Introduction
Solitary fibrous tumor (SFT) is a rare spindle cell
tumor of mesenchymal origin, initially reported by Klempere and
Rabin (1) in 1931. SFT is commonly
found in the mediastinum and visceral pleura; however, it also
occurs in the pleura external sites, such as the head and neck,
pericardium, peritoneum, liver, thyroid, mesentery, and sinuses and
orbits (2). Due to the lack of a
true connective tissue component in the central nervous system
(CNS), primary SFT of the CNS is rare, accounting for ~1% of all
primary CNS tumors (3,4). Most CNS SFTs occur in the cranial
cavity and just over one-fifth were intraspinal (5–7).
Primary spinal SFT may occur at any age (5). The mean age of onset was 40.9 years
for males and 35.0 years for females (5). There was no significant difference in
morbidity between males and females (5). Primary spinal SFT usually occurs in
the thoracic spinal cord, followed by the cervical and lumbar
spinal cord, and the sacral spinal cord is rarely affected
(5). Intracranial SFT occurs most
commonly in adults aged 20–70 years, with similar incidence rates
in males and females (8). When CNS
SFTs occur intracranially, they are frequently extra-axially
located (9,10). Hemangiopericytomas (HPCs) are also
rare mesenchymal tumors that exhibit similar clinical, radiological
and histological features to SFTs (11). The NGFI-A-binding protein (NAB2) and
signal transducer and activator of transcription 6 (STAT6) gene
fusion was identified as a driver mutation of SFT (12,13).
Previous pathological findings demonstrated that SFT and HPCs
contain identical genetic abnormalities and these prompted the
World Health Organization (WHO) to classify the two tumor types as
a new combined entity in 2016 (14). This classification described three
grades of SFT/HPC, namely grade I, II and III. Of note, the
distinction between the two types was no longer clinically
significant due to the pronounced clinical and histopathological
overlap. In the 2021 WHO classification of CNS tumors, the term
‘hemangiopericytoma’ was removed and replaced with SFT (15).
A solid lesion located in the CNS distinct from
fibrous meningioma, termed primary SFT, was initially reported by
Carneiro et al (16) in
1996. Intracranially, SFT may occur at the cerebellopontine angle,
spinal dura, parasagittal region, meninges and the intraventricular
region (17). The present article
reports on a 44-year-old male patient with SFT. The SFT originated
from the superior sagittal sinus and not only penetrated through
the skull, but also invaded the bilateral occipital lobes distally.
This is the first case of SFT completely penetrating the skull, to
the best of our knowledge. The imaging data, histopathological
features and treatment of SFT were briefly reviewed and the imaging
features of this case were discussed.
Case report
A 44-year-old male patient presented at the
neurology outpatient clinic of Xiaolan People's Hospital of
Zhongshan (Zhongshan, China) in August 2020 due to dizziness and
blurred vision for one month. Neurological examination of the
patient appeared normal; however, a visual field defect was
observed below the central visual field of both eyes. Bone window
computed tomography angiography (Ingenuity CT; Philips Medical
Systems, Inc.; slice thickness, 0.8 mm; center, 450; width, 1,600)
of the head demonstrated that the mass had invaded and penetrated
the skull (Fig. 1A).
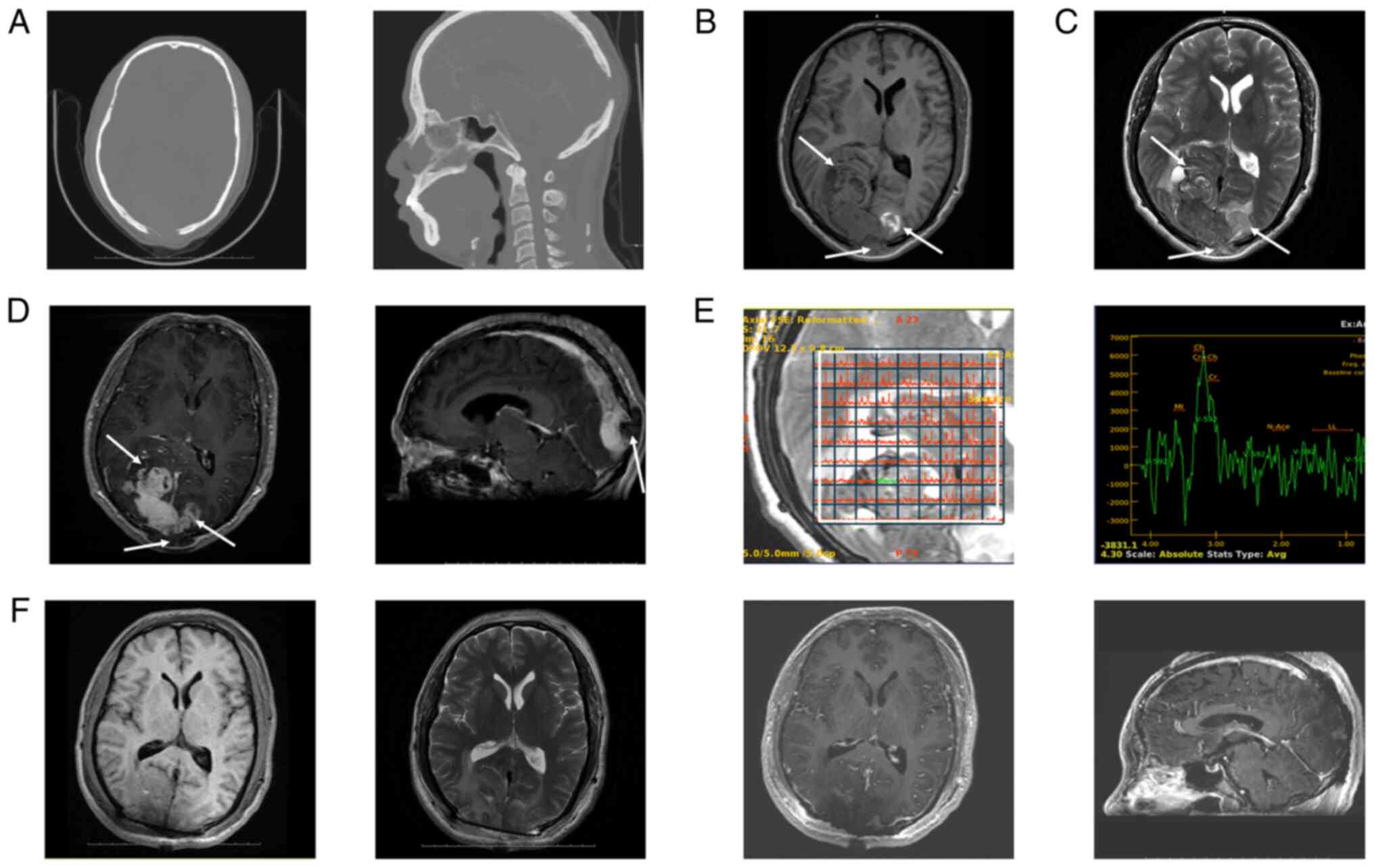 | Figure 1.Preoperative and postoperative
imaging profiles of the tumor. (A) The axial (left panel) and
sagittal (right panel) images of the bone window of the head
computed tomography angiogram demonstrated that the mass invaded
and penetrated the skull. MRI on admission demonstrated a mass
shadow near the occipital cerebral falx, with an irregular shape
and unclear boundaries. The mass was ~64×44×64 mm in size. (B) The
majority of the intracranial and subcutaneous tumors demonstrated
uneven low signals on T1WI, while the tumors at the far end of the
left occipital lobe demonstrated high signals on T1WI. (C) The
majority of the intracranial tumors demonstrated low mixed signals
on T2WI, while the tumors at the far end of the left occipital lobe
and under the scalp demonstrated high signals on T2WI. (D) The
axial (left panel) demonstrated that the tumor crossed both sides
of the cerebral falx. There were unclear boundaries with the
