Introduction
Modern science has acquired understanding of the
innate control of cell immunity; however, understanding of adaptive
immune mechanisms in cancer is relatively limited. Despite a long
history of cancer immunotherapy, strategies to restore
antitumor-immunity have not delivered satisfactory results
(1). A specific pro-tumor part of
adaptive immunity following the escape phase of the immune
surveillance process promotes tumor growth and cannot be
reprogrammed (2). Novel
immunotherapies targeting adaptive immunity by inhibiting certain
immune checkpoints are a key breakthrough in oncology, providing
therapeutic strategies that improve the outcome of various types of
cancer, such as melanoma, renal cell and urothelial carcinoma and
lung cancer (3,4). A new approach based on specific
inhibition of checkpoints is different to the previous strategies
aimed at boosting anti-tumor immunity.
The mechanisms underlying the effectiveness of
immune checkpoint inhibitors (ICIs) may be associated with the role
of T cells in tumor development. Tumor-infiltrating immune cells,
namely regulatory T cells (Tregs), serve as a cellular basis for
cancer immunotherapy (5). A better
understanding of their role in the tumor microenvironment is key to
determine mechanisms underlying immunotherapy and identify
prognostic biomarkers. Treatment strategies aim to deplete or block
Tregs, resulting in significant intratumoral Treg depletion,
coinciding with long-term antitumor activity in solid tumor models;
the success of aforementioned approach however has been limited to
a subset of respondents (6).
Immunotherapy targeting cytotoxic T lymphocyte-associated protein 4
(CTLA-4) and programmed cell death protein-1 (PD-1) has achieved
long-term remission in patients with multiple types of solid
tumors, continuously revolutionizing treatment strategies for many
malignancies.
Response to immunotherapy is often unpredictable,
even with known starting levels of predictive biomarkers (7). Despite clinical success, the response
rate is 20–40% and biomarkers for favorable response are still
being investigated (8,9). Consequently, obstacles to clinical
application of immunotherapeutic regimens include limited response
rate, inability to predict clinical efficacy and potential side
effects. A better understanding of the biological events following
checkpoint blockade is necessary to identify reliable predictive
biomarkers of successful PD-1/PD-L1 (programmed cell death ligand
1) blockade and tailor immune therapies for specific clinical
conditions. The present review aimed to summarize the history of
the development of ICIs and to analyze the underlying mechanism of
this type of immunotherapy in the context of tumor-associated
adaptive immunity. Understanding of the basic principles,
advantages and limitations of novel immunotherapy-based techniques
may improve development of novel strategies and clinical
efficacy.
Immune surveillance: Understanding
host-tumor interaction
The immune system serves a key role in the host
response to tumors (10). However,
immune surveillance is a controversial issue in tumor immunology.
In the early 20th century, Paul Ehrlich proposed immune
surveillance, according to which the immune system scans tissue for
transformed cells and eradicates them using immune mechanisms
(11). Sir MacFarlane Burnet
proposed clonal selection to explain self-tolerance by deleting
self-reactive clones in 1957 (12). The 1960 Nobel Prize was awarded to
Burnet and Peter Medawar for immunological tolerance. According to
the theory of clonal selection, the concept of immune control of a
cell types heterogeneity is based on existing mechanisms of
antitumor immunity acting under the condition of permanent
appearance of altered cells in the body (13). Thomas (14) suggested that lymphocytes serve as
sentinels in recognizing and eliminating continuously arising,
nascent transformed cells. Cancer immune surveillance is a key host
mechanism to prevent cancer via inhibition of carcinogenesis and
regular monitoring of tissue homeostasis (15). Two problems with Burnet's theory
were formulated by Hodgkin (16).
The first is the cell type dilemma, which states that novel T cell
subtypes may still be discovered due to technological advances, and
understanding of the behavior of different types of T cell is not
complete. The second issue is the complexity of cooperation between
immune cells via modifying signals that elicit different responses.
Thus, the immune surveillance hypothesis underlying cancer
immunology is important for understanding how the immune system
functions in this case. However, the theory has contributed little
to attempts to treat cancer via immunological mechanisms (17). It has been suggested that immune
surveillance primarily functions as a component of a more general
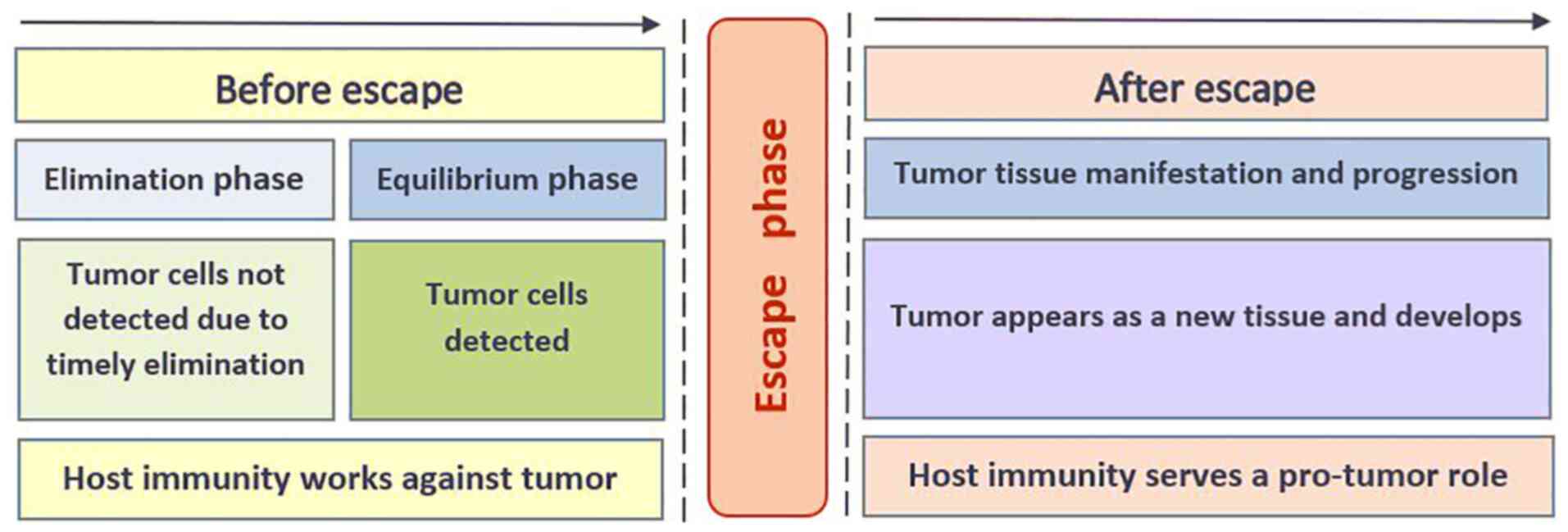
dynamic process of ‘cancer immunoediting’ that has three phases:
Elimination, equilibrium and escape (18,19).
As long as the elimination and equilibrium phases continue,
immunity serves to protect against tumors. Escape from immune
surveillance leads to manifestation of tumorous tissue and a change
in the direction of immune reactions from anti-tumor to pro-tumor
by which the escaped cells survive in immunocompetent hosts. The
escape phenomenon is an event that leads to tumor formation;
therefore, it is an attractive but very challenging target for
immunotherapy, since evasion is only a transient moment between the
completed phases of elimination and equilibrium and manifestation
of tumor tissue (Fig. 1). The
elimination phase assumes the predominance of effector cytotoxic
mechanisms for the eradication of malignant cells, while the
equilibrium phase exists due to tolerance to the emerging pool of
tumor cells. The manifestation of a tumor as tissue and its
development in the presence of immune cells in the microenvironment
indicates the failure of previous mechanisms and the formation of a
new type of interaction between immune and tumor cells. The
development of immunoediting theory determined that the primary
site of action in tumor-associated immunity is interactions between
immune and tumor cells. The expression of checkpoint molecules on
immune cells suggests the possibility of interaction with other
populations of intratumoral cells. These functional receptors serve
an essential role in the control of cell fate and tissue
homeostasis (20). Therefore,
receptors that determine these antigen-driven interactions, on both
immune and tumor cells, have become research target for effective
antitumor strategies (21).
