Introduction
Head and neck squamous cell carcinoma (HNSC) is one
of the most common types of malignant tumors worldwide, and its
onset is closely associated with alcohol consumption, tobacco use
and human papillomavirus (1). HNSC
has high morbidity and mortality rates, and >90% of patients are
susceptible to oral squamous cell carcinoma (OSCC). OSCC is the
most devastating and common oral malignancy that accounts for 95%
of all oral cancer types and causes 500,000 deaths/year (2). OSCC tends to occur in the tongue,
cheeks and gums; however, in the advanced stages, it can also
involve the whole tongue, pharynx, jawbone, and vital blood vessels
and nerves in the neck and skull base. This results in numbness,
pain and the significant impairment of speech and swallowing
(3,4). The development of OSCC occurs via a
multi-stage process, which is accompanied by invasion, metastasis
and precancerous lesions. Furthermore, OSCC development is a
consequence of multiple genes, such as HNRNPA2B1, UBE2C and Rab31
(5–7). Moreover, environmental and genetic
factors can regulate the occurrence and progression of OSCC;
however, the specific etiology of the disease remains unclear
(8). Early detection and treatment
are important for patient prognosis, and OSCC is considered to be a
preventable disease (9).
Therefore, it is crucial to deeply understand the underlying
mechanisms in the occurrence and progression of OSCC.
Human hepatocellular carcinoma-related protein 1
(HCRP-1), which is also known as vacuolar protein
sorting-associated protein 37A, is a subunit of the endosomal
sorting complexes required for the transport I protein family,
which mediates the internalization process of membrane protein
ubiquitination in cells (10).
HCRP-1 regulates the cell cycle, proliferation, migration and
apoptosis, and maintains the survival of precursor cells prior to
cell differentiation. Previous studies have demonstrated that
HCRP-1 is a tumor suppressor gene that affects tumor progression,
with low expression in various tumors, including prostate (11), breast (12), liver (13) and non-small cell lung (14) cancer. For example, expression
levels of HCRP-1 are decreased in colon cancer tissues and its
knockdown promotes cell invasion and migration (15). Furthermore, HCRP-1 has been
reported to significantly inhibit cell proliferation, invasion and
the epithelial-mesenchymal transition in esophageal squamous cell
carcinoma (16). To date, the
expression pattern of HCRP-1 in OSCC and its clinical significance
remain to be elucidated.
Epithelial growth factor receptor (EGFR) is widely
distributed on the cell surface of mammalian epithelial cells,
including fibroblasts and glial cells; it is mainly composed of the
extracellular ligand-binding region, the transmembrane region and
the intracellular region kinase domain (17). EGFR is a glycoprotein that belongs
to the tyrosine kinase-type receptor family and is a receptor for
EGF, which is responsible for cell proliferation and signal
transduction (18). It has been
reported that EGFR is abnormally expressed in certain solid tumors
and serves a role in tumor proliferation, angiogenesis, metastasis
and apoptosis (19,20).
Therefore, in the present study, the expression
pattern of HCRP-1 in OSCC is discussed. Moreover, whether HCRP-1
regulates EGFR expression levels, along with its downstream
effectors, is investigated to determine the regulatory mechanism of
OSCC tumor cell behavior.
Materials and methods
Cell culture and transfection
Human immortalized oral epithelial cells were
purchased from Qingqi (Shanghai) Biotechnology Development Co.,
Ltd. OSCC CAL-27, Fadu, SCC-4 and SCC-15 cell lines were purchased
from the American Type Culture Collection, and immortalized HUVECs
were purchased from Cobioer Biosciences Co., Ltd. Cells were
incubated in DMEM (Gibco; Thermo Fischer Scientific, Inc.) with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Shanghai Grammar Biotechnology Co., Ltd.)
at 37°C with 5% CO2. Exogenous EGF (30 ng/ml,
Sigma-Aldrich; Merck KGaA) (21)
and colivelin (1 µM; Selleck Chemicals) (22) were used to treat CAL-27 cells to
activate EGFR and STAT3, respectively.
CAL-27 cells were transfected with 4 µg specific
pcDNA3.1 plasmids (VectorBuilder, Inc.) to overexpress HCRP-1
(ov-HCRP-1 group). Cells transfected with 4 µg empty pcDNA3.1
plasmids were used as negative controls (ov-NC group). Transfection
was performed using FuGENE HD reagent [Roche Diagnostics (Shanghai)
Co., Ltd.] at 37°C for 24 h and transfection efficiency was
confirmed via reverse transcription-quantitative PCR (RT-qPCR) and
western blotting. Cells were collected for subsequent experiments
after 24 h co-culture at 37°C.
RT-qPCR
Total RNA was extracted from untransfected oral
epithelial cells and OSCC cells and complementary DNA was produced
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and the PrimeScript RT Reagent Kit (Takara
Biotechnology Co., Ltd.), respectively, according to the
manufacturers' protocols. qPCR was performed using the QuantiTect
SYBR Green PCR Kit (Qiagen China Co., Ltd.). The following
thermocycling conditions were used: Initial denaturation at 95°C
for 10 min; 40 cycles of denaturation at 95°C for 10 sec, annealing
at 60°C for 20 sec and elongation at 72°C for 30 sec; and a final
extension at 72°C for 7 min. Relative HCRP-1 mRNA expression levels
were normalized against GAPDH and quantified using the
2−∆∆Cq method (23).
HCRP-1 forward, 5′-CTGGCTTTTTCCCCTGACCA-3′ and reverse
5′-AGTGTGAGTTCCGGAGGGA-3′; and GAPDH forward,
5′-GACTCATGACCACAGTCCATGC-3′ and reverse,
5′-AGAGGCAGGGATGATGTTCTG-3′.
Western blotting
Proteins were extracted from untransfected oral
epithelial cells and OSCC cells or transfected OSCC cells using
RIPA lysis buffer (Beyotime Institute of Biotechnology) and total
protein was quantified using Nanodrop spectrophotometer (Thermo
Fisher Scientific, Inc.). Total protein (40 µg per lane) was
separated on a 10% gel using SDS-PAGE on a polyacrylamide gel and
then the separated proteins were transferred to a PVDF membrane
[Roche Diagnostics (Shanghai) Co., Ltd.]. The membranes were
blocked using skimmed milk for 1 h at room temperature.
