Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent types of cancer and the second leading cause of
cancer-related death worldwide (1). The independent risk factors for the
occurrence and development of HCC include excessive intake of
alcohol, smoking and obesity (2).
Surgery is the preferred option for the treatment of HCC, although
it is associated with a poor prognosis and a low 5-year survival
rate (3). Furthermore, most
patients are diagnosed at an advanced stage, which increases the
complexity of surgery (4).
Therefore, there is an urgent need to understand the pathogenesis
of HCC in order to develop effective therapies against this
disease.
Glycosylphosphatidylinositol (GPI) anchor attachment
1 (GPAA1) is one of the subunits of the GPI transferase complex,
which serves as a link between GPI anchor sites and proteins
(5). It has been reported that
GPAA1 is upregulated in various types of cancer and that GPAA1
promotes disease progression by regulating C-Myc in childhood acute
lymphoblastic leukemia (6).
Moreover, overexpression of GPAA1 has been shown to promote
tumorigenicity and invasiveness of breast cancer cells in nude mice
(7). GPAA1 may also promote the
metastasis and invasion of gastric cancer (5). Although GPAA1 has been reported to be
upregulated in HCC, in-depth studies on its potential role in HCC
are still lacking (8).
The starBase database suggested that the RNA-binding
protein splicing factor (SF)3B4 may interact with GPAA1.
Alternative splicing is an important step during gene transcription
that allows the generation of multiple mRNA transcripts from one
specific gene (9). Alternative
splicing factors, which have been extensively studied in a wide
variety of disorders and tumors, serve an essential role in the
progression of cancer and the occurrence of chemoresistance
(10–12). RNA splicing is modulated by U2 and
U12 small nuclear ribonucleoprotein (snRNP)-dependent spliceosomes,
and the U2 snRNP consists of U2 snRNA and the SF3A/SF3B complex
(13). It has been identified
that, among the six subunits of the SF3B complex, SF3B4 is
upregulated in patients with HCC (14). SF3B4 can also be used as a
diagnostic marker for HCC. Compared with the current diagnostic
markers used for HCC (GPC3, GS and HSP70), SF3B4 combined with
BANF1 and PLOD3 has been reported to exhibit stronger diagnostic
efficacy for early HCC (15).
Furthermore, this protein serves an oncogenic role in other types
of cancer, such as pancreatic cancer and esophageal squamous cell
carcinoma (16,17). Therefore, it was hypothesized in
the present study that the upregulation of GPAA1, which may be
regulated by SF3B4, could promote the progression of HCC.
Materials and methods
Cell culture
The normal human liver MIHA cell line, and HCC
Hep10, HuH-7, SNU-387 and Hep 3B2.1-7 cell lines were obtained from
The Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences. The cells were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and streptomycin in a
humidified atmosphere at 37°C with 5% CO2.
Cell transfection
To knock down the expression of GPAA1 and SF3B4,
small interfering RNA (siRNA) molecules targeting GPAA1 [si-GPAA1#1
(siG000008733A-1-5) and si-GPAA1#2 (siG000008733B-1-5)] and SF3B4
[si-SF3B4#1 (siG000010262A-1-5) and si-SF3B4#2 (siG000010262B-1-5)]
were purchased from Guangzhou RiboBio Co., Ltd. In addition, a
scrambled siRNA negative control (si-NC; cat. no. A06001) was
designed and synthesized by Shanghai GenePharma Co., Ltd. The SF3B4
overexpression (oe-SF3B4) plasmid was constructed by cloning the
full length of the SF3B4 sequence into the pcDNA3.1 vector obtained
from Shanghai GenePharma Co., Ltd. The empty vector pcDNA3.1 is
referred to as the control (oe-NC) plasmid. HuH-7 cells were plated
in 24-well dishes at a density of 1×106 cells/well, and
plasmid transfection was performed at a concentration of 50 ng/ml
using Lipofectamine™ 2000 transfection reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) for 48 h at 37°C according to the
manufacturer's protocols. The transfection efficiency was detected
48 h post-transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from MIHA, Hep10, HuH-7,
SNU-387 and Hep3B cells using TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc.) and cDNA was synthesized using the
PrimeScript™ RT MasterMix kit (Takara Bio, Inc.) according to the
manufacturer's protocol. qPCR was performed using the LightCycler
480 Probes Master kit (Roche Applied Science) on a LightCycler 480
system (Roche Applied Science). The following thermocycling
conditions were used for qPCR: 95°C for 10 min; followed by 40
cycles of denaturation at 95°C for 10 sec and annealing/extension
at 60°C for 60 sec. The primer sequences were as follows: GPAA1,
forward 5′-CTCCCGCTTCGTCTCCATC-3′ and reverse
5′-CACTGGCAGGACATAGAGGG-3′; SF3B4, forward 5′-AGACGGCGGGATCTCTTT-3′
and reverse 5′-CACGTACACAGTGGCATCCT-3′; and GAPDH, forward
5′-CATCACTGCCACCCAGAAGA-3′ and reverse 5′-CCACCTGGTGCTCAGTGTAG-3′.
