarian cancer, the expression levels of IL-33 and ST2 were
measured using by immunoblot assays and RT-qPCR. IL-33 and ST2
expression in cancerous tissues was significantly higher compared
with para-carcinoma tissues (n=10; both P<0.001; Fig. 1A and B). To investigate whether
higher expression of IL-33 and ST2 was associated with prognosis,
survival analysis in a prospective cohort was performed, including
156 patients according to the expression levels measured by
immunohistochemical staining. It was reported that 67 out of 156
patients with an increased expression of IL-33 had significantly
poorer overall survival time (OS) (P=0.010) and progression-free
survival time (PFS) (P=0.012) compared with those with a lower
expression of IL-33 (Fig. 1C and D).
Higher expression levels of ST2 in cancerous tissue were also
associated with poor 3-year OS (P<0.001) and PFS (P=0.002)
(Fig. 1E and F). To determine the
cellular source of IL-33 in the cancerous tissues, double
immunohistochemical staining using IL-33 and cellular markers of
ovarian epithelial cancer cells, inflammatory cells and
cancer-associated fibroblasts, including PAX8, CD45 and α-SMA, was
performed. There was prominent co-location of α-SMA with IL-33 and
rare IL-33-producing CD45+ and PAX8+ cells in
cancerous tissues (Fig. 1G). The
results suggested that cancer-associated fibroblasts could produce
more IL-33 compared with ovarian epithelial cancer cells and
CD45+ inflammatory cells.
Human recombined IL-33 and
supernatants from cultured primary cancer-associated fibroblasts
promote the differentiation of blood-derived monocytes into M2-like
macrophages
Primary CAFs were isolated and cultured from five
patients with ovarian cancer recurrence after surgical resection
and chemotherapy. Then NOFs were isolated from five patients with
benign ovarian tumors. After 7 days, the supernatant of NOFs and
CAFs was extracted and used to obtain blood-derived monocytes from
the same individuals. Significantly elevated expression of M2
markers was observed, including CD209, mannose receptor C-Type 1
(CD206; MRC1), solute carrier family 40 member 1 (SLC40A1) and
folate receptor β (n=5; P<0.05; Fig.
2A) in blood-derived monocytes cultured with supernatant from