Subsequently, the membranes were incubated at 4°C overnight with
the following primary antibodies against: HCRP-1 (cat. no.
ab251760; 1:5,000), MMP9 (cat. no. ab283575; 1:1,000), MMP14 (cat.
no. ab51074; 1:5,000), EGFR (cat. no. ab32077; 1:5,000),
phosphorylated (p)-EGFR (cat. no. ab40815; 1:2,000), STAT3 (cat.
no. ab109085; 1:1,000) and p-STAT3 (cat. no. ab267373; 1:1,000).
Following the primary incubation, the membranes were incubated with
HRP-conjugated secondary antibody (cat. no. ab6721; 1:5,000) for 2
h at room temperature. All antibodies were purchased from Abcam.
Blots were visualized using an ECL detection reagent
(MilliporeSigma) and data were analyzed using ImageJ 1.52 software
(National Institutes of Health).
Cell counting kit-8 (CCK-8) assay
Transfected CAL-27 cells (3×103
cells/well) were seeded into a 96-well plate, treated with EGF and
colivelin, and incubated for 24, 48 or 72 h. At each time point,
each well was supplemented with 10 µl CCK-8 solution (Dojindo
Laboratories, Inc.). Absorbance was assessed using a microplate
reader (Perlong Medical Equipment Co., Ltd.) at 450 nm following
incubation for 2 h.
Colony formation assay
Transfected CAL-27 cells (500 cells/dish) were
seeded into culture dishes, treated with EGF and colivelin, and
cultured at 37°C for 2 weeks. Subsequently, the cells were fixed
with 4% paraformaldehyde (Shanghai Aladdin Biochemical Technology
Co. Ltd.) for 15 min and stained with crystal violet (Shanghai
Yeasen Biotechnology Co., Ltd.) for 30 min, both at room
temperature. Images were captured of the results and a cluster of
>50 cells was regarded as a colony. The number of colonies was
counted manually.
Wound healing assay
Transfected CAL-27 cells (5×105
cells/well) were seeded into a 6-well plate, treated with EGF and
colivelin, and cultured at 37°C until an 80% confluent monolayer
formed. A sterile pipette tip was used to generate a wound in the
middle of the monolayer. Cells were washed with serum free medium
to remove floating cells and then incubated in DMEM supplemented
with 1% FBS at 37°C. Images were captured at 0 and 24 h using a
light microscope (magnification, ×100; Olympus Corporation). The
relative migration rate was calculated as (0 h scratch width-24 h
scratch width)/0 h scratch width ×100%.
Transwell assay
To assess the invasion of transfected CAL-27 cells
or the migration of HUVECs following EGF and colivelin treatment, a
cell suspension (1×105 cells) was added to the upper
chamber (8 µm; Corning, Inc.) of 24-well Transwell plates (8.0-µm
PET membrane; Corning, Inc.) in 400 µl serum-free DMEM precoated
with or without Matrigel (BD Biosciences) as described previously
(24). RPMI-1640 with 20% FBS was
added to the lower chamber. Following 24 h of incubation at 37°C,
the cells on the lower surface were fixed with 90% ethanol solution
for 30 min at 37°C and stained with 0.1% crystal violet for 10 min
at room temperature. Images were captured using a light microscope
(magnification, ×100; Olympus Corporation).
Angiogenesis assay
HUVECs (8×104 cells/well) were seeded
into a 24-well plate precoated with Matrigel as described
previously (25) and cultured
until adherence. The original culture medium was substituted with
the culture medium from untransfected CAL-27 cells, CAL-27 cells
transfected with Ov-NC, Ov-CHRP-1 plasmids and transfected CAL-27
cells treated with EGF and colivelin followed by incubation at 37°C
for 6 h. The structure of the tubes was observed using a light
microscope (magnification, ×4; Olympus Corporation) and the images
were analyzed using ImageJ 1.52 software (National Institutes of
Health).
Statistical analysis
Experiments were independently performed three
times. Data are presented as the mean ± SD and statistical analysis
was performed using Prism 8.0 software (GraphPad Software, Inc.).
One-way ANOVA followed by Tukey's post hoc test was used to compare
the statistical differences between three or more groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
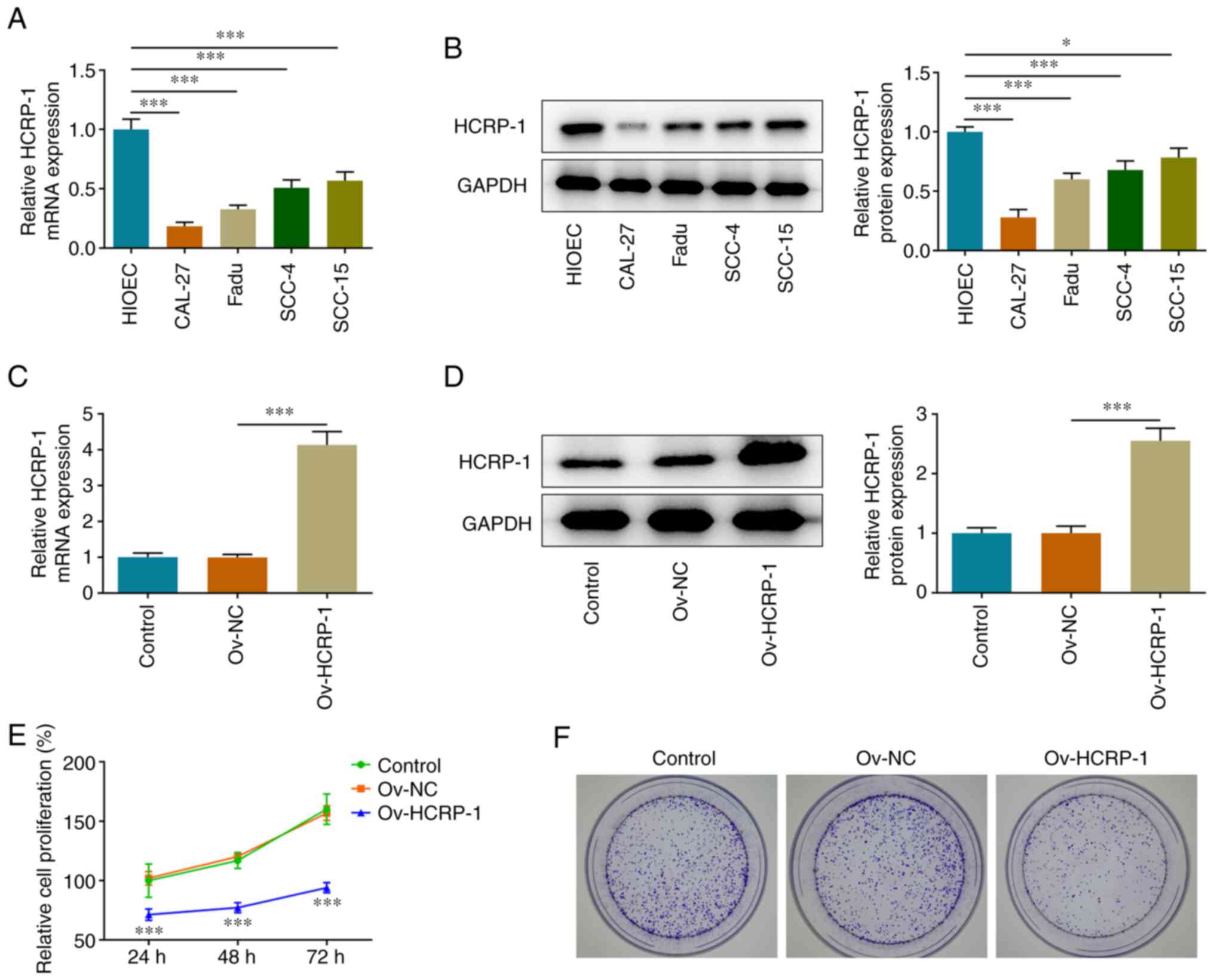
HCRP-1 overexpression inhibits OSCC
cell proliferation
The expression levels of HCRP-1 in oral epithelial
and OSCC cells were determined using RT-qPCR and western blotting.
