Introduction
Multiple myeloma (MM) is characterized by an
excessive accumulation of plasma cells in the bone marrow. The
diagnosis of MM requires monoclonal immunoglobulin and bone marrow
examination or biopsy evidence (1). CD38 and CD138 are expressed by plasma
cells in myeloma (2). Studies have
found that FGF-R3 and CD138 regulate autocrine and paracrine
signals in MM and osteoprotegerin (OPG) has a role in myeloma bone
disease through the receptor activator of nuclear factor-κB (RANK)
ligand/RANK/OPG system (3–5). In addition to alkylating agents and
corticosteroids, a number of new drugs have been used to treat MM
in recent years. For example, thalidomide, bortezomib and
daratumumab belong to immunomodulator proteasome inhibitors and
monoclonal antibodies to CD38 are used for MM treatment (1,6). The
etiology of MM is remains to be elucidated.
Non-small cell lung carcinoma (NSCLC) accounts for
~80–85% of all carcinomas of the lungs (7). The pathogenesis of lung cancer
remains to be elucidated. EGFR is a common gene mutation in NSCLC.
However, drug treatment of NSCLC often leads to drug resistance
through the acquisition of the EGFR TM790 mutation in the later
stage (8,9). Hematopoietic and solid cancers have
effects on the function of T cells (10). Sporadic cases of the co-existence
of MM and NSCLC have been reported. Patients with co-existing MM
and lung cancer have a poor prognosis (11) and a standard treatment for these
patients is lacking. However, the link between MM and NSCLC remains
to be elucidated.
The present study described a case of MM that was
diagnosed with NSCLC. The findings help expand the awareness of MM
combined with NSCLC and provide a reference for strategies for
early diagnosis and treatment.
Case report
A 52-year-old man was treated in a local hospital
because of lower limb pain, swelling and weakness for more than
half a year. He developed sore limbs and weakness in the last two
months and was transferred to Hebei General Hosipital
(Shijiazhuang, China) in August 2018. Detailed physical examination
information was collected: Body temperature 36.5°C, pulse 80/min,
respiration 20/min, blood pressure 126/86 mmHg, clear
consciousness, normal skin color, no damage to mucosa and no
swelling of spleen, liver or lymph nodes. The laboratory results at
admission are presented in Table
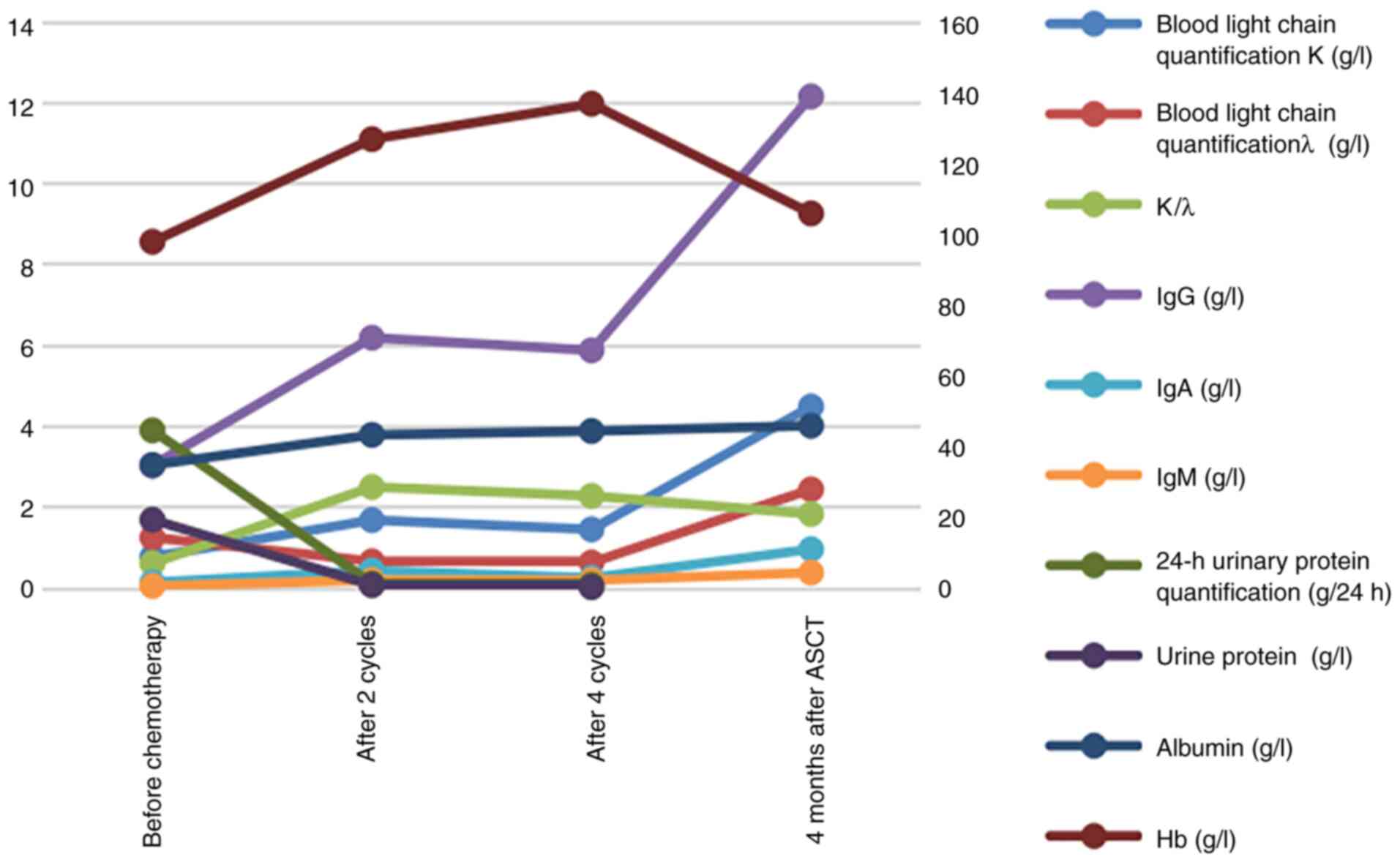
I. The levels of blood immunoglobulin (Ig) A, IgG and IgM were
lower than normal (Fig. 1).
