Introduction
Cervical cancer is the third most common cancer in
women with around 0.5 million cases worldwide (1). There is an annual increase of 0.6% in
new cases (2). Approximately 76%
of recent cases occur in low-resources nations, with numbers
increasing in high-income countries (3). In Germany, there is an incidence of
2.2% of all new cases of cervical cancer in women. It belongs to
the less common malignancies in Germany. There is a marked decline
in the incidence, because of cytological screening with
Papanicolaou of 80% (4). It is
also recognized that cervical cancer is a rare long-term outcome of
persistent infection of the lower genital tract by one of about 15
high-risk HPV types. In Germany, there was the use of Papanicolaou
classification system in conventional cytology until 1975. After
that there was the muenchener classifications II, which has been
used until 2014 and then muenchener classification III. In the last
classification, there was more details and subgroups than the
muenchener classification II, like IIp (abnormal squamous cells).
Group IIp (ASC-US) was for a long time under controversial
discussions. It may be due to the irritation of cells with
inflammation or mechanical irritation, which leads to metaplasia
but it does not mean that it is dysplasia. With the beginning of
the new screening program for women in Germany for early detection
of cervical cancer, there are new controversial discussions about
the importance of this group and about the importance of
conventional cytological smears. The screening program based on the
following: all women under the age of 30 years old should only be
examined using conventional cytological examination. Women between
the age of 30 and 34 years old should be examined with the
conventional cytological examination and if the sample showed
abnormal suspicious cells, then this sample should be processed for
HPV-Test for high-risk subtypes after 6 months from the cytological
examination. Women at or above 35 years old should be examined
every 3 years with both conventional cytological examination and
HPV-HR-Test. Some researchers think that it is enough to protect
women from cervix carcinoma by examining women only with subtyping
the HPV high-risk without doing the conventional cytological
examination or immunocytochemistry. Our work is focusing on the
group IIp (ASC-US) in women at or above 35 years old, to evaluate
the importance of conventional cytological examination with the
optional use of immunocytochemistry (CINtecPlus and L1-capsid) to
evaluate the abnormal cells and grading the dysplasia, if present,
in comparison with the HPV-HR-Test.
Materials and methods
Data collection
In the Institute for Pathology and
Cytology-Schuettorf-Leer-Germany were at the beginning of 2020
until the beginning of year 2021 approximately 146.800 samples of
women above 35 years old. These samples have been processed for
both the cytological examination with Surepath-liquid
based-technique (BD) in parallel with processing for HPV-HR-Test
(BD Viper), according to advices and protocol of
BD-manufacture.
Data analysis and examination
Among these samples, there were 555 cases, which
have been subgrouped as IIp (Synonym for ASC-US in Germany) from
certified Cytological technical assistants (CTA) and certified
Cytopathologists (JJ, BT and MA). In about 135 cases (24.3%), there
was a need to perform immunocytochemistry (IC) to confirm or to
exclude the dysplasia. 112936 women (77%) have no HPV-vaccine, 296
women (0.2%) have had vaccine and only one woman in this age group
with IIp (ASC-US) has had HPV–Vaccine.
Immunocytochemistry
Immunocytochemistry has been applied in Dako-Automat
with manual kit from Roche, CINtecPlus cytology kit under advices
and protocol of the manufacturer. Immunocytochemical staining of
L1-capsid antibody was performed following manufactures instruction
(Virofem, dilution 1:10). The results of immunocytochemistry have
been subdivided into negative, suspect of positivity, positive and
technically not suitable to be judged and negative. If there is any
weakness of the immunocytochemical reaction such as weak red signal
of L1-capsid or weakness of brown signal in the cytoplasm of
CINtecPlus, we have to call it suspect of positivity. Surely
positive results are when the dysplastic cells clearly react to
L1-capsid or to CINtecPlus or to both without element of
suspicious. All immunocytochemical results were confirmed from both
the CTA and the Cytopathologist.
HPV-Test for high-risk subtypes
(HPV-HR-Test)
The results of HPV-HR-Test were subdivided into
negative, positive and technically not suitable to be judged.
