Introduction
Colon cancer is a common type of digestive system
malignancy worldwide (1,2). Cancer statistics from 185 countries
show that 1,104,100 novel cases and 883,200 mortalities were caused
by colon cancer in 2018 (3). The
incidence rate was ranked fourth and the mortality rate was ranked
second among all types of cancer worldwide (3). With improved living standards and
diet, the incidence of colon cancer has increased owing to high-fat
diet and insufficient food fiber (4). Colon cancer is most common where the
rectum and sigmoid colon connect, and it is more common in male
compared with female patients (5).
Prognosis and treatment of colon cancer is important. Current
clinical treatment of colon cancer is based on surgery,
radiotherapy and chemotherapy, but the five-year survival rate has
not greatly improved (6).
Mutations in Kirsten rat sarcoma virus (KRAS) is a diagnostic
biomarker and therapeutic target of colon cancer (7). Therefore, determining the molecular
mechanism of colon cancer prognosis is crucial to develop gene
therapy.
The only dNTP hydrolase in eukaryotes, SAMHD1, is
involved in multiple pathological processes (8). SAMHD1 serves a key role in preventing
virus transcription and replication by hydrolyzing dNTP, thus
protecting host cellular genome integrity (9). As an intrinsic host restriction
factor, SAMHD1 exploits deoxynucleoside triphosphohydrolase
(dNTPase) activity to resist human immunodeficiency virus 1
(10). Literature has shown that
SAMHD1 is primarily involved in apoptosis and proliferation through
hydrolyzing nucleic acids (11,12).
Although some consider SAMHD1 to serve only as a dNTPase, others
hypothesize that SAMHD1 can be used not only as dNTPase but also as
RNase. Therefore, this remains controversial (8,13).
SAMHD1 is also known as Aicardi-Goutières syndrome (AGS) gene
because multipoint mutations in SAMHD1 cause familial autoimmune
AGS (14–17). SAMHD1 is affected by
post-translational modifications, such as phosphorylation,
acetylation and methylation, which primarily affect its ability to
prevent viral infection (18–22).
For example, phosphomimetic mutation of T592E causes notable
destabilization of the active tetrameric form of SAMHD1 and a
~3-fold decline in dNTPase activity (9). However, the T592V variant does not
disrupt the crystal structure of SAMHD1, thus its dNTPase activity
is not affected (9). Furthermore,
SAMHD1 is acetylated on K405 by acetyltransferase arrest defective
protein 1, which increases its dNTPase activity in vitro
(21).
Previous studies have shown that SAMHD1 is
associated with development of several types of cancer, including
lung and colon cancer (20,23).
SAMHD1 mRNA and protein expression levels have been shown to be
decreased in lung adenocarcinoma tissue compared with adjacent
normal controls, which suggests that the SAMHD1 promoter is highly
methylated in lung adenocarcinoma, leading to decreased SAMHD1
expression (20). The decrease of
SAMHD1 activity is caused by frequent mutations of SAMHD1 gene in
colon cancer cells (23), which
suggests that the decrease of SAMHD1 activity is associated with an
increased risk for colon cancer compared with other types of
cancer. A total of 164 unique mutations in SAMHD1 from a variety of
cancer tissues are listed in the catalogue of cancer somatic
mutations, which indicates that SAMHD1 mutation is more frequent in
cancer (24). SAMHD1 mutations at
residues 123, 143, 145, 201, 209, 254, 369 and 385 lead to
decreased function of endogenous SAMHD1 protein and disrupt
nucleotide metabolism in myeloid cells, leading to carcinogenesis
(15). However, the effect of
SAMHD1 on colon cancer prognosis is not clear. The present study
aimed to determine expression of SAMHD1 in colon cancer and the
effect of its dNTPase function on cell apoptosis and proliferation
to provide potential biomarkers for prognosis of colon cancer.
Materials and methods
Dataset access and information
The Gene Expression Omnibus (GEO) database was
searched (ncbi.nlm.nih.gov/geo; GSE41258). Perl
(strawberry-perl-5.32.1.1-64bit; strawberryperl.com/releases.html)
command was used to convert the genetic probe IDs in the matrix
documents to the platform's genetic symbols to acquire a matrix
document encompassing formal symbols. All datasets were normalized
using the limma R package (R for Windows 4.1.0 Setup and http://www.rstudio.com). The entire genetic expression
data underwent log2 transformation.
Dataset analysis
Gene differential analysis [|LogFC|>1, adjusted
P-value (FDR) <0.05] was performed by comparing tumor tissue
with controls using limma R package. A volcano plot (R for Windows
4.1.0 Setup; rstudio.com) was created to present the fold-change
and P-values of differentially expressed genes (DEGs) using limma R
package.
Protein-protein interaction (PPI)
network construction
Core genes were obtained from PPI network [Search
Tool for the Retrieval of Interacting Genes/Proteins (STRING);
string-db.org/] and Cytoscape software
(Cytoscape_3_8_2_windows_64bit; cytoscape.org/download.html) was
used to plot the net diagram. Each node denotes a protein or gene
(25); edges between nodes denote
molecular interactions.
Patient information
The present study was approved by the Ethics
Committee of Wuxi No. 2. People's Hospital (Wuxi, China; approval
no. 20180706). The patients were recruited between January and
November 2020. For all patient-derived tissues, written informed
consent was obtained. A total of 184 patients (sex, 98 male and 86
female; age, 19–87 years) with colon cancer and 45 adjacent normal
tissues (sex, 23 male and 22 female; age, 19–85 years) were
included.
Colon cancer tissue were collected to assess tumor
morphology as part of routine surgery. Inclusion criteria were as
follows: i) Colon cancer diagnosed by histopathological
examination; ii) imaging examination confirmed that there was one
or more measurable lesions; iii) routine blood examination, liver
and kidney function and ECG were normal and iv) estimated survival
time >3 months. Exclusion criteria were as follows: i) Patients
with other types of tumor or ii) serious underlying disease.
Immunohistochemistry (IHC)
IHC was performed routinely on all primary colon
cancer samples, as previously described (26,27).
All samples were fixed in 10% neutral buffered formalin at room
temperature for 1 h and maintained at 4°C with 30% saccharose
overnight. Subsequently, all biopsy samples were embedded in
optimal cutting temperature compound and sectioned in 4-µm thick
slices, followed by blocking with 10% normal goat serum (cat. no.
abs933; Absin) at 37°C for 60 min. The sections were incubated with
SAMHD1 antibody (cat. no. ab128107; Abcam; 1:150) at 37°C for 0.5
h, followed by incubation with HRP-Polymer anti-Mouse/Rabbit IHC
kit (cat. no. KIT-5020; MXB; 1:100) at 37°C for 30 min. To
visualize the antigen-antibody complexes, sections were
counterstained with 0.01% DAB at room temperature for 5 min and
hematoxylin at room temperature for 3 min. Slides were washed for
10 min with PBS after every step. All samples were imaged using a
CX31 light microscope (Olympus Corporation).