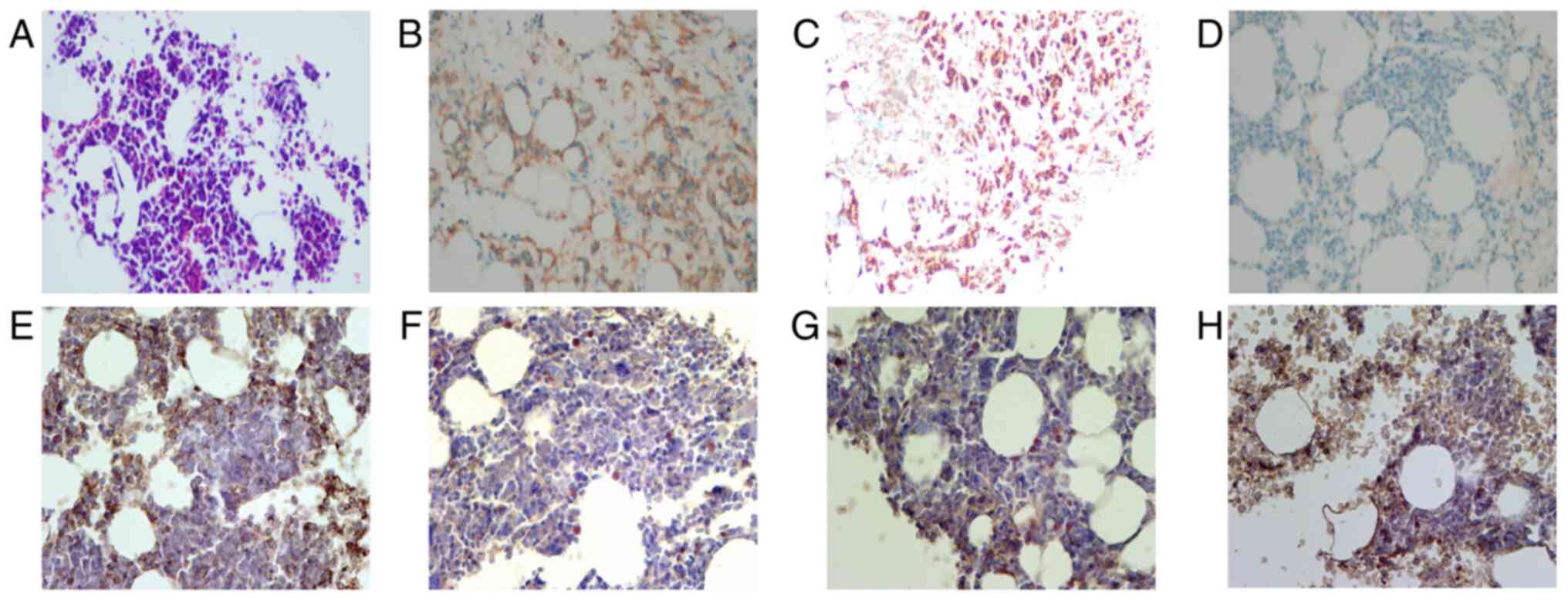
Bence-Jones protein was positive. Bone marrow biopsy confirmed
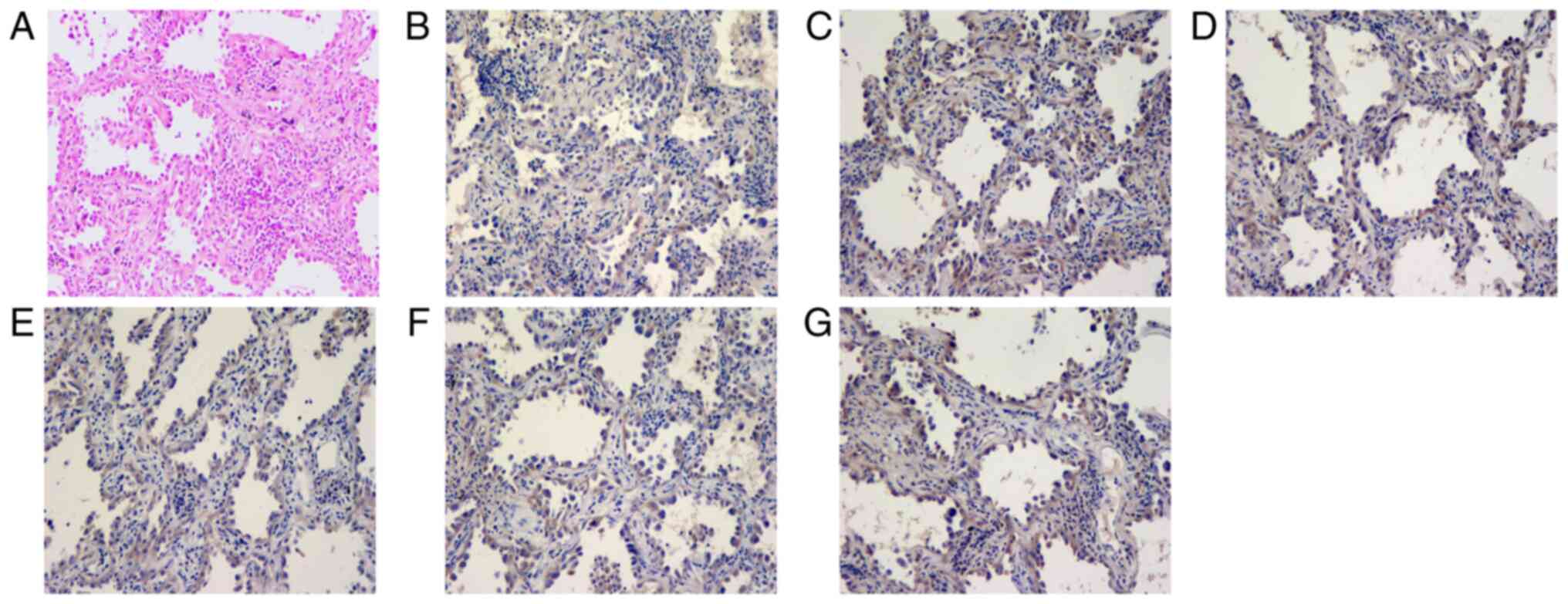
myeloma-like cells and immunostaining revealed positive staining
for CD38, CD138, CD39, CD203a, TNF-α, CD6, CD7, MPO, CD3, CD20 and
PAS and negative staining for CD34, CD73 and pan-cytokeratin
(Fig. 2). The Lambda
involvement/Kappa non-involved light chain ratio was >100.
