Introduction
Tumors are the product of gene mutations. Their
malignant capacity usually derives from abnormal function of mutant
oncoproteins and aberrant signal transduction (1). Under abnormal molecular signal
transmission, certain functional proteins would be modified by
incorrect glycosylation under the action of the Golgi apparatus,
and this abnormal glycosylation is closely associated with the
malignant proliferation of tumor cells (2,3).
Moreover, aberrant glycosylation of functionally membrane
glycoproteins would affect adhesion or motility of tumor cells,
resulting in invasion and metastasis. For example, high expression
of sialoglycoconjugates in colorectal cancer is significantly
associated with poor prognosis and lymph node metastasis (4). Altered sialylation of cancer cells
was found to be closely associated with the malignant properties of
invasiveness and metastasis (5).
Plant lectin is a kind of protein molecule derived
from natural plants, which has been described as a class of toxic
protein that exerts biological effects by binding specifically to
glycan (6). As a toxic protein,
plant lectin has been found to have anti-proliferative activity
against tumor cells (7,8). In addition, based on the specific
sugar-binding ability, plant lectin has also been applied to
diagnose malignant and benign tumors with different degrees of
glycosylation (9,10).
Liver cancer is the most frequent fatal malignancy.
Previous studies showed that Concanavalin A (Con A), a plant lectin
from Jack bean seeds, has a potent anti-liver cancer effect
(11,12). Con A, after binding to the mannose
moiety on the cell membrane glycoprotein, is internalized
preferentially to the mitochondria, and then autophagy is
triggered, which can lead to cell death (13). Moreover, Con A, as a T cell
mitogen, can activate the immune response in the liver, which
results in the eradication of the tumor in a murine in situ
hepatoma model (14). However,
there are limited studies on the effect of Con A on liver cancer
cell migration, particularly the molecular mechanism based on the
sugar-binding ability of Con A.
The current study explored the effect of Con A on
the viability and migration of three human liver cancer cell lines
with different metastatic ability, and further analyzed the
possible sugar sites and related molecular mechanisms of the
interaction between Con A and human liver cancer cells.
Materials and methods
Cell culture and Con A solution
The human liver cancer cell lines HCCLM3 (cat. no.
C6303), MHCC97L (cat. no. C6586) and HepG2 (cat. no. C6346) were
obtained from Beyotime Institute of Biotechnology, while the normal
hepatocyte cell line MIHA was obtained from American Type Culture
Collection (ATCC; cat. no. AC340123). Human liver cancer cells and
hepatocytes were cultured in 25-cm2 gas-permeable cell
culture flasks (Wuhan HealthCare Biotechnology Co., Ltd.) in a cell
incubator with an atmosphere of 5% CO2 at 37°C. Culture
medium was changed every 3 days. High-glucose DMEM (Gibco; Thermo
Fisher Scientific, Inc.) and standard RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) were used for cell culture, and
were supplemented with 10% fetal bovine serum (HyClone; Cytiva),
100 U/ml streptomycin and 100 U/ml penicillin (Biosharp Life
Sciences). All cell lines were authenticated periodically by cell
morphology monitoring and growth curve analysis, according to the
ATCC cell line verification test recommendations.
The Con A powder used in the current study was
purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.
Con A powder (10 mg) was dissolved in 1 ml PBS to prepare a stock
solution of a concentration of 10 µg/µl, which was then
diluted into several 2-µg/µl working solutions with
PBS at a ratio of 1:4, and stored at −20°C until use in subsequent
experiments.
Cell viability assay
Cell Counting Kit (CCK)-8 assay was used to detect
cell viability. First, cells were inoculated into 96-well plates at
a density of 5×103 cells/well. After the cell adhered
and returned to their normal shape, they were serum-starved
overnight and then treated with different concentrations of Con A
(0, 1, 3, 5 and 10 µg/ml) for 12, 24 or 48 h. Following the
indicated treatment time, 5 mg/ml CCK-8 reagent (0.5 mg/ml;
Sigma-Aldrich; Merck KGaA) was added to each well and incubated
with cells for 2 h at 37°C. Lastly, the absorbance of the cells at
a wavelength of 450 nm was measured with a spectrophotometer
(Molecular Devices, LLC) to quantify the cell viability.
Cell migration assay
Transwell assay was used to detect cell migration.
The experiment was performed in a 24-well plate, and the Transwell
chamber (pore diameter, 8 µm; MilliporeSigma) was placed
above the plate. After the cells were starved overnight in a cell
culture flask, the cell suspension was prepared by adding
serum-free medium, and subsequently inoculated into the upper layer
of the chamber at a density of 5×104 cells/100
µl. Next, 600 µl complete medium was added to the
bottom of the plate to induce cell migration, and different
concentrations of Con A (0, 1, 3, 5 and 10 µg/ml) were then
added to the upper layer of the chamber containing the cell
suspension for the cell migration assay. After 6 h, the chamber was
removed from the well plate. The remaining upper cell suspension
was adsorbed with a piece of cotton, and the cells in the upper
chamber that failed to migrate were wiped slightly. Lastly, the
cells that migrated to the lower layer of the chamber were fixed
with 4% paraformaldehyde (Biosharp Life Sciences) for 10 min at
room temperature, stained with 0.1% crystal violet (Shanghai
Beyotime Biotechnology Co., Ltd.) for 30 min at room temperature
and images were captured with an inverted light microscope (Olympus
Corporation).
Sugar inhibition assay
D-glucose and D-mannose (Shanghai Aladdin
Biochemical Technology Co., Ltd.) were used to detect the
sugar-binding site between Con A and human liver cancer cells. A
Con A + DMEM control group to verify whether D-glucose in
high-glucose DMEM had an effect on the glucose binding ability of
Con A. A total of 20 mg powdered glucose or mannose were dissolved
in 10 ml sterile deionized water to generate working solutions with
a concentration of 2 µg/µl, which were aliquoted and stored
at −80°C. Prior to cell viability and migration assays,
high-glucose DMEM or the aforementioned sugar solutions were
pre-incubated with Con A at a ratio of 1:1 at room temperature for
30 min, and cell viability or migration assays was then carried
out.
Western blot analysis
Western blotting was used to detect protein
expression of mitogen-activated protein kinases (MAPK) pathways in
HCCLM3 cells. First, 5×104 cells were inoculated on
six-well plates. When the cell density reached ~70%, the cells were
starved overnight and then treated with Con A (10 µg/ml) for
0, 15, 30, 60 or 120 min. After Con A treatment, total protein was
extracted using cell lysis buffer (Shanghai Beyotime Biotechnology
Co., Ltd.). The protein concentration was determined by the Lowry's
method using bovine serum albumin (BSA; Shanghai Beyotime
Biotechnology Co., Ltd.) as standard. Then, 50 µg total protein was
loaded per lane. Upon electrophoretic separation by 12% SDS-PAGE,
the proteins were electrotransferred onto 0.22-µm-pore
Polyvinylidene fluoride (PVDF) membranes (MilliporeSigma). Next,
TBS containing 0.1% Tween-20 (TBST) and 5% BSA were used to block
the PVDF membranes at room temperature for 1 h. To analyze the
effect of Con A on individual phosphorylated proteins in the MAPK
pathway, the following antibodies were incubated with the membranes
at 4°C overnight according to the corresponding manufacturer's
protocol: Anti-ERK1 [phosphorylated (p)T202/pY204] + ERK2
(pT185/pY187) rabbit monoclonal antibody [(mAb); 1:10,000, cat. no.
ab76299; Abcam], anti-ERK1 + ERK2 rabbit mAb (1:10,000; cat. no.
ab184699; Abcam), anti-JNK1 + JNK2 + JNK3 (pT183 + T183 + T221)
rabbit mAb (1:10,000; cat. no. ab124956; Abcam), anti-JNK1 + JNK2 +
JNK3 rabbit mAb (1:10,000; cat. no. ab179431; Abcam),
anti-p38α/MAPK14 (pY322) rabbit polyclonal antibody [(pAb);
1:1,000; cat. no. 4511T; Cell Signaling Technology, Inc.],
anti-p38α/MAPK14 rabbit mAb (1:1,000; cat. no. ab182453; Abcam) and
anti-GAPDH rabbit mAb (1:10,000; cat. no. AP0063, Bioworld
Technology, Inc.). Subsequently, the membranes were incubated with
an HRP-conjugated antibody (1:10,000; cat. no. 511203; Zen-Bio) for
1 h at room temperature. Upon washing with TBST, the bands were
visualized with an enhanced chemiluminescence kit (Thermo Fisher
Scientific, Inc.) using a ChemiDoc Gel Imaging System (Clinx
Science Instruments Co., Ltd.). ImageJ software (version 1.53e;
National Institutes of Health) was used to analyze the optical
density of the bands. GAPDH was used as a loading control for
normalization.