bilateral occipital lobe brain tissue. The majority of intracranial
tumors and the tumors at the far end of the left occipital lobe
were significantly enhanced following enhanced scanning, while the
tumors under the scalp were not significantly enhanced following
enhanced scanning. The sagittal (right panel) images of the
contrast-enhanced MRI demonstrated that tumors filled the posterior
section of the superior sagittal sinus to form a filling defect,
invading and penetrating the occipital bone. (E) The voxel (left
panel) and corresponding magnetic resonance spectroscopy map (right
panel) demonstrated no notable N-acetylaspartic acid peaks in the
mass collected by multivoxel, elevated choline/creatine peaks in
the solid area, and no notable abnormalities in the spectral lines
of tissues around the mass. (F) Postoperative MRI demonstrated that
the tumor was completely removed. The bilateral occipital lobes
surrounding the surgical area demonstrated T1WI low signal (left
panel) and T2WI high signal (second left panel), and the axial
(second right panel) and sagittal (right panel) images of enhanced
scanning demonstrated obvious enhancement along the edges of the
surgical area. White arrows indicate the tumor. MRI, magnetic
resonance imaging; T1W1, T1-weighted imaging; T2W1, T2-weighted
imaging. |
Head magnetic resonance imaging (MRI) findings
demonstrated a mass shadow near the occipital cerebral falx, with
an irregular shape and unclear boundaries. The mass was ~64×44×64
mm in size and stretched across both sides of the cerebral falx.
The mass filled the posterior portion of the superior sagittal
sinus to form a filling defect, invading and penetrating the
occipital bone, and the boundary between the mass and bilateral
occipital lobe brain tissue was unclear. Of note, the majority of
the intracranial tumors exhibited uneven low signals on T1-weighted
imaging (T1WI) and low mixed signals on T2WI, with notable
enhancements following enhanced scanning. The distal part of the
left occipital lobe demonstrated hypersignals on T1WI and T2WI,
with significant enhancements following enhanced scanning. In
addition, the lower part of the scalp exhibited low signals on T1WI
and high signals on T2WI, and there were not notably enhanced
following enhanced scanning (Fig.
1B-D).
Results of the magnetic resonance spectroscopy (MRS)
demonstrated a multi voxel collection with no notable
N-acetylaspartic acid peak in the mass. The choline/creatine peak
in the solid area was increased; however, no notable abnormalities
were found in the spectral lines of the tissues surrounding the
mass (Fig. 1E).
A craniotomy was performed for tumor resection and
follow-up MRI demonstrated complete tumor resection (Fig. 1F). During the operation, the tumor
was gray and red in color with an abundant blood supply (Fig. S1A). Foci indicative of previous
bleeding far from the origin occurred in the tumors in both the
occipital lobe and the scalp. In addition, the tumor texture was
uneven, with both soft and tough sections, with an incomplete
capsule and lobulated invasive growth. The tumor broke through the
brain tissue of the occipital lobe and the demarcation between the
tumor and the brain tissue of the occipital lobe was unclear. The
occipital pia mater was markedly edematous. The adjacent occipital
skull demonstrated osteolytic bone destruction, the tumor
penetrated the occipital bone to form a local mass under the scalp
and the local scalp thickened reactively.
Pathological examination revealed that the tumor was
composed of alternately distributed cell-rich areas and cell-sparse
areas. The tumor cells in the cell-rich area were short-spindle or
oval, with little cytoplasm and uniform nuclear chromatin. There
was no notable atypia in the two areas. The frequency of mitotic
figures was 1/10 high-power fields (HPF) and the tumor cells were
arranged in sheets and striae. These were hemangiopericytoma-like,
with abundant blood vessels in the tumor. Thus, these were labeled
‘staghorn-shaped blood vessels’ (Fig.
2A). Tumor cells presented with diffuse strong immunoreactivity
to CD34, vimentin and STAT6 (Fig.
2B-D). The Ki-67 labeling index was ~10% with no signs of
necrosis (Fig. 2E). The tumor cells
presented as weakly positive for epithelial membrane antigen (EMA)
and negativity for progesterone receptor (PR) (Fig. S1B and C). Grade I SFT cells are
fusiform, with lower cell density and higher collagen content.
