Introduction
Spinal metastases are most common in patients with
advanced disease among all cancer types (1–5). They
frequently cause vertebral body collapse (VBC) and malignant spinal
cord compression (MSCC), resulting in pain and paralysis. VBC is
caused by the destruction of the vertebral body. It often
accompanies pain and sometimes has paralysis when spinal cord is
compressed by collapsed vertebral body (1,2). MSCC
is usually caused by the compression of spinal cord by metastatic
tumor which extends into the vertebral column. Its common symptoms
are radicular pain, motor weakness, sensory complaints and bladder
dysfunction (3). These spinal
skeletal-related events (SREs) drastically reduce patients'
activities of daily living (ADL) and quality of life (QOL)
(1–3). If the patient has symptoms of VBC
and/or SREs, radiotherapy (RT) and surgery would be preferred to
chemotherapy because of their direct local effect such as shrinkage
of the tumor, decompression with the removal of lamina or pedicle,
and removal of the compressing tumor (4–6). In
patients with paralysis, decompression and fixation are the first
treatment choices (6–9). However, conservative treatment using
orthoses is often preferred in patients without paralysis (10). In addition, VBC can progress even
after RT (10,11). Although conventional RT is most
commonly used for spinal SREs, the rate of occurrence of new VBC
and its progression at RT initiation has not been fully
investigated (10,11).
Rief et al reported the occurrence of new VBC
following RT in 2% of patients diagnosed with various cancer types
(10). Among colorectal cancer
patients, new VBC occurred in 9% of patients after RT completion
(11). However, in these studies,
the time points chosen to examine potential VBC manifestations were
inconsistent in terms of interval frequencies and lengths, making
an accurate and comparative evaluation of VBC development over time
extremely difficult. Moreover, investigation of VBC occurrence in
these studies is limited due to a lack of information on the degree
of VBC prior to RT.
To the best of our knowledge, no study has focused
on the evaluation of VBC occurrences and progression in patients
with vertebral bone metastases without paralysis by MSCC.
Therefore, development of an approach that allows for a more
detailed evaluation of VBC development were performed. In this
regard, the patients were divided based on their degree of VBC at
RT initiation and investigated for changes in VBC for up to 6
months after RT. In addition, potential risk factors for VBC in
patients with painful spinal metastases without paralysis were also
examined. The study specifically focused to answer the following
two questions with respect to the new VBC cases: (1) What are the incidence rates, timing,
and degree of new VBC cases, and when does it cease? (2) What are the potential risk factors for
the occurrence of new VBC? In addition, the study also attempted to
answer the following questions with regard to VBC before RT:
(3) What are the incidence rates
and degree of VBC progression, and when does it occur and cease to
occur? (4) What are the risk
factors for the progression of VBC?.
Patients and methods
Study population
The records of patients who received RT for
palliation of painful vertebral bone metastases at our institution
between July 2012 and November 2016 were retrospectively
investigated. The last follow-up time point for the evaluation of
patients involved in this study was January 2017. The patients who
underwent treatment for metastatic lesion at the same irradiated
vertebrae, including surgery, RT or other local interventional
therapies were excluded. The patients with clinical MSCC, sacral
lesions, and those who were followed up for less than one month
were also excluded. In the same period of this study, there were
two patient who developed paralysis for MSCC during the follow-up
period. These cases were resistant to RT and their pain got worse
again. Then, they were excluded in our study.
This retrospective chart review study involving
human participants was conducted in accordance with the ethical
standards of the institutional and national research committee and
with the 1964 Helsinki Declaration and its later amendments or
comparable ethical standards. The Human Investigation Committee
(IRB) of the Shikoku Cancer Ethics Committee approved this study
(Approval No. 2017-26). All the participants provided written
informed consent for this study.
Assessment of pain at metastatic
vertebrae
A numeric rating scale (NRS) was used to evaluate
the degree of pain at the metastatic vertebrae at the time of
movement (mechanical pain). NRS is a patient-based assessment tool
that evaluates pain intensity on a scale of 0 (no pain) to 10
(worst pain) (12). Based on the
National Comprehensive Cancer Network guidelines, the level of pain
was determined as none (0), mild (1–3),
moderate (4–6), or severe (7–10)
(13).
Radiological assessment
The status of the vertebral bone was evaluated using
CT (Aquilion, Canon) at 120 kV and a slice thickness of 5 mm. All
images were viewed with routine bone window settings (window level
200 HU, window width 2000 HU) with axial, coronal, and sagittal
planes. Bone quality was classified as lytic, mixed, or blastic at
RT initiation. There was no patients with intra-trabeculae
metastases.
