Introduction
Gastric cancer (GC) is one of the most common
cancers and the third leading cause of cancer-related deaths
worldwide, primarily because of rapid disease progression to
advanced stages and highly malignant potential (1,2).
Despite recent progress in the diagnosis and treatment of GC
patients, one-third of diagnosed patients have already an advanced
stage disease characterized by extensive infiltration, lymph node
metastasis, or distant metastasis (3,4).
Therefore, the discovery of novel biomarkers to identify high-risk
populations with regards to their survival outcomes is desperately
needed to assist physicians in selecting GC patients who require
intensive post-treatment surveillance for early recurrence.
Methyltransferase-like 3 (METTL3) is a
representative RNA methyltransferase that maintains the homeostasis
of m6A methylation, and controls cell differentiation
and proliferation through methylation of its target mRNAs (5). Accumulating evidence has revealed a
pivotal role of METTL3 in the pathogeneses of various human
malignancies, including breast and ovarian cancer (6–8).
Interestingly, regardless of its m6A catalytic activity,
the oncogenic potential of METTL3 is mediated by the translation
process of target oncogenes in lung cancer, suggesting that METTL3
promotes cancer cell growth, survival, and invasion (6). Although a growing number of studies
have demonstrated the functions of METTL3 in experimental systems,
the clinical impact of METTL3 expression in GC remains poorly
understood.
Our previous studies have shown that several
metastasis-associated genes are differentially expressed in
advanced GC and can be used as biomarkers for prognosis of in this
malignancy (9–15). In this study, we systematically
investigated the prognostic impact and biomarker potential of
METTL3 expression using multiple cohorts of clinical specimens
including both FFPE and fresh frozen samples, and clarified the
clinical significance of METTL3 expression in GC patients. We also
assessed the functional role of METTL3 in GC development by
conducting a series of in vitro experiments.
Materials and methods
Tissue samples and patient
characteristics
This study included analysis of a total of 158
patients (111 men and 47 women, median age: 69 years, age range:
18–88 years) who received surgery for primary GC from 2005 to 2011
at the Department of Gastrointestinal Surgery, Mie University
Hospital, Japan for immunohistochemistry analysis.
Immunohistochemical analysis of METTL3 protein expression was
performed using Formalin-fixed, paraffin-embedded (FFPE) samples
with primary GC (FFPE cohort). We used the tumor node metastasis
(TNM) classification to assess clinicopathological findings. This
cohort included 70 GC patients with a stage I disease, 24 with
stage II, 26 with stage III and 38 with stage IV tumors.
Histological findings indicated that 86 patients exhibited an
intestinal type GC while 72 were diffuse type. Post-operative
follow-up data was obtained from all enrolled patients, and the
median follow-up duration was 24.5 months (range: 1–83 months).
In addition, we analyzed tissue specimens from a
fresh frozen cohort of 57 GC patients, (44 men, 13 women, median
age: 69 years, age range: 48–85 years) who received surgery for
primary GC from 2011 to 2015 at the Department of Gastrointestinal
Surgery, Mie University Hospital, Japan. To investigate METTL3 gene
expression by quantitative polymerase chain reaction (qPCR).
Fifty-seven gastric specimens were preserved immediately after
surgical resection in RNAlater (Qiagen) and stored at −80°C
until RNA extraction to investigate METTL3 gene expression through
qPCR. There were 10 patients with stage I, 11 with stage II, 23
with stage III, and 13 with stage IV GCs. Histological findings for
these fresh frozen samples identified 29 patients had
intestinal-type GC and 28 had the diffuse type. Post-operative
follow-up data were obtained from all patients, and the median
follow-up duration was 17.2 months (range: 1–98 months).
In both cohorts, patients who underwent endoscopic
mucosal resection, neoadjuvant therapy, or had non-gastric
carcinomas were excluded. All enrolled patients were followed up
after their initial hospital discharge with physical examinations,
tumor marker assays (carcinoembryonic antigen and carbohydrate
antigen 19-9) performed every 1–3 months, and computed tomography
every 6 months. Endoscopic examinations were performed when
necessary. All participants provided written informed consent. The
study protocol was approved by the Institutional Review Board of
Mie University (approval no. H2019-197). This study was performed
in accordance with The Declaration of Helsinki.
Immunohistochemistry
For the immunohistochemical measurement of METTL3
expression, we used FFPE sections (2–3 µm thick) from 158 GC
patients. Following deparaffinization and dehydration of the cells,
the specimens were boiled in 10 mM sodium citrate buffer to
retrieve antigens, as described previously (13). These specimens were then blocked
and incubated with the primary antibody overnight at 4°C. The
primary antibody against METTL3 (Abcam) was diluted at a 1:500
ratio. Antibody binding was detected by a horseradish peroxidase
Envision kit (Dako Cytomation), and all sections were
counterstained with hematoxylin.
Assessment of METTL3 expression using
immunohistochemistry
The expression of METTL3 was first assessed by
scanning the entire tissue specimen under low power magnification
(×40), and then confirmed under high power (×200 and ×400). A
scoring system for immunoreactivity was used, as described
previously (13,16): (A) fraction of positively stained
cells: 0, ≤5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; and 4, >75%; (B)
intensity of staining: 0, colorless; 1, pale yellow; 2, yellow; and
3, brown. Scores of immunoreactivities were defined as (A)
multiplied by (B) (the fraction of positive stained cells
multiplied by the intensity of staining). Assessment of METTL3
expression in stained FFPE sections were separately conducted by
two experts without knowledge of the clinicopathological or
survival data of any of the patients, to ensure confidence in
histopathological analysis. METTL3 expression in FFPE sections was
re-evaluated if scores of immunoreactivities by the two experts
differed by more than 3.
Total RNA extraction and cDNA
synthesis
We used Mixer Mill MM 300 homogenizer (Qiagen) to
homogenize the fresh frozen specimens. Total RNA from fresh frozen
tissues and cell lines was isolated using a RNeasy mini kit
(Qiagen), as previously described (13). UV absorbance at 260 and 280 nm were
used for measurement of the concentration and quality of RNA, and
OD260/280 ratios of 1.8–2.1 were considered adequate.
Five µg of total RNA with random hexamers and Superscript III
Reverse Transcriptase (Invitrogen, Carlsbad, CA) were used for cDNA
synthesis.
Reverse transcription-qPCR
(RT-qPCR)
StepOne™ Real Time PCR System (Applied Biosystems)
was used for RT-qPCR analysis, as previously described (10,13).
Primers for METTL3 and GAPDH were created by Primer 3 software
(Biology Workbench version 3.2, San Diego Supercomputer Center,
University of California). The designed sequences were as follows:
METTL3: forward, ACATGCTGCCTCAGATGTTG; reverse,
GGATTGTTCCTTGGCTGTTG; GAPDH: forward, GGAAGGTGAAGGTCGGAGTC;
reverse, AATGAAGGGGTCATTGATGG. qPCR was performed with Power SYBR
Green PCR Master Mix (×2) (Applied Biosystems), and the following
cycling conditions were used: 95°C for 10 min, 40 cycles at 95°C
for 15 sec, and 60°C for 1 min. Relative gene expression levels of
METTL3 were determined by the standard curve method, and
quantitative normalization was conducted using GAPDH gene
expression as an internal control, as previously described
(16).