Statistical analysis
Significant results will be considered if the
P-Value is <0.05. Statistics were calculated with GraphPad Prism
(GraphPad Software, Inc.) with non-parametric Kruskal-Wallis test
and Dunn's post hoc test (Prism 5-2007). Data are presented as the
mean ± SD of three experimental repeats.
Ethics statement
The approval was granted by the ethics committee
(Ethics Committee of the medical association-Hannover-Germany. An
informed consent for inclusion into the study was waived, as
patient records were anonymized and retrospectively analysed. The
samples were anonymous with respect to measurements of data
protection.
Results
Immunocytochemistry (IC)
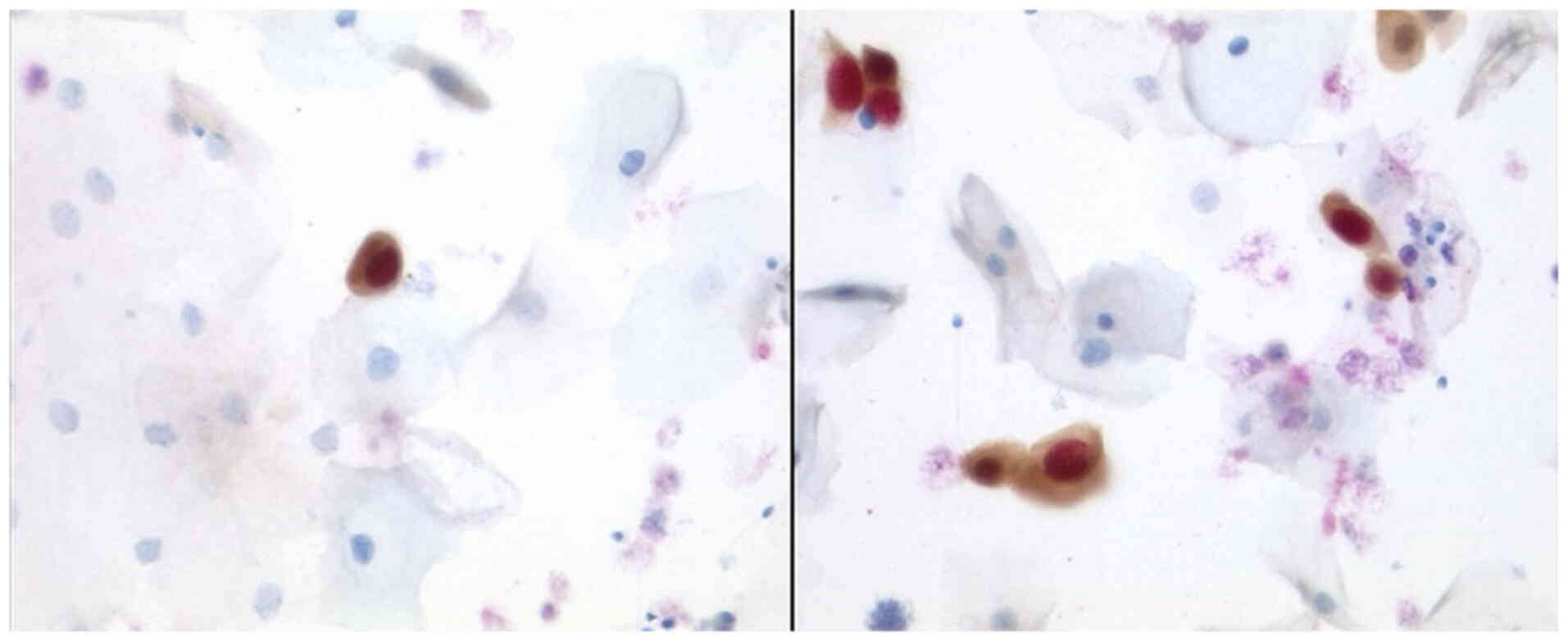
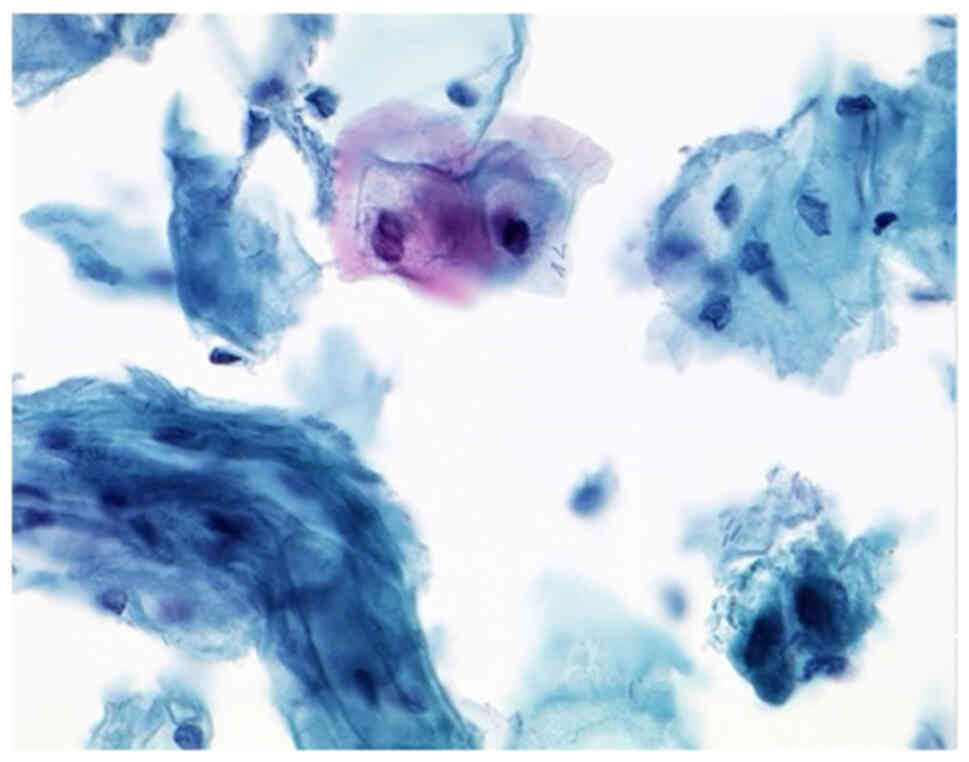
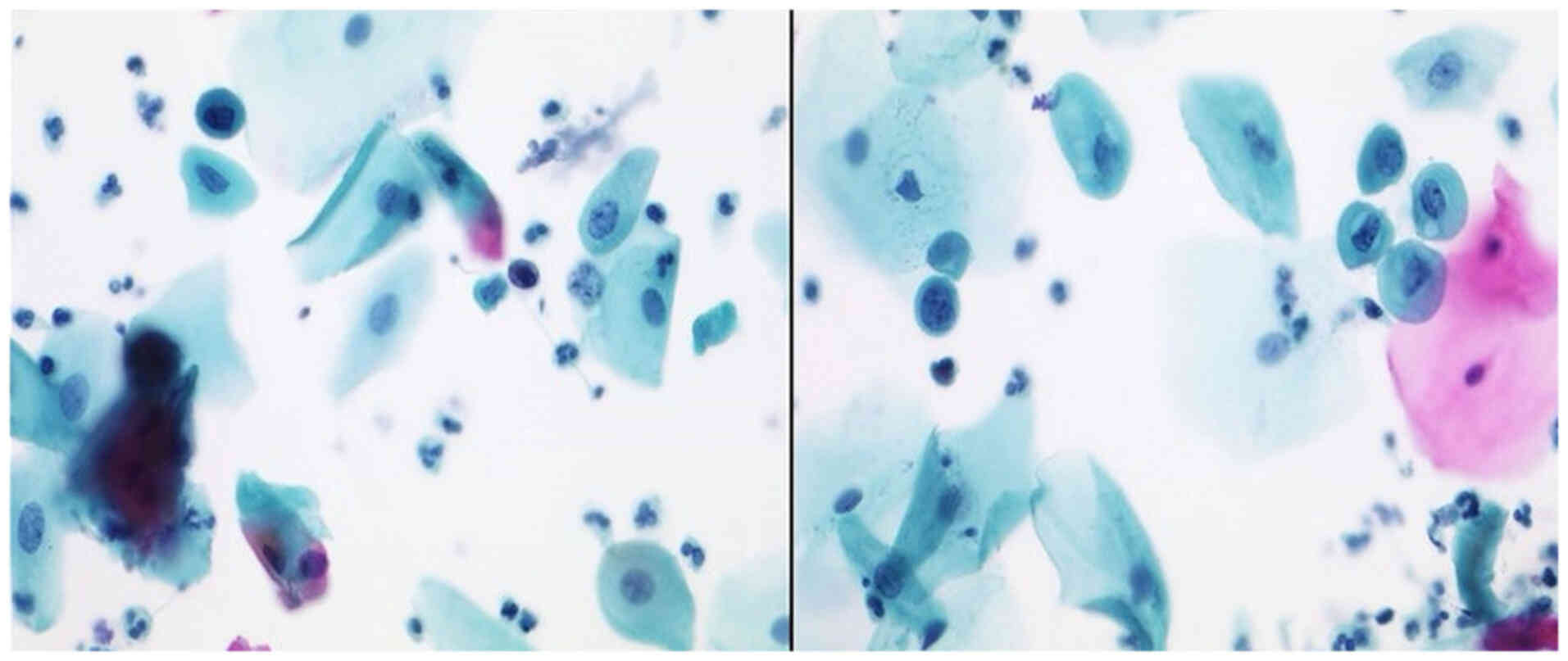
After performing the immunocytochemistry (IC) using
P16/Ki67 (CINtecPlus, Fig. 1),