Target mRNA expression was calculated using the 2−ΔΔCq
method and normalized to GAPDH levels (18).
Western blotting
MIHA, Hep10, HuH-7, SNU-387 and Hep3B cells were
lysed in cell lysis buffer (Beijing Solarbio Science &
Technology Co., Ltd.), and protein concentration was determined
using a BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.). The
cell lysates containing equal amounts of protein (30 µg per lane)
were resolved by 10% SDS-PAGE and were then transferred to PVDF
membranes (Bio-Rad Laboratories, Inc.). The membranes were blocked
with 5% skimmed milk powder for 2 h at room temperature in 0.1%
TBS-Tween (TBST) buffer, then incubated with the primary antibodies
against GPAA1 (cat. no. PA5-100548; dilution, 1:1,000; Thermo
Fisher Scientific, Inc.), MMP2 (cat. no. ab92536; dilution,
1:1,000; Abcam), MMP9 (cat. no. ab76003; dilution, 1:1,000; Abcam),
SF3B4 (cat. no. ab157117; dilution, 1:1,000; Abcam) and GAPDH (cat.
no. ab9485; dilution, 1:2,500; Abcam) at 4°C overnight. After
washing in TBST three times, the membranes were incubated with a
secondary antibody (cat. no. ab6721; dilution, 1:2,000; Abcam) at
room temperature for 1 h. GAPDH was used as the loading control.
The protein bands were developed using a chemiluminescence
detection kit (Cytiva) and the band densities of the target
proteins were semi-quantified using ImageJ 1.51 (National
Institutes of Health).
Cell proliferation
To examine proliferation, HuH-7 cells were seeded at
a density of 4×103 cells/well in 24-well plates. After
24, 48 and 72 h of culture, the cells were incubated with 10 µl
Cell Counting Kit-8 reagent (Thermo Fisher Scientific, Inc.) at
37°C for 4 h. Subsequently, the absorbance was measured at 450 nm
using a microplate reader. The cell proliferation rate (%) was
calculated via optical density (OD) using the following formula:
(Experimental OD-control OD)/control OD ×100.
Colony formation assay
The transfected HuH-7 cells were collected and
inoculated into 24-well dishes at a density of 4×103
cells/well. The medium was replaced every 4 days. After 2 weeks,
the colonies that had formed were washed in PBS three times, fixed
with 1% paraformaldehyde for 15 min at room temperature, then
stained with 0.1% crystal violet for 30 min at room temperature.
After washing and drying the colonies, images were captured using a
light microscope.
Cell migration
HuH-7 cells were seeded at a density of
2×104 cells/well into 6-well plates. After 24 h of cell
culture, when cells were cultured to 100% confluence, a wound was
made in the cell monolayer using a 200-µl pipette tip. After
washing, the medium was replaced with serum-free medium. Images
were captured under an inverted light microscope at 0 and 24 h
using the following equation: (Initial width at 0 h-final width at
24 h)/initial width at 0 h.
Cell invasion
The invasion of HuH-7 cells was determined using
24-well Transwell chambers with 8-µm pores (Corning, Inc.) coated
with Matrigel® (BD Biosciences) at room temperature for
24 h. HuH-7 cells were suspended in serum-free DMEM at a density of
2×104 cells/well in the upper chamber of the Transwell.
The lower chamber contained DMEM supplemented with 10% FBS. After
24 h of incubation at 37°C, the cells that had invaded to the lower
surface of the membranes were fixed with 4% paraformaldehyde for 10
min at room temperature, then stained with 0.2% crystal violet at
room temperature for 30 min. Images were captured under an inverted
light microscope (Olympus CX23; Olympus Corporation).
RNA immunoprecipitation (RIP)
assay
The RIP assay was conducted using a Magna RIP
RNA-Binding Protein Immunoprecipitation Kit (MilliporeSigma)
according to the manufacturer's protocol. After transfection, HuH-7
cells (1×107) were inoculated and lysed in 100 µl RIP
lysis buffer. The cell lysate (100 µl) was then incubated with 50
µl magnetic beads coupled with anti-SF3B4 antibody (cat. no.
ab157117; Abcam) or control IgG (cat. no. ab172730; Abcam) in RIP
buffer. The expression of GPAA1 was analyzed by RT-qPCR as
previously described.
Detection of RNA stability
Transfected HuH-7 cells (6×105
cells/well) were plated into 24-well plates and cultured for 24 h.
Subsequently, the cells were treated with 5 µg/ml actinomycin D
(MedChemExpress) at 37°C and collected after 20, 40 or 60 min.
Total RNA was extracted using the miRNeasy Kit (Qiagen GmbH), and
GPAA1 expression was analyzed using RT-qPCR and western
blotting.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 7 (GraphPad Software, Inc.). Data were generated from
three independent experimental repeats. The results are presented
as the mean ± standard deviation. Differences between groups were
compared using Student's t-test or using one-way ANOVA followed by
Tukey's post hoc test. Mantel-Cox test was to determine the overall
survival rate of HCC patients. Pearson's correlation analysis was
utilized to confirm the correlation between SF3B4 and GPAA1.