VBC was defined as a reduction in vertebral body
height compared to the height of the upper and lower vertebral
bodies. The degree of VBC was determined as severe (≥50% collapse)
or mild (>0 and <50% collapse) based on the approach of a
previous report assessing VBC development (14). The progression of VBC was defined as
the advancement of the collapse of the vertebral body in the
irradiated vertebral bone with collapse at RT initiation.
Progression of VBC in patients who presented with VBC at RT
initiation were evaluated at RT initiation and 1, 2, 3, 4, and 6
months after RT.
The patients without VBC were also divided as
follows: no collapse with ≥50% body involvement of the tumor and no
collapse with <50% body involvement of the tumor, based on the
approach of a previous report assessing VBC development (14). The ‘body involvement’ was defined as
the occupation of the tumors in the vertebral body. The rate of the
occupation of the tumors in the vertebral body was evaluated in the
axial view of CT. For them, radiological evaluations were performed
at RT initiation and 1, 2, 3, 4, and 6 months after RT. The new VBC
was defined as a reduction in vertebral body height compared to the
height of the upper and lower vertebral bodies in the irradiated
vertebral bone.
Statistical analyses
The potential risk factors in patients with new VBC,
in patients without VBC, and progression of VBC at RT initiation
and one month after RT were assessed. The clinical data of the
patients included information on age, sex, primary cancer site,
radiation site, chemotherapy before RT, chemotherapy after RT, the
overall dose of RT, degree of pain as measured by NRS, bone
quality, lung metastases, vertebral body collapse, and tumor
involvement of posterolateral elements of the spine. The
progression of vertebral body collapse was estimated by CT at 1, 2,
3, 4, and 6 months after RT. The rates of cease of the progression
of the collapse at each time point were estimated by the
Kaplan-Meier method. The endopoint was the time to the stop of the
progression of vertebral body collapse. Those who had dead was
ceased.
Univariate analysis was performed using the
chi-square test, and multivariate analysis was performed using
logistic regression. For all analyses, associations were considered
significant if the P-value was <0.05. The COX hazard model
analysis was thought to be inappropriate due to the low power of
detection because the time units are months instead of days. All
statistical analyses were performed using the statistical computing
software R (R version 3.5.0, R Core Team, Vienna, Austria).
Results
Patients' characteristics
A total of 177 patients were included in this study,
of whom 95 were males and 82 were females, with a median age of 67
years (range, 30–91) (Table I). The
primary tumor sites in the participants were the lung (n=58),
breast (n=39), prostate (n=22), colorectum (n=17), stomach (n=10),
liver (n=9), pancreas (n=4), and others (n=18). The spine locations
were the cervical (n=14), thoracic (n=91), and lumbar (n=72)
regions. They were divided into the junctional level (C1, C2, C7 to
T2, T11 to L1, and L5) (n=57), mobile segments (C3 to C6 and L2 to
L4) (n=58), and rigid segments (T3 to T10) (n=62). The types of
metastases were lytic (n=64), mixed (n=74), and blastic (n=39). All
patients underwent RT. Chemotherapy was administered to 88 patients
(50%) before RT. All patients were treated conservatively. The
decompression and spine stabilization was performed for patients
with paralysis by metastatic spinal cord compression. There was one
patient who had paralysis during the study period. The patient also
had severe pain who cannot get out of bed with instability of the
spine as measured by spine instability neoplastic score (6). The surgery was performed (laminecomy
and spine stabilization) for the patient.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Value |
|---|
| Sex |
|
| Male | 95 |
|
Female | 82 |
| Median age
(range) | 67 (30–91) |
| Primary tumor
sites |
|
| Lung | 58 |
|
Breast | 39 |
|
Prostate | 22 |
|
Colorectum | 17 |
|
Stomach | 10 |
|
Liver | 9 |
|
Pancreas | 4 |
|
Others | 18 |
| Spine locations |
|
|
Cervical | 14 |
|
Thoracic | 91 |
|
Lumbar | 72 |
| Spine locations
(based on segments) |
|
|
Junctional level (C1, C2, C7
to T2, T11 to L1 and L5 | 57 |
| Mobile
segments (C3 to C6 and L2 to L4) | 58 |
| Rigid
segments (T3 to T10) | 62 |
| Types of
metastases |
|
|
Lytic | 64 |
|
Mixed | 74 |
|
Blastic | 39 |
| Chemotherapy before
RT |
|
| Yes | 88 |
| No | 89 |
| Chemotherapy after
RT |
|
| Yes | 111 |
| No | 66 |
| Lung metastases |
|
| Yes | 79 |
| No | 98 |
Assessment of pain at metastatic
vertebrae
All patients experienced reduced pain during the
follow-up period. None of them required surgery to alleviate the
pain. The level of pain at RT initiation was none in 72, mild in
46, moderate in 29, and severe in 30 patients.