Immunity in maintaining tissue
homeostasis
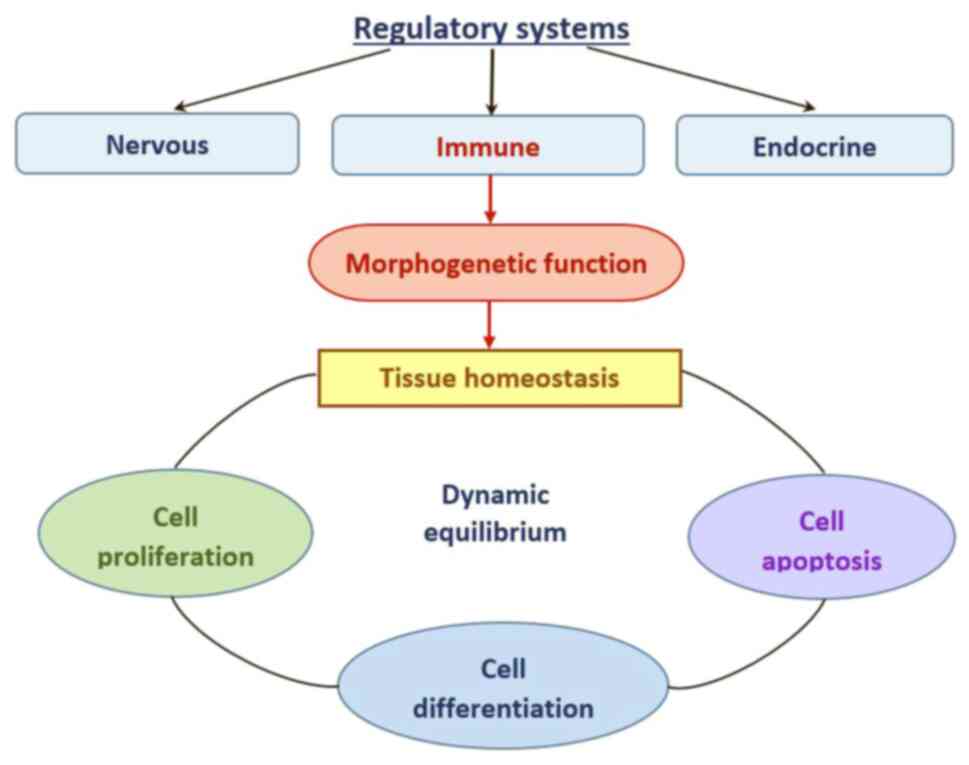
Tissue homeostasis is achieved when the behaviors of
constituent cells, including proliferation, differentiation and
apoptosis, are in balance (22).
This dynamic equilibrium between cells and their environment
requires constant control of regulatory systems at different levels
(Fig. 2). Under physiological
conditions, the function of the immune system, in addition to
protecting against infections, is also morphogenetic-monitoring
morphological and genetic tissue homeostasis to protect against
malignant transformation (23).
However, the innate immune reactions of host defense against
pathogens cannot be applied to mechanisms that protect against
cancer involving adaptive immunity (24). Immune surveillance is the ability
of the immune system to detect cellular imbalances and respond by
activating adaptive cellular immunity to restore tissue homeostasis
(25). The immune system
contributes to permanent tissue renewal and remodeling following
damage (26,27). To maintain tissue equilibrium, key
elements of the central part of the immune system, namely,
thymus-derived lymphocytes, are functionally represented in each
developing tissue; to ensure this representation, immune cells have
unique ability to move between compartments and realize feedback
mechanisms for inverse correlation with the thymus as a central
organ (20,28). Thymus-derived regulatory T cells,
Tregs, are considered to have a homeostatic function (23,29).
The mechanism of transforming autoreactive thymocytes into Tregs,
which do not induce inflammatory reactions in self body tissue, is
involves ‘education and differentiation’ in response to
autoantigens (30,31). Accordingly,
self-antigen-recognition by a specific T cell receptor (TCR) is the
predominant requirement for the induction of thymic Tregs (32). Due to their homing capacity that
allows them to target tissue-specific migration, T lymphocytes
monitor tissue homeostasis constantly. Migrating cells are
continuously recycled between the core and peripheral compartments
of the immune system, penetrating tissue through post-capillary
vessel walls (33). This
immunological process of tissue ‘patrolling’ has biological
rationality in the monitoring and controlling of antigenic
constancy. TCRs are not only involved in specific T cell clone
induction and maturation, but also determine the destination of
migrating lymphocytes in a particular tissue. Interaction of TCRs
with ligands in the endothelium of post-capillary venules allows
entry into tissue in a controlled manner (26). TCRs recognize molecules of the
major histocompatibility complex (MHC), which are tissue-specific
and ubiquitously expressed on cells of different tissue (34). The binding specificity of the TCR
is a key component in MHC recognition (35). Migration of T lymphocytes, directed
by TCRs, occurs due to ligand-integrin interaction of T cells with
adhesive molecules on the endothelium of blood vessels (34,36).
Capillary endothelial cells select lymphocytes for active movement
into tissue according to recognition of the ‘homing’ receptors
(33).
Movement of lymphocytes through lymph and blood and
their migration into tissue is targeted (20). The trafficking of T cells to
peripheral tissue is performed in response to homeostatic
chemokines, which are permanently expressed by cells of the
microvascular endothelium to attract the corresponding clones of
lymphoid cells to certain tissue sites (36). Lymphocytes leave the bloodstream,
entering tissue between adjacent endothelial cells (33). Recirculating lymphocytes are a
highly mobile population of cells able to enter through vessel
walls into tissue and back into circulation. Thus, migrating
lymphocytes control tissue homeostasis via bidirectional cell
transfer, which is beneficial for tissue development (37–39).
When immunocompetent T cells appear in the tissue, they become part
of the local microenvironment. Complex interactions between
lymphocytes, extracellular matrix proteins, sedentary immune cells
and tissue cells determine the outcome of immune responses at the
tissue level (28). Functioning
tissue exhibits an immune regulatory compartment-zone located
around the post-capillary venules where interactions between
endothelial cells, lymphocytes and tissue structures occur
(40,41). Tissue lymphocytes are represented
primarily by T cells, which are defined as a regulatory population,
originating from double-positive helper/suppressor cells (42,43).
Lymphatic tissue constantly maintains a recirculating pool of T
lymphocytes, which are generated in the thymus and undergo T
lymphocytes during their circulation cycle reside in non-lymphoid
tissues where they complete differentiation and acquire
immunological specificity (44).
The selectivity of the migration of T cell clones, termed ‘homing’,
determines formation of a functional complex, which consists of a
specific T cells clone tissue, and regional lymph nodes (26,45).
Targeted migration of regulatory T lymphocytes into the tissue
compartment is necessary for tissue development and renewal
(46). Consequently, renewing
tissue under non-inflammatory conditions favors preferential
recruitment of a highly restricted repertoire of specific Tregs for
development (27,42).
Nevertheless, mechanisms underlying tissue
homeostasis regulation by the immune system have not been
completely elucidated and require further clarification.
Multicellular organisms function as stable integrated systems due
to physiological intercellular interactions (47). The substrate for storing and
transmitting information in biological systems is nucleic acid
sequences in the form of RNA and DNA. Previous studies have shown
that cells communicate via direct exchange of genetic patterns in
the form of extracellular vesicles, such as exosomes, microvesicles
and apoptotic bodies that deliver functional RNA/DNA molecules
(37,48). The majority of cells secrete
exosomes into the extracellular environment and exosomes have been
observed in the cytoplasm of primary T lymphocytes (49). Exosomes fuse via phagocytosis with
recipient cells, including macrophages and dendritic cells, which
internalize exosomes (50,51). Fusion of exosomes with membranes of
recipient cells has also been described in cancer cells (52). Intercellular communication via
exosomes is a potential driver of phenotypical changes and cell
plasticity during tissue regeneration (53). Targeted transfer of genetic
information is key in tissue development and this mechanism
underlies the immune-editing function of the T cell arm of the
immune system (47). Specific
genetic messages are designed for incorporation into acceptor cells
to promote tissue-specific differentiation. The exosomes necessary
for induction of differentiation are provided by apoptosis of
immune cells, which underlies their mechanism of action (54). microRNAs within exosomes regulate
innate immune responses; exosomes from T cells directly fuse with
host tissue cells, releasing miRNAs (55). The genetic information contained in
exosomes affects target cells in various ways, inducing activation,
differentiation or apoptosis (47).
