Introduction
Nasopharyngeal carcinoma (NPC) is a rare cancer type
that accounts for 0.7% of all cancer cases worldwide, according to
2018 estimates of cancer incidence by the International Agency for
Research on Cancer (1). However,
the incidence of NPC in Southeast Asia and Taiwan is much higher
due to epidemiologic factors, such as Epstein-Barr virus infection,
environmental factors, host genetics and smoking (2,3).
Cisplatin-based concurrent chemoradiotherapy is now the standard
treatment for locoregionally advanced disease and contributes to
good prognoses as follows: 5-year failure-free survival rates of
>70% and 5-year overall survival (OS) rates of >80% (4). Compared with patients with other head
and neck cancers, patients with NPC are much younger and achieve
higher distant metastatic rates. For metastatic disease, cisplatin
plus fluorouracil has been the first-line treatment of choice;
however, outcomes have been disappointing, with a 40–60% response
rate, leading to a median OS of 10–15 months (2). Certain patients with metastatic
disease have poor response, particularly when previously exposed to
cisplatin treatment. Furthermore, rare treatment choices may be
considered for progressive disease refractory to cisplatin-based
treatments. There is currently no targeted therapy available for
patients with advanced NPC. Exploring biomarkers for predicting
cisplatin resistance and determining the resistance mechanisms are
important research goals for NPC.
Protein degradation via ubiquitination is an
important mechanism of cellular metabolism, during which the
ubiquitin-activating enzyme E1 cooperates with the
ubiquitin-conjugating enzyme E2 and ubiquitin ligase E3 to complete
protein ubiquitination (5).
Several literature studies have demonstrated that the intracellular
ubiquitination system may be implicated in oncogenic process
through cell cycle control, metabolic pathway regulation and DNA
damage response (6,7). The DNA repair enzyme,
O6-methylguanine-DNA methyltransferase (MGMT), modulates the
cytotoxicity of several alkylating agents (such as cisplatin) in
relation to the DNA alkylation of the O6-position of guanine.
According to a previous study, the potential regulator of MGMT,
ubiquitin-conjugating E2 enzyme B (UBE2B), has the strongest
ability to bind to MGMT and regulate MGMT ubiquitination mediated
by alkylating agents (8).
Furthermore, a previous study by our group demonstrated that MGMT
repairs platinum-DNA adducts and MGMT proficiency contributes to
the poor prognosis of patients with NPC (9). These results suggest a role of UBE2B
expression in predicting survival outcomes for patients with NPC
receiving cisplatin-based therapy.
The present study aimed to determine UBE2B
expression in the malignant tissue of patients with NPC and
evaluate UBE2B as a risk factor contributing to poor treatment
outcomes of cisplatin-based chemoradiotherapy. First, UBE2B
expression was compared between normal nasopharyngeal mucosa and
NPC tissues by reappraising two datasets from a public database.
The prognostic value of UBE2B was further examined in a Taiwanese
NPC cohort comprising 124 patients receiving cisplatin-based
chemoradiotherapy.
Materials and methods
Analysis of published NPC
datasets
UBE2B expression levels were analyzed in clinical
NPC specimens and compared with nasopharynx mucosa tissue by
searching for profiling datasets of gene expression in the Gene
Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/). A total of two gene
expression datasets satisfying these requirements, GSE12452
(Affymetrix-GPL570 platform) and GSE68799 (GPL11154 Illumina HiSeq
2000 platform), were reappraised for comparison (10). Regarding the microarray data of
GSE12452, the preprocessed gene expression data were downloaded,
whereas for the RNA-sequencing data of GSE68799, fragments per
kilobase per million mapped reads were downloaded. All probe sets
were analyzed without pre-selection or filtering. The UBE2B
expression levels in NPC tissues were compared with those in
nasopharynx tissues using the Wilcoxon rank-sum test. When multiple
probe sets for the same gene symbol exhibited statistical
significance, the probe values with the highest statistical
difference between mucosa and tumor tissues were presented as UBE2B
expression values.
Cell culture
The human NPC cell line TW01 and a human oral
epithelial cell line (Dysplastic Oral Keratinocyte; DOK) used in
the present study were obtained from the cell bank of the Taiwanese
National Institute of Cancer Research, National Health Research
Institutes. NPC cells were authenticated by the Authentication
Services of Topgen Biotechnology. TW01 cells were incubated with
α-MEM medium (Gibco; Thermo Fisher Scientific, Inc.) (8). DOK cells were routinely cultured in
DMEM medium (Gibco; Thermo Fisher Scientific, Inc.). All the
culture media were supplemented with 10% fetal bovine serum (Thermo
Fisher Scientific, Inc.) and 1% antibiotic-antimycotic solution
(GeneDireX). All cells were cultured at 37°C in a humidified
incubator with 5% CO2.
Antibodies and reagents
The rabbit polyclonal antibody against UBE2B (cat.
no. GTX100416; 1:1,000 dilution) and rabbit monoclonal antibody
against β-actin (cat. no. GTX109639; 1:10,000 dilution) were
purchased from GeneTex. The mouse monoclonal antibody against MGMT
(cat. no. MGMT2015#5; 1:5,000 dilution) was obtained from LTK
BioLaboratories. The horseradish peroxidase-conjugated secondary
antibodies (cat. no. sc-2004 and sc-2005; 1:10,000 dilution) and
the rabbit polyclonal antibodies against enhanced green
fluorescence protein (EGFP) (cat. no. sc-8334; 1:1,000 dilution)
were obtained from Santa Cruz Biotechnology, Inc. Other
experimental chemicals were described in a previous report
(9).
Western blot analysis
The detailed experimental conditions of western blot
analysis were as previously described (8). In brief, protein was quantitated by a
protein assay kit (cat. no. 5000006; Bio-Rad Laboratories, Inc.).
Equal amounts of protein (30 µg) from the cell extract prepared
with RIPA lysis buffer (Merck Millipore) were separated through 10%
SDS-PAGE and transferred to polyvinylidene fluoride membranes
(Immobilon®-P; Merck Millipore). After blocking with 5%
non-fat milk (Fonterra) at ambient temperature for 1 h, the
membranes were probed with primary antibodies against UBE2B, MGMT
and actin at 4°C overnight. The membrane was then hybridized with
horseradish peroxidase-conjugated secondary antibody at ambient
temperature for 1 h. The immunoreactivities of the membranes were
detected using the Western Lightning™ Plus-ECL Enhanced
Chemiluminescence Substrate (PerkinElmer, Inc.). The protein levels
were measured using densitometric analysis with ImageJ 1.53
(National Institutes of Health) and normalized to actin
signals.
Small interfering (si)RNA
transfection
For UBE2B silencing, an siRNA technique was used as
described previously (8). In
brief, NPC cells in the exponential growth phase were transfected
with control siRNA (cat. no. 12935146; Thermo Fisher Scientific,
Inc.) or siUBE2B (cat. no. 4390824; Thermo Fisher Scientific, Inc.)
using Lipofectamine® 2000 reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
After transfection for 24 h, the cells were subjected to
pertinent experiments.