Patients with or without VBC at RT
initiation
The number of patients that presented without and
with VBC at RT initiation was 68 (38%) and 109 (62%), respectively
(Table II). Of 68 patients without
VBC, 19 presented with ≤50% body involvement of the tumor and 49
with >50% body involvement of the tumor. Of 109 patients with
VBC at RT initiation, 8 presented with ≥50% collapse, and 101
presented with >0 and <50% collapse. The number of patients
with or without VBC decreased during the follow-up period due to
death from the disease (Table
II).
 | Table II.Vertebral body collapse at the
beginning of RT and at 1, 2, 3, 4 and 6 months after RT. |
Table II.
Vertebral body collapse at the
beginning of RT and at 1, 2, 3, 4 and 6 months after RT.
| Before RT | RT | 1 month | 2 months | 3 months | 4 months | 6 months |
|---|
| ≥50% collapse
(n=8) | ≥50% collapse | 8 | 7 (88%) | 5 (63%) | 5 (63%) | 3 (37%) |
|
| Dead | 0 | 1 | 3 | 3 | 5 |
| >0<50%
collapse (n=101) | >0<50%
collapse | 93 | 66 (65%) | 54 (53%) | 43 (43%) | 33 (33%) |
|
| ≥50% collapse | 8 | 6 (6%) | 5 (5%) | 5 (5%) | 4 (4%) |
|
| Dead | 0 | 29 | 42 | 53 | 64 |
| No collapse with
>50% body involved of the tumor (n=49) | No collapse with
>50% body involved of the tumor | 42 | 36 | 28 | 25 | 22 |
|
| >0<50%
collapse | 7 | 6 | 5 | 3 | 2 |
|
| ≥50% collapse | 0 | 0 | 0 | 0 | 0 |
|
| Dead | 0 | 7 | 16 | 21 | 25 |
| No collapse with
≤50% body involved of the tumor (n=19) | No collapse with
≤50% body involved of the tumor | 19 | 13 (68%) | 8 (42%) | 7 (37%) | 6 (32%) |
|
| >0<50%
collapse | 0 | 0 | 0 | 0 | 0 |
|
| ≥50% collapse | 0 | 0 | 0 | 0 | 0 |
|
| Dead | 0 | 6 | 11 | 12 | 13 |
| Total number of the
patients |
| 177 | 134 | 105 | 88 | 70 |
Analysis of patients without collapse
before RT initiation
New VBC occurred in 8 patients (12%) without
collapse at RT initiation. New VBC did not occur in any patient
without collapse and ≤50% body involvement of the tumor. New VBC
occurred in 8 of 49 patients (16%) without collapse and >50%
body involvement of the tumor. All new VBC advanced to <50%
collapse, occurred briefly after the initiation of RT until a
median of one month [1st month (5 patients) and 2nd month (2
patients)]. Among them, there were 2 patients in whom VBC occurred
in asymptomatic patients after RT. Univariate analysis revealed
that primary cancer site (lungs), bone quality (lytic metastases),
NRS score (≥4), and tumor involvement of posterolateral elements of
the spine were risk factors for new VBC (Table III). Multivariate analysis
revealed that NRS score (≥4) [Relative risk (RR), 27.100; 95%
confidence interval (CI), 1.859 to 394.884; P=0.016] was associated
with the occurrence of new VBC at the one-month follow-up time
point.
 | Table III.Risk factors for new vertebral body
collapse at 1 month after RT. |
Table III.
Risk factors for new vertebral body
collapse at 1 month after RT.