Hematuria immunofixation by electrophoresis, blood free light chain
quantitative and urine free light chain results are shown in
Table I. Several tumor markers
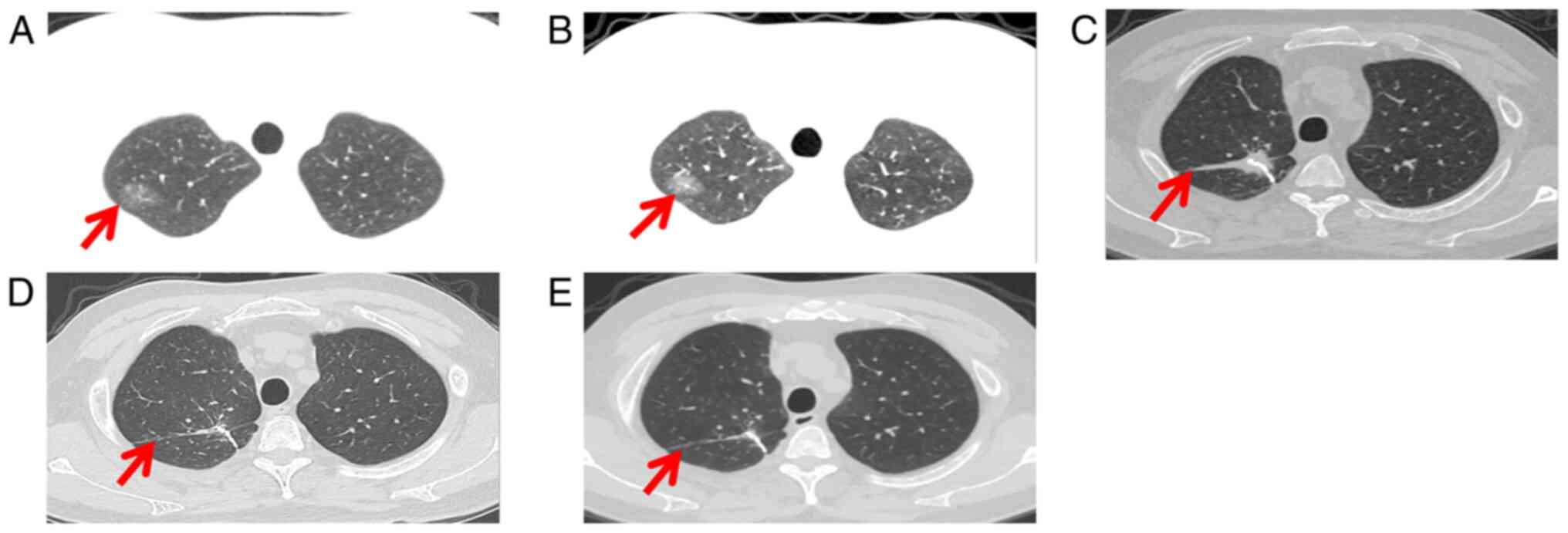
were normal. Lung computed tomography (CT) images showed ground
glass shadow in the upper lobe of the right lung (Fig. 3A). Multi-Disciplinary Treatment
analysis suggested that the lung lesions were more likely to be