P<0.05 was considered to indicate a statistically significant
difference.
Bioinformatics tools
GPAA1 and SF3B4 expression in HCC tissues, and the
correlation between GPAA1 or SF3B4 expression and the overall
survival rate of patients with HCC were analyzed based on Gene
Expression Profiling Interactive Analysis 2 (GEPIA2) database
(http://gepia2.cancer-pku.cn/#index).
The binding between SF3B4 and GPAA1 and the correlation between
SF3B4 and GPAA1 in HCC was predicted by starBase database
(https://starbase.sysu.edu.cn/).
Results
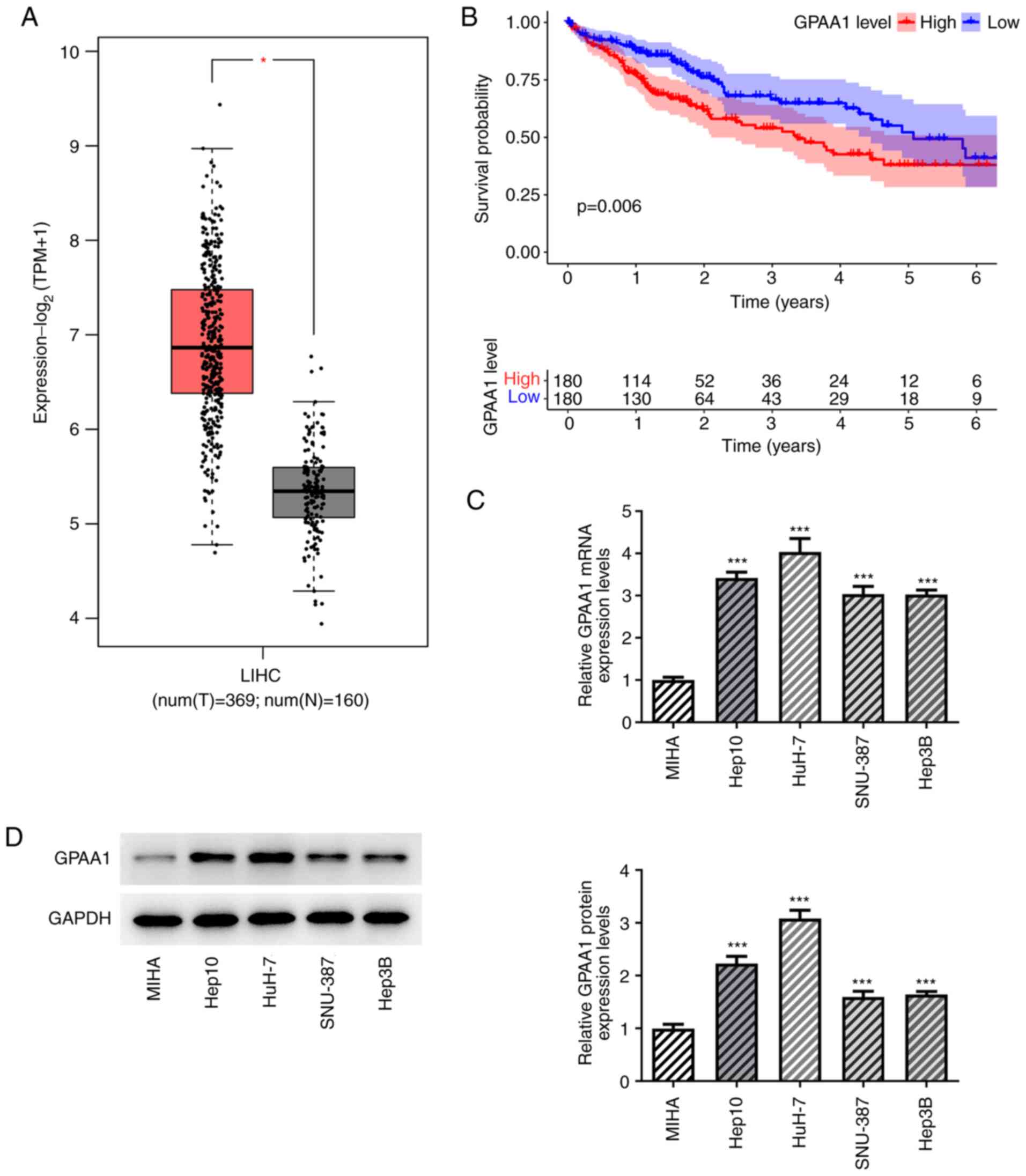
GPAA1 is upregulated in HCC cells
To examine the role of GPAA1 in HCC, the expression
of GPAA1 was examined using the GEPIA2 database. The results
suggested that GPAA1 was upregulated in HCC, which may be
associated with poor overall survival in patients with HCC (180
patients in high GPAA1 group and 180 patients in low GPAA1 group;
divided using median expression value) (Fig. 1A and B). To validate this finding,
RT-qPCR and western blotting were carried out on HCC cell lines.
The expression level of GPAA1 was increased in HCC cells compared
with that in the MIHA cell line, and the HuH-7 cell line exhibited
the highest mRNA and protein expression levels of GPAA1 (Fig. 1C and D). Therefore, HuH-7 cells
were used in subsequent experiments.