there were cases of Group ASC-US (IIp, Figs. 2 and 3) with suspect of positivity in 79%,
negative in 17% and surely positive in 1.48%. Positivity in these
cases of IIp leads to sure diagnosis of IIID2 or IVa-p (highly
squamous intraepithelial lesion, HSIL). After performing the
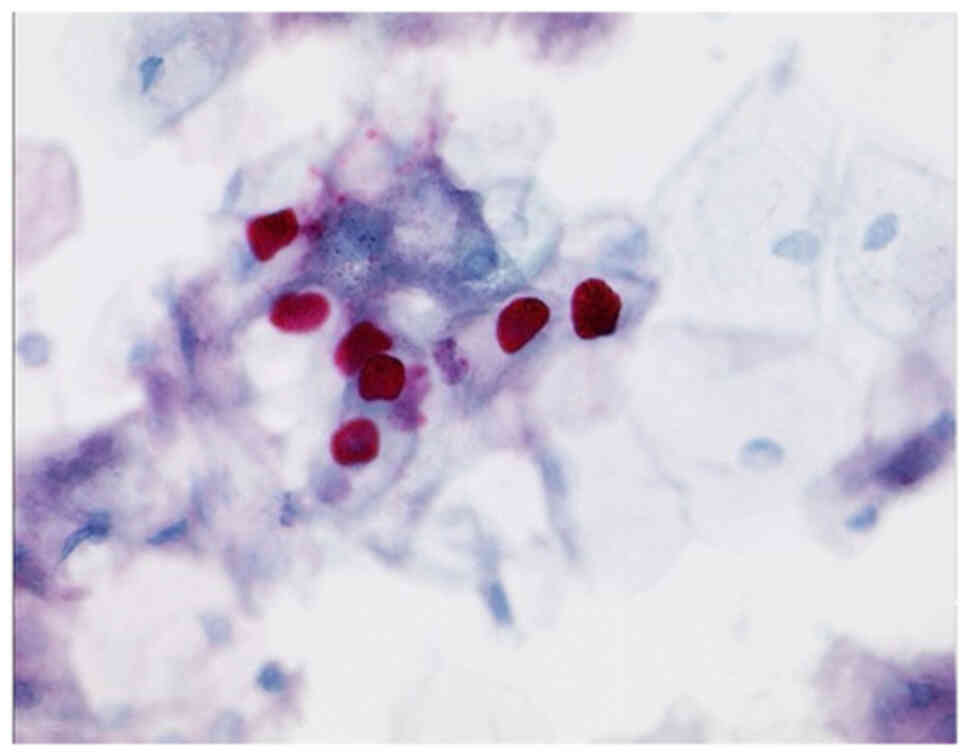
immunocytochemistry (Tables I and
II) using L1-capsid (Fig. 4; Table III), there were 95.3% negative
cases, 0.7% suspect of positivity and 3% surely positive.