malignant tumors, but the patient's family disagreed with the
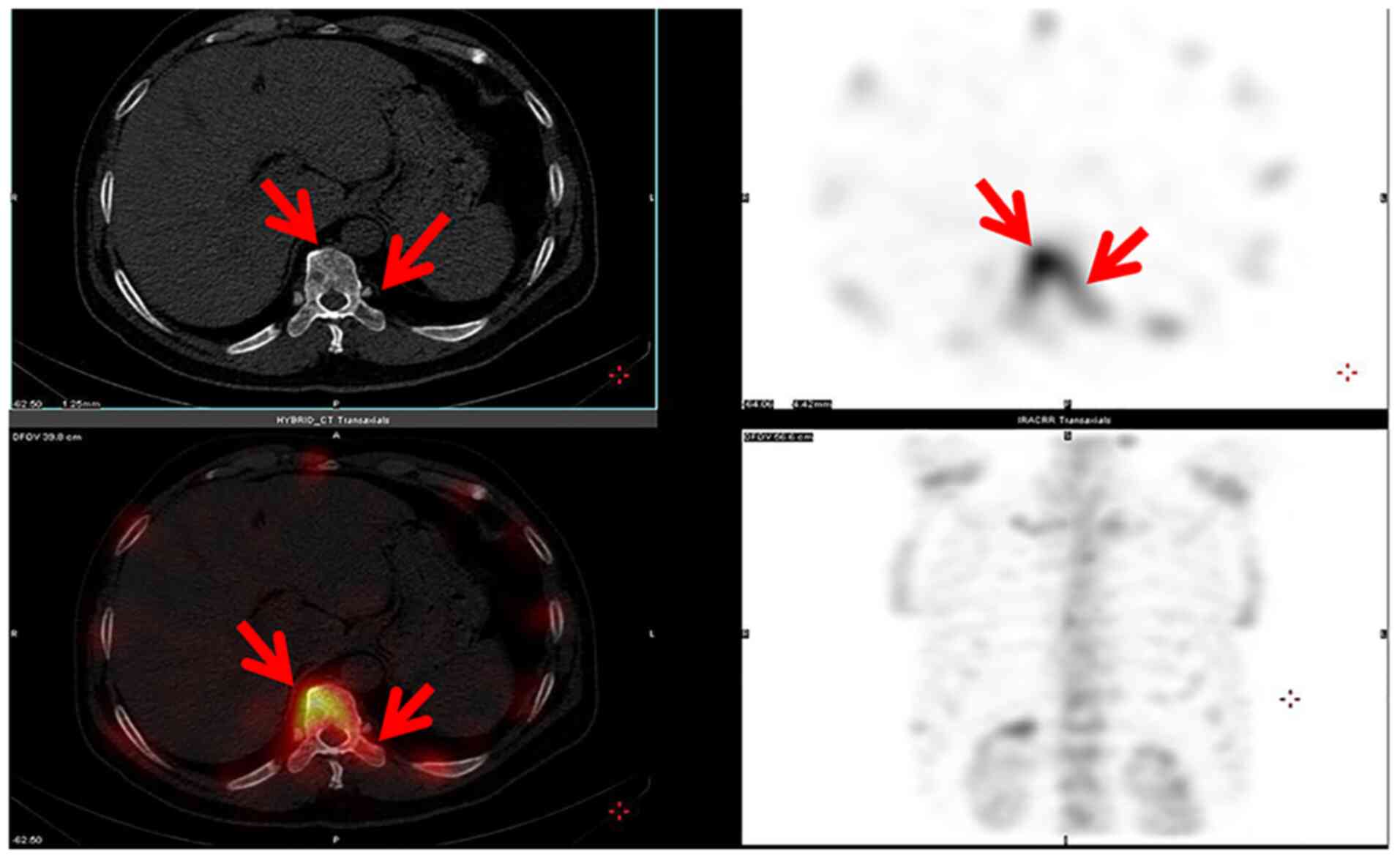
operation. Dynamic lung CT evaluation was performed. In addition,
Radionuclide Bone Scans was performed (Fig. 4). Based on the laboratory results,
the patient was diagnosed as MM (IgD-λ type).
 | Table I.Laboratory results at admission. |
Table I.
Laboratory results at admission.