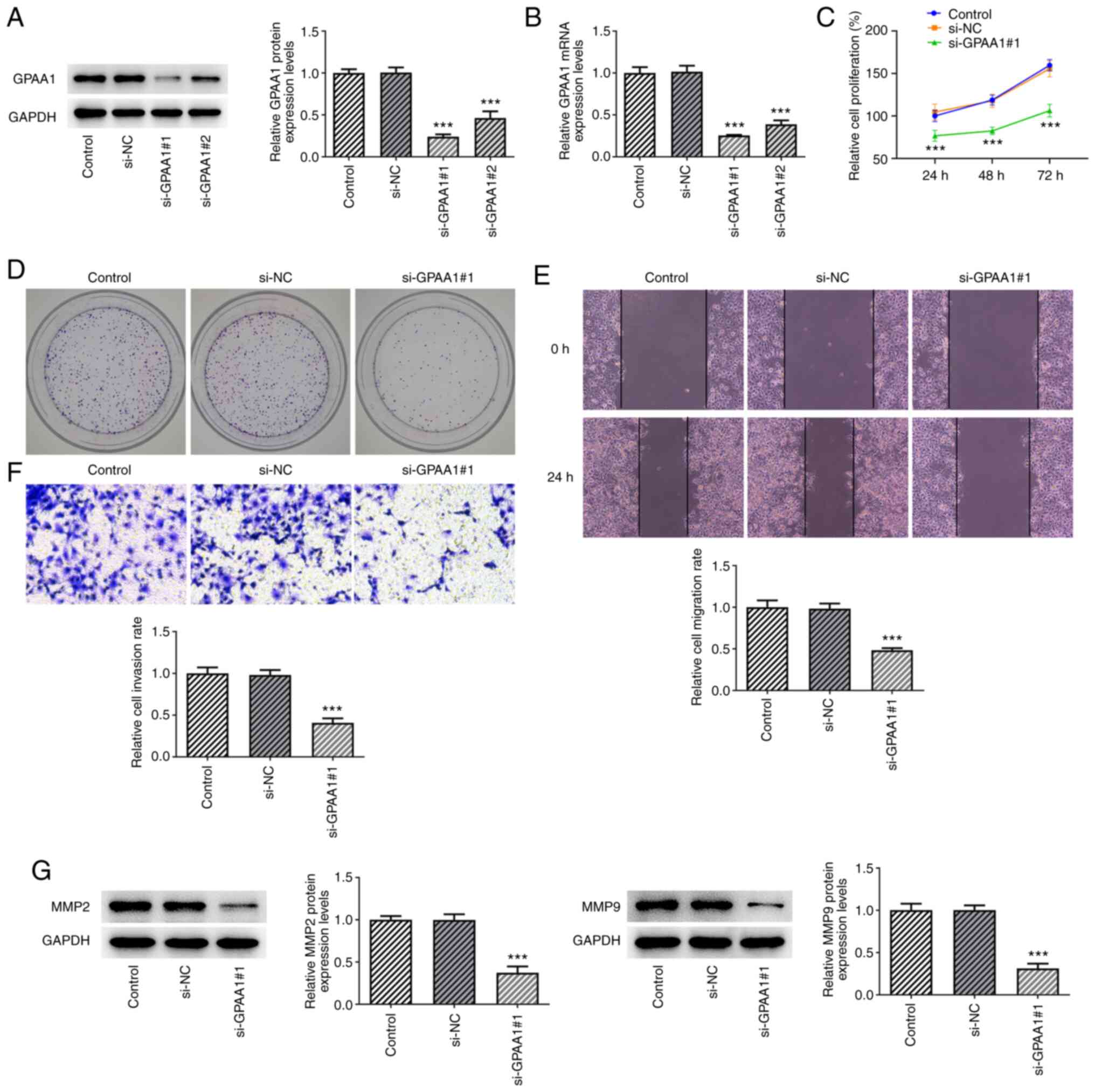
GPAA1 regulates the proliferation,
migration and invasion of HCC cells
As GPAA1 was upregulated in HCC cells, its
expression was knocked down by transfecting siRNA targeting GPAA1
into these cells. The expression levels of GPAA1 were significantly
downregulated after transfection of si-GPAA1#1/2 plasmids, and the
interference efficiency of si-GPAA1#1 was greater than that of
si-GPAA1#2; thus, si-GPAA1#1 was used in subsequent experiments
(Fig. 2A and B). In addition, the
proliferation and colony formation abilities of HuH-7 cells were
reduced following si-GPAA1#1 transfection (Fig. 2C and D). Furthermore, transfection
with si-GPAA1#1 inhibited the migration and invasion of HuH-7
cells, which was accompanied by MMP2 and MMP9 downregulation
(Fig. 2E-G). These findings
indicated that GPAA1 could regulate the proliferation, migration
and invasion of HCC cells.