The results demonstrated that HCRP-1 expression levels were
significantly downregulated in OSCC cells compared with HIOEC
cells. The most significant decline in HCRP-1 expression was noted
in CAL-27 cells and these cells were therefore selected for use in
the subsequent assays to highlight the underlying role of HCRP-1
(Fig. 1A and B). CAL-27 cells were
transfected to overexpress HCRP-1, and RT-qPCR and western blotting
were performed to verify transfection efficiency (Fig. 1C and D). The proliferation of
transfected cells was subsequently assessed using the CCK-8 and
colony formation assays. HCRP-1 overexpression significantly
reduced the proliferation of CAL-27 cells (Fig. 1E) and the number of colonies formed
was also markedly reduced compared with the ov-NC group (Fig. 1F).
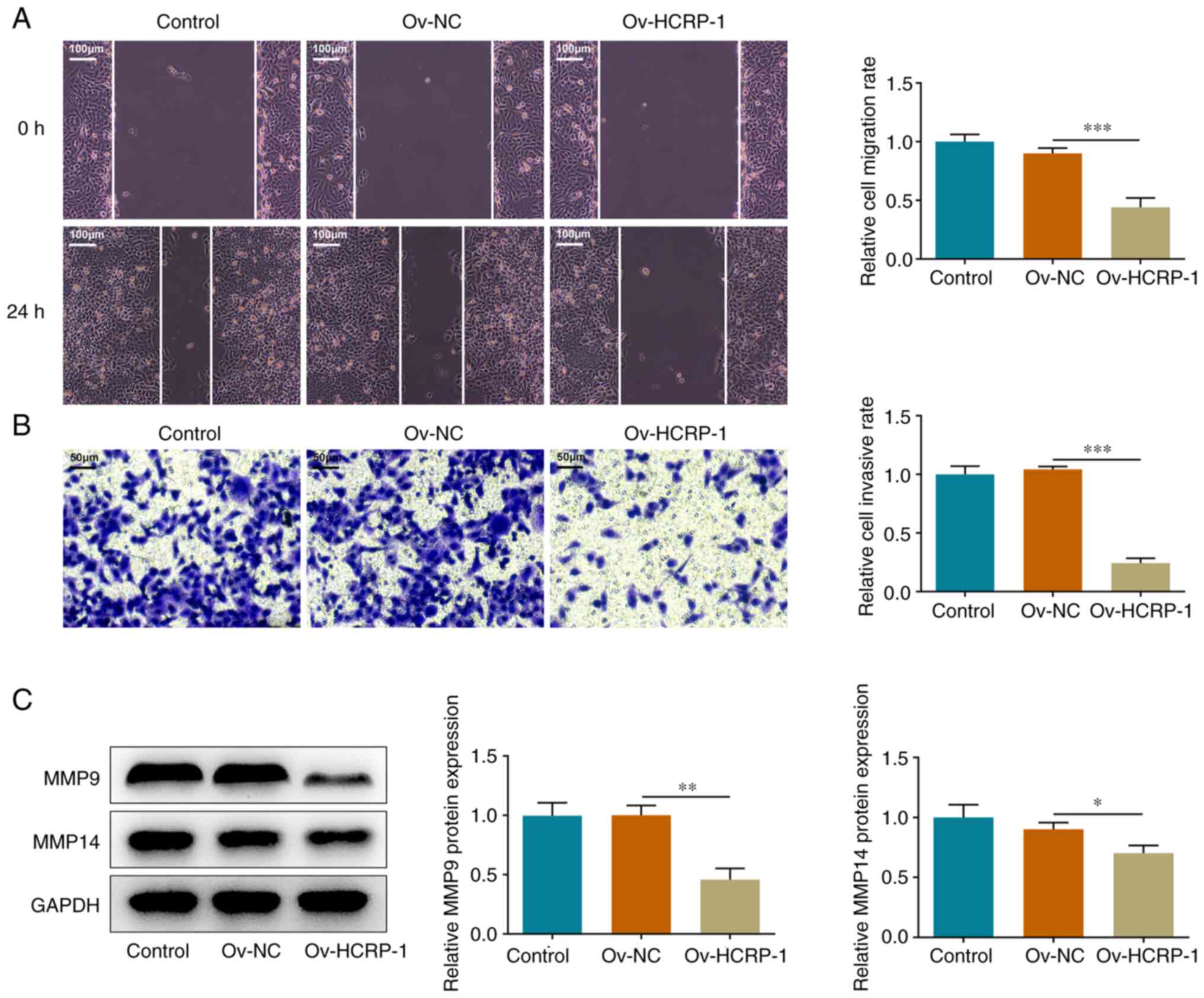
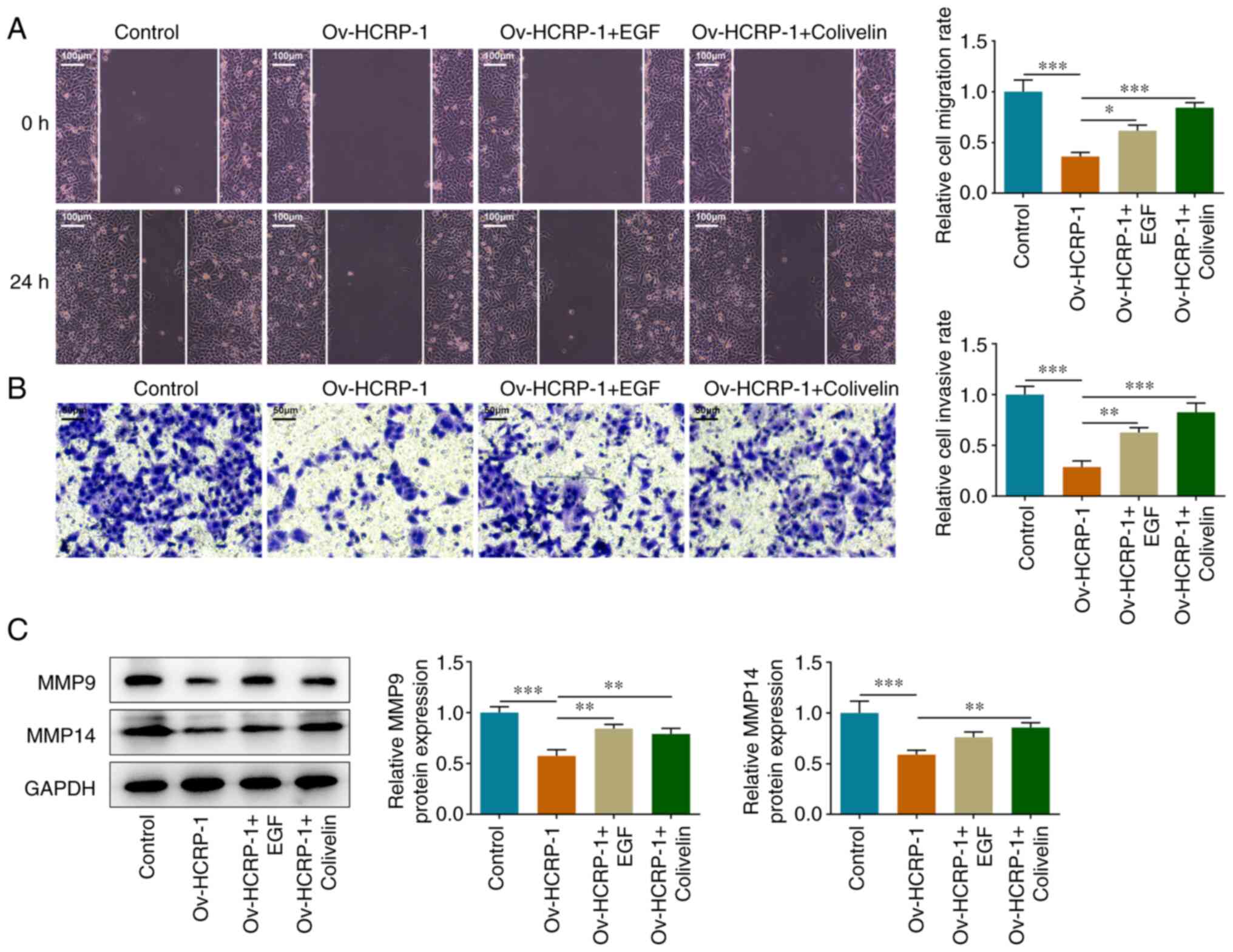