Grade II SFT has more cells, less collagen, no specific cell
arrangement and typical staghorn-shaped blood vessels. There are
more than 4 mitotic figures per 10 HPF of grade III SFT (14). Intracranial SFT is mainly
differentiated from meningioma because they are similar in clinical
presentation and pathological diagnosis. The histopathological
features of SFT are sparse and dense areas separated by fibrous
stroma, with hemangiopericytoma branching vessels (18). The phenotype of SFT is characterized
by a patternless architecture or a short fascicular pattern
(14). SFT is histopathologically
characterized by alternating low-cell and high-cell areas and thick
collagen bands (14).
Microscopically, the meningioma cells are nested, with abundant
cytoplasm and unclear cells (syncytioid) (19). Pseudo-inclusions are common in
meningioma nuclei, where cells have weakly defined cell boundaries
(syncyti-like) (19). In SFT, STAT6
is positive in almost all patients, while CD34 is positive to
varying degrees (20–22). However, all forms of meningiomas
characteristically expressed EMA and PR; CD34 reactivity was patchy
and weak; STAT6 was not expressed (21,22).
Histopathological examination confirmed WHO grade II SFT.
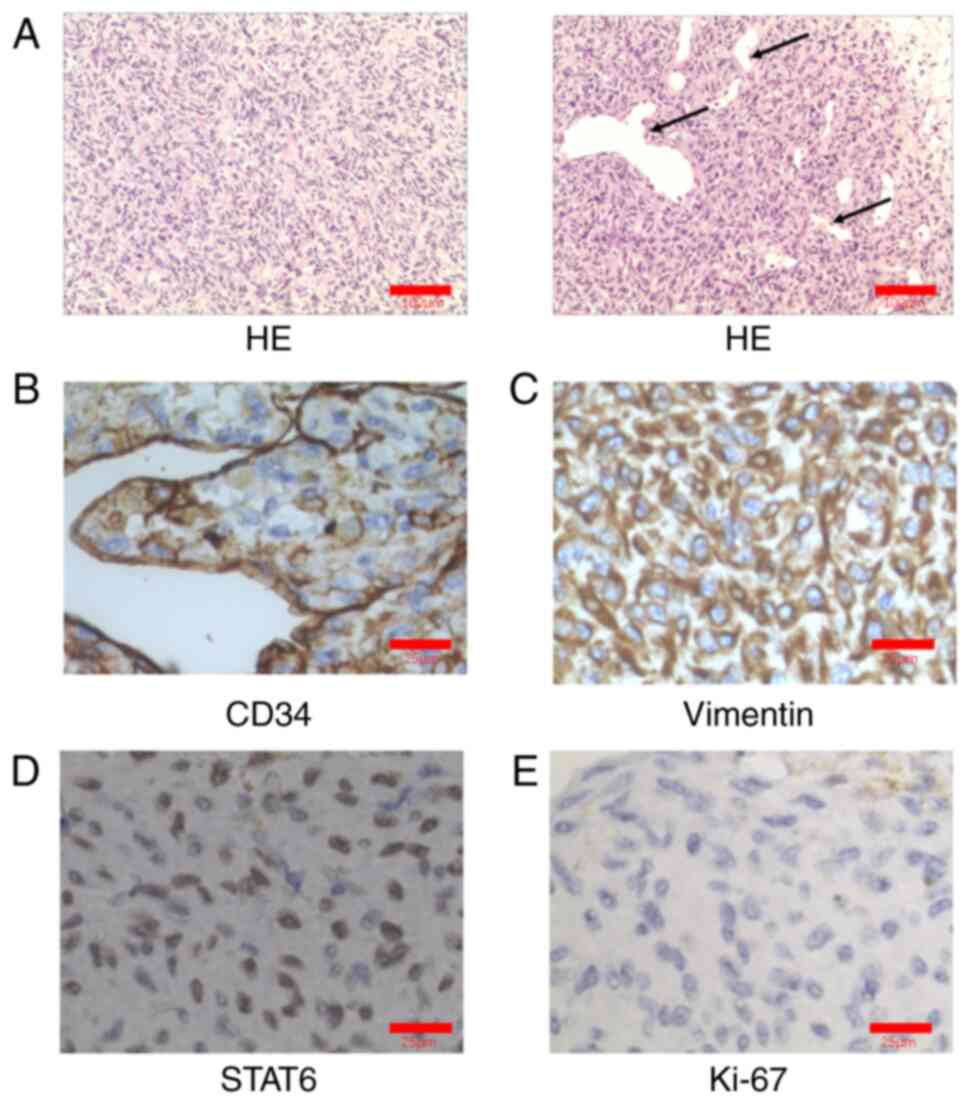 | Figure 2.Histopathological and
immunohistochemical features of the tumor. (A) The tumor was
composed of alternately distributed cell-rich areas and cell-sparse
areas (left panel). The tumor cells in the cell-rich area were
short spindle- or oval-shaped, with little cytoplasm and uniform
nuclear chromatin (left panel). There was no notable atypia in the
two areas (left panel). The frequency of mitotic figures was 1/10
high-power fields and the tumor cells were arranged in sheets and
striae (left panel). These were hemangiopericytoma-like with
abundant blood vessels in the tumor (right panel). Thus, these were
labeled ‘staghorn-shaped blood vessels’, indicated using black
arrows (magnification, ×100; scale bar, 100 µm; H&E staining).
Immunohistochemical examination demonstrated strong expression of
(B) CD34, (C) vimentin and (D) STAT6. (E) The Ki-67 labeling index
was ~10% (magnification, ×400; scale bar, 25 µm). A brown color in
the cells indicated positive staining for CD34, vimentin, STAT6 and
Ki-67. STAT6, signal transducer and activator of transcription
6. |
The protocols of the imaging examinations,
histopathological staining and immunohistochemistry (IHC) are
provided in the supplemental data.