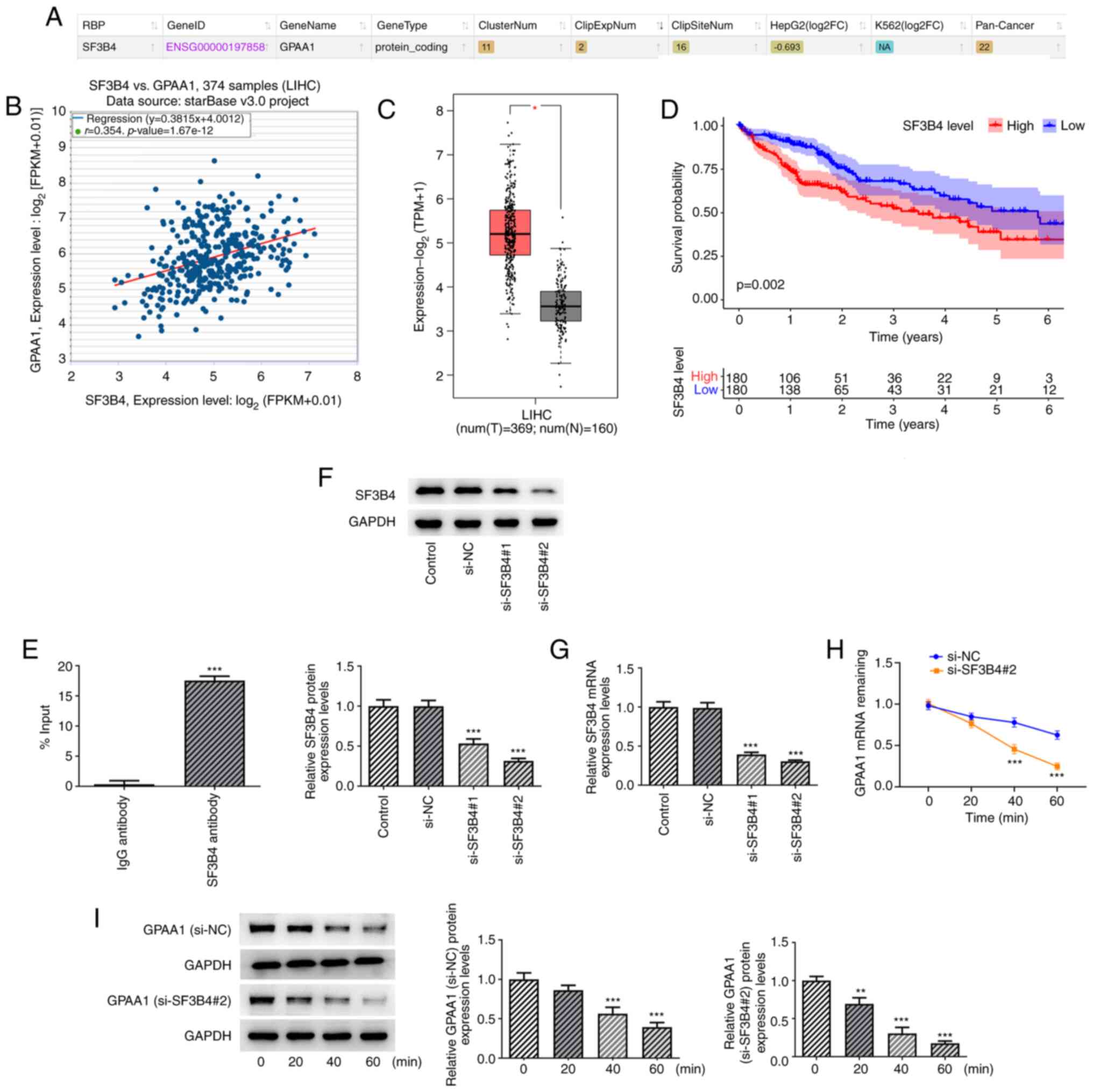
SF3B4 binds to and stabilizes GPAA1
mRNA
As predicted by starBase, GPAA1 was predicted to
bind to SF3B4 and the expression levels of GPAA1 were positively
correlated with those of SF3B4 (Fig.
3A and B). Further analysis using GEPIA2 indicated that SF3B4
displayed high expression in HCC tissues and high expression levels
of SF3B4 were associated with poor overall survival in patients
with HCC (180 patients in high SF3B4 group and 180 patients in low
SF3B4 group) (Fig. 3C and D).
Thus, it was hypothesized that SF3B4 may modulate the progression
of HCC by interacting with GPAA1. The results of the RIP assay
confirmed that these two proteins could interact with each other
(Fig. 3E). Subsequently, si-SF3B4
was transfected into HCC cells. The expression levels of SF3B4 were
lowest in the si-SF3B4#2 group; therefore, this siRNA was used for
subsequent experiments (Fig. 3F and
G). Following treatment with actinomycin D, si-SF3B4
transfection reduced the mRNA and protein stability of GPAA1
(Fig. 3H and I). These findings
suggested that SF3B4 could bind to and stabilize GPAA1 mRNA.
