Introduction
Prostate cancer (PCa) is one of the most frequently
diagnosed malignancies and remains the second leading cause of
cancer-associated mortality in men worldwide (1). Despite recent advancements in early
diagnosis and the therapeutic treatment of PCa, the 5-year survival
rate of this malignancy remains <30% (2). Thus, it is important to determine its
underlying molecular mechanisms to identify novel diagnostic
markers and develop targeted therapies to improve the prognosis of
patients with PCa.
Long non-coding RNAs (lncRNAs) are a class of RNA
molecules >200 nucleotides in length, without protein-encoding
function (3). lncRNAs are
dysregulated in PCa and can modulate multiple physiological and
pathological processes, including cell proliferation,
differentiation, migration, invasion and drug-resistance (4–7). For
example, lncAMPC promotes metastasis and immunosuppression of PCa
via the lncAMPC/LIF/LIFR axis (8).
Furthermore, lncRNA FOXP4-AS1 can promote tumorigenesis and
progression of PCa by regulating the microRNA
(miRNA/miR)-3184-5p/FOXP4 axis (9). lncRNA625 is upregulated in PCa cells
and modulates PCa progression via the miR-432/Wnt/β-catenin
signaling pathway (10).
Recently, a newly identified lncRNA, small nucleolar
RNA host gene 3 (SNHG3), was reported to be dysregulated in
different types of cancer (11–14).
However, the functions of SNHG3 are inconsistent. SNHG3 has been
reported to act as an oncogene in colorectal cancer and glioma
(13,15); however, it acts as a tumor
suppressor in papillary thyroid carcinoma (12). This may be due to different
techniques or indicates the versatility of SNHG3. Although SNHG3
has been reported to accelerate PCa progression via the
miR-577/SMURF1 axis (14), its
molecular mechanism in PCa remains unclear.
The present study aimed to investigate the
expression and function of SNHG3 in PCa cells, and determine the
potential molecular mechanisms of SNHG3 in regulating the
phenotypes of PCa cells.
Materials and methods
Human clinical samples
A total of 30 paired PCa tissues and adjacent normal
tissues (5 cm away from tumor tissues) were collected from 30
patients during radical prostatectomy at the Affiliated Nanhai
Hospital, Southern Medical University between January 2016 and May
2019. All samples were stored at −80°C until subsequent
experimentation. The mean age of patients with PCa 62.3 years (age
range, 48–78 years) The present study was approved by the Medical
Ethics Committee of Affiliated Nanhai Hospital, Southern Medical
University (Foshan, China, IRB no. 2020-027-1 and no.
IRB-NY-2020-018) and written informed consent was provided by all
patients prior to the study start.
Bioinformatics analysis
The Cancer Genomic Atlas (TCGA, http://www.cancer.gov) prostate adenocarcinoma cohort,
comprising 492 patients, was used for the expression and survival
analyses. Briefly, 492 patients with PCa, with mRNA expression data
from TCGA, were enrolled in the present study. Among these, 52
patients had SNHG3 expression data in both PCa tumor tissues and
normal tissues. The DIANA (http://diana.imis.athena-innovation.gr/DianaTools/)
and StarBase (http://starbase.sysu.edu.cn) databases were used to
predict the target genes.
Cell culture
The human prostatic epithelial cell line (RWPE-1)
and PCa cell lines (PC-3, DU145, VCaP and LNCaP) were purchased
from the Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences. Cells were maintained in Dulbecco's Modified
Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum and
1% antibiotics (all purchased from Gibco; Thermo Fisher Scientific,
Inc.), at 37°C with 5% CO2.
Cell transfection
Cells (2×105) were seeded into 6-well
plates at a density of 30–50%. Transient transfection with 20 nM
small interfering si-SNHG3, miR-1827 mimics
(3′-UAAGUUAGAUGACGGAGU-5′), miR-1827 inhibitor
(3′-ACUCCGUCAUCUAACUUA-5′), miR-mimics-NC
(3′-AACAUGAUGUGUUUUCAUGAC-5′) or miR-inhibitor-NC
(3′-UUUGCACUGUGCAAGC-5′) was performed when cell reached 50–60%
confluence using Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.) at room temperature for 24 h, according to
the manufacturer's instructions. For overexpression, lncSNHG3 was
cloned into mammalian expression vector pcDNA3.1 (Invitrogen;
Thermo Fisher Scientific, Inc.) to construct overexpressing plasmid
pcDNA3.1- lncSNHG3. After transfection for 48 h, the transfected
cells were used for subsequent experiments. si-SNHG3 (#1 and #2),
SNHG3 overexpression plasmid, miR-1827 mimics, miR-1827 inhibitor
and their respective negative controls (si-NC for si-SNHG3, empty
vector of pcDNA3.1 for SNHG3 overexpression plasmid, miR-mimics-NC
for miR-1827 mimics and miR-inhibitor-NC for miR-1827 inhibitor)
were purchased from Guangzhou RiboBio Co., Ltd.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from tissues or PCa cells
using the TRIzol® RNA Purification kit (Invitrogen;
Thermo Fisher Scientific, Inc.) and reverse transcribed into cDNA
using PrimeScript RT Master Mix (Takara Biotechnology Co., Ltd.) at
37°C for 15 min. qPCR was subsequently performed using SYBR Premix
Ex Taq (Takara Bio, Inc.) in a Roche LightCycler ®480II
PCR instrument (Roche Diagnostics). The following primer sequences
were used for qPCR: SNHG3 forward, 5′-GGAAATAAAGCTGGGCCTCG-3′ and
reverse, 5′-AACAGAGCGACTCCATCTCC-3′; miR-1827 forward,
5′-GGTGAGGCAGTAGATTGAATCTC-3′ and reverse,
5′-CTCAACTGGTGTCGTGGAGTC-3′; GAPDH forward,
5′-AATGGGCAGCCGTTAGGAAA-3′ and reverse, 5′-GCGCCCAATACGACCAAATC-3′;
and U6 forward, 5′-TGCGGGTGCTCGCTTCGGCAGC-3′ and reverse,
5′-CCAGTGCAGGGTCCGAGGT-3′. The thermocycling conditions for qPCR
were as follows: Initial denaturation 95°C for 30 sec, 40 of cycles
of denaturation 95°C for 5 sec, annealing and extension 60°C for 30
sec and final extension 4°C for 5 min. Relative expression levels
were calculated using the 2−ΔΔCq method and U6 and GAPDH
were used as the internal reference controls (16).
Cell viability assay
The Cell Counting Kit-8 (CCK-8, Dojindo Molecular
Technologies, Inc.) assay was performed to assess cell viability,
according to the manufacturer's instructions. Transfected cells
were seeded into 96-well plates (2×103) and treated with
10 µl CCK-8 reagent at 0, 24, 48 and 72 h. After 1.5 h, the
absorbance values were measured at a wavelength of 450 nm, using a
microplate reader (Thermo Fisher Scientific, Inc.).