Cell lines
Human GC cell lines MKN7 (intestinal type), MKN74
(intestinal type), KATO III (diffuse type), NUGC3 (diffuse type),
and NUGC4 (diffuse type) were obtained from the Cell Resource
Center for Biomedical Research, Institute of Development, Aging and
Cancer (Tohoku University, Sendai, Japan). The cell lines were
maintained in RPMI-1640 medium supplemented with 10% fetal bovine
serum and antibiotics, and cultured at 37°C in a humidified
atmosphere with 5% CO2, as described previously
(13).
METTL3 siRNA interference
METTL3-specific siRNA (Silencer® Select
Validated siRNA (Assay ID: s32143, s32141), standard purity) and
negative control siRNA (Silencer™ Negative Control siRNA: Catalog
Number: 4390843) were purchased from Ambion (Austin, TX). The
sequences used were as follows: METTL3-specific siRNA(Assay ID:
s32143): Sense sequence: GCAGUUCCUGAAUUAGCUATT; Antisense sequence:
UAGCUAAUUCAGGAACUGCTG; METTL3-specific siRNA(Assay ID: s32141):
Sense sequence: GAACGGGUAGAUGAAAUUATT; Antisense sequence:
UAAUUUCAUCUACCCGUUCAT; negative control siRNA: Forward
transfections were conducted by mixing siRNA oligonucleotides (50
nM) with Lipofectamine RNAiMAX (Invitrogen) and Opti-MEM I
(Invitrogen) and applying the mixture to cells at 24 h after
plating. A series of in vitro assays were conducted after 48
h of incubation.
Cell proliferation assay
A WST-8
[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
monosodium salt] colorimetric assay was performed to assess cell
proliferation, as previously described (13). METTL3 siRNA-transfected and
negative control siRNA-transfected cells (5,000 cells/well) were
seeded in 96-well plates (Becton Dickinson Labware) in 100 µl
culture medium. After 0–72 h of culture, the medium was discarded
and replaced with 90 µl fresh medium, followed by the addition of
10 µl WST-8 reagent solution (Cell Counting Kit; Dojindo
Laboratories). The cells were then incubated for 2 h at 37°C. Each
independent experiment was conducted three times. Cell
proliferation was investigated by colorimetric comparison by
reading OD values using a microplate reader (SoftMax; Molecular
Devices) at an absorption wavelength of 450 nm.
Cell invasion and migration assay
Cell invasion and migration were assessed using
Biocoat Matrigel invasion chambers and control inserts (Becton
Dickinson Labware), as described previously (13,16).
A total of 5×104 transfected cells/well were seeded in
both the invasion and control chambers. We used 10% fetal bovine
serum as the chemoattractant, and seeding cells were incubated for
48 h at 37°C. The incubation medium containing cells was removed
from the top chamber using cotton swabs and serum-free medium. The
membranes were fixed in methanol, stained with Mayer's hematoxylin,
dehydrated in ethanol, and mounted on glass slides. The number of
cells that invaded the underside of the membrane was then counted.
Each independent experiment was conducted three times.
Anoikis assay
We used six-well Costar Ultra-Low Attachment
Microplates for Anoikis assays, as described previously (9,13). A
total of 1×106 transfected cells/2ml/well were seeded in
each well and incubated for 48 h in a humidified atmosphere (37°C
with 5% CO2). After induction of anoikis, an MTT assay
was performed with cells seeded at 5×103 cells/well in
microtiter plates (96 wells, flat bottom) in a final volume of 100
µl culture medium per well. The spectrophotometric absorbance of
the samples was measured as described above. Each independent
experiment was conducted three times.
Statistical methods
Statistical analysis was conducted using Medcalc
version 19.3.1 (Broekstraat 52, 9030), as previously described
(13). Results were expressed as
the mean ± standard deviation (SD). Differences between groups were
estimated by one-way ANOVA, Wilcoxon's signed rank test, the
Mann-Whitney U-test, or Kruskal-Wallis test as appropriate based on
the normality of distribution determined by using the Shapiro-Wilk
test and equality of variance for comparable groups using Levene's
test. When multiple hypothesis testing was performed, Tukey-Kramer
method was used for post-hoc analysis after ANOVA, and Dunn method
was used after the Kruskal-Wallis test. Differences between
categorized groups were estimated by the χ2 test.
Cochran-Armitage test for trend was used in place of χ2
test if the variable had two columns and three or more rows. For
time-to-event analyses, survival estimates were calculated by
Kaplan-Meier analysis, and groups were compared by the log-rank
test. Receiver operating characteristic (ROC) curves were
established to determine cutoff values for analysis of survival
outcomes by Youden's index in each cohort. Overall survival (OS)
was measured from the date the patient underwent surgery until the
date of death from any cause, (cancer-unrelated deaths were not
censored) or the last known follow-up for patients that were still
alive. Disease-free survival (DFS) was measured from the date the
patient underwent curative surgery to the date of disease
recurrence, death from any cause (cancer unrelated deaths were not
censored), or until the last contact with the patient. Cox's
proportional hazards models were used to estimate hazard ratios
(HR) for death or recurrence. Assumption of proportionality was
confirmed for the Cox proportional hazards analyses by generating
Kaplan-Meier survival curves (e.g., high vs. low METTL3 expression
groups) and ensuring that the two curves did not intersect each
other. Variables with P<0.05 in the univariate analysis were
included in the multivariate analysis. Clinical variables that were
considered for univariate and multivariate analyses, in addition to
the target METTL3 expression status, were previously identified
confounding factors that affected the prognosis of patients with
gastric cancer: sex, age at diagnosis, histological type
(intestinal or diffuse), T stage (T1/2 or T3/4), venous invasion
(present or absent), lymphatic vessel invasion (present or absent),
nerve invasion (present or absent), lymph node metastasis (present
or absent), and distant metastasis (presence or absence).
To clarify the prognostic value of METTL3 expression
in GC patients, we conducted propensity score matching (PSM)
analysis (13). High or low
expression of METTL3 protein in GC tissues was designated as the
objective factor. By applying logistic regression analysis, a
continuous propensity score ranging from 0 to 1 was generated.
Matched covariates included gender (male or female), T
classification (T1/2 or T3/4), venous invasion (presence or
absence), lymphatic vessel invasion (presence or absence), lymph
node metastasis (presence or absence), and distant metastasis
(presence or absence), in accordance with the results of the
univariate analysis for the risk of high METTL3 expression in GC
tissues. Matching on the estimated propensity scores with the
maximum allowable difference of 0.001 yielded 74 matched pairs with
high or low METTL3 expression (37 patients in each group, 95% CI:
−0.000039 to 0.000033, P=0.87). All P-values were two-sided, and
values of <0.05 were considered statistically significant.
Results
METTL3 protein was mainly expressed in
GC cells compared with cancer stroma and adjacent normal
mucosa
At first, we evaluated the cellular distribution of
METTL3 protein expression in GC tissues using immunohistochemical
analysis. METTL3 protein expression was primarily expressed in the
nuclei of tumor cells and significantly increased in GC cells
compared with cancer stroma and adjacent normal gastric mucosa
(P<0.0001; Figs. 1A, S1A and B).