Cell culture and transfection
To eliminate background interference, HT-29
(cas9-SAMHD1) cells were constructed. NCM460 was purchased from
American Tissue Culture Colection (Manassas, VA, USA), HT-29 and
HT-29 (Cas9-SAMHD1) cells were purchased from Cell Bank of Chinese
Academy of Sciences (Shanghai, China) and verified by STR DNA
profiling. HT-29 (Cas9-SAMHD1) cells were derived from HT-29 cells,
which had previously undergone SAMHD1 gene knockout using
CRISPR/Cas9 system. All cells were cultured in McCoy's 5A medium
(cat. no. 12330031; Thermo Fisher Scientific, Inc.) supplemented
with 10% FBS (cat. no. 16140071; Thermo Fisher Scientific, Inc.),
0.5 mmol/l L-glutamine (cat. no. 25030-024; Thermo Fisher
Scientific, Inc.), 0.01 mg/ml insulin (Novo Nordisk A/S), 100 IU/ml
penicillin and 100 mg/ml streptomycin (cat. no. DE17-602E; Lonza
Group, Ltd.), and all cells were cultured in 37°C and 5%
CO2. 100 cm2 Flasks and plates were purchased
from Nunc (Thermo Fisher Scientific, Inc.) and 6-well plates were
purchased from Corning Life Sciences.
HT-29 (Cas9-SAMHD1) cells were inoculated into a
6-well plate (3×105) and incubated for 12 h prior to
transfection in 37°C and 5% CO2. Cells were separated
into experimental and control groups with each group containing 3
µg plasmid + 5 µl Lipofectamine® 3000 (cat. no.
L3000015, Thermo Fisher Scientific). All plasmids, including
Flag-SAMHD1 (WT), Flag-SAMHD1 (Q548A), Flag-SAMHD1 (D137N) and
Flag-SAMHD1 (D311A), were purchased from Shanghai GenePharma Co.,
Ltd. The vector for all plasmids involved in this study was
PCNDA4.0. Cells were transfected at 70% confluence at room
temperature. A total of 5 µl Lipofectamine 3000 was added to 125 µl
DMEM/F-12 and mixed well. In addition, 3 µg DNA plasmid and 5 µl
P3000™ reagent were added to 125 µl DMEM/F-12 and mixed well. The
two solutions were then mixed, maintained at room temperature for
20 min and added into the wells in a drop-wise manner. Cells were
transfected in DMEM/F-12 for 6 h, then DMEM/F-12 was replaced with
McCoy's 5A for 18 h. Cells were then harvested for subsequent
experiments. According to preliminary results, 20 µg/ml was the
most suitable concentration of 5-FU for tumor cells (data not
shown).
Immunofluorescence (IF)
After placing the cell slide on the 24 well plate,
NCM460 normal colon epithelial and HT-29 colorectal cancer cells
were inoculated in 24 well plates at a density of 1×105
cells/well. Following 24 h culture at 37°C and 5% CO2,
cells were centrifuged and washed with PBS and centrifuged two
times at room temperature and 1,000 × g for 5 min. 4%
paraformaldehyde was fixed for 20 min and washed with PBS and
centrifuged two times at room temperature for 5 min. Cells were
fixed with punched with 0.1% Triton X-100 for 10 min and washed
with PBS for 5 min at room temperature. Then, 0.1% BSA (cat. no.
A8010, Solarbio) was used for blocking at room temperature for 1 h,
followed by incubation with SAMHD1 antibody (cat. no. ab128107;
Abcam; 1:100) at 4°C overnight and rinsing three times with PBS for
5 min each. Incubation with goat Anti-Mouse IgG H&L
(FITC-conjugated; cat. no. ab6785, Abcam) was performed at room
temperature in the dark for 1 h, followed by rinsing with PBS three
times for 5 min each. The cell nuclei were stained with 10 ug/ml
DAPI (cat. no. C0065; Beijing Solarbio Science & Technology
Co., Ltd.) for 5 min at room temperature. Next, a drop of sealing
agent was added, the film was sealed and imaging with a
fluorescence microscope was performed as previously described
(28,29). Images were analyzed using Cellsens
Standard version 1.9 software (Olympus Corporation;
olympus-lifescience.com.cn/zh/software/cellsens/).
Western blotting (WB)
Treated cells were harvested and lysed in RIPA
buffering solution (Beyotime Institute of Biotechnology). Protein
levels were determined using BCA (Beyotime Institute of
Biotechnology). Protein samples (50 µg/lane) from each group were
separated using 10% SDS-PAGE, transferred onto PVDF membranes and
blocked in BSA. Next, the membranes were immersed in primary
antibody at 4°C overnight. The primary antibodies (all Abcam) were
as follows: Bax (cat. no. ab53154; 1:1,000), Cleaved-caspase3 (cat.
no. ab32042; 1;500), Caspase3 (cat. no. ab32351; 1:1,000), Bcl-2
(cat. no. ab32124; 1:1,000) and Flag (cat. no. ab205606; 1:1,000).
The normal band density of GAPDH (cat. no. ab8245; Abcam; 1:1,000)
at room temperature for 2 h was used as an internal reference. Then
they were rinsed with TBST (0.1% Tween-20, cat. no. ST671,
beyotime) three times for 10 min each. Incubation with Rabbit
Anti-Mouse IgG H&L (HRP-conjugated; cat. no. ab6728, Abcam,
1:5,000) or goat Anti-Rabbit IgG H&L (HRP, ab6721; Abcam,
1:5,000) was performed at room temperature for 1 h, followed by
rinsing with TBST three times for 10 min each. Protein detection
was performed using BeyoECL Star (cat. no. P0018AM, beyotime). The
bands were quantified using the Image Lab System version 6.1
(Bio-Rad, bio-rad.com/SearchResults?search_api_fulltext=Image+Lab).
These assays were performed at least three times, as previously
described (30,31).
Cell counting kit-8 (CCK-8) assay
To detect cell proliferation, CCK-8 assays (cat. no.
C0037; Beyotime Institute of Biotechnology) were performed. HT-29
(Cas9-SAMHD1) cells at the logarithmic growth stage were collected
and digested to prepare HT-29 (Cas9-SAMHD1) cell suspension at room
temperature for 1 min by trypsin digestion (cat. no. 25200072,
Gibco). Next, 1×104 cells/well were inoculated into
96-well plates. Following 24 h incubation at 37°C and 5%
CO2, cell viability in each group was detected prior to
transfection. The 200 ng Flag-SAMHD1 (WT), Flag-SAMHD1 (Q548A),
Flag-SAMHD1 (D137N) and Flag-SAMHD1 (D311A) plasmids were then
transfected into HT-29 (Cas9-SAMHD1) cells. Cell viability was
detected 24 and 48 h after transfection. A total of 10 µl CCK-8
solution was added to 90 µl culture medium. The medium was replaced
in each well for testing, and cells were incubated at 37°C for 1 h.
A microplate reader was used to measure optical density in each
well at a wavelength of 450 nm, as previously described (32).
Following 24 h transfection at 37°C and 5%
CO2, cell viability in the WT and D137N groups was
detected. Cell viability was detected for 24 and 48 h following 20
µg/ml 5-FU treatment in 37°C and 5% CO2, as
aforementioned.