Wound healing assay
The wound healing assay was performed to assess cell
migration. Transfected cells were seeded into 6-well plates
(2×105). After 24 h, once the cells reached >90%
confluence, the monolayers were scratched using sterile pipette
tips. Cells were cultured in serum-free medium (DMEM, Gibco; Thermo
Fisher Scientific, Inc.) at 37°C for 12 h. The wound healing
process was observed under an inverted light microscope (Olympus
Corporation, ×20 magnification), and migration was evaluated by the
percentage of wound width that were healed.
Invasion assay
The Transwell assay was performed to assess cell
invasion, using BioCoat Matrigel Invasion Chambers (Corning, Inc.),
according to the manufacturer's instructions. Briefly, transfected
cells (2×105) in 0.2 ml serum-free DMEM were plated in
the upper chambers of Transwell plates coated with Matrigel
(Corning, Inc.) at 37°C for 30 min, while DMEM supplemented with
20% FBS was plated in the lower chambers. Following incubation for
24 h at 37°C, invasive cells in the lower chambers were fixed with
4% PFA and stained with 0.1% crystal violet for 5 min at room
temperature. Stained cells were observed under an IX71 inverted
light microscope (Olympus Corporation, ×20 magnification).
Nuclear/cytoplasmic fractionation
assay
The PARIS kit (Thermo Fisher Scientific, Inc.) was
used for nuclear/cytoplasmic fractionation, according to the
manufacturer's instructions. The expression levels of SNHG3 from
the cell nuclear and cytoplasmic fractions were detected via
RT-qPCR analysis. U6 and GAPDH served as the internal controls,
respectively.
Dual-luciferase reporter assay
Wild-type (WT) or mutant (MUT) SNHG3 sequences were
cloned and inserted into the pGL3-control vector (Promega
Corporation) to construct SNHG3-WT and SNHG3-MUT. PC-3 cells were
seeded into 24-well plates and co-transfected with SNHG3-WT or
SNHG3-MUT along with miR-1827 mimics and miR-1827 inhibitor, using
Lipofectamine® 3000 reagent for 48 h at 37°C. Following
incubation for 48 h, luciferase activities were detected using the
luciferase reporter assay system (Promega Corporation), according
to the manufacturer's instructions. Renilla luciferase
activity (Promega Corporation) was used for normalization.
RNA immunoprecipitation (RIP)
assay
The RIP assay was performed using the EZ-Magna RIP
RNA-Binding Protein Immunoprecipitation kit (MilliporeSigma),
according to the manufacturer's instructions. Cells
(5×106) which transfected with siRNAs were collected by
centrifugation at 800 × g for 5 min at 4°C and then lysed with
complete lysis buffer (cat. no. KT102-01; GZSCBio Co. Ltd.)
containing RNase (Thermo Fisher Scientific, Inc.) and protease
inhibitor cocktail (Roche Diagnostics). Subsequently, cell extracts
(100 µl) were co-immunoprecipitated with protein G Sepharose beads
(40 µl, included in the kit, according to the manufacturer's
instructions) pre-coated with Argonaute2 (Ago2) antibody (dilution,
1:150, cat. no. P10502500, Otwo Biotech Inc.) or IgG (dilution,
1:150, Sigma-Aldrich; Merck KGaA). Following incubation for 2 h at
4°C. The beads were centrifuged at 1,500 × g for 30 sec. The
co-precipitated SNHG3 was isolated from the beads using the
TRIzol® RNA Purification kit (Invitrogen; Thermo Fisher
Scientific, Inc.) and assessed via RT-qPCR analysis.
Western blotting
Total proteins of the cells were isolated using RIPA
buffer (cat. no. P0013B; Beyotime Institute of Biotechnology).
After that, a BCA protein assay kit (Beyotime Institute of
Biotechnology) was used to measure the protein concentration. Then,
40 µg proteins were loaded on the 10% sodium dodecyl
sulfate-polyacrylamide gel and separated by electrophoresis. The
proteins were transferred to polyvinylidene difluoride (PVDF)
membranes and blocked for 2 h using 5% skimmed non-fat milk (Yili)
at room temperature. Next, the corresponding primary antibodies,
cleaved PARP (CST; cat. no. 5625), N-cadherin (CST; cat. no.
13116), MMP9 (CST; cat. no. 13667), β-catenin (CST; cat. no. 8480),
Akt (CST; cat. no. 4691), phosphorylated (p-)Akt (CST; cat. no.
4060) mTOR (CST; cat. no. 2983), p-mTOR (CST; cat. no. 5536) and
GAPDH (CST; cat. no. 5174) were used to incubate the membranes at a
dilution ratio of 1:1,000 with PBST containing 5% BSA overnight at
4°C. After incubation, the membranes were treated with the
secondary antibodies (anti-rabbit, CST; cat. no. 3900; dilution
ratio of 1:5,000 with PBST containing 5% non-fat milk and 0.05%
Tween) for 1 h at room temperature. Finally, the protein bands were
developed with enhanced chemiluminescence (cat. no. 407207; EMD
Millipore; Merck KGaA) on the Tanon 4600 imaging system (Tanon
Science and Technology Co., Ltd.).
Tumor xenograft model in nude
mice
A total of 12 male BALB/c nude mice (6-weeks-old;
16–20 g; n=6 for each group) were obtained from the Chinese Academy
of Sciences. All mice were housed in cages with no more than 5
mice/cage. The room temperature was maintained at 20–25% and the
humidity was at 40–55%, with a 12 h light/dark cycle. All mice were
fed commercially available mouse food and sterilized water.
PC-3 cells transfected with si-SNHG3#1 or si-NC were
subcutaneously injected into the right flanks of mice. Tumor volume
was measured every week using the following formula: V=(tumor
length × tumor width2)/2. After 5 weeks, the mice were
euthanized via cervical dislocation, and the absence of fluctuation
in the chest cavity, breathing and heartbeat confirmed mortality.
Subsequently, RNA was isolated from the tumor to detect the
expression levels of SNHG3 and miR-1827. All animal experiments
were reviewed and approved by the Animal Welfare Committee of
Affiliated Nanhai Hospital, Southern Medical University (Foshan,
China; IRB no. 2020-027-1).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 7.0 software (GraphPad Software, Inc.) and SPSS 22.0 software
(IBM Corp.). All experiments were performed in triplicate and data
are presented as the mean ± standard error of the mean. A paired
Student's t-test was used to compare differences between tumor and
adjacent normal tissues, and an unpaired Student's t-test was used
to compare differences between the experimental and control groups.