The thymus undergoes notable decline in both size
and function during the postnatal period but does not undergo
atrophy and continues to perform a key role in T cell arrangement
in adulthood by maintaining the development and homeostasis of the
T cell arm of immunity (56). This
is supported by the fact that thymus epithelium stem cells are
constantly generated and their pool is dynamically regulated by
signals from the periphery in response to tissue needs (57–59).
Maturation of T cells in the thymus requires a constant supply of T
cell progenitors from bone marrow (60). The role of lymphocytes in
regulation of cell differentiation is confirmed by their presence
in the microenvironment of differentiating hematopoietic stem cells
in bone marrow (61). Skin and
mucous membranes must be constantly monitored to ensure homeostasis
as they serve as a barrier to the external environment. Thus, these
tissues are most predisposed to developing cancer (62).
Tumor as a new tissue
Malignant tumors evolve by developing mechanisms to
evade antitumor immune-based programs and conscripting them to
promote carcinogenesis (2,62). Having passed through the escape
phase of immune surveillance, the tumor appears as a new tissue and
develops according to self-regulating mechanisms and is subject to
the same central regulatory rules as a normal tissue. This new
tissue evolves as what appears to be a unique tissue and the immune
system continues to maintain homeostasis in the newly appeared
anatomical area, allowing tumor development instead of targeting
the new tissue (63,64). This is supported by the fact that
developing and progressive tumor tissue can exist as a symbiont of
an organ (65). The paradoxical
role of adaptive and innate lymphocytes is that they serve as key
regulators in cancer development and progression (66). Tumor progression is not a random
process and it follows internal rules and mechanisms of central
regulation in the host-tumor system that are still under
investigation (67–69).
There is evidence to support the importance of a
central mechanism in regulating tumor progression. The formation of
metastatic niches in the form of stromal restructuring in tissue
remote from the site of the primary tumor begins before
dissemination of malignant cells (70). Tumor cells acquire metastatic
properties before they migrate from the primary tumor site
(71) and systemic circulation of
tumor cells may remain latent or unproductive for an extended
period (72). Additionally,
increased formation of blood and lymphatic vessels in tumors
contributes to metastasis (73,74).
Stimulation of vascular growth in tumors occurs via the same
mechanism as that in normal tissue (75). For blood vessels, a tumor lesion is
an extra cell mass that requires nutrition and elimination of
tissue metabolic products (65).
The aforementioned processes support the hypothesis that the effect
of host regulatory systems on formation of the tumor
microenvironment is a factor initiating metastasis.
The tumor microenvironment includes heterogeneous
immune cell populations (10).
Advances in single-cell characterization have provided insight into
involvement of T cells in the tumor microenvironment. Solid tumors
typically contain functionally active antigen-specific
tumor-infiltrating lymphocytes that paradoxically do not interfere
with tumor growth and progression (76,77).
During each phase of the metastatic process, tumor cells are
targeted by immune cells, which recognize them as harmful and
restrict their development (78).
However, numerous studies have shown that tumor-infiltrating immune
cells promote the metastatic cascade (2,64).
Tregs may serve a role in increasing the number of surviving tumor
cells in the circulation and at sites of metastasis (2). Tregs have been found in lymph nodes
containing micrometastases (79);
moreover, the detection of this population of lymphocytes precedes
detection of metastatic lesions in regional lymph nodes (80).
Leukocytes infiltrating tumor tissue are
predominantly Tregs with a CD4+CD25+ forkhead
box P3 (Foxp3+) phenotype that serve a key role in
immune editing (81,82). Phenotype, differentiation status
and function of regulatory immune cells differ depending on the
anatomical compartment in which they reside; this shows that immune
cells that originate from the same precursors but reside in
different types of tissue are affected by organ-specific factors
(20,45). Consequently, each tissue possesses
its own antigens to activate the immune system and generate local
immune responses that are associated with homeostasis of that
tissue (83,84). The features of Treg behavior make
it possible to understand the mechanisms underlying immune-mediated
regulation of tissue homeostasis as well as to assess the role of
the thymus as a central organ controlling the location and function
of T cells populations in the periphery (58).
In solid tumors, the density of Tregs in tumor
lesions and their imbalances in blood are associated with clinical
outcomes (85–87). Experimental data are consistent
with the hypothesis that tumor-specific Tregs originate in the
thymus during T cell development and are preferentially recruited
into tumor tissue compared with the diverse systemic Treg pool
(88,89). Clinical data reporting an increase
in the population of Tregs in peripheral blood of patients with
solid tumors are consistent with experimental data on increased
yield of mature thymocytes and migration into the peripheral
circulation (85,90). The direction of target migration of
T lymphocyte clones determines their destination and recruitment in
the tumor tissue (5,81). According to clinical and
experimental studies, tumor infiltrating leukocytes are mainly
represented by the same regulatory subpopulation of thymic
lymphocytes (88,91,92).
Tumor tissue development is accompanied by formation
of clones of T lymphocytes designed for that tissue.
Tumor-infiltrating regulatory lymphocytes undergo early
differentiation in the thymus and complete differentiation in the
tumor tissue, which promotes in generation of immune signals to
support tumor development (87).
Therefore, tumor tissue can also be considered a peripheral
compartment of the immune system, consisting of post-capillary
vessels endothelial cells, a circulating and settled pool of Tregs,
tumor cells and active components of the extracellular matrix;
however, the function of this compartment is not conducive with
physiological function of the immune system. Accordingly, in an
organ affected by a tumor, each type of tissue (tumor and normal)
has a regulatory zone infiltrated by T lymphocytes from the
regulatory population; meanwhile, in the peripheral circulation of
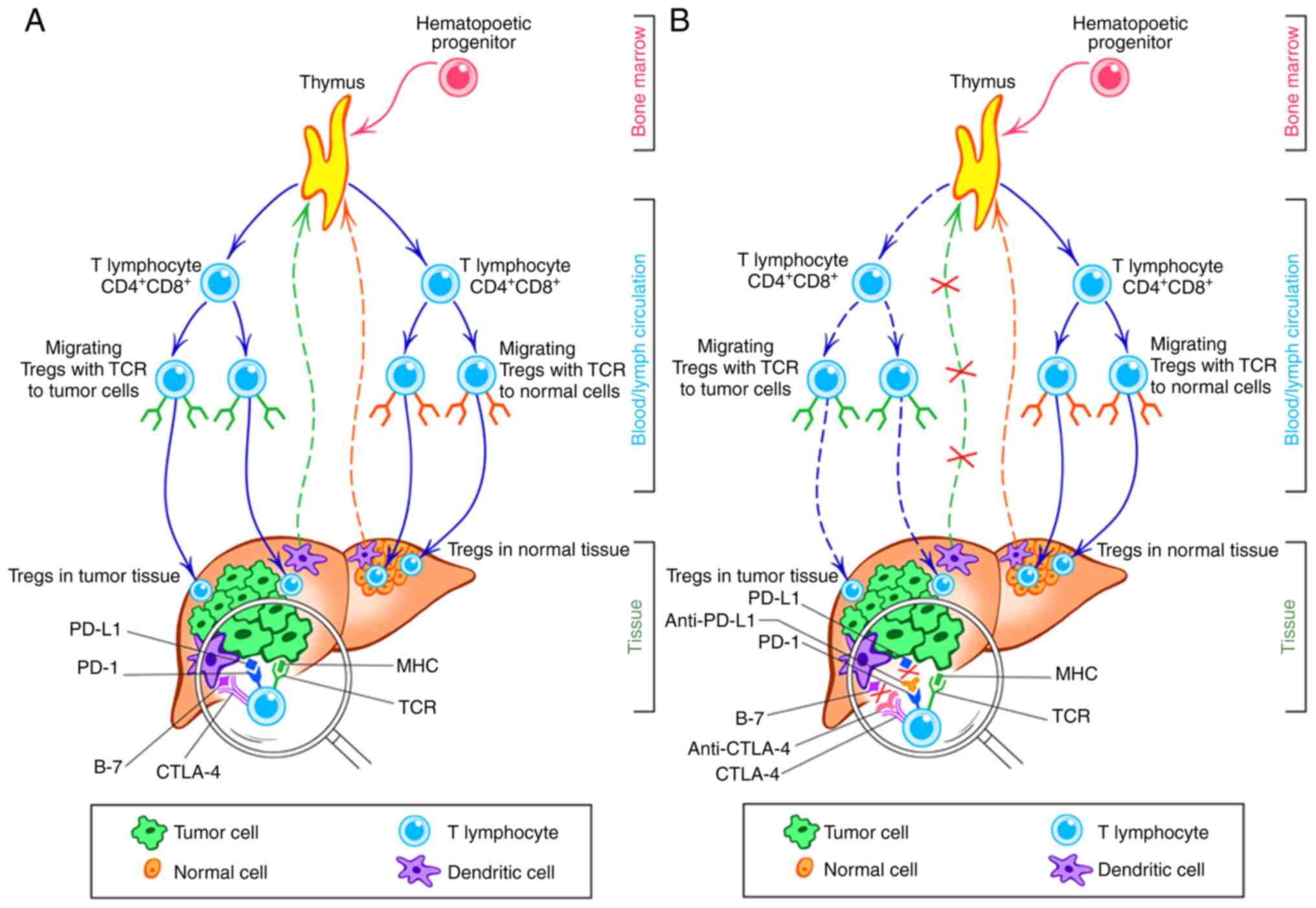
the host, T cell clones intended both for tumor and normal tissue.