HCRP-1 overexpression inhibits OSCC
cell migration and invasion
The effects of HCRP-1 overexpression on cell
migration and invasion were subsequently assessed. The migration
rate was quantified using the wound healing assay. The results
demonstrated that cell migration in the Ov-HCRP-1 group was
suppressed compared with that in the Ov-NC group (Fig. 2A). The results from the Transwell
assay also indicated that HCRP-1 overexpression suppressed the
invasion ability of the CAL-27 cells (Fig. 2B). Furthermore, the protein
expression levels of MMP9 and MMP14 were determined using western
blotting. Compared with the Ov-NC group, the protein expression
levels of MMP9 and MMP14 were significantly decreased in the
Ov-HCRP-1 group (Fig. 2C).
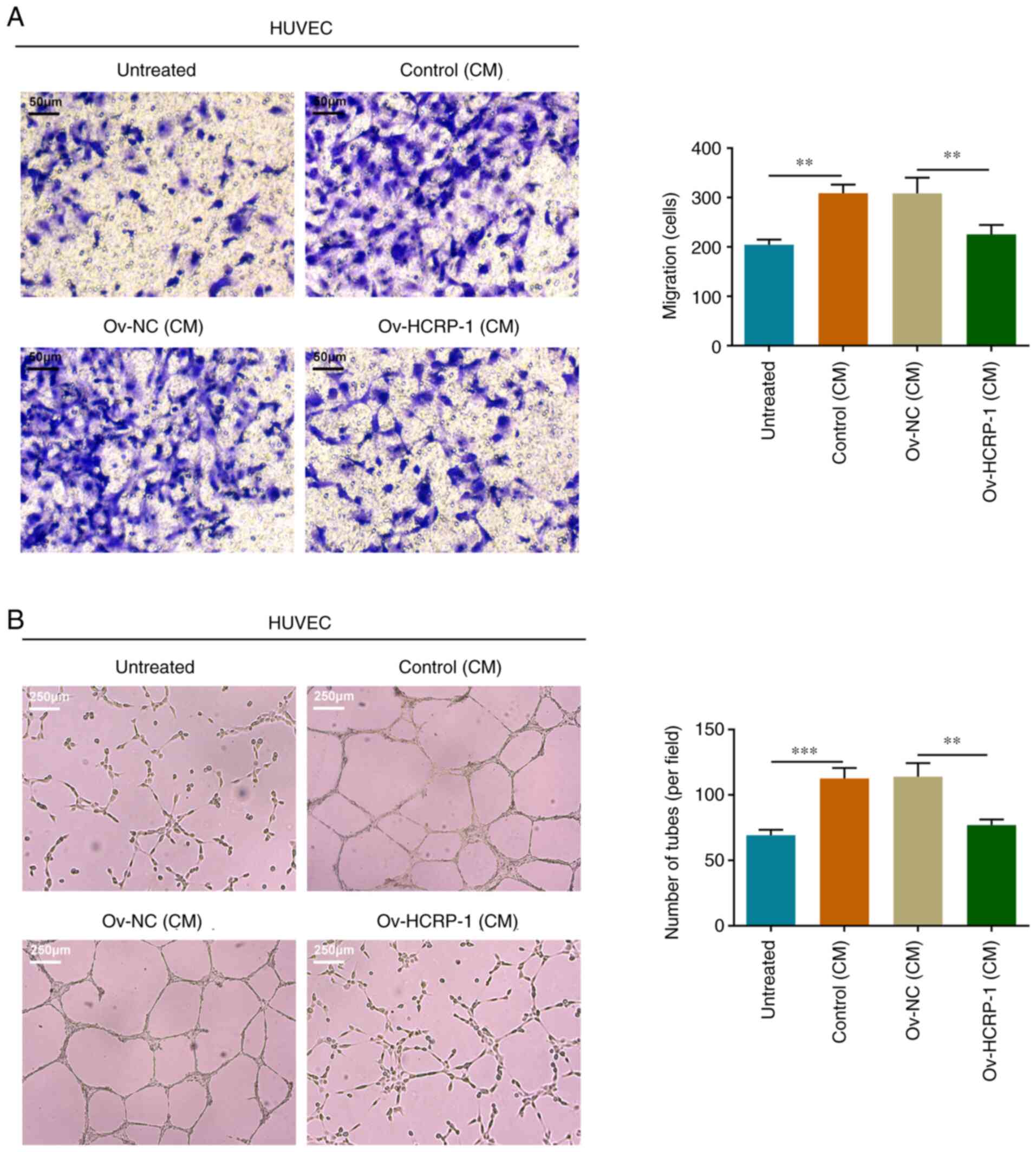
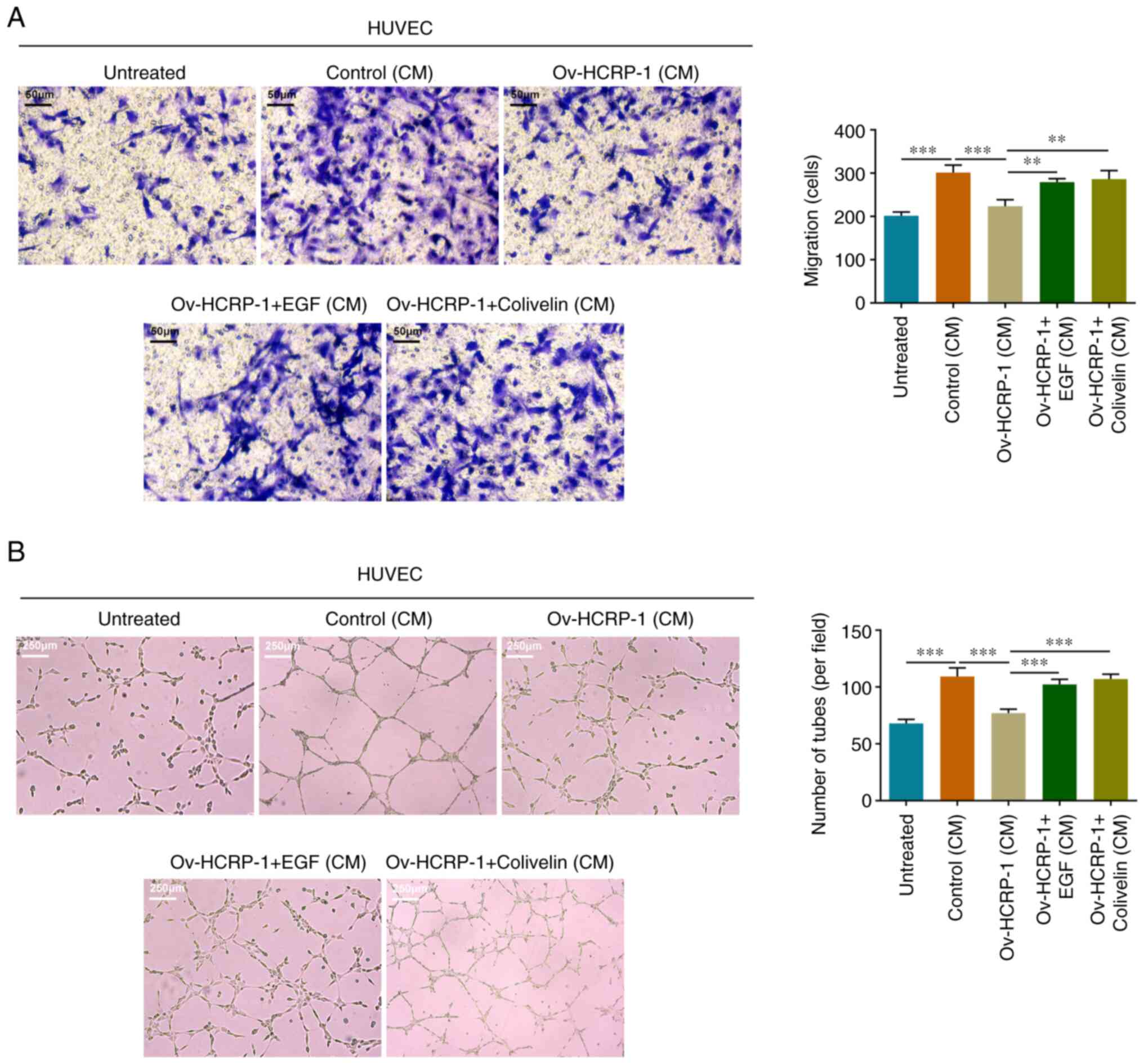
HCRP-1 overexpression inhibits HUVEC
migration and angiogenesis
The culture medium from the control, Ov-NC and
Ov-HCRP-1 groups of CAL-27 cells was separately supplemented into
the HUVEC culture and the migration ability of the HUVECs was
assessed using the Transwell assay. HUVECs in the culture medium
from the control group possessed a relatively strong migratory
capacity, whereas the migratory capacity of the cells in the
culture medium from the Ov-HCRP-1 group was inhibited (Fig. 3A). Moreover, the tube formation
ability in these groups of HUVECs was assessed using the
angiogenesis assay. Similar to the aforementioned results, the
culture medium from the control group promoted the angiogenesis of
HUVECs, whereas the culture medium from the Ov-HCRP-1 group
markedly suppressed angiogenesis, which resulted in a smaller
number of junctions (Fig. 3B).
