Introduction
Merkel cell carcinoma (MCC) is a rare and highly
aggressive neuroendocrine malignancy thought to be arising from
mechanoreceptors in the basal epidermis. It has also been discussed
in the literature that Merkel cells might not be the cell of origin
in MCC, but instead derived from epidermal stem cells or other
primitive totipotent stem cells that under malignant transformation
gain neuroendocrine characteristics (1). This theory is partly considered due
to various expression patterns of immunohistochemically markers,
and in some patients MCC are found concomitant with other
epithelial lesions like basal cell carcinoma or squamous cell
carcinoma in the same area (2).
Therefore, the term ‘primary neuroendocrine carcinoma of the skin
(PNECS)’ has been suggested as an alternative nomenclature. MCC is
associated with Merkel cell polyomavirus (MCPyV) and UV exposure,
and other risk factors include age, Caucasian skin type, and
immunosuppression (e.g. HIV or transplant recipients) (3). It most commonly presents as rapidly
growing and painless nodules in the skin of the face and neck, but
lymph node metastases without a primary localization have also been
reported (4). MCC belongs to the
small cell carcinoma family shared with small cell lung carcinoma
(SCLC), carcinoids and medullary carcinoma of the thyroid, but
despite the similarity, paraneoplastic syndromes are rarely seen in
MCC, and instead are more commonly reported in SCLC (5). It spreads rapidly to distant lymph
nodes and has a high propensity for recurrence following treatment,
with an overall 5-year survival rate of 0–18% in patients with
distant metastases (6). With
better results in survival rate, avelumab (FDA-approved 2017) is
now used as a monotherapy for adults with metastatic MCC (6). It targets the programmed death-ligand
(PD-L1), which in several cases is upregulated on tumor cells to
inactivate T-cells, and underlies the mechanism by which the tumor
cells evade the immune system. PD-L1 inhibition with avelumab makes
it possible for the continued recognition of tumor cells as foreign
by T-cells and thus for effective elimination of the tumor
(7).
Lambert-Eaton myasthenic syndrome (LEMS) is a
disorder of neuromuscular transmission, which is caused by
antibodies against the P/Q-type voltage-gated calcium channels
(VGCC) on the presynaptic nerve terminals. This impairs the release
of acetylcholine and results in a poorly transmitted action
potential with ensuing muscle weakness. LEMS usually presents with
areflexia, proximal muscle weakness (especially in the lower limbs)
and autonomic dysfunction (8).
More than 50% of cases are associated with underlying malignancies
(primarily SCLC), which express functional VGCCs (9). Diagnosis is confirmed using
electromyography, clinical examination, and detection of
antibodies; however, ~15% of patients with LEMS lack these
antibodies, thus these criteria alone cannot be used to exclude a
diagnosis (8). Treatment of
underlying malignancy can often reduce the muscular symptoms, but
complementing treatment with 3,4-diaminopyridine (and sometimes
also immunosuppressants and pyridostigmine) is usually essential
(10).
Case report
The patient presented has provided written informed
consent for publishing his data and associated images (documented
in his patient files), and all reporting and operational procedures
were performed in accordance with the Declaration of Helsinki. In
January 2018, a 67-year-old man presented with around a 1-year
history of progressive muscle weakness and involuntary weight loss.
The weakness was most prominent in the lower limbs, which had made
him wheelchair-bound over the last few months. He also reported dry
mouth, erectile dysfunction, and constipation as signs of autonomic
dysfunction. There was no previous history of malignancy, but he
was an ex-smoker with 25 previous pack-years and was obese with
hypertension at the time of presentation.