Statistical analysis
SPSS 19.0 (IBM Corp.) was used to analyze the data.
Differences between multiple groups were analyzed using one-way
ANOVA followed by Tukey's honestly significant difference post hoc
test. Data are expressed as mean ± SEM. An independent samples
t-test was used to assess differences between two groups;
χ2 test was conducted to determine differences in
proportion. Progression-free survival was determined using the
Kaplan-Meier method and survival differences were analyzed using
log-rank test. Cox proportional hazards model was used for
multivariate analysis of prognostic factors. Two-sided P<0.05
was considered to indicate a statistically significant difference.
All assays were performed at least three times.
Results
Identification of DEGs in colon
cancer
GSE41258 genetic expression matrix and corresponding
annotated files were acquired from GEO database. The dataset, which
included 187 patients with colon cancer and 45 adjacent normal
tissue, was analyzed using the GPL596 Affymetrix Human Genome U133
Plus 2.0 Array. A total of 6,905 DEGs were identified from the
GSE41258 dataset using the R package. DEGs were identified using
microarray volcano plots (Fig.
1A). STRING database and Cytoscape were used to construct and
visualize a PPI network of DEGs. The location of DEG was visualized
by PPI analysis. The KRAS mutation is common in colon cancer
(occurring in 30–50% of cases) and has been recognized as a key
molecular marker for predicting response to anti-epidermal growth
factor receptor monoclonal antibodies, cetuximab and panitumumab in
metastatic colon cancer (33).
Using the PPI network, SAMHD1 was found to be associated with KRAS
and the expression of SAMHD1 is upregulated in colon cancer. The
red is up and green is downregulation. (Fig. 1B). A total of 187 patients with
colon cancer from the GEO database were divided into high and low
KRAS expression groups. SAMHD1 expression was negatively associated
with KRAS (Fig. 1C). Cox
proportional hazards model was used to analyze the effect of
multivariate data (SAMHD1 expression, age, sex and TNM) on patient
mortality. Cox proportional hazards regression analysis and
survival analysis found that low SAMHD1 expression was a factor
that led to patient mortality (Fig.
1D). The survival time of patients with low SAMHD1 expression
was significantly shorter compared with that of patients with high
SAMHD1 expression (Fig. 1E).
According to bioinformatics, low expression of SAMHD1 was
associated with poor prognosis.
Low expression of SAMHD1 in colon
cancer
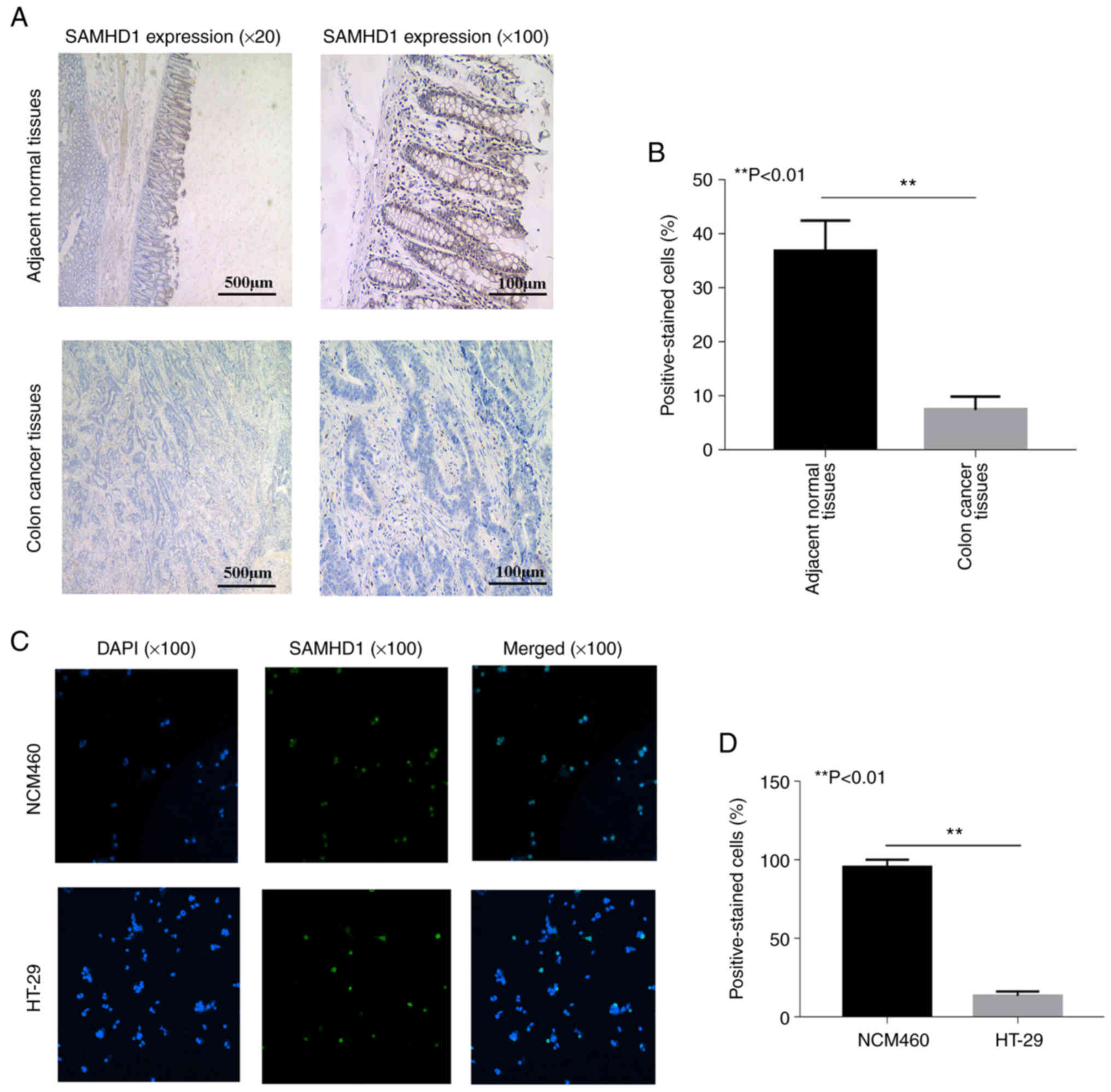
IHC analysis of colon cancer tissue revealed lower
expression of SAMHD1 compared with expression in adjacent normal
tissue (Fig. 2A and B). The
nucleus of healthy tissue exhibited diffuse brown staining,
indicating the presence of SAMHD1. However, low SAMHD1 expression
was detected in patients with colon cancer. To verify these
results, expression levels of SAMHD1 were measured in HT-29
colorectal cancer cells. IF revealed lower SAMHD1 expression in
HT-29 compared with NCM460 healthy colonic epithelial cells
(Fig. 2C and D), which was
consistent with IHC results.
To determine the association between SAMHD1
expression and clinical features, patients were divided into two
groups: Positive (≥5%) and negative (<5%) SAMHD1 expression
(Table I). No significant
differences in age and sex were observed between the two groups.