Protein signaling inhibition
assay
The ERK1/2 inhibitor U0126 (Dalian Meilun Biology
Technology Co., Ltd.), the JNK1/2/3 inhibitor SP600125 (Shanghai
Beyotime Biotechnology Co., Ltd.) and the p38 inhibitor SB203580
(Dalian Meilun Biology Technology Co., Ltd.) were used in the
present study. The concentration of these three inhibitors was set
to 5, 10 or 20 µM in order to determine the optimal
effective concentration of inhibitors on HCCLM3 cells. Next, cells
were pre-treated with U0126, SP600125 or/and SB203580 for 60 min at
37°C before the addition of Con A (10 µg/ml) in the cell
viability, cell migration or western blot assays.
Detection of fibrous actin (F-actin)
filaments and cell spreading area
Immunofluorescence staining was used to detect the
effect of Con A on the F-actin of cells. Cells were inoculated on
24-well plates, and when the cell density reached ~50%, the cells
were starved overnight, and then treated with Con A (10
µg/ml) for 60 min. To block ERK1/2, JNK1/2/3 or/and p38
signaling, the cells were incubated with U0126, SP600125 or/and
SB203580 for 60 min at 37°C before Con A treatment. Subsequently,
the cells were fixed with 4% formaldehyde for 30 min, treated with
a permeabilization solution (0.25% Triton X-100; Beijing Solarbio
Science & Technology Co., Ltd.) for 10 min and blocked with
blocking solution (5% BSA; Beijing Solarbio Science &
Technology Co., Ltd.) for 60 min at room temperature. Next, FITC
phalloidin (Shanghai Beyotime Biotechnology Co., Ltd.) was added to
stain F-actin in HCCLM3 cells overnight at 4°C in the dark.
Subsequently, the nuclei were labeled with 2 µg/ml DAPI (Shanghai
Beyotime Biotechnology Co., Ltd.) for 10 min at room temperature.
Lastly, images of the cells were captured using an inverted
fluorescence trichromatic microscope (Olympus Corporation). The
F-actin fluorescence intensity and spreading area of individual
cells were semi-quantitatively evaluated by ImageJ software
(version 1.53e; National Institutes of Health).
Gelatin zymography
Gelatin zymogram was used to detect the effect of
Con A on the activities of matrix metalloproteinase-2 (MMP-2) and
matrix metalloproteinase-9 (MMP-9). The HCCLM3 cells were
inoculated on six-well plates. When the cell density reached ~90%,
the cells were serum-starved overnight and then treated with Con A
(10 µg/ml) for 6 h. Next, the supernatants were collected and
centrifuged at 4,000 × g for 5 min at 4°C. A BCA protein
quantification kit (Nanjing KeyGen Biotech Co., Ltd.) was used to
measure the protein concentration, and equal protein quantities (50
µg) were loaded per lane in the gel. A pre-stained protein
molecular weight marker (Thermo Fisher Scientific, Inc.) was used
as a size standard for protein electrophoresis. Gelatinase was
concentrated and separated by 10% SDS-PAGE containing pigskin
gelatin (Shanghai Beyotime Biotechnology Co., Ltd.). The separated
gelatin containing MMP-2 and MMP-9 was then collected, eluted using
2.5% Triton-X-100 solution for 60 min and incubated in developing
buffer (Tris 0.5 M, Brij35 0.2%, NaCl 2 M and CaCl2 50
mM, pH 7.6) for 16 h at 37°C. Next, gelatin was stained by
Coomassie Brilliant Blue (Beijing Solarbio Science & Technology
Co., Ltd.) for 30 min at room temperature. Lastly, images of the
gelatin were captured with a ChemiDoc Imaging System (Clinx Science
Instruments Co., Ltd.). ImageJ software (version 1.53e; National
Institutes of Health) was used to analyze the optical density of
the bands.
Statistical analysis
SPSS software (version 21.0; IBM Corp.) was used for
statistical analysis. Data are presented as the mean ± SD of ≥3
independent experiments, and were analyzed using one-way ANOVA
followed by Bonferroni post hoc test for the comparison of multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
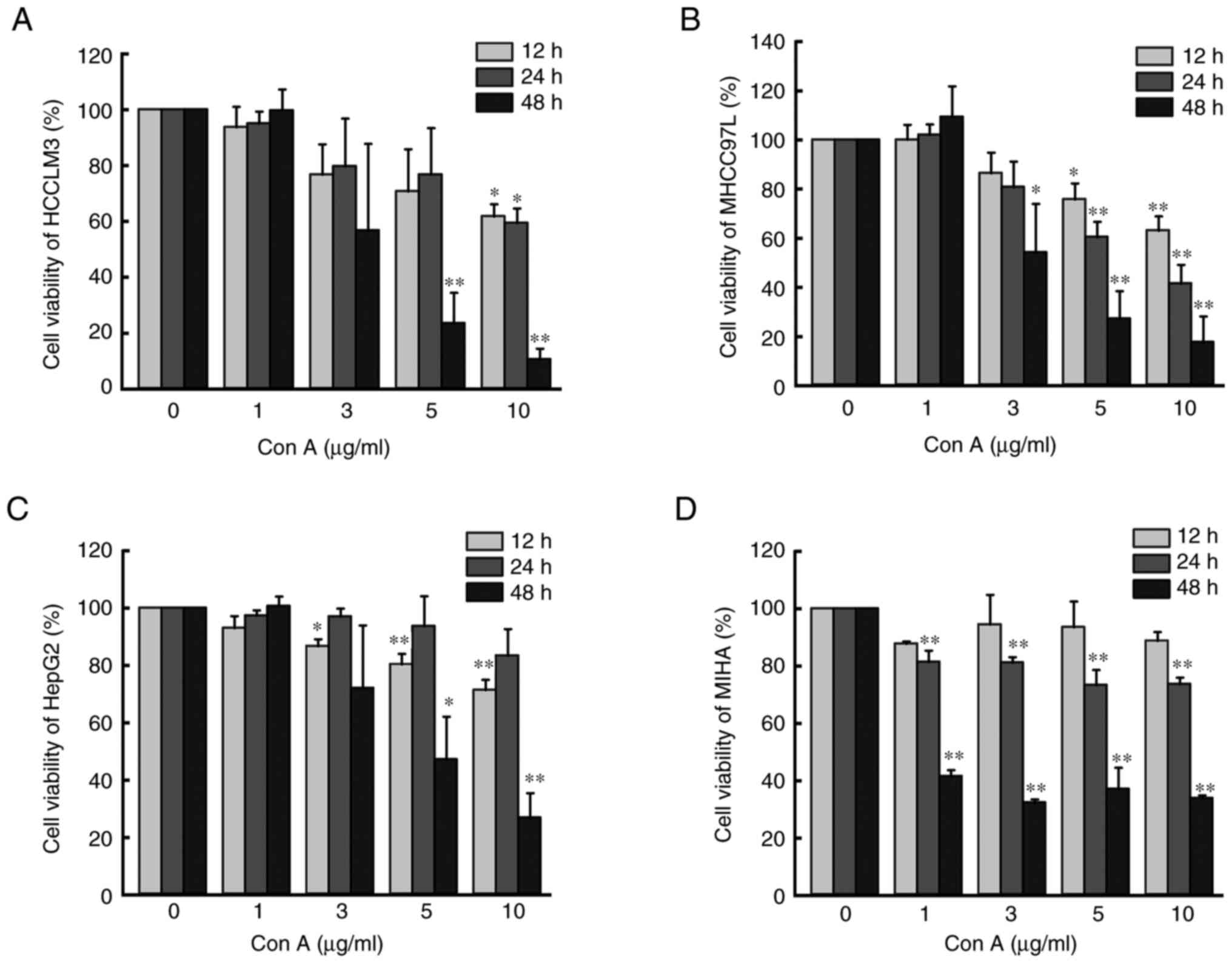
Con A reduces the viability of human
liver cancer cells and hepatocytes
CCK-8 assay was used to assess cell viability. As
shown in Fig. 1, Con A inhibited
the viability of three types of human liver cancer cells (HCCLM3,
MHCC97L and HepG2) as well as hepatocytes (MIHA) in a time and
concentration-dependent manner. When the concentration of Con A
reached 10 µg/ml and was incubated with the cells for 48 h, the
viability of the aforementioned four cell lines was inhibited to
~20% (P<0.05).
