Introduction
Endoscopic mucosal dissection has become the
first-choice treatment for early esophageal cancer. Any invasive
exercises are challenging in patients with decompensated cirrhosis
due to poor coagulation and a poor condition. Liver cirrhosis has
been shown to be a risk factor for esophageal cancer surgery;
however, endoscopic treatment appears to be safe, particularly in
patients without severe liver dysfunction (1). Simultaneously, stenosis of early
esophageal cancer after endoscopic mucosal dissection is a
noteworthy problem. A circumferential mucosal defect more than
three-fourths the circumference of the esophagus, mucosal defects
longer than 40 mm and an infiltration depth greater than the lamina
propria of the mucosa are independent risk factors for stenosis
after endoscopic mucosal dissection (2–4). The
patient in the present case report had independent risk factors for
the postoperative stenosis of esophageal cancer, including an
esophageal dissection circumference ≥3/4, cirrhosis with esophageal
and gastric varices, ascites and other complications, which
increased the difficulty of this treatment. The main objective of
this study was to explore the feasibility of endoscopic treatment
of decompensated cirrhosis with early cancer and to provide a
protocol for the prevention of stenosis after endoscopic mucosal
dissection.
Case report
A 73-year-old man was admitted to Xiangyang Central
Hospital (Xiangyang, China) in April 2022 due to swallowing
difficulties that had persisted for the past 1 month. At the end of
the first month, progressive dysphagia gradually developed without
obvious induction, particularly while consuming dry rice and rough
food. No signs of obvious obstruction were observed while consuming
liquid or semi-liquid food. He reported no symptoms of pharyngeal
pain, a burning sensation behind the sternum, vomiting after
eating, fatigue, abdominal pain, abdominal distension, diarrhea,
nausea, acid regurgitation or belching. Gastroscopy at Xiangyang
Central Hospital demonstrated an irregularly raised mass 30 cm away
from the incisors, suspected to be esophageal cancer. The patient
was then admitted to the Department of Surgery of Tongji Hospital
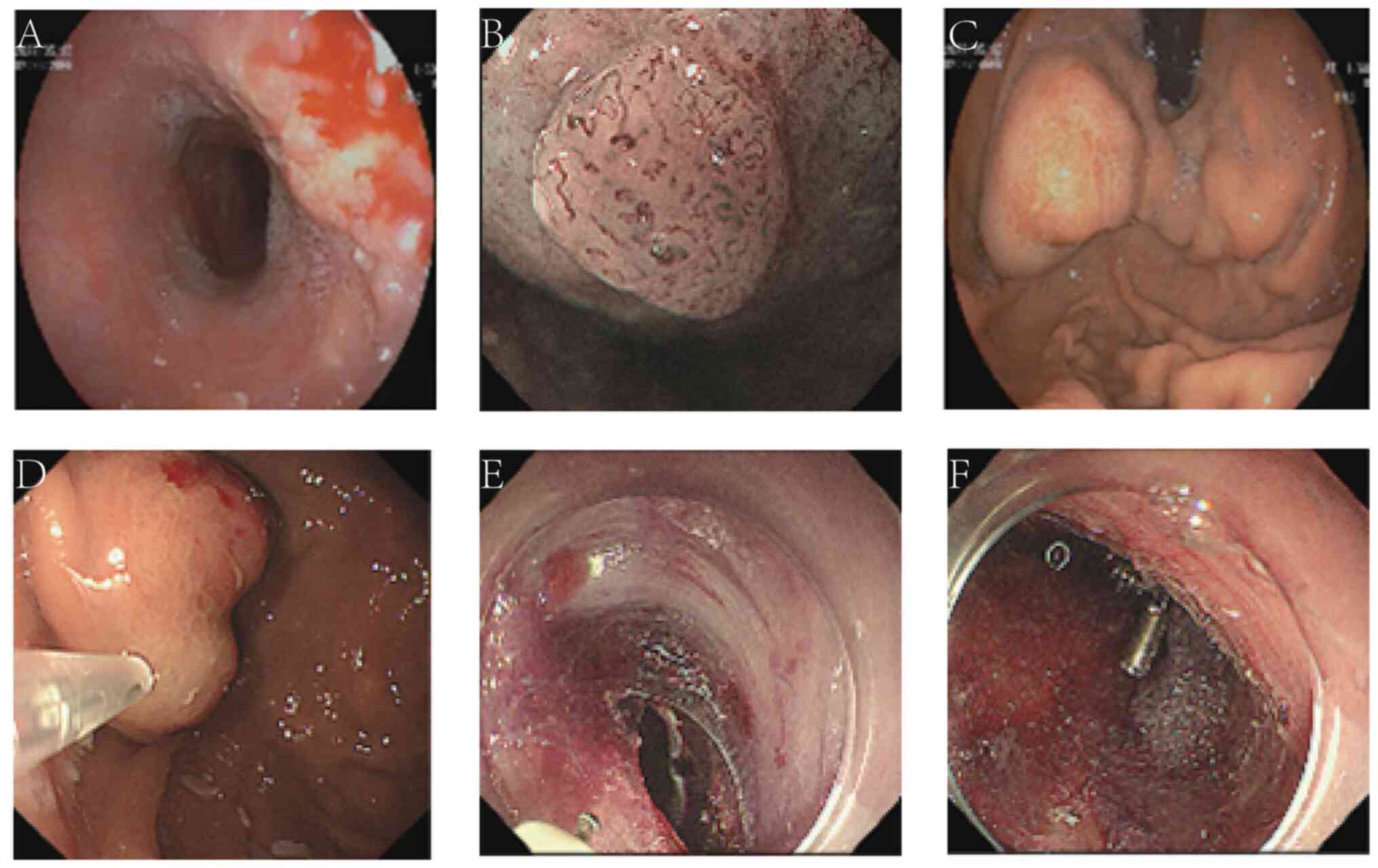
(Wuhan, China), where an endoscopic examination was conducted
(Fig. 1A). The examination showed a