Three-dimensional emphasis radiotherapy was
performed 18 days after surgery [tumor absorbed dose: Planning
target volume (PTV)1, 60.2 Gray/28 fractions; PTV2, 54.6 Gray/28
fractions]. At the follow-up 3 months after the surgery, the
patient reported that the headache and dizziness symptoms had
gradually disappeared after the surgery. The patient's visual field
was examined using a Humphrey II 740 Visual Field (Carl Zeiss
Meditec), indicating that the patient's visual field was
significantly improved compared with that prior to surgery. During
the two-year follow-up, the patient experienced no recurrence of
SFT.
Discussion
The majority of intracranial SFTs are dural masses
originating predominantly from thick collagen bands, which are
produced by fibroblasts, most frequently occurring in the
parasagittal sinus and spinal canal (3,23–25).
In the present study, the patient experienced SFT originating in
the superior sagittal sinus, consistent with previous reports
(3,23–25).
Symptoms of SFT vary and patients may present with
several non-specific symptoms associated with elevated intracranial
pressure or tumor location. These include headache, nausea,
vomiting, dizziness, gait disturbance, hemiplegia, hearing loss and
memory disturbance (26).
Sugiyama et al (27) reported on an 86-year-old male with
SFT, which was located in the right parietal lobe and invaded the
parietal bone, who presented with sustained progressive motor
weakness in the left lower extremity for 1 month. Another study
reported on a 30-year-old male with SFT, which was located near the
right temporal lobe and led to the thickening of the temporal bone
of its neighbor; the patient developed left facial nerve paralysis
and dysarthria, and decreased muscle strength of the left upper and
lower limbs (28). SFT in the
present case reported was located in the parietal occipital area
and invaded and penetrated the skull and the patient presented with
headache, dizziness and blurred vision. Headache and dizziness are
mainly caused by increased intracranial pressure. Blurred vision is
caused by a tumor pressing on the visual center. The patient of the
present study had no symptoms of limb weakness, facial nerve
paralysis or dysarthria.
The differential diagnosis of SFT via imaging is
difficult due to its variable signal intensity on MRI scans
(29). Differentiation from
meningioma, schwannoma, neurofibroma, metastases and lymphoma was
required (30–33).
Computed tomography and MRI are important imaging
techniques for the diagnosis of SFT. The medical imaging of
intracranial SFT reveals numerous characteristics and previous
imaging revealed that intracranial SFT is more likely to occur at
the base of the skull (34),
sagittal sinus (35), falx cerebri
and peritentorium cerebelli (36),
or near the venous sinus (37). In
addition, intracranial SFT is characterized by extracranial tumors,
which are lobular or irregular, and some may appear oval- or
dumbbell-shaped (38–40). Previous CT scans demonstrated high
or equal density. The majority of boundaries were clear; however, a
small number of the boundaries with the brain tissue were not clear
(27,28,40,41).
Cystic degeneration and necrosis in the tumor were common, but
there was no calcification (27,28,40,41).
The density of the tumor following necrosis and cystic degeneration
was uneven and destruction of the skull adjacent to the tumor may
occur (27,28,40,41).
In general, SFT appears as isointense to slightly high on T1WI and
isointense on T2WI, compared with gray matter. T1WI demonstrated
isointense to slightly high signals (27,28,36)
and isointense mixed signals (36)
in the case of cystic degeneration and necrosis. In addition, T2WI
demonstrated slightly high or isointense mixed signals (27,36),
and isointense mixed signals in the case of cystic degeneration and
necrosis (36). Following enhanced
MRI scanning, the tumor appeared significantly strengthened, and
those with cystic degeneration demonstrated heterogeneous
enhancement (36,38,40,42–44).
Peritumoral edema is often mild (41,44,45).
The imaging findings of SFT were similar to those of
meningioma and MRS may be used to distinguish SFT from meningioma.
The relative ratios of choline and myo-inositol are increased in
SFT compared with meningioma (40,46).
Chen et al (47) also
reported that the normalized apparent diffusion coefficient ratios
and intratumoral susceptibility signal intensity are useful for
differentiating SFT/HPC from meningioma. In the present case
reported, SFT occurred near the cerebral falx. STF was irregular,
exhibited unclear boundaries with the occipital lobe brain tissue,
displayed notable enhancements in the intracranial section
following enhanced scanning and exhibited an elevated choline peak
in the MRS analysis, which was consistent with previous reports
(40,46). SFT in the present case reported was
located in the distal part of the left occipital lobe, demonstrated
high signals on T1WI and T2WI, and was significantly enhanced
following enhanced scanning. These results were also consistent
with those previously reported (27,28,36,38,40,42–44).
The majority of the intracranial tumors in the
patient in the present report demonstrated uneven low signal
intensity on T1WI and low mixed signal intensity on T2WI, which
differed from the results obtained from previous reports. Due to
the intraoperative situation, it was hypothesized that the tumor
demonstrated low signal intensity on T1WI and T2WI due to
intra-tumor hemorrhage. SFT may cause skull destruction when
adjacent to the skull, which is manifested as hyperostosis, bone
erosion or bone destruction (27,28,40,41,48).
To the best of our knowledge, this is the first case of SFT that
completely penetrated the skull. The SFT signal penetrating the
skull under the scalp demonstrated a low signal on T1WI and a high
signal on T2WI, and no notable enhancements were observed following
enhanced scanning. There was no notable enhancement of SFT under
the scalp following enhanced scanning, which was also inconsistent
with the results obtained from previous reports (36,38,40,42–44).
Thus, the tumor was considered heterogeneous. As tumor cells were
dense, the interstitial components were relatively sparse with few
vascular components. Of note, the sub-scalp tumor was not enhanced
in the conventional enhancement time window. Thus, SFT under the
scalp may require delayed enhancement for accurate development. In
addition, for SFT located inside and outside the skull, and under
the scalp, dynamic enhancement of multiple time windows is required
in MRI to fully display the scope and nature of the tumor, and to
avoid miscalculation.