| Covariates | Patients without
new collapse | Patients with new
collapse | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
<65 | 25 | 3 |
|
|
|
|
|
≥65 | 35 | 5 | 1.19
(0.260-5.446) | >0.999 |
|
|
| Sex |
|
|
|
|
|
|
|
Male | 36 | 7 |
|
|
|
|
|
Female | 24 | 1 | 0.214
(0.025-1.854) | 0.242 |
|
|
| Primary cancer
site |
|
|
|
|
|
|
|
Lung | 15 | 6 |
|
|
|
|
|
Others | 45 | 2 | 0.111
(0.020-0.610) | 0.009a | 6.947
(0.889-54.312) | 0.065 |
| Radiation site |
|
|
|
|
|
|
|
Junctional level | 17 | 4 |
|
|
|
|
| Mobile
segments/rigid segments | 43 | 4 | 0.395
(0.089-1.764) | 0.240 |
|
|
| Chemotherapy before
RT |
|
|
|
|
|
|
|
Yes | 39 | 2 |
|
|
|
|
| No | 21 | 6 | 5.571
(1.032-30.072) | 0.051 |
|
|
| Chemotherapy after
RT |
|
|
|
|
|
|
|
Yes | 44 | 4 |
|
|
|
|
| No | 16 | 4 | 2.750
(0.614-12.317) | 0.221 |
|
|
| Overall dose
(RT) |
|
|
|
|
|
|
|
≤35 | 10 | 1 |
|
|
|
|
|
>35 | 50 | 7 | 1.400
(0.155-12.667) | >0.999 |
|
|
| NRS score |
|
|
|
|
|
|
|
<4 | 49 | 3 |
|
|
|
|
| ≥4 | 11 | 5 | 0.135
(0.028-0.650) | 0.015a | 27.100
(1.859-394.884) | 0.0158a |
| Bone Quality |
|
|
|
|
|
|
|
Lytic | 16 | 6 |
|
|
|
|
| Mixed
or blastic | 44 | 2 | 0.121
(0.022-0.663) | 0.024a | 9.305
(0.935-92.564) | 0.057 |
| Lung
metastases |
|
|
|
|
|
|
|
Yes | 25 | 4 |
|
|
|
|
| No | 35 | 4 | 0.714
(0.163-3.131) | 0.714 |
|
|
| Vertebral body
collapse |
|
|
|
|
|
|
| No
collapse with <50% body involved of the tumor | 19 | 0 |
|
|
|
|
| No collapse with
>50% body involved of the tumor | 41 | 8 | - | 0.094 |
|
|
| Posterolateral
involvement of spinal elements |
|
|
|
|
|
|
|
Bilateral/unilateral | 5 | 3 |
|
|
|
|
| No
involvement | 55 | 5 | 0.152
(0.028-0.829) | 0.046a | 10.990
(0.687-175.753) | 0.090 |
Analysis of patients with collapse
before RT initiation
VBC progression occurred in 56 patients (51%) with
collapse and 50 out of 101 patients (50%) who presented with mild
collapse at RT initiation. VBC occurred briefly after the
initiation of RT until a median of one month [1st month (38
patients), 2nd month (10 patients), 3rd month (2 patients), and no
patient in 4th and 6th month]. Among these patients, VBC progressed
to ≥50% collapse in 11 patients (12%) at a median of one month [1st
month (8 patients), 2nd month (2 patients), 3rd month (1 patient),
and no patient in 4th and 6th month]. VBC progression occurred in 6
out of 8 patients (75%) who presented with severe collapse at RT
initiation and briefly after the initiation of RT until a median of
one month [1st month (3 patients), 2nd month (3 patients), and no
patient in 3rd, 4th and 6th month].
Univariate analysis revealed that bone quality
(lytic metastases), NRS score (≥4), and tumor involvement of
posterolateral elements of the spine were risk factors for the
progression of VBC at the one-month follow-up time point (Table IV). Multivariate analysis revealed
that bone quality (lytic metastases) (RR, 3.138; 95% CI, 1.280 to
7.698; P=0.013), NRS score (≥4) (RR, 2.963; 95% CI, 1.179 to 7.446;
P=0.021), and tumor involvement of posterolateral elements of the
spine (RR, 2.735; 95% CI, 1.026 to 7.294; P=0.044) were associated
with the progression of VBC at the one-month follow-up time
point.
 | Table IV.Risk factors for progression of VBC
at 1 month after RT. |
Table IV.
Risk factors for progression of VBC
at 1 month after RT.