In March of the same year, clinical examinations
were performed, and together with elevated titers of P/Q-type
VGCC-antibodies in serum (65.2 pmol/l, ref <40), a diagnosis of
LEMS was confirmed and symptomatic treatment with
3,4-diaminopyridine and pyridostigmine was initiated. Since LEMS is
strongly associated with malignancy, CT thorax/abdomen, ultrasound
of scrotum, and 18-FDG-PET/CT were performed. No primary tumors on
the skin were identified. Slightly enlarged nodes were observed in
the left axilla and mediastinum, and more prominent nodes were
observed in the right groin area. The 18-FDG-PET/CT presented
increased activity in a small area of the prostate, but urological
examinations ruled out malignancy. Additionally, four areas with
increased metabolism were also detected in both the right groin and
iliac lymph nodes: A 3.5 cm node along the right external iliac
artery, a 1.7 cm node deeply located, a 3 cm node superficially
located, and lastly a node in an area dorsally and adjacent to the
left ischial bone.
Extirpation of one of the nodes in the right groin
was performed, and immunohistochemically stained preparations
showed markers associated with neuroendocrine tumors, with the
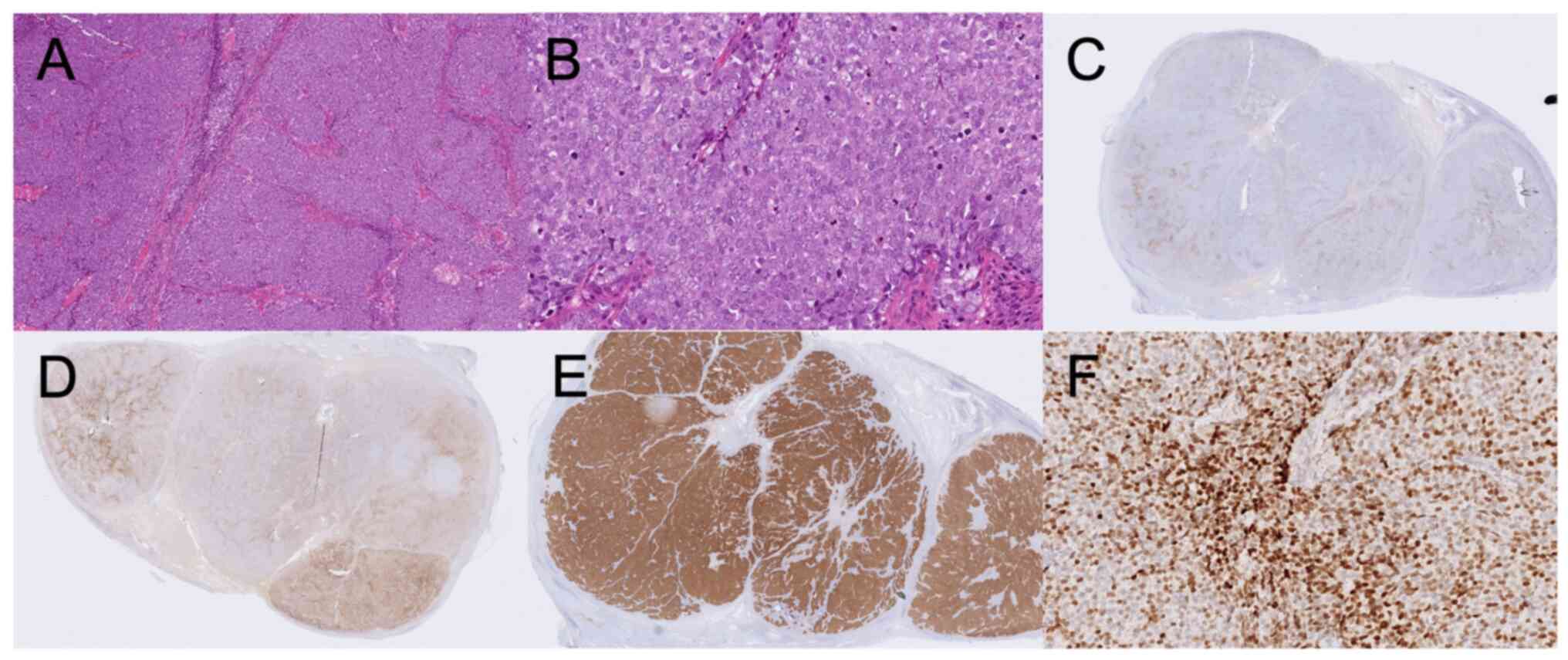
exception of chromogranin-A. Staining was positive for AE1/AE3
(Dako M3515, clone AE1/AE3, dilution 1:50), synaptophysin
(Novocastra NCL-L-synap-299, clone 27G12, dilution 1:50), CD56
(Cellmarque 156R-94, clone MRQ42, dilution 1:500), and TTF-1
(Novocastra NCL TTF-1, clone SPT24, dilution 1:100), and dot-like
positive for CK20 (Dako M7010, clone Ks20.8, dilution 1:25).