Both these sets of T cell possess the same phenotype
(CD4+Foxp3+) but they differ in the direction
of TCR-driven migration into corresponding tissue, either normal or
tumor, due to each of them carrying unique histocompatible antigens
(Fig. 3A) (91,93).
Numerous studies have confirmed the role of Tregs in
regulating the pace of solid tumor progression: Increased
quantities of Tregs in tumor tissue are associated with a higher
degree of tumor differentiation and favorable prognosis (5,85,90).
A decrease in Treg content among tumor-infiltrating lymphocytes
following effective neoadjuvant chemotherapy in patients with
breast cancer has been found in a comparative study of
postoperative tumor samples (94).
Clinical studies have demonstrated an association between tumor
regression in response to chemotherapy and a decrease in Treg
population, indicating that Treg levels may serve as a prognostic
marker (5,95). The role of Tregs in cancer
development provides a rationale for targeting Tregs for future
therapeutic strategies.
Discovery of ICIs
The microenvironment of tumor cells has a complex,
heterogeneous and dynamic nature (96). The region of interaction between
cancer and immune cells is decisive for tumor tissue progression
(10). Previous cancer
immunotherapies (such as immunostimulatory cytokines, vaccination
with tumor-specific antigens, stem cell therapy) aimed at
stimulating the immune system has not yielded satisfactory results
(1,97), although novel immune therapies
(such as immune checkpoints inhibitors) that target precise
blockades rather than non-specific stimulation have shown promising
efficacy. This type of immunotherapy focuses on the tumor
microenvironment rather than tumor cells. The effectiveness of ICIs
has shown that tissue regulation by the immune system serves a key
role in tumor progression and Tregs are important for tumor
development (23,81,98).
The ambiguous dual role of Tregs in cancer development has been
shown as Treg inactivation and depletion may initiate an antitumor
immune response (99). Thus,
investigation of Treg behavior and their association with tumor
cells may support methods to modulate host response to malignant
tissue transformation. Targeted immunotherapies should aim to
either enhance the antitumor properties and/or prohibit the
pro-tumor properties of immune cells (100).
Research on the association between tumor cells and
activated immune cells led to the discovery of immune checkpoint
blockade, a novel form of immunotherapy. James Ellison and Tasuku
Honjo discovered functional receptors, CTLA-4 and PD-1, on
activated T lymphocytes, which are key to developing
checkpoint-blockade immunotherapeutic agents, revolutionized cancer
treatment and was awarded the 2018 Nobel Prize in Physiology or
Medicine (101). The clinical
efficacy of checkpoint blockade in various types of cancer, e.g. in
melanomas, kidney cancers, urothelial carcinoma and non-small cell
lung cancers (102–105), proves the universality of its
mechanism of action (106,107).
Current status of immunotherapy with
ICIs
Targeted immunotherapy against CTLA-4 and PD-1
lymphocyte receptors in clinical practice has demonstrated efficacy
in a subgroup of patients with aggressive solid tumors, including
disseminated melanoma, renal cell carcinoma and non-small cell lung
cancer (3,8,21,108). The patients who respond to
immunotherapy show a pronounced and enduring clinical response that
persists following treatment discontinuation and may resume in
response to a similar treatment in case of disease progression
(102). Successful clinical
application of ICIs has contributed to development of novel
immunotherapeutics for treatment of metastatic and locally advanced
disease to application in a neo- and adjuvant setting in earlier
stages of high-risk disease (109,110). Currently, ICIs are the standard
treatment for numerous types of solid tumor (111) and their application is expanding
following study of their effects in combination with radiotherapy,
chemotherapy and targeted therapy (4).
New immunotherapies have a notable effect on Treg
subpopulations (107).
Immunotherapeutic agents targeting lymphocytes alter the
immunological tumor microenvironment, thereby decreasing the
promoting effect and facilitating induction of immunological
tolerance to tumor tissue (82,87).
Immunological tolerance may be considered as a deprivation of tumor
support from the host (112).
Tumor-infiltrating Tregs are functional and their activity is
mediated by apoptosis (93,113,114).
A subset of regulatory T cells with high expression of functional
receptors CTLA-4 and PD-1 realize effector functions, such as
motility, migration and apoptosis (115). These Tregs express similar
receptors (PD-1, lymphocyte-activating 3 and T cell immunoreceptor
with Ig and ITIM domains) (116).
Based on the association between decreased number of
Tregs and tumor regression, it is hypothesized that tumor undergoes
involution, becoming deprived of Treg-mediated signaling due to
successful blockade of the PD-1/PD-L1 axis (6). The functional failure of Tregs
resulting from PD-1/PD-L1 checkpoint blocking is the initial step
in a series of reactions in the host-tumor system that leads to
tumor regression (21). Activated
mononuclear phagocytes/macrophages are required in PD-1-based
therapy in experimental models, because destroyed/damaged tumor
cells trigger the process of phagocytosis (117).
The self-regulatory loop, provided by feedback
mechanisms from the peripheral to the central immune system,
ensures functional activity of Tregs in tumor tissue (116). The generation of adaptive
immunity to cancer is a cyclical process that can be
self-sustaining, leading to amplification and broadening of the T
cell response (118). Regulatory
feedback mechanisms of the immune cycle can promote or limit
development of immune reactions (119). Experimental data have confirmed
the participation of regulatory T cells in tumor progression and
the presence of central immune-mediated mechanisms that regulate
tumor spread (95,120). Moreover, according to
experimental and clinical data, the central mechanisms of immune
regulation determine the nature, direction and results of cell
interactions at the tissue level (2,121).
A novel therapeutic ICI strategy has shown that only functional
blockade of lymphocytes regulating homeostasis in tumor tissue
leads to significant tumor regression due to deprivation of
immunological support from the host (21).
Discussion
The therapeutic effect of immune checkpoint
inhibition can successfully cure certain patients with solid
tumors. However, it is unclear why PD-LI blockade yields a complete
response in only certain patients, especially given all ICIs have a
common focus of action in targeting inhibition of the immune
checkpoints of the PD-1/PD-L1 axis. Improving the effectiveness of
ICI therapy and expanding the population of responders requires
clarification of mechanisms underlying successful response to
inhibition of immune checkpoints. However, theoretical explanation
of the actions of ICIs at the cellular level does not allow
clinicians to evaluate their integral implementation at the
organism level (107).
Further research is required to determine the best
predictors of response, how to distinguish between real progression
and atypical patterns of response, such as pseudo- or hyper
progression; and how to avoid dangerous side effects to achieve the
feasibility of tailored treatment regimens and the optimal duration
of treatment (9). To address the
aforementioned issues, comprehensive insight is needed concerning
the events initiated by ICIs at cell, tissue and organ levels. The
mechanisms underlying cross-interactions between tumor and immune
cells in their environment have been studied (10); however, the role of central
regulatory organs and circulatory systems remains to be
investigated with regard to interactions in the tumor-host system.
The lack of clear understanding of the mechanisms underlying ICIs
complicates identification of clinically useful predictive
biomarkers (122). Therefore,
additional studies are required to uncover the immune mechanisms
underlying tissue homeostasis.