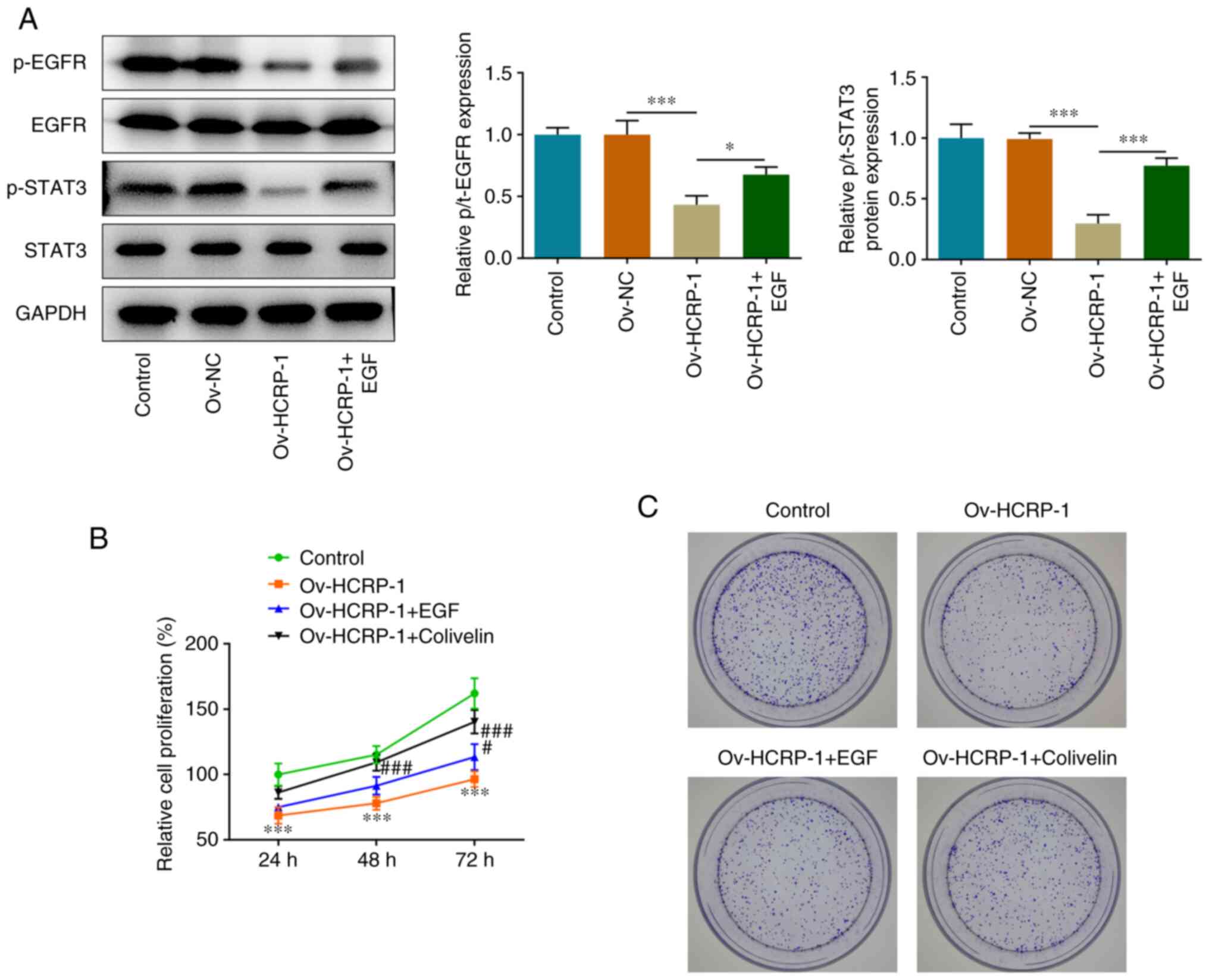
HCRP-1 overexpression inhibits the
EGFR/STAT3 signaling pathway in OSCC cells
The expression levels of proteins associated with
the EGFR/STAT3 signaling pathway were determined using western
blotting. The protein expression levels of p-EGFR and p-STAT3 were
significantly decreased in the Ov-HCRP-1 group, whereas EGF
treatment reversed this decrease (Fig.
4A). To assess the roles of EGFR and STAT3 in the regulation of
HCRP-1, EGF and colivelin were used to treat CAL-27 cells. The
proliferation of cells was determined using the CCK-8 and colony
formation assays. EGF and colivelin treatment both promoted
proliferation compared with the Ov-HCRP-1 group (Fig. 4B and C).
HCRP-1 functions via the regulation of
the EGFR/STAT3 signaling pathway
Subsequently, the effects of EGF and colivelin on
the migration and invasion of CAL-27 cells were assessed. The
results from the wound healing and Transwell assays demonstrated
that EGF and colivelin treatment accelerated cell migration and
invasion (Fig. 5A and B). These
results were supported by the increase exhibited in the MMP9 and
MMP14 protein expression levels (Fig.
5C). Furthermore, the migration and angiogenesis in each group
of HUVECs were assessed. EGF and colivelin treatment facilitated
the migration and angiogenesis of HUVECs, which indicated that the
activation of EGFR/STAT3 signaling reversed the effects of HCRP-1
overexpression on cells (Fig.
6).
Discussion
The special anatomical structure and environment of
the oral cavity, the abundant blood supply of the maxillofacial
region, and the abundant lymph node tissue of the maxillofacial
region and neck have led to the limitation of conventional surgical
treatment and radio-chemotherapy for OSCC (26). The 5-year survival rate of patients
has not significantly improved and remains at only 50% (27,28).
Moreover, for patients with advanced OSCC with recurrence or
distant metastasis, the 5-year survival rate is <50% (29). With the development of molecular
targeted therapy and individualized treatment, clinical
multidisciplinary comprehensive treatment has gradually emerged,
which provides a novel opportunity for the treatment of OSCC
(30–32). A recent study by Yokokawa et
al (33) reported that,
according to data from 208 patients with OSCC following
post-surgical treatment, EGFR overexpression can be regarded as an
indicator to evaluate patient prognosis. Moreover, the molecular
targeted drug cetuximab has also been approved for the clinical
treatment of OSCC and has previously been shown to achieve
significant efficiency (34).
These aforementioned studies have therefore demonstrated the use of
EGFR as an important therapeutic target.
However, the application of cetuximab still has
certain difficulties including mutations, toxicity/side effects,
drug resistance and an optimal dosage to administer (35–38).