One-way ANOVA followed by Bonferroni's post hoc test were used to
compare differences between multiple groups. Survival analysis was
performed using the Kaplan-Meier method and log-rank test.
Correlations were analyzed by Pearson's correlation test. P<0.05
was considered to indicate a statistically significant
difference.
Results
lncRNA SNHG3 is upregulated in PCa
tissues and cell lines
To evaluate SNHG3 expression in PCa, the mRNA data
of 492 patients with PCa from TCGA dataset were analyzed. The
results demonstrated that SNHG3 expression was significantly
elevated in PCa tumor tissues compared with normal tissues
(P<0.05; Fig. 1A). Kaplan-Meier
survival analysis was subsequently performed to assess the
cumulative survival time. As presented in Fig. 1B, patients with high SNHG3
expression had a shorter overall survival time than those with low
SNHG3 expression (P=0.0022). To eliminate the possibility that the
differential expression levels of SNHG3 in TCGA patients were
caused by the uneven sample size, SNHG3 expression was also
detected in 30 paired PCa tumor tissues and adjacent normal
tissues. The results confirmed that SNHG3 expression was
significantly upregulated in PCa (P<0.01; Fig. 1C). RT-qPCR analysis was performed
to detect SNHG3 expression in PCa cell lines. The results
demonstrated that SNHG3 expression was significantly upregulated in
PCa cell lines compared with the normal human prostate epithelial
cell line, RWPE-1 (P<0.05; Fig.
1D). Taken together, these results suggest that SNHG3 is an
oncogene in the tumorigenesis of PCa, and is associated with a poor
prognosis.
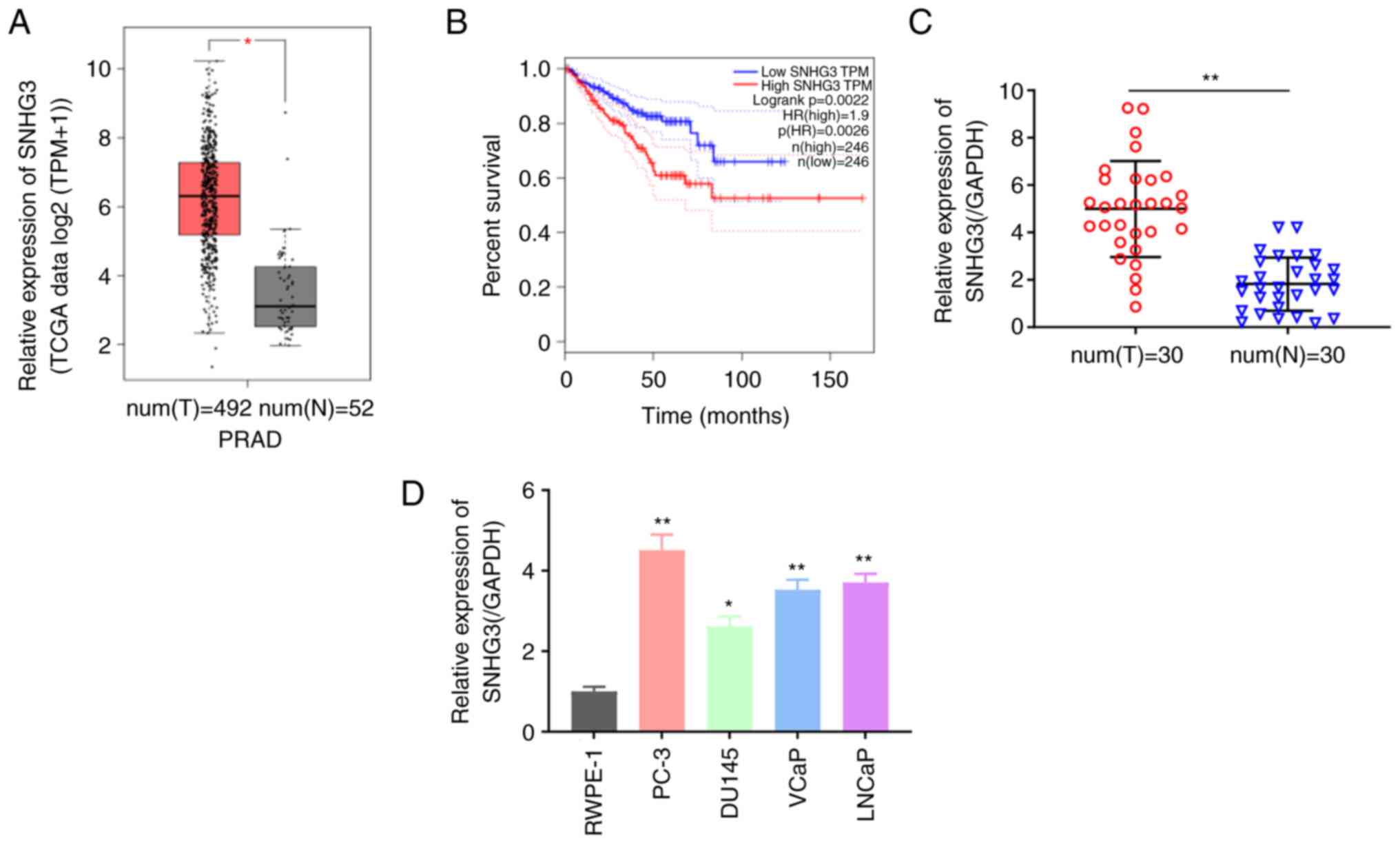 | Figure 1.lncRNA SNHG3 expression in PCa tumor
tissues and its clinical significance. (A) The expression of lncRNA
SNHG3 in PCa tumor tissues (n=492) compared with normal tissues
(n=52) in TCGA dataset. (B) Kaplan-Meier survival analysis of
patients with high (n=246) and low (n=246) SNHG3 expression levels
in TCGA dataset. (C) RT-qPCR analysis was performed to detect SNHG3
expression in PCa tumor tissues (n=30) compared with adjacent
normal tissues (n=30). (D) RT-qPCR analysis was performed to detect
SNHG3 expression in PCa cell lines (PC-3, DU145, VCaP and LNCaP)
and the human prostatic epithelial cell line, RWPE-1. Data are
presented as the mean ± standard error of the mean (n≥3).