Approximately 2.2% of the cases were due to a small number of cells
or due to technical problems unsuitable to be judged.
 | Table I.Results of IC after performing
CINtecPlus and L1-capsid in cases of ASC-US (IIp). |
Table I.
Results of IC after performing
CINtecPlus and L1-capsid in cases of ASC-US (IIp).
| ASC-US (IIp)/IC | Suspicion of
positivity, n (%) | Negative, n (%) | Positive, n (%) | Technically not
analyzable, n (%) |
|---|
| CINtecPlus | 105 (77.70) | 20 (15.30) | 2 (1.48) | 8 (5.52) |
| L1-capsid | 1 (0.77) | 124 (95.38) | 4 (3.08) | 1 (0.77) |
 | Table II.Association between HPV 16 and 18
results and the results of histopathology after colposcopy. |
Table II.
Association between HPV 16 and 18
results and the results of histopathology after colposcopy.
| HPV16 and
18/histopathology | Without biopsy, n
(%) | Without dysplasia, n
(%) | CIN I, n (%) | CIN II, n (%) | CIN III, n (%) | Clinically without
dysplasia |
|---|
| HPV-16-positive cases
(n=19/147; 12.9%) | 12 (63.30) | 2 (10.50) | 0 (0.00) | 2 (10.50) | 1 (5.20) | 2 (10.50) |
| HPV-16-negative cases
(n=22/147; 14.9%) | 15 (68.40) | 2 (9.00) | 1 (4.50) | 3 (13.60) | 0 (0.00) | 1 (4.50) |
| HPV-18-positive cases
(n=1/147; 0.7%) | 1 (100.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| HPV-18-negative cases
(n=21/147; 14.6%) | 14 (66.90) | 2 (9.50) | 1 (4.70) | 3 (14.20) | 0 (0.00) | 1 (4.70) |
| HPV-16 and
18-negative cases (n=107/555; 19.2%) | 49 (54.54) | 25 (23.30) | 7 (6.45) | 9 (8.25) | 6 (5.60) | 2 (1.86) |
 | Table III.Association between results of
L1-capsid and results of histopathology after colposcopy. |
Table III.
Association between results of
L1-capsid and results of histopathology after colposcopy.
| L1-capsid | Without biopsy, n
(%) | Without dysplasia, n
(%) | CIN I, n (%) | CIN II, n (%) | CIN III, n (%) | Clinically without
dysplasia, n (%) |
|---|
| Suspicion of
positivity | 1 (100.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Negative | 112 (90.40) | 5 (4.00) | 0 (0.00) | 2 (1.60) | 2 (1.60) | 3 (2.40) |
| Positive | 2 (50.00) | 0 (0.00) | 1 (25.00) | 1 (25.00) | 0 (0.00) | 0 (0.00) |
HPV-Test for high-risk subtypes
All cases were parallel processed to HPV-HR-Test.
41.6% were negative for HPV-HR, 26.4% were positive and
approximately 31.8% were unsuitable to be judged. As a rule of this
screening, the cases with IIp and negative for HPV-HR, histological
biopsy should not be done, although 33.7% of these cases were
immunocytochemically evaluated as suspect of positivity and
approximately 0.5% were surely positive (Table IV). In cases with IIp and
positivity of HPV-HR, there were 12.9% positive for HPV-16 and 0.7%
for HPV-18. In cases with HPV-16-positivity, there was sure
histological diagnosis of CINII and CINIII (HSIL) in 15.7%. In
cases of HPV-16-negativity, there was histological diagnosis of
HSIL in 13.6%. There was interestingly approximately 18.1% of these
cases (IIp and negative HPV-16) with surely histological diagnosis
of CINI (LSIL), CINII and III (HSIL). In cases with
HPV-18-negativity and IIp, there were 21 cases (14.6%). 14.2% of
them with HSIL and 18.9% of them with LSIL and HSIL. There were 107
cases (19.2%) in this group of cases (ASC-US) with negativity of
both HPV-16 and HPV-18. After performing the colposcopy and biopsy,
there were 6.5% with CIN I, 8.4% with CIN II and 5.6% with CIN III
(Table II).