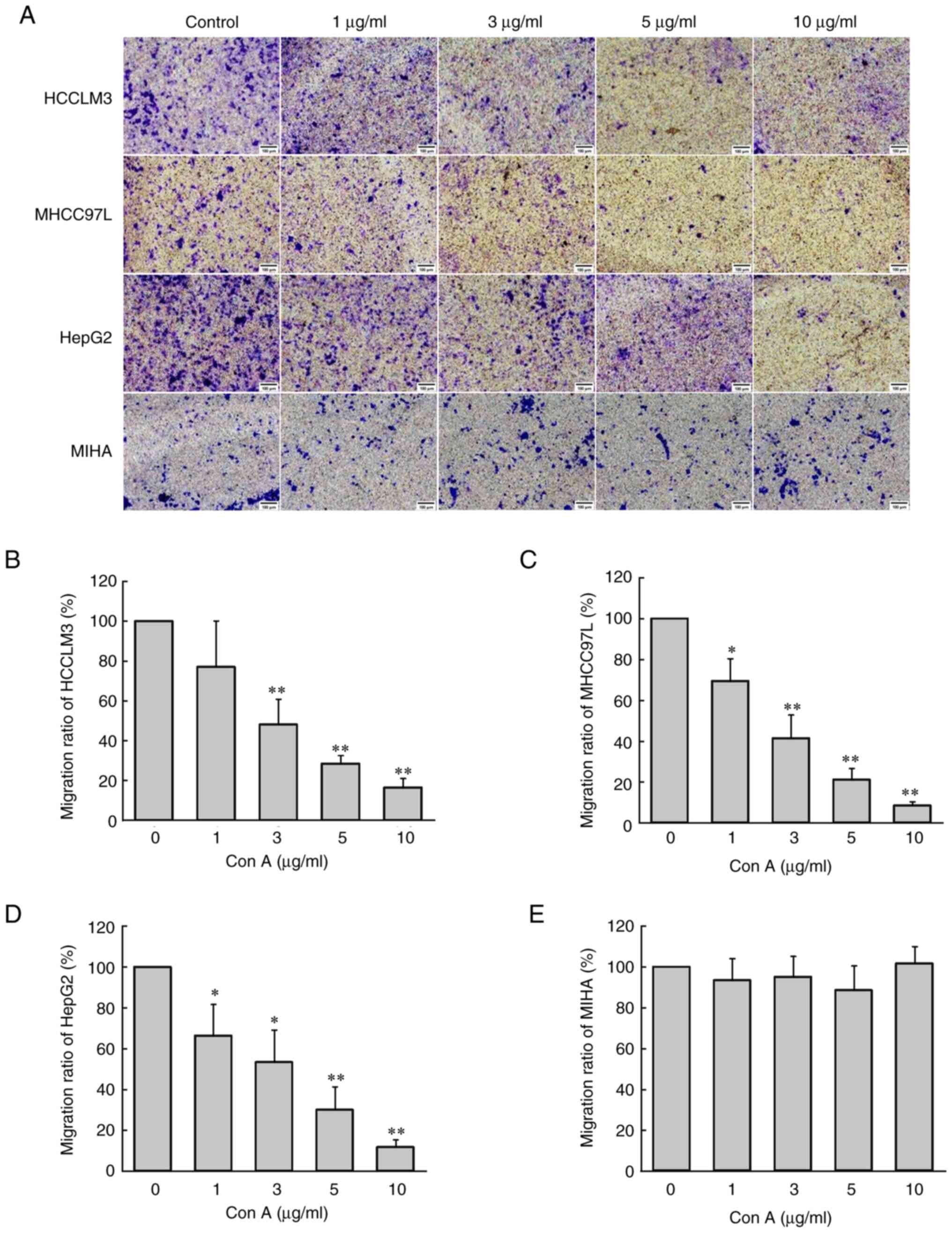
Con A inhibits the migration of three
human liver cancer cell types, but had no effect on normal
hepatocytes
Transwell assay was used to evaluate the effect of
Con A on the migration of human liver cancer cells and normal
hepatocytes. As revealed in Fig.
2A-D, Con A significantly inhibited human liver cancer cell
migration (P<0.05) in a concentration-dependent manner. When the
concentration of Con A reached 10 µg/ml and was incubated with the
cells for 6 h, the migration of human liver cancer cells was almost
completely inhibited. However, different concentrations of Con A
(0, 1, 3, 5 and 10 µg/ml) had no significant effect on
hepatocyte migration (Fig.
2E).