primary CAFs compared with NOFs. The concentrations of IL-10 and
TGF-β were also significantly elevated after stimulation with IL-33
or the supernatant from primary CAFs compared with the NOFs (n=5;
P<0.05; Fig. 2B). Blood-derived
monocyte cells were harvested after 3 days and flow cytometry
analysis was performed to measure the frequency of M2-macrophages.
There was a significantly higher frequency of
CD163highCD209high M2-macrophages in the
blood-derived monocytes stimulated by human recombined IL-33 and
supernatant of CAFs compared with the control (n=5; P<0.001;
Fig. 2C). The expression of
proinflammatory cytokines, including IL-1β, TNF-α and IL-6, were
significantly decreased in the blood-derived monocytes cultured by
human recombined IL-33 and the supernatant of CAFs compared with
controls (n=5; P<0.01 or P<0.05; Fig. 2D). Meanwhile, NF-κB signaling-related
proteins were significantly decreased in the blood-derived
monocytes treated with human recombined IL-33 and the supernatant
of CAFs compared with the control (n=5; P<0.001 or P<0.05;
Fig. 2E and F). These results
demonstrated that IL-33 could lead to the differentiation of
M2-like macrophages and the secretion of anti-inflammatory
cytokines.
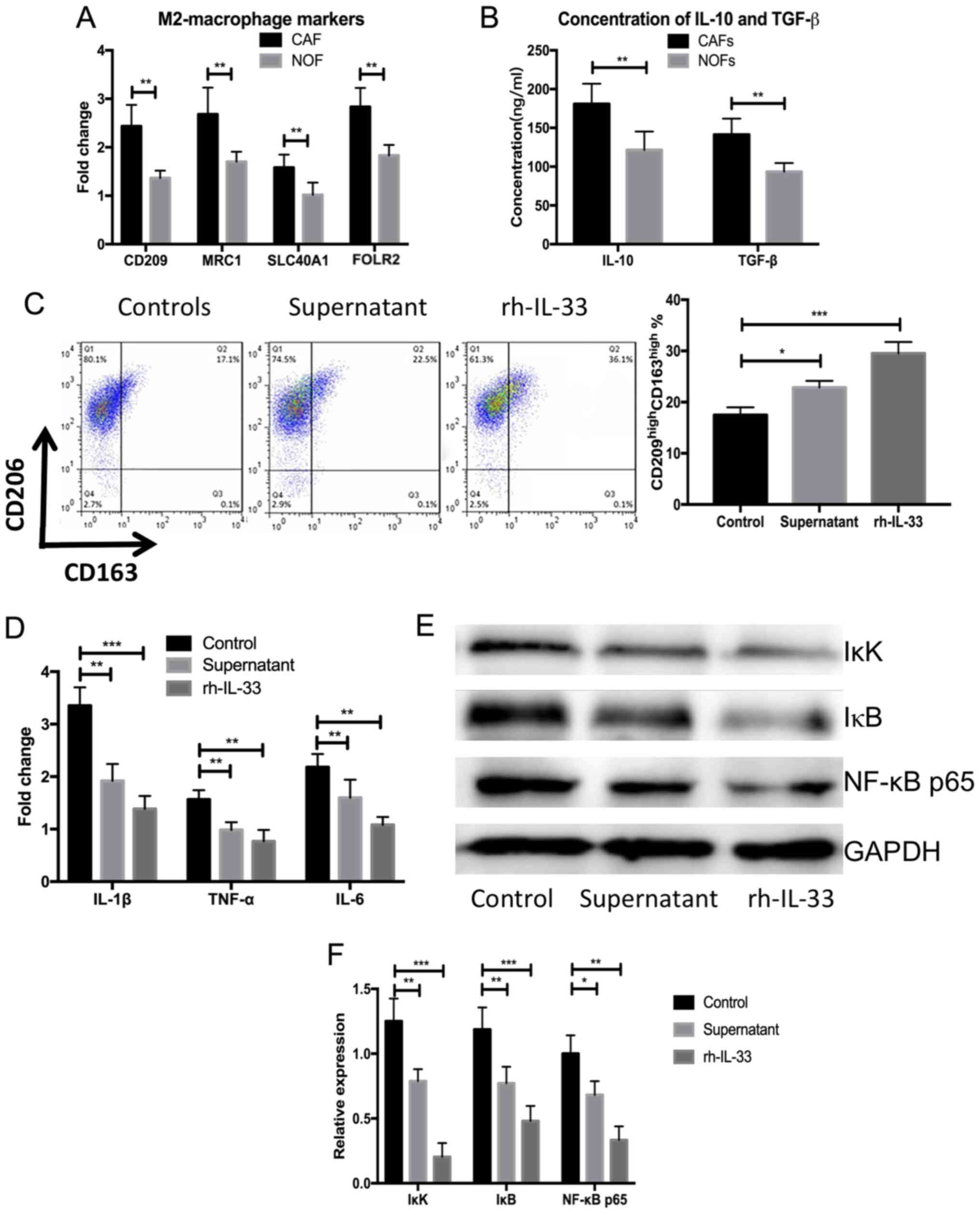 | Figure 2.rh-IL-33 and supernatant from cultures
primary cancer-associated fibroblasts promote the differentiation
of blood-derived monocyte towards M2-like macrophages. (A) Higher
expression of M2 markers, including CD209, MRC1, SLC40A1 and FOLR2,
in the blood-derived stimulated by supernatant of NOFs and CAFs
measured using western blot assays. (B) Higher concentration of
IL-10 and TGF-β in the supernatant of NOFs and CAFs after
stimulation with IL-33 or the supernatant from primary CAFs. (C)
Higher frequency of CD163high CD209high
M2-macrophages in the blood-derived monocytes stimulated with human
recombined IL-33 and supernatant of CAMs. (D) Expression of
proinflammatory cytokines including IL-1β, TNF-α and IL-6 in the
blood-derived monocytes cultured with human recombined IL-33 and
supernatant of CAMs. (E and F) Expressions of NK-κB
signaling-related proteins measured using western blot assays.
*P<0.05, **P<0.01 and ***P<0.001. SLC40A1, solute carrier
family 40 member 1: NOF, normal ovarian fibroblasts; CAF,