There was a significant association between SAMHD1 expression and
N, T and M stage. These data indicated that SAMHD1 may be a
biological marker for diagnosis and prognosis of patients with
colon cancer.
 | Table I.SAMHD1 expression in colon
cancer. |
Table I.
SAMHD1 expression in colon
cancer.
| Clinicopathological
characteristic | SAMHD1 negative
(n=148) | SAMHD1 positive
(n=36) | χ2 | P-value |
|---|
| Sex |
|
|
|
|
|
Male | 76 | 22 | 0.353 | 0.1930 |
|
Female | 72 | 14 |
|
|
| Age, years |
|
|
|
|
|
≤65 | 76 | 20 | 0.205 | 0.6510 |
|
>65 | 72 | 16 |
|
|
| T |
|
|
|
|
|
1+2 | 37 | 21 | 14.905 | <0.0001 |
|
3+4 | 111 | 15 |
|
|
| N |
|
|
|
|
| 0 | 38 | 13 |
|
|
| 1 | 44 | 16 | 7.677 | 0.0220 |
| 2 | 66 | 7 |
|
|
| M |
|
|
|
|
| 0 | 59 | 21 | 4.019 | 0.0450 |
| 1 | 89 | 15 |
|
|
D137N mutation of SAMHD1 inhibits
apoptosis and promotes proliferation of HT-29 (Cas9-SAMHD1)
Post-translational modification and associated sites
of SAMHD1 are presented in Table
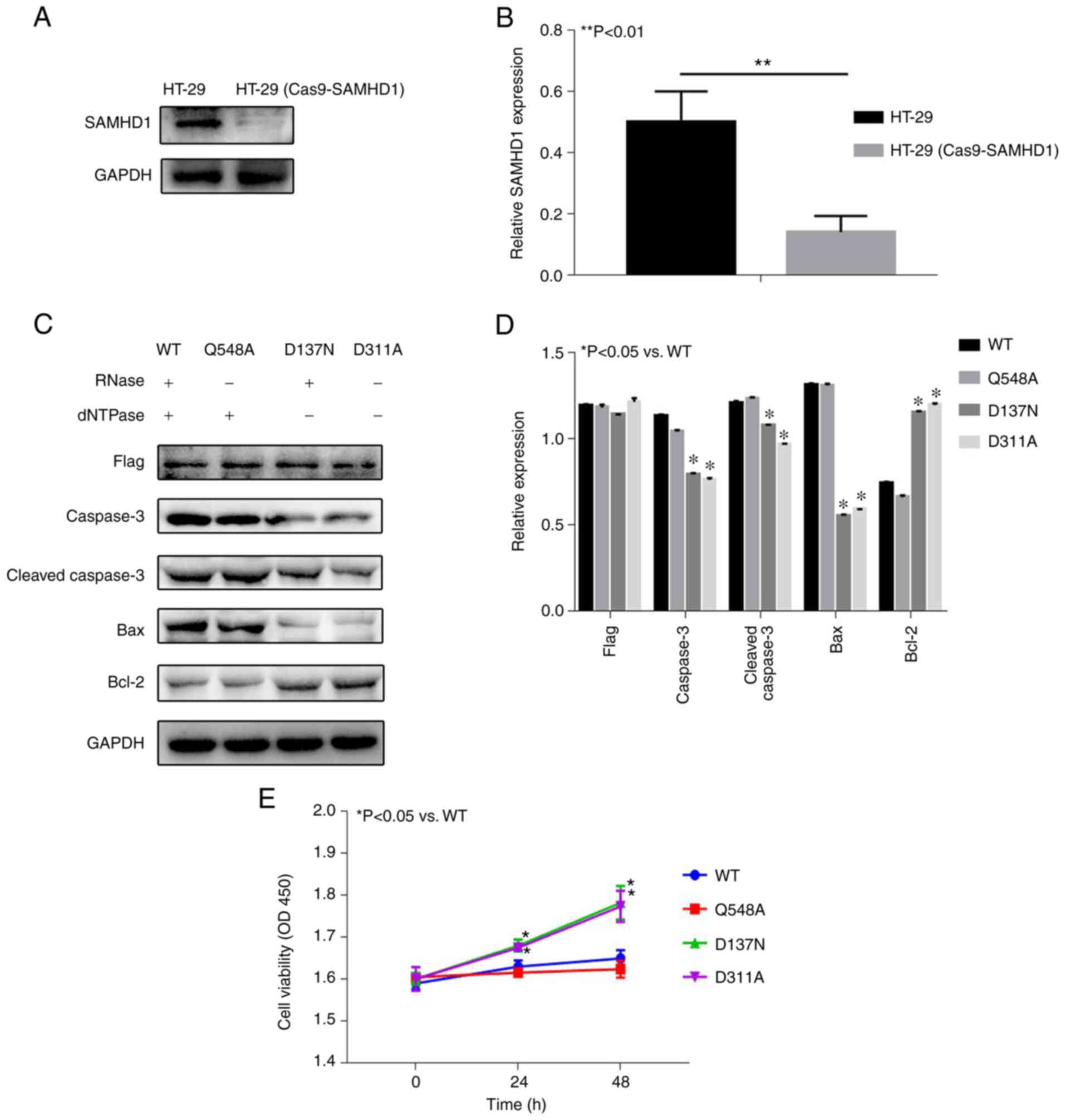
II. WB showed SAMHD1 expression was significantly lower in the
HT-29 (Cas9-SAMHD1) group compared with HT-29 (Fig. 3A and B), which indicated the HT-29
(Cas9-SAMHD1) cell model was successfully constructed. Then, three
classical mutation sites of SAMHD1 were selected: Q548A, D137N and
D311A (11,12). Q548A is RNase deletion, D137N is
dNTPase deletion, D311A is RNase deletion and dNTPase deletion
(11,12). WB analysis showed that there was no
significant difference in Caspase3, Cleaved-caspase3, Bax and Bcl-2
expression levels between the Q548A and WT groups (Fig. 3C and D). Compared with the WT
group, expression levels of Caspase3, Cleaved-caspase3 and Bax
significantly decreased, whereas Bcl-2 expression significantly
increased in the D137N and D311A groups, which indicated that D137N
and D311A groups significantly inhibited the expression of
apoptosis-related proteins. CCK-8 results showed that the
difference in proliferation between the Q548A and WT groups was not
statistically significant (Fig.
3E). Compared with WT group, proliferation increased in the
D137N and D311A groups.
 | Table II.Modifications in SAMHD1 in point
mutation. |
Table II.