*P<0.05, **P<0.01 vs. RWPE-1 cells. lncRNA, long non-coding
RNA; SNHG3, small nucleolar RNA host gene 3; PCa, prostate cancer;
TCGA, The Cancer Genome Atlas; RT-qPCR, reverse
transcription-quantitative PCR; T, tumor; N, normal. |
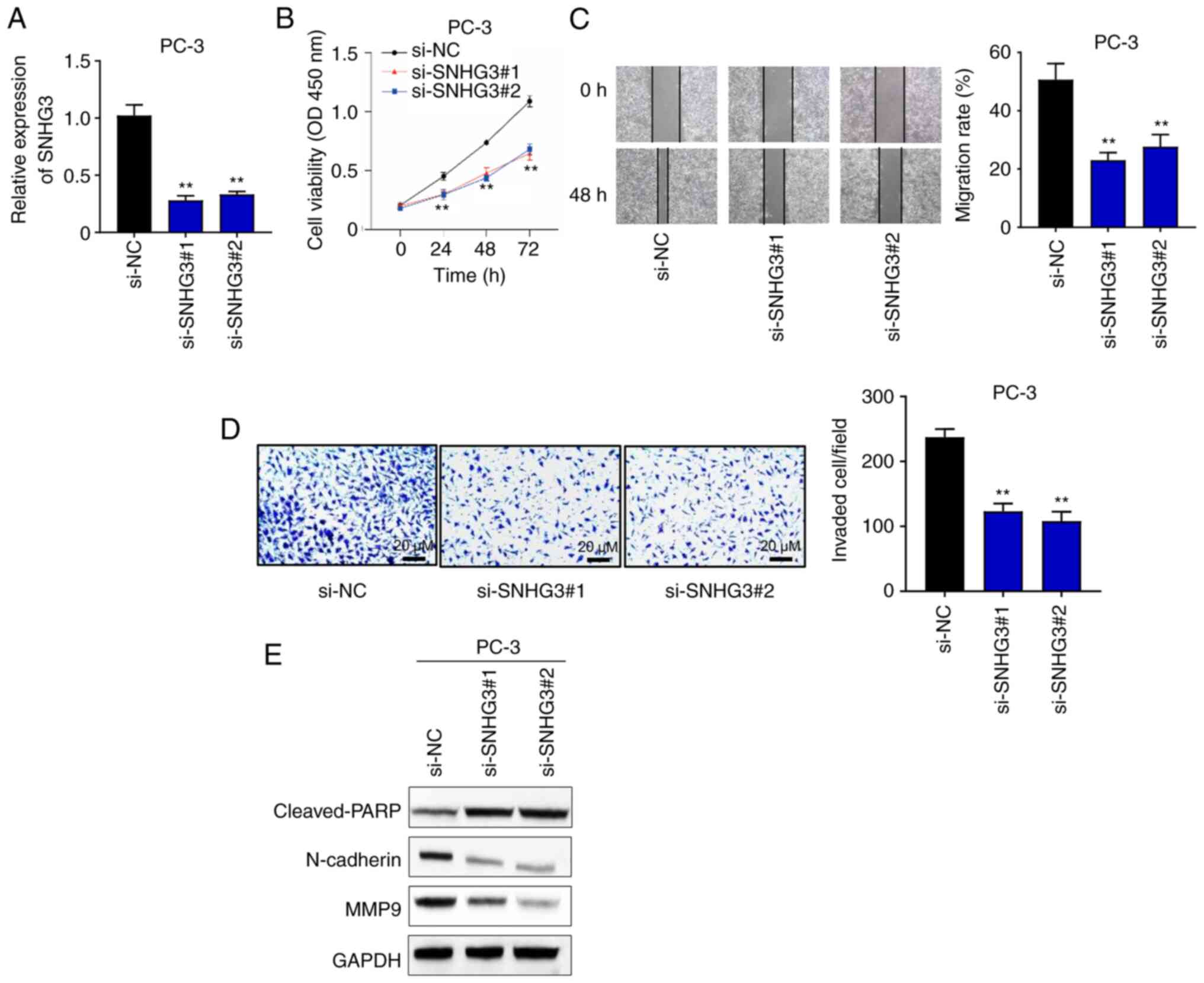
lncRNA SNHG3 knockdown inhibits PC-3
cell proliferation and migration in vitro
To further investigate the biological functions of
SNHG3 in PCa, PC-3 cells were selected for subsequent
experimentation as they express high expression levels of SNHG3
(Fig. 1D). PC-3 cells were
transfected with si-SNHG3 and cell viability was assessed via the
CCK-8 assay. The results demonstrated that SNHG3 expression
significantly decreased following transfection compared with the
control group (Fig. 2A). In
addition, SNHG3 knockdown significantly inhibited the proliferation
of PC-3 cells (Fig. 2B). The
migratory and invasive abilities of PC-3 cells following
transfection were subsequently assessed. SNHG3 knockdown
significantly suppressed the migratory and invasive abilities of
PC-3 cells (Fig. 2C and D). The
expression levels of the protein markers of apoptosis
(cleaved-PARP) and epithelial-to-mesenchymal transition [EMT;
N-cadherin and matrix metalloproteinase (MMP)9] were detected
following SNHG5 knockdown via western blotting. The results
demonstrated that cleaved-PARP expression significantly increased
following SNHG5 knockdown, which suggests that impaired cell
proliferation may be induced by cell apoptosis (Fig. 2E). Conversely, the expression
levels of N-cadherin and MMP9 decreased following SNHG5 knockdown
(Fig. 2E), suggesting that EMT
inhibits the migration of PCa cells. Collectively, these results
suggest that lncRNA SNHG3 promotes malignancy of PCa in
vitro.