SF3B4 overexpression reverses the
effects of GPAA1 knockdown on the proliferation, migration and
invasion of HCC cells
To investigate whether SF3B4 exerted effects on the
progression of HCC cells by binding to GPAA1, SF3B4 was
overexpressed in HCC cells; the transfection efficiency of oe-SF3B4
was confirmed by western blotting and RT-qPCR (Fig. 4A and B). As shown in Fig. 4C and D, the si-GPAA1-induced
inhibition of GPAA1 was abrogated by oe-SF3B4. In addition, SF3B4
overexpression promoted the expression levels of GPAA1 (Fig. 4E and F), whereas SF3B4 knockdown
suppressed the expression levels of GPAA1 (Fig. 4G and H). Moreover, the reduction in
proliferation and colony formation of HuH-7 cells mediated by
si-GPAA1 was abolished by SF3B4 overexpression (Fig. 4I and J). Additionally, GPAA1
knockdown reduced the migration and invasion of HuH-7 cells, which
was reversed following oe-SF3B4 transfection (Fig. 4K and L). The expression levels of
MMP2 and MMP9 were also suppressed following si-GPAA1 transfection,
which was reversed by oe-SF3B4 transfection (Fig. 4M). These findings indicated that
the overexpression of SF3B4 reversed the effects of GPAA1 knockdown
on the proliferation, migration and invasion of HCC cells.
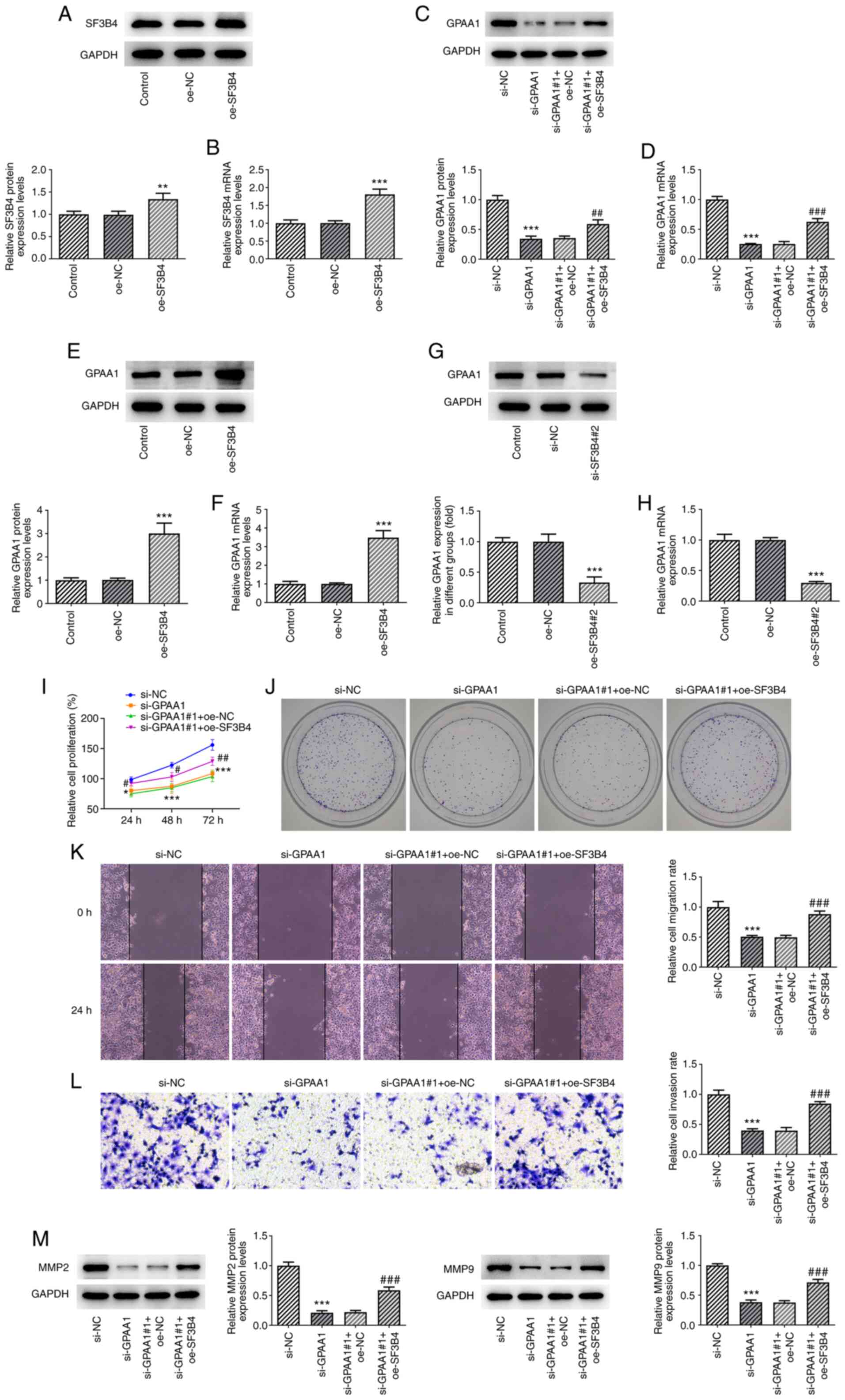 | Figure 4.Overexpression of SF3B4 reverses the
effects of GPAA1 knockdown on the proliferation, migration and
invasion of HCC cells. (A) Protein and (B) mRNA expression levels
of SF3B4 after transfection with oe-SF3B4. (C) Protein and (D) mRNA
expression levels of GPAA1 after transfection with si-GPAA1 and
oe-SF3B4. (E) Protein and (F) mRNA expression levels of GPAA1 after
transfection with oe-SF3B4. (G) Protein and (H) mRNA expression
levels of GPAA1 after transfection with si-SF3B4#2. (I)
Proliferation, (J) colony formation, (K) migration (×100
magnification), (L) invasion (×100 magnification), and (M) MMP2 and
MMP9 expression in HCC cells transfected with si-GPAA1 and
oe-SF3B4. *P<0.05, **P<0.01 and ***P<0.001 vs. si-NC;
#P<0.05, ##P<0.01 and
###P<0.001 vs. si-GPAA1 + oe-NC. GPAA1,
glycosylphosphatidylinositol anchor attachment 1; HCC,
hepatocellular carcinoma; NC, negative control; oe, overexpression;
SF3B4, splicing factor 3B4; si, small interfering. |
Discussion
Several studies have focused on GPAA1, due to its
functional role in cancer development (8). It has been proposed that GPAA1 may
increase the activity of the GPI transamidase complex, which
mediates the transfer of a GPI anchor to the C-terminus of target
proteins without transmembrane domain proteins (19,20).