Chromogranin A (Roche 760–2519, clone LK2H10, no dilution) was
negative and Ki-67 (Roche 790–4286, clone 30–9, no dilution) was
positive with 48% positive cells. All antibodies were stained in a
Benchmark Ultra using either Ulatrview or Optiview kit. No negative
controls were used for the routine stainings. The
immunohistochemical stainings considered relevant for the final
diagnostic workup are shown in Fig.
1 (stainings for the other markers not shown). The most common
staining pattern of MCC is Chromogranin A and CK20 positivity, and
TTF-1 negativity. Since the results from our immunohistochemical
staining did not follow the typical pattern (and only metastases,
but no primary tumor was found), the MCC diagnosis was considered,
but not fully clarified at this stage. Further investigation with
MCPyV PCR was performed, amplifying the small T and viral protein 1
regions with primers and probes described in Table I. The detailed experimental
procedure of the PCR has been described previously (11). Presence of MCPyV was confirmed, and
together with the ELECTHIP criteria (discussed further below), MCC
was considered the most plausible diagnosis. Due to the dot-like
positive staining of TTF-1, the conclusion from the
multidisciplinary team meeting was to consider the staining as
positive, but the antibody clone used at that time in our lab is
known to crossreact with MCC with this staining pattern. Thus, the
diagnosis of MCC was still considered the most likely despite that
TTF-1 positivity very rarely is seen in MCC (12).
 | Table I.Primer and probe sequences (5′-3′)
used for detection of MCPyV. |
Table I.
Primer and probe sequences (5′-3′)
used for detection of MCPyV.
| Primer
description | Primer name | Sequence (5′-3′) |
|---|
| Forward primer of
small T region | MCPyV-5009.F |
GTCTCGCCAGCATTGTAGTCT |
| Reverse primer of
small T region | MCPyV-4890.R |
AAACTTTCCCAAGTAGGAGGAA |
| Probe of small T
region | MCPyV STp |
GCTATCAGTGCTTTATTCTTTGG |
| Forward primer of
viral protein 1 region | MCPyV-1682.F |
AGGCCTAGTTTTAGATTACCAGAC |
| Reverse primer of
viral protein 1 region | MCPyV-1780.R |
CTTGTGGATCTAGGCCCTGAT |
| Probe of viral
protein 1 region | MCPyV VP1p |
ACAAATGGTGGGCCTATTAC |
In April 2018, 3 months after the first radiological
examinations, the patient had difficulty breathing. CT imaging
showed no signs of lung metastases or pulmonary embolism, but a
discrete progression of the largest node adjacent to the right
acetabulum was observed. At this point, the patient was sent to the
Oncology Department to receive local radiation therapy (3 Gy × 13
fractions) directed towards the enlarged iliac node and the groin
area. About 1 month later, the patient exhibited some regression of
the symptoms, but still experienced morning stiffness and muscle
weakness. In June 2018, during the final weeks of radiation
therapy, the muscle weakness gradually improved, and the
VGCC-antibody titers were reduced (47.7 pmol/l, ref <40).