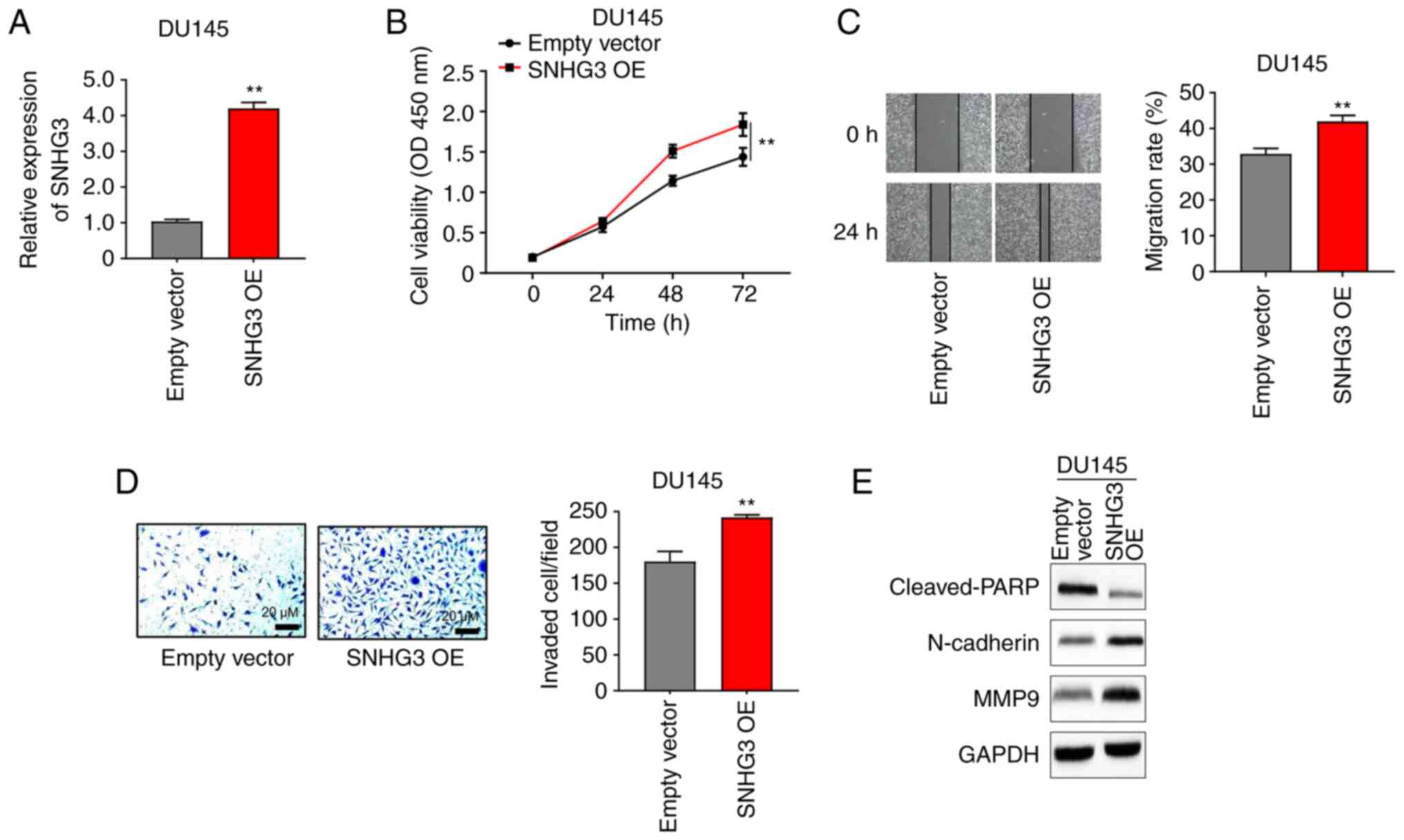
Overexpression of lncRNA SNHG3
promotes DU145 cell proliferation and migration in vitro
DU145 cells were selected for the overexpression
experiments as they express low levels of SNHG3 (Fig. 1D). SNHG3 expression was
significantly upregulated in DU145 cells following transfection
with SNHG3 overexpression plasmid (OE; Fig. 3A). In addition, transfection with
SNHG3 OE promoted the proliferation of DU145 cells (Fig. 3B). The migratory and invasive
abilities of DU145 cells were assessed following transfection with
SNHG3 OE. The results demonstrated that overexpression of SNHG3
significantly promoted the migratory and invasive abilities of
DU145 cells (Fig. 3C and D). The
expression levels of protein markers of apoptosis and EMT were also
investigated following overexpression of SNHG3. The results
demonstrated that overexpression of SNHG3 increased the protein
expression levels of N-cadherin and MMP9 but decreased cleaved-PARP
expression (Fig. 3E). Taken
together, these results suggest that overexpression of SNHG3
promotes the proliferation and EMT of PCa cells.
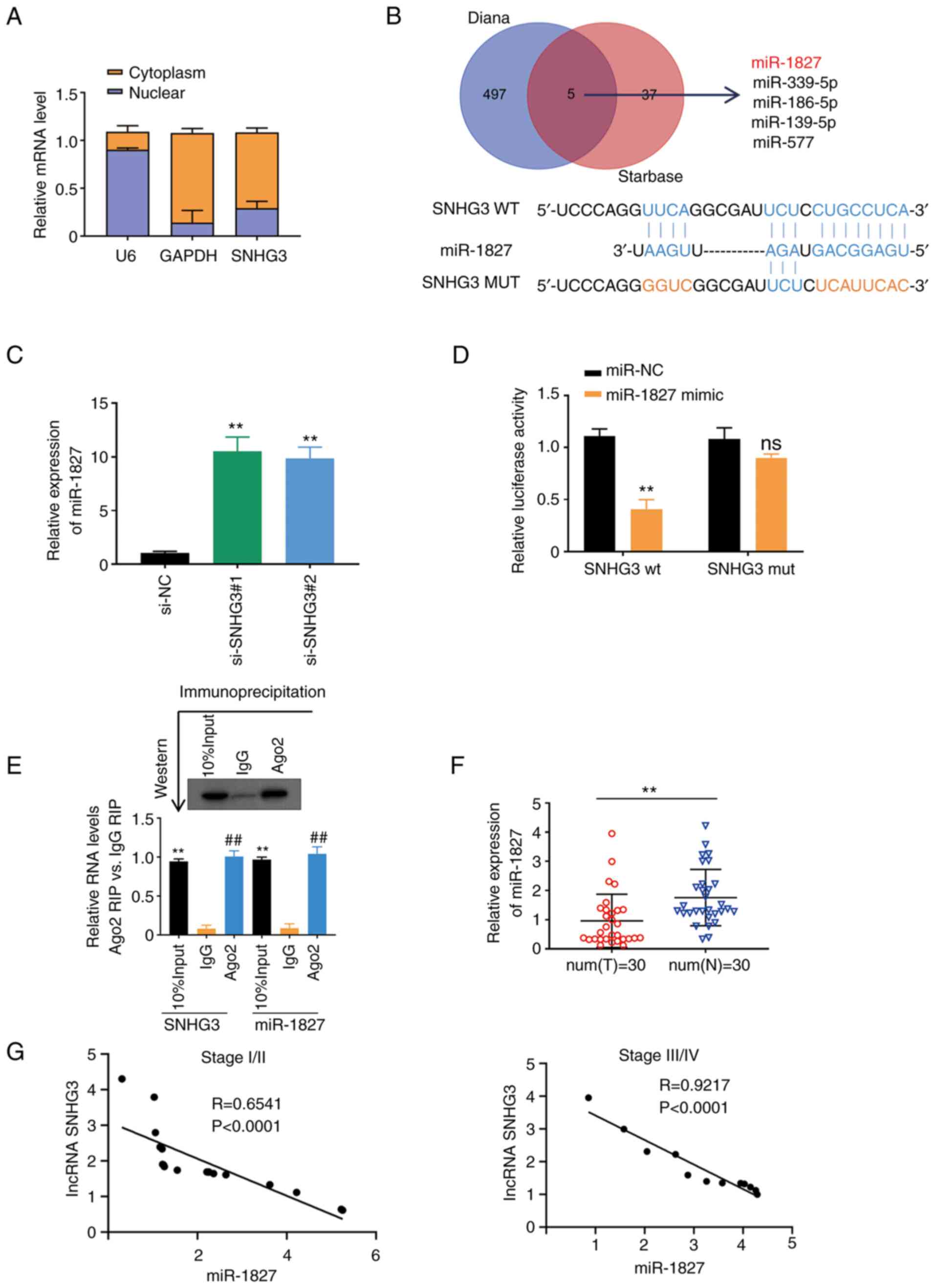
miRNA-1827 is a direct target of
lncRNA SNHG3 in PC-3 cells
Having demonstrated the association between SNHG3
and PCa progression, the present study investigated the oncogenic
mechanisms of lncRNA SNHG3. The results of the nuclear/cytoplasmic
fractionation assay demonstrated that lncRNA SNHG3 was
predominantly located in the cytoplasm of PC-3 cells (Fig. 4A). The potential interactive
targets of lncRNA SNHG3 were identified using the DIANA and
StarBase databases. A total of five overlapped downstream miRNAs
were predicted (Fig. 4B). Among
these overlapped targets, only miR-1827 expression increased
following SNHG3 knockdown in PC-3 cells (Figs. 4C and S1). The dual-luciferase reporter and RIP
assays were performed to verify the direct interaction between
SNHG3 and miR-1827. The results of the dual-luciferase reporter
assay demonstrated that overexpression of miR-1827 significantly
inhibited the relative luciferase activity of SNHG3-WT, but had no
effect on the SNHG3-MUT group (Figs.