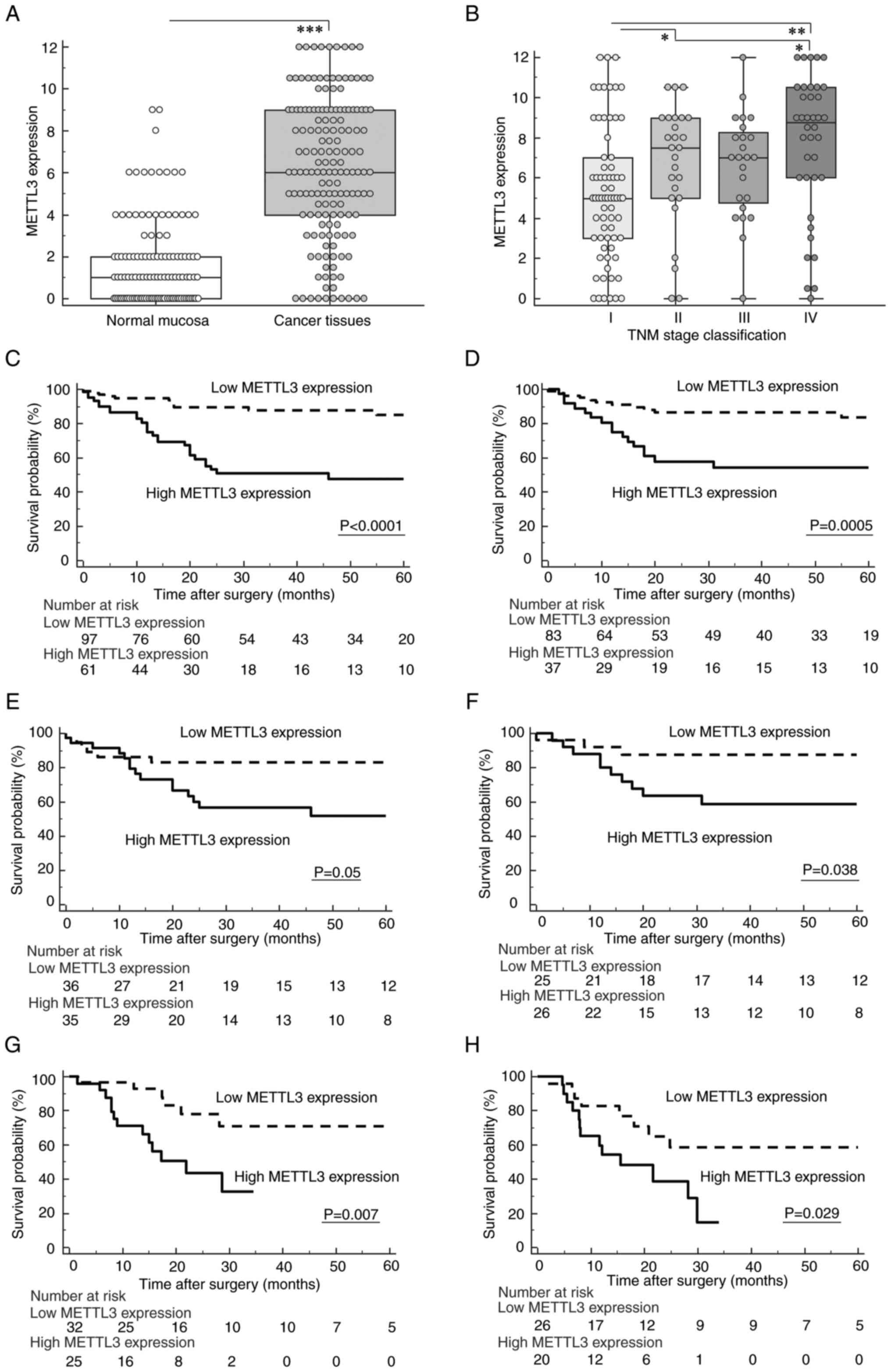 | Figure 1.Dysregulation pattern of
Methyltransferase-like 3 (METTL3) expression and its prognostic
potential in overall survival (OS) and disease-free survival (DFS)
of gastric cancer (GC) patients. (A) METTL3 expression was
significantly increased in GC tissues compared with adjacent normal
mucosa. (B) Scattergrams of METTL3 expression according to the UICC
classification in GC patients. (C and D) Kaplan-Meier survival
curves for OS (C) and DFS (D) of GC patients based on METTL3
expression in GC tissues of the formalin-fixed, paraffin-embedded
cohort. Patients with high expression of METTL3 exhibited a
significantly poorer survival compared with those in the low
expression group (OS: P<0.0001; DFS: P=0.0005, log-rank test).
(E and F) Survival curve analysis subdivided by METTL3 expression
in GC tissues using PSM analysis demonstrated that high METTL3
expression was significantly correlated with poor OS (E) (P=0.05,
log-rank test) and DFS (F) (P=0.038, log-rank test). (G, H)
Kaplan-Meier survival curves for OS (G) and DFS (H) of GC patients
based on METTL3 expression in the fresh frozen cohort. Elevated
expression of METTL3 in GC tissues was significantly associated
with poor oncological outcomes in the fresh frozen cohort (OS:
P=0.007; DFS: P=0.029, log-rank test). All statistical tests were
two-sided. ***P<0.001, **P<0.01, *P<0.05. METTL3,
methyltransferase-like 3; OS, overall survival; DFS, disease-free
survival; GC, gastric cancer. |
METTL3 expression was associated with
disease development in the FFPE cohort of GC patients
Next, we investigated associations between
clinicopathological factors and METTL3 expression in the FFPE
cohort. We defined a cutoff value of >7.5 as the high staining
group (n=61) and ≤7.5 as the low staining group (n=97) based on ROC
analyses with Youden's index correction for METTL3 expression. The
high staining group was significantly associated with males
(P=0.029), an advanced T stage (P=0.0002), the presence of venous
invasion (P<0.0001), lymphatic vessel invasion (P=0.011), lymph
node metastasis (P=0.004), distant metastasis (P=0.0004), and
advanced TNM stage classification (P=0.0001) in the FFPE cohort of
GC patients (Table I; Fig. 1B).
 | Table I.Clinicopathological variables and
METTL3 protein expression in gastric cancer patients. |
Table I.
Clinicopathological variables and
METTL3 protein expression in gastric cancer patients.
| Characteristic | All patients | Highb (n=61) | Low (n=97) | P-value |
|---|
| Sex |
|
|
| 0.029c,d |
|
Male | 111 | 49 | 62 |
|
|
Female | 47 | 12 | 35 |
|
| Age, years |
|
|
| 0.05d |
|
<69a | 88 | 28 | 60 |
|
|
≧69 | 70 | 33 | 37 |
|
| Histological
type |
|
|
| 0.06d |
|
Intestinal type | 86 | 39 | 47 |
|
| Diffuse
type | 72 | 22 | 50 |
|
| Pathological T
category |
|
|
| 0.0002c,d |
|
pT1/2 | 79 | 19 | 60 |
|
|
pT3/4 | 79 | 42 | 37 |
|
| Vessel
invasion |
|
|
|
<0.0001c,d |
|
Present | 86 | 48 | 38 |
|
|
Absent | 72 | 13 | 59 |
|
| Lymphovascular
invasion |
|
|
| 0.011c,d |
|
Present | 117 | 52 | 65 |
|
|
Absent | 41 | 9 | 32 |
|
| Lymph node
metastasis |
|
|
| 0.004c,d |
|
Present | 73 | 37 | 36 |
|
|
Absent | 85 | 24 | 61 |
|
| Distant
metastasis |
|
|
| 0.0004c,d |
|
Present | 38 | 24 | 14 |
|
|
Absent | 120 | 37 | 83 |
|
| UICC TNM
classification |
|
|
| 0.0001c,e |
| Stage
I | 70 | 16 | 54 |
|
| Stage
II | 26 | 12 | 14 |
|
| Stage
III | 24 | 9 | 15 |
|
| Stage
IV | 38 | 24 | 14 |
|
Increased expression of METTL3 protein
is an independent prognostic factor for both OS and DFS in the FFPE
cohort of GC patients
To investigate the potential use of METTL3
expression as a prognostic biomarker, we performed time-to-event
analysis. GC Patients with increased expression of METTL3 showed
poorer prognosis in terms of both OS and DFS compared to those with
low expression (OS: P<0.0001, Fig.