rough and uneven esophageal mucosa, with varicose veins at the
fundus eminence. The patient suffered from chronic erosive
gastritis [grade II, Kyoto classification (5)] and chronic atrophic gastritis (grade
I). Biopsy specimens from the esophagus and the gastric angle
confirmed high-grade squamous intraepithelial neoplasia with focal
suspicious infiltration and (horn) gastric mucosa showing chronic
inflammatory changes with mild intestinal metaplasia. Thereafter,
the patient was transferred to the Department of Gastroenterology
for further treatment.
The patient's medical history reported hepatitis B
viral cirrhosis. A physical examination revealed a body temperature
of 36.3°C (normal range, 36.2-37.2°C), a heart rate of 72 beats/min
(normal range, 60–100 beats/min), a blood pressure of 159/89 mmHg
(normal ranges, systolic blood pressure ≥90 and <140 mmHg;
diastolic blood pressure ≥60 and <90 mmHg) and a respiratory
rate of 20 breaths/min (normal range, 12–20 breaths/min). The
abdomen was flat and soft with audible bowel sounds 2–3 times/min.
The liver, spleen and the mass were not palpable, and there was no
tenderness present. There was no edema or restriction in the
movement of either of the lower limbs. Serological examination
revealed hypersplenism, mild liver dysfunction, mild coagulation
dysfunction, active hepatitis B virus replication and no
abnormalities in renal function or electrolyte levels (Table I). Additionally, abdominal Doppler
ultrasound showed signs of liver cirrhosis and intrahepatic bile
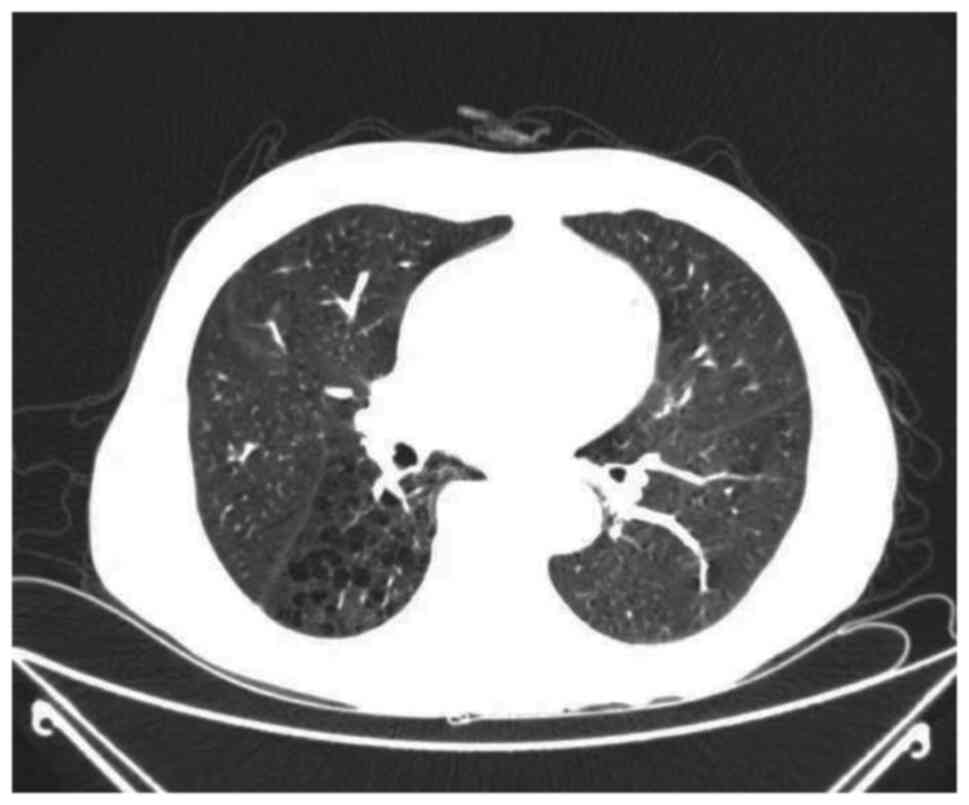
duct stones. Furthermore, contrast-enhanced chest computed
tomography (CT) (Fig. 2) revealed
micronodules in the left lower lobe of the lung, bilateral
emphysema, and mediastinal lymph nodes increased and some of them
enlarged. Based on a consultation with imaging experts, it was
considered that the left lower lung lobe likely had a benign
lesion, and dynamic observations were planned during the follow-up.
CT-hepatic portal vein angiography demonstrated cirrhosis,
splenomegaly, lower esophageal varices and minor ascites.
Magnification endoscopy (Fig. 1B and
C) revealed esophageal grade 0-IIb+IIa lesions [Paris
endoscopic classification (6)]
indicating early cancer (suspected deep infiltration of submucosa),
gastric varices (severe) and chronic atrophic gastritis (grade I).