A further CT thorax/abdomen showed some regression
of two of the nodes, but the results were not satisfactory enough
and further treatment options were required. Chemotherapy with
carboplatin/etoposide was considered, but this was based primarily
on the indications to treat the paramalignant phenomenon and not to
treat the malignancy itself. Because of its recommendations as an
effective treatment for metastatic MCC, avelumab was determined to
be the optimal treatment of choice. Due to the unavailability of
avelumab at the time of initiation of therapy,
carboplatin/etoposide was given at full dose (carboplatin AUC5 day
1 and etoposide 100 mg/m2 days 1–3 in 21 day cycles)
instead. Shortly after the first dose, the patient developed
neutropenic fever and required antibiotic treatment. Due to the
neutropenia, the next chemotherapy was therefore reduced to 80% of
the dose, and the third and fourth doses were further reduced to
75%. After ~3 months of chemotherapy, the neutrophil count remained
low, and unfortunately, the overall results from the chemotherapy
were not satisfactory in terms of symptom reduction of LEMS. The
muscular symptoms gradually improved with chemotherapy and the
P/Q-type VGCC antibodies were not detectable anymore on follow-up
tests, but at the end of treatment the symptoms started to recur
and progress again. Shortness of breath was once again reported,
but this time a pulmonary embolism was detected.
These side effects highlighted the need for
alternative treatment options, and at this point, in October 2018,
avelumab was available. A total of 800 mg avelumab was given
intravenously at 2-week intervals, with paracetamol 500 mg ×2 and
cetirizine 10 mg ×1 as pre-medications 1 h before treatment. In
December 2018, the patient experienced further improvement in
muscle strength and no side effects. Another 5 months later he was
able to walk without aids. In June 2019, after 8 months and
administration of 16 doses, a new radiological examination showed a
distinct reduction in the size of the metastases, and it was
decided that the treatment with avelumab should continue every
second week for as long as no serious side effects occurred.
Unfortunately, after about 1 year of avelumab infusions, the
patient was diagnosed with suspected drug-induced hypothyroidism
after presenting with high TSH and low T4 values. Levothyroxine was
prescribed and treatment with avelumab was continued. A CT
thorax/abdomen follow-up after 10 weeks presented lung
infiltrations in the lower right lobe, as well as lateral and
subpleural infiltrates in both upper lobes. There was no metastatic
suspicion, but findings consistent with pneumonitis provided
suspicion of another inflammatory drug-induced side effect. Since
the treatment at this time had been administered for almost 1.5
years with no signs of recurrence, but with upcoming most likely
drug-induced inflammatory side-effects such as hypothyroidism and
pneumonitis, the decision was made to end the treatment.
3,4-diaminopyridine and pyridostigmine were to be continued, and
radiological follow-up with 3-month intervals.
In April 2022, more than 2 years after the last dose
of avelumab, the patient had no clinical or radiological (CT
thorax/abdomen) signs of recurrence. The pulmonary infiltrates were
gone, but a new case of pulmonary embolism had arisen during the
follow-up period. With this exception, the patient was healthy with
few signs of recurrent muscle weakness. As a follow-up, clinical
and radiological examinations with CT thorax/abdomen are from now
on to be performed every 4–6 months.
Discussion
In the present report, the case of a 67-year-old
patient with pronounced paraneoplastic muscle symptoms arising from
an uncommon cutaneous neuroendocrine tumor is described. MCC can
present as a lymph node metastasis without a primary tumor or a
cutaneous tumor, and it can therefore be hard to distinguish MCC
from other neuroendocrine carcinomas. The distinction in diagnosis
is important due to the completely different approaches to
therapeutic management and outcomes (13,14).
In a large study published in 2017, it was found that MCC (both
with and without a primary tumor) could be distinguished from lymph
node metastases caused by other neuroendocrine carcinomas using
seven different criteria: Elderly age, Location of the tumor,
Extent of the disease, Cytokeratin expression, TTF-1 expression,
Histologic type and Merkel cell Polyomavirus detection, abbreviated
as ELECTHIP (13). The study
concluded that all patients with MCC had at least five of these
criteria, while almost everyone (except one patient) with other
neuroendocrine tumors had three or fewer. However, several MCC
tumors without a known primary tumor had four criteria, and this
was taken into consideration when diagnosing our patient (13). To summarize these criteria, the
‘normal’ pattern of MCC is: Age >70 years, tumors localized to
the inguinal or parotid area, disease extent localized to a single
lymph node area rather than systemic spread, positive staining for
CK20, and most commonly negative staining for TTF-1 (15). MCC is a small cell carcinoma, while
other neuroendocrine carcinomas may be small cell, large cell, or
well-differentiated. A total of 80% are positive for MCPyV
(16). Interestingly,
MCPyV-positive tumors are associated with better 5-year survival
rates and are less likely to have spread at diagnosis compared with
MCPyV-negative tumors (17).