cancer-associated fibroblasts; rh-IL-33, recombination human
interleukin-33; FOLR2, folate receptor β. |
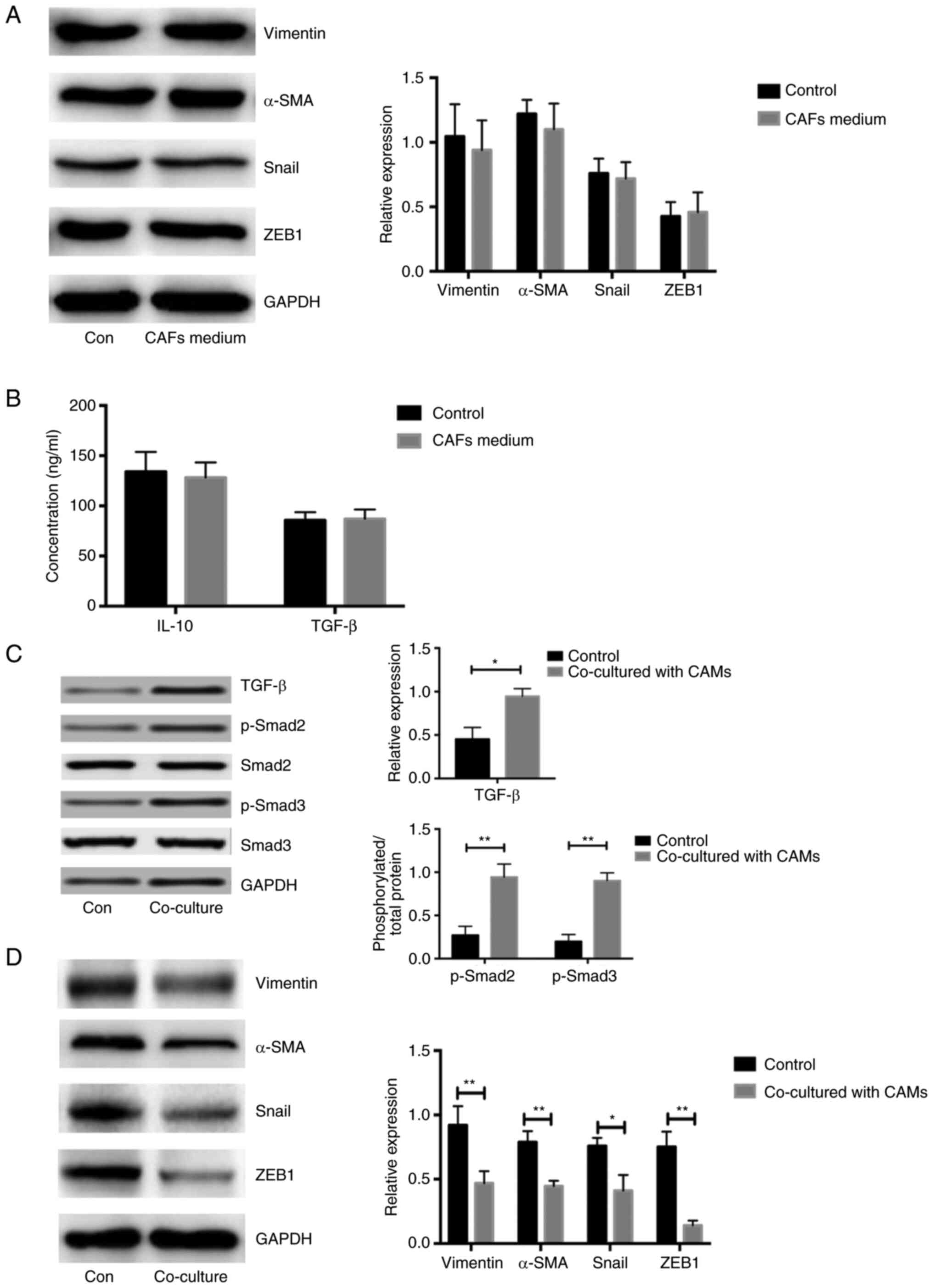
Increased EMT in ovarian cancerous
cells co-cultured with CAF-induced CAMs
To explore the expression of marker genes associated
with EMT in COC1 cells, the levels of vimentin, Snail, α-SMA and
ZEB1 were analyzed. Notably, similar expression levels of EMT
markers in COC1 cells treated with CAF and NOF medium were observed
(n=5; P<0.05; Fig. 3A). The
concentrations of IL-10 and TGF-β were similar between the media
directly derived from CAFs and NOFs, respectively (n=5; P<0.05;
Fig. 3B). Due to the increased
concentration of TGF-β in the CAMs stimulated with CAF supernatant,
the expression of proteins associated with TGF-β/Smad signaling
were measured. The expression of TGF-β and phosphorylation of
Smad2/3 was significantly higher in the COC1 cells co-cultured with
CAF-induced CAMs compared with that in controls, respectively,
(n=5; P<0.05; Fig. 3C). The
expression of genes associated with the EMT process in COC1 cells
co-cultured with CAF-induced CAMs was significantly higher compared
with that in the controls (n=5; P<0.05; Fig. 3D). Considering that there was a
higher concentration of TGF-β and IL-10 in the CAF-induced CAMs
medium aforementioned, TGF-β/Smad signaling might be activated only
in the COC1 cells co-cultured with CAF-induced CAMs. These results
demonstrated that the CAM-derived medium led to the activation of
TGF-β/Smad and increased the expression of EMT associated
molecules.
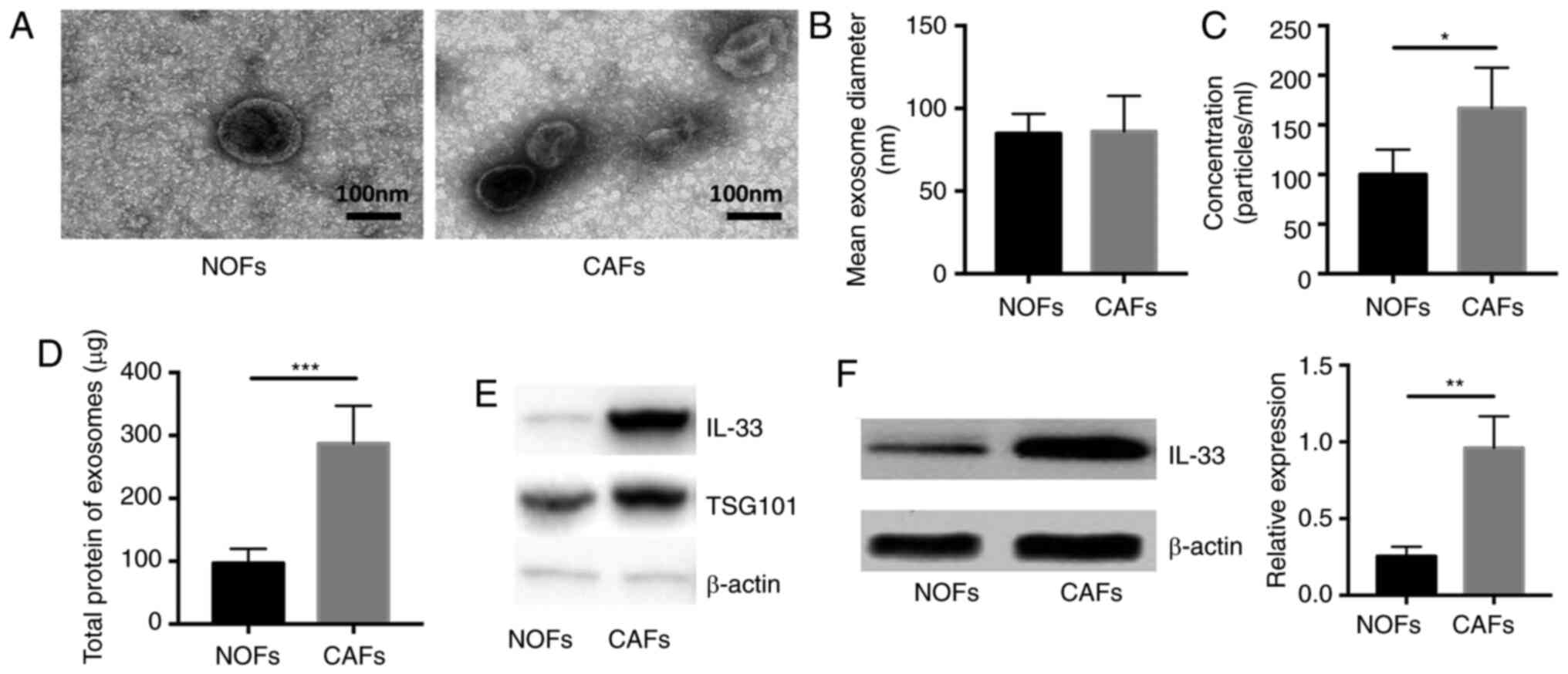
Significantly increased expression of
IL-33 in CAFs and exosomes from CAFs compared with NOFs
To verify that the extracellular vesicles could load
and transport secretory proteins from CAFs to other cells, the
exosomes were isolated and then the concentration of total proteins
wrapped in these extracellular vesicles was measured. The shape and
size of exosomes in the supernatant of NOFs and CAFs was assessed
using transmission electron microscopy (Fig. 4A). The mean diameter and
concentration per milliliter of exosomes were calculated and it was
reported that the exosomes had a similar mean diameter between the
supernatant of NOFs and CAFs and a significantly higher