1C; DFS: P=0.0005, Fig. 1D;
log-rank test).
Next, we determined the potential of the METTL3
expression status as a predictive biomarker for recurrence and
prognosis in GC patients using multivariate Cox regression
analysis. These analyses demonstrated that increased METTL3
expression in GC tissues was an independent prognostic factor for
both OS [hazard ratio (HR), 3.24; 95% confidence interval (CI),
1.57–6.68; P=0.001, Table IIA)
and DFS (HR, 2.4; 95% CI, 1.12–5.16; P=0.025, Table IIB) in the FFPE cohort of GC
patients.
 | Table II.Multivariate analysis for predictors
of survival in the GC cohort using immunohistochemical
analysis. |
Table II.
Multivariate analysis for predictors
of survival in the GC cohort using immunohistochemical
analysis.
| A, Overall
survival |
|---|
|
|---|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender (male) | 1.75 | 0.83–3.68 | 0.14 |
|
|
|
| Age (≧69 years
old)a | 2.44 | 1.29–4.6 | 0.006b | 2.57 | 1.33–4.98 | 0.005b |
| Histological type
(intestinal type) | 0.99 | 0.53–1.84 | 0.97 |
|
|
|
| T classification
(pT3/4) | 3.25 | 1.63–6.46 | 0.0008b | 0.63 | 0.23–1.72 | 0.37 |
| Vessel involvement
(present) | 2.89 | 1.38–6.09 | 0.005b | 0.84 | 0.34–2.06 | 0.7 |
| Lymphatic vessel
involvement (present) | 1.52 | 0.7–3.31 | 0.29 |
|
|
|
| Lymph node
metastasis (present) | 5.21 | 2.52–10.8 |
<0.0001b | 3.95 | 1.55–10.1 | 0.004b |
| Distant metastasis
(present) | 5.96 | 3.06–11.6 |
<0.0001b | 3.28 | 1.47–7.34 | 0.004b |
| High METTL3
expressionc | 4.39 | 2.23–8.65 |
<0.0001b | 3.24 | 1.57–6.68 | 0.001b |
|
| B, Disease-free
survival |
|
|
|
Univariate |
Multivariate |
|
|
|
|
|
Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| Gender (male) | 2.24 | 0.92–5.5 | 0.08 |
|
|
|
| Age (≧69 years
old)a | 2.41 | 1.16–5.02 | 0.019b | 1.7 | 0.79–3.67 | 0.18 |
| Histological type
(intestinal type) | 0.85 | 0.41–1.78 | 0.67 |
|
|
|
| T classification
(pT3/4) | 3.94 | 1.86–8.37 | 0.0003b | 1.64 | 0.67–4.01 | 0.28 |
| Vessel involvement
(present) | 1.98 | 0.93–4.19 | 0.08 |
|
|
|
| Lymphatic vessel
involvement (present) | 1.24 | 0.57–2.7 | 0.6 |
|
|
|
| Lymph node
metastasis (present) | 5 | 2.37–10.6 |
<0.0001b | 3.36 | 1.39–8.12 | 0.007b |
| High METTL3
expressionc | 3.4 | 1.63–7.09 | 0.001b | 2.4 | 1.12–5.16 | 0.025b |
PSM analysis validated the prognostic
impact of METTL3 expression in GC patients
PSM analysis has come into the limelight as a new
statistical method to overcome selection bias and different patient
characteristics to elevate the evidence level of a non-randomized
observational study (17). To
clarify the potential of METTL3 expression as a prognostic
biomarker in GC patients, we performed PSM analysis using FFPE
cohort and categorized 74 GC patients (37 patients in each group)
for further analysis. No differences in patient characteristics
between high- and low-staining group were found following PSM
analysis (Table SI). Kaplan-Meier
survival curve analysis demonstrated that high expression of METTL3
was significantly associated with poor prognosis in terms of both
OS and DFS (OS: P=0.05, Fig. 1E;
DFS: P=0.038, Fig. 1F; log-rank
test) in the PSM cohort of GC patients. Collectively, these
findings clearly indicated that METTL3 protein expression in GC
tissues might be used as a prognostic biomarker in GC patients.
The prognostic impact of METTL3
expression was successfully validated in fresh frozen cohort of GC
patients
We confirmed the biomarker potential of METTL3
expression using FFPE specimens to identify GC patients with
high-risk for oncological outcomes. In a clinical setting,
assessment of METTL3 expression using preoperative biopsy specimens
could provide valuable information for physicians to decide the
treatment course for GC patients. From the aspect that preoperative
biopsy specimens are usually preserved in a fresh frozen specimen,
we further evaluated METTL3 gene expression using fresh frozen
specimens from an independent GC cohort to clarify the prognostic
impact of METTL3 gene expression. We quantified expression levels
of METTL3 in 57 GC tissues using RT-qPCR, and the cut-off threshold
for METTL3 expression were consistent method of the FFPE cohort and
performed ROC analyses with Youden's index to determine prognosis
of GC patients. Interestingly, in accordance with METTL3 protein
expression in the FFPE cohort, the high expression group was
significantly associated with the presence of venous invasion
(P=0.001) in the fresh frozen cohort (Table III).
 | Table III.Clinicopathological variables and
METTL3 mRNA expression in gastric cancer patients. |
Table III.
Clinicopathological variables and
METTL3 mRNA expression in gastric cancer patients.
|
|
| METTL3
expression |
|
|---|
|
|
|
|
|
|---|
| Variable | n | Highb (n=25) | Low (n=32) | P-value |
|---|
| Sex |
|
|
|
|
|
Male | 44 | 20 | 24 | 0.66c |
|
Female | 13 | 5 | 8 |
|
| Age, years |
|
|
|
|
|
<69a | 25 | 10 | 15 | 0.61c |
|
≧69 | 32 | 15 | 17 |
|
| Histological
type |
|
|
|
|
|
Intestinal type | 29 | 12 | 17 | 0.7c |
| Diffuse
type | 28 | 13 | 15 |
|
| Pathological T
category |
|
|
|
|
|
pT1/2 | 12 | 6 | 6 | 0.63c |
|
pT3/4 | 45 | 19 | 26 |
|
| Vessel
invasion |
|
|
|
|
|
Present | 35 | 19 | 16 | 0.047c,d |
|
Absent | 22 | 6 | 16 |
|
| Lymphovascular
invasion |
|
|
|
|
|
Present | 48 | 20 | 28 | 0.45c |
|
Absent | 9 | 5 | 4 |
|
| Lymph node
metastasis |
|
|
|
|
|
Present | 38 | 18 | 20 | 0.67c |
|
Absent | 17 | 7 | 10 |
|
| Distant
metastasis |
|
|
|
|
|
Present | 13 | 7 | 6 | 0.41c |
|
Absent | 44 | 18 | 26 |
|
| UICC TNM
classification |
|
|
|
|
| Stage
I | 10 | 5 | 5 | 0.62e |
| Stage
II | 11 | 3 | 8 |
|
| Stage
III | 23 | 10 | 13 |
|
| Stage
IV | 13 | 7 | 6 |
|
To further confirm the prognostic potential of
METTL3 expression in GC patients, we investigated whether
assessment of METTL3 gene expression in fresh frozen specimens
could identify GC patients with high-risk for poor survival
outcomes. Survival curve analysis showed that high METTL3 gene
expression in GC tissues was significantly associated with poorer
prognosis in terms of both OS and DFS in the fresh frozen cohort
(OS: P=0.007, Fig. 1G; DFS:
P=0.029, Fig. 1H). Surprisingly,
multivariate Cox regression analysis also revealed that increased
METTL3 expression was an independent prognostic factor, especially
for OS (OS: HR, 3.38; 95% CI, 1.17–9.73; P=0.024, Table IVA; DFS: HR, 2.69; 95% CI,
0.97–7.45; P=0.058, Table IVB),
in the fresh frozen cohort of GC patients. Collectively, our
findings highlighted that quantification of METTL3 gene expression
in fresh frozen specimens could also provide valuable information
for oncologists to identify patients at high-risk for recurrence
and poor prognosis in GC patients.