Although the presence of MCPyV was used as one of the criteria, the
possibility of a false positive PCR should not be overlooked
(18). To minimize for this
uncertainty, appropriate positive (plasmid pMCV-R17a) and negative
(material from empty paraffin-block treated in the same way as the
patient sample) controls where used when analyzing the sample. This
uncertainty was also considered during the diagnostic workup by the
multidisciplinary team meeting. Our patient matched four of these
seven criteria: Tumor localized to the inguinal area, CK20
positivity, small cell histology, and detectable MCPyV. The
slightly enlarged nodes in the axilla and mediastinum were not
confirmed as malignant, thus the extent of the disease was most
likely localized to two adjacent lymph node areas (Table II). In the present case, markers
including CK20 and TTF-1 (normally used to distinguish MCC from
other small cell carcinomas like SCLC) did not provide concrete
guidance for an MCC diagnosis. TTF-1 is selectively expressed in
the thyroid and pulmonary epithelial cells, while CK20 stains
positive for Merkel cells and some other cells in the GI tract.
SCLC is therefore usually CK20 negative and TTF-1 positive, whereas
MCC exhibits the opposite pattern (negative for TTF-1 and positive
for CK20) (19). Chromogranin A is
also a typical positive neuroendocrine marker, that in our case,
was negatively stained, and together with TTF-1 positivity, made
the conclusion of diagnosis more difficult. The ELECTHIP-criteria
were therefore a useful tool in this case when normal
immunohistochemical staining patterns were absent, and no primary
tumor was located. However, it should be noted that the diagnosis
of MCC in this case remains uncertain since there is no evidence of
primary cutaneous tumor. The diagnostic workup could possibly have
been completed with Gallium-DOTATOC PET/CT which could have added
valuable information. This was not done in our patient. It would
possibly not have added much information regarding the diagnosis,
but rather function as a marker for presence of somatostatin
receptors, and thus guide in choice of treatment with somatostatin
receptor analogues and/or radionucleotide therapy (20).
 | Table II.ELECTHIP criteria. |
Table II.
ELECTHIP criteria.
| Criteria | Indicator | Current case |
|---|
| Elderly age | >70 years | No |
| Location of the
tumor | Inguinal or
parotid | Yes |
| Extent of
disease | Restricted to one
lymph node area | No |
| Cytokeratin
expression | CK20
immunopositivity | Yes |
| TTF-1 expression | Immunonegativity | No |
| Histological
type | Small cell
carcinoma | Yes |
| Merkel cell
polyomavirus detection | Detection on PCR | Yes |
The Nobel Prize in 2018 was awarded to Tasuku Honjo
and James Allison for the discovery of the PD-1 molecule on
T-cells. PD-1 is involved in immune suppression, which is one of
the most important escape mechanisms used by certain tumor cells.
The inhibition of inhibitory mechanisms leads to a more active
immune defense against the cancer and prolongs overall survival
significantly in cancer forms that previously had long-term
survival rates in single-digit percentages (21). The use of these immune checkpoint
inhibitors provides hope to patients that previously only had
months left to live and lays the foundation for further research on
how to modulate and strengthen our immune response, as the most
effective treatment against cancer (21). Until recently, cytotoxic
chemotherapy has been part of the standard treatment for patients
with MCC, even though there is no scientific support for the
effectiveness of this treatment, and the rates of relapse are high
(3). MCC is considered a
chemosensitive carcinoma, but a survival benefit has not been shown
and responses to chemotherapy are usually not durable (22). In 2016, a foundational study was
published regarding the use of avelumab as on treatment naïve
patients and patients who had received previous chemotherapy with
metastatic MCC (6). The results
were promising; 32% objective responses (9% complete and 23%
partial responses), no treatment-related grade 4 events, and
(compared to the relatively high incidence of toxicity-related
morbidity of chemotherapy) acceptable side effects. The study also
showed almost twice as good results in patients with just one
previous line of treatment, compared to the patients that did
receive two or more.