| Item | Units | Value | Normal
range/limit |
|---|
| Biochemical
indicators |
|
|
|
| Serum
total protein | g/l | 56.1↓ | 65-85 |
| Serum
albumin | g/l | 34.9↓ | 40-55 |
|
Albumin | g/l | 32.5↓ | 40-55 |
|
Globulins | g/l | 20.30 | 20-40 |
| Serum
urea | mmol/l | 4.59 | 2.5-7.1 |
|
Creatinine | mmol/l | 66.48 | 53-132 |
| Uric
acid | µmol/l | 311.68 | 208-428 |
| Lactic
dehydrogenase | IU/l | 164.6 | 120-250 |
|
Potassium | mmol/l | 4.4 | 3.5-5.3 |
|
Calcium | mmol/l | 2.36 | 2.11-2.52 |
| Complete blood
count |
|
|
|
|
Hemoglobin | g/l | 98.00↓ | 130-175 |
| White
blood cell | 109/l | 5.18 | 3.5-9.5 |
|
Platelets | 109/l | 196.00 | 125-350 |
|
Erythrocyte sedimentation
rate | mm/h | 28↑ | 0-15 |
| Immunofixation
electrophoresis |
|
|
|
| IgD-λ
type M protein |
| Positive | Negative |
| Urine
protein qualitative |
| Positive | Negative |
| Blood free light
chain quantitative | mg/l |
|
|
| Free
light chain λ |
| 3,650 | 6-26 |
|
Kappa/lambda |
| 0.0018 | 0.26-1.65 |
| Urine free light
chain quantitation | mg/l |
|
|
| Free
light chain λ |
| >3,675 | <5 |
|
Kappa/lambda |
| <0.0035 |
|
The patient was treated with VDT (bortezomib 1.3
mg/m2 day 1 (D1), 4, 8, 11; dexamethasone 20 mg ivgtt
D1, 2, 4, 5, 8, 9, 11 and 12) and thalidomide (100 mg oral qd).
After one cycle, there was no significant change in lung CT. After
the second cycle of treatment, lung CT showed that the density of
ground glass shadow increased, with a size of 22×16×12 mm (Fig. 3B). The patient received surgery and
was diagnosed with stage IA lung adenocarcinoma by thoracoscopic
right pulmonary nodule resection without chemotherapy and
radiotherapy (Fig. 5). At the same
time, tumor markers were monitored (Table II). After the fifth cycle of VDT,
the patient underwent autologous stem cell transplantation and was
rechecked regularly in the outpatient department.
 | Table II.Analysis of the tumor markers. |
Table II.
Analysis of the tumor markers.
| Marker | Before
treatment | After two
cycles | After four
cycles | Three months after
ASCT | Reference
values |
|---|
| CEA, ng/ml | 1.14 | 0.930 | 1.150 | 1.510 | <5.5 |
| NSE, ng/ml | 14.49 | 14.49 | 10.100 | 14.160 | 0-15 |
| CYFRA21-1,
ng/ml | 2.11 | 2.50 | 3.640↑ | 2.950 | <3.3 |
| SCC, ng/ml | 0.798 | 1.402 | 0.84 | 1.131 | <2.5 |
| AFP, ng/ml | None | None | None | 5.250 | <7 |
| CA199, U/ml | None | None | None | 10.380 | <34 |
| CA125, U/ml | None | None | None | 36.180↑ | <35 |
| CA153, U/ml | None | None | None | 16.670 | <25 |
| TPSA, ng/ml | None | None | None | 1.590 | <4.4 |
At the time of writing, the patient showed a
beneficial therapeutic response to VDT and autologous stem cell
transplantation, After the lung lesions were resected, no invasion
was found at the cut edge of the tissue and no recurrence and new
lesions were found in postoperative dynamic lung CT evaluation.
Lung tumor markers were stable. No evidence of the lung cancer was
detected.
Discussion
MM is a genetically complex hematopoiesis
malignancy, comprising 10% of all hematological malignancies
(12). MM occurs alone as well as
simultaneously or secondary with other tumors, such as lung cancer.
When suffering from lung cancer and MM, the chronological
occurrence of the two cancers needs to be clarified. Fewer than 20
cases of MM with lung cancer have been reported so far; four of
these cases were lung adenocarcinoma cases and MM and lung cancer
occurred simultaneously in only two cases (11,13–20).
Zuo et al (17) reported a
rare case of simultaneous MM and pulmonary adenocarcinoma; the
patient was treated with bortezomib and is stable. The patient in
the present study received thalidomide and was stable. It was
hypothesized that MM and lung cancer appeared at the same time in
the current case. From the comprehensive examination of laboratory,
CT, whole-body bone scanning, immunohistochemistry and tumor marker
analyses, it was possible to clearly diagnose MM. Prior to the
diagnosis of MM, the lung CT showed ground glass shadow. At two
months following MM treatment, CT evaluation of the lung revealed
changes of the lesions; the lung lesions were removed by surgery
and the pathological diagnosis was stage IA lung adenocarcinoma
without any lesions found in other organs; thus, it was assumed
that none of the cancers were caused by metastasis of the other. In
a case reported by Marinopoulos et al (20) MM was found 11 months after
chemotherapy for lung cancer. Lin et al (15) report a case of MM diagnosed with
lung cancer 18 months after treatment. The patient in the present
study underwent systemic examination at admission and no lesions
were found in other locations. The probability of lung tumor after
only two months of chemotherapy is very low.
The pathological mechanisms of MM with lung cancer
remain unclear. EGFR gene mutation is detected in the majority of
solid tumors and also observed in MM cells (12,21).
Studies have found that MM acquires resistance to EGFR inhibitor
via induction of the pentose phosphate pathway (22–24).
Dasatinib, a tyrosine kinase inhibitor, has been shown to increase
the sensitivity of anticancer drugs in RANK (+) MM cells (25). Kaiser et al (18) questioned whether CD38 blocking of
lung tumor cells by dalamab would enhance the function of cytotoxic
T cells.
The imbalance of the immune system is a focus of
research in cancer studies. TNF-α serves an important role in the
function, differentiation and transformation of B lymphocytes in
MM, but it can also induce the apoptosis of myeloma cells (26). It is widely known that immune
imbalance and proliferation are linked with carcinogenesis, and
there is an association between the high expression of CD 38 and
the degree of damage to immune system cells (27,28).