Clonogenic assay
After NPC cells were seeded at 5×104
cells/well in 6-well plates overnight, the cells were transfected
with control siRNA or siUBE2B for 24 h. The cells were then
reseeded at 1,000 cells/well into 6-well plates and allowed to grow
for 7–14 days, dependent on the growth rates of individual NPC
cells. Following the application of methylene blue staining, the
numbers of colonies formed, which consisted of at least 50 tumor
cells, were manually recorded.
Patients and tumor specimens
All formalin-fixed and paraffin-embedded NPC tissues
were approved for the present study by the institutional review
board of Chi-Mei Medical Center in Taiwan (IRB10710-L01). The
specimens were obtained from the BioBank of Chi-Mei Medical Center
(Tainan, Taiwan) and were anonymously processed for research
purposes that excluded the identification of the participants'
personal information. Informed consent was obtained from all
subjects involved in the study. Available paraffin-embedded tissue
blocks were retrieved from 124 patients with NPC diagnosed between
January 1993 and December 2002. No signs or events of distant
metastases were identified at the initial diagnosis. A total of two
pathologists reappraised the histological subtypes according to the
criteria of the current World Health Organization (WHO)
classification (11). The tumor
staging of these samples was re-examined using the 7th American
Joint Committee on Cancer system (12).
Immunohistochemistry and assessment of
UBE2B expression
Tissue sections (3 µm) were obtained from
paraffin-embedded blocks, as previously described (13). The slides were deparaffinized,
rehydrated with ethanol and heated in a 10 mM citrate buffer (pH
6.0) by microwave for 7 min to retrieve antigens. Endogenous
peroxidase was blocked with 3% hydrogen peroxide for 15 min at
ambient temperature. The slides were then washed with tris-buffered
saline and incubated with a primary rabbit polyclonal antibody
against UBE2B (1:100 dilution; GeneTex, Inc.) for 1 h. A ChemMate
EnVision kit (DAKO; Agilent Technologies, Inc.) was applied to
detect the primary antibody. Tissue sections incubated with rabbit
IgG in place of primary antibody served as negative controls. A
total of two pathologists who were blinded to this study examined
the UBE2B expression and scored the intensity and distribution with
a multiheaded microscope to reach a consensus on the histology
(H)-score using the following equation: H-score=ΣPi (i+1) where i
represents the intensity of stained tumor cells, and Pi is the
percentage of stained tumor cells, ranging from 0 to 100%. Tumors
with H-scores higher than the median value for all examined samples
were classified as having high UBE2B expression levels.
Treatment and follow-up
All 124 patients with NPC completed the radiotherapy
course, including a daily fractionation of 180–200 cGy and 5
fractions weekly to achieve a total dose of no less than 7,000 cGy.
Patients with stage II–IV NPC also underwent at least 3 cycles of
cisplatin-based chemotherapy, with all treatments performed
according to the published protocol (14). The patients' responses were
classified according to the WHO criteria (15). In total, there were 110 complete
and 7 partial tumor regressions.
Plasmid construction
The pEGFPc1-MGMT plasmid was constructed as
described previously (8). In
brief, human MGMT cDNA was amplified from the previously
constructed pTRE2hyg-MGMT plasmid (Clontech; Takara Bio, USA),
which was kindly gifted by Professor Jang-Yang Chang (Institute of
Biotechnology and Pharmaceutical Research, National Health Research
Institutes, Miaoli, Taiwan) (16).
After performing PCR, the amplified products of MGMT cDNA (0.624
kb) were then cloned into the pEGFPc1 vector (Clontech; Takara Bio
USA) (8,16). The clones were verified by
sequencing with the BigDye Terminator Cycle Sequencing Ready
Reaction Kit (Applied Biosystems; Thermo Fisher Scientific, Inc.)
on an AB 3130×l Genetic Analyzer (Applied Biosystems; Thermo Fisher
Scientific, Inc.).
Cell viability assay
After seeding 1×104 cells/well into
24-well plates overnight, NPC cells were transfected with 20 nM
control siRNA, siUBE2B or siUBE2B with 0.5 µg/well pEGFPc1-MGMT for
an additional 24 h. To evaluate drug sensitivity, NPC cells were
subsequently exposed to indicated concentrations of cisplatin (0,
0.125, 0.25, 0.5 and 1 µM cisplatin) for 3 growth generations.
After the cells were fixed and stained using methylene blue,
cisplatin cytotoxicity was measured as described previously
(8). In brief, the survival rates
of NPC cells with the indicated treatments were calculated from the
absorbance (A) of methylene blue-stained cells as follows: Survival
rate=(Atested cells-Abackground
controls)/(Amatched control cells-Abackground
controls) ×100%. The IC50 values (50% inhibition
of cell viability) were determined based on the dose-response
curves.
Statistical analysis
SPSS 16.0 statistics software (SPSS, Inc.) was
employed for statistical analysis. To compare the mean or median of
each group with that of the control group, the Mann-Whitney U-test
was used. Data from the cell viability assay and clonogenic assay
were examined using analysis of variance (ANOVA). If statistical
significance of a treatment effect was obtained using ANOVA,
Tukey's multiple-comparisons test was applied to determine the
difference between treatment groups. To evaluate the associations
between UBE2B expression and various clinicopathological variables,
the χ2 test or Fisher's exact test was performed,
depending on the group size. For the patient cohort, the following
three endpoints were calculated: Disease-specific survival (DSS),
distant metastasis-free survival (DMeFS), and local recurrence-free
survival (LRFS), which were determined from the start date of the
radiotherapy to the onset of an event. The Kaplan-Meier method with
the log-rank test was applied to compare survival between groups.
For multivariate analysis of factors influencing survival, linear
logistic regression with the Cox proportional hazards model was
applied. Two-sided tests were used for all analyses and P<0.05
was considered to indicate statistical significance.
Results
High UBE2B expression is frequently
observed in NPC tissues
A total of two public datasets (GSE12452 and
GSE68799) were analyzed in order to explore the importance of UBE2B
expression in the carcinogenesis of NPC. The GSE12452 dataset
provided mRNA expression profiling from 31 NPC and 10 normal
healthy nasopharyngeal tissue specimens. The subject information
regarding age and sex was not available for the GSE12452 dataset.