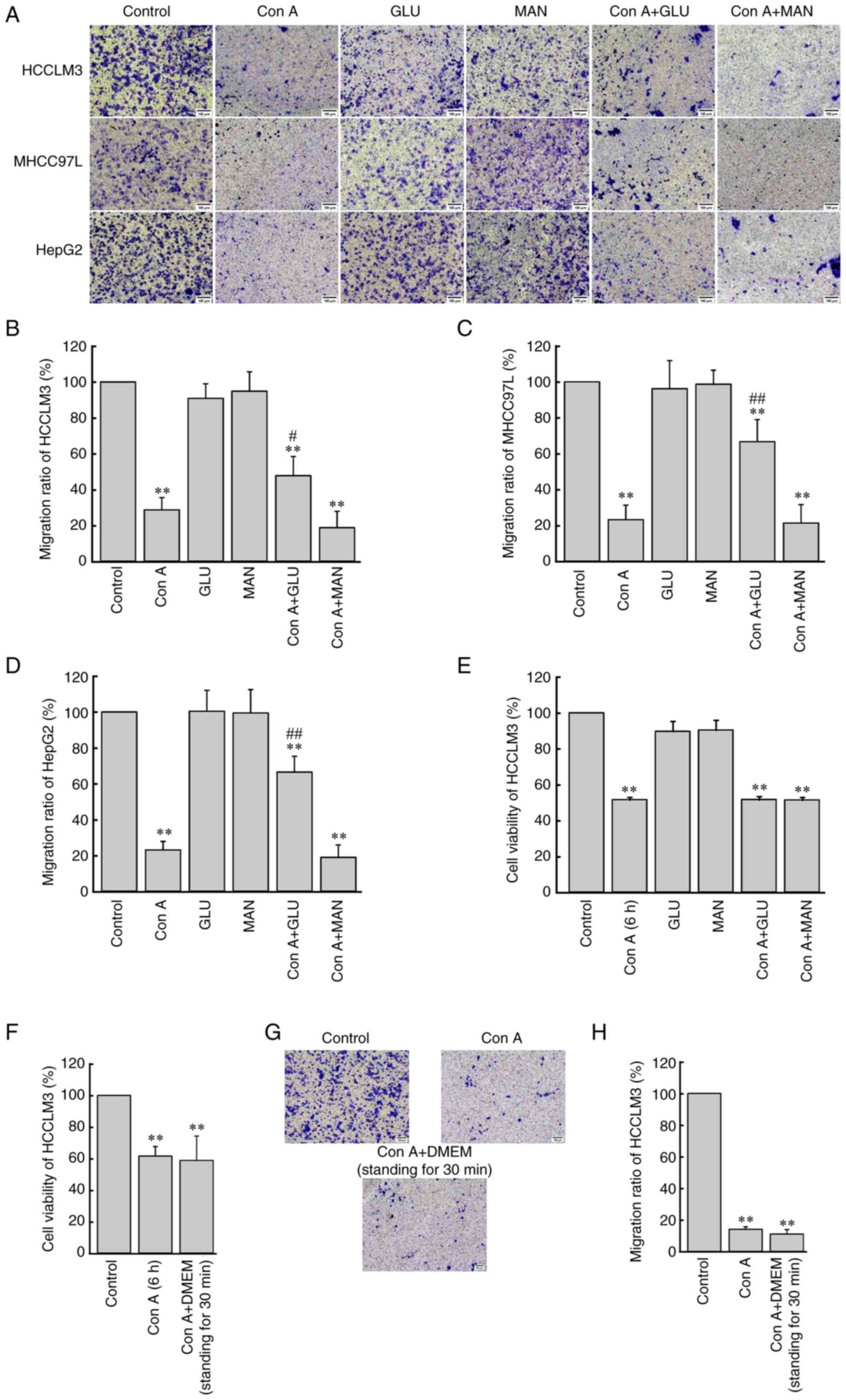
Con A inhibits human liver cancer cell migration
through glucose-related sugar binding sites. To determine the
glycobinding site between Con A and liver cancer cells, exogenous
glucose and mannose were incubated with Con A prior to cell
migration assay. Since DMEM also contained glucose, a co-incubation
experiment of DMEM with Con A was also performed. As shown in
Fig. 3A-D, the addition of glucose
(10 µg/ml) or mannose (10 µg/ml) alone had no significant effect on
human liver cancer cell migration, but when Con A (10 µg/ml) was
first incubated with glucose or mannose for 30 min, the migration
level of human liver cancer cells in the Con A + glucose group was
slightly restored (P<0.05), while the migration level of human
liver cancer cells in the Con A + mannose group was the same as
that of the Con A group.
In addition, compared with the almost complete
inhibition of HCCLM3 cell migration in the migration assay
(Fig. 2B), Con A (10 µg/ml)
could only inhibit the viability of HCCLM3 cells to ~50% within 6 h
(Fig. 3E). Fig. 3E also showed that co-incubation of
Con A (10 µg/ml) with glucose (10 µg/ml) or mannose
(10 µg/ml) did not restore the HCCLM3 cell viability reduced
by Con A. As revealed in Fig.
3F-H, when Con A (10 µg/ml) was first co-incubated with
DMEM, the effect of Con A on HCCLM3 viability and migration was not
different from that of the Con A direct treatment group.
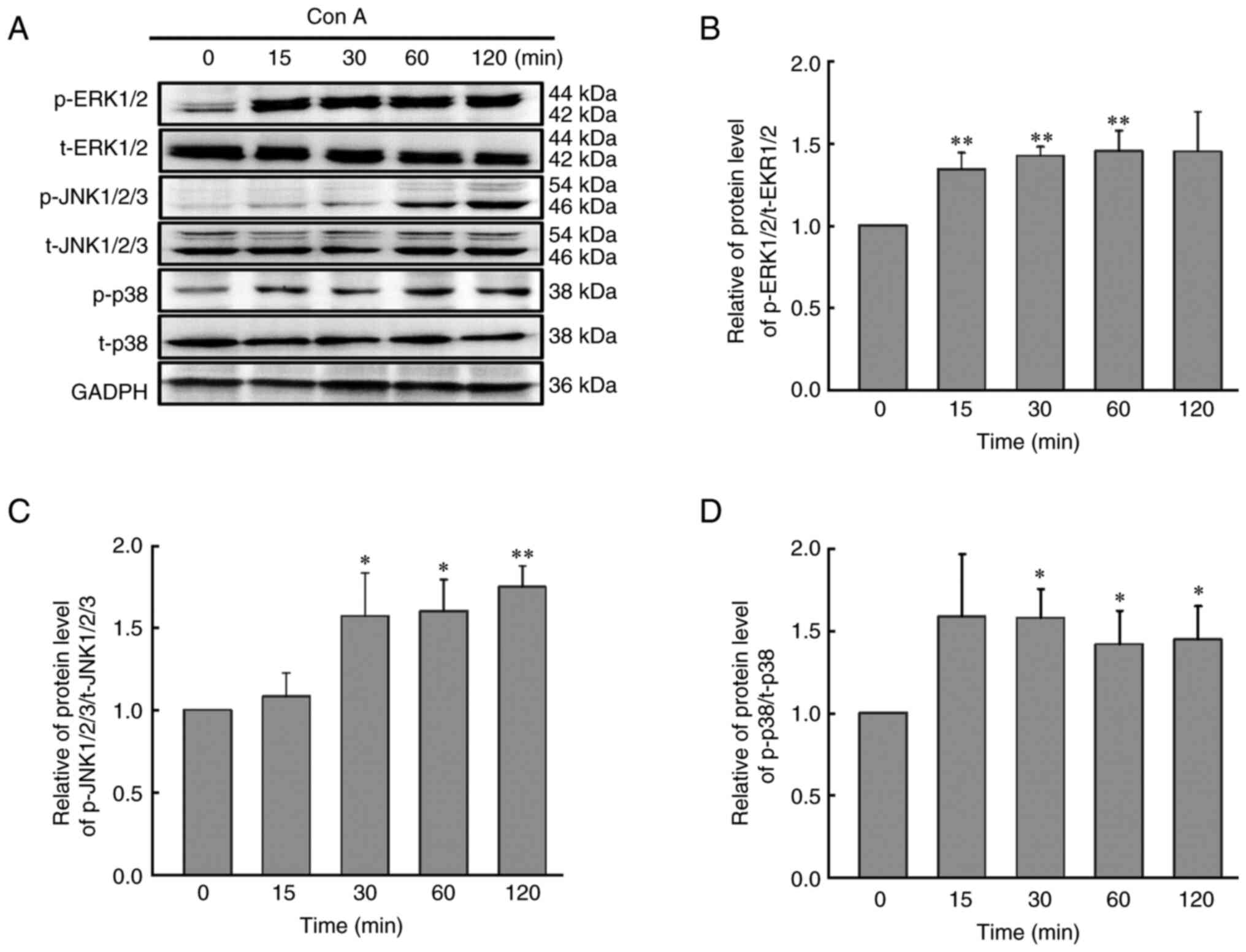
Con A inhibits HCCLM3 cell migration
via upregulation of the phosphorylation of ERK1/2, JNK1/2/3 and
p38
As demonstrated in Fig.