 | Table IV.Association between results of
CINtecPlus and results of histopathology after colposcopy. |
Table IV.
Association between results of
CINtecPlus and results of histopathology after colposcopy.
| CINtecPlus | Without biopsy, n
(%) | Without dysplasia, n
(%) | CIN I, n (%) | CIN II, n (%) | CIN III, n (%) | Clinically without
dysplasia, n (%) |
|---|
| Suspicion of
positivity | 94 (89.67) | 5 (4.70) | 0 (0.00) | 3 (2.80) | 1 (0.93) | 2 (1.90) |
| Positive | 1 (50.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (50.00) | 0 (0.00) |
| Negative | 20 (91.40) | 0 (0.00) | 1 (4.30) | 0 (0.00) | 0 (0.00) | 1 (4.30) |
Statistical analysis
There was no significant difference (P=0.3679)
between the frequency of a HSIL diagnosis between the
HPV-16-positive and HPV-16-negative cases. Statistics were
calculated with GraphPad Prism with Kruskal-Wallis test (Prism
5-2007). Significant results will be considered if the P-Value is
<0.05.
Discussion
After applying the Munich nomenclature III in
Germany in 2015, there were annually approximately 0.59% of
patients with group IIp (ASC-US) as reported Hilal Z. and
colleagues in 2015 (5). In our
work, we have only 0.37% of IIp in approximately 146.800 cases. In
the literature so far, there is no study with this number of cases
focusing on group IIp (ASC-US). This little number of cases with
group IIp in our collection may be due to the extensive training of
our certified CTAs and our Cytopathologists and after adding the
immunocytochemistry other research groups like Rokita et al, 2012
(6) and Wentzensen et al, 2012
(7) as well as Dupin et al, 2015
(8) have added the
immunocytochemistry (CINtecPlus). They have reported respectively
increased sensitivity to detect dysplasia in the cervix or anus of
78, 92.3 and 64%. In our collection, there is approximately 1.48%
positivity for CINtecPlus, which means approximately 3.7% detection
of high-grade lesions (CINII and CINIII). If we added the cases
with assumed (suspect of) positivity, we will get approximately
80.5%. Up to date we do not find research groups that have
investigated L1-capsid in the group IIp. In our collection, there
are four (3%) definitely positive cases, which confirms the
diagnosis of CINI.
The sensitivity of HPV-subtyping to detect dysplasia
was approximately 87.2% in the work of Gilani et al, 2014 (9), and approximately 84.1% in Pichon et
al, 2019 (10). In our work, there
is HPV-HR-positivity in 26.4% in Group IIP. We have also focused on
the HPV-HR-subtypes 16 and 18 in the cases of ASC-US. 12.9% of
these cases were positive for HPV-16 and 0.7% were positive for
HPV-18. We have also analyzed the HPV-HR-negative cases, which were
41.6%. We have found that in 13.6% in the cases with IIp and
HPV-16-negativity, histologically certain CINII and III (HSIL), and
22.9% with LSIL and HSIL. We have also found that 14.6% with
HPV-18-negativity, histologically certain HSIL (CIN II) and
approximately 19.5% with LSIL and HSIL. Interestingly, there was in
general 19.2% of these cases with negativity for both HPV-16 and
HPV-18. 14% of them were diagnosed later as HSIL [CIN II (8.4%) and
CIN III (5.6%)].