 | Table IV.Multivariate analysis for predictors
of survival in GC cohort using qPCR analysis. |
Table IV.
Multivariate analysis for predictors
of survival in GC cohort using qPCR analysis.
| A, Overall
survival |
|---|
|
|---|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender (male) | 2.41 | 0.56–10.4 | 0.24 |
|
|
|
| Age (≧69 years
old)a | 2.98 | 1.07–8.31 | 0.036b | 3.09 | 0.98–9.73 | 0.05 |
| Histological type
(diffuse type) | 1.46 | 0.59–3.65 | 0.41 |
|
|
|
| T classification
(pT3/4) | 5.77 | 0.77–43.3 | 0.09 |
|
|
|
| Vessel involvement
(present) | 2.32 | 0.77–7.01 | 0.13 |
|
|
|
| Lymphatic vessel
involvement (present) | 3.83 | 0.51–28.8 | 0.19 |
|
|
|
| Lymph node
metastasis (present) | 10.1 | 1.34–76.6 | 0.025b | 10.5 | 1.33–82.4 | 0.026b |
| Distant metastasis
(present) | 2.48 | 0.97–6.35 | 0.06 |
|
|
|
| High METTL3
expressionc | 3.54 | 1.34–9.37 | 0.011b | 3.38 | 1.17–9.73 | 0.024b |
|
| B, Disease-free
survival |
|
|
|
Univariate |
Multivariate |
|
|
|
|
|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| Gender (male) | 1.01 | 0.37–2.77 | 0.98 |
|
|
|
| Age (≧69 years
old)a | 3.06 | 1.22–7.68 | 0.017b | 3.55 | 1.24–10.2 | 0.018b |
| Histological type
(diffuse type) | 1.69 | 0.71–4.01 | 0.24 |
|
|
|
| T classification
(pT3/4) | 2.74 | 0.81–9.33 | 0.11 |
|
|
|
| Vessel involvement
(present) | 2.88 | 0.97–8.56 | 0.06 |
|
|
|
| Lymphatic vessel
involvement (present) | 2.71 | 0.63–11.7 | 0.18 |
|
|
|
| Lymph node
metastasis (present) | 3.35 | 1.1–10.2 | 0.033b | 4 | 1.25–12.8 | 0.02b |
| High METTL3
expressionc | 2.61 | 1.07–6.36 | 0.035b | 2.69 | 0.97–7.45 | 0.058 |
siRNA transfection led to significant
inhibition of METTL3 expression in GC cell lines
Considering the prognostic burden of METTL3
expression in GC tissues as described above, we further evaluated
the biological role of METTL3 in GC development. At first, we
assessed METTL3 expression by RT-qPCR analysis in established GC
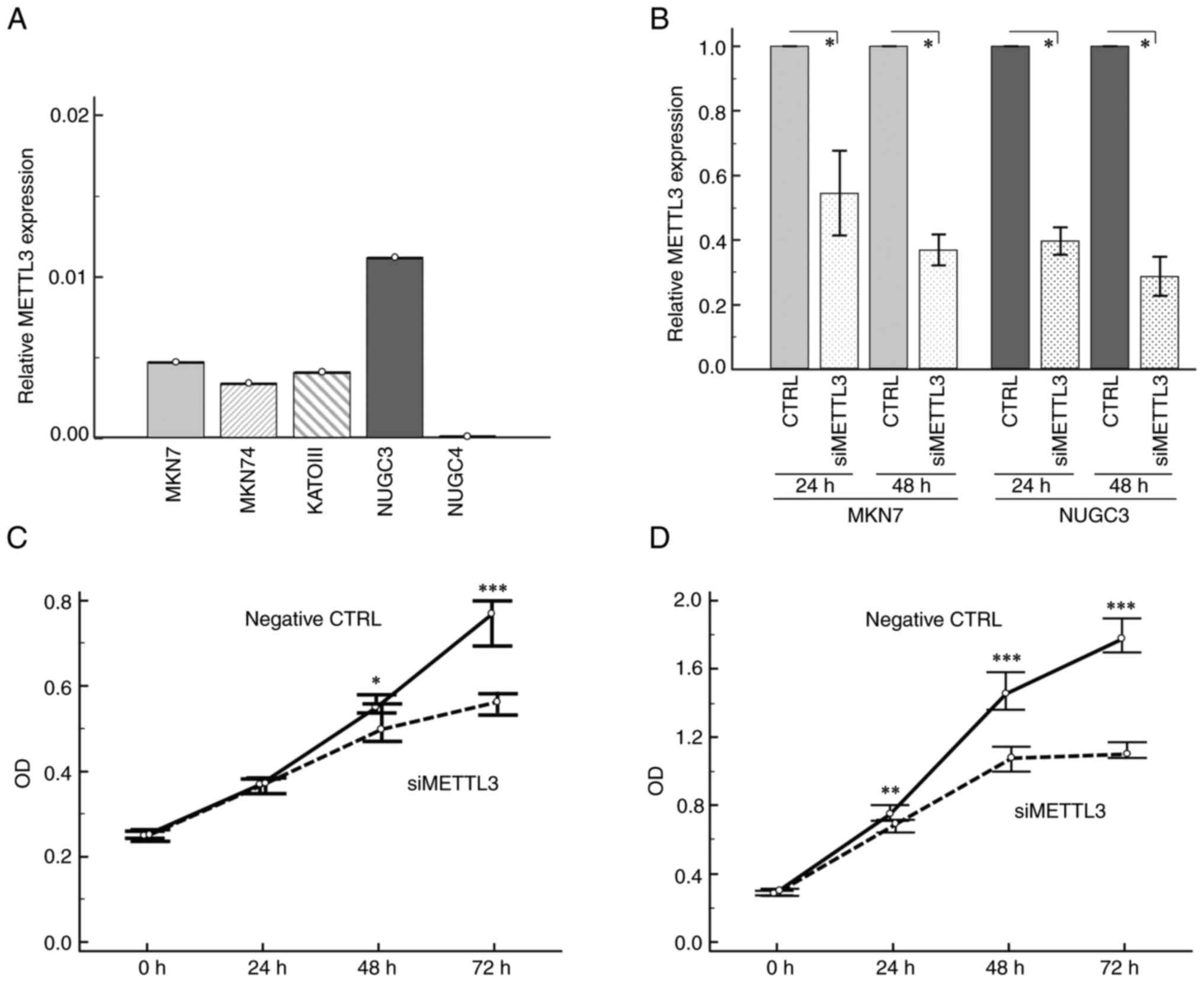
cell lines (Fig. 2A) and
demonstrated that METTL3 was highly expressed in MKN7 and NUGC3
cells compared to all other GC cell lines. Therefore, we decided to
use MKN7 and NUGC3 cell lines for further knockdown experiments.