After repeated communication with the patient and their family,
based on the results of the magnification endoscopy, the patient
was prescribed open surgical treatment. However, this was declined
owing to the patient's age, and therefore, endoscopic surgery was
planned. Since esophageal and gastric varices were present, the
patient was at a high risk of stenosis after endoscopic mucosal
dissection for early esophageal cancer, and so with consent, both
treatment for endoscopic varices and polyglycolic acid (PGA) sheet
attachment for the prevention of esophageal stenosis were
scheduled. Endoscopic treatment was performed at the end of May
2022 (Fig. 1D-F). Tissue glue
injection for the esophageal and gastric varices, and mucosal
dissection for the early esophageal cancer, were successively
performed. Simultaneously, multiple PGA sheets were attached to the
wound on the esophageal dissection surface and fixed with titanium
clips. The clips did not need to be removed after surgery, and
would fall off automatically after a period of time. The patient
was treated with proton-pump inhibitors (for 4 weeks), somatostatin
analogs (for 7 days), hemostasis and fluid rehydration (for 3
days), and was also treated for a fever on the first day after the
operation. Anti-infection therapy (for 4 days) was also initiated.
On the third day post-surgery, the patient's body temperature
gradually decreased and a liquid diet was introduced. On the sixth
day post-surgery, the pathological results revealed that the
esophageal squamous cell carcinoma (high to moderate
differentiation) had invaded the submucosa of the esophageal wall
(submucosal infiltration depth of ~1.1 mm); no tumor thrombus was
found in the gastric mucosa and submucosal vessels, with negative
horizontal and vertical margins. Treatment with surgery or
chemoradiotherapy was recommended; however, the patient and their
family refused this treatment. The patient was discharged on the
seventh day. Oral hormones (which were discontinued within 12 weeks
of discharge) and hepatitis B virus replication inhibitors (to be
taken for life) with supplementation for stomach lining protection
(for 12 weeks) with calcium supplementation (for 12 weeks), and
anticoagulant therapy (for 12 weeks), were prescribed.
 | Table I.Laboratory findings of the first visit
in May 2022. |
Table I.