The question was therefore raised if it may be more
effective to start the treatment earlier with fewer previous cycles
of cytotoxic treatment, since a functional immune system is
necessary for the best possible response. For example, it has been
reported that MCC developed shortly after the use of the TNF-a
inhibitor adalimumab, which also suggests the importance of
immunosuppression. In our case, chemotherapy was initiated due to
the unavailability of avelumab. Chemotherapy treatment had to be
stopped after four cycles due to neutropenia. Since the patient
still had symptoms and the disease was progressing at the time, we
decided to administer additional treatment with avelumab.
Inflammatory side effects that presented after initiation of
avelumab included pneumonitis, pulmonary embolism, and
hypothyroidism. Most side effects are well known and tolerable,
except for the neutropenia experienced by our patient. Pneumonitis
occurred after ~1 year with avelumab and resulted in the
termination of the treatment, but disappeared almost immediately
after that. Pulmonary embolism, on the other hand, occurred after
the end of treatment.
In 2020, the first publication of a case with MCC
and paraneoplastic LEMS treated with avelumab was reported, to the
best of our knowledge (23). In
this report, the patient had a severe reduction in vital capacity,
and the muscular symptoms initially worsened instead of improved
after infusion. Additional immunoglobulins had to be added to
manage these severe immunological side effects, but after that, the
LEMS improved and the MCC went into remission. In our patient, even
though he previously had received both chemotherapy and radiation
therapy, there was no need for additional immunoglobulins, and the
LEMS continued to improve after the introduction of avelumab.
Previously, there have also been concerns that treatment with drugs
potentiating the immune system may worsen the paramalignant
symptoms, that was, however, not noted in our patient.
In the literature, we found four case reports with
an association between MCC and LEMS (24–27).
To summarize, in most cases treatment of the tumor reduced the
LEMS-associated symptoms but none of these cases received treatment
with avelumab. Some other case reports raises the concern regarding
whether immunotherapy may cause LEMS (28–32),
none of these are reported having MCC. The majority of the cases
described had lung cancer and out of the case reports one cannot
say whether LEMS was caused by the immunotherapy, only that it
presented after initiation of treatment. However, those patients
with onset after start of immunotherapy generally had less effect
when treating the LEMS-symptoms.
In conclusion, signs indicative of LEMS should
always form the basis for a thorough malignancy screening even if
lung examinations appear normal. The effect of the combined
treatment with radiotherapy, chemotherapy, and avelumab, as well as
the tolerance in our patient suggests that this might be a suitable
treatment strategy for other patients with MCC combined with
LEMS.
Acknowledgements
The authors would like to thank Dr Daniel Nosek
(Department of Medical Biosciences, Umeå University, Umeå, Sweden)
for his technical assistance with Figure 1.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CG, MIM, CK, TR, TD, PI and DL contributed to
acquisition, analysis and interpretation of the patient data
presented in this case report. CG and DL drafted the manuscript.
All authors have made critical revisions. All authors read and
approved the final manuscript. CG and DL confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The patient has provided written informed consent to
participate.