Among the GSE68799 dataset, which is a collection of mRNA profiles
of 42 patients with NPC and 4 non-NPC tissues, the number of males
was 35 and the mean age was 54 years. The clinicopathological data
of these two datasets downloaded from the GSE database were
summarized in Tables SI and
SII. By examining mRNA expression
levels in NPC tissues (n=31 and 42 for GSE12452 and GSE68799,
respectively) and nontumor nasopharyngeal tissues (n=10 and 4 for
GSE12452 and GSE68799, respectively) in these datasets, it was
determined that UBE2B expression levels were increased in cancer
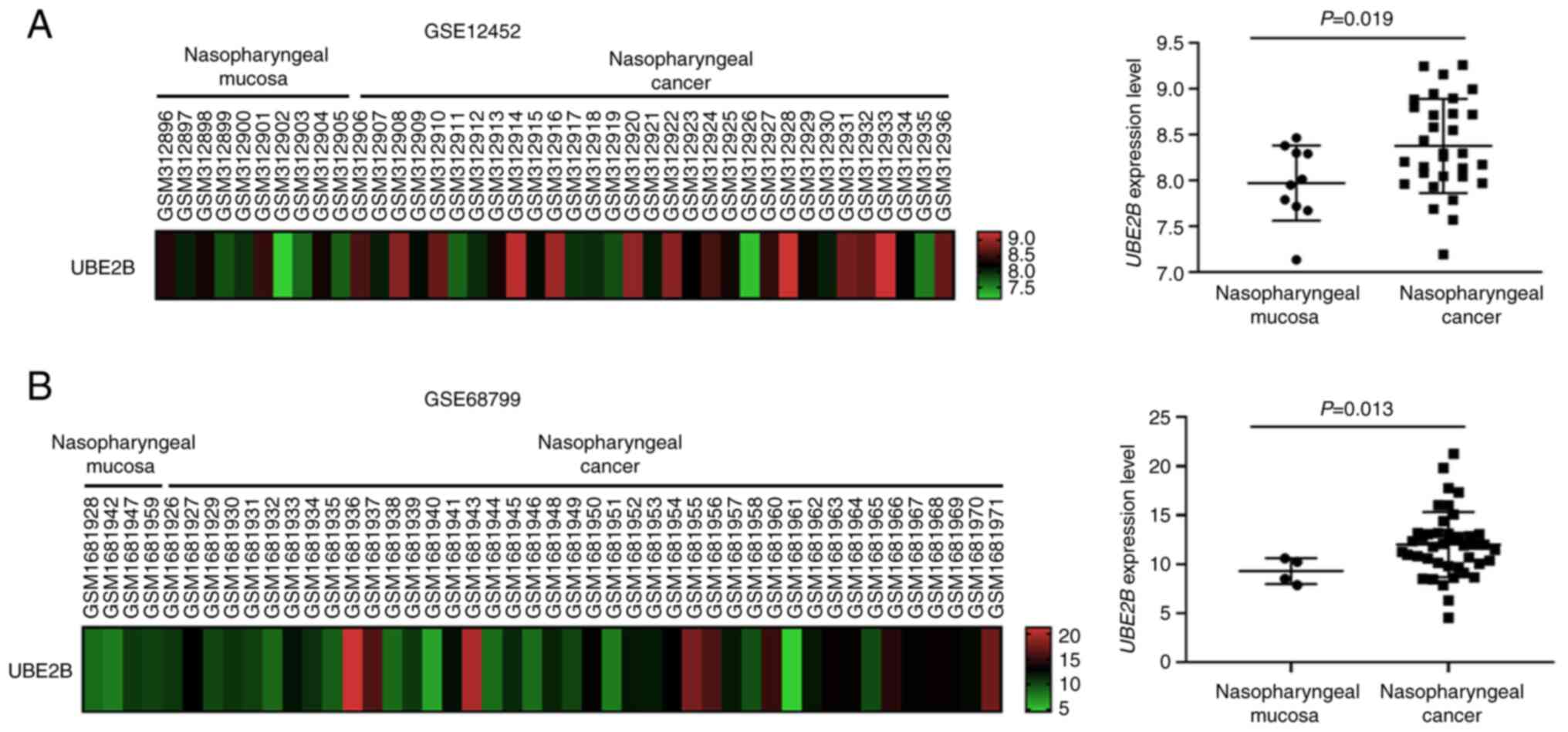
tissues. As presented in Fig. 1,
UBE2B expression levels in NPC tissues were significantly higher
than those in nontumor nasopharyngeal tissues (P=0.019 for
GSE12452; Fig. 1A; P=0.013 for
GSE68799; Fig. 1B). To validate
whether UBE2B is involved in NPC carcinogenesis, UBE2B expression
levels were also compared between non-cancerous epithelial cells
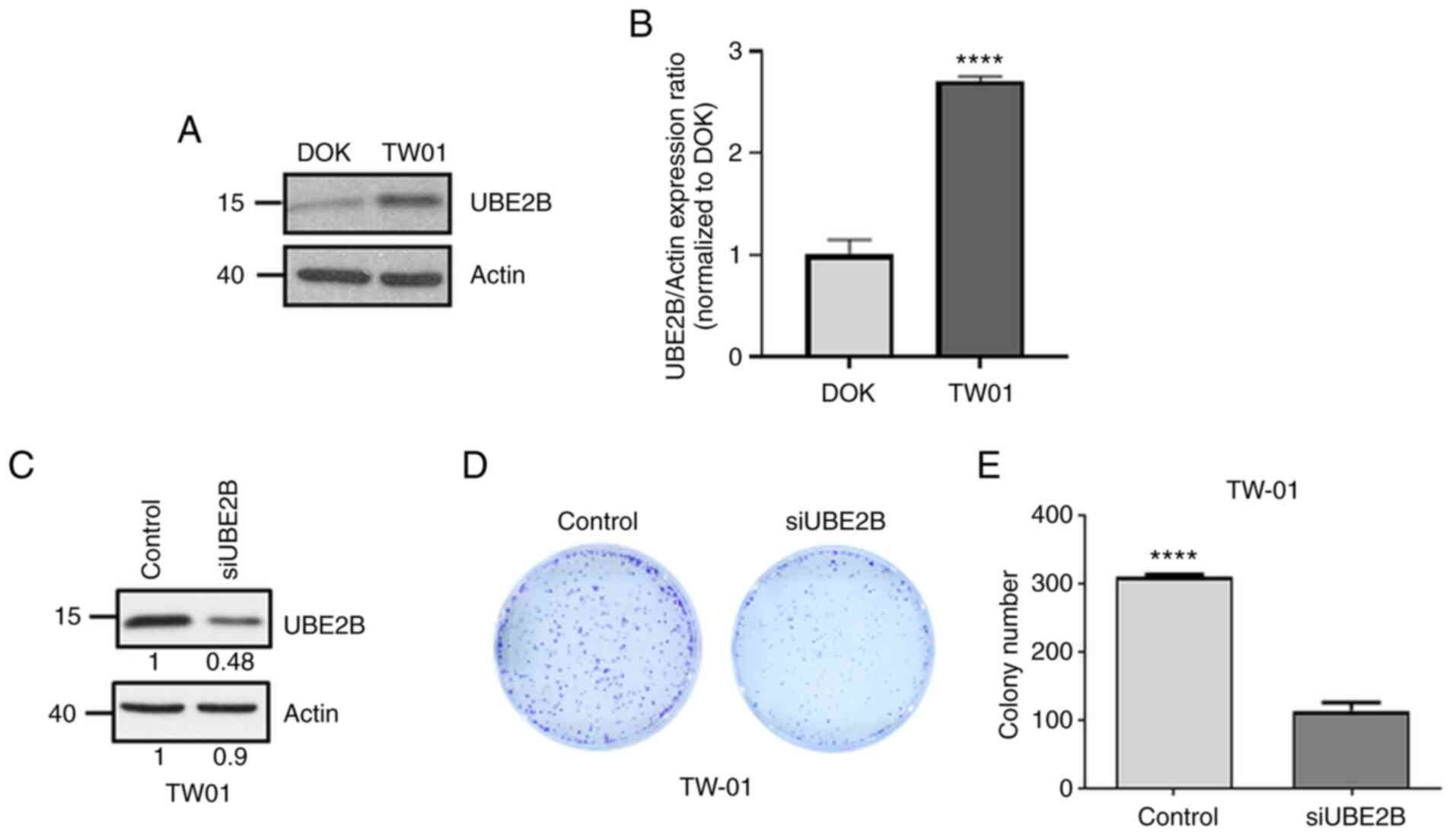
(DOK cells) and NPC cells (TW01 cells). As presented in Fig. 2A and B, UBE2B levels in TW01 cells
were increased by 2.7-fold compared with those in DOK cells.