Laboratory findings of the first visit
in May 2022.
| Laboratory test | Actual value (normal
range) |
|---|
| White blood cell
count, 109/l | 2.87 (3.50-9.50) |
| Red blood cell count,
1012/l | 2.54 (4.30-5.80) |
| Platelet count,
109/l | 55.0
(125.0-350.0) |
| Hemoglobin, g/l | 78.0
(130.0-175.0) |
| Prothrombin time,
sec | 20.7 (11.5-14.5) |
| Internationalization
standard value | 1.75 (0.80-1.20) |
| Activated partial
thromboplastin time, sec | 55.3 (29.0-42.0) |
| α-fetal protein,
ng/ml | 12.79 (≤7.00) |
| Aspartate
aminotransferase, U/l | 60 (≤40) |
| Alanine
aminotransferase, U/l | 42 (≤41) |
| HBV-DNA, IU/ml |
2.22×106 |
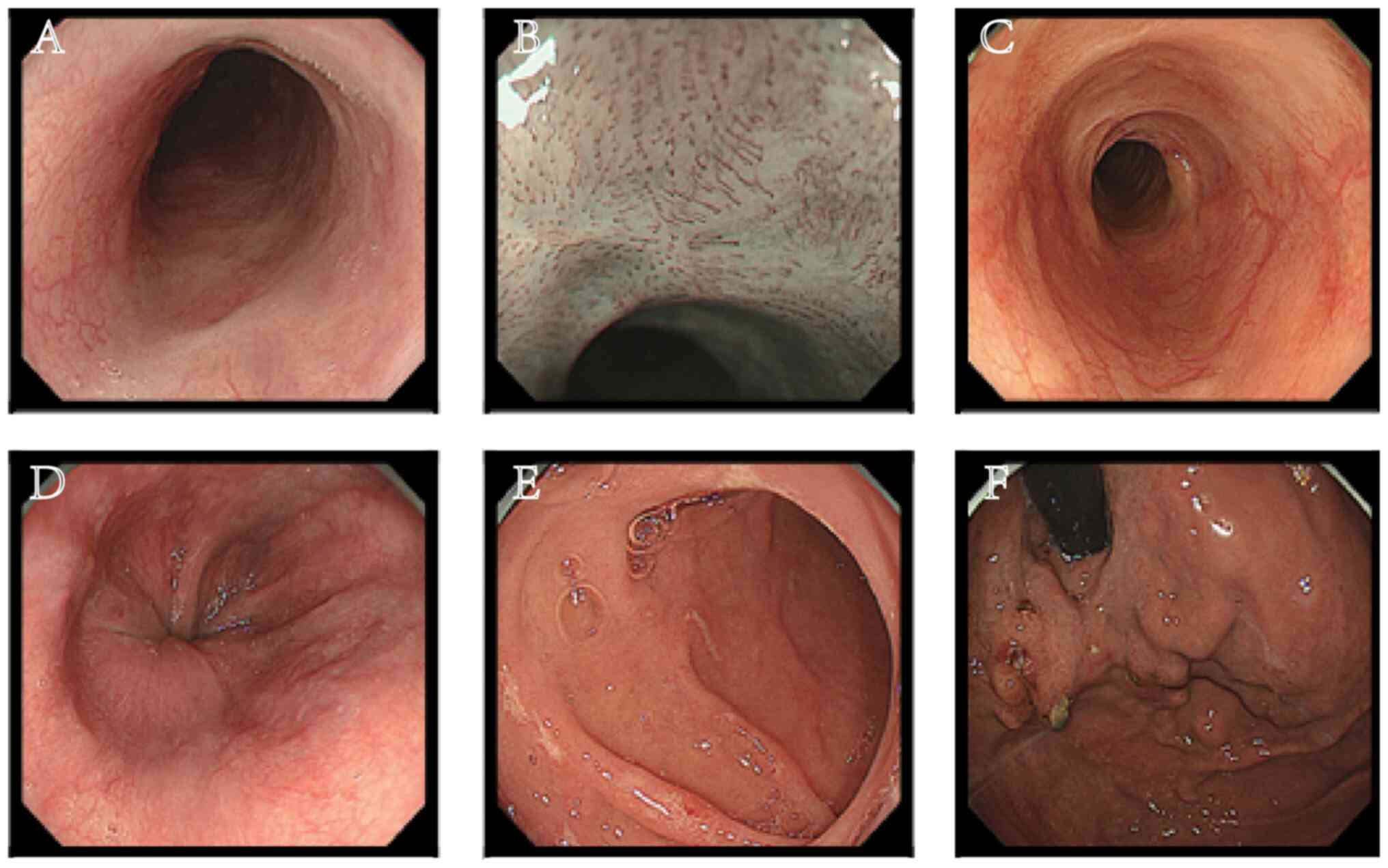
Gastroscopy was performed in September 2022
(Fig. 3). There was no resistance
on ordinary endoscopy, the esophageal mucosa had healed well and no
residual polyglycolic acid sheet was found. There were no
gastrointestinal bleeding, fracture, infection, new-onset diabetes
or other complications during medication administration.