concentration in the exosomes isolated from CAFs compared with NOFs
(Fig. 4B and C; P<0.05 and
P<0.01 respectively). In addition, the amount of total protein
in exosomes from CAFs was significantly higher compared with that
in NOFs (n=5, P<0.05; Fig. 4D).
Next, we measured the expression of IL-33 in exosomes and cells.
There was a significantly higher expression of IL-33 in exosomes
from CAFs and CAMs compared with NOFs (n=5; both P<0.05;
Fig. 4E and F). These data
demonstrated that the exosomes released by CAFs indeed act as a
carrier of IL-33 and might deliver secretory proteins to other
cells.
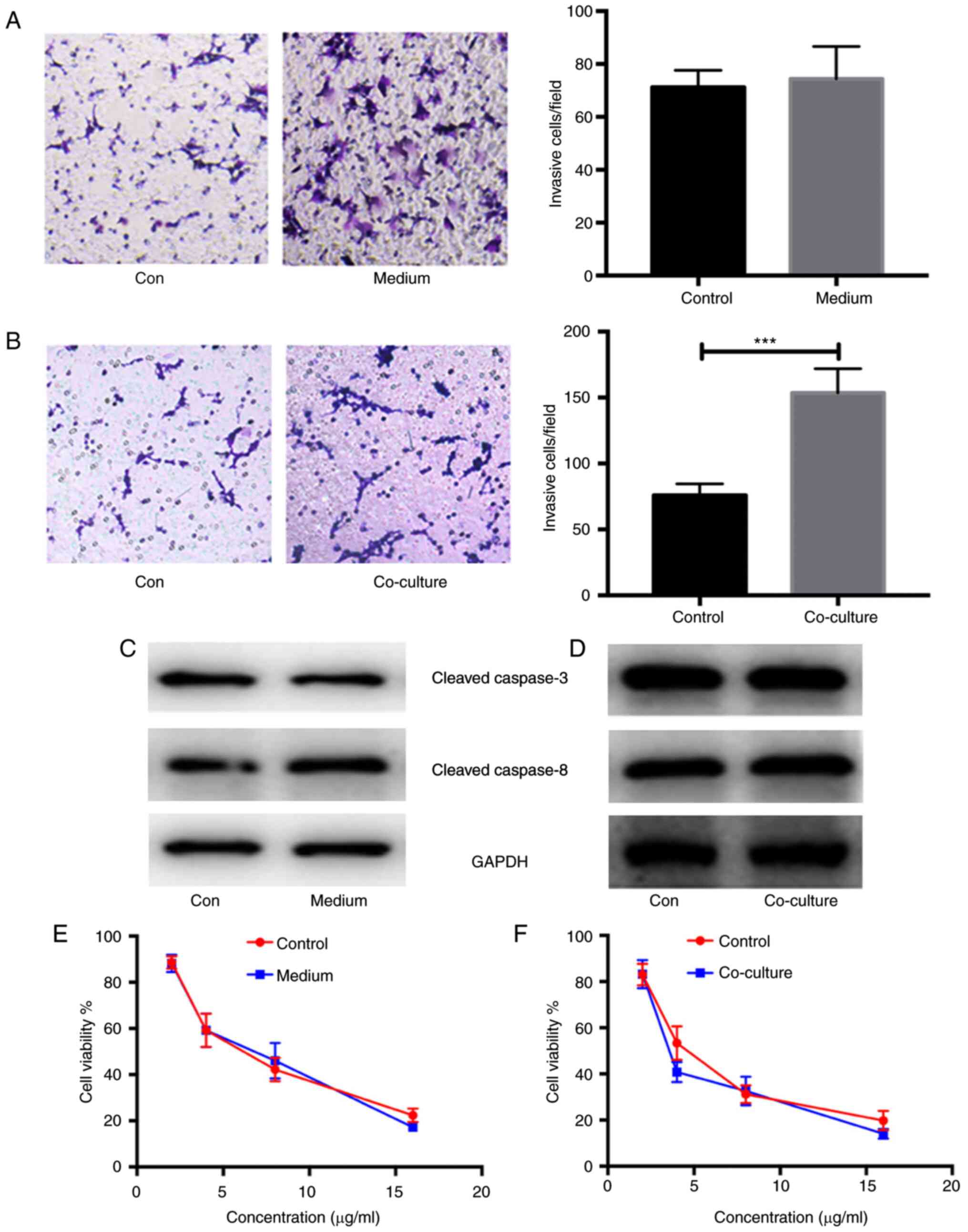
Enhanced invasion and metastasis in
ovarian cancer cells co-cultured with CAF-induced CAMs
In order to verify the alteration in cancerous
biological phenotypes, the invasion and migration of COC1 cells
stimulated by CAF-derived medium and co-cultured with CAF-induced
CAMs were measured using Transwell assays. As expected, there were
no significant differences in the invasion and migration between
COC1 cells stimulated with CAF-derived medium or not (n=5;
P>0.05; Fig. 5A). However, there
were significant differences in the invasion and migration between
COC1 cells co-cultured with CAF-induced CAMs or not (n=5;
P<0.001; Fig. 5B). In addition,
the expression of apoptosis-related proteins, such as cleaved
caspase-3 and −8, was detected in COC1 cells stimulated with
CAF-derived medium and co-cultured with CAF-induced CAMs. No
differences in the expression of apoptosis-related proteins were
found in COC1 cells under either condition compared with the
controls (n=5; P>0.05; Fig. 5C and
D). The chemotherapeutic resistance in COC1 cells stimulated
with CAF-derived medium and co-cultured with CAF-induced CAMs was
also analyzed. Using MTT assays, similar cell viabilities of COC1
cells were reported after the administration of cisplatin at
concentrations of 4, 8 and 16 µg/ml in the COC1 cells stimulated
with CAF-derived medium and controls (n=5; Fig. 5E). Similar results were obtained for
COC1 cells co-cultured with CAF-induced CAMs. A similar viability
of COC1 cells was also found after administration of cisplatin at
concentrations of 4, 8 and 16 µg/ml in COC1 cells co-cultured with