| Covariates | Patients without
progression of VBC | Patients with
progression of VBC | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
<65 | 20 | 26 |
|
|
|
|
|
≥65 | 33 | 30 | 1.430
(0.666-3.071) | 0.439 |
|
|
| Sex |
|
|
|
|
|
|
|
Male | 20 | 26 |
|
|
|
|
|
Female | 33 | 30 | 1.401
(0.659-2.980) | 0.445 |
|
|
| Primary cancer
site |
|
|
|
|
|
|
|
Lung | 16 | 21 |
|
|
|
|
|
Others | 37 | 35 | 1.388
(0.625-3.081) | 0.544 |
|
|
| Radiation site |
|
|
|
|
|
|
|
Junctional level | 16 | 20 |
|
|
|
|
| Mobile
segments/rigid segments | 37 | 36 | 1.285
(0.576-2.864) | 0.550 |
|
|
| Chemotherapy before
RT |
|
|
|
|
|
|
|
Yes | 27 | 20 |
|
|
|
|
| No | 26 | 36 | 0.535
(0.248-1.152) | 0.125 |
|
|
| Chemotherapy after
RT |
|
|
|
|
|
|
|
Yes | 32 | 31 |
|
|
|
|
| No | 21 | 25 | 0.814
(0.380-1.743) | 0.699 |
|
|
| Overall dose
(RT) |
|
|
|
|
|
|
|
≤35 | 44 | 53 |
|
|
|
|
|
>35 | 9 | 3 | 0.277
(0.071-1.085) | 0.069 |
|
|
| NRS score |
|
|
|
|
|
|
|
<4 | 41 | 25 |
|
|
|
|
| ≥4 | 12 | 31 | 4.237
(1.845-9.731) |
<0.001a | 2.963
(1.179-7.446) | 0.021a |
| Bone Quality |
|
|
|
|
|
|
|
Lytic | 12 | 30 |
|
|
|
|
| Mixed
or blastic | 41 | 26 | 3.942
(1.718-9.045) | 0.002a | 3.138
(1.280-7.698) | 0.013a |
| Lung
metastases |
|
|
|
|
|
|
|
Yes | 26 | 24 |
|
|
|
|
| No | 27 | 32 | 0.779
(0.366-1.657) | 0.567 |
|
|
| VBC |
|
|
|
|
|
|
| <50%
collapse | 2 | 6 |
|
|
|
|
| ≥50%
collapse | 51 | 50 | 3.060
(0.589-15.888) | 0.272 |
|
|
| Posterolateral
involvement of spinal elements |
|
|
|
|
|
|
|
Bilateral/Unilateral | 9 | 25 |
|
|
|
|
| No
involvement | 44 | 31 | 3.943
(1.619-9.599) | 0.002a | 2.735
(1.026-7.294) | 0.044a |
The collapse progression-free rates estimated by the
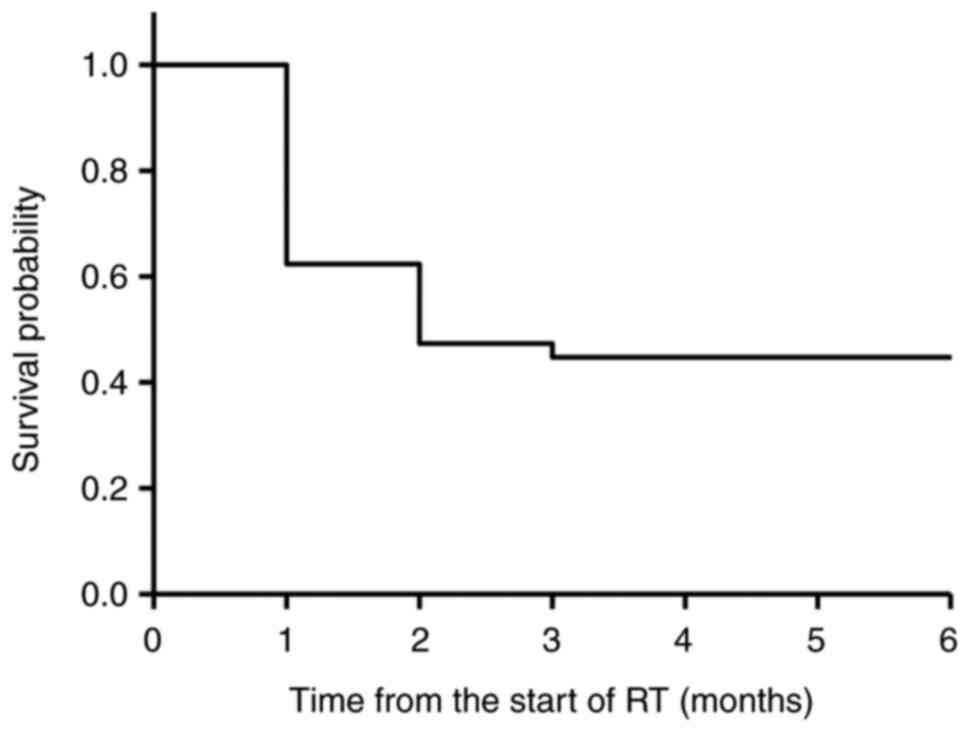
Kaplan-Meier method were 62, 47, 44, 44, and 44% at the 1-, 2-, 3-,
4-, and 6-month time points, respectively (Fig. 1).