The diagnosis of SFT mainly relies on pathological
examination. Histological staining demonstrates that the tumor
tissue is rich in spindle-shaped or polygonal cells. In typical
cases, a large number of ‘staghorn-shaped’ blood vessels and
collagen fibers may be observed. The tumor cells are arranged in
concentric circles around the blood vessels, and these may form
dense or sparse areas (49). IHC
staining demonstrated that CD34, vimentin and STAT6 are positive in
SFT tissues, and the Ki-67 proliferation index is frequently
indicative of patient prognosis (50). Various studies recommended that high
Ki-67 (>5%) should be included as an adverse prognostic
parameter in assessing the prognosis of SFT of the CNS (7,51). At
present, CD34 is considered the most consistent marker in SFT and
positive staining is reported in 95–100% of patients; however, its
absence does not rule out this tumor (52,53).
STAT6 is positive in almost all patients with intracranial SFT
(22,54). STAT6 may be associated with the
fusion of the NAB2-STAT6 gene caused by 12q chromosome
rearrangement (25). Thus,
detection of STAT6 or the NAB2-STAT6 fusion gene is recommended for
the diagnosis of intracranial SFT (54–56).
NAB2 and STAT6 are neighbour genes localized on the long arm of
chromosome 12 and transcribed in opposite directions (57). In SFT, an intrachromosomal inversion
places the genes in the same orientation, which results in an
in-frame fusion transcribed from the NAB2 promoter, leading to
STAT6 nuclear expression that may be detected by IHC (14,57).
The expression of STAT6 in intracranial SFT tissue was detected
using IHC staining, and the NAB2-STAT6 fusion gene was accurately
detected with both high specificity and sensitivity (54–56).
STAT6 IHC is both a highly specific and sensitive surrogate for
NAB2-STAT6 gene fusions, and the specificity and sensitivity of
nuclear STAT6 for SFT/HPCs were 100 and 96.6%, respectively
(20). In the present study, STAT6
expression was detected by IHC instead of detecting the NAB2-STAT6
fusion gene. Different NAB2-STAT6 fusion variants may be related to
clinical pathology and prognosis (12,58–60).
Therefore, the lack of NAB2-STAT6 fusion gene detection was a
possible limitation of the present report. The SFT tissue of the
patient described in the present study was positive for CD34,
vimentin and STAT6, which was consistent with the results of
previous reports (50,52–54).
The patient experienced no tumor recurrence following surgery.
SFT is characterized by high rates of local and
extracranial metastases (61).
Results of previous studies demonstrated that in patients with SFT
for a prolonged period, there is a risk of recurrence, even after
10 years of the initial resection (62–64).
Therefore, patients with SFT require active treatment and long-term
follow-up. As the tumor described in the present study is rare,
treatment and prognosis require further investigation.
Yu et al (65) retrospectively studied patients
treated for intracranial SFT between January 2009 and June 2019.
Their results demonstrated reduced WHO grading, and patients who
underwent gross total resection and adjuvant therapy, such as Gamma
Knife surgery, exhibited prolonged progression-free survival (PFS)
(65). Of note, the aforementioned
previous study was retrospective in nature, with a small sample
size and selection bias, leading to biased results. Results of a
multi-center study demonstrated that postoperative radiotherapy,
including 2-dimensional conventional radiotherapy, 3-dimensional
conformal radiotherapy and intensity-modulated radiotherapy, may
significantly improve the PFS of patients with SFT, irrespective of
the surgical extent and grade (61). Of note, the present study did not
investigate the effects of different radiotherapy techniques on
SFT. At present, there are no standardized treatment guidelines for
intracranial malignant SFT. Surgical resection and postoperative
radiotherapy are not effective in the treatment of intracranial
malignant SFT. Anlotinib, a newly multitargeted tyrosine kinase
inhibitor with anti-neoplastic and anti-angiogenic activities,
inhibits tumor angiogenesis and proliferation (66). Anti-angiogenesis may be a potential
option for the treatment of SFT (67–69).
Surgery, radiotherapy and anlotinib alone are effective in the
treatment of malignant intracranial SFT (13). However, the present article reports
one case and further research and larger randomized controlled
trials are required to verify its findings. Pazopanib, a
multi-target receptor tyrosine kinase inhibitor with potent
anti-angiogenic properties, is approved for the treatment of
advanced renal cell carcinoma and certain subtypes of advanced soft
tissue sarcoma (70). Of note,
pazopanib is effective in treating patients with metastatic or
unresectable SFT (69,71). The present study demonstrated that
surgical resection is the optimal choice for the treatment of SFT,
and postoperative radiotherapy may significantly improve PFS in
patients. Molecular targeted therapy, such as tyrosine kinase
inhibitors anlotinib and pazopanib, is a promising approach for
malignant, unresectable or metastatic SFT.
In conclusion, SFT is a rare tumor type. Due to the
rarity and similarity to other more common brain tumors, SFTs
exhibit a high rate of misdiagnosis following imaging. Of note,
histopathological testing is critical for differentiating SFT from
other CNS disorders. In addition, complete tumor resection is the
preferred treatment option for SFT. The indications for adjuvant
therapy following surgery remain to be elucidated. Due to the
potential for recurrence, rigorous long-term follow-up, including
periodic imaging surveillance, is recommended.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL and XZ designed the study and drafted the
manuscript. JZ collected and analyzed the clinical data. XZ
critically revised the manuscript. All authors have read and
approved the final manuscript. QL and XZ confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the present manuscript, including the
medical data and any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Klemperer P and Rabin CB: Primary
neoplasms of the pleura. A report of five cases. Arch Pathol.
11:385–412. 1931.
|
|
2
|
Goodlad JR and Fletcher CD: Solitary
fibrous tumour arising at unusual sites: Analysis of a series.