4D), which confirms the direct association between SNHG3 and
miR-1827. The results of the RIP assay demonstrated that SNHG3 and
miR-1827 was enriched in Ago2-immunoprecipitated complexes,
confirming the interaction between SNHG3 and miR-1827 in PC-3 cells
(Fig. 4E). To further assess the
interaction between SNHG3 and miR-1827 in PC-3 cells, the following
experiments were performed. The present study first evaluated the
effect of miR-1827 on SNHG3 levels in PC3. The results demonstrated
that lncRNA SNHG3 was marginally altered following transfection
with miR-1827 mimics or miR-1827 inhibitor (Fig. S2A-S2C). The effect of SNHG3 on
miR-1827 in MCF-7 cells was also assessed. The results demonstrated
that miR-1827 expression changed marginally following transfection
of MCF-7 cells with si-SNHG3 (Fig.
S2D).
miR-1827 expression was detected in 30 paired PCa
tumor tissues and adjacent normal tissues. The results demonstrated
that miR-1827 expression was significantly higher in adjacent
normal tissues compared with tumor tissues (Fig. 4F). Pearson's correlation analysis
demonstrated that SNHG3 expression was negatively correlated with
miR-1827 expression in tissues from PCa patients with stage I/II or
stage III/IV assessed by the tumor, node and metastasis (TNM)
system (17) (Fig. 4G). Collectively, these results
suggest that SNHG3 binds to miR-1827 and modulates miR-1827
expression in tissues from patients with PCa (Fig. 4G). Collectively, these results
suggest that SNHG3 binds to miR-1827 and modulates miR-1827
expression.
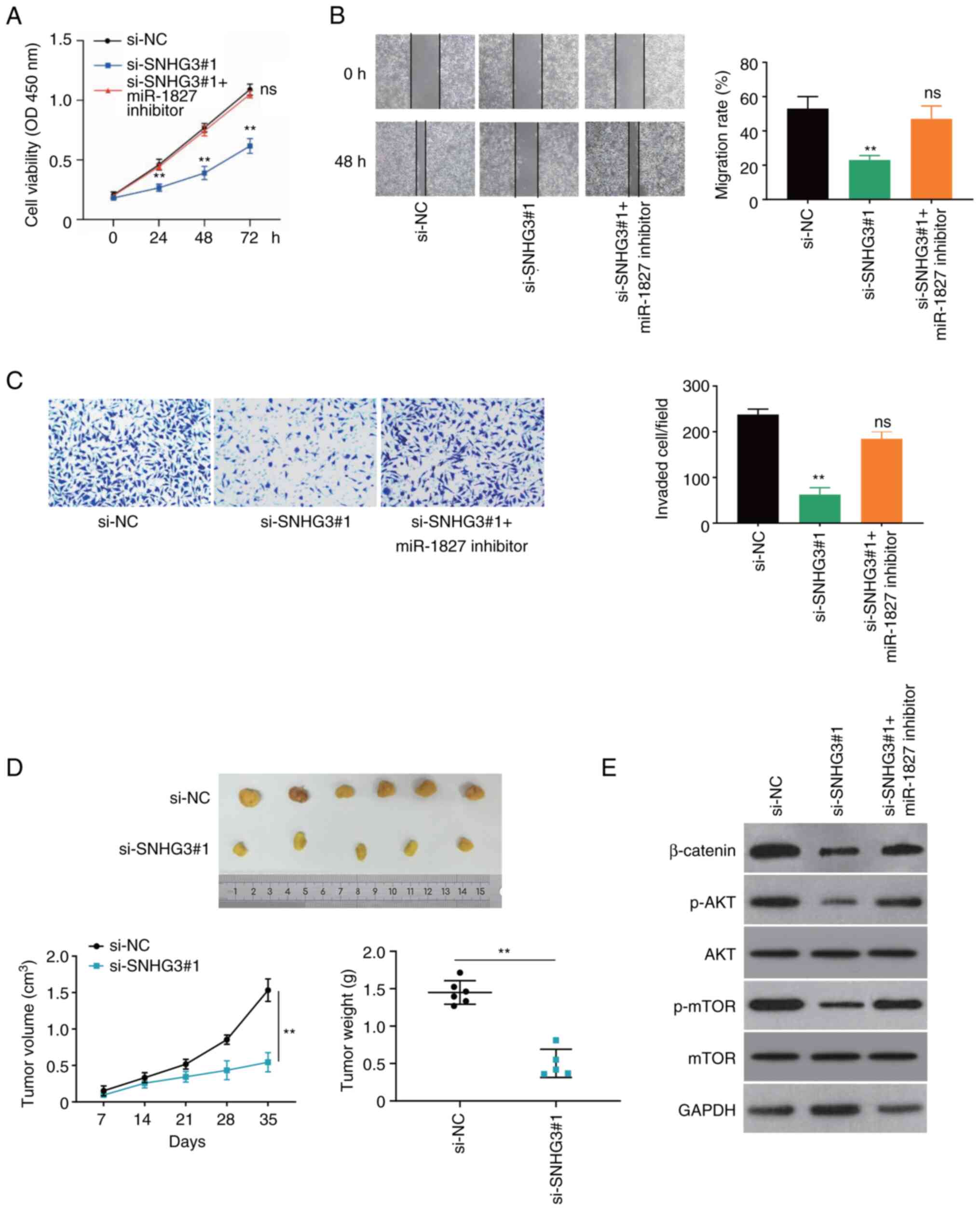
miR-1827 mediates the
tumor-suppressive effects of lncRNA SNHG3 knockdown on PC-3
cells
The rescue assay was performed to further
investigate the association between SNHG3 and miR-1827 in PC-3
cells. Notably, the decreased cell viability of PC-3 cells
following SNHG3 knockdown was recovered following inhibition of
miR-1827 expression (Fig. 5A). In
addition, the wound healing and Transwell assays indicated that the
reduced migration and invasion of PC-3 cells following SNHG3
knockdown was also recovered following miR-1827 knockdown (Fig. 5B and C).