Discussion
Although conventional RT is most commonly utilized
for spinal SREs, the occurrence of new VBC during RT has not been
fully investigated previously (10,11,15–23).
Shi et al reported that a total of 51 out of 250 (20.4%)
lesions subsequently developed new fracture or progression of
existing fracture after RT for spinal metastasis (23). Of these new or worsened fractures,
30 (58.8%) were asymptomatic, and 21 (41.2%) were painful
fractures. Rief et al reported the occurrence of a new VBC
in 2% of patients at the 6-month timepoint after conventional RT in
various cancer types (10). In
addition, they reported that the thoracic spine showed
significantly more fractures than the other vertebrae. However,
they did not perform a radiological evaluation to investigate the
degree and timing of VBC, especially in the acute period of 1–3
months after RT initiation during which the patients need the most
intense clinical care for pain and VBC. Lee et al
investigated VBC every 2–4 months and reported the occurrence of
new VBC in 18% of patients with colorectal cancer who received
conventional RT (11). In addition,
they also reported that previously performed irradiation and
pre-existing compression fracture were independent risk factors for
VBC using the multivariate analysis. However, the application of
inconsistent examination time points has led to difficulties in
interpreting their outcomes. In this study, the new VBC occurred in
12% of patients that presented without collapse at RT initiation.
The study by Lee et al did not find pain as the risk factor
for VBC in patients with colorectal cancer who received
conventional RT (11). However, the
present study reports that the degree of pain was a predictor of
VBC, as found that moderate or severe pain (NRS (≥4)) was
associated with the risk of the occurrence of new VBC. Thus,
clinicians should pay attention to moderate or severe pain (NRS
(≥4)) to predict the occurrence of new VBC in patients without VBC
at RT initiation. Furthermore, it was found that its degree was
mild (<50% collapse), occurred within one month after RT
initiation and did not progress any further after two months.
In patients presenting with VBC at RT initiation,
VBC progressed in 51% of them upon RT treatment. The VBC occurred
one month after RT and ceased within two months in most patients
with collapse progression-free rates of 62, 47, 44, 44, and 44% at
the 1-, 2-, 3-, 4-, and 6-month(s) time points, respectively. In
patients with mild collapse at RT initiation, VBC progression
occurred in 50%. The collapse occurred briefly after the start of
RT until a median of one month. Among them, the VBC progressed to
become severe (≥50% collapse) in 12% of patients until a median of
one month. However, in patients with severe collapse at RT
initiation, VBC progressed within a median of one month in 75% of
patients.
Precise assessment of risk factors for the potential
progression of VBC is critical during RT initiation to determine
patients who require close observation. Multivariate analysis
revealed that bone quality (lytic metastases), NRS score (≥4), and
tumor involvement of posterolateral elements of the spine were
associated with the progression of VBC at the one-month follow-up
time point. In the vertebral bones, posterolateral elements of the
spine (facet, pedicle, or costovertebral joint) play an essential
role in spinal stability (24,25)
which was previously reported by Taneichi et al in patients
with lytic vertebral metastases (26). They reported that the risk factors
for vertebral body fractures were costovertebral joint destruction
in the thoracic region (T1-T10) and pedicle destruction in the
thoracolumbar and lumbar region (T10-L5). Therefore, clinicians
should pay close attention to the destruction of the posterolateral
elements of the spine for the assessment and prediction of
potential VBC progression. In previous studies, lytic metastases
were reported to be associated with spinal instability (14,27).
The present study also found an association between bone lesions
(lytic metastases) and the progression of VBC. Lytic metastases
without bone formation can be at a higher risk for compression
since they cannot withstand axial load. This study demonstrated
that moderate or severe pain (NRS (≥4)) was associated with the
risk of both new VBC occurrence and progression of VBC. Pain can be
easily measured at the bedside and is often used in the treatment
of bone metastasis. Therefore, the study findings suggest that NRS
is a useful index for predicting the occurrence and progression of
VBC.
The present study had a few limitations. First, not
all the patients were followed up for 6 months. However, this is a
common limitation of studies involving patients with bone
metastases, given their relatively shorter survival time. Second,
the inherent bias in the choice of fractionation used, where
radiotherapy with fewer dose fractions was given for patients with
greater metastatic burden or for the histologies known to be
predictive of shorter survival.