Histopathology. 19:515–522. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y and Zhang J, Liu Q, Liu F, Zhu X
and Zhang J: Solitary fibrous tumor of the pineal region with
delayed ectopic intracranial metastasis: A case report and review
of the literature. Medicine (Baltimore). 98:e157372019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shukla P, Gulwani HV, Kaur S and
Shanmugasundaram D: Reappraisal of morphological and
immunohistochemical spectrum of intracranial and spinal solitary
fibrous tumors/hemangiopericytomas with impact on long-term
follow-up. Indian J Cancer. 55:214–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L, Yu J, Shu D, Huang B, Wang Y and
Zhang L: Primary endodermal hemangiopericytoma/solitary fibrous
tumor of the cervical spine: A case report and literature review.
BMC Surg. 21:4052021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Apra C, El Arbi A, Montero AS, Parker F
and Knafo S: Spinal solitary fibrous tumors: An original
multicenter series and systematic review of presentation,
management, and prognosis. Cancers (Basel). 14:28392022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bisceglia M, Galliani C, Giannatempo G,
Lauriola W, Bianco M, D'angelo V, Pizzolitto S, Vita G, Pasquinelli
G, Magro G and Dor DB: Solitary fibrous tumor of the central
nervous system: A 15-year literature survey of 220 cases (August
1996-July 2011). Adv Anat Pathol. 18:356–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thway K, Ng W, Noujaim J, Jones RL and
Fisher C: The current status of solitary fibrous tumor: Diagnostic
features, variants, and genetics. Int J Surg Pathol. 24:281–292.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marcó Del Pont F, Ries Centeno T,
Villalonga JF, Giovannini SJM, Caffaratti G, Lorefice E and Cervio
A: Results in the treatment of intracranial hemangiopericytomas.
Case series. Neurocirugia (Astur: Engl Ed). 32:62–68. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Claus E, Seynaeve P, Ceuppens J, Vanneste
A and Verstraete K: Intracranial solitary fibrous tumor. J Belg Soc
Radiol. 101:112017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeng L, Wang Y, Wang Y, Han L, Niu H,
Zhang M, Ke C, Chen J and Lei T: Analyses of prognosis-related
factors of intracranial solitary fibrous tumors and
hemangiopericytomas help understand the relationship between the
two sorts of tumors. J Neurooncol. 131:153–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barthelmeß S, Geddert H, Boltze C,
Moskalev EA, Bieg M, Sirbu H, Brors B, Wiemann S, Hartmann A,
Agaimy A and Haller F: Solitary fibrous tumors/hemangiopericytomas
with different variants of the NAB2-STAT6 gene fusion are
characterized by specific histomorphology and distinct
clinicopathological features. Am J Pathol. 184:1209–1218. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang DY, Su L and Wang YW: Malignant
solitary fibrous tumor in the central nervous system treated with
surgery, radiotherapy and anlotinib: A case report. World J Clin
Cases. 10:631–642. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO classification of tumors of the
central nervous system: A summary. Neuro Oncol. 23:1231–1251. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carneiro SS, Scheithauer BW, Nascimento
AG, Hirose T and Davis DH: Solitary fibrous tumor of the meninges:
A lesion distinct from fibrous meningioma. A clinicopathologic and
immunohistochemical study. Am J Clin Pathol. 106:217–224. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu SW, Tsai KB, Yang SF, Lee KS and Chai
CY: Unusual solitary fibrous tumors in the central nervous system:
A report of two cases. Kaohsiung J Med Sci. 21:179–184. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang SC and Huang HY: Solitary fibrous
tumor: An evolving and unifying entity with unsettled issues.
Histol Histopathol. 34:313–334. 2019.PubMed/NCBI
|
|
19
|
Buerki RA, Horbinski CM, Kruser T,
Horowitz PM, James CD and Lukas RV: An overview of meningiomas.
Future Oncol. 14:2161–2177. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Q, Zhang C and Li Z: Delayed pulmonary
metastasis and recurrence of intracranial malignant solitary
fibrous tumor/hemangiopericytoma: Case report and literature
review. Oncol Lett. 24:2552022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perry A, Scheithauer BW and Nascimento AG:
The immunophenotypic spectrum of meningeal hemangiopericytoma: A
comparison with fibrous meningioma and solitary fibrous tumor of
meninges. Am J Surg Pathol. 21:1354–1360. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Macagno N, Figarella-Branger D, Mokthari
K, Metellus P, Jouvet A, Vasiljevic A, Loundou A and Bouvier C:
Differential diagnosis of meningeal SFT-HPC and meningioma: Which
immunohistochemical markers should be used? Am J Surg Pathol.
40:270–278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bertero L, Anfossi V, Osella-Abate S,
Disanto MG, Mantovani C, Zenga F, Rudà R, Garbossa D, Soffietti R,
Ricardi U, et al: Pathological prognostic markers in central
nervous system solitary fibrous tumour/hemangiopericytoma: Evidence
from a small series. PLoS One. 13:e02035702018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Keraliya AR, Tirumani SH, Shinagare AB,
Zaheer A and Ramaiya NH: Solitary fibrous tumors: 2016 Imaging
update. Radiol Clin North Am. 54:565–579. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim BS, Kim Y, Kong DS, Nam DH, Lee JI,
Suh YL and Seol HJ: Clinical outcomes of intracranial solitary
fibrous tumor and hemangiopericytoma: Analysis according to the
2016 WHO classification of central nervous system tumors. J
Neurosurg. 129:1384–1396. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang XQ, Zhou Q, Li ST, Liao CL, Zhang H
and Zhang BY: Solitary fibrous tumors of the central nervous
system: Clinical features and imaging findings in 22 patients. J
Comput Assist Tomogr. 37:658–665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sugiyama H, Tsutsumi S, Hashizume A, Inaba
T and Ishii H: Are bone erosion and peripheral feeding vessels
hallmarks of intracranial solitary fibrous
tumor/hemangiopericytoma? Radiol Case Rep. 17:2702–2707. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chikasue T, Uchiyama Y, Tanoue S, Komaki
S, Sugita Y and Abe T: Intracranial solitary fibrous
tumor/hemangiopericytoma mimicking cystic meningioma: A case report
and literature review. Radiol Case Rep. 16:1637–1642. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim JH, Yang KH, Yoon PH and Kie JH:
Solitary fibrous tumor of central nervous system: A case report.