Depletion of Treg pools is associated with
successful antitumor therapy (90,94,95).
Tregs have a similar phenotype irrespective of tumor location but
are highly heterogeneous due to MHC-specific clonality (34). The cross-talk between Tregs and
tumor cells is important because inhibition of this axis may lead
to tumor shrinkage or progression (123). Due to Tregs being tissue-specific
(antigen-experienced T cells), irrespective of them being a similar
phenotype, the central mechanisms of tissue competency formation
define interactions in the ‘tumor-host’ system (121). Despite the promoting role of
tumor-infiltrating immune cells at each stage of the metastatic
cascade (2,77,124), interactions between tumor and
immune cells are traditionally considered in the context of
tolerance or suppression only at the tissue level, without
assessing the role of immune cell-mediated central thymic
regulation. As Tregs are thymus-derived and operate in a
tissue-specific manner (125), it
is necessary to determine the centrally driven mechanisms
underlying tumor regression following checkpoint inhibition and
formation of self-regulatory loops that provide feedback mechanisms
from central regulatory structures.
Tumor-infiltrating Tregs are functional and their
activity is mediated by apoptosis via activation of PD-1 receptors
(93,113). Apoptosis, essentially altruistic
cell suicide that necessary for transmitting information to
acceptor cells, underlies the effector mechanisms of immune cells
(54). The aforementioned
association between tumor regression and decreased numbers of Tregs
supports the hypothesis that tumors deprived of essential signals
from Tregs due to successful checkpoint blockage of the PD-1/PD-L1
axis undergo involution (112).
Tregs may exert a regulatory effect and deliver key information to
tumor tissue (126).
Tregs serve a key role in maintaining homeostasis in
normal (127) and tumor tissue
(29) and interactions between
tumor cells and Tregs are targets for ICIs. Moreover, interactions
between Tregs and tumor cells via the PD-1/PD-L1 axis are targeted
by ICIs, which can act on both the lymphocyte receptor (PD-1) and
the tumor cell ligand (PD-L1). When starting immunotherapy,
quantitative assessment of tumor cells, active lymphocytes and the
rate of generation of novel antigen-specific T cells are typically
unknown. The conventional practice consists in initial measuring of
only tumor markers such as PD-L1 expression and microsatellite
instability, while it is also possible to quantify fluctuations of
tumor-specific Tregs in the blood at varying time points during
treatment to establish a host pattern.
The function of monoclonal antibody (mAb) is
realized by Ab binding affinity with a specific antigen (128). Accordingly, a defined quantity of
mAbs targeting PD-1 or PD-L1 decreases receptor/ligand ratio on the
target cell by a specific amount. A number of specific Tregs serve
a key role in treatment outcome (129), but it is difficult to
quantitatively assess the interaction between Tregs and tumor
cells, calculate a patient-specific mAb dose and determine the
optimal duration of therapy, taking into consideration the distinct
tumor mass, tumor burden and number of tumor-specific Tregs in
patients of various ages and T cell response.
Through the in-depth dissection of existing data and
inferences using Hermann Hesse's ‘Glass Bead Game’ principles (for
example, intellectual synthesis of scientific facts and evidence of
all ages to make them into organic whole), a multilevel mechanism
of action of ICIs that better reflects real-life situations with
various clinical outcomes is envisaged. The thymus generates T cell
clones tailored to tumor tissue in response to tissue-specific
signals. Lymphocytes infiltrating the tumor are predominately Treg
with tumor-promoting activity. During effective therapy, a direct
association between tumor regression and a decrease in Treg
population is observed; blockade of immune checkpoints causes a
functional failure of Tregs, resulting in tumor deprivation of
co-stimulation signals. Depletion of Tregs as a result of
checkpoint inhibition is the first step in a series of reactions in
the host-tumor system leading to tumor decrease or progression. The
dynamics of self-regulatory mechanisms preferentially maintain one
of the processes at a given time: either growth or regression of
tumor tissue.
The goal of effective inhibition of immune
checkpoints is to reverse tumor progression, including by changing
the direction of self-regulatory mechanisms (130,131). The interaction between receptors
on immune and tumor cells and rate of generation of new clones of
tumor-associated T lymphocytes determine the effects of ICIs at the
tissue level (Fig. 3B).
Conclusion
Present antitumor immunotherapy-based strategies,
namely immune checkpoints inhibitors, aimed at specific blockade of
the tumor-associated part of the adaptive immunity show promising
results. Neutralizing pro-tumor activity of immune cells in a case
of effective treatment leads to deprivation of tumor tissue
activity and provides a chance for preferential development of the
essential normal tissue program. Novel immune therapies with ICIs
demonstrate that regulation of the immune system serves a key role
in tumor growth and metastatic spread (2,77,124). The aforementioned treatment
options have highlighted potential mechanisms that affect solid
tumors by targeting tumor cells through the microenvironment via
immune-regulated mechanisms (21).
Therefore, it is imperative to identify the population of
tumor-associated immune cells that can be exploited for selective
therapeutic intervention without affecting cells elsewhere in the
body. Successful therapy aims to switch growth and development of
tumor tissue to tumor shrinkage and involution. ICIs induce this
change by blocking tumor-associated immune cells. Methodologies
combining imaging-based biomarkers with tumor markers and host's
tumor-specific immune characteristics are needed to improve patient
selection and monitoring during immunotherapy. Enhanced imaging
modalities and laboratory-determined predictive markers may allow
development of criteria to predict patient response to
immunotherapy. Clarifying the underlying mechanisms of
immunotherapy may identify the point at which pro-tumor activity of
immune cells occurs and improve patient outcomes by inhibiting or
reversing tumor growth.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
NL has analyzed and systematized data and evidence
related to the immune checkpoints to create a more comprehensive
picture of their mechanism of action. The author has read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rosenberg SA: Raising the bar: The
curative potential of human cancer immunotherapy. Sci Transl Med.
4:127ps82012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kitamura T, Qian BZ and Pollard JW: Immune
cell promotion of metastasis. Nat Rev Immunol. 15:73–86. 2015.
View Article : Google Scholar
|
|
3
|
Ribas A and Wolchok JD: Cancer
immunotherapy using checkpoint blockade. Science. 359:1350–135.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun L, Zhang L, Yu J, Zhang Y, Pang X, Ma
C, Shen M, Ruan S, Wasan HS and Qiu S: Clinical efficacy and safety
of anti-PD-1/PD-L1 inhibitors for the treatment of advanced or
metastatic cancer: A systematic review and meta-analysis. Sci Rep.
10:20832020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Curiel TJ: Regulatory T cells and
treatment of cancer. Curr Opin Immunol. 20:241–246. 2008.
View Article : Google Scholar
|
|
6
|
Shergold AL, Millar R and Nibbs RJB:
Understanding and overcoming the resistance of cancer to PD-1/PD-L1
blockade. Pharmacol Res. 145:1042582019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davis AA and Patel VG: The role of PD-L1
expression as a predictive biomarker: An analysis of all US food
and drug administration (FDA) approvals of immune checkpoint
inhibitors. J Immunother Cancer. 7:2782019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Postow MA, Callahan MK and Wolchok JD:
Immune checkpoint blockade in cancer therapy. J Clin Oncol.
33:1974–1982. 2015. View Article : Google Scholar
|
|
9
|
Wu M, Zhang Y, Zhang Y, Liu Y, Wu M and Ye
Z: Imaging-based Biomarkers for predicting and evaluating cancer
immunotherapy response. Radiol Imaging Cancer. 1:e1900312019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lei X, Lei Y, Li JK, Du WX, Li RG, Yang J,
Li J, Li F and Tan HB: Immune cells within the tumor
microenvironment: Biological functions and roles in cancer
immunotherapy. Cancer Lett. 470:126–133. 2020. View Article : Google Scholar
|
|
11
|
Ehrlich P: Über den jetzigen stand der
karzinomforschung. Ned Tijdshr Geneeskd. 5:273–290. 1909.
|
|
12
|
Burnet M: Cancer: A biological approach.
III. Viruses associated with neoplastic conditions. IV. Practical
applications. Br Med J. 1:841–847. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dunn GP, Old LJ and Schreiber RD: The
immunobiology of cancer immunosurveillance and immunoediting.