Among the published cases of MM complicated with
lung cancer, the IgG type was dominant; one case was IgD type with
poor prognosis, there was one case each of CD38 positive and CD138
positive and one case was strongly positive for CD38 and CD138, as
shown in Table III. IgD multiple
myeloma (IgD MM) is rare, accounting for ~1–2% of myeloma cases
(29). Wang et al (19) reported a case of IgD MM complicated
with lung cancer that was treated with surgery and anti-CD38
monoclonal antibody (daratumumab); the patient achieved complete
remission. The case in the present study was also IgD MM and lung
cancer was treated surgically, but the VDT scheme was adopted,
autologous stem cell transplantation and long-term thalidomide
treatment after operation. The patient has survived for >3
years.
 | Table III.Analysis of multiple myeloma
complicated with lung cancer. |
Table III.
Analysis of multiple myeloma
complicated with lung cancer.
| First author
(year) | Diagnosis | Immunohistochemical
index | Treatment | (Refs.) |
|---|
| Ji (2004) | SCLC with MM (IgG-λ
stage I) | IgG (+), λ light
chain (+) | Radiotherapy,
carboplantin, etoposide | (13) |
| Agarwal (2008) | MM (λ stage IIIA)
with lung adenocarcinoma (stage IV) | CD38 (++), CD138
(++), CD56 (+), λ light chains (+), CD19 (−), κ light chains
(−) | Radiotherapy,
carboplatins, taxanes | (11) |
| Marinopoulos
(2008) | MM (IgG-κ) with
NSCLC | A1/A3 (+), TTF1
(+), IgG-κ (+) | Surgery, cisplatin,
docetaxel, vinorelbine, topotecan | (20) |
| Goto (2010) | SCC (stage IB) with
MM (IgG-λ) | CD38 (+), IgG (+),
λ-light chain (+) | Surgery,
dexamethasone | (16) |
| Lin (2010) | MM (IgA-λ) with SCC
(stage IA) | IgA-λ (+), p53 (−),
VEGF (±), p16 (−), CEA (−) | Cellular
immunotherapy with CIK cells | (15) |
| Zuo (2017) | MM (IgG-κ stage
IIB) with lung adenocarcinoma (stage I) | CD138 (+), IgG (+),
κ light chain (+) | surgery,
bortezomib, lenalidomide | (17) |
| Kaiser (2020) | MM with squamous
subtype non-small cell lung cancer | None | Venetoclax,
daratumumab, dexamethasone, pembrolizumab | (18) |
| Wang (2021) | MM (IgD) with lung
cancer | IgD (+) | Daratumumab,
surgery | (19) |
In the patient, the immunohistochemical staining of
CD38, CD138, CD39 and TNF-α were positive in both bone marrow and
lung lesion.CD38 is one of the prognostic factors in hematological
cancers and a high level of CD38 has been detected in other cancers
(30,31). Hogan et al (32) demonstrates a role for CD38 in
immune modulation and confirms the multifaceted role of CD38 in the
immune response in MM and lung cancer. Adenosine is important in
immune regulation. CD38 regulates extracellular adenosine, consumes
NAD+ and synthesizes adenosine through
NAD+/CD203a/CD73, similar to ATP catabolism mediated by
CD39/CD73 (32–34). Horenstein et al (35) also confirms this finding. Studies
have shown that CD133+ CXCR4+ lung cancer
stem cells evade immune monitoring by increasing the expression of
CD38 and CD73 (36,37). Gao et al (38) confirm that lung cancer cells
promote tumor progression by CD38-catalyzed cyclic ADP-ribose.
These results indicate a role for CD38 in MM with lung cancer and
provide an experimental basis for its use as a potential target. In
addition, Bu et al (39)
noted CD38 over-expression in lung cancer cells and tissues and
that knockout of the CD38 gene reduced the occurrence of tumor in
mice.
The present case expands our understanding of MM
combined with NSCLC. CD38 may serve a role in MM and lung cancer by
adenosine. Only one patient was reported in the present study and
more studies of additional patients are required. In addition,
further research is needed to explore the potential pathogenesis of
the MM with lung cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YL is the principal responsible person of the study
and substantially contributed to the conception and the design of
the study. HHD and YL were accountable for all aspects of the work
and contributed to the analysis and interpretation of the data, and
also contributed to manuscript drafting critical revisions on the
intellectual content. JL acquired and analyzed the data. LK and QW
analyzed and interpreted the data. HHD, JL, LK, QW and YL confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All procedures were approved by the ethics committee
of Hebei General Hospital (Shijiazhuang, China; approval no.
2022058). Written informed consent was obtained from the
patient.
Patient consent for publication
The publication of the article is with the informed
consent of the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rajkumar SV: Multiple myeloma: Every year
a new standard? Hematol Oncol. 37 (Suppl 1):S62–S65. 2019.