Modifications in SAMHD1 in point
mutation.
|
|
|
| Records, n |
|---|
|
|
|
|
|
|---|
| Mutation | Flanking
sequence | Modification | LTP | HTP |
|---|
| S6 | MQRADsEQPSkRP |
Phosphorylation | 0 | 4 |
| K11 |
ADsEQPSkRPRCDDs | Ubiquitylation | 0 | 4 |
| S18 |
kRPRCDDsPRtPsNt |
Phosphorylation | 3 | 62 |
| T21 |
RCDDsPRtPsNtPsA |
Phosphorylation | 4 | 106 |
| S23 |
DDsPRtPsNtPsAEA |
Phosphorylation | 0 | 15 |
| N24 |
DsPRtPsntPsAEAD |
Phosphorylation | 1 | 18 |
| T25 |
sPRtPsNtPsAEADW |
Phosphorylation | 3 | 45 |
| S27 |
RtPsNtPsAEADWsP |
Phosphorylation | 0 | 8 |
| E29 |
PsNtPsAeADWsPGL |
Phosphorylation | 0 | 10 |
| S33 |
PsAEADWsPGLELHP |
Phosphorylation | 4 | 50 |
| V63 |
RGGFEEPvLLkNIRE | Ubiquitylation | 0 | 1 |
| K66 |
FEEPVLLkNIRENEI | Ubiquitylation | 0 | 10 |
| S93 |
FENLGVSsLGERkkL |
Phosphorylation | 0 | 1 |
| K98 |
VSsLGERkkLLsyIQ | Ubiquitylation | 0 | 1 |
| K99 |
SsLGERkkLLsyIQR | Ubiquitylation | 0 | 3 |
| S102 |
GERkkLLsyIQRLVQ |
Phosphorylation | 0 | 2 |
| Y103 |
ERkkLLsyIQRLVQI |
Phosphorylation | 0 | 1 |
| T138 |
LLVRIIDtPQFQRLR |
Phosphorylation | 0 | 1 |
| Y146 |
PQFQRLRyIkQLGGG |
Phosphorylation | 0 | 1 |
| K148 |
FQRLRyIkQLGGGYy | Ubiquitylation | 0 | 1 |
| Y155 |
kQLGGGYyVFPGASH |
Phosphorylation | 0 | 1 |
| E255 |
NGIKPVMeQYGLIPE | Acetylation | 0 | 1 |
| K269 |
EEDICFIkEQIVGPL | Ubiquitylation | 0 | 1 |
| S278 |
QIVGPLEsPVEDSLW |
Phosphorylation | 1 | 24 |
| E281 |
GPLEsPVeDSLWPYk | Ubiquitylation | 0 | 1 |
| S283 |
LEsPVEDsLWPYkGR |
Phosphorylation | 0 | 1 |
| K288 |
EDSLWPYkGRPENkS | Ubiquitylation | 0 | 1 |
| K294 |
YkGRPENkSFLYEIV | Acetylation | 0 | 1 |
| K294 |
YkGRPENkSFLYEIV | Ubiquitylation | 0 | 1 |
| K304 |
LYEIVSNkRNGIDVD | Ubiquitylation | 0 | 5 |
| K312 |
RNGIDVDkWDyFARD | Ubiquitylation | 0 | 3 |
| Y315 |
IDVDkWDyFARDCHH |
Phosphorylation | 0 | 3 |
| K332 |
IQNNFDYkRFIKFAR | Sumoylation | 0 | 2 |
| E346 |
RVCEVDNeLRICARD | Ubiquitylation | 0 | 1 |
| Y360 |
DKEVGNLyDMFHTRN |
Phosphorylation | 0 | 1 |
| T384 |
KVGNIIDtMItDAFL |
Phosphorylation | 0 | 2 |
| T387 |
NIIDtMItDAFLKAD |
Phosphorylation | 0 | 2 |
| K405 |
EITGAGGkKYRISTA |
Phosphorylation | 0 | 1 |
| Y419 |
AIDDMEAyTKLTDNI |
Phosphorylation | 0 | 1 |
| Y432 |
NIFLEILySTDPKLK |
Phosphorylation | 0 | 1 |
| K446 |
KDAREILkQIEYRNL | Ubiquitylation | 0 | 9 |
| K455 |
IEYRNLFkYVGETQP | Ubiquitylation | 0 | 4 |
| K467 |
TQPTGQIkIkREDYE | Ubiquitylation | 0 | 1 |
| K469 |
PTGQIkIkREDYESL | Sumoylation | 0 | 3 |
| K478 |
EDYESLPkEVAsAKP | Ubiquitylation | 0 | 1 |
| S482 |
SLPkEVAsAKPkVLL |
Phosphorylation | 0 | 1 |
| K484 |
PkEVAsAkPkVLLDV | Ubiquitylation | 0 | 1 |
| K486 |
EVAsAKPkVLLDVKL |
Phosphorylation | 0 | 2 |
| Y507 |
VDVINMDyGMQEKNP |
Phosphorylation | 0 | 1 |
| S519 |
KNPIDHVsFYCKTAP |
Phosphorylation | 0 | 1 |
| K534 |
NRAIRITkNQVSQLL | Ubiquitylation | 0 | 1 |
| K544 |
VSQLLPEkFAEQLIR | Ubiquitylation | 0 | 4 |
| Y553 |
AEQLIRVyCKKVDRk |
Phosphorylation | 0 | 1 |
| K560 |
yCKKVDRkSLYAARQ |
Phosphorylation | 0 | 1 |
| T579 |
WCADRNFtKPQDGDV |
Phosphorylation | 0 | 1 |
| T592 |
DVIAPLItPQkkEWN |
Phosphorylation | 15 | 64 |
| K595 |
APLItPQkkEWNDsT | Sumoylation | 1 | 2 |
| K596 |
PLItPQkkEWNDsTS | Ubiquitylation | 0 | 1 |
| S601 |
QkkEWNDsTSVQNPt |
Phosphorylation | 0 | 1 |
| T608 |
sTSVQNPtRLREASK |
Phosphorylation | 0 | 1 |
| S616 |
RLREASKsRVQLFkD |
Phosphorylation | 0 | 2 |
| K622 | KsRVQLFkDDPM | Sumoylation | 0 | 2 |
5-FU treatment promotes apoptosis and
inhibits proliferation of HT-29 (Cas9-SAMHD1)
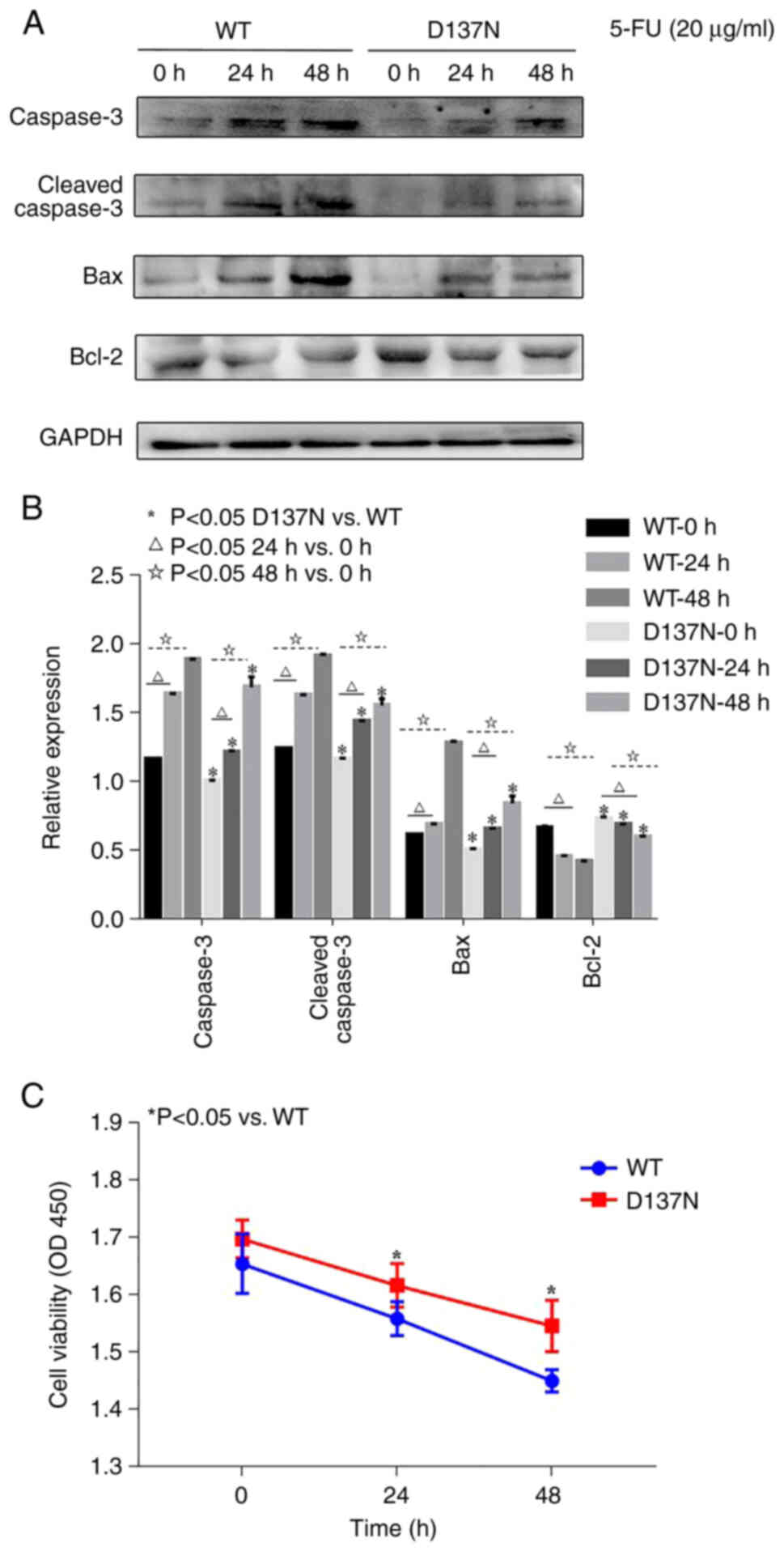
WB analysis showed that in the WT and D137N groups,
Caspase3, Cleaved-caspase3 and Bax expression significantly
increased and Bcl-2 significantly decreased over time following 20
µg/ml 5-FU treatment (Fig. 4A and
B), potentially promoting cell apoptosis. However, compared
with the WT group, Caspase3, Cleaved-caspase3 and Bax expression
levels were significantly lower and the Bcl-2 level was
significantly higher in the D137N group, suggesting a potentially
inhibiting effect on the expression of apoptosis-related proteins.
CCK-8 results showed that, following 20 µg/ml 5-FU treatment,
proliferation in the WT and D137N groups was significantly
decreased over time (Fig. 4C).
However, compared with the WT group, viability in the D137N group
was significantly higher at each time point.
Discussion
The human SAMHD1 gene was first cloned from human
dendritic cell cDNA in 2000 (34).
Its protein expression is induced by IFN-γ (34,35).
In addition to being associated with occurrence and development of
lung and colon cancer, low-level exogenous SAMHD1 expression leads
to a notable decrease in development, proliferation and colony