Immunity. 21:137–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thomas L: On immunosurveillance in human
cancer. Yale J Biol Med. 55:329–333. 1982.PubMed/NCBI
|
|
15
|
Kim R, Emi M and Tanabe K: Cancer
immunoediting from immune surveillance to immune escape.
Immunology. 121:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hodgkin PD: Modifying clonal selection
theory with a probabilistic cell. Immunol Rev. 285:249–262. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ribatti D: The concept of immune
surveillance against tumors. The first theories. Oncotarget.
8:7175–7180. 2017. View Article : Google Scholar
|
|
18
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: From immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar
|
|
19
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu W and Pasare C: Location, location,
location: Tissue-specific regulation of immune responses. J Leukoc
Biol. 94:409–421. 2013. View Article : Google Scholar
|
|
21
|
Bersanelli M and Buti S: From targeting
the tumor to targeting the immune system: Transversal challenges in
oncology with the inhibition of the PD-1/PD-L1 axis. World J Clin
Oncol. 8:37–53. 2017. View Article : Google Scholar
|
|
22
|
Liang J, Balachandra S, Ngo S and O'Brien
LE: Feedback regulation of steady-state epithelial turnover and
organ size. Nature. 548:588–591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lui PP, Cho I and Ali N: Tissue regulatory
T cells. Immunology. 161:4–17. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Klose CSN and Artis D: Innate lymphoid
cells control signaling circuits to regulate tissue-specific
immunity. Cell Res. 30:475–491. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schutte B and Ramaekers FC: Molecular
switches that govern the balance between proliferation and
apoptosis. Prog Cell Cycle Res. 4:207–217. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shechter R, London A and Schwartz M:
Orchestrated leukocyte recruitment to immune-privileged sites:
Absolute barriers versus educational gates. Nat Rev Immunol.
13:206–218. 2013. View Article : Google Scholar
|
|
27
|
Burzyn D, Benoist C and Mathis D:
Regulatory T cells in nonlymphoid tissues. Nat Immunol.
14:1007–1013. 2013. View Article : Google Scholar
|
|
28
|
Dong J, Chen Y, Xu X, Jin R, Teng F, Yan
F, Tang H, Li P, Sun X, Li Y, et al: Homeostatic properties and
phenotypic maturation of murine CD4+ pre-thymic emigrants in the
thymus. PLoS One. 8:e563782013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dobrzanski MJ: Expanding roles for CD4 T
cells and their subpopulations in tumor immunity and therapy. Front
Oncol. 3:632013. View Article : Google Scholar
|
|
30
|
Lee HM, Bautista JL, Scott-Browne J, Mohan
JF and Hsieh CS: A broad range of self-reactivity drives thymic
regulatory T cell selection to limit responses to self. Immunity.
37:475–486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Geenen V: The thymus and the science of
self. Semin Immunopathol. 43:5–14. 2021. View Article : Google Scholar
|
|
32
|
Hsieh CS, Lee HM and Lio CW: Selection of
regulatory T cells in the thymus. Nat Rev Immunol. 12:157–167.
2012. View Article : Google Scholar
|
|
33
|
Muller WA: How endothelial cells regulate
transmigration of leukocytes in the inflammatory response. Am J
Pathol. 184:886–896. 2014. View Article : Google Scholar
|
|
34
|
Singh NK, Riley TP, Baker SCB, Borrman T,
Weng Z and Baker BM: Emerging concepts in TCR specificity:
Rationalizing and (Maybe) predicting outcomes. J Immunol.
199:2203–2213. 2017. View Article : Google Scholar
|
|
35
|
Sethna Z, Elhanati Y, Dudgeon CR, Callan
CG Jr, Levine AJ, Mora T and Walczak AM: Insights into immune
system development and function from mouse T-cell repertoires. Proc
Natl Acad Sci USA. 114:2253–2258. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Anders HJ, Romagnani P and Mantovani A:
Pathomechanisms: Homeostatic chemokines in health, tissue
regeneration, and progressive diseases. Trends Mol Med. 20:154–165.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Corrado C, Raimondo S, Chiesi A, Ciccia F,
De Leo G and Alessandro R: Exosomes as intercellular signaling
organelles involved in health and disease: Basic science and
clinical applications. Int J Mol Sci. 14:5338–5366. 2013.
View Article : Google Scholar
|
|
38
|
Senovilla L, Galluzzi L, Zitvogel L and
Kroemer G: Immunosurveillance as a regulator of tissue homeostasis.
Trends Immunol. 34:471–481. 2013. View Article : Google Scholar
|
|
39
|
Munoz MA, Biro M and Weninger W: T cell
migration in intact lymph nodes in vivo. Curr Opin Cell Biol.
30:17–24. 2014. View Article : Google Scholar
|
|
40
|
Mai J, Virtue A, Shen J, Wang H and Yang
XF: An evolving new paradigm: Endothelial cells-conditional innate
immune cells. J Hematol Oncol. 6:612013. View Article : Google Scholar
|
|
41
|
Ruddle NH: Lymphatic vessels and tertiary
lymphoid organs. J Clin Invest. 124:953–959. 2014. View Article : Google Scholar
|
|
42
|
Schaerli P, Ebert L, Willimann K, Blaser
A, Roos RS, Loetscher P and Moser B: A skin-selective homing
mechanism for human immune surveillance T cells. J Exp Med.
199:1265–1275. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Smigiel KS, Srivastava S, Stolley JM and
Campbell DJ: Regulatory T-cell homeostasis: Steady-state
maintenance and modulation during inflammation. Immunol Rev.
259:40–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Thiault N, Darrigues J, Adoue V, Gros M,
Binet B, Perals C, Leobon B, Fazilleau N, Joffre OP, Robey EA, et
al: Peripheral regulatory T lymphocytes recirculating to the thymus
suppress the development of their precursors. Nat Immunol.
16:628–634. 2015. View Article : Google Scholar
|
|
45
|
Fu H, Ward EJ and Marelli-Berg FM:
Mechanisms of T cell organotropism. Cell Mol Life Sci.
73:3009–3033. 2016. View Article : Google Scholar
|
|
46
|
Rothstein DM and Camirand G: New insights
into the mechanisms of Treg function. Curr Opin Organ Transplan.
20:376–384. 2015. View Article : Google Scholar
|
|
47
|
Mittelbrunn M and Sánchez-Madrid F:
Intercellular communication: Diverse structures for exchange of
genetic information. Nat Rev Mol Cell Biol. 13:328–335. 2012.
View Article : Google Scholar
|
|
48
|
Kouwaki T, Okamoto M, Tsukamoto H,
Fukushima Y and Oshiumi H: Extracellular vesicles deliver host and
virus RNA and regulate innate immune response. Int J Mol Sci.
18:6662017. View Article : Google Scholar
|
|
49
|
Villarroya-Beltri C, Gutiérrez-Vázquez C,
Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N,
Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M and
Sánchez-Madrid F: Sumoylated hnRNPA2B1 controls the sorting of
miRNAs into exosomes through binding to specific motifs. Nat
Commun. 4:29802013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ,
Chang LF, Zhou Q and Sui SF: Cellular internalization of exosomes
occurs through phagocytosis. Traffic. 11:675–687. 2010. View Article : Google Scholar
|
|
51
|
Morelli AE, Larregina AT, Shufesky WJ,
Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z,
Watkins SC, et al: Endocytosis, intracellular sorting, and
processing of exosomes by dendritic cells. Blood. 104:3257–3266.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Colombo M, Raposo G and Théry C:
Biogenesis, secretion, and intercellular interactions of exosomes
and other extracellular vesicles. Annu Rev Cell Dev Biol.
30:255–289. 2014. View Article : Google Scholar
|
|
53
|
Mao X and Jin F: The exosome and breast
cancer cell plasticity. Onco Targets Ther. 12:9817–9825. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Green DR, Droin N and Pinkoski M:
Activation-induced cell death in T cells. Immunol Rev. 193:70–81.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ventimiglia LN and Alonso MA: Biogenesis
and function of T cell-derived exosomes. Front Cell Dev Biol.