Treatment of GC cell lines with METTL3 siRNA transfection
(Silencer® Select Validated siRNA (Assay ID: s32143))
showed significant inhibition of this methyl transferase mRNA
expression (up to 60%) compared to those with negative control
siRNA at 48 h post-transfection (Fig.
2B).
METTL3 knockdown could inhibit
proliferation, invasion, and migration of GC cells
To determine whether METTL3 siRNA transfection
affected cell proliferation in human GC cell lines, we used MTT
assays. METTL3 knockdown significantly suppressed tumor cell growth
at 48 and 72 h after cell transfection in both MKN7 and NUGC3 cell
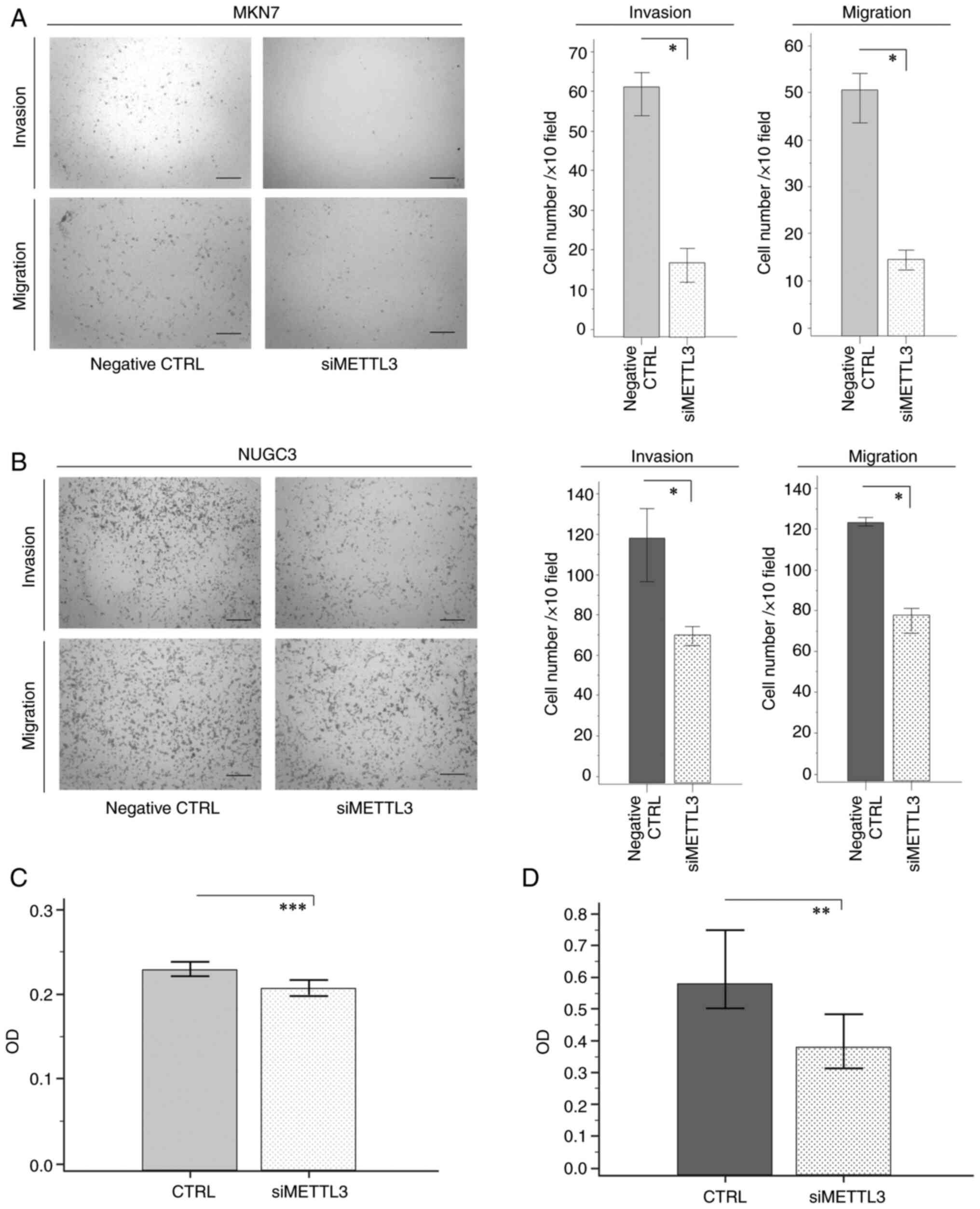
lines (Fig. 2C and D). We further
conducted in vitro invasion and migration assays to reveal
that METTL3 siRNA transfection of MKN7 and NUGC3 GC cells resulted
in significant inhibition of the invasive and migratory potentials
compared with cells transfected with control siRNA (Fig. 3A and B).
METTL3 siRNA transfection suppressed
anoikis resistance in GC cells
Anoikis is an apoptotic process induced by loss of
cell adhesion (18), and
resistance to anoikis is a pivotal metastatic process in
malignancies (19). To clarify
whether METTL3 knockdown promoted anoikis, we assessed the number
of viable suspended MKN7 and NUGC3 cells in low attachment plates
through MTT assays. Interestingly, both GC cell lines with METTL3
inhibition demonstrated significant decreases in the number of
viable cells compared with cells transfected with control siRNA
(Fig. 3C and D). Collectively,
these findings suggest that METTL3 is involved in the pathogenesis
of GC by enhancing cell growth, invasive and migratory potentials,
and anoikis resistance in GC cells.
METTL3 inhibition using different
METTL3-siRNAs suppressed proliferation in GC cells
To further clarify whether these results are
siRNA-specific phenomena, we performed additional in vitro
analysis using different METTL3-siRNA transfection. Treatment of GC
cell lines with METTL3 siRNA transfection [Silencer®
Select Validated siRNA (Assay ID: s32141)] also showed significant
inhibition of METTL3 expression (up to 60%) compared to those with
negative control siRNA at 48 h post-transfection (Fig. S2A). We further conducted MTT assay
after treatment of GC cell lines with METTL3 siRNA transfection,
and successfully verified that inhibition of METTL3 expression
significantly suppressed tumor cell growth at 48 and 72 h after
cell transfection in both MKN7 and NUGC3 cell lines (Fig. S2B and C).