formation of HUT78 human T lymphocyte leukemia cells by promoting
apoptosis, and SAMHD1 may be a therapeutic target against cancer
involving T lymphocytes (20,23,36).
The number of SAMHD1 mutations and expression levels vary between
types of so lid cancer, such as lung adenocarcinoma and colon
cancer, and affect the efficiency of clinical first-line
chemotherapy drug ara-C in leukemia (20,23,37).
Multipoint gene mutations of SAMHD1 were detected in leukemia
cells, resulting in decreased mRNA and protein expression levels of
SAMHD1 (38,39). The sensitivity of THP-1 human
myeloid leukemia monocytes, which do not exhibit dNTPase activity
of SAMHD1, to anti-metabolites, such as fludarabine, decitabine,
cytarabine and clofarabine, has improved (40). SAMHD1 protects cancer cells from
anti-nucleoside metabolites, such as ara-C (37,40–42).
Certain SAMHD1 mutation results in loss of dNTPase activity,
leading to abnormal dNTP accumulation, which causes rapid cancer
cell proliferation (43–46) and immune system dysfunction
(44). In addition, SAMHD1 may
protect cancer cells from DNA replication inhibitors, such as
antimetabolite antineoplastic agents (43,47).
As a result, SAMHD1 is considered a potential biomarker for the
stratification of patients with AML based on response to ara-C and
a potential therapeutic agent against ara-C-refractory AML
(37). It is hypothesized that a
similar phenomenon may occur in colon cancer. Previous studies have
found that SAMHD1 mutations increase whole mutation rates in colon
cancer cells, and SAMHD1 may be a prognostic marker of colorectal
cancer (23,48). D137N (dNTPase deletion) and D311A
mutation (RNase and dNTPase deletion) prevent the antiviral
activity of SAMHD1, whereas the Q548A mutation (RNase deletion)
does not significantly inhibit its antiviral activity (11). The present study analyzed the
association between SAMHD1 and KRAS and found that SAMHD1 was
expressed at low levels in colon cancer. The effects of three
classic SAMHD1 mutations (Q548A, D137N and D311A) on apoptosis and
proliferation of colon cancer cells were subsequently assessed. The
effect treatment with first-line drug 5-FU against colon cancer
cells with D137N mutation is time-dependent.
The incidence and mortality rate of colon cancer in
2018 ranked fourth (3) in all
types of cancer worldwide, which showed that the present research
has high clinical value. In addition, a review of The Cancer Genome
Atlas found that, among 15 types of cancer with frequent SAMHD1
mutations, mutation rate of SAMHD1 in colon cancer ranked third and
frequent mutations in SAMHD1 gene that result in its downregulation
have also been found in colon cancer (23). Here, SAMHD1 was differentially
expressed between the adjacent normal tissue group and the
colorectal cancer group. Therefore, the present study investigated
expression of SAMHD1 in colon cancer. In the present study, 6,905
DEGs were identified using R software firstly. The classical
colorectal cancer gene KRAS was selected and found different SAMHD1
expression levels in KRAS-L and KRAS-H in colorectal cancer. SAMHD1
and KRAS were found to be negatively associated. Cox proportional
hazards regression and survival analyses demonstrated that low
expression of SAMHD1 was associated with increased patient
mortality. This is consistent with the conclusions of previous
studies in AML (49). Therefore,
it was hypothesized that SAMHD1 may serve a key role in colorectal
cancer. The expression of SAMHD1 in colorectal cancer was assessed
in tissue and cells; its mRNA expression was lower in tumor
compared with that in adjacent normal tissue. Similarly, compared
with NCM460 normal colon epithelial cells, SAMHD1 expression in
HT-29 colorectal cancer cells was decreased, which was consistent
with the results of bioinformatic analysis. The effect of mutations
in the dNTPase functional site of SAMHD1 on proliferation and
apoptosis of colon cancer was investigated. Therefore, three
previously reported mutation sites of SAMHD1 were selected: Q548A,
D137N and D311A (11,12). The Q548A mutation had no
significant effect on proliferation and apoptosis of HT-29 cells
compared with WT group, whereas D137N and D311A notably promoted
proliferation and inhibited apoptosis of HT-29 cells. WT and D137N
groups were treated with 20 µg/ml 5-FU for 24 and 48 h. The results
showed cell proliferation was inhibited and apoptosis-related
protein expressions were promoted in WT and D137N groups over time
following 20 µg/ml 5-FU treatment. However, compared with the WT
group, cell proliferation in the D137N group was higher and
apoptosis-associated protein expression levels were lower following
20 µg/ml 5-FU treatment.