View Article : Google Scholar
|
|
2
|
Vozella F, Fazio F, Lapietra G, Petrucci
MT, Martinelli G and Cerchione C: Monoclonal antibodies in multiple
myeloma. Panminerva Med. 63:21–27. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Čolović M, Jurisic V, Bila J, Čolović N
and Palibrk V: FGF-R3 and OPG expression in patient with multiple
myeloma following systemic sclerosis: Case report and review of the
literature. Int J Hematol. 93:228–231. 2011. View Article : Google Scholar
|
|
4
|
St-Germain JR, Taylor P, Tong J, Jin LL,
Nikolic A, Stewart II, Ewing RM, Dharsee M, Li Z, Trudel S and
Moran MF: Multiple myeloma phosphotyrosine proteomic profile
associated with FGFR3 expression, ligand activation, and drug
inhibition. Proc Natl Acad Sci USA. 106:20127–20132. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deligiorgi MV, Panayiotidis MI, Griniatsos
J and Trafalis DT: Harnessing the versatile role of OPG in bone
oncology: Counterbalancing RANKL and TRAIL signaling and beyond.
Clin Exp Metastasis. 37:13–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel DS, Schiller GJ, Samaras C, Sebag
M, Berdeja J, Ganguly S, Matous J, Song K, Seet CS, Talamo G, et
al: Pomalidomide, dexamethasone, and daratumumab in relapsed
refractory multiple myeloma after lenalidomide treatment. Leukemia.
34:3286–3297. 2020. View Article : Google Scholar
|
|
7
|
Osmani L, Askin F, Gabrielson E and Li QK:
Current WHO guidelines and the critical role of immunohistochemical
markers in the subclassification of non-small cell lung carcinoma
(NSCLC): Moving from targeted therapy to immunotherapy. Semin
Cancer Biol. 52:103–109. 2018. View Article : Google Scholar
|
|
8
|
Jurišić V, Obradovic J, Pavlović S and
Djordjevic N: Epidermal growth factor receptor gene in
non-small-cell lung Cancer: The importance of promoter polymorphism
investigation. Anal Cell Pathol (Amst). 2018:61921872018.
|
|
9
|
Suda K, Onozato R, Yatabe Y and Mitsudomi
T: EGFR T790M mutation: A double role in lung cancer cell survival?
J Thorac Oncol. 4:1–4. 2009. View Article : Google Scholar
|
|
10
|
Montironi C, Muñoz-Pinedo C and Eldering
E: Hematopoietic versus Solid Cancers and T cell dysfunction:
Looking for similarities and distinctions. Cancers (Basel).
13:2842021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agarwal R, Gupta R, Bhaskar A, Sharma A,
Thulkar S and Kumar L: Synchronous presentation of multiple myeloma
and lung cancer. J Clin Oncol. 26:5814–5816. 2008. View Article : Google Scholar
|
|
12
|
Castaneda O and Baz R: Multiple myeloma
genomics-A concise review. Acta Med Acad. 48:57–67. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ji SH, Park JO, Lee J, Oh MJ, Lim DH, Park
BB, Park KW, Lee SH, Kim K, Kim WS, et al: Three cases of
synchronous solid tumor and multiple myeloma. Cancer Res Treat.
36:338–340. 2004. View Article : Google Scholar
|
|
14
|
Osipova MV and Men'shakova SN: A case of a
combination of multiple myeloma and lung cancer. Ter Arkh.
69:64–65. 1997.(In Russian). PubMed/NCBI
|
|
15
|
Lin J, Zhu H, Lu X, Yang B, Han W, Dai H
and Wang Y: Autologous cytokine-induced killer cells in the
treatment of multiple myeloma concomitant with lung cancer and
paraneoplastic dermatoses. Intern Med. 49:2341–2346. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goto T, Maeshima A, Oyamada Y and Kato R:
Definitive diagnosis of multiple myeloma from rib specimens
resected at thoracotomy in a patient with lung cancer. Interact
Cardiovasc Thorac Surg. 10:1051–1053. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zuo W, Zhu X, Yang J, Mei Z, Deng M, Lin
Q, Song Y and Yin Q: Bortezomib combined with lenalidomide as the
first-line treatment for the rare synchronous occurrence of
multiple myeloma and pulmonary adenocarcinoma: A case report.
Medicine (Baltimore). 96:e57872017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaiser MF, Croft J, Shah P, Yousaf N and
Popat S: Durable response of multiple myeloma and non-small cell
lung cancer with simultaneous, biologically targeted treatment. Br
J Haematol. 189:e1–e3. 2020. View Article : Google Scholar
|
|
19
|
Wang Z, Deng H, Zhang L, Dai Y, Li X, Lin
X, Wei J and Zou X: Daratumumab for Refractory IgD multiple myeloma
with lung cancer and persistent thrombocytopenia: A Case Report.
Clin Lab. Nov 1–2021.(Epub ahead of print). doi:
10.7754/Clin.Lab.2021.210405. View Article : Google Scholar
|
|
20
|
Marinopoulos S, Skorda L, Karatapanis S
and Rasidakis A: Multiple myeloma emerging after chemotherapy for
non-small-cell lung cancer. Med Oncol. 25:415–418. 2008. View Article : Google Scholar
|
|
21
|
Rao L, Giannico D, Leone P, Solimando AG,
Maiorano E, Caporusso C, Duda L, Tamma R, Mallamaci R, Susca N, et
al: HB-EGF-EGFR signaling in bone marrow endothelial cells mediates
angiogenesis associated with multiple myeloma. Cancers (Basel).