4, Con A (10 µg/ml) could significantly upregulate the
phosphorylation level of ERK1/2, JNK1/2/3 and p38 (P<0.05). To
further identify the association between the activation of the MAPK
signaling pathway and Con A-mediated inhibition of HCCLM3 cell
migration, the ERK1/2 inhibitor U0126, the JNK1/2/3 inhibitor
SP600125 and the p38 inhibitor SB203580 were used to detect the
roles of the ERK1/2, JNK1/2/3 and p38 signaling, respectively. As
revealed in Fig. 5, when the
concentration of U0126, SP600125 or SB203580 reached 5 µM,
the phosphorylation level of ERK1/2, JNK1/2/3 or p38 they could be
restored to that of the control group. Furthermore, inhibition of
ERK1/2, JNK1/2/3 or/and p38 signaling also suppressed the Con
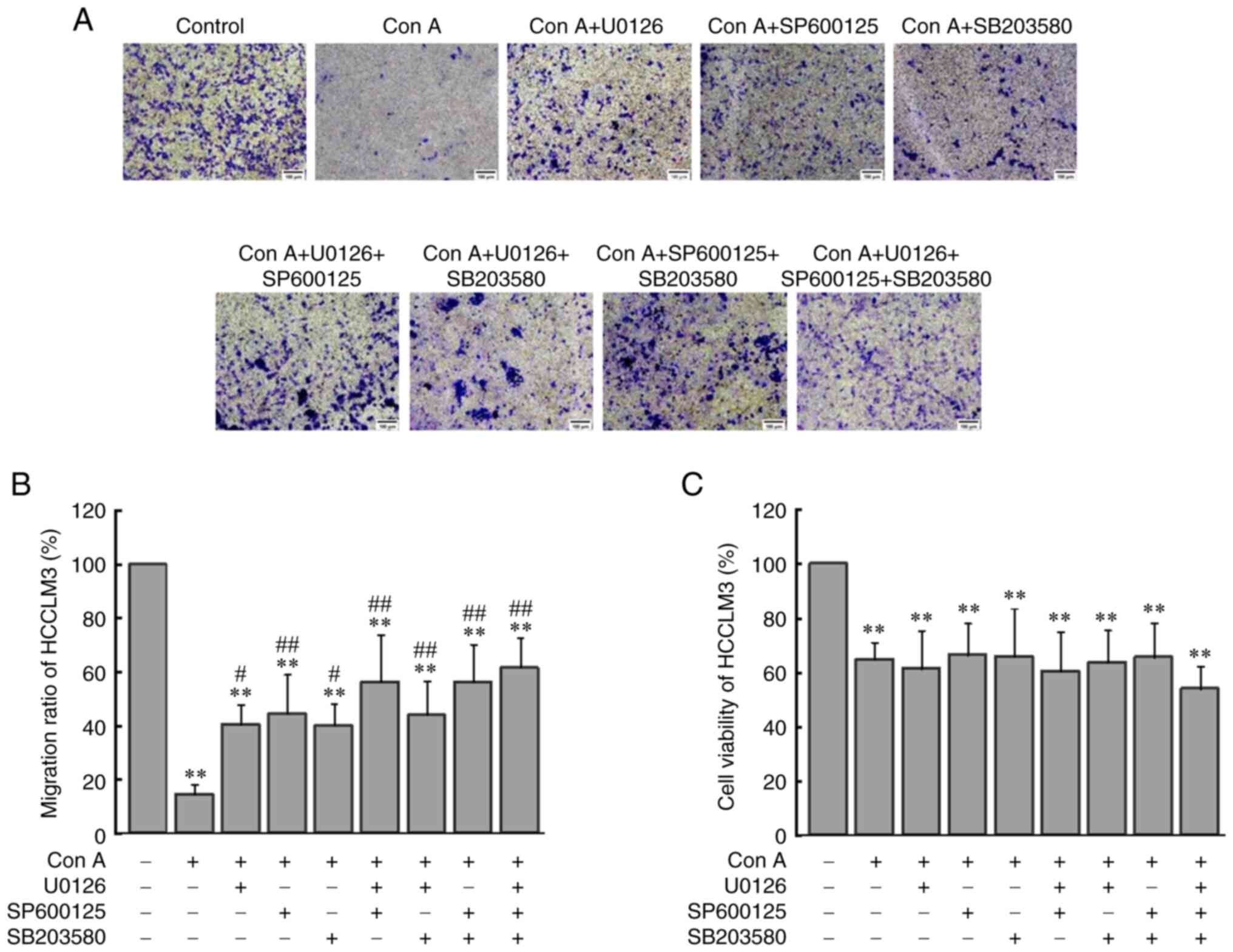
A-mediated reduction in cell migration, and the cell migration rate
recovered from 15% to ~50% (Fig. 6A
and B). However, it was found that inhibition of ERK1/2,
JNK1/2/3 or/and p38 signaling had no effect on the Con A-mediated
inhibition of HCCLM3 cell viability (Fig. 6C).
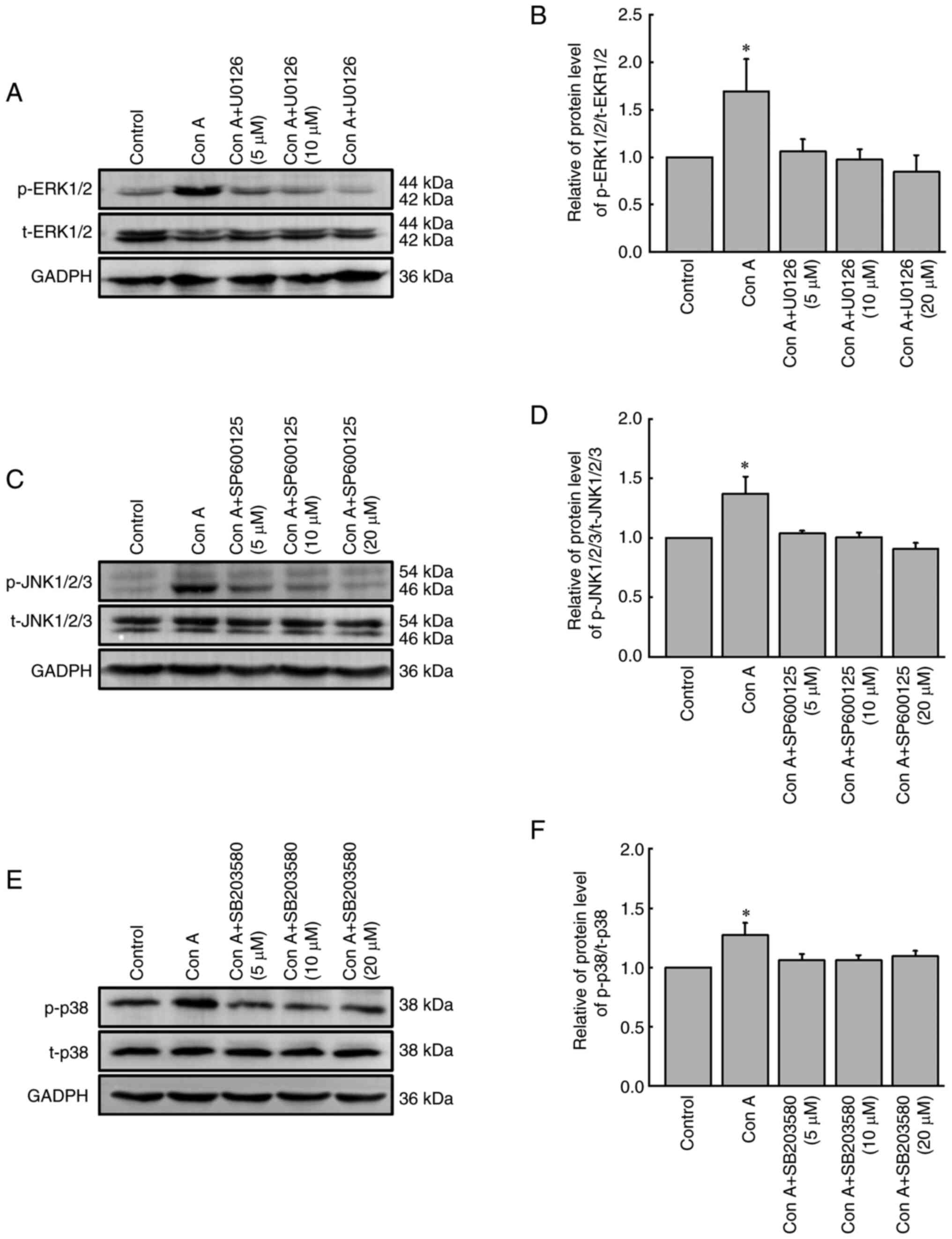 | Figure 5.Effect of inhibitors of ERK1/2,
JNK1/2/3 and p38 on the activation of ERK1/2, JNK1/2/3 and p38 in
HCCLM3 cells. HCCLM3 cells were pre-treated with the ERK1/2
inhibitor U0126, the JNK1/2/3 inhibitor SP600125 or the p38
inhibitor SB203580 for 60 min, and then the expression levels of
(A) ERK1/2, (C) JNK1/2/3 and (E) p38 in HCCLM3 cells were analyzed
by western blotting. Relative protein level of (B)
p-ERK1/2/t-ERK1/2, (D) p-JNK1/2/3/t-JNK1/2/3 and (F) p-p38/t-p38 in
HCCLM3 cells. The results of the densitometric analysis of ERK1/2,
JNK1/2/3 and p38 activation were normalized to the levels of GAPDH.