In conclusion, the results of this study with this
number of cases ensure the need of conventional cytological
examination as well as the additive immunocytochemistry in
suspicious cases of group IIp to confirm the diagnosis and to
exclude the higher dysplasia. 3.7% of the cases of IIP will have
high-grade dysplasia (HSIL). The sensitivity of conventional
cytological examination and the added immunocytochemistry was up to
80.5%. The limitation by this examination is the need of human
power (CTAs and certified cytopathologist) as well as the
continuous training. 33.7% of cases with IIp and HPV-HR-negative
were immunocytochemically evaluated as suspect of positivity and
approximately 0.5% were surely positive. The advantage of
HPV-subtyping is that machinery work with screening too much number
of cases in little time but it is not accurate in indicating the
presence of dysplasia and can be misleading, especially in negative
cases as other high- or low-risk subtypes of HPV not included in
the HPV-HR test may be present.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The dataset used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MA developed the idea of the work and study design,
interpreted the results and drafted the manuscript. OB collected
data, interpreted the results and provided final approval. IE was
involved in sample selection, collection and analysis of results.
JDJ interpreted the results and was involved in analysis of
figures. MA and OB confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The approval was granted by the ethics committee
(Ethics Committee of the medical association, Hannover, Germany).
The samples were anonymous with respect to measurements of data
protection. An informed consent for inclusion into the study was
waived, as patient records were anonymized and retrospectively
analyzed. The samples were anonymous with respect to measurements
of data protection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arbyn M, Bergeron C, Klinkhamer P,
Martin-Hirsch P, Siebers AG and Bulten J: Liquid compared with
conventional cervical cytology: A systematic review and
meta-analysis. Obstet Gynecol. 111:167–177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forouzanfar MH, Foreman KJ, Delossantos
AM, Lozano R, Lopez AD, Murray CJ and Naghavi M: Breast and
cervical cancer in 187 countries between 1980 and 2010: A
systematic analysis. Lancet. 378:1461–1484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robert Koch Institute, . Malignancy in
Germany 2007/2008. Epidemiology. 8. Berlin: 2012
|
|
5
|
Hilal Z, Tempfer C, Schiermeier S,
Reinecke J, Ruppenkamp C and Hilal Z: Progression or
regression?-strengths and weaknesses of the new munich nomenclature
III for cervix cytology. Geburtshilfe Frauenheilkd. 75:1051–1057.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rokita W, Skawiński D,
Zmelonek-Znamirowska A, Kedzia W, Karowicz-Bilińska A, Spaczyński R
and Spaczyński M: Results of pap smears and immunocytochemical
detection of the p16 and Ki67 proteins in women with cervical
intraepithelial neoplasia and cervical cancer. Ginekol Pol.
83:822–826. 2012.(In Polish). PubMed/NCBI
|
|
7
|
Wentzensen N, Schwartz L, Zuna RE, Smith
K, Mathews C, Gold MA, Allen RA, Zhang R, Dunn ST, Walker JL and
Schiffman M: Performance of p16/Ki-67 immunostaining to detect
cervical cancer precursors in a colposcopy referral population.
Clin Cancer Res. 18:4154–4162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dupin C, Siproudhis L, Henno S, Minjolle
S, Arvieux C and Tattevin P: Use of human papillomavirus genotyping
and biomarkers for targeted screening of anal dysplasia in human
immunodeficiency virus-infected patients. Dig Liver Dis.
47:423–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gilani SM, Tashjian R and Fathallah L:
Cervical cytology with a diagnosis of atypical squamous cells,
cannot exclude high-grade squamous intraepithelial lesion (ASC-H):
A follow-up study with corresponding histology and significance of
predicting dysplasia by human papillomavirus (HPV) DNA testing.
Arch Gynecol Obstet. 289:645–648. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pichon M, Joly M, Lebreton F, Benchaïb M,
Mekki Y and Devouassoux-Shisheboran M: Evaluation of p16/Ki-67 dual
staining compared with HPV genotyping in anal cytology with
diagnosis of ASC-US for detection of high-grade anal
intraepithelial lesions. J Cytol. 36:152–156. 2019. View Article : Google Scholar : PubMed/NCBI
|