Furthermore, UBE2B-deficient NPC cells were established by using
the siRNA technique (Fig. 2C). The
results of the clonogenic assay demonstrated that the number of
colonies formed was decreased by 67% in UBE2B-deficient TW01 cells,
as compared with that in their control counterpart (Fig. 2D and E). All of these results
suggested that UBE2B is involved in the carcinogenesis of NPC.
High UBE2B expression correlates with
poor clinical outcome for patients with NPC receiving
cisplatin-based concurrent chemoradiotherapy
To validate UBE2B as a biomarker for predicting
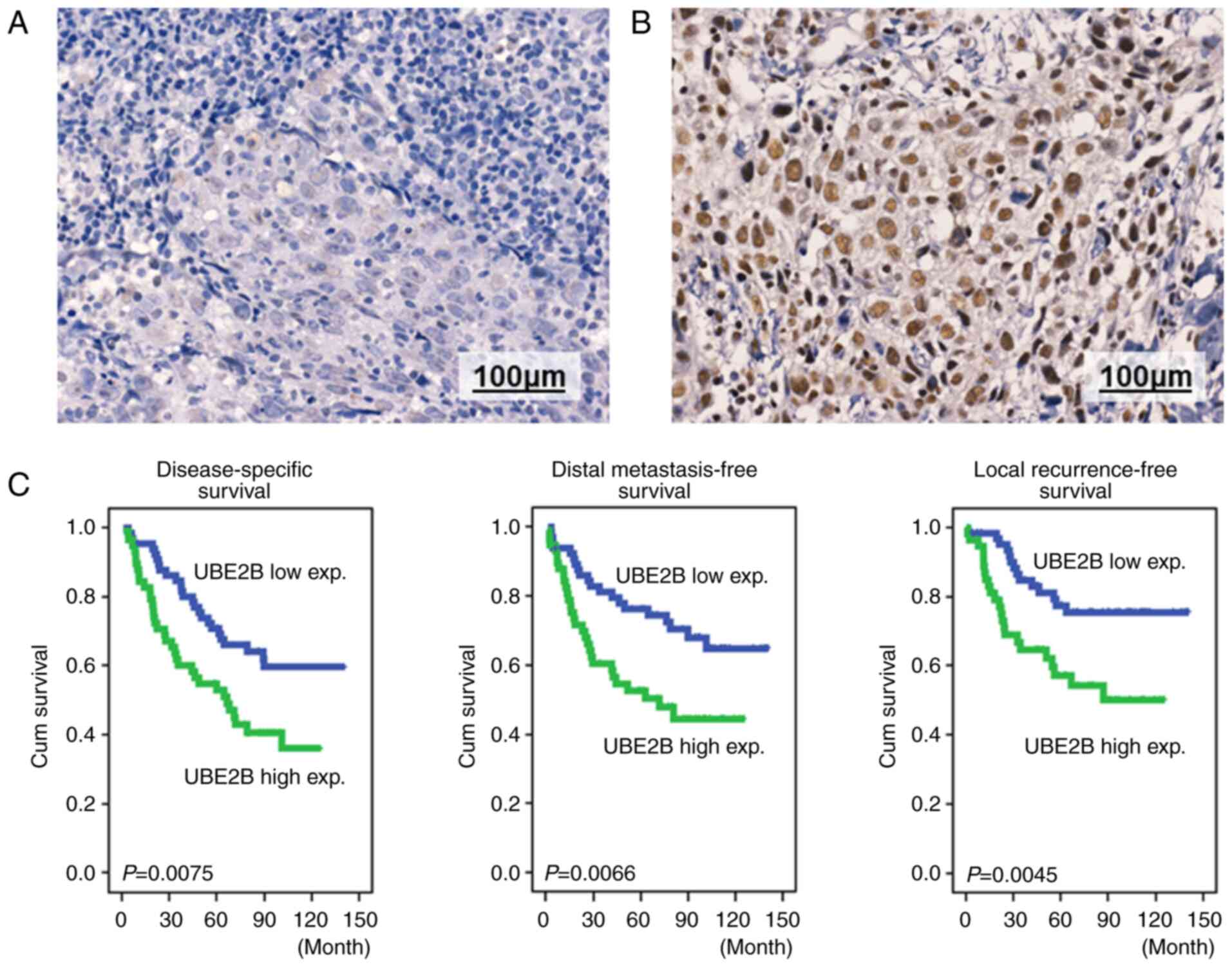
clinical outcome for patients with NPC, immunohistochemical
staining of NPC tissues using anti-UBE2B monoclonal antibodies was
employed (Fig. 3A and B). Based on
the nuclear and cytoplasmic staining intensity and percentage of
the tumor cells analyzed by two pathologists to determine the
H-score of NPC tumor tissues, patients were stratified into high
and low UBE2B expression groups. The median H-score was used as the
cut-off point for distinguishing high and low UBE2B expression in
NPC tumor tissues. The UBE2B expression was analyzed in tumor
tissues from patients treated with cisplatin-based
chemoradiotherapy at the Chi-Mei Medical Center (Tainan, Taiwan)
from January 1993 to December 2002. Among the 124 collected NPC
cases, the number of males was 95 and the mean age was 48.6 years.
The H-scores in the low expression group were significantly lower
than those in the high expression group according to the
Mann-Whitney U-test (median H-score=200 and 300 for low- and
high-expression group, respectively; P<0.001). In this patient
population, the clinicopathological variables, such as sex, age,
primary tumor status, nodal status, clinical stage or histological
grade, exhibited no difference between the high and low UBE2B
expression populations (Table I).
However, the patient group with high UBE2B expression had poorer
DSS, DMeFS and LFRS (Fig. 3C and
Table II). The log-rank analysis
also demonstrated that advanced primary tumor status, nodal status
and clinical stage were associated with poor DSS, DMeFS and LRFS.