The data were expressed as the mean ± SD (n=3). *P<0.05 vs. the
control group. Con A, concanavalin A (10 µg/ml); p, phosphorylated;
t, total. |
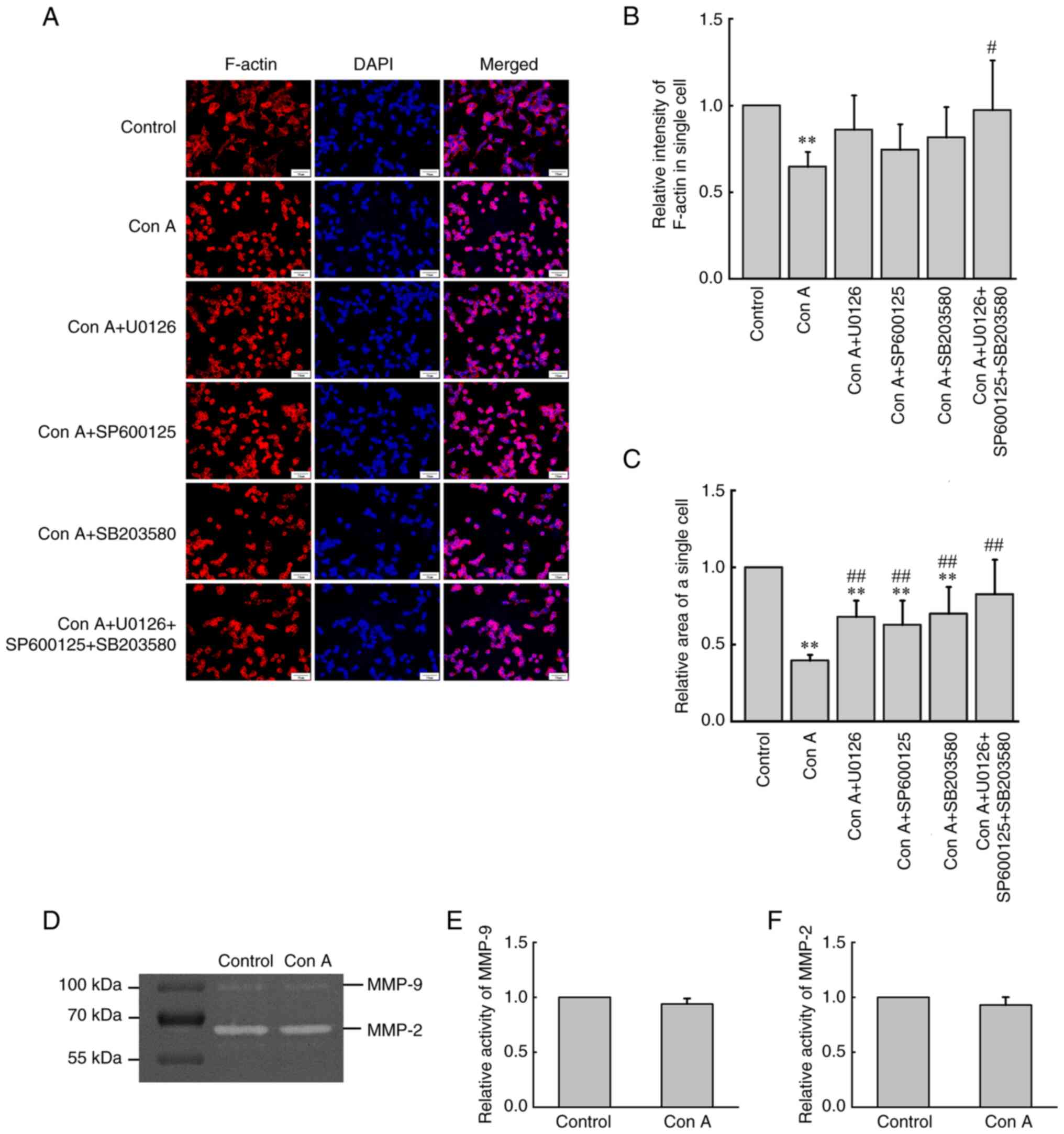
Con A regulates F-actin redistribution
and assembly via ERK1/2, JNK1/2/3 and p38 signaling
Immunofluorescence staining was used to detect
F-actin. F-actin was stained red with FITC phalloidin, while the
cell nucleus was stained blue with DAPI. As shown in Fig. 7A and B, when analyzing the
fluorescence intensity of single cells, it was found that the
content of F-actin of individual cells decreased significantly
(P<0.05) under the influence of Con A, while inhibition of
ERK1/2, JNK1/2/3 or/and p38 signaling could partly recover the
content of F-actin decreased by Con A. The spreading area of
individual cells was also analyzed. As revealed in Fig. 7A and C, under the influence of Con
A, HCCLM3 cells contracted and changed from fibrous to round shape.
In addition, inhibition of ERK1/2, JNK1/2/3 or/and p38 signaling
could also partly reverse the changes in contraction caused by Con
A.
Con A has no effect on the activation
of MMP-2 or MMP-9 in HCCLM3 cells
The effect of Con A on the activation of MMP-2 and
MMP-9 in HCCLM3 cells was detected by gelatin zymography. In the
control group, activated gelatinase MMP-2 and MMP-9 in HCCLM3 cells
could degrade gelatin in a polyacrylamide gel, which manifested as
white bands. However, after the addition of Con A (10
µg/ml), the optical density of the white bands did not
change significantly compared with that of the control group
(Fig. 7D-F).
Discussion
In previous studies, Con A was mainly used to
establish mouse liver injury models for hepatitis-related research
(15,16). By contrast, the present study
mainly focused on the anticancer function of Con A and its
molecular mechanisms. In the cell viability assay, Con A had a
strong inhibitory effect on the viability of human liver cancer
cells and normal hepatocytes, which was consistent with the results
of previous studies (12,13). In the cell migration assay, Con A
could inhibit the migration of HCCLM3, MHCC97L and HepG2 cells
completely within 6 h, but had no significant inhibitory effect on
MIHA cells. These results demonstrated that Con A had a specific
inhibitory effect on liver cancer cell migration. It was
hypothesized that, since HepG2 cells have a different tissue origin
and degree of malignant metastasis from those of HCCLM3 and MHCC97L
cells (17,18), HepG2 cells may have different
response patterns to Con A; however, the cell migration assay
showed that there was no significant difference in the effects of
Con A on HCCLM3, MHCC97L or HepG2 cells. These results suggested
that Con A may affect the migration of these cells in a similar
way.