Patient consent for publication
The patient has provided written informed consent
for publication of the data in this manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hoefler H, Kerl H, Rauch HJ and Denk H:
New immunocytochemical observations with diagnostic significance in
cutaneous neuroendocrine carcinoma. Am J Dermatopathol. 6:525–530.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walsh NM: Primary neuroendocrine (Merkel
cell) carcinoma of the skin: Morphologic diversity and implications
thereof. Hum Pathol. 32:680–689. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Becker JC, Stang A, DeCaprio JA, Cerroni
L, Lebbe C, Veness M and Nghiem P: Merkel cell carcinoma. Nat Rev
Dis Primers. 3:170772017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pavolucci L, Giannini G, Giannoccaro MP,
Foschini MP, Lang B, Avoni P, Tinuper P, Vincent A and Liguori R:
Paraneoplastic cerebellar degeneration and lambert-eaton myasthenia
in a patient with merkel cell carcinoma and voltage-gated calcium
channel antibodies. Muscle Nerve. 56:998–1000. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eggers SD, Salomao DR, Dinapoli RP and
Vernino S: Paraneoplastic and metastatic neurologic complications
of Merkel cell carcinoma. Mayo Clin Proc. 76:327–330. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaufman HL, Russell J, Hamid O, Bhatia S,
Terheyden P, D'Angelo SP, Shih KC, Lebbé C, Linette GP, Milella M,
et al: Avelumab in patients with chemotherapy-refractory metastatic
Merkel cell carcinoma: A multicentre, single-group, open-label,
phase 2 trial. Lancet Oncol. 17:1374–1385. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Collins JM and Gulley JL: Product review:
Avelumab, an anti-PD-L1 antibody. Hum Vaccin Immunother.
15:891–908. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kesner VG, Oh SJ, Dimachkie MM and Barohn
RJ: Lambert-eaton myasthenic syndrome. Neurol Clin. 36:379–394.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Neill JH, Murray NM and Newsom-Davis J:
The lambert-eaton myasthenic syndrome. A review of 50 cases. Brain.
111:577–596. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Skeie GO, Apostolski S, Evoli A, Gilhus
NE, Illa I, Harms L, Hilton-Jones D, Melms A, Verschuuren J, Horge
HW, et al: Guidelines for treatment of autoimmune neuromuscular
transmission disorders. Eur J Neurol. 17:893–902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gustafsson B, Priftakis P, Rubin J, Giraud
G, Ramqvist T and Dalianis T: Human polyomaviruses were not
detected in cerebrospinal fluid of patients with neurological
complications after hematopoietic stem cell transplantation. Future
Virol. 8:809–814. 2013. View Article : Google Scholar
|
|
12
|
Llombart B, Monteagudo C, Lopez-Guerrero
JA, Carda C, Jorda E, Sanmartin O, Almenar S, Molina I, Martín JM
and Llombart-Bosch A: Clinicopathological and immunohistochemical
analysis of 20 cases of Merkel cell carcinoma in search of
prognostic markers. Histopathology. 46:622–634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kervarrec T, Zaragoza J, Gaboriaud P, Le
Gouge A, Beby-Defaux A, Le Corre Y, Hainaut-Wierzbicka E, Aubin F,
Bens G, Michenet P, et al: Differentiating Merkel cell carcinoma of
lymph nodes without a detectable primary skin tumor from other
metastatic neuroendocrine carcinomas: The ELECTHIP criteria. J Am
Acad Dermatol. 78:964–972. e32018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garcia-Carbonero R, Sorbye H, Baudin E,
Raymond E, Wiedenmann B, Niederle B, Sedlackova E, Toumpanakis C,
Anlauf M, Cwikla JB, et al: ENETS consensus guidelines for
high-grade gastroenteropancreatic neuroendocrine tumors and
neuroendocrine carcinomas. Neuroendocrinology. 103:186–194. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuwamoto S: Recent advances in the biology
of Merkel cell carcinoma. Hum Pathol. 42:1063–1077. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng H, Shuda M, Chang Y and Moore PS:
Clonal integration of a polyomavirus in human Merkel cell
carcinoma. Science. 319:1096–1100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sihto H, Kukko H, Koljonen V, Sankila R,
Bohling T and Joensuu H: Clinical factors associated with Merkel
cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer
Inst. 101:938–945. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duncavage EJ, Le BM, Wang D and Pfeifer
JD: Merkel cell polyomavirus: A specific marker for Merkel cell
carcinoma in histologically similar tumors. Am J Surg Pathol.