Combined with UBE2B expression status and clinicopathological
variables, the multivariate survival analysis revealed that the
clinical stage remained an independent prognostic factor for DSS
[hazard ratio (HR)=2.743, P=0.004], DMeFS (HR=2.564, P=0.011) and
LFRS (HR=3.884, P=0.005), as presented in Table III. Furthermore, high UBE2B
expression was also associated with shorter DSS (HR=1.955,
P=0.011), DMeFS (HR=2.141, P=0.009) and LRFS (HR=2.557,
P=0.006).
 | Table I.Associations between UBE2B expression
levels and important clinicopathological variables. |
Table I.
Associations between UBE2B expression
levels and important clinicopathological variables.
|
| UBE2B expression,
n |
|
|---|
|
|
|
|
|---|
| Parameter | Low | High | P-value |
|---|
| Sex |
|
| 0.506 |
|
Male | 49 | 46 |
|
|
Female | 17 | 12 |
|
| Age, years |
|
| 0.416 |
|
<60 | 54 | 44 |
|
|
≥60 | 12 | 14 |
|
| Primary tumor
(T) |
|
| 0.363 |
|
T1-T2 | 45 | 35 |
|
|
T3-T4 | 21 | 23 |
|
| Nodal status
(N) |
|
| 0.666 |
|
N0-N1 | 31 | 25 |
|
|
N2-N3 | 35 | 33 |
|
| Stage |
|
| 0.486 |
|
I–II | 22 | 16 |
|
|
III–IV | 44 | 42 |
|
| Histological
grade |
|
| 0.346 |
|
Keratinizing | 4 | 1 |
|
|
Non-keratinizing | 26 | 28 |
|
|
Undifferentiated | 36 | 29 |
|
 | Table II.Univariate log-rank analyses. |
Table II.
Univariate log-rank analyses.
|
|
| DSS | DMeFS | LRFS |
|---|
|
|
|
|
|
|
|---|
| Parameter | No. of cases | No. of events | P-value | No. of events | P-value | No. of events | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
Male | 95 | 45 | 0.7870 | 38 | 0.6128 | 30 | 0.3240 |
|
Female | 29 | 14 |
| 11 |
| 7 |
|
| Age, years |
|
|
|
|
|
|
|
|
<60 | 98 | 48 | 0.8600 | 42 | 0.3091 | 29 | 0.8206 |
|
≥60 | 26 | 11 |
| 7 |
| 8 |
|
| Primary tumor
(T) |
|
|
|
|
|
|
|
|
T1-T2 | 80 | 32 | 0.0289 | 25 | 0.0085 | 19 | 0.0180 |
|
T3-T4 | 44 | 27 |
| 24 |
| 18 |
|
| Nodal status
(N) |
|
|
|
|
|
|
|
|
N0-N1 | 56 | 18 | 0.0008 | 17 | 0.0132 | 12 | 0.0160 |
|
N2-N3 | 68 | 41 |
| 32 |
| 25 |
|
| Stage |
|
|
|
|
|
|
|
|
I–II | 38 | 10 | 0.0020 | 9 | 0.0072 | 5 | 0.0026 |
|
III–IV | 86 | 49 |
| 40 |
| 32 |
|
| Histological
grade |
|
|
|
|
|
|
|
|
Keratinizing/non-keratinizing | 47 | 20 | 0.1980 | 17 | 0.2753 | 15 | 0.9521 |
|
Undifferentiated | 77 | 39 |
| 32 |
| 22 |
|
| UBE2B
expressiona |
|
|
|
|
|
|
|
|
Low | 66 | 25 | 0.0075 | 20 | 0.0066 | 9 | 0.0045 |
|
High | 58 | 34 |
| 29 |
| 28 |
|
 | Table III.Multivariate survival analyses. |
Table III.
Multivariate survival analyses.
|
| DSS | DMeFS | LRFS |
|---|
|
|
|
|
|
|---|
| Parameter | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Stage |
|
|
|
|
|
|
|
|
|
|
I–II | 1 | - | 0.004 | 1 | - | 0.011 | 1 | - | 0.005 |
|
III–IV | 2.743 | 1.387-5.422 |
| 2.564 | 1.241-5.294 |
| 3.884 | 1.510-9.990 |
|
| UBE2B
expressiona |
|
|
|
|
|
|
|
|
|
|
Low | 1 | - | 0.011 | 1 | - | 0.009 | 1 | - | 0.006 |
|
High | 1.955 | 1.164-3.282 |
| 2.141 | 1.206-3.801 |
| 2.557 | 1.313-4.981 |
|
MGMT is involved in UBE2B-mediated
cisplatin sensitivity
To evaluate the role of UBE2B in cisplatin
sensitivity, the siRNA technique was used to silence UBE2B
expression in NPC cells. When NPC cells were treated with 0.5
µM cisplatin, the results of cell viability assays indicated
that cell cytotoxicity was increased by 26% in UBE2B-depleted TW01
cells as compared to control cells (Fig. 4). In a clonogenic assay, the
IC50 value of cisplatin was decreased by 64% in
UBE2B-depleted TW01 cells as compared to control cells (Table IV). Furthermore, western blot
analysis indicated cisplatin treatment (10 µM for 8 h)
reduced UBE2B expression levels by 14% in TW01 cells as compared
with the control cells (Fig. S1).
These results suggested that UBE2B expression modulates cisplatin
sensitivity in NPC cells. Previous studies have reported that UBE2B
is able to regulate MGMT activity, which is also a determinant of
cisplatin sensitivity in NPC cells (8,9). To
examine whether MGMT is involved in UBE2B-mediated cisplatin
sensitivity, NPC cells were transfected with MGMT-overexpression
plasmids (Fig. S2). As presented
in Fig. 4 and Table IV, MGMT expression attenuated
cisplatin cytotoxicity in UBE2B-depleted NPC cells. The
IC50 value of cisplatin was increased by 100% in
UBE2B-depleted TW01 cell cells transfected with MGMT-overexpression
plasmid as compared with UBE2B-depleted NPC cells. These results
indicate the participation of MGMT in UBE2B-mediated cisplatin
sensitivity of NPC cells.