4:842016. View Article : Google Scholar
|
|
56
|
Chinn IK, Blackburn CC, Manley NR and
Sempowski GD: Changes in primary lymphoid organs with aging. Semin
Immunol. 24:309–320. 2012. View Article : Google Scholar
|
|
57
|
Ucar O, Li K, Dvornikov D, Kreutz C,
Timmer J, Matt S, Brenner L, Smedley C, Travis MA, Hofmann TG, et
al: A thymic epithelial stem cell pool persists throughout ontogeny
and is modulated by TGF-β. Cell Rep. 17:448–457. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Geenen V, Trussart C, Michaux H, Halouani
A, Jaïdane H, Collée C, Renard C, Daukandt M, Ledent P and Martens
H: The presentation of neuroendocrine self-peptides in the thymus:
An essential event for individual life and vertebrate survival. Ann
NY Acad Sci. 1455:113–125. 2019. View Article : Google Scholar
|
|
59
|
Caton AJ, Kropf E, Simons DM, Aitken M,
Weissler KA and Jordan MS: Strength of TCR signal from self-peptide
modulates autoreactive thymocyte deletion and Foxp3(+) Treg-cell
formation. Eur J Immunol. 44:785–793. 2014. View Article : Google Scholar
|
|
60
|
Saran N, Łyszkiewicz M, Pommerencke J,
Witzlau K, Vakilzadeh R, Ballmaier M, von Boehmer H and Krueger A:
Multiple extrathymic precursors contribute to T-cell development
with different kinetics. Blood. 115:1137–1144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tang Q, Jiang D, Harfuddin Z, Cheng K, Moh
MC and Schwarz H: Regulation of myelopoiesis by CD137L signaling.
Int Rev Immunol. 33:454–469. 2014. View Article : Google Scholar
|
|
62
|
Medler TR and Coussens LM: Duality of the
immune response in cancer: Lessons learned from skin. J Invest
Dermatol. 134:E23–E28. 2014. View Article : Google Scholar
|
|
63
|
Egeblad M, Nakasone ES and Werb Z: Tumors
as organs: Complex tissues that interface with the entire organism.
Dev Cell. 18:884–901. 2010. View Article : Google Scholar
|
|
64
|
de Visser KE, Eichten A and Coussens LM:
Paradoxical roles of the immune system during cancer development.
Nat Rev Cancer. 6:24–37. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Re RN and Cook JL: An intracrine view of
angiogenesis. Bioessays. 28:943–953. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kumar P, Bhattacharya P and Prabhakar BS:
A comprehensive review on the role of co-signaling receptors and
Treg homeostasis in autoimmunity and tumor immunity. J Autoimmun.
95:77–99. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Seyfried TN and Huysentruyt LC: On the
origin of cancer metastasis. Crit Rev Oncog. 18:43–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Keskinov AA and Shurin MR: Myeloid
regulatory cells in tumor spreading and metastasis. Immunobiology.
220:236–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kovács KA, Hegedus B, Kenessey I and Tímár
J: Tumor type-specific and skin region-selective metastasis of
human cancers: Another example of the ‘seed and soil’ hypothesis.
Cancer Metastasis Rev. 32:493–499. 2013. View Article : Google Scholar
|
|
70
|
Caixeiro NJ, Kienzle N, Lim SH, Spring KJ,
Tognela A, Scott KF, de Souza P and Becker TM: Circulating tumour
cells-a bona fide cause of metastatic cancer. Cancer Metastasis
Rev. 33:747–756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ben-Baruch A: Organ selectivity in
metastasis: Regulation by chemokines and their receptors. Clin Exp
Metastasis. 25:345–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Satelli A, Mitra A, Brownlee Z, Xia X,
Bellister S, Overman MJ, Kopetz S, Ellis LM, Meng QH and Li S:
Epithelial-mesenchymal transitioned circulating tumor cells capture
for detecting tumor progression. Clin Cancer Res. 21:899–906. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sceneay J, Smyth MJ and Möller A: The
pre-metastatic niche: Finding common ground. Cancer Metastasis Rev.
32:449–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Paduch R: The role of lymphangiogenesis
and angiogenesis in tumor metastasis. Cell Oncol (Dordr).
39:397–410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Spinella F, Caprara V, Cianfrocca R,
Rosanò L, Di Castro V, Garrafa E, Natali PG and Bagnato A: The
interplay between hypoxia, endothelial and melanoma cells regulates
vascularization and cell motility through endothelin-1 and vascular
endothelial growth factor. Carcinogenesis. 35:840–848. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Horton BL and Gajewski TF: Back from the
dead: TIL apoptosis in cancer immune evasion. Br J Cancer.
118:309–311. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Smith HA and Kang Y: The
metastasis-promoting roles of tumor-associated immune cells. J Mol
Med (Berl). 91:411–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lee JH, Chen Y, Chan JL, Qian YW and
Goydos JS: Molecular analysis of melanoma-induced sentinel lymph
node immune dysfunction. Cancer Immunol Immunother. 60:685–692.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lagios MD: Clinical significance of
immunohistochemically detectable epithelial cells in sentinel lymph
node and bone marrow in breast cancer. J Surg Oncol. 83:1–4. 2003.
View Article : Google Scholar
|
|
81
|
Nishikawa H and Sakaguchi S: Regulatory T
cells in tumor immunity. Int J Cancer. 127:759–767. 2010.PubMed/NCBI
|
|
82
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008. View Article : Google Scholar
|
|
83
|
Brzostek J and Gascoigne NRJ: Thymic
origins of T cell receptor alloreactivity. Transplantation.
101:1535–1541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ariotti S, Beltman JB, Chodaczek G,
Hoekstra ME, Van Beek AE, Gomez-Eerland R, Ritsma L, van Rheenen J,
Marée AF, Zal T, et al: Tissue-resident memory CD8+ T cells
continuously patrol skin epithelia to quickly recognize local
antigen. Proc Natl Acad Sci USA. 109:19739–19744. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Deleeuw RJ, Kost SE, Kakal JA and Nelson
BH: The prognostic value of FoxP3+ tumor-infiltrating lymphocytes
in cancer: A critical review of the literature. Clin Cancer Res.
18:3022–3029. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Aggarwal S, Sharma SC and N Das S:
Dynamics of regulatory T cells (Tregs) in patients with
oral squamous cell carcinoma. J Surg Oncol. 116:1103–1113. 2017.
View Article : Google Scholar
|
|
87
|
Protti MP, De Monte L and Di Lullo G:
Tumor antigen-specific CD4+ T cells in cancer immunity: From
antigen identification to tumor prognosis and development of
therapeutic strategies. Tissue Antigens. 83:237–246. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Mailloux AW and Young MR: Regulatory
T-cell trafficking: From thymic development to tumor-induced immune
suppression. Crit Rev Immunol. 30:435–447. 2010. View Article : Google Scholar
|
|
89
|
Huang X, Chen Z, Zhang N, Zhu C, Lin X, Yu
J, Chen Z, Lan P and Wan Y: Increase in
CD4+FOXP3+ regulatory T cell number and
upregulation of the HGF/c-Met signaling pathway during the liver
metastasis of colorectal cancer. Oncol Lett. 20:2113–2118. 2020.
View Article : Google Scholar
|
|
90
|
Hamidinia M, Ghafourian Boroujerdnia M,
Talaiezadeh A, Solgi G, Roshani R, Iranprast S and Khodadadi A:
Increased P-35, EBI3 transcripts and other treg markers in
peripheral blood mononuclear cells of breast cancer patients with
different clinical stages. Adv Pharm Bull. 5:261–267. 2015.
View Article : Google Scholar
|
|
91
|
Vasco C, Canazza A, Rizzo A, Mossa A,
Corsini E, Silvani A, Fariselli L, Salmaggi A and Ciusani E:
Circulating T regulatory cells migration and phenotype in
glioblastoma patients: An in vitro study. J Neurooncol.
115:353–363. 2013. View Article : Google Scholar
|
|
92
|
Zhang X, Kelaria S, Kerstetter J and Wang
J: The functional and prognostic implications of regulatory T cells
in colorectal carcinoma. J Gastrointest Oncol. 6:307–313. 2015.
|
|
93
|
Chen X and Oppenheim JJ: Resolving the
identity myth: Key markers of functional CD4+FoxP3+ regulatory T
cells. Int Immunopharmacol. 11:1489–1496. 2011. View Article : Google Scholar
|
|
94
|
Ladoire S, Arnould L, Apetoh L, Coudert B,
Martin F, Chauffert B, Fumoleau P and Ghiringhelli F: Pathologic
complete response to neoadjuvant chemotherapy of breast carcinoma
is associated with the disappearance of tumor-infiltrating foxp3+
regulatory T cells. Clin Cancer Res. 14:2413–2420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Teng MW, Ngiow SF, von Scheidt B,
McLaughlin N, Sparwasser T and Smyth MJ: Conditional regulatory
T-cell depletion releases adaptive immunity preventing
carcinogenesis and suppressing established tumor growth. Cancer
Res. 70:7800–7809. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Neophytou CM, Panagi M, Stylianopoulos T
and Papageorgis P: The role of tumor microenvironment in cancer
metastasis: Molecular mechanisms and therapeutic opportunities.