CAF-induced CAMs (n=5; Fig. 5F).
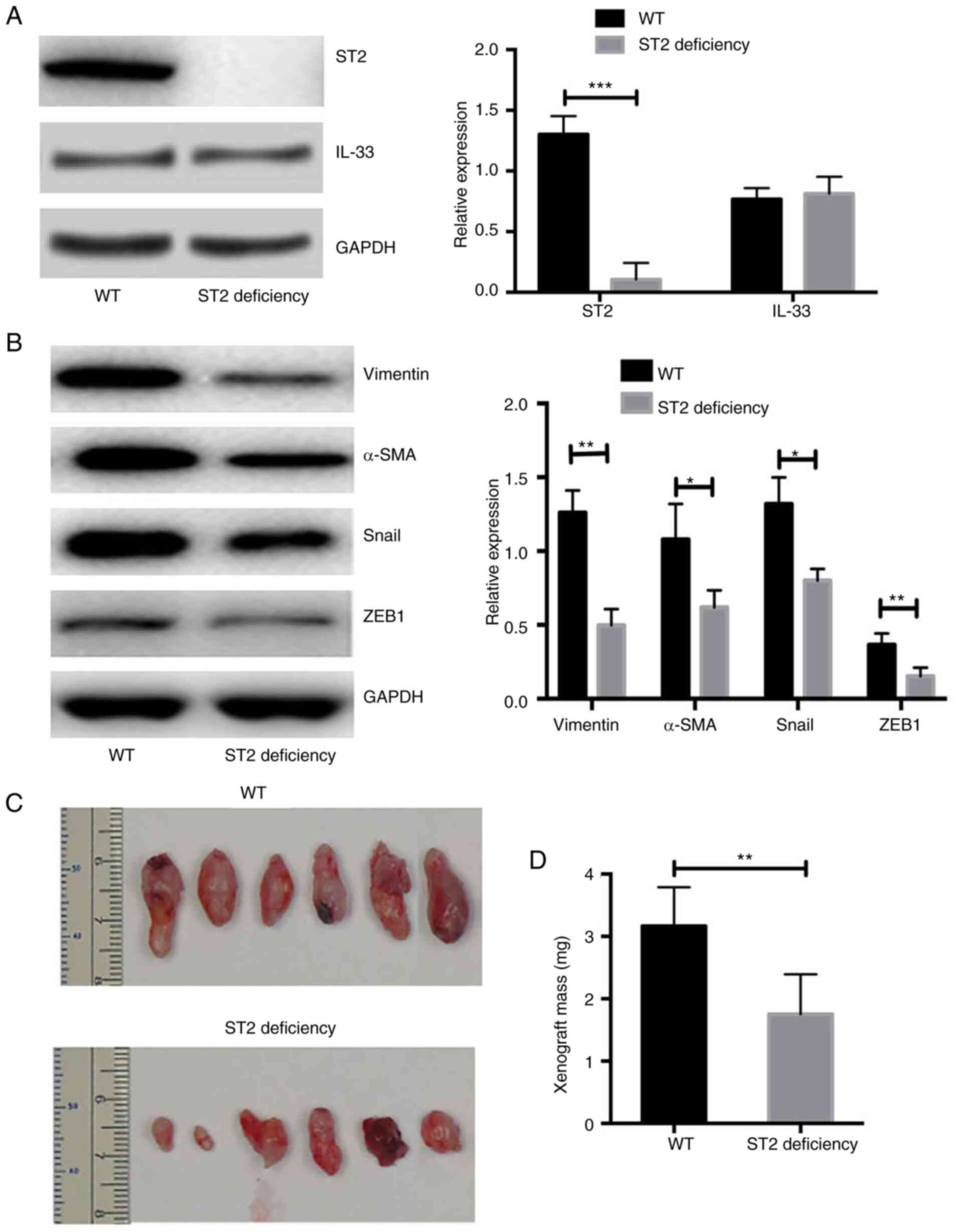
Mice with ST2-deficiency show a
significantly reduced volume of xenografts after transplantation of
human-derived primary ovarian cancer cells
Xenograft transplantation was performed in NOD-SCID
and NOD-SCID ST2-deficient mice. The expression of IL-33 in ST2
transplant tissues was also measured. It was demonstrated that the
expression of IL-33 was similar between transplant tissues from WT
and ST2-deficient mice (n=6; P>0.05; Fig. 6A). However, the expression of ST2 in
the xenograft tissues was almost undetectable from the NOD-SCID
ST2-deficient mice and was significantly lower compared with that
in NOD-SCID WT mice (n=6; P<0.01; Fig. 6A). In addition, the expression of EMT
markers was significantly lower in ST2-deficient mice compared with
that in WT mice (n=6; Fig. 6B). The
volume of xenografts from NOD-SCID ST2-deficient mice was
significantly smaller compared with that of NOD-SCID WT mice (n=6;
both P<0.05; Fig. 6C and D). The
data from in vivo experiments demonstrated that the blockade
of IL-33/ST2 signaling repressed the EMT process and reduced the
volume of xenografts of human-derived primary ovarian cancer
cells.
Discussion
Interactions between cancer exosomes secreted from
cancer-associated fibroblasts and the tumor microenvironment have
been brought into focus in recent years. There is evidence that the
exosomes secreted from fibroblasts could impact the biological
processes of cancer cells, induce immune repression and drive the
formation of a disease-associated microenvironment (19,20).
Although such data are lacking for ovarian cancer currently,
studies have shown that exosomes derived from CAFs could regulate
the function of immune cells in various cancer types, such as
breast cancer, pancreatic cancer and laryngeal squamous cell
carcinoma (19–22). The main goal of the present study was
to investigate the effect of exosomes isolated from cultured
primary CAFs on blood-derived monocytes and their secondary
influence on ovarian cancer cells.
The interplay between cancer-associated inflammatory
cells and CAFs is complex, and some investigations have uncovered
underlying mechanisms related to tumor invasion and metastasis
(23–25). A previous study reported that primary