In conclusion, new VBC with a mild degree (<50%
collapse) occurred in 12% of patients without collapse within a
month. Moderate or severe pain (NRS (≥4)) was the predictor of the
occurrence of new VBC. However, progression of VBC after RT
occurred in 51% of patients with collapse at RT initiation. Bone
quality (lytic metastases), NRS score (≥4), and tumor involvement
of posterolateral elements of the spine were associated with the
progression of VBC at the one-month follow-up time point. This
ensures proper evaluation of the effectiveness of conservative
treatment and facilitates the determination of patients who require
close monitoring.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
EN, SS, and TO designed the study. EN and SS
collected and analyzed data. EN and RN confirm the authenticity of
the raw data. RN, HK and TI analyzed the data. EN and SS treated
the patients presented in this manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This retrospective chart review study involving
human participants was conducted in accordance with the ethical
standards of the institutional and national research committee and
with the 1964 Helsinki Declaration and its later amendments or
comparable ethical standards. The Human Investigation Committee
(IRB) of the Shikoku Cancer Ethics Committee approved this study
(approval No. 2017-26). Written informed consent was obtained from
every participant included in this study.
Patient consent for publication
All the participants provided written informed
consent for this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Van den Brande R, Cornips EM, Peeters M,
Ost P, Billiet C and Van de Kelft E: Epidemiology of spinal
metastases, metastatic epidural spinal cord compression and
pathologic vertebral compression fractures in patients with solid
tumors: A systematic review. J Bone Oncol. 35:1004462022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sciubba DM, Pennington Z, Colman MW,
Goodwin CR, Laufer I, Patt JC, Redmond KJ, Saylor P, Shin JH,
Schwab JH, et al: Spinal metastases 2021: A review of the current
state of the art and future directions. Spine J. 21:1414–1429.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rades D, Segedin B, Schild SE, Lomidze D,
Veninga T and Cacicedo J: Identifying patients with malignant
spinal cord compression (MSCC) near end of life who can benefit
from palliative radiotherapy. Radiat Oncol. 17:1432022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bahouth SM, Yeboa DN, Ghia AJ, Tatsui CE,
Alvarez-Breckenridge CA, Beckham TH, Bishop AJ, Li J, McAleer MF,
North RY, et al: Advances in the management of spinal metastases:
What the radiologist needs to know. Br J Radiol. 24:202202672023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oldenburger E, Brown S, Willmann J, van
der Velden JM, Spałek M, van der Linden YM, Kazmierska J, Menten J,
Andratschke N and Hoskin P: ESTRO ACROP guidelines for external
beam radiotherapy of patients with complicated bone metastases.
Radiother Oncol. 173:240–253. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Serratrice N, Faddoul J, Tarabay B, Attieh
C, Chalah MA, Ayache SS and Abi Lahoud GN: Ten Years After SINS:
Role of surgery and radiotherapy in the management of patients with
vertebral metastases. Front Oncol. 12:8025952022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu X, Lu J, Xu H, Tang Q, Song G, Deng C,
Wu H, Xu Y, Chen H and Wang J: A comparative study between
minimally invasive spine surgery and traditional open surgery for
patients with spinal metastasis. J Spine (Phila Pa 1976). 46:62–68.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pranata R, Lim MA, Vania R and Bagus
Mahadewa TG: Minimal invasive surgery instrumented fusion versus
conventional open surgical instrumented fusion for the treatment of
spinal metastases: A systematic review and meta-analysis. World
Neurosurg. 148:e264–e274. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patchell RA, Tibbs PA, Regine WF, Payne R,
Saris S, Kryscio RJ, Mohiuddin M and Young B: Direct decompression
surgical resection in the treatment of spinal cord compression
caused by metastatic cancer: A randomized trial. Lancet.
366:643–648. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rief H, Förster R, Rieken S, Bruckner T,
Schlampp I, Bostel T and Debus J: The influence of orthopedic
corsets on the incidence of pathological fractures in patients with
spinal bone metastases after radiotherapy. BMC Cancer. 15:7452015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee J, Rhee WJ, Chang JS, Chang SK and
Koom WS: Evaluation of predictive factors of vertebral compression
fracture after conventional palliative radiotherapy for spinal
metastasis from colorectal cancer. J Neurosurg Spine. 28:333–340.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fallon M, Hoskin PJ, Colvin LA,
Fleetwood-Walker SM, Adamson D, Byrne A, Murray GD and Laird BJ:
Randomized double-blind trial of pregabalin versus placebo in
conjunction with palliative radiotherapy for cancer-induced bone
pain. J Clin Oncol. 34:550–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Swarm RA, Abernethy AP, Anghelescu DL,
Benedetti C, Buga S, Cleeland C, Deleon-Casasola OA, Eilers JG,
Ferrell B, Green M, et al: Adult cancer pain. J Natl Compr Canc
Netw. 11:992–1022. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fisher CG, DiPaola CP, Ryken TC, Bilsky
MH, Shaffrey CI, Berven SH, Harrop JS, Fehlings MG, Boriani S, Chou
D, et al: A novel classification system for spinal instability in
neoplastic disease: An evidence-based approach and expert consensus
from the Spine Oncology Study Group. Spine (Phila Pa 1976).