To verify whether the effect of Con A on inhibiting
the migration of HCCLM3 cells was associated with its effect on
inhibiting the viability of HCCLM3 cells, a cell viability assay
was conducted within 6 h. The results showed that Con A could
reduce the viability of HCCLM3 cells by ~50% within 6 h, while
incubation with Con A for a similar time could completely inhibit
the migration of HCCLM3 cells. These results indicated that Con
A-mediated inhibition of HCCLM3 cell migration was not completely
caused by a decline in HCCLM3 cell viability.
Glucose and mannose are isomers, and they both can
bind Con A (11). The present
study found that Con A inhibits the migration of liver cancer cells
only through glucose-related sugar sites. In addition, the effect
of glucose or mannose on Con A-mediated inhibition of HCCLM3 cell
viability was verified. The results showed that prior co-incubation
of glucose or mannose with Con A could not affect the Con
A-mediated inhibition of HCCLM3 cell viability, suggesting that Con
A did not inhibit the viability of liver cancer cells through
glucose or mannose-related pathways. Con A may inhibit liver cancer
cell viability via other unknown glycoprotein-related or unrelated
molecular pathways. The molecular mechanism of its toxicity needs
to be further investigated.
Tumor cells are generally involved in aerobic
glycolysis, which leads to a higher demand for glucose in the tumor
environment (19,20). When tumor cells were cultured in
high-glucose DMEM, in addition to sufficient glucose for
biosynthesis, excessive glucose may affect the effect of Con A on
tumor cells due to the high affinity of Con A for glucose.
Therefore, Con A was co-cultured with DMEM to verify the effect of
glucose in DMEM on the viability and migration behavior of HCCLM3
cells. The results showed that co-incubation of Con A with DMEM did
not affect the inhibitory effect of Con A on HCCLM3 cell viability
or migration. It could be hypothesized that the concentration of
glucose in the culture medium may be markedly lower than the
glucose-binding quantity of Con A, or the optical activity or/and
structure of exogenous glucose may be different from that of the
glucose in the culture medium.
The MAPK signaling pathway is one of the most widely
studied pathway, and numerous biological behaviors of tumor cells
have been associated with the MAPK signaling pathway and its
downstream effectors (21–23). Liu et al (24) demonstrated that Polygonatum
cyrtonema lectin induces the apoptosis and autophagy of human lung
adenocarcinoma A549 cells through the MAPK signaling pathway. The
current study found that Con A could upregulate the phosphorylation
levels of ERK1/2, JNK1/2/3 and p38 within 2 h. Moreover, inhibition
of ERK1/2, JNK1/2/3 or/and p38 signaling also suppressed the Con
A-mediated reduction in cell migration. These results indicated
that Con A inhibited HCCLM3 cell migration through the ERK1/2,
JNK1/2/3 and p38 signaling pathways. In addition, it was found that
the addition of inhibitors of the aforementioned three signaling
pathways did not affect the Con A-mediated inhibition of HCCLM3
cell viability. Combined with the results of the sugar inhibition
assay, it was concluded that the molecular mechanism of Con
A-mediated altered HCCLM3 cell viability was different from that of
Con A-mediated altered HCCLM3 cell migration.
In tumor cells, the depolymerization and
polymerization of F-actin play important roles in cell migration
and invasion (25,26). In addition, changes in cell
morphology or contractility can also affect tumor cell metastasis
(27). In the present study, when
HCCLM3 cells were exposed to Con A for 6 h, the spreading area of
individual cells decreased significantly, and the cells changed
shape from long spindle to round. Upon analyzing and quantifying
the fluorescence intensity of individual cells, the content of
F-actin in HCCLM3 cells was observed to decrease significantly
compared with that of the control group. Although the fluorescence
intensity of F-actin in the Con A group appeared visually to be
stronger than that of the control group, considering that the cell
spreading area of HCCLM3 cells in the Con A group was also reduced,
the relative fluorescence intensity of F-actin of single cells in
the Con A group was lower than that of the control group. Moreover,
inhibition of ERK1/2, JNK1/2/3 or/and p38 signaling could partly
restore the Con A-mediated reduction in cell spreading area and
F-actin content. These results showed that Con A could regulate
F-actin redistribution and assembly via the MAPK signaling pathway.
Previous studies also showed that the MAPK signaling pathway could
affect the polymerization of F-actin, thus affecting the metastasis
of tumor cells (28,29).
The migration ability of tumor cells is usually
associated with the activities of the gelatinases MMP-2 and MMP-9
(30,31). Jian et al (32) found that Bandeiraea simplicifolia
lectin could bind to N-acetylgalactosamine in hepatoma cells, thus
affecting the expression of MMP-2 and MMP-9 to inhibit cell
migration. However, the present study found that Con A had no
significant effect on the activities of MMP-2 or MMP-9 in HCCLM3
cells, indicating that there were significant differences in the
response mechanism of different plant lectins.
In summary, the present study reported the effect of
Con A on the viability and migration of human liver cancer cells
and hepatocytes, and preliminary discussed the molecular mechanism
of Con A affecting HCCLM3 cell migration. Studying the association
between the specific sugar-binding ability of different plant
lectins and the glycosylation variation of different tumor cells
can increase the understanding of tumor cells and their
glycosylation changes, as well as provide a new insight for plant
lectins as potential anticancer agents.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and Technology
Research Program of Chongqing Municipal Education Commission (grant
no. KJQN201800601) and the Natural Science Foundation of Chongqing
(grant no. cstc2020jcyj-msxmX0760).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BZ and HJ designed the study. HJ, XW and XZ
performed the experiments. HJ analyzed the data and drafted the
initial manuscript. BZ supervised the present study and revised the
manuscript. BZ and HJ confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yuan H, Ji J, Shi M, Shi Y, Liu J, Wu J,
Yang C, Xi W, Li Q, Zhu W, et al: Characteristics of pan-cancer
patients with ultrahigh tumor mutation burden. Front Oncol.
11:6820172021. View Article : Google Scholar
|
|
2
|
Li Y, Zhou Y, Mao F, Shen S, Zhao B, Xu Y,
Lin Y, Zhang X, Cao X, Xu Y, et al: mir-452 reverses abnormal
glycosylation modification of ERα and estrogen resistance in TNBC
(triple-negative breast cancer) through targeting UGT1A1. Front
Oncol. 10:15092020. View Article : Google Scholar
|
|
3
|
Fan C, Tu C, Qi P, Guo C, Xiang B, Zhou M,
Li X, Wu X, Li X, Li G, et al: GPC6 promotes cell proliferation,
migration, and invasion in nasopharyngeal carcinoma. J Cancer.
10:3926–3932. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kannagi R: Carbohydrate antigen sialyl
Lewis 1- its pathophysiological significance an induction mechanism
in cancer progression. Chang Gung Med J. 30:189–209.
2007.PubMed/NCBI
|
|
5
|
Miyagi T, Takahashi K, Hata K, Shiozaki K
and Yamaguchi K: Sialidase significance for cancer progression.