12:1732020. View Article : Google Scholar
|
|
22
|
Chen Y, Huang R, Ding J, Ji D, Song B,
Yuan L, Chang H and Chen G: Multiple myeloma acquires resistance to
EGFR inhibitor via induction of pentose phosphate pathway. Sci Rep.
5:99252015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang X, Ye H, He M, Zhou X, Sun N, Guo W,
Lin X, Huang H, Lin Y, Yao R and Wang H: lncRNA PDIA3P interacts
with c-Myc to regulate cell proliferation via induction of pentose
phosphate pathway in multiple myeloma. Biochem Biophys Res Commun.
498:207–213. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu R, Li W, Tao B, Wang X, Yang Z, Zhang
Y, Wang C, Liu R, Gao H, Liang J and Yang W: Tyrosine
phosphorylation activates 6-phosphogluconate dehydrogenase and
promotes tumor growth and radiation resistance. Nat Commun.
10:9912019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mashimo K, Tsubaki M, Takeda T, Asano R,
Jinushi M, Imano M, Satou T, Sakaguchi K and Nishida S:
RANKL-induced c-Src activation contributes to conventional
anti-cancer drug resistance and dasatinib overcomes this resistance
in RANK-expressing multiple myeloma cells. Clin Exp Med.
19:133–141. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jurisić V and Colović M: Correlation of
sera TNF-alpha with percentage of bone marrow plasma cells, LDH,
beta2-microglobulin, and clinical stage in multiple myeloma. Med
Oncol. 19:133–139. 2002. View Article : Google Scholar
|
|
27
|
Jurisic V, Colovic N, Konjevic G, Minic I
and Colovic M: An aggressive extramedullary cutaneous plasmacytoma
associated with extreme alterations in the innate immune system.
Onkologie. 33:113–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iqbal J, Kumar K, Sun L and Zaidi M:
Selective upregulation of the ADP-ribosyl cyclases CD38 and CD157
by TNF but not by RANK-L reveals differences in downstream
signaling. Am J Physiol Renal Physiol. 291:F557–F566. 2006.
View Article : Google Scholar
|
|
29
|
Chen J, Fang M, Chen X, Yi C, Ji J, Cheng
C, Wang M, Gu X, Sun Q and Gao C: N-glycosylation of serum proteins
for the assessment of patients with IgD multiple myeloma. BMC
Cancer. 17:8812017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Van de Donk NWCJ, Richardson PG and
Malavasi F: CD38 antibodies in multiple myeloma: Back to the
future. Blood. 131:13–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ehlerding EB, England CG, Jiang D, Graves
SA, Kang L, Lacognata S, Barnhart TE and Cai W: CD38 as a PEt
imaging target in lung cancer. Mol Pharm. 14:2400–2406. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hogan KA, Chini CCS and Chini EN: The
Multi-faceted Ecto-enzyme CD38: Roles in Immunomodulation, Cancer,
Aging, and Metabolic Diseases. Front Immunol. 10:11872019.
View Article : Google Scholar
|
|
33
|
Yang R, Elsaadi S, Misund K, Abdollahi P,
Vandsemb EN, Moen SH, Kusnierczyk A, Slupphaug G, Standal T, Waage
A, et al: Conversion of ATP to adenosine by CD39 and CD73 in
multiple myeloma can be successfully targeted together with
adenosine receptor A2A blockade. J Immunother Cancer.
8:e0006102020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Giatromanolaki A, Kouroupi M, Pouliliou S,
Mitrakas A, Hasan F, Pappa A and Koukourakis MI: Ectonucleotidase
CD73 and CD39 expression in non-small cell lung cancer relates to
hypoxia and immunosuppressive pathways. Life Sci. 259:1183892020.
View Article : Google Scholar
|
|
35
|
Horenstein AL, Bracci C, Morandi F and
Malavasi F: CD38 in adenosinergic pathways and metabolic
Re-programming in human multiple myeloma cells: In-tandem insights
from basic science to therapy. Front Immunol. 10:7602019.
View Article : Google Scholar
|
|
36
|
Fortunato O, Belisario DC, Compagno M,
Giovinazzo F, Bracci C, Pastorino U, Horenstein A, Malavasi F,
Ferracini R, Scala S, et al: CXCR4 inhibition counteracts
immunosuppressive properties of metastatic NSCLC stem cells. Front
Immunol. 11:021682020. View Article : Google Scholar
|
|
37
|
Vaisitti T, Aydin S, Rossi D, Cottino F,
Bergui L, D'Arena G, Bonello L, Horenstein AL, Brennan P, Pepper C,
et al: CD38 increases CXCL12-mediated signals and homing of chronic
lymphocytic leukemia cells. Leukemia. 24:958–969. 2010. View Article : Google Scholar
|
|
38
|
Gao L, Liu Y, Du X, Ma S, Ge M, Tang H,
Han C, Zhao X, Liu Y, Shao Y, et al: The intrinsic role and
mechanism of tumor expressed-CD38 on lung adenocarcinoma
progression. Cell Death Dis. 12:6802021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bu X, Kato J, Hong JA, Merino MJ, Schrump
DS, Lund FE and Moss J: CD38 knockout suppresses tumorigenesis in
mice and clonogenic growth of human lung cancer cells.
Carcinogenesis. 39:242–251. 2018. View Article : Google Scholar : PubMed/NCBI
|