33:1771–1777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ordonez NG: Value of thyroid transcription
factor-1 immunostaining in distinguishing small cell lung
carcinomas from other small cell carcinomas. Am J Surg Pathol.
24:1217–1223. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taralli S, Sollini M, Milella M, Perotti
G, Filice A, Menga M, Versari A and Rufini V: (18)F-FDG and
(68)Ga-somatostatin analogs PET/CT in patients with Merkel cell
carcinoma: A comparison study. EJNMMI Res. 8:642018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishida Y: PD-1: Its discovery, involvement
in cancer immunotherapy, and beyond. Cells. 9:13762020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Desch L and Kunstfeld R: Merkel cell
carcinoma: Chemotherapy and emerging new therapeutic options. J
Skin Cancer. 2013:3271502013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dohrn MF, Schone U, Kuppers C, Christen D,
Schulz JB, Gess B and Tauber S: Immunoglobulins to mitigate
paraneoplastic lambert eaton myasthenic syndrome under checkpoint
inhibition in Merkel cell carcinoma. Neurol Res Pract. 2:522020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agostini A, Merli M, Avallone G, Burzi L,
Mastorino L, Parisi M, Bertuzzo D, Ferrero B, Cerrato M, Badellino
S, et al: Lambert-eaton myasthenic syndrome and paraneoplastic
cerebellar degeneration associated with merkel cell carcinoma with
unknown primary: A case report. Acta Derm Venereol.
101:adv004522021.PubMed/NCBI
|
|
25
|
Nguyen ND, Simmons DB, Bersabe AR,
Duginski TM, Sladky JH, Walton D, Will M and Renshaw JS:
Lambert-Eaton myasthenic syndrome and merkel cell carcinoma. Cutis.
103:E19–E23. 2019.PubMed/NCBI
|
|
26
|
Iyer JG, Parvathaneni K, Bhatia S,
Tarabadkar ES, Blom A, Doumani R, McKenzie J, Asgari MM and Nghiem
P: Paraneoplastic syndromes (PNS) associated with Merkel cell
carcinoma (MCC): A case series of 8 patients highlighting different
clinical manifestations. J Am Acad Dermatol. 75:541–547. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bombelli F, Lispi L, Calabro F, Corsi FM
and Petrucci A: Lambert-Eaton myasthenic syndrome associated to
Merkel cell carcinoma: Report of a case. Neurol Sci. 36:1491–1492.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee JH, Baek SK, Han JJ, Kim HJ, Lee YA,
Yoo D and Maeng CH: Lambert-Eaton myasthenic syndrome (LEMS) in a
patient with lung cancer under treatment with pembrolizumab: A case
study. J Chemother. 13:1–6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sakaguchi T, Kokubo Y, Furuhashi K,
Nakamura Y, Suzuki Y, Ito K, Fujiwara K, Nishii Y, Taguchi O and
Hataji O: An extensive-stage small-cell lung cancer case with
preexisting lambert-eaton myasthenic syndrome successfully treated
with an immune checkpoint inhibitor. Clin Lung Cancer.
23:e273–e275. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gill AJ, Gandhy S and Lancaster E:
Nivolumab-associated lambert-eaton myasthenic syndrome and
cerebellar dysfunction in a patient with a neuroendocrine tumor.
Muscle Nerve. 63:E18–E21. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kunii E, Owaki S, Yamada K, Yoshihara M,
Yamaba Y, Takakuwa O, Toyoda T and Akita K: Lambert-eaton
myasthenic syndrome caused by atezolizumab in a patient with
small-cell lung cancer. Intern Med. 61:1739–1742. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakatani Y, Tanaka N, Enami T, Minami S,
Okazaki T and Komuta K: Lambert-eaton myasthenic syndrome caused by
nivolumab in a patient with squamous cell lung cancer. Case Rep
Neurol. 10:346–352. 2018. View Article : Google Scholar : PubMed/NCBI
|