human monocytes have a tendency to develop into immunosuppressive
myeloid cells, characterized by the expression of the
myeloid-derived suppressor cell marker S100A9, in a triple-negative
(TN) breast cancer environment (25). This results in the activation of
cancer-associated fibroblasts and the expression of CXCL16, which
is a monocyte chemoattractant (26).
This feedback loop promotes the formation of a reactive stroma,
contributing to the aggressive and invasive phenotype of TN breast
tumors (26). In colorectal cancer,
molecular subgroups and microenvironmental signatures are highly
correlated (26). In contrast to the
good-prognosis microsatellite instability-enriched subgroup
characterized by overexpression of genes specific to cytotoxic
lymphocytes, the poor-prognosis mesenchymal subgroup (CMS4)
expresses markers of lymphocytes and cells of monocytic origin.
Pathological examination of CRC tissues also revealed that the
mesenchymal subtype is characterized by a prominent infiltration of
fibroblasts that likely produce chemokines and cytokines that favor
tumor-associated inflammation and support angiogenesis, resulting
in a poor prognosis (27).
Another study investigated the role of prostate CAFs
in tumor biology. CAFs in prostate cancer could recruit monocytes
into cancerous tissues and enhance their trans-differentiation
toward the M2 macrophage phenotype. The study demonstrated that M2
macrophages and CAFs interact with each other, as M2 macrophages
could promote the mesenchymal-mesenchymal transition of
fibroblasts, leading to increased recruitment and the effects of M2
macrophage differentiation regulation on recruited monocytes. On
the other hand, prostate cancer cells also participate in crosstalk
through secretion of monocyte chemotactic protein-1, facilitating
monocyte recruitment and M2 polarization. Finally, this complicated
crosstalk among cancer cells, CAFs and M2 macrophages leads to an
enhancement of tumor cell motility, which promotes cancer cells
metastasis from the primary tumor, as well as angiogenesis
(28). The study illustrated that
the interplay between CAFs and CAMs could influence the tumor
biological phenotypes in prostate cancer by promoting the
differentiation of M2 macrophages.