Cancers (Basel). 13:20532021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Baxevanis CN, Perez SA and Papamichail M:
Combinatorial treatments including vaccines, chemotherapy and
monoclonal antibodies for cancer therapy. Cancer Immunol
Immunother. 58:317–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Bhatia A and Kumar Y: Cellular and
molecular mechanisms in cancer immune escape: A comprehensive
review. Expert Rev Clin Immunol. 10:41–62. 2014. View Article : Google Scholar
|
|
99
|
Sasidharan Nair V and Elkord E: Immune
checkpoint inhibitors in cancer therapy: A focus on T-regulatory
cells. Immunol Cell Biol. 96:21–33. 2018. View Article : Google Scholar
|
|
100
|
Ruffell B, Denardo DG, Affara NI and
Coussens LM: Lymphocytes in cancer development: Polarization
towards pro-tumor immunity. Cytokine Growth Factor Rev. 21:3–10.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Rotte A, D'Orazi G and Bhandaru M: Nobel
committee honors tumor immunologists. J Exp Clin Cancer Res.
37:2622018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Dolan DE and Gupta S: PD-1 pathway
inhibitors: Changing the landscape of cancer immunotherapy. Cancer
Control. 21:231–237. 2014. View Article : Google Scholar
|
|
103
|
Sharma P, Wagner K, Wolchok JD and Allison
JP: Novel cancer immunotherapy agents with survival benefit: Recent
successes and next steps. Nat Rev Cancer. 11:805–812. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Sharma A, Suleyman N, Jones O and Vasdev
N: Immunotherapy in urological tumors. Rev Urol. 21:15–20.
2019.
|
|
105
|
Steven A, Fisher SA and Robinson BW:
Immunotherapy for lung cancer. Respirology. 21:821–833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Dobosz P and Dzieciątkowski T: The
intriguing history of cancer immunotherapy. Front Immunol.
10:29652019. View Article : Google Scholar
|
|
107
|
Fritz JM and Lenardo MJ: Development of
immune checkpoint therapy for cancer. J Exp Med. 216:1244–1254.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Pennock GK and Chow LQ: The evolving role
of immune checkpoint inhibitors in cancer treatment. Oncologist.
20:812–822. 2015. View Article : Google Scholar
|
|
109
|
Ahern E, Solomon BJ, Hui R, Pavlakis N,
O'Byrne K and Hughes BGM: Neoadjuvant immunotherapy for non-small
cell lung cancer: Right drugs, right patient, right time? J
Immunother Cancer. 9:e0022482021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Stege H, Haist M, Nikfarjam U, Schultheis
M, Heinz J, Pemler S, Loquai C and Grabbe S: The status of adjuvant
and neoadjuvant melanoma therapy, new developments and upcoming
challenges. Target Oncol. 16:537–552. 2021. View Article : Google Scholar
|
|
111
|
Signorelli D, Giannatempo P, Grazia G,
Aiello MM, Bertolini F, Mirabile A, Buti S, Vasile E, Scotti V,
Pisapia P, et al: Patients selection for immunotherapy in solid
tumors: Overcome the Naïve vision of a single biomarker. Biomed Res
Int. 2019:90564172019. View Article : Google Scholar
|
|
112
|
Zhu J, Powis de Tenbossche CG, Cané S,
Colau D, van Baren N, Lurquin C, Schmitt-Verhulst AM, Liljeström P,
Uyttenhove C and Van den Eynde BJ: Resistance to cancer
immunotherapy mediated by apoptosis of tumor-infiltrating
lymphocytes. Nat Commun. 8:14042017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Horton BL, Williams JB, Cabanov A,
Spranger S and Gajewski TF: Intratumoral CD8+ T-cell
apoptosis is a major component of T-cell dysfunction and impedes
antitumor immunity. Cancer Immunol Res. 6:14–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chaoul N, Tang A, Desrues B, Oberkampf M,
Fayolle C, Ladant D, Sainz-Perez A and Leclerc C: Lack of MHC class
II molecules favors CD8+ T-cell infiltration into tumors
associated with an increased control of tumor growth.
Oncoimmunology. 7:e14042132017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Brunner-Weinzierl MC and Rudd CE: CTLA-4
and PD-1 control of T-cell motility and migration: Implications for
tumor immunotherapy. Front Immunol. 9:27372018. View Article : Google Scholar
|
|
116
|
Balkhi MY: Receptor signaling,
transcriptional, and metabolic regulation of T cell exhaustion.
Oncoimmunology. 9:17473492020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Salmon H, Idoyaga J, Rahman A, Leboeuf M,
Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J,
Tung N, et al: Expansion and activation of CD103(+) dendritic cell
progenitors at the tumor site enhances tumor responses to
therapeutic PD-L1 and BRAF inhibition. Immunity. 44:924–938. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Li KP, Shanmuganad S, Carroll K, Katz JD,
Jordan MB and Hildeman DA: Dying to protect: Cell death and the
control of T-cell homeostasis. Immunol Rev. 277:21–43. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Paludan SR, Pradeu T, Masters SL and
Mogensen TH: Constitutive immune mechanisms: Mediators of host
defence and immune regulation. Nat Rev Immunol. 21:137–150. 2021.
View Article : Google Scholar
|
|
120
|
Fusi A and Dalgleish A: The importance for
immunoregulation for long-term cancer control. Future Oncol.
13:1619–1632. 2017. View Article : Google Scholar
|
|
121
|
Lisovska N and Shanazarov N: Tumor
progression mechanisms: Insights from the central immune regulation
of tissue homeostasis. Oncol Lett. 17:5311–5318. 2019.
|
|
122
|
Bruni D, Angell HK and Galon J: The immune
contexture and Immunoscore in cancer prognosis and therapeutic
efficacy. Nat Rev Cancer. 20:662–680. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Lecis D, Sangaletti S, Colombo MP and
Chiodoni C: Immune checkpoint ligand reverse signaling: Looking
back to go forward in cancer therapy. Cancers (Basel). 11:6242019.
View Article : Google Scholar
|
|
124
|
Rei M, Pennington DJ and Silva-Santos B:
The emerging protumor role of γδ T lymphocytes: Implications for
cancer immunotherapy. Cancer Res. 75:798–802. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Savage PA, Leventhal DS and Malchow S:
Shaping the repertoire of tumor-infiltrating effector and
regulatory T cells. Immunol Rev. 259:245–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Gourzones C, Barjon C and Busson P:
Host-tumor interactions in nasopharyngeal carcinomas. Semin Cancer
Biol. 22:127–136. 2012. View Article : Google Scholar
|
|
127
|
Fidler IJ: Lymphocytes are not only
immunocytes. Biomedicine. 32:1–3. 1980.PubMed/NCBI
|
|
128
|
Thomas VA and Balthasar JP: Understanding
inter-individual variability in monoclonal antibody disposition.
Antibodies (Basel). 8:562019. View Article : Google Scholar
|
|
129
|
Wolf D, Sopper S, Pircher A, Gastl G and
Wolf AM: Treg(s) in cancer: Friends or foe? J Cell Physiol.
230:2598–2605. 2015. View Article : Google Scholar
|
|
130
|
Sanmamed MF and Chen L: A paradigm shift
in cancer immunotherapy: From enhancement to normalization. Cell.
175:313–326. 2018. View Article : Google Scholar
|
|
131
|
Bedognetti D, Ceccarelli M, Galluzzi L, Lu
R, Palucka K, Samayoa J, Spranger S, Warren S, Wong KK, Ziv E, et
al: Toward a comprehensive view of cancer immune responsiveness: A
synopsis from the SITC workshop. J Immunother Cancer. 7:1312019.
View Article : Google Scholar : PubMed/NCBI
|