35:E1221–E1229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim YR, Lee CH, Yang SH, Hyun SJ, Kim CH,
Park SB, Kim KJ and Chung CK: Accuracy and precision of the spinal
instability neoplastic score (SINS) for predicting vertebral
compression fractures after radiotherapy in spinal metastases: A
meta-analysis. Sci Rep. 11:55532021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Westhoff PG, de Graeff A, Monninkhof EM,
Pomp J, van Vulpen M, Leer JW, Marijnen CA and van der Linden YM;
Dutch Bone Metastasis Study Group, : Quality of life in relation to
pain response to radiation therapy for painful bone metastases. Int
J Radiat Oncol Biol Phys. 93:694–701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Howell DD, James JL, Hartsell WF,
Suntharalingam M, Machtay M, Suh JH, Demas WF, Sandler HM, Kachnic
LA and Berk LB: Single-fraction radiotherapy versus multifraction
radiotherapy for palliation of painful vertebral bone
metastases-equivalent efficacy, less toxicity, more convenient: A
subset analysis of Radiation Therapy Oncology Group trial 97–14.
Cancer. 119:888–896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soliman M, Taunk NK, Simons RE, Osborne
JR, Kim MM, Szerlip NJ and Spratt DE: Anatomic and functional
imaging in the diagnosis of spine metastases and response
assessment after spine radiosurgery. Neurosurg Focus. 42:E52017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kouloulias V, Liakouli Z, Zygogianni A,
Mystakidou K and Kouvaris JR: Bone density as a marker of response
to radiotherapy in bone metastatic lesions: A review of the
published data. Int J Mol Sci. 17:13912016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakata E, Sugihara S, Kataoka M, Yamashita
N, Furumatsu T, Takigawa T, Tetsunaga T and Ozaki T: Early response
assessment of re-ossification after palliative conventional
radiotherapy for vertebral bone metastases. J Orthop Sci.
24:332–336. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rief H, Bischof M, Bruckner T, Welzel T,
Askoxylakis V, Rieken S, Lindel K, Combs S and Debus J: The
stability of osseous metastases of the spine in lung cancer-a
retrospective analysis of 338 cases. Radiat Oncol. 8:2002013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Quinn RH, Randall RL, Benevenia J, Berven
SH and Raskin KA: Contemporary management of metastatic bone
disease: Tips and tools of the trade for general practitioners. J
Bone Joint Surg Am. 95:1887–1895. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi DD, Hertan LM, Lam TC, Skamene S, Chi
JH, Groff M, Cho CH, Ferrone ML, Harris M, Chen YH and Balboni TA:
Assessing the utility of the spinal instability neoplastic score
(SINS) to predict fracture after conventional radiation therapy
(RT) for spinal metastases. Pract Radiat Oncol. 8:e285–e294. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen J, Wu C, Hong H, Wang X, Zhang J, Xue
P, Jiang J, Wang D and Cui Z: Simplified Chinese version of the
spinal instability neoplastic score in evaluating patients with
metastatic spinal tumor: A cross-cultural adaptation and
validation. Orthop Surg. 14:1630–1637. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Widmer J, Cornaz F, Scheibler G, Spirig
JM, Snedeker JG and Farshad M: Biomechanical contribution of spinal
structures to stability of the lumbar spine-novel biomechanical
insights. Spine J. 20:1705–1716. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taneichi H, Kaneda K, Takeda N, Abumi K
and Satoh S: Risk factors and probability of vertebral body
collapse in metastases of the thoracic and lumbar spine. Spine
(Phila Pa 1976). 22:239–245. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weber MH, Burch S, Buckley J, Schmidt MH,
Fehlings MG, Vrionis FD and Fisher CG: Instability and impending
instability of the thoracolumbar spine in patients with spinal
metastases: A systematic review. Int J Oncol. 38:5–12.
2011.PubMed/NCBI
|