The present study demonstrated that IL-33 could play
a role in migration and invasion in the progression of ovarian
cancer as a secretion cytokine, which might be attributed to the
interplay between CAFs and CAMs. CAMs exhibit an M2-like phenotype
in the presence of IL-33 from secreted exosomes and ultimately
alter the tumor microenvironment (28). Liu et al (29) revealed that IL-33 could have a direct
tumor-promoting effect on COC1 cells, which could increase cell
proliferation and inhibit apoptosis in vitro. IL-33 also
predicts poor prognosis and promotes the proliferation of cancerous
cells through the ERK and JNK signaling pathways. Previous studies
have demonstrated the association between IL-33 and tumorigenesis,
but only the direct influence of IL-33/ST2 signaling on cancerous
cells was investigated (30,31). The current study demonstrated that
IL-33 could influence the tumor microenvironment and integrate the
biological roles of CAMs and CAFs in the development of ovarian
cancer.
The present study reported that exosomes from CAFs
in ovarian cancer promoted M2 macrophage polarization, which led to
enhanced EMT by TGF-β/Smad signaling and a deteriorative invasive
phenotype in cancerous cells. The indirect effect of CAFs on
cancerous cells in ovarian cancer could be dependent on M2
macrophage polarization. It was demonstrated that exosomes derived
from CAFs did not directly affect the biological phenotype of
cancerous cells but could remodel the microenvironment by
regulating immune cell function and differentiation. Therefore,
CAFs and the interplay between CAFs and CAMs might be potential
therapeutic targets in the inhibition of invasion and metastasis in
ovarian cancer.
Acknowledgements
The authors would like to thank Dr Jing Ren
(Department of Biochemistry and Molecular Biology, Xi'an Medical
College, Xi'an, Shaanxi, China) for help with the experimental
design of the study.
Funding
The study was supported by The National Natural Science
Foundation of China (grant no. 81615924).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CF and LK participated in the experimental design
and data analysis. PY performed some of the experiments. YJ and CF
conceived the project, performed most of the experiments and wrote
the manuscript. CF and YJ confirm the authenticity of all the raw
data. All the authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
Baoji People's Hospital Ethics Committee and with the 1964 Helsinki
declaration and its later amendments or comparable ethical
standards. The protocols for animal experiments were approved by
the Baoji People's Hospital Ethics Committee on the Use of Live
Animals in Teaching and Research (Baoji, China) (approval no.
2018-129). Informed consent was obtained from all individual
participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Machida H, Matsuo K, Yamagami W, Ebina Y,
Kobayashi Y, Tabata T, Kanauchi M, Nagase S, Enomoto T and Mikami
M: Trends and characteristics of epithelial ovarian cancer in Japan
between 2002 and 2015: A JSGO-JSOG joint study. Gynecol Oncol.
153:589–596. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rose PG, Java JJ, Salani R, Geller MA,
Secord AA, Tewari KS, Bender DP, Mutch DG, Friedlander ML, Van Le
L, et al: Nomogram for predicting individual survival after
recurrence of advanced-stage, high-grade ovarian carcinoma. Obstet
Gynecol. 133:245–254. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Silva EG, Sood AK, et al: Ovarian
epithelial carcinogenesis[M]//gynecologic and obstetric pathology,
Volume 2. Springer; Singapore: pp. 121–139. 2019
|
|
4
|
Hwang JY, Lim WY, Tan CS, Lim SL, Chia J,
Chow KY and Chay WY: Ovarian cancer incidence in the multi-ethnic
asian city-state of Singapore 1968–2012. Asian Pac J Cancer Prev.
20:3563–3569. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sahai E, Astsaturov I, Cukierman E,
DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR,
Hunter T, et al: A framework for advancing our understanding of
cancer-associated fibroblasts. Nat Rev Cancer. 20:174–186. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsang M, Quesnel K, Vincent K,
Hutchenreuther J, Postovit LM and Leask A: Insights into fibroblast
plasticity: Cellular communication network 2 is required for
activation of cancer-associated fibroblasts in a murine model of
melanoma. Am J Pathol. 190:206–221. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ling J and Chiao PJ: Two birds with one
stone: Therapeutic targeting of IL-1β signaling pathways in
pancreatic ductal adenocarcinoma and the cancer-associated
fibroblasts. Cancer Discov. 9:173–175. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Neri S, Hashimoto H, Kii H, Watanabe H,
Masutomi K, Kuwata T, Date H, Tsuboi M, Goto K, Ochiai A and Ishii
G: Cancer cell invasion driven by extracellular matrix remodeling
is dependent on the properties of cancer-associated fibroblasts. J
Cancer Res Clin Oncol. 142:437–446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teng F, Tian WY, Wang YM, Zhang YF, Guo F,
Zhao J, Gao C and Xue FX: Cancer-associated fibroblasts promote the
progression of endometrial cancer via the SDF-1/CXCR4 axis. J
Hematol Oncol. 9:82016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ogawa K, Lin Q, Li L, Bai X, Chen X, Chen
H, Kong R, Wang Y, Zhu H, He F, et al: Prometastatic secretome
trafficking via exosomes initiates pancreatic cancer pulmonary
metastasis. Cancer Lett. 481:63–75. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arai K, Ishimatsu H, Iwasaki T, Tsuchiya
C, Sonoda A and Ohata K: Membranous S100A10 involvement in the
tumor budding of colorectal cancer during oncogenesis: Report of
two cases with immunohistochemical analysis. World J Surg Oncol.