Glycoconj J. 29:567–577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Estrada-Martínez LE, Moreno-Celis U,
Cervantes-Jiménez R, Ferriz-Martínez RA, Blanco-Labra A and
García-Gasca T: Plant lectins as medical tools against digestive
system cancers. Int J Mol Sci. 18:14032017. View Article : Google Scholar
|
|
7
|
Oliveira I, Nunes A, Lima A, Borralho P,
Rodrigues C, Ferreira RB and Ribeiro AC: New lectins from
mediterranean flora. Activity against ht29 colon cancer cells. Int
J Mol Sci. 20:30592019. View Article : Google Scholar
|
|
8
|
Lawanprasert A, Guinan CA, Langford EA,
Hawkins CE, Sloand JN, Fescemyer HW, Aronson MR, Halle JA, Marden
JH and Medina SH: Discovery of antitumor lectins from rainforest
tree root transcriptomes. PLoS One. 15:e02294672020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bloise N, Okkeh M, Restivo E, Della PC and
Visai L: Targeting the ‘sweet side’ of tumor with glycan-binding
molecules conjugated-nanoparticles: Implications in cancer therapy
and diagnosis. Nanomaterials. 11:2892021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He Z, Chen Q, Chen F, Zhang J, Li H and
Lin JM: DNA-mediated cell surface engineering for multiplexed
glycan profiling using MALDI-TOF mass spectrometry. Chem Sci.
7:5448–5452. 2016. View Article : Google Scholar
|
|
11
|
Cavada BS, Pinto-Junior VR, Osterne VJ and
Nascimento KS: ConA-like lectins: High similarity proteins as
models to study structure/biological activities relationships. Int
J Mol Sci. 20:302018. View Article : Google Scholar
|
|
12
|
Lei HY and Chang CP: Lectin of
Concanavalin A as an anti-hepatoma therapeutic agent. J Biomed Sci.
16:102009. View Article : Google Scholar
|
|
13
|
Lai YC, Chuang YC, Chang CP and Yeh TM:
Macrophage migration inhibitory factor has a permissive role in
concanavalin A-induced cell death of human hepatoma cells through
autophagy. Cell Death Dis. 6:e20082015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang CP, Yang MC, Liu HS, Lin YS and Lei
HY: Concanavalin A induces autophagy in hepatoma cells and has a
therapeutic effect in a murine in situ hepatoma model. Hepatology.
45:286–296. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai AC, Chi PY, Thio CL, Han YC, Kao HN,
Hsieh HW, Gervay-Hague J and Chang YJ: α-lactosylceramide protects
against iNKT-mediated murine airway hyperreactivity and liver
injury through competitive inhibition of Cd1d binding. Front Chem.
7:8112019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li S, Bi Y, Wang Q, Xu M, Ma Z, Yang Y,
Chang Y, Chen S, Liu D, Duan Z, et al: Transplanted mouse liver
stem cells at different stages of differentiation ameliorate
concanavalin A-induced acute liver injury by modulating tregs and
Th17 cells in mice. Am J Trans Res. 11:7324–7337. 2019.
|
|
17
|
Luo G, Chao YL, Tang B, Li BS, Xiao YF,
Xie R, Wang SM, Wu YY, Dong H, Liu XD and Yang SM: miR-149
represses metastasis of hepatocellular carcinoma by targeting
actin-regulatory proteins PPM1F. Oncotarget. 6:37808–37823. 2015.
View Article : Google Scholar
|
|
18
|
Zhang Y, Hu MY, Wu WZ, Wang ZJ, Zhou K,
Zha XL and Liu KD: The membrane-cytoskeleton organizer ezrin is
necessary for hepatocellular carcinoma cell growth and
invasiveness. J Cancer Res Clin Oncol. 132:685–697. 2006.
View Article : Google Scholar
|
|
19
|
Yang LN, Ning ZY, Wang L, Yan X and Meng
ZQ: HSF2 regulates aerobic glycolysis by suppression of FBP1 in
hepatocellular carcinoma. Am J Cancer Res. 9:1607–1621.
2019.PubMed/NCBI
|
|
20
|
Liu Y, Zhang Y, Xiao B, Tang N, Hu J,
Liang S, Pang Y, Xu H, Ao J, Yang J, et al: MiR-103a promotes
tumour growth and influences glucose metabolism in hepatocellular
carcinoma. Cell Death Dis. 12:6182021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du P, Liang H, Fu X, Wu P, Wang C, Chen H,
Zheng B, Zhang J, Hu S, Zeng R, et al: SLC25A22 promotes
proliferation and metastasis by activating MAPK/ERK pathway in
gallbladder cancer. Cancer Cell Int. 19:332019. View Article : Google Scholar
|
|
22
|
Chen J, Ji T, Wu D, Jiang S, Zhao J, Lin H
and Cai X: Human mesenchymal stem cells promote tumor growth via
MAPK pathway and metastasis by epithelial mesenchymal transition
and integrin α5 in hepatocellular carcinoma. Cell Death Dis.
10:4252019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang YP, Liu KL, Yang Z, Lu BS, Qi JC,
Han ZW, Yin YW, Zhang M, Chen DM, Wang XW, et al: The involvement
of FBP1 in prostate cancer cell epithelial mesenchymal transition,
invasion and metastasis by regulating the MAPK signaling pathway.
Cell Cycle. 18:2432–2446. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu T, Wu L, Wang D, Wang H, Chen J, Yang
C, Bao J and Wu C: Role of reactive oxygen species-mediated MAPK
and NF-κB activation in polygonatum cyrtonema lectin-induced
apoptosis and autophagy in human lung adenocarcinoma A549 cells. J
Biochem. 160:315–324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang X, Xu L and Yang T: miR-31 modulates
liver cancer HepG2 cell apoptosis and invasion via ROCK1/F-actin
pathways. OncoTargets Ther. 13:877–888. 2020. View Article : Google Scholar
|
|
26
|
Schneiderman RS, Giladi M, Zeevi E,
Voloshin T, Shteingauz A, Porat Y, Munster M, Kirson E, Weinberg U
and Palti Y: Angi-11. tumor treating fields (ttfields) inhibit
cancer cell migration and invasion by inducing reorganization of
the actin cytoskeleton and formation of cell adhesions. Neuro
Oncol. 20 (Suppl 6):vi302018. View Article : Google Scholar
|
|
27
|
Gkretsi V and Stylianopoulos T: Cell
adhesion and matrix stiffness: Coordinating cancer cell invasion
and metastasis. Front Oncol. 8:1452018. View Article : Google Scholar
|
|
28
|
Rudzka DA, Mason S, Neilson M, McGarry L,
Kalna G, Hedley A, Blyth K and Olson MF: Selection of established
tumour cells through narrow diameter micropores enriches for
elevated Ras/Raf/MEK/ERK MAPK signalling and enhanced tumour
growth. Small GTPases. 12:294–310. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ni Y, Wang X, Yin X, Li Y, Liu X, Wang H,
Liu X, Zhang J, Gao H, Shi B and Zhao S: Plectin protects podocytes
from adriamycin-induced apoptosis and F-actin cytoskeletal
disruption through the integrin α6β4/FAK/p38 MAPK pathway. J Cell
Mol Med. 22:5450–5467. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kwon Y, Park SJ, Nguyen BT, Kim MJ, Oh S,
Lee H, Park N, Kim HS, Kang MJ, Min BS, et al: Multi-layered
proteogenomic analysis unravels cancer metastasis directed by MMP-2
and focal adhesion kinase signaling. Sci Rep. 11:171302021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu J, Wang Z, Li S, Xin Q, Yuan M, Li H,
Song X, Gao H, Pervaiz N, Sun X, et al: Quercetin inhibits the
migration and invasion of HCCLM3 cells by suppressing the
expression of p-Akt1, matrix metalloproteinase (MMP) MMP-2, and
MMP-9. Med Sci Monit. 24:2583–2589. 2018. View Article : Google Scholar
|
|
32
|
Jian Q, Yang Z, Shu J, Liu XW, Zhang J and
Zheng L: Lectin BS-I inhibits cell migration and invasion via
AKT/GSK-3β/β-catenin pathway in hepatocellular carcinoma. J Cell
Mol Med. 22:315–329. 2018. View Article : Google Scholar : PubMed/NCBI
|