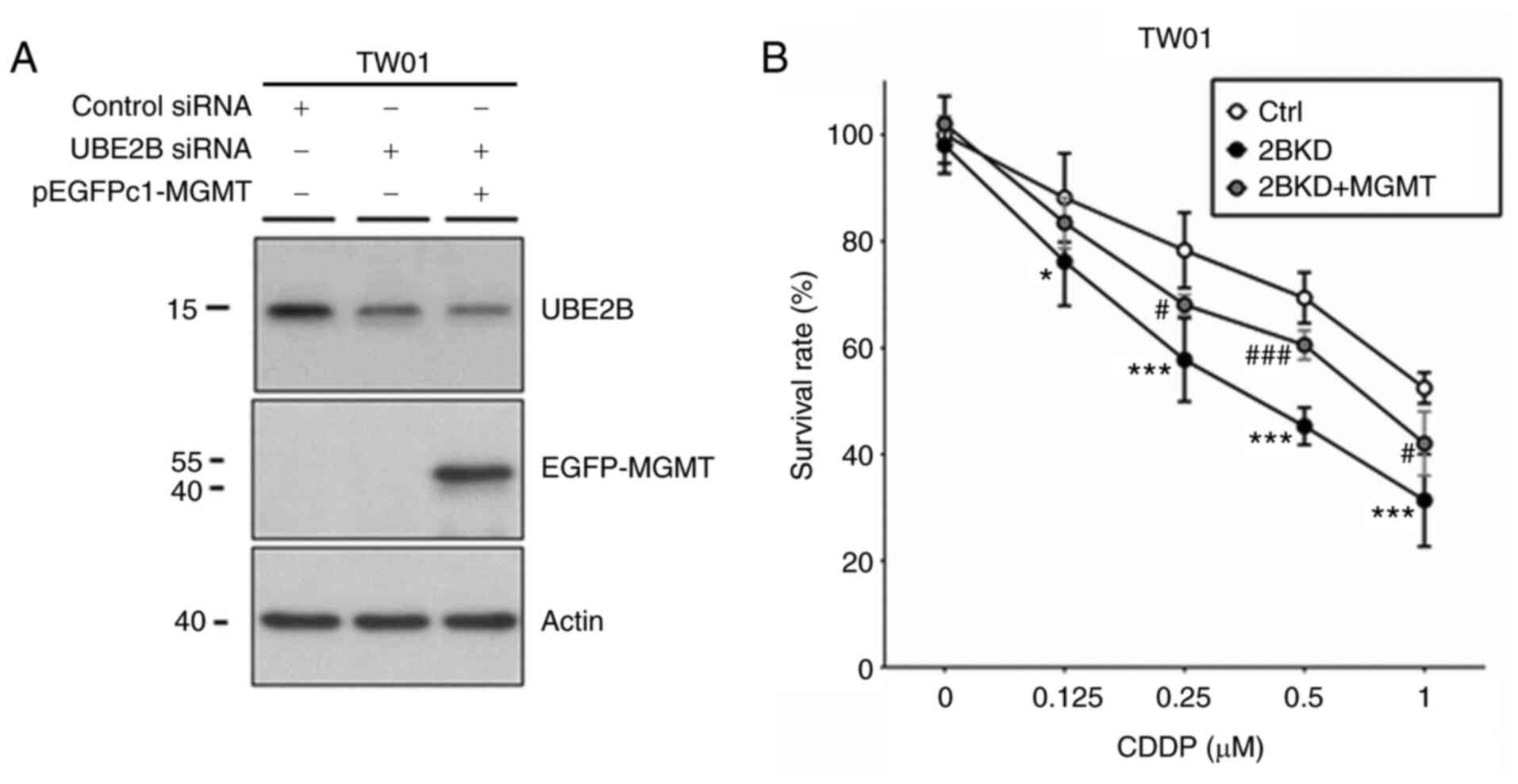 | Figure 4.UBE2B modulates cisplatin
cytotoxicity in nasopharyngeal carcinoma cells by targeting MGMT
expression. (A) Western blot analysis demonstrated UBE2B and MGMT
expression in TW01 cells with distinctive siRNA or plasmid
transfection. (B) Cell viability assays were performed with TW01
cells to analyze the role of UBE2B and MGMT in cisplatin-induced
cell death by using methylene blue staining. At least three
independent experiments were performed. Cell survival results were
presented as the mean ± standard deviation and compared using
analysis of variance with Tukey's post-hoc test. *P<0.01,
***P<0.0001, 2BKD group vs. control group;
#P<0.01, ###P<0.0001, 2BKD + MGMT group
vs. 2BKD group. Ctrl, cells transfected with scrambled siRNA; 2BKD,
cells transfected with siUBE2B; 2BKD + MGMT, cells transfected with
siUBE2B plus pEGFPc1-MGMT. UBE2B, ubiquitin-conjugating enzyme E2
B; siRNA, small interfering RNA; MGMT, O6-methylguanine-DNA
methyltransferase; pEGFP, plasmid expressing enhanced green
fluorescence protein. |
 | Table IV.IC50 values of cisplatin
in cells transfected with scrambles ctrl siRNA, siUBE2B or siUBE2B
plus pEGFPc1-MGMT (µM). |
Table IV.
IC50 values of cisplatin
in cells transfected with scrambles ctrl siRNA, siUBE2B or siUBE2B
plus pEGFPc1-MGMT (µM).
| Cell type | Ctrl | 2BKD | 2BKD + MGMT |
|---|
| TW01 | 1.1±0.1 |
0.4±0.1a |
0.8±0.1b |
Discussion
The global incidence and mortality rate of NPC have
gradually declined, particularly in endemic areas (17,18),
due to improvements in multidisciplinary cooperation and lifestyle
changes (2). Patients with
early-stage NPC are treated with radiotherapy alone with curative
intent, while locoregionally advanced diseases require chemotherapy
combined with radiotherapy. However, certain patients are at
advanced stages at initial presentation and exhibit poor response
to standard cisplatin-based chemoradiotherapy. Exploring the risk
stratification for poor responders and developing novel targets for
NPC treatment may help to improve survival outcomes for these
patients. Several studies have examined the genetic landscape of
NPC and explored the potential targetable pathways to improve
treatment in NPC, including NF-κB pathway dysregulation, cell-cycle
alteration and the PI3K/MAPK signaling pathway (2,19–21).
Numerous theories have discussed drug resistance in NPC treatment,
such as the targeting p53-MDM2 interaction for NPC treatment
(22), stem cell-like properties
induced by the leucyl-tRNA synthetase-mitotic arrest deficient 1
like 1 fusion gene through the far upstream element binding protein
1/c-Myc axis (23), cisplatin
resistance contributed by higher expression levels of never in
mitosis associate related kinase 2 (24), and transforming growth factor beta
signaling pathway alteration induced by microRNA-449b (25). However, these theoretical
mechanisms of drug resistance have not been established and applied
in clinical practice.
Protein ubiquitination and degradation via the
proteasome, and localization or interaction with other proteins,
have an important role in oncogenic signaling (6). A crucial step in ubiquitination is
the cooperation with the ubiquitin-activating enzyme E1, the
ubiquitin-conjugating enzyme E2 and the ubiquitin ligase E3.
Several cellular processes implicated in cancer progression, such
as cell-cycle progression, receptor downregulation, apoptosis and
gene transcription, are regulated by the ubiquitination process
(7). Therefore, the dysregulated
ubiquitination processes may lead to mechanisms of anticancer agent
resistance, such as E3 ubiquitin ligases contributing to the
resistance mechanism by modulating pluripotent cancer stem cells
(26). A previous study also
revealed that E3-ligase Skp2 is able to regulate the cancer stem
cell pool and predict poor prognosis in NPC (27).