18:2892020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brunetto E, De Monte L, Balzano G, Camisa
B, Laino V, Riba M, Heltai S, Bianchi M, Bordignon C, Falconi M, et
al: The IL-1/IL-1 receptor axis and tumor cell released
inflammasome adaptor ASC are key regulators of TSLP secretion by
cancer associated fibroblasts in pancreatic cancer. J Immunother
Cancer. 7:452019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi H, Enomoto A, Woods SL, Burt AD,
Takahashi M and Worthley DL: Cancer-associated fibroblasts in
gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 16:282–295.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qian L, Tang Z, Yin S, Mo F, Yang X, Hou
X, Liu A and Lu X: Fusion of dendritic cells and cancer-associated
fibroblasts for activation of anti-tumor cytotoxic T lymphocytes. J
Biomed Nanotechnol. 14:1826–1835. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suklabaidya S, Dash P and Senapati S:
Pancreatic fibroblast exosomes regulate survival of cancer cells.
Oncogene. 36:3648–3649. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanada M, Bachmann MH and Contag CH:
Signaling by extracellular vesicles advances cancer hallmarks.
Trends Cancer. 2:84–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang F, Ning Z, Ma L, Liu W, Shao C, Shu Y
and Shen H: Exosomal miRNAs and miRNA dysregulation in
cancer-associated fibroblasts. Mol Cancer. 16:1482017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ringuette Goulet C, Bernard G, Tremblay S,
Chabaud S, Bolduc S and Pouliot F: Exosomes induce fibroblast
differentiation into Cancer-associated fibroblasts through TGF-β
signaling. Mol Cancer Res. 16:1196–1204. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Wei H, Wang J, Li L, Chen A and Li
Z: MicroRNA-181d-5p-containing exosomes derived from CAFs promote
EMT by regulating CDX2/HOXA5 in breast cancer. Mol Ther Nucleic
Acids. 19:654–667. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yeon JH, Jeong HE, Seo H, Cho S, Kim K, Na
D, Chung S, Park J, Choi N and Kang JY: Cancer-derived exosomes
trigger endothelial to mesenchymal transition followed by the
induction of cancer-associated fibroblasts. Acta Biomater.
76:146–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin X, Guo H, Wang X, Zhu X, Yan M, Wang
X, Xu Q, Shi J, Lu E, Chen W and Zhang J: Exosomal miR-196a derived
from cancer-associated fibroblasts confers cisplatin resistance in
head and neck cancer through targeting CDKN1B and ING5. Genome
Biol. 20:12–14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li K, Chen Y, Li A, Tan C and Liu X:
Exosomes play roles in sequential processes of tumor metastasis.
Int J Cancer. 144:1486–1495. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu T and Kong J: CAF-derived exosomes
remodel ECM by targeting lung fibroblasts via integrin α2β1 at the
pre-metastatic niche. J Extracellular Vesicles. 7:234–235.
2018.
|
|
24
|
Guo H, Ha C, Dong H, Yang Z, Ma Y and Ding
Y: Cancer-associated fibroblast-derived exosomal microRNA-98-5p
promotes cisplatin resistance in ovarian cancer by targeting
CDKN1A. Cancer Cell Int. 19:3472019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu HJ, Hao M, Yeo SK and Guan JL: FAK
signaling in cancer-associated fibroblasts promotes breast cancer
cell migration and metastasis by exosomal miRNAs-mediated
intercellular communication. Oncogene. 39:2539–2549. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Allaoui R, Bergenfelz C, Mohlin S,
Hagerling C, Salari K, Werb Z, Anderson RL, Ethier SP, Jirström K,
Påhlman S, et al: Cancer-associated fibroblast-secreted CXCL16
attracts monocytes to promote stroma activation in triple-negative
breast cancers. Nat Commun. 7:130502016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Becht E, de Reyniès A, Giraldo NA, Pilati
C, Buttard B, Lacroix L, Selves J, Sautès-Fridman C, Laurent-Puig P
and Fridman WH: Immune and stromal classification of colorectal
cancer is associated with molecular subtypes and relevant for
precision immunotherapy. Clin Cancer Res. 22:4057–4066. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Comito G, Giannoni E, Segura CP,
Barcellos-de-Souza P, Raspollini MR, Baroni G, Lanciotti M, Serni S
and Chiarugi P: Cancer-associated fibroblasts and M2-polarized
macrophages synergize during prostate carcinoma progression.
Oncogene. 33:2423–2431. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu X, Hansen DM, Timko NJ, Zhu Z, Ames A,
Qin C, Nicholl MB, Bai Q, Chen X, Wakefield MR, et al: Association
between interleukin-33 and ovarian cancer. Oncol Rep. 41:1045–1050.
2019.PubMed/NCBI
|
|
30
|
Tong X, Barbour M, Hou K, Gao C, Cao S,
Zheng J, Zhao Y, Mu R and Jiang HR: Interleukin-33 predicts poor
prognosis and promotes ovarian cancer cell growth and metastasis
through regulating ERK and JNK signaling pathways. Mol Oncol.
10:113–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Larsen KM, Minaya MK, Vaish V and Peña
MMO: The role of IL-33/ST2 pathway in tumorigenesis. Int J Mol Sci.
19:26762018. View Article : Google Scholar : PubMed/NCBI
|