The regulatory role of GPAA1 in the progression of numerous types
of cancer has been well documented in previous years. For example,
GPAA1 has been reported to regulate the expression of GPI-anchored
proteins and promote the ERBB signaling pathway, thus contributing
to tumor growth in gastric cancer (5). Moreover, the upregulation of GPAA1 in
patients with colorectal cancer has highlighted its potential
significance in regulating the proliferation, invasion and
metastasis of this type of cancer (21). It has been previously reported that
GPAA1 is expressed at high levels in HCC compared with in matched
adjacent non-tumor tissue samples, suggesting that GPAA1 expression
may be associated with HCC progression and poor survival rate
(8). In the present study, it was
predicted by the GEPIA2 database and further confirmed by
subsequent experiments that GPAA1 was highly expressed in HCC
cells. Transfection with si-GPAA1 resulted in significantly reduced
cell proliferation, fewer numbers of colonies, and decreased
migratory and invasive capacities in HuH-7 cells.
It is well established that alternative splicing of
pre-mRNA is a common phenomenon that governs the diversity of the
proteome (22). Dysregulation of
alternative splicing, which is usually seen in tumor cells, can
regulate the malignant behavior of cells, including proliferation,
angiogenesis, invasion and metastasis (23). Alternative splicing is one of the
most important processes that can affect cellular functions; it is
mediated by splicing factors, which are regulatory proteins
expressed intracellularly (23). A
previous study also indicated the importance of alternative
splicing as a source of HCC prognostic markers (24). SF3B4 has been demonstrated to serve
an oncogenic role in various tumor types and is associated with a
poor prognosis (13,14,16).
In the present study, it was demonstrated that the expression of
GPAA1 was positively correlated with that of SF3B4, and was
associated with a poor prognosis in patients with HCC. RIP
experiments confirmed the interaction between GPAA1 and SF3B4.
Furthermore, SF3B4 knockdown reduced the mRNA stability of GPAA1.
Comprehensive meta-analyses on gene profiles have suggested that
upregulation of SF3B4 in HCC may be linked to a poor prognosis in
patients with HCC, consistent with previous findings regarding the
role of GPAA1 in HCC (25). Thus,
it was hypothesized that SF3B4 may exert its effects on HCC cells
by binding to GPAA1. In comparison to the HuH-7 cells transfected
with si-GPAA1 alone, co-transfection with si-GPAA1 and oe-SF3B4
resulted in increased mRNA and protein expression levels of GPAA1,
demonstrating the participation of SF3B4 in the mechanism
underlying the pathogenesis of HCC. As anticipated, the
proliferation, migration and invasion of HuH-7 cells, which were
significantly inhibited by GPAA1 knockdown, were increased
following SF3B4 overexpression.
In conclusion, SF3B4 may promote the proliferation,
invasion and migration of HCC cells by binding to GPAA1. This
finding provides novel insight into the pathogenesis of HCC.
Further studies are required to confirm this conclusion in in
vivo models.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contribution
SG and XY conceptualized and designed the present
study. SG and QZ acquired, analyzed and interpreted data. SG
drafted the manuscript and XY revised it critically for important
intellectual content. SQ, QZ and XY confirm the authenticity of all
the raw data. All authors approved the final manuscript for
submission.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schultheiß M, Bengsch B and Thimme R:
Hepatocellular carcinoma. Dtsch Med Wochenschr. 146:1411–1420.