Reexamination of the serology showed that the white blood cell
count, red blood cell count and platelet count in the blood routine
test results were close to the normal value ranges, the liver
function was improved and hepatitis B virus replication was low
(Table II). The patient will
continue to undergo follow-up.
 | Table II.Laboratory findings of the subsequent
visit in September 2022. |
Table II.
Laboratory findings of the subsequent
visit in September 2022.
| Laboratory test | Actual value (normal
range) |
|---|
| White blood cell
count, 109/l | 4.77 (3.5-9.5) |
| Red blood cell count,
1012/l | 3.95 (4.30-5.80) |
| Platelet count,
109/l | 131.0
(125.0-350.0) |
| Hemoglobin, g/l | 104.0
(130.0-175.0) |
| Aspartate
aminotransferase, U/l | 52 (≤40) |
| Alanine
aminotransferase, U/l | 40 (≤41) |
| HBV-DNA, IU/ml |
<1.00×102 |
Discussion
Cirrhosis is considered an independent risk factor
for esophageal cancer surgery. Particularly for patients with
decompensated cirrhosis who have poor coagulative function and poor
esophageal vascular conditions, surgical treatment is challenging.
Studies have shown that patients with liver cirrhosis are more
likely to have pulmonary complications, ascites and anastomotic
leakage or fistula within 30 days of surgery for esophageal cancer
(7,8). Furthermore, some studies report that
patients with cirrhosis have a higher mortality rate after surgery
than those without cirrhosis (9,10).
However, use of endoscopic treatment for liver cirrhosis with early
esophageal cancer appears to be evidentially safer (11). To the best of our knowledge, no
studies have reported a patient experiencing endoscopic mucosal
dissection-related esophageal perforation, postoperative bleeding
or death (1,12). In patients with gastroesophageal
varices, the varices must be treated before endoscopic mucosal
dissection, as serious adverse events have been reported to occur
after endoscopic mucosal dissection (13). Endoscopic treatment of liver
cirrhosis complicated by early esophageal or gastric cancer has
been reported to be safe (14,15)
Another retrospective study showed no significant difference in the
incidence of complications after endoscopic mucosal dissection and
no deterioration of liver function after endoscopic mucosal
dissection in liver cirrhosis complicated with gastric tumors in
different liver function groups (16). However, further research with more
cases is needed to corroborate this.
Currently, there is no standard prevention program
regarding stenosis after endoscopic mucosal dissection for early
esophageal cancer. In China, most doctors prefer to use oral
hormones to prevent stenoses. However, previous studies (17,18)
have shown that oral steroids have a minute effect, which is not
sufficient for patients with high-risk factors for stenosis,
especially for patients with a defect ≥3/4 the circumferential
range of the esophagus after esophagectomy. A case study of a
73-year-old man from South Korea in which PGA was used to prevent
strictures after esophageal endoscopic submucosal dissection
surgery reported no evidence of recurrence at the 1-year follow-up
(19). In addition, a Japanese
study (20) showed that the
patients with circumferential range more than 1/2 had a
postoperative stenosis rate of only 1/13. Another study showed that
PGA combined with fibrin glue had a stenosis rate of 3/8 in
patients with a circumferential range >3/4 (21). Moreover, a 2017 retrospective study
(22) showed that the stenosis rate
of PGA combined with fibrin glue was 3/33. A domestic prospective
study (23) in 2018 showed that the
stenosis rate of PGA combined with esophageal stents was 7/34,
which was significantly lower than the 15/32 recorded for the stent
group. Consequently, these studies have shown that PGA has a good
effect on the prevention of esophageal cancer strictures after
endoscopic submucosal dissection. Furthermore, PGA can promote
wound healing and additionally serve as a physical support for the
postoperative esophagus. Due to the physical properties of PGA, it
degrades in ~1 month, eliminating the need for a second endoscopic
resection, which significantly reduces endoscopic pain and surgical
costs for the patients.