Discussion
In the last decade, treatment options for GC have
progressed drastically to improve the prognosis of GC patients
(2). However, GC is an aggressive
cancer, and the survival rate of advanced GC remains poor (1). Currently, the pathological TNM stage
classification is the best available prognostic indicator. However,
the differences in oncological outcomes of GC patients largely
depend on the underlying molecular heterogeneity, and the TNM stage
classification is inadequate at predicting survival outcomes
accurately for individual GC patients. Therefore, identification of
high-risk populations may help physicians to decide the treatment
course and improve the prognosis of GC. In this study, we
systemically investigated the clinical value and potential role of
METTL3 in GC and made several novel discoveries. First, METTL3
protein expression was significantly elevated in tumor cells of GC
tissues compared with adjacent normal mucosa. Second, increased
expression of METTL3 was significantly correlated with
well-established disease development factors and was an independent
prognostic factor for both OS and DFS in the FFPE cohort. Third,
PSM analysis clearly demonstrated the biomarker potential of METTL3
expression in GC tissues to identify poor oncological outcomes of
GC patients. Fourth, we confirmed the prognostic value of METTL3
expression using fresh frozen specimens from GC patients. Finally,
knockdown of METTL3 expression inhibited various oncological
functions of GC cells, including proliferation, invasion, migration
and anoikis resistance.
Medical treatments for GC patients have advanced
tremendously over the last decade, and several clinical trials have
revealed the therapeutic impact of various chemotherapies and
molecular targeted therapies, including trastuzumab (20), ramucirumab (21,22),
nivolumab (23),
trifluridine/tipiracil (24), on
oncological outcomes, especially in unresectable GC patients. In
the adjuvant setting, a recent study demonstrated significant
survival benefits of docetaxel combined with S-1 in post-operative
patients with stage III GC (25).
Furthermore, a recent randomized controlled trial showed no
survival benefit of additional gastrectomy over chemotherapy alone
in patients with non-curable advanced GC (26). Considering such evidence,
identification of a high-risk population for recurrence and
survival using pre-operative or post-operative specimens may
provide feasible information for physicians to decide the best
treatment course possible for GC patients with chemotherapeutic
regimens. This could considerably improve survival rates of GC
patients.
METTL3 plays a central functional role in
oncogenesis (27,28), and several studies have
corroborated its potential as a prognostic biomarker in various
cancers (29,30). For instance, Lin and co-workers
assessed METTL3 protein expression through immunohistochemical
analysis in 100 patients with hepatocellular carcinoma (HCC)
(29). They demonstrated that
METTL3 protein was significantly upregulated in the nuclei of HCC
cells compared with normal liver tissues, and that HCC patients
with strong expression of METTL3 protein had poorer survival than
those with weak expression, similar to our findings in GC cells.
Another study conducted immunohistochemical analysis of surgical
specimens from 162 ovarian carcinoma patients (30). METTL3 was frequently upregulated in
ovarian carcinoma and high expression of METTL3 protein was
significantly associated with a poor survival rate. Consistent with
this evidence, a major finding of this study was the prognostic
impact of METTL3 expression in FFPE specimens of GC patients.
Increased expression of METTL3 was significantly associated with
well-established disease development factors and an independent
prognostic factor for both the DFS and OS of GC patients.
Interestingly, the PSM analysis clearly revealed and confirmed the
prognostic value of METTL3 expression even after adjustment of
covariate factors based on the different GC patient
characteristics. In addition, we quantified METTL3 gene expression
in fresh frozen specimens, assuming pre-operative biopsy specimens,
and validated the prognostic value of METTL3 by RT-qPCR analysis.
Collectively, these findings suggested that assessment of METTL3
expression in fresh frozen or surgical specimens can be used to
decide the treatment course or chemotherapeutic regimens for GC
patients.
Accumulating evidence has continued to reveal the
pivotal role of epigenetic regulation, including DNA methylation,
histone modification, and non-coding RNAs, in disease development
of malignancies (31). RNA
methylation was classically identified in the 1970s (32,33),
and the biological function of this RNA modification was recently
recognized as a pivotal epigenetic alteration that is deeply
involved in the post-transcriptional regulation of major genes
associated with human cancers (34). N6-methyladenosine (m6A)
modification is the most prevalent internal modification in
eukaryotic cells (35), and
several lines of evidence have demonstrated that dysregulation of
m6A methylation regulates cancer development (27,28).
METTL3 is a representative methyltransferase responsible for
m6A modification, and its catalytic subunit forms the
m6A methyltransferase complex with METTL14, WTAP, and
RBM15 (36). Furthermore, Lin and
coworkers found that the N-terminal of METTL3 is sufficient to
promote translation, while the catalytic domain containing the
C-terminus of METTL3 has no effect on promoting translation,
further confirming that METTL3 promotes translation of oncogenes
independently of its catalytic activity and m6A readers
(6). Based on these findings,
emerging studies have demonstrated the oncogenic role of METTL3 in
various processes of disease development, including cell
proliferation, invasion, and migration in malignancies including
lung cancer, liver cancer, breast cancer, bladder cancer, and
myeloid leukemia progression (6–8,37–39).
Chen et al conducted a series of in vitro and in
vivo analyses, and demonstrated that knockdown of METTL3
significantly inhibited cell proliferation, migration, and
tumorigenicity via suppressor of cytokine signaling 2 mRNA
m6A modification in hepatocellular cell carcinoma
(38). Another study showed that
METTL3 knockdown reduced proliferation, invasion, and the
anti-apoptotic potential via regulation of AKT phosphorylation in
ovarian cancer cells (8). Not only
is this evidence consistent with our findings, we went a step
further by confirming that METTL3 knockdown inhibited various
oncogenic phenotypes related to oncogenesis, including
proliferation, invasion, migration, and anoikis resistance in GC
cell lines. Collectively, these findings suggest that METTL3 plays
critical roles in the malignant potential of GC, which may be a
therapeutic target.
In conclusion, our study highlights that assessment
of METTL3 expression in fresh frozen or FFPE specimens may provide
pivotal information for the identification of high-risk GC
patients. In addition, METTL3 might be a novel therapeutic target
in GC patients.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors thank Mrs. Yuki Orito and Dr Amphone
Okada (Gastrointestinal and Pediatric Surgery, Division of
Reparative Medicine, Institute of Life Sciences, Mie University
Graduate School of Medicine) for providing excellent technical
assistance.
Funding
This work was supported in part by Grants-in-Aid for Scientific
Research (grant nos. 18K08566, 18K08700, 18K08674, 18K08591 and
20K09004) from the Ministry of Education, Culture, Sports, Science
and Technology, Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YO, YT, MO and KN conceived and designed the study.
TI, HI, TK, TS, MK, HY, HF, TY and MO provided the samples. YO, YT,
CY, MR, TI, HI, TK, TS, MK, TY, and IM acquired the data. YO, YT,
AG, MR, HY, HF, IM, MO and KN analyzed and interpretated the data.
YO and YT performed the statistical analysis. YO, YT, AG, MO and KN
drafted the manuscript. YO and YT confirm the authenticity of raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Mie University (approval no. H2019-197). The
requirement for informed consent was waived, and an information
disclosure statement was uploaded onto the homepage of our hospital
website for opt-out.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta GP and Massague J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meyer KD and Jaffrey SR: The dynamic
epitranscriptome: N6-methyladenosine and gene expression control.
Nat Rev Mol Cell Biol. 15:313–326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin S, Choe J, Du P, Triboulet R and
Gregory RI: The m(6)A Methyltransferase METTL3 promotes translation
in human cancer cells. Mol Cell. 62:335–345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cai X, Wang X, Cao C, Gao Y, Zhang S, Yang
Z, Liu Y, Zhang X, Zhang W and Ye L: HBXIP-elevated
methyltransferase METTL3 promotes the progression of breast cancer
via inhibiting tumor suppressor let-7g. Cancer Lett. 415:11–19.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang S, Guan H, Lin X, Li N, Geng F and
Li J: METTL3 serves an oncogenic role in human ovarian cancer cells
partially via the AKT signaling pathway. Oncol Lett. 19:3197–3204.