Furthermore, EGFR level detection needs to be performed prior to
the administration of cetuximab. At present, each detection method
has both advantages and disadvantages, and there is no unified
standard, which leads to different results in the same patient
(39). Alternatively, downstream
or upstream regulation from a known target can also be used as a
potential approach. Therefore, in the present study, compared with
that in oral epithelial cells, the expression of HCRP-1 was
downregulated in OSCC cells and HCRP-1 overexpression could inhibit
cell proliferation, migration, invasion and angiogenesis. These
results suggested that HCRP-1 may potentially act as a tumor
suppressor in OSCC as well as in the aforementioned types of cancer
(prostate, breast, liver, colon and non-small cell lung cancer).
Subsequently, it was demonstrated that HCRP-1 overexpression
inhibited EGFR phosphorylation and that EGF treatment markedly
increased EGFR expression in cells, which reversed the effects of
HCRP-1 overexpression. EGFR signaling is involved in the malignant
process of OSCC (40) and it can
therefore be hypothesized that HCRP-1 can alleviate the malignant
phenotype of cells via the inhibition of EGFR.
EGFR binds to EGF and undergoes homodimerization or
heterodimerization, which results in the phosphorylation of
intracellular tyrosine residues. Subsequently activated receptors
recruit signaling complexes, activate downstream signaling
proteins, and finally regulate tumor cell proliferation and
metastasis (41,42). Therefore, in the present study, the
protein expression levels of downstream STAT3 signaling pathway
proteins were investigated. The results demonstrated that HCRP-1
also inhibited the phosphorylation of STAT3. Moreover, colivelin
treatment reversed the inhibition of HCRP-1 overexpression on cell
malignant progression, which suggested that STAT3 signaling
potentially mediated the regulatory mechanism of HCRP-1 on cells.
The present study therefore demonstrated the regulatory pattern of
HCRP-1 in OSCC cells. However, a limitation of the present study is
that only in vitro experiments were included, and therefore,
animal experiments should be performed in future work. In the
present study, HCRP-1 was overexpressed to explore its role in
OSCC, and knocking it down could be used to verify the present
study findings in the future. Data is yet to be located that
mentions HRCP-1 expression in patients with OSCC samples in public
databases; therefore, this will be an area for further
research.
In conclusion, in the present study, HCRP-1
potentially alleviated the malignant phenotype and angiogenesis of
OSCC cells via the downregulation of EGFR/STAT3 signaling. The
present study may have determined the expression pattern and
regulatory pathway of HCRP-1 in OSCC cells and may have further
elucidated certain aspects of OSCC pathology.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and LH contributed to concept, experiments and
analysis. YC contributed to the manuscript draft. YC and LH have
read and approved the final manuscript, and confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh P, Rai A, Verma AK, Alsahli MA,
Rahmani AH, Almatroodi SA, Alrumaihi F, Dev K, Sinha A, Sankhwar S
and Dohare R: Survival-based biomarker module identification
associated with oral squamous cell carcinoma (OSCC). Biology
(Basel). 10:7602021.PubMed/NCBI
|
|
3
|
Romer CAE, Broglie Daeppen MA, Mueller M,
Huber GF, Guesewell S and Stoeckli SJ: Long-term speech and
swallowing function after primary resection and sentinel node
biopsy for early oral squamous cell carcinoma. Oral Oncol.
89:127–132. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jethwa AR and Khariwala SS:
Tobacco-related carcinogenesis in head and neck cancer. Cancer
Metastasis Rev. 36:411–423. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu F, Yang T, Yao M, Shen T and Fang C:
HNRNPA2B1, as a m6A reader, promotes tumorigenesis and
metastasis of oral squamous cell carcinoma. Front Oncol.
11:7169212021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin Z, Zhao X, Cui L, Xu X, Zhao Y, Younai
F, Messadi D and Hu S: UBE2C promotes the progression of head and
neck squamous cell carcinoma. Biochem Biophys Res Commun.
523:389–397. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Zhu F, Liu Z, Tang X, Han Y, Jiang
J, Ma C and He Y: High expression of Rab31 confers a poor prognosis
and enhances cell proliferation and invasion in oral squamous cell
carcinoma. Oncol Rep. 45:1182–1192. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hasegawa K, Fujii S, Matsumoto S, Tajiri
Y, Kikuchi A and Kiyoshima T: YAP signaling induces PIEZO1 to
promote oral squamous cell carcinoma cell proliferation. J Pathol.
253:80–93. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rahimi S: HPV-related squamous cell
carcinoma of oropharynx: A review. J Clin Pathol. 73:624–629. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tomasich E, Topakian T, Heller G, Udovica
S, Krainer M and Marhold M: Loss of HCRP1 leads to upregulation of
PD-L1 via STAT3 activation and is of prognostic significance in
EGFR-dependent cancer. Transl Res. 230:21–33. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun L, Lü J, Ding S, Bi D, Ding K, Niu Z
and Liu P: HCRP1 regulates proliferation, invasion, and drug
resistance via EGFR signaling in prostate cancer. Biomed
Pharmacother. 91:202–207. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang W, Wang JG, Wang Q, Qin Y, Lin X,
Zhou D, Ren K, Hou C, Xu J and Liu X: Decreased HCRP1 promotes
breast cancer metastasis by enhancing EGFR phosphorylation. Biochem
Biophys Res Commun. 477:222–228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu J, Zhang X, Wang H, Ge S, Gao T, Song
L, Wang X, Li H, Qin Y and Zhang Z: HCRP1 downregulation promotes
hepatocellular carcinoma cell migration and invasion through the
induction of EGFR activation and epithelial-mesenchymal transition.
Biomed Pharmacother. 88:421–429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du Y, Wang P, Sun H, Yang J, Lang X, Wang
Z, Zang S, Chen L, Ma J and Sun D: HCRP1 is downregulated in
non-small cell lung cancer and regulates proliferation, invasion,
and drug resistance. Tumour Biol. Oct 13–2016.(Epub ahead of
print). View Article : Google Scholar
|
|
15
|
Chen F, Zhang L, Wu J, Huo F, Ren X, Zheng
J and Pei D: HCRP-1 regulates EGFR-AKT-BIM-mediated anoikis
resistance and serves as a prognostic marker in human colon cancer.
Cell Death Dis. 9:11762018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Y, Yang Y and Xian YS: HCRP1 inhibits
cell proliferation and invasion and promotes chemosensitivity in
esophageal squamous cell carcinoma. Chem Biol Interact.