Brain Tumor Res Treat. 3:127–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Deng J, Sun Q, Xue C, Li S, Zhou Q,
Huang X, Liu H and Zhou J: Differentiation of intracranial solitary
fibrous tumor/hemangiopericytoma from atypical meningioma using
apparent diffusion coefficient histogram analysis. Neurosurg Rev.
45:2449–2456. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yue X, Huang J, Zhu Y and Du Y: Solitary
fibrous tumor/hemangiopericytoma in the cerebellopontine angle
mimicking vestibular schwannoma: A case report and literature
review. Medicine (Baltimore). 99:e196512020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mondal SK, Mallick MG, Bandyopadhyay R and
Mondal PK: Neurofibroma of kidney: An uncommon neoplasm and
diagnostic dilemma with solitary fibrous tumor. J Cancer Res Ther.
6:388–390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Smith AB, Horkanyne-Szakaly I, Schroeder
JW and Rushing EJ: From the radiologic pathology archives: Mass
lesions of the dura: Beyond meningioma-radiologic-pathologic
correlation. Radiographics. 34:295–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peng Z, Wang Y, Wang Y, Li Q, Fang Y, Fan
R, Zhang H and Jiang W: Hemangiopericytoma/solitary fibrous tumor
of the cranial base: A case series and literature review. BMC Surg.
22:2892022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kalani MY, Martirosyan NL, Eschbacher JM,
Nakaji P, Albuquerque FC and Spetzler RF: Large hemangiopericytoma
associated with arteriovenous malformations and dural arteriovenous
fistulae. World Neurosurg. 76:592.e7–e10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bai LC, Luo TY, Zhu H and Xu R: MRI
features of intracranial anaplastic hemangiopericytoma. Oncol Lett.
13:2945–2948. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen Z, Ye N, Jiang N, Yang Q, Wanggou S
and Li X: Deep learning model for intracranial hemangiopericytoma
and meningioma classification. Front Oncol. 12:8395672022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma L, Wang L, Fang X, Zhao CH and Sun L:
Diagnosis and treatment of solitary fibrous
tumor/hemangiopericytoma of central nervous system. Retrospective
report of 17 patients and literature review. Neuro Endocrinol Lett.
39:88–94. 2018.PubMed/NCBI
|
|
39
|
Yi X, Xiao D, He Y, Yin H, Gong G, Long X,
Liao W, Li X, Sun L, Zhang Y and Zhang B: Spinal solitary fibrous
tumor/hemangiopericytoma: A clinicopathologic and radiologic
analysis of eleven cases. World Neurosurg. 104:318–329. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Clarençon F, Bonneville F, Rousseau A,
Galanaud D, Kujas M, Naggara O, Cornu P and Chiras J: Intracranial
solitary fibrous tumor: Imaging findings. Eur J Radiol. 80:387–394.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Al Armashi AR, Alkrekshi A, Al Zubaidi A,
Somoza-Cano FJ, Hammad F, Elantably D, Patell K and Ravakhah K:
Grade III solitary fibrous tumor/hemangiopericytoma: An enthralling
intracranial tumor-A case report and literature review. Radiol Case
Rep. 17:3792–3796. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Y, Wang Q, Zhang T, Yang L and Liang
WJ: MR imaging of intracranial solitary fibrous tumor: A
retrospective study of 7 cases. Afr Health Sci. 18:799–806. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Z, Li Y, She L, Wang X, Yan Z, Sun
S, Antony A and Zhang H: A footprint-like intracranial solitary
fibrous tumor/hemangiopericytoma with extracranial extension and
acute intratumoral hemorrhage. J Craniofac Surg. 31:e682–e685.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhou JL, Liu JL, Zhang J and Zhang M:
Thirty-nine cases of intracranial hemangiopericytoma and anaplastic
hemangiopericytoma: A retrospective review of MRI features and
pathological findings. Eur J Radiol. 81:3504–3510. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He L, Li B, Song X and Yu S: Signal value
difference between white matter and tumor parenchyma in T1- and
T2-weighted images may help differentiating solitary fibrous
tumor/hemangiopericytoma and angiomatous meningioma. Clin Neurol
Neurosurg. 198:1062212020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ohba S, Murayama K, Nishiyama Y, Adachi K,
Yamada S, Abe M, Hasegawa M and Hirose Y: Clinical and radiographic
features for differentiating solitary fibrous
tumor/hemangiopericytoma from meningioma. World Neurosurg.
130:e383–e392. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen T, Jiang B, Zheng Y, She D, Zhang H,
Xing Z and Cao D: Differentiating intracranial solitary fibrous
tumor/hemangiopericytoma from meningioma using diffusion-weighted
imaging and susceptibility-weighted imaging. Neuroradiology.
62:175–184. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nagai Yamaki V, de Souza Godoy LF, Alencar
Bandeira G, Tavares Lucato L, Correa Lordelo G, Fontoura Solla DJ,
Santana Neville I, Jacobsen Teixeira M and Silva Paiva W:
Dural-based lesions: Is it a meningioma? Neuroradiology.
63:1215–1225. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sun LJ, Dong J, Gao F, Chen DM, Li K, Liu
J, Zhang C, Tohti M and Yang XP: Intracranial solitary fibrous
tumor: Report of two cases. Medicine (Baltimore). 98:e153272019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zuo Z, Zhou H, Sun Y, Mao Q, Zhang Y and
Gao X: Rapidly growing solitary fibrous tumors of the pleura: A
case report and review of the literature. Ann Transl Med.
8:8902020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Macagno N, Vogels R, Appay R, Colin C and
Mokhtari K; French CNS SFT/HPC Consortium; Dutch CNS SFT/HPC
Consortium, . Küsters B, Wesseling P, Figarella-Branger D, et al:
Grading of meningeal solitary fibrous tumors/hemangiopericytomas:
analysis of the prognostic value of the marseille grading system in
a cohort of 132 patients. Brain Pathol. 29:18–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Vogels RJ, Vlenterie M, Versleijen-Jonkers
YM, Ruijter E, Bekers EM, Verdijk MA, Link MM, Bonenkamp JJ, van
der Graaf WT, Slootweg PJ, et al: Solitary fibrous
tumor-clinicopathologic, immunohistochemical and molecular analysis
of 28 cases. Diagn Pathol. 9:2242014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Davanzo B, Emerson RE, Lisy M, Koniaris LG
and Kays JK: Solitary fibrous tumor. Transl Gastroenterol Hepatol.