In conclusion, SAMHD1 was expressed at low levels
and was associated with prognosis in colon cancer. The dNTPase
function of SAMHD1 may inhibit proliferation and promote apoptosis
in colon cancer cells. In addition, first-line chemotherapy with
5-FU had a time-dependent effect on colon cancer cells, which may
provide novel options for clinical treatment of colon cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Wuxi Health Commission
Scientific Research Project (Wuxi, China; grant no. MS201930).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PS conceived and designed the experiments. ZZ and PL
performed the experiments, analyzed the data and wrote the
manuscript. All authors have read and approved the final
manuscript. ZZ and PS confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Wuxi No. 2. People's Hospital (Wuxi, China; approval
no. 20180706). Written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marley AR and Nan H: Epidemiology of
colorectal cancer. Int J Mol Epidemiol Genet. 7:105–114. 2016.
|
|
2
|
Labianca R, Beretta GD, Kildani B, Milesi
L, Merlin F, Mosconi S, Pessi MA, Prochilo T, Quadri A, Gatta G, et
al: Colon cancer. Crit Rev Oncol Hematol. 74:106–133. 2010.
View Article : Google Scholar
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chiang CJ, Lo WC, Yang YW, You SL, Chen CJ
and Lai MS: Incidence and survival of adult cancer patients in
Taiwan, 2002–2012. J Formos Med Assoc. 115:1076–1088. 2016.
View Article : Google Scholar
|
|
5
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lemmens V, van Steenbergen L,
Janssen-Heijnen M, Martijn H, Rutten H and Coebergh JW: Trends in
colorectal cancer in the south of the Netherlands 1975–2007: Rectal
cancer survival levels with colon cancer survival. Acta Oncol.
49:784–796. 2010. View Article : Google Scholar
|
|
7
|
Wu C: Systemic therapy for colon cancer.
Surg Oncol Clin N Am. 27:235–242. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Z, Zheng L, Yu Y, Wu J, Yang F, Xu
Y, Guo Q, Wu X, Cao S, Cao L and Song X: Involvement of SAMHD1 in
dNTP homeostasis and the maintenance of genomic integrity and
oncotherapy (review). Int J Oncol. 56:879–888. 2020.
|
|
9
|
Tang C, Ji X, Wu L and Xiong Y: Impaired
dNTPase activity of SAMHD1 by phosphomimetic mutation of Thr-592. J
Biol Chem. 290:26352–26359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldstone DC, Ennis-Adeniran V, Hedden JJ,
Groom HC, Rice GI, Christodoulou E, Walker PA, Kelly G, Haire LF,
Yap MW, et al: HIV-1 restriction factor SAMHD1 is a deoxynucleoside
triphosphate triphosphohydrolase. Nature. 480:379–382. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sommer AF, Rivière L, Qu B, Schott K,
Riess M, Ni Y, Shepard C, Schnellbächer E, Finkernagel M,
Himmelsbach K, et al: Restrictive influence of SAMHD1 on hepatitis
B virus life cycle. Sci Rep. 6:266162016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ryoo J, Choi J, Oh C, Kim S, Seo M, Kim
SY, Seo D, Kim J, White TE, Brandariz-Nuñez A, et al: The
ribonuclease activity of SAMHD1 is required for HIV-1 restriction.
Nat Med. 20:936–941. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Antonucci JM, St Gelais C, de Silva S,
Yount JS, Tang C, Ji X, Shepard C, Xiong Y, Kim B and Wu L:
SAMHD1-mediated HIV-1 restriction in cells does not involve
ribonuclease activity. Nat Med. 22:1072–1074. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leshinsky-Silver E, Malinger G, Ben-Sira
L, Kidron D, Cohen S, Inbar S, Bezaleli T, Levine A, Vinkler C, Lev
D and Lerman-Sagie T: A large homozygous deletion in the SAMHD1
gene causes atypical Aicardi-Goutiéres syndrome associated with
mtDNA deletions. Eur J Hum Genet. 19:287–292. 2011. View Article : Google Scholar
|
|
15
|
Rice GI, Bond J, Asipu A, Brunette RL,
Manfield IW, Carr IM, Fuller JC, Jackson RM, Lamb T, Briggs TA, et
al: Mutations involved in Aicardi-Goutières syndrome implicate
SAMHD1 as regulator of the innate immune response. Nat Genet.
41:829–832. 2009. View
Article : Google Scholar
|
|
16
|
Thiele H, du Moulin M, Barczyk K, George
C, Schwindt W, Nürnberg G, Frosch M, Kurlemann G, Roth J, Nürnberg
P and Rutsch F: Cerebral arterial stenoses and stroke: Novel
features of Aicardi-Goutières syndrome caused by the Arg164X
mutation in SAMHD1 are associated with altered cytokine expression.
Hum Mutat. 31:E1836–E1850. 2010. View Article : Google Scholar
|
|
17
|
Dale RC, Gornall H, Singh-Grewal D,
Alcausin M, Rice GI and Crow YJ: Familial Aicardi-Goutières
syndrome due to SAMHD1 mutations is associated with chronic
arthropathy and contractures. Am J Med Genet A. 152A:938–942. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Silva S, Hoy H, Hake TS, Wong HK, Porcu
P and Wu L: Promoter methylation regulates SAMHD1 gene expression
in human CD4+ T cells. J Biol Chem. 288:9284–9292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Silva S, Wang F, Hake TS, Porcu P, Wong
HK and Wu L: Downregulation of SAMHD1 expression correlates with
promoter DNA methylation in Sézary syndrome patients. J Invest
Dermatol. 134:562–565. 2014. View Article : Google Scholar
|
|
20
|
Wang JL, Lu FZ, Shen XY, Wu Y and Zhao LT:
SAMHD1 is down regulated in lung cancer by methylation and inhibits
tumor cell proliferation. Biochem Biophys Res Commun. 455:229–233.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee EJ, Seo JH, Park JH, Vo TTL, An S, Bae
SJ, Le H, Lee HS, Wee HJ, Lee D, et al: SAMHD1 acetylation enhances
its deoxynucleotide triphosphohydrolase activity and promotes
cancer cell proliferation. Oncotarget. 8:68517–68529. 2017.