2021.(In German). PubMed/NCBI
|
|
3
|
Bruix J, Sherman M, Llovet JM, Beaugrand
M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M
and Rodés J; EASL Panel of Experts on HCC, : Clinical management of
hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL
conference. European association for the study of the liver. J
Hepatol. 35:421–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Llovet JM, Bru C and Bruix J: Prognosis of
hepatocellular carcinoma: The BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang XX, Ni B, Li Q, Hu LP, Jiang SH, Li
RK, Tian GA, Zhu LL, Li J, Zhang XL, et al: GPAA1 promotes gastric
cancer progression via upregulation of GPI-anchored protein and
enhancement of ERBB signalling pathway. J Exp Clin Cancer Res.
38:2142019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang JX, Wang JH, Sun XG and Hou TZ:
GPAA1 promotes progression of childhood acute lymphoblastic
leukemia through regulating c-myc. Eur Rev Med Pharmacol Sci.
24:4931–4939. 2020.PubMed/NCBI
|
|
7
|
Wu G, Guo Z, Chatterjee A, Huang X, Rubin
E, Wu F, Mambo E, Chang X, Osada M, Sook Kim M, et al:
Overexpression of glycosylphosphatidylinositol (GPI) transamidase
subunits phosphatidylinositol glycan class T and/or GPI anchor
attachment 1 induces tumorigenesis and contributes to invasion in
human breast cancer. Cancer Res. 66:9829–9836. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ho JC, Cheung ST, Patil M, Chen X and Fan
ST: Increased expression of glycosyl-phosphatidylinositol anchor
attachment protein 1 (GPAA1) is associated with gene amplification
in hepatocellular carcinoma. Int J Cancer. 119:1330–1337. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baralle FE and Giudice J: Alternative
splicing as a regulator of development and tissue identity. Nat Rev
Mol Cell Biol. 18:437–451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang GS and Cooper TA: Splicing in
disease: Disruption of the splicing code and the decoding
machinery. Nat Rev Genet. 8:749–761. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grosso AR, Martins S and Carmo-Fonseca M:
The emerging role of splicing factors in cancer. EMBO Rep.
9:1087–1093. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim E, Goren A and Ast G: Insights into
the connection between cancer and alternative splicing. Trends
Genet. 24:7–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen Q and Nam SW: SF3B4 as an early-stage
diagnostic marker and driver of hepatocellular carcinoma. BMB Rep.
51:57–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iguchi T, Komatsu H, Masuda T, Nambara S,
Kidogami S, Ogawa Y, Hu Q, Saito T, Hirata H, Sakimura S, et al:
Increased copy number of the gene encoding SF3B4 indicates poor
prognosis in hepatocellular carcinoma. Anticancer Res.
36:2139–2144. 2016.PubMed/NCBI
|
|
15
|
Shen Q, Eun JW, Lee K, Kim HS, Yang HD,
Kim SY, Lee EK, Kim T, Kang K, Kim S, et al: Barrier to
autointegration factor 1, procollagen-lysine, 2-oxoglutarate
5-dioxygenase 3, and splicing factor 3b subunit 4 as early-stage
cancer decision markers and drivers of hepatocellular carcinoma.
Hepatology. 67:1360–1377. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kidogami S, Iguchi T, Sato K, Yoshikawa Y,
Hu Q, Nambara S, Komatsu H, Ueda M, Kuroda Y, Masuda T, et al:
SF3B4 plays an oncogenic role in esophageal squamous cell
carcinoma. Anticancer Res. 40:2941–2946. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou W, Ma N, Jiang H, Rong Y, Deng Y,
Feng Y, Zhu H, Kuang T, Lou W, Xie D and Wang D: SF3B4 is decreased
in pancreatic cancer and inhibits the growth and migration of
cancer cells. Tumour Biol. 39:10104283176959132017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vainauskas S, Maeda Y, Kurniawan H,
Kinoshita T and Menon AK: Structural requirements for the
recruitment of Gaa1 into a functional glycosylphosphatidylinositol
transamidase complex. J Biol Chem. 277:30535–30542. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Capurro M, Wanless IR, Sherman M, Deboer
G, Shi W, Miyoshi E and Filmus J: Glypican-3: A novel serum and
histochemical marker for hepatocellular carcinoma.
Gastroenterology. 125:89–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen G, Li SY, Cai HY and Zuo FY: Enhanced
expression and significance of glycosylphosphatidylinositol anchor
attachment protein 1 in colorectal cancer. Genet Mol Res.
13:499–507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xing S, Li Z, Ma W, He X, Shen S, Wei H,
Li ST, Shu Y, Sun L, Zhong X, et al: DIS3L2 promotes progression of
hepatocellular carcinoma via hnRNP U-Mediated alternative splicing.
Cancer Res. 79:4923–4936. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oltean S and Bates DO: Hallmarks of
alternative splicing in cancer. Oncogene. 33:5311–5318. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee SE, Alcedo KP, Kim HJ and Snider NT:
Alternative splicing in hepatocellular carcinoma. Cell Mol
Gastroenterol Hepatol. 10:699–712. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu W, Huang H, Yu L and Cao L:
Meta-analysis of gene expression profiles indicates genes in
spliceosome pathway are up-regulated in hepatocellular carcinoma
(HCC). Med Oncol. 32:962015. View Article : Google Scholar : PubMed/NCBI
|