In conclusion, the present study reports the
successful treatment a patient with decompensated cirrhosis and
early esophageal cancer, and the prevention of stenosis after
endoscopic mucosal dissection, resulting in a considerable
improvement in the patient's quality of life. Close follow-up of
the patient will continue.
Acknowledgements
Not applicable.
Funding
The study was supported in part by the National Natural Science
Foundation of China (grant no. 82002609).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WT collected patient data and designed the study. ML
and XXF performed the surgery. XXF participated in the analysis and
interpretation of the data. WT and XXF both contributed to
manuscript drafting, writing and final correction of the
manuscript. WT, ML and XXF read and approved the final manuscript,
and confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The study was conducted according to the guidelines
of the Declaration of Helsinki and was approved by the Ethics
Committee of Huazhong University of Science and Technology (Wuhan,
China).
Patient consent for publication
The patient provided written informed consent for
the publication of this case report and all accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tsou YK, Liu CY, Fu KI, Lin CH, Lee MS, Su
MY, Ohata K and Chiu CT: Endoscopic submucosal dissection of
superficial esophageal neoplasms is feasible and not riskier for
patients with liver cirrhosis. Digest Dis Sci. 61:3565–3571. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katada C, Muto M, Manabe T, Boku N, Ohtsu
A and Yoshida S: Esophageal stenosis after endoscopic mucosal
resection of superficial esophageal lesions. Gastrointest Endosc.
57:165–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ono S, Fujishiro M, Niimi K, Goto O,
Kodashima S, Yamamichi N and Omata M: Predictors of postoperative
stricture after esophageal endoscopic submucosal dissection for
superficial squamous cell neoplasms. Endoscopy. 41:661–665. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi Q, Ju H, Yao LQ, Zhou PH, Xu MD, Chen
T, Zhou JM, Chen TY and Zhong YS: Risk factors for postoperative
stricture after endoscopic submucosal dissection for superficial
esophageal carcinoma. Endoscopy. 46:640–644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugano K, Tack J, Kuipers EJ, Graham DY,
El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N and Malfertheiner
P; faculty members of Kyoto Global Consensus Conference, : Kyoto
global consensus report on Helicobacter pylori gastritis. Gut.
64:1353–1367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
The Paris endoscopic classification of
superficial neoplastic lesions, . esophagus, stomach, and colon:
November 30 to december 1, 2002. Gastrointest Endosc. 58 (Suppl
6):S3–S43. 2003.PubMed/NCBI
|
|
7
|
Schizas D, Giannopoulos S, Vailas M,
Mylonas KS, Giannopoulos S, Moris D, Rouvelas I, Felekouras E and
Liakakos T: The impact of cirrhosis on esophageal cancer surgery:
An up-to-date meta-analysis. Am J Surg. 220:865–872. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Valmasoni M, Pierobon ES, De Pasqual CA,
Zanchettin G, Moletta L, Salvador R, Costantini M, Ruol A and
Merigliano S: Esophageal cancer surgery for patients with
concomitant liver cirrhosis: A single-center matched-cohort study.
Ann Surg Oncol. 24:763–769. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang ZQ, Deng HY, Yang YS, Wang Y, Hu Y,
Yuan Y, Wang WP and Chen LQ: Can oesophagectomy be performed for
patients with oesophageal carcinoma and concomitant liver
cirrhosis? A retrospective study based on a propensity-matched
cohort. Interact Cardiovasc Thorac Surg. 25:442–447. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tachibana M, Kotoh T, Kinugasa S, Dhar DK,
Shibakita M, Ohno S, Masunaga R, Kubota H, Kohno H and Nagasue N:
Esophageal cancer with cirrhosis of the liver: Results of
esophagectomy in 18 consecutive patients. Ann Surg Oncol.