lncRNA SNHG3 knockdown inhibits PCa
tumorigenesis in vivo
The promoting role of SNHG3 in tumorigenesis in
vivo was verified. The results demonstrated that tumor volume
and weight decreased following SNHG3 knockdown (P<0.01; Figs. 5D and S2E). These results suggest that SNHG3 is
essential for tumor progression of PCa in vivo.
The present study investigated the Wnt/β-catenin and
PI3K/AKT/mTOR signaling pathways in PCa cells transfected with
si-SNHG3 or a combination of si-SNHG3 and miR-1827 inhibitor by
western blotting. The expression levels of β-catenin, p-AKT and
p-mTOR significantly decreased following transfection with
si-SNHG3, which were partially restored following transfection with
both si-SNHG3 and miR-1827 inhibitor (Fig. 5E). Taken together, these results
suggest that lncRNA SNHG3 promotes PCa progression through miR-1827
via the Wnt/AKT/mTOR pathway.
Discussion
lncRNAs play crucial roles in several biological
processes of PCa, including cell proliferation, migration, invasion
and apoptosis (4,6,8,9).
lncAMPC has been reported to promote metastasis and
immunosuppression of PCa via the lncAMPC/LIF/LIFR axis (8). lncRNA FOXP4-AS1 has been demonstrated
to promote the tumorigenesis and progression of PCa by regulating
the miR-3184-5p/FOXP4 axis (9).
The present study reported that a novel lncRNA, SNHG3, was
significantly upregulated in PCa tissues, which was associated with
a shorter survival time of patients. Furthermore, the results
demonstrated that SNHG3 knockdown suppressed proliferation,
migration and invasion of PCa cells in vitro. The oncogenic
mechanisms of SNHG3 in PCa were investigated in the present study
using both in vitro and in vivo experiments.
lncRNA SNHG3 is located at 1p36.1 (18). SNHG3 was first reported to
dysregulate translational machinery and ribosome biogenesis during
the neurodegeneration of Alzheimer's disease (18). Recently, lncRNA SNHG3 has been
reported to be dysregulated in different types of cancer, with
varied functional roles (11–13,15).
In colorectal cancer, SNHG3 acts as a competing endogenous RNA
molecule to promote cancer progression (15). In glioma, SNHG3 facilitates
malignant behavior via the KLF2/p21 axis (13). However, in papillary thyroid
carcinoma, SNHG3 acts as a tumor suppressor via the AKT/mTOR/ERK
pathway (12). Its diversity may
be caused by inadequate research on SNHG3 expression patterns and
functional mechanisms. A previous study suggested that SNHG3 can
promote PCa progression via the miR-577/SMURF1 axis (14). However, this conclusion was drawn
solely based on the experiments using cell lines, which, by no
means, can accurately reflect the situation in clinical specimens.
The present study investigated the expression pattern and
functional role of SNHG3 using TCGA PCa patient cohort and PCa
tissues. The results demonstrated that SNHG3 expression was
significantly upregulated in tumor tissues compared with normal
tissues from both TCGA cohort and hospitalized patients. In
addition, high SNHG3 expression was associated with a shorter
overall survival time. The present study further verified that
SNHG3 knockdown significantly inhibited cell viability,
proliferation, migration and invasion of PCa cells, the effects of
which were reversed following overexpression of SNHG3 in PCa cells
in vitro. Tumor growth in nude mice was investigated
following SNHG3 knockdown. The results demonstrated that SNHG3
knockdown significantly decreased tumor volume and weight in
vivo. Overall, the results of the present study are consistent
with previous findings (14,19,20),
that SNHG3 acts as an oncogene in PCa.
Increasing evidence suggest that lncRNAs can
function as competitive endogenous RNAs in different types of
cancer (3). The competitive
endogenous RNA can reduce the stability of target miRNAs, thereby
modulating the expression of the miRNA-targeted genes (21). For examples, SNHG3 can sponge
miR-577 to regulate the SMURF1 expression to promote PCa
progression (14). The results of
the present study demonstrated that miR-1827 contains a putative
binding site of SNHG3. miR-1827 is dysregulated in different types
of cancer, including colorectal cancer and lung cancer, and
exhibits varied biological functions (22,23).
In colorectal cancer, miR-1827 acts as an oncogene and promotes the
progression of colorectal cancer by elevating Wnt/β-catenin
activity (22). In lung cancer,
miR-1827 is downregulated in high invasive lung cancer cell lines,
and suppresses cancer cell migration by targeting CRKL (23). However, the role of miR-1827 in PCa
remains largely unknown. The results of the present study
demonstrated that SNHG3 can directly interact with miR-1827. In
addition, SNHG3 knockdown significantly decreased miR-1827
expression, while SNHG3 expression was not significantly altered
following transfection with miR-1827 mimics or inhibitors. Notably,
miR-1827 expression was downregulated in tumor tissues compared
with normal tissues, and SNHG3 expression was negatively correlated
with miR-1827 expression at different stages of PCa. Furthermore,
decreased malignant phenotypes of PC-3 cells caused by SNHG3
knockdown were recovered following miR-1827 knockdown. These
results confirm that miR-1827 is the downstream target of SNHG3 and
is negatively regulated by SNHG3. The results of the present study
also demonstrated that the expression levels of β-catenin, p-AKT
and p-mTOR significantly decreased following transfection with
si-SNHG3, which was partially reversed following transfection with
a combination of si-SNHG3 and miR-1827 inhibitor. Collectively,
these results suggest that lncRNA SNHG3 promotes PCa progression
through miR-1827 via the Wnt/AKT/mTOR pathway.
The present study is not without limitations. First,
the downstream targets of miR-1827 were not investigated. Thus,
further studies are required to investigate the downstream targets
of miR-1827 in PCa. Furthermore, the association between miR-1827
and AKT/mTOR requires further investigation.