3:942018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yamashita D, Suehiro S, Kohno S, Ohue S,
Nakamura Y, Kouno D, Ohtsuka Y, Nishikawa M, Matsumoto S, Bernstock
JD, et al: Intracranial anaplastic solitary fibrous
tumor/hemangiopericytoma: Immunohistochemical markers for
definitive diagnosis. Neurosurg Rev. 44:1591–1600. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chenhui Z, He G, Wu Z, Rong J, Ma F, Wang
Z, Fang J, Gao W, Song H, Zhang F, et al: Intracranial solitary
fibrous tumor/hemangiopericytomas: A clinical analysis of a series
of 17 patients. Br J Neurosurg. 1–8. 2021.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sahoo N, Mohapatra D, Panigrahi S, Lenka
A, Das P and Mohapatra SS: Intracranial solitary fibrous
tumor/hemangiopericytoma: A clinicoradiological poorly recognized
entity-an institutional experience. Turk Neurosurg. Nov
19–2020.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Georgiesh T, Namløs HM, Sharma N, Lorenz
S, Myklebost O, Bjerkehagen B, Meza-Zepeda LA and Boye K: Clinical
and molecular implications of NAB2-STAT6 fusion variants in
solitary fibrous tumour. Pathology. 53:713–719. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Akaike K, Kurisaki-Arakawa A, Hara K,
Suehara Y, Takagi T, Mitani K, Kaneko K, Yao T and Saito T:
Distinct clinicopathological features of NAB2-STAT6 fusion gene
variants in solitary fibrous tumor with emphasis on the acquisition
of highly malignant potential. Hum Pathol. 46:347–356. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Huang SC, Li CF, Kao YC, Chuang IC, Tai
HC, Tsai JW, Yu SC, Huang HY, Lan J, Yen SL, et al: The
clinicopathological significance of NAB2-STAT6 gene fusions in 52
cases of intrathoracic solitary fibrous tumors. Cancer Med.
5:159–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tai HC, Chuang IC, Chen TC, Li CF, Huang
SC, Kao YC, Lin PC, Tsai JW, Lan J, Yu SC, et al: NAB2-STAT6 fusion
types account for clinicopathological variations in solitary
fibrous tumors. Mod Pathol. 28:1324–1335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lee JH, Jeon SH, Park CK, Park SH, Yoon
HI, Chang JH, Suh CO, Kang SJ, Lim DH, Kim IA, et al: The role of
postoperative radiotherapy in intracranial solitary fibrous
tumor/hemangiopericytoma: A multi-institutional retrospective study
(KROG 18-11). Cancer Res Treat. 54:65–74. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sonabend AM, Zacharia BE, Goldstein H,
Bruce SS, Hershman D, Neugut AI and Bruce JN: The role for adjuvant
radiotherapy in the treatment of hemangiopericytoma: A
surveillance, epidemiology, and end results analysis. J Neurosurg.
120:300–308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Vuorinen V, Sallinen P, Haapasalo H,
Visakorpi T, Kallio M and Jääskeläinen J: Outcome of 31
intracranial haemangiopericytomas: Poor predictive value of cell
proliferation indices. Acta Neurochir (Wien). 138:1399–1408. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Guthrie BL, Ebersold MJ, Scheithauer BW
and Shaw EG: Meningeal hemangiopericytoma: Histopathological
features, treatment, and long-term follow-up of 44 cases.
Neurosurgery. 25:514–522. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yu Y, Hu Y, Lv L, Chen C, Yin S, Jiang S
and Zhou P: Clinical outcomes in central nervous system
solitary-fibrous tumor/hemangiopericytoma: A STROBE-compliant
single-center analysis. World J Surg Oncol. 20:1492022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shen G, Zheng F, Ren D, Du F, Dong Q, Wang
Z, Zhao F, Ahmad R and Zhao J: Anlotinib: A novel multi-targeting
tyrosine kinase inhibitor in clinical development. J Hematol Oncol.
11:1202018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Maruzzo M, Martin-Liberal J, Messiou C,
Miah A, Thway K, Alvarado R, Judson I and Benson C: Pazopanib as
first line treatment for solitary fibrous tumours: The Royal
Marsden Hospital experience. Clin Sarcoma Res. 5:52015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ebata T, Shimoi T, Bun S, Miyake M,
Yoshida A, Shimomura A, Noguchi E, Yonemori K, Shimizu C, Fujiwara
Y, et al: Efficacy and safety of pazopanib for recurrent or
metastatic solitary fibrous tumor. Oncology. 94:340–344. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Martin-Broto J, Stacchiotti S, Lopez-Pousa
A, Redondo A, Bernabeu D, de Alava E, Casali PG, Italiano A,
Gutierrez A, Moura DS, et al: Pazopanib for treatment of advanced
malignant and dedifferentiated solitary fibrous tumour: A
multicentre, single-arm, phase 2 trial. Lancet Oncol. 20:134–144.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Schutz FA, Choueiri TK and Sternberg CN:
Pazopanib: Clinical development of a potent anti-angiogenic drug.
Crit Rev Oncol Hematol. 77:163–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Martin-Broto J, Cruz J, Penel N, Le Cesne
A, Hindi N, Luna P, Moura DS, Bernabeu D, de Alava E,
Lopez-Guerrero JA, et al: Pazopanib for treatment of typical
solitary fibrous tumours: A multicentre, single-arm, phase 2 trial.
Lancet Oncol. 21:456–466. 2020. View Article : Google Scholar : PubMed/NCBI
|
