7:758–763. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Endlicher E, Gelbmann C, Schlottmann K,
Herfarth H, Rümmele P, Friedrich A, Schölmerich J and Kullmann F:
Endoscopic mucosal resection for early esophageal cancer with
esophageal varices. Z Gastroenterol. 42:609–613. 2004.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Liu Y, He S, Zhang Y, Dou L, Sun
L, et al: Endoscopic submucosal dissection for early esophageal
squamous cell carcinoma with esophageal-gastric fundal varices
caused by liver cirrhosis: a case report. Transl Cancer Res.
11:2433–2437. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sawaguchi M, Jin M, Matsuhashi T, Ohba R,
Hatakeyama N, Koizumi S, Onochi K, Yamada Y, Kanazawa N, Kimura Y,
et al: The feasibility of endoscopic submucosal dissection for
superficial esophageal cancer in patients with cirrhosis (with
video). Gastrointest Endosc. 79:681–685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kolb JM, Wani S, Soetikno R, Edmundowicz
SA and Hammad H: Endoscopic submucosal dissection for early
esophageal and gastric neoplasia in decompensated cirrhosis with
varices. Endoscopy. 53:E128–E129. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang AY, Smith EZ, Sauer BG, Henry ZH,
Shah NL and Caldwell SH: A pilot experience of endoscopic
submucosal dissection of Barrett's dysplasia despite esophageal
varices and decompensated cirrhosis. Hepatology. 70:2225–2227.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choe WH, Kim JH, Park JH, Kim HU, Cho DH,
Lee SP, Lee TY, Lee SY, Sung IK, Park HS and Shim CS: Endoscopic
submucosal dissection of early gastric cancer in patients with
liver cirrhosis. Digest Dis Sci. 63:466–473. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kadota T, Yoda Y, Hori K, Shinmura K, Oono
Y, Ikematsu H and Yano T: Prophylactic steroid administration
against strictures is not enough for mucosal defects involving the
entire circumference of the esophageal lumen after esophageal
endoscopic submucosal dissection (ESD). Esophagus. 17:440–447.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Isomoto H, Yamaguchi N, Nakayama T,
Hayashi T, Nishiyama H, Ohnita K, Takeshima F, Shikuwa S, Kohno S
and Nakao K: Management of esophageal stricture after complete
circular endoscopic submucosal dissection for superficial
esophageal squamous cell carcinoma. BMC Gastroenterol. 11:462011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim YJ, Park JC, Chung H, Shin SK, Lee SK
and Lee YC: Polyglycolic acid sheet application to prevent
esophageal stricture after endoscopic submucosal dissection for
recurrent esophageal cancer. Endoscopy. 48:E319–E320. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iizuka T, Kikuchi D, Yamada A, Hoteya S,
Kajiyama Y and Kaise M: Polyglycolic acid sheet application to
prevent esophageal stricture after endoscopic submucosal dissection
for esophageal squamous cell carcinoma. Endoscopy. 47:341–344.
2015.PubMed/NCBI
|
|
21
|
Sakaguchi Y, Tsuji Y, Ono S, Saito I,
Kataoka Y, Takahashi Y, Nakayama C, Shichijo S, Matsuda R,
Minatsuki C, et al: Polyglycolic acid sheets with fibrin glue can
prevent esophageal stricture after endoscopic submucosal
dissection. Endoscopy. 47:336–340. 2015.PubMed/NCBI
|
|
22
|
Iizuka T, Kikuchi D, Hoteya S, Kajiyama Y
and Kaise M: Polyglycolic acid sheet and fibrin glue for preventing
esophageal stricture after endoscopic submucosal dissection: A
historical control study. Dis Esophagus. 30:1–8. 2017. View Article : Google Scholar
|
|
23
|
Chai NL, Feng J, Li LS, Liu SZ, Du C,
Zhang Q and Linghu EQ: Effect of polyglycolic acid sheet plus
esophageal stent placement in preventing esophageal stricture after
endoscopic submucosal dissection in patients with early-stage
esophageal cancer: A randomized, controlled trial. World J
Gastroentero. 24:1046–1055. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oyama T, Inoue H, Arima M, Momma K, Omori
T, Ishihara R, Hirasawa D, Takeuchi M, Tomori A and Goda K:
Prediction of the invasion depth of superficial squamous cell
carcinoma based on microvessel morphology: magnifying endoscopic
classification of the Japan esophageal society. Esophagus.
14:105–112. 2017. View Article : Google Scholar : PubMed/NCBI
|