In conclusion, the results of the present study
demonstrated that lncRNA SNHG3 expression was significantly
upregulated in PCa samples, which was associated with poor survival
outcomes. Mechanistically, SNHG3 promoted cell proliferation,
migration and invasion by inhibiting miR-1827 expression in the
cytoplasm. The inhibited malignance of PCa cells induced by SNHG3
knockdown was recovered following miR-1827 knockdown. Taken
together, these results suggest that lncRNA SNHG3 promotes PCa
progression through miR-1827 via the Wnt/AKT/mTOR pathway. Thus,
the lncRNA SNHG3/miR-1827/Wnt/AKT/mTOR axis may be used as a
diagnostic biomarker for patients with PCa, and may serve as a
promising therapeutic target for the treatment of PCa.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
HG designed the present study. MH, MR, XC, and MS
performed the cell experiments. MH, ZZ and YY performed the in
vivo experiments. MH and HG analyzed the data. HG and MH
drafted the initial manuscript. MH and HG confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Affiliated Nanhai Hospital, Southern Medical
University (Foshan, China; IRB no. 2020-027-1) and written informed
consent was provided by all patients prior to the study start. All
animal experiments were reviewed and approved by the Animal Welfare
Committee of Affiliated Nanhai Hospital, Southern Medical
University (Foshan, China; IRB no. IRB-NY-2020-018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shoag JE, Nyame YA, Gulati R, Etzioni R
and Hu JC: Reconsidering the trade-offs of prostate cancer
screening. N Engl J Med. 382:2465–2468. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao G, Yao J, Kong D, Ye C, Chen R, Li L,
Zeng T, Wang L, Zhang W, Shi X, et al: The long noncoding RNA
TTTY15, which is located on the Y chromosome, promotes prostate
cancer progression by sponging let-7. Eur Urol. 76:315–326. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Profumo V, Forte B, Percio S, Rotundo F,
Doldi V, Ferrari E, Fenderico N, Dugo M, Romagnoli D, Benelli M, et
al: LEADeR role of miR-205 host gene as long noncoding RNA in
prostate basal cell differentiation. Nat Commun. 10:3072019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang C, Wang GX, Fu B, Zhou XC, Li Y and
Li YY: LncRNA CASC15 promotes migration and invasion in prostate
cancer via targeting miR-200a-3p. Eur Rev Med Pharmacol Sci.
24:72152020.PubMed/NCBI
|
|
7
|
Jiang Z, Zhang Y, Chen X, Wu P and Chen D:
Long non-coding RNA LINC00673 silencing inhibits proliferation and
drug resistance of prostate cancer cells via decreasing KLF4
promoter methylation. J Cell Mol Med. 24:1878–1892. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang W, Shi X, Chen R, Zhu Y, Peng S,
Chang Y, Nian X, Xiao G, Fang Z, Li Y, et al: Novel long non-coding
RNA lncAMPC promotes metastasis and immunosuppression in prostate
cancer by stimulating LIF/LIFR expression. Mol Ther. 28:2473–2487.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu X, Xiao Y, Zhou Y, Zhou Z and Yan W:
LncRNA FOXP4-AS1 is activated by PAX5 and promotes the growth of
prostate cancer by sequestering miR-3184-5p to upregulate FOXP4.
Cell Death Dis. 10:4722019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li JB, Liu F, Zhang BP, Bai WK, Cheng W,
Zhang YH and Yu LJ: LncRNA625 modulates prostate cancer cells
proliferation and apoptosis through regulating the Wnt/β-catenin
pathway by targeting miR-432. Eur Rev Med Pharmacol Sci.
21:2586–2595. 2017.PubMed/NCBI
|
|
11
|
Dai G, Huang C, Yang J, Jin L, Fu K, Yuan
F, Zhu J and Xue B: LncRNA SNHG3 promotes bladder cancer
proliferation and metastasis through miR-515-5p/GINS2 axis. J Cell
Mol Med. 24:9231–9243. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duan Y, Wang Z, Xu L, Sun L, Song H, Yin H
and He F: lncRNA SNHG3 acts as a novel tumor suppressor and
regulates tumor proliferation and metastasis via AKT/mTOR/ERK
pathway in papillary thyroid carcinoma. J Cancer. 11:3492–3501.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fei F, He Y, He S, He Z, Wang Y, Wu G and
Li M: LncRNA SNHG3 enhances the malignant progress of glioma
through silencing KLF2 and p21. Biosci Rep. 38:BSR201804202018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li T, Xing Y, Yang F, Sun Y, Zhang S, Wang
Q and Zhang W: LncRNA SNHG3 sponges miR-577 to up-regulate SMURF1
expression in prostate cancer. Cancer Med. 9:3852–3862. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dacheng W, Songhe L, Weidong J, Shutao Z,
Jingjing L and Jiaming Z: LncRNA SNHG3 promotes the growth and
metastasis of colorectal cancer by regulating miR-539/RUNX2 axis.
Biomed Pharmacother. 125:1100392020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mocellin S, Rossi CR, Pilati P, Nitti D
and Marincola FM: Quantitative real-time PCR: A powerful ally in
cancer research. Trends Mol Med. 9:189–195. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng L, Montironi R, Bostwick DG,
Lopez-Beltran A and Berney DM: Staging of prostate cancer.
Histopathology. 60:87–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arisi I, D'Onofrio M, Brandi R, Felsani A,
Capsoni S, Drovandi G, Felici G, Weitschek E, Bertolazzi P and
Cattaneo A: Gene expression biomarkers in the brain of a mouse
model for Alzheimer's disease: Mining of microarray data by logic
classification and feature selection. J Alzheimers Dis. 24:721–738.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu L and Ren Y: Long noncoding RNA small
nucleolar RNA host gene 3 mediates prostate cancer migration,
invasion, and epithelial-mesenchymal transition by sponging
miR-487a-3p to regulate TRIM25. Cancer Biother Radiopharm. Jan
7–2021.(Epub ahead of print). View Article : Google Scholar
|
|
20
|
Gong X and Ning B: Five lncRNAs associated
with prostate cancer prognosis identified by coexpression network
analysis. Technol Cancer Res Treat. 19:15330338209635782020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thomson DW and Dinger ME: Endogenous
microRNA sponges: Evidence and controversy. Nat Rev Genet.
17:272–283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fasihi A, B MS, Atashi A and Nasiri S:
Introduction of hsa-miR-103a and hsa-miR-1827 and hsa-miR-137 as
new regulators of Wnt signaling pathway and their relation to
colorectal carcinoma. J Cell Biochem. 119:5104–5117. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ho CS, Noor SM and Nagoor NH: MiR-378 and
MiR-1827 regulate tumor invasion, migration and angiogenesis in
human lung adenocarcinoma by targeting RBX1 and CRKL, respectively.
J Cancer. 9:331–345. 2018. View Article : Google Scholar : PubMed/NCBI
|