View Article : Google Scholar
|
|
22
|
Cribier A, Descours B, Valadao AL,
Laguette N and Benkirane M: Phosphorylation of SAMHD1 by cyclin
A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep.
3:1036–1043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rentoft M, Lindell K, Tran P, Chabes AL,
Buckland RJ, Watt DL, Marjavaara L, Nilsson AK, Melin B, Trygg J,
et al: Heterozygous colon cancer-associated mutations of SAMHD1
have functional significance. Proc Natl Acad Sci USA.
113:4723–4728. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Forbes SA, Beare D, Boutselakis H, Bamford
S, Bindal N, Tate J, Cole CG, Ward S, Dawson E, Ponting L, et al:
COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids
Res. 45(D1): D777–D783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Doncheva NT, Morris JH, Gorodkin J and
Jensen LJ: Cytoscape StringApp: Network analysis and visualization
of proteomics data. J Proteome Res. 18:623–632. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Felip E, Gutiérrez-Chamorro L, Gómez M,
Garcia-Vidal E, Romeo M, Morán T, Layos L, Pérez-Roca L,
Riveira-Muñoz E, Clotet B, et al: Modulation of DNA damage response
by SAM and HD domain containing deoxynucleoside triphosphate
triphosphohydrolase (SAMHD1) determines prognosis and treatment
efficacy in different solid tumor types. Cancers (Basel).
14:6412022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Janardhan KS, Jensen H, Clayton NP and
Herbert RA: Immunohistochemistry in investigative and toxicologic
pathology. Toxicol Pathol. 46:488–510. 2018. View Article : Google Scholar
|
|
28
|
Xu C, Zhang W, Liu S, Wu W, Qin M and
Huang J: Activation of the SphK1/ERK/p-ERK pathway promotes
autophagy in colon cancer cells. Oncol Lett. 15:9719–9724.
2018.
|
|
29
|
Yu X, Du Z, Sun X, Shi C, Zhang H and Hu
T: Aberrant Cosmc genes result in Tn antigen expression in human
colorectal carcinoma cell line HT-29. Int J Clin Exp Pathol.
8:2590–2602. 2015.
|
|
30
|
Kim SJ, Kim HJ, Kim HR, Lee SH, Cho SD,
Choi CS, Nam JS and Jung JY: Antitumor actions of baicalein and
wogonin in HT-29 human colorectal cancer cells. Mol Med Rep.
6:1443–1449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Q, Zhou X, Fang Z and Zhou H: Knockdown
of KLK12 inhibits viability and induces apoptosis in human
colorectal cancer HT-29 cell line. Int J Mol Med. 44:1667–1676.
2019.PubMed/NCBI
|
|
32
|
Guo H, Wu L, Zhao P and Feng A:
Overexpression of long non-coding RNA zinc finger antisense 1 in
acute myeloid leukemia cell lines influences cell growth and
apoptosis. Exp Ther Med. 14:647–651. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Siddiqui AD and Piperdi B: KRAS mutation
in colon cancer: A marker of resistance to EGFR-I therapy. Ann Surg
Oncol. 17:1168–1176. 2010. View Article : Google Scholar
|
|
34
|
Li N, Zhang W and Cao X: Identification of
human homologue of mouse IFN-gamma induced protein from human
dendritic cells. Immunol Lett. 74:221–224. 2000. View Article : Google Scholar
|
|
35
|
Kueck T, Cassella E, Holler J, Kim B and
Bieniasz PD: The aryl hydrocarbon receptor and interferon gamma
generate antiviral states via transcriptional repression. Elife.
7:e388672018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kodigepalli KM, Li M, Liu SL and Wu L:
Exogenous expression of SAMHD1 inhibits proliferation and induces
apoptosis in cutaneous T-cell lymphoma-derived HuT78 cells. Cell
Cycle. 16:179–188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schneider C, Oellerich T, Baldauf HM,
Schwarz SM, Thomas D, Flick R, Bohnenberger H, Kaderali L, Stegmann
L, Cremer A, et al: SAMHD1 is a biomarker for cytarabine response
and a therapeutic target in acute myeloid leukemia. Nat Med.
23:250–255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Clifford R, Louis T, Robbe P, Ackroyd S,
Burns A, Timbs AT, Wright Colopy G, Dreau H, Sigaux F, Judde JG, et
al: SAMHD1 is mutated recurrently in chronic lymphocytic leukemia
and is involved in response to DNA damage. Blood. 123:1021–1031.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rossi D: SAMHD1: A new gene for CLL.
Blood. 123:951–952. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Herold N, Rudd SG, Sanjiv K, Kutzner J,
Bladh J, Paulin CBJ, Helleday T, Henter JI and Schaller T: SAMHD1
protects cancer cells from various nucleoside-based
antimetabolites. Cell Cycle. 16:1029–1038. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rudd SG, Schaller T and Herold N: SAMHD1
is a barrier to antimetabolite-based cancer therapies. Mol Cell
Oncol. 4:e12875542017. View Article : Google Scholar
|
|
42
|
Zhu KW, Chen P, Zhang DY, Yan H, Liu H,
Cen LN, Liu YL, Cao S, Zhou G, Zeng H, et al: Association of
genetic polymorphisms in genes involved in Ara-C and dNTP
metabolism pathway with chemosensitivity and prognosis of adult
acute myeloid leukemia (AML). J Transl Med. 16:902018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kohnken R, Kodigepalli KM and Wu L:
Regulation of deoxynucleotide metabolism in cancer: Novel
mechanisms and therapeutic implications. Mol Cancer. 14:1762015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mauney CH and Hollis T: SAMHD1: Recurring
roles in cell cycle, viral restriction, cancer, and innate
immunity. Autoimmunity. 51:96–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang Z, Bhattacharya A, Villacorta J,
Diaz-Griffero F and Ivanov DN: Allosteric activation of SAMHD1
protein by deoxynucleotide triphosphate (dNTP)-dependent
tetramerization requires dNTP concentrations that are similar to
dNTP concentrations observed in cycling T cells. J Biol Chem.
291:21407–21413. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bonifati S, Daly MB, St Gelais C, Kim SH,
Hollenbaugh JA, Shepard C, Kennedy EM, Kim DH, Schinazi RF, Kim B
and Wu L: SAMHD1 controls cell cycle status, apoptosis and HIV-1
infection in monocytic THP-1 cells. Virology. 495:92–100. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Herrmann A, Wittmann S, Thomas D, Shepard
CN, Kim B, Ferreirós N and Gramberg T: The SAMHD1-mediated block of
LINE-1 retroelements is regulated by phosphorylation. Mob DNA.
9:112018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang CA, Huang HY, Chang YS, Lin CL, Lai
IL and Chang JG: DNA-sensing and nuclease gene expressions as
markers for colorectal cancer progression. Oncology. 92:115–124.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jiang H, Li C and Liu Z: Shengjing
Hospital and Hu: Expression and relationship of SAMHD1 with other
apoptotic and autophagic genes in acute myeloid leukemia patients.
Acta Haematol. 143:51–59. 2020. View Article : Google Scholar
|