The ubiquitination process conducted by UBE2B, which
is highly conserved in eukaryotic cells, has several important
cellular mechanisms, such as facilitating DNA methyltransferase
DNMT3a ubiquitination and leading to gene promotor demethylation
(28). In cancer cells, MGMT is a
DNA repair enzyme that is able to disrupt the cytotoxicity of
alkylating agents. The results of a previous study by our group
demonstrated that UBE2B cooperates with RAD18 to ubiquitinate MGMT,
which is then degraded by the proteasome (8). Immunofluorescence studies
demonstrated the co-localization of MGMT and UBE2B with a greater
entry into nuclei in 1,3-bis(2-chloroethyl)-1-nitrosourea
(BCNU)-treated NPC cancer cells, supporting the interaction
hypothesis. Of note, a previous study by our group suggested that
UBE2B downregulation reduced MGMT ubiquitination but led to
increased BCNU cytotoxicity (8).
The deactivated MGMT protein, caused by BCNU or established by MGMT
mutant mimics, was accumulated in cancer cells through
UBE2B-mediated ubiquitination. These deactivated MGMT proteins may
induce cell stress and lead to cell death (8). It was therefore hypothesized that
higher UBE2B expression may be a prognostic factor for poor
clinical prognosis in patients with NPC.
The present study confirmed that ubiquitination
mediated by UBE2B is an important biological process in NPC.
According to two public datasets, tumor tissues from patients with
NPC had higher UBE2B mRNA expression levels than healthy
nasopharyngeal tissues. Furthermore, in vitro assays
suggested that UBE2B expression levels in NPC cells were higher
than those in oral epithelial cells. The results of the clonogenic
assay demonstrated that the cell growth ability was decreased in
UBE2B-deficient NPC cells as compared with UBE2B-proficient cells.
These findings support the role of UBE2B in NPC tumorigenesis. In
the present NPC cohort of Chi-Mei Medical Center, no difference in
important clinicopathological variables and UBE2B expression levels
was observed between the high and low UBE2B expression groups.
However, higher UBE2B expression was associated with poor DSS,
DMeFS and LRFS. The results of the present survival analyses also
support the role of higher UBE2B expression in regulating
resistance to alkylating agents, such as cisplatin. Accordingly,
high UBE2B expression may be employed as a risk factor for
predicting poor prognosis of patients with NPC, particularly those
undergoing cisplatin-based chemoradiotherapy. Cisplatin belongs to
the group of alkylating chemotherapy drugs, which exert their
anticancer activity by causing extensive DNA damage in tumor cells
(29). Although several mechanisms
of action may contribute to cisplatin resistance, DNA damage
responses in tumor cells have a major role in the regulation of
cisplatin-induced cytotoxicity. Accordingly, UBE2B may be involved
in DNA damage responses in tumor cells, particularly those of cells
exposed to alkylating chemotherapy drugs. In the mechanistic study
performed in the present study, cisplatin cytotoxicity was
increased in UBE2B-depleted NPC cells; however, MGMT expression
attenuated cisplatin cytotoxicity induced by UBE2B knockdown. These
results provide evidence that UBE2B may regulate cisplatin
sensitivity by targeting MGMT activity in NPC cells. In addition to
the interaction between UBE2B and MGMT, a recent study demonstrated
that treatment with UBE2B inhibitor is able to prolong the
formation of γ-H2AX foci and recruitment of DNA repair proteins,
such as 53BP1 and RAD51 (30). The
present study also indicates that UBE2B downregulation may result
in impairment of the DNA repair pathway. Taken together, high UBE2B
expression levels would therefore lead to an offset in the effect
of the alkylating agent, contribute to cisplatin resistance and
predict poor clinical prognosis as a biomarker in patients with NPC
treated with cisplatin-based chemoradiotherapy.
In the present study, clinical information and tumor
tissues of 124 Taiwanese patients with NPC were compiled, and
therefore, a relatively small sample size is the major limitation
of the present study. In addition, selection bias may hinder the
evaluation of the study results due to the retrospective nature of
this study. However, the in vitro experiments indicated the
role of UBE2B in the regulation of cisplatin cytotoxicity via MGMT
activity in NPC cells. These findings may provide novel insight
into therapeutic strategies for patients with NPC by targeting
UBE2B expression.
In conclusion, the present study suggested that high
UBE2B expression is a biological marker for poor prognosis in
patients with NPC. The results of the present study support the
role of UBE2B contributing to resistance mechanisms of alkylating
agents in tumor cells, particularly NPC. Further research is an
unmet requirement to overcome cisplatin resistance in patients with
NPC and high UBE2B expression levels.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors are grateful for the pTRE2hyg-MGMT
plasmid provided by Professor Jang-Yang Chang (Institute of
Biotechnology and Pharmaceutical Research, National Health Research
Institutes, Miaoli, Taiwan).
Funding
This work was supported by grants from Chi Mei Medical Center,
Liouying (grant nos. CLFHR10806 and CLFHR10929) and the Ministry of
Science and Technology, Taiwan (grant no. MOST
109-2314-B-384-009).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
WCK, SHC and SYH designed and conceived the study.
SHH and CLL performed the experiments. CLL, CYL, SWC, YXC and CHC
contributed the resources. CYL, SWC, YXC and CHC collected and
analyzed the clinical data. SWL and WTH interpreted the
experimental data. CJT performed statistical analyses. CJT, SHC and
SYH confirmed the authenticity of all the raw data. WCK and SYH
wrote the manuscript. CJT, WTH and SHC revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Chi Mei Medical Center in Taiwan (approval no. 10710-L01). Informed
consent was obtained from all subjects involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors indicate that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
|
MGMT
|
O6-methylguanine-DNA
methyltransferase
|
|
UBE2B
|
ubiquitin-conjugating E2 enzyme B
|
|
BCNU
|
1,3-bis(2-chloroethyl)-1-nitrosourea
|
|
DSS
|
disease-specific survival
|
|
DMeFS
|
distant metastasis-free survival
|
|
LRFS
|
local recurrence-free survival
|
|
RFS
|
recurrence-free survival
|
|
OS
|
overall survival
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen YP, Chan ATC, Le QT, Blanchard P, Sun
Y and Ma J: Nasopharyngeal carcinoma. Lancet. 394:64–80. 2019.
View Article : Google Scholar
|
|
3
|
Huang CY, Chang WS, Tsai CW, Hsia TC, Shen
TC, Bau DT and Shui HA: The contribution of interleukin-8 genotypes
and expression to nasopharyngeal cancer susceptibility in Taiwan.