308:357–363. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferguson KM, Hu C and Lemmon MA: Insulin
and epidermal growth factor receptor family members share parallel
activation mechanisms. Protein Sci. 29:1331–1344. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Talukdar S, Emdad L, Das SK and Fisher PB:
EGFR: An essential receptor tyrosine kinase-regulator of cancer
stem cells. Adv Cancer Res. 147:161–188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sabbah DA, Hajjo R and Sweidan K: Review
on epidermal growth factor receptor (EGFR) structure, signaling
pathways, interactions, and recent updates of EGFR inhibitors. Curr
Top Med Chem. 20:815–834. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Masood A, Kancha RK and Subramanian J:
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors
in non-small cell lung cancer harboring uncommon EGFR mutations:
Focus on afatinib. Semin Oncol. 46:271–283. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu YC, Tsai JJ, Weng YS and Hsu FT:
Regorafenib suppresses epidermal growth factor receptor
signaling-modulated progression of colorectal cancer. Biomed
Pharmacother. 128:1103192020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu S, Fan HW, Li K and Fan XD: Suppression
of Elp2 prevents renal fibrosis and inflammation induced by
unilateral ureter obstruction (UUO) via inactivating
Stat3-regulated TGF-β1 and NF-κB pathways. Biochem Biophys Res
Commun. 501:400–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng HW, Chen YF, Wong JM, Weng CW, Chen
HY, Yu SL, Chen HW, Yuan A and Chen JJ: Cancer cells increase
endothelial cell tube formation and survival by activating the
PI3K/Akt signalling pathway. J Exp Clin Cancer Res. 36:272017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Dong L, Zhong H, Yang L, Li Q, Su
C, Gu W and Qian Y: Extracellular vesicles (EVs) from lung
adenocarcinoma cells promote human umbilical vein endothelial cell
(HUVEC) angiogenesis through yes kinase-associated protein (YAP)
transport. Int J Biol Sci. 15:2110–2118. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shintani T, Takatsu F, Rosli SNZ, Usui E,
Hamada A, Sumi K, Hayashido Y, Toratani S and Okamoto T:
Eldecalcitol (ED-71), an analog of
1α,25(OH)2D3, inhibits the growth of squamous
cell carcinoma (SCC) cells in vitro and in vivo by down-regulating
expression of heparin-binding protein 17/fibroblast growth
factor-binding protein-1 (HBp17/FGFBP-1) and FGF-2. In Vitro Cell
Dev Biol Anim. 53:810–817. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sciubba JJ and Larian B: Oral squamous
cell carcinoma: Early detection and improved 5-year survival in 102
patients. Gen Dent. 66:e11–e16. 2018.PubMed/NCBI
|
|
28
|
Chaturvedi AK, Anderson WF,
Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, Rosenberg PS,
Bray F and Gillison ML: Worldwide trends in incidence rates for
oral cavity and oropharyngeal cancers. J Clin Oncol. 31:4550–4559.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sá JO, Trino LD, Oliveira AK, Lopes AFB,
Granato DC, Normando AGC, Santos ES, Neves LX, Carnielli CM and
Paes Leme AF: Proteomic approaches to assist in diagnosis and
prognosis of oral cancer. Expert Rev Proteomics. 18:261–284. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mirza S, Hadi N, Pervaiz S, Zeb Khan S,
Mokeem SA, Abduljabbar T, Al-Hamoudi N and Vohra F: Expression of
HER-2/neu in oral squamous cell carcinoma. Asian Pac J Cancer Prev.
21:1465–1470. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pillai J, Chincholkar T, Dixit R and
Pandey M: A systematic review of proteomic biomarkers in oral
squamous cell cancer. World J Surg Oncol. 19:3152021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wittekindt C, Wagner S, Sharma SJ,
Würdemann N, Knuth J, Reder H and Klußmann JP: HPV-a different view
on head and neck cancer. Laryngorhinootologie. 97 (Suppl
1):S48–S113. 2018.PubMed/NCBI
|
|
33
|
Yokokawa M, Morita KI, Oikawa Y, Oikawa Y,
Kayamori K, Sakamoto K, Ikeda T and Harada H: Co-expression of EGFR
and MET has a synergistic effect on the prognosis of patients with
oral squamous cell carcinoma. J Oral Pathol Med. 49:235–242. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chai AWY, Lim KP and Cheong SC:
Translational genomics and recent advances in oral squamous cell
carcinoma. Semin Cancer Biol. 61:71–83. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin P, Cui S, Liao X and Yao X: Galectin-3
blockade suppresses the growth of cetuximab-resistant human oral
squamous cell carcinoma. Mol Med Rep. 24:6852021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Uzawa K, Kasamatsu A, Saito T, Kita A,
Sawai Y, Toeda Y, Koike K, Nakashima D, Endo Y, Shiiba M, et al:
Growth suppression of human oral cancer cells by candidate agents
for cetuximab-side effects. Exp Cell Res. 376:210–220. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ding L, Ren J, Zhang D, Li Y, Huang X, Ji
J, Hu Q, Wang H, Ni Y and Hou Y: The TLR3 agonist inhibit drug
efflux and sequentially consolidates low-dose cisplatin-based
chemoimmunotherapy while reducing side effects. Mol Cancer Ther.
16:1068–1079. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Russo A, Franchina T, Ricciardi G,
Battaglia A, Picciotto M and Adamo V: Heterogeneous responses to
epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors
(TKIs) in patients with uncommon EGFR mutations: New insights and
future perspectives in this complex clinical scenario. Int J Mol
Sci. 20:14312019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eskilsson E, Røsland GV, Solecki G, Wang
Q, Harter PN, Graziani G, Verhaak RGW, Winkler F, Bjerkvig R and
Miletic H: EGFR heterogeneity and implications for therapeutic
intervention in glioblastoma. Neuro Oncol. 20:743–752. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tseng YK, Chen CF, Shu CW, Lee CH, Chou
YT, Li YJ, Liou HH, Cheng JT, Chen CL, Ger LP and Liu PF: Effect of
EGFR on SQSTM1 expression in malignancy and tumor progression of
oral squamous cell carcinoma. Int J Mol Sci. 22:122262021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tanaka T, Zhou Y, Ozawa T, Okizono R,
Banba A, Yamamura T, Oga E, Muraguchi A and Sakurai H:
Ligand-activated epidermal growth factor receptor (EGFR) signaling
governs endocytic trafficking of unliganded receptor monomers by
non-canonical phosphorylation. J Biol Chem. 293:2288–2301. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun N, Zhang X, Zhang X and Kim KM: The
EGF receptor inhibits the signaling of dopamine D3
receptor through the phosphorylation of GRK2 on tyrosine residues.
Biochem Biophys Res Commun. 489:515–522. 2017. View Article : Google Scholar : PubMed/NCBI
|