2020.PubMed/NCBI
|
|
9
|
Okugawa Y, Toiyama Y, Hur K, Toden S,
Saigusa S, Tanaka K, Inoue Y, Mohri Y, Kusunoki M, Boland CR and
Goel A: Metastasis-associated long non-coding RNA drives gastric
cancer development and promotes peritoneal metastasis.
Carcinogenesis. 35:2731–2739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okugawa Y, Tanaka K, Inoue Y, Kawamura M,
Kawamoto A, Hiro J, Saigusa S, Toiyama Y, Ohi M, Uchida K, et al:
Brain-derived neurotrophic factor/tropomyosin-related kinase B
pathway in gastric cancer. Br J Cancer. 108:121–130. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okugawa Y, Inoue Y, Tanaka K, Kawamura M,
Saigusa S, Toiyama Y, Ohi M, Uchida K, Mohri Y and Kusunoki M: Smad
interacting protein 1 (SIP1) is associated with peritoneal
carcinomatosis in intestinal type gastric cancer. Clin Exp
Metastasis. 30:417–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okugawa Y, Toiyama Y, Tanaka K, Matsusita
K, Fujikawa H, Saigusa S, Ohi M, Inoue Y, Mohri Y, Uchida K and
Kusunoki M: Clinical significance of Zinc finger E-box Binding
homeobox 1 (ZEB1) in human gastric cancer. J Surg Oncol.
106:280–285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ichikawa T, Okugawa Y, Toiyama Y, Tanaka
K, Yin C, Kitajima T, Kondo S, Shimura T, Ohi M, Araki T and
Kusunoki M: Clinical significance and biological role of L1 cell
adhesion molecule in gastric cancer. Br J Cancer. 121:1058–1068.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okugawa Y, Toiyama Y, Shigeyasu K,
Yamamoto A, Shigemori T, Yin C, Ichikawa T, Yasuda H, Fujikawa H,
Yoshiyama S, et al: Enhanced AZIN1 RNA editing and overexpression
of its regulatory enzyme ADAR1 are important prognostic biomarkers
in gastric cancer. J Transl Med. 16:3662018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okugawa Y, Mohri Y, Tanaka K, Kawamura M,
Saigusa S, Toiyama Y, Ohi M, Inoue Y, Miki C and Kusunoki M:
Metastasis-associated protein is a predictive biomarker for
metastasis and recurrence in gastric cancer. Oncol Rep.
36:1893–1900. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Toiyama Y, Yasuda H, Saigusa S, Tanaka K,
Inoue Y, Goel A and Kusunoki M: Increased expression of Slug and
Vimentin as novel predictive biomarkers for lymph node metastasis
and poor prognosis in colorectal cancer. Carcinogenesis.
34:2548–2557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Austin PC: An introduction to propensity
score methods for reducing the effects of confounding in
observational studies. Multivariate Behav Res. 46:399–424. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frisch SM and Francis H: Disruption of
epithelial cell-matrix interactions induces apoptosis. J Cell Biol.
124:619–626. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eccles SA and Welch DR: Metastasis: Recent
discoveries and novel treatment strategies. Lancet. 369:1742–1757.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry
DR, et al: Ramucirumab monotherapy for previously treated advanced
gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An
international, randomised, multicentre, placebo-controlled, phase 3
trial. Lancet. 383:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shitara K, Doi T, Dvorkin M, Mansoor W,
Arkenau HT, Prokharau A, Alsina M, Ghidini M, Faustino C, Gorbunova
V, et al: Trifluridine/tipiracil versus placebo in patients with
heavily pretreated metastatic gastric cancer (TAGS): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.
19:1437–1448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshida K, Kodera Y, Kochi M, Ichikawa W,
Kakeji Y, Sano T, Nagao N, Takahashi M, Takagane A, Watanabe T, et
al: Addition of docetaxel to oral fluoropyrimidine improves
efficacy in patients with stage III Gastric cancer: Interim
Analysis of JACCRO GC-07, a randomized controlled trial. J Clin
Oncol. 37:1296–1304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujitani K, Yang HK, Mizusawa J, Kim YW,
Terashima M, Han SU, Iwasaki Y, Hyung WJ, Takagane A, Park DJ, et
al: Gastrectomy plus chemotherapy versus chemotherapy alone for
advanced gastric cancer with a single non-curable factor (REGATTA):
A phase 3, randomised controlled trial. Lancet Oncol. 17:309–318.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen XY, Zhang J and Zhu JS: The role of
m(6)A RNA methylation in human cancer. Mol Cancer. 18:1032019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lan Q, Liu PY, Haase J, Bell JL,
Hüttelmaier S and Liu T: The critical Role of RNA m(6)A Methylation
in cancer. Cancer Res. 79:1285–1292. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin Y, Wei X, Jian Z and Zhang X: METTL3
expression is associated with glycolysis metabolism and sensitivity
to glycolytic stress in hepatocellular carcinoma. Cancer Med.
9:2859–2867. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hua W, Zhao Y, Jin X, Yu D, He J, Xie D
and Duan P: METTL3 promotes ovarian carcinoma growth and invasion
through the regulation of AXL translation and epithelial to
mesenchymal transition. Gynecol Oncol. 151:356–365. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okugawa Y, Grady WM and Goel A: Epigenetic
alterations in colorectal cancer: Emerging biomarkers.
Gastroenterology. 149:1204–1225.e12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adams JM and Cory S: Modified nucleosides
and bizarre 5′-Termini in mouse myeloma mRNA. Nature. 255:28–33.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Desrosiers R, Friderici K and Rottman F:
Identification of methylated nucleosides in messenger RNA from
Novikoff hepatoma cells. Proc Natl Acad Sci USA. 71:3971–3975.
1974. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao BS, Roundtree IA and He C:
Post-transcriptional gene regulation by mRNA modifications. Nat Rev
Mol Cell Biol. 18:31–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wei W, Ji X, Guo X and Ji S: Regulatory
role of N6-methyladenosine (m6A) methylation
in rna processing and human diseases. J Cell Biochem.
118:2534–2543. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang
L, Jia G, Yu M, Lu Z, Deng X, et al: A METTL3-METTL14 complex
mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem
Biol. 10:93–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng M, Sheng L, Gao Q, Xiong Q, Zhang H,
Wu M, Liang Y, Zhu F, Zhang Y, Zhang X, et al: The m6A
methyltransferase METTL3 promotes bladder cancer progression via
AFF4/NF-κB/MYC signaling network. Oncogene. 38:3667–3680. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen M, Wei L, Law CT, Tsang FH, Shen J,
Cheng CL, Tsang LH, Ho DW, Chiu DK, Lee JM, et al: RNA
N6-methyladenosine methyltransferase-like 3 promotes liver cancer
progression through YTHDF2-dependent posttranscriptional silencing
of SOCS2. Hepatology. 67:2254–2270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Barbieri I, Tzelepis K, Pandolfini L, Shi
J, Millán-Zambrano G, Robson SC, Aspris D, Migliori V, Bannister
AJ, Han N, et al: Promoter-bound METTL3 maintains myeloid leukaemia
by m6A-dependent translation control. Nature.
552:126–131. 2017. View Article : Google Scholar : PubMed/NCBI
|