Medicine (Baltimore). 97:e121352018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB,
Sun Y, Li WX, Chen YY, Xie FY, Liang SB, et al: Adjuvant
chemotherapy in patients with locoregionally advanced
nasopharyngeal carcinoma: Long-term results of a phase 3
multicentre randomised controlled trial. Eur J Cancer. 75:150–158.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hershko A and Ciechanover A: The ubiquitin
system. Annu Rev Biochem. 67:425–479. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mansour MA: Ubiquitination: Friend and foe
in cancer. Int J Biochem Cell Biol. 101:80–93. 2018. View Article : Google Scholar
|
|
7
|
Hoeller D and Dikic I: Targeting the
ubiquitin system in cancer therapy. Nature. 458:438–444. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu SH, Chen SH, Kuo CC and Chang JY:
Ubiquitin-conjugating enzyme E2 B regulates the ubiquitination of
O6-methylguanine-DNA methyltransferase and BCNU sensitivity in
human nasopharyngeal carcinoma cells. Biochem Pharmacol.
158:327–338. 2018. View Article : Google Scholar
|
|
9
|
Chen SH, Huang WT, Kao WC, Hsiao SY, Pan
HY, Fang CW, Shiue YL, Chou CL and Li CF: O6-methylguanine-DNA
methyltransferase modulates cisplatin-induced DNA double-strand
breaks by targeting the homologous recombination pathway in
nasopharyngeal carcinoma. J Biomed Sci. 28:22021. View Article : Google Scholar
|
|
10
|
Sengupta S, den Boon JA, Chen IH, Newton
MA, Dahl DB, Chen M, Cheng YJ, Westra WH, Chen CJ, Hildesheim A, et
al: Genome-wide expression profiling reveals EBV-associated
inhibition of MHC class I expression in nasopharyngeal carcinoma.
Cancer Res. 66:7999–8006. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thompson L: World health organization
classification of tumours: Pathology and genetics of head and neck
tumours. Ear Nose Throat J. 85:742006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edge SB and Compton CC: The American Joint
Committee on cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar
|
|
13
|
Lee YY, Chao TB, Sheu MJ, Tian YF, Chen
TJ, Lee SW, He HL, Chang IW, Hsing CH, Lin CY and Li CF: Glutamate
Decarboxylase 1 overexpression as a poor prognostic factor in
patients with nasopharyngeal carcinoma. J Cancer. 7:1716–1723.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wolden SL, Zelefsky MJ, Kraus DH,
Rosenzweig KE, Chong LM, Shaha AR, Zhang H, Harrison LB, Shah JP
and Pfister DG: Accelerated concomitant boost radiotherapy and
chemotherapy for advanced nasopharyngeal carcinoma. J Clin Oncol.
19:1105–1110. 2001. View Article : Google Scholar
|
|
15
|
Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS
and Wang WY: Phase III study of concurrent chemoradiotherapy versus
radiotherapy alone for advanced nasopharyngeal carcinoma: Positive
effect on overall and progression-free survival. J Clin Oncol.
21:631–637. 2003. View Article : Google Scholar
|
|
16
|
Kuo CC, Liu JF and Chang JY: DNA repair
enzyme, O6-methylguanine DNA methyltransferase, modulates
cytotoxicity of camptothecin-derived topoisomerase I inhibitors. J
Pharmacol Exp Ther. 316:946–954. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang LL, Chen WQ, Xue WQ, He YQ, Zheng RS,
Zeng YX and Jia WH: Global trends in incidence and mortality of
nasopharyngeal carcinoma. Cancer Lett. 374:22–30. 2016. View Article : Google Scholar
|
|
18
|
Lee AW, Foo W, Mang O, Sze WM, Chappell R,
Lau WH and Ko WM: Changing epidemiology of nasopharyngeal carcinoma
in Hong Kong over a 20-year period (1980–99): An encouraging
reduction in both incidence and mortality. Int J Cancer.
103:680–685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin DC, Meng X, Hazawa M, Nagata Y, Varela
AM, Xu L, Sato Y, Liu LZ, Ding LW, Sharma A, et al: The genomic
landscape of nasopharyngeal carcinoma. Nat Genet. 46:866–871. 2014.
View Article : Google Scholar
|
|
20
|
Zheng H, Dai W, Cheung AK, Ko JM, Kan R,
Wong BW, Leong MM, Deng M, Kwok TC, Chan JY, et al: Whole-exome
sequencing identifies multiple loss-of-function mutations of NF-κB
pathway regulators in nasopharyngeal carcinoma. Proc Natl Acad Sci
USA. 113:11283–11288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li YY, Chung GT, Lui VW, To KF, Ma BB,
Chow C, Woo JK, Yip KY, Seo J, Hui EP, et al: Exome and genome
sequencing of nasopharynx cancer identifies NF-κB pathway
activating mutations. Nat Commun. 8:141212017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yee-Lin V, Pooi-Fong W and Soo-Beng AK:
Nutlin-3, A p53-Mdm2 antagonist for nasopharyngeal carcinoma
treatment. Mini Rev Med Chem. 18:173–183. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong Q, Liu ZH, Lin ZR, Hu ZD, Yuan L,
Liu YM, Zhou AJ, Xu LH, Hu LJ, Wang ZF, et al: The RARS-MAD1L1
fusion gene induces cancer stem cell-like properties and
therapeutic resistance in nasopharyngeal carcinoma. Clin Cancer
Res. 24:659–673. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu H, Zeng L, Guan Y, Feng X, Zhu Y, Lu Y,
Shi C, Chen S, Xia J, Guo J, et al: High NEK2 confers to poor
prognosis and contributes to cisplatin-based chemotherapy
resistance in nasopharyngeal carcinoma. J Cell Biochem.
120:3547–3558. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bissey PA, Law JH, Bruce JP, Shi W,
Renoult A, Chua MLK, Yip KW and Liu FF: Dysregulation of the
MiR-449b target TGFBI alters the TGFβ pathway to induce cisplatin
resistance in nasopharyngeal carcinoma. Oncogenesis. 7:402018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gallo LH, Ko J and Donoghue DJ: The
importance of regulatory ubiquitination in cancer and metastasis.
Cell Cycle. 16:634–648. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Huang Y, Guan Z, Zhang JL, Su HK,
Zhang W, Yue CF, Yan M, Guan S and Liu QQ: E3-ligase Skp2 predicts
poor prognosis and maintains cancer stem cell pool in
nasopharyngeal carcinoma. Oncotarget. 5:5591–5601. 2014. View Article : Google Scholar
|
|
28
|
Chen ZG, Wang YJ, Chen RS, Geng F, Gan CL,
Wang WS, Liu X, Zhou H, He L, Hu G and Liu JG: Ube2b-dependent
degradation of DNMT3a relieves a transcriptional brake on
opiate-induced synaptic and behavioral plasticity. Mol Psychiatry.
26:1162–1177. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nagy Á, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep. 8:92272018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang WL, Luo CW, Chou CL, Yang CC, Chen
TJ, Li CF and Pan MR: High expression of UBE2B as a poor prognosis
factor in patients with rectal cancer following chemoradiotherapy.
Anticancer Res. 40:6305–6317. 2020. View Article : Google Scholar : PubMed/NCBI
|