Introduction
Esophageal cancer (ESC) is ranked sixth in terms of
cancer-associated mortality rates globally and was responsible for
~544,000 deaths in 2020 (1). In
terms of management options, surgery with curative intent remains
the most viable treatment for patients diagnosed with early ESC
(2,3). However, >50% of patients with ESC
are diagnosed at a locally advanced stage when the tumor has become
inoperable, leaving them with limited management options and a poor
prognosis (4,5). Recently, due to the application of
multimodality approaches, neoadjuvant therapy has assumed a
critical role in ESC management (6–11). For
example, neoadjuvant therapy has achieved a reduction in the tumor
burden in patients with unresectable ESC, thereby providing them
with the opportunity to undergo surgery (9). Furthermore, neoadjuvant therapy has
been shown to improve the R0 resection rate, leaving tumor residues
in fewer cases and thereby causing a reduction in the postoperative
recurrence of ESC in patients following surgery (10,11).
Neoadjuvant chemoradiotherapy (nCRT) is a common
type of neoadjuvant therapy, which has been shown to achieve a high
pathological complete response (pCR) rate, as well as longer
disease-free survival (DFS) and higher overall survival (OS) rates,
in patients who received surgery and nCRT compared with those who
underwent surgery alone (12,13).
It is generally recommended that nCRT is completed
within a 6–8-week period prior to surgery for patients with ESC;
however, the optimal time interval between nCRT and surgery remains
controversial, and the time interval that is most beneficial to the
patient requires further investigation (14,15).
For example, several studies have illustrated that a prolonged time
interval is associated with a higher pCR rate in patients with ESC,
whereas other studies have found no association between the time
interval and the pCR rate (15–17).
Similarly, in terms of the survival profile, certain studies
observed that a longer time interval was not associated with
recurrence-free survival (RFS) or OS (15,17),
whereas another study suggested that a prolonged time interval is
indeed associated with a shorter OS in patients with ESC (18).
These inconsistent previous findings are the
rationale for the exploration of the association of the time
interval between nCRT and surgery with the treatment response and
survival profile in patients with ESC in the present study. The aim
of the study is to provide more evidence to support clinicians when
making decisions regarding the optimal time interval between nCRT
and surgery.
Materials and methods
Patients
The present study retrospectively reviewed a total
of 161 patients with resectable ESC who were treated with nCRT
followed by surgical resection at West China Hospital, Sichuan
University (Chengdu, China) between July 2017 and June 2020. The
screening of patients was performed, and patients were included in
the present study if they met the following criteria: i) The
patient was diagnosed with ESC based on gastroscopy and
pathological examinations; ii) the patient was >18 years old;
iii) the patient received nCRT followed by surgical resection in
line with National Comprehensive Cancer Network (NCCN) Clinical
Practice Guidelines for Esophageal and Esophagogastric Junction
Cancers (19); iv) the patient had
at least one measurable tumor, as evaluated by contrast-enhanced
computed tomography (CT) scan according to the Response Evaluation
Criteria in Solid Tumors (RECIST) guidelines (20); and v) clinical data and accessible
follow-up data for study use were available for the patient.
Patients who met the following criteria were excluded from the
study: i) Previously received emergency surgery; ii) had salvage
resection; and iii) were unresectable due to the involvement of the
heart, great vessels, trachea or adjacent organs including the
liver, pancreas, lung and spleen (19). The study was approved by the
Institutional Review Board of West China Hospital, Sichuan
University.
Data collection
The clinical characteristics of the patients were
collected from the database of West China Hospital, Sichuan
University. These characteristics included age, sex, tumor
location, pathological type, tumor size, tumor-node-metastasis
(TNM) stage according to the eighth edition of the American Joint
Committee on Cancer (AJCC) TNM Staging System (21) and nCRT information. In addition, the
time interval between nCRT and surgery were obtained, which was
defined as the time interval from the end of the last dosage of
nCRT to the day of surgery. In addition, follow-up data of the
patients were collected, and the final date of follow-up was
February 24, 2021. The median duration of follow-up was 15.8 months
(range, 0.4-41.4 months).
Treatment procedures
All patients underwent nCRT followed by surgical
resection and none of the patients received conversion surgery. The
nCRT regimens included synchronous chemoradiotherapy and sequential
chemoradiotherapy. The appropriate treatment regimen was selected
for each patient according to the patient's disease condition. The
chemotherapy protocols were platinum-based doublet chemotherapy
regimens, including albumin-bound paclitaxel + carboplatin (AC),
albumin-bound paclitaxel + nedaplatin (AN), albumin-bound
paclitaxel + cisplatin (AP), fluorouracil + cisplatin (FP), taxol +
carboplatin (TC), taxol + nedaplatin (TN) and taxol + cisplatin
(TP). The radiotherapy schedules differed slightly according to the
specific conditions of the patients. In all cases, a planned total
radiation dosage of 40.0-50.4 Gy was administered in 20–28
fractions of 1.8 or 2.0 Gy on 5 days of each week; no radiation was
administered at weekends. The timing of surgery was dependent upon
the performance status and nutrient status of the patient as well
as the availability of operating rooms. The accessibility of
surgery in patients after nCRT was determined in line with NCCN
guidelines (19).
Outcome assessment
A contrast-enhanced CT scan was performed to assess
the clinical response of the patients at the end of the neoadjuvant
treatment, according to the RECIST guidelines (20). The clinical response was classified
as complete response (CR), partial response (PR), stable disease
(SD) and progressive disease (PD). In addition, the pathological
response was evaluated according to the tumor regression grade
(TRG) system and classified as follows: TRG1, 0% residual tumor
cells per tumor bed; TRG2, 1–50% residual tumor cells per tumor
bed; and TRG3, >50% residual tumor cells per tumor bed (22). Patients who were classified as TRG1
were considered to have a pCR. In addition, DFS and OS were
calculated based on the follow-up data; DFS was defined as the time
interval between surgery and disease relapse or death, and OS was
defined as the time interval between surgery and death. Patients
who did not experience DFS or OS events at the time of the final
analysis were censored at their last date of contact.
Statistical analysis
SPSS 22.0 (IBM Corp.) was used to perform the
statistical analysis, and GraphPad Prism 6.1 (GraphPad Software,
Inc.) was used to plot figures. The median time interval between
nCRT and surgery was 66 days (range, 0–196 days), and the patients
were divided into two groups based on the median value: <66 days
(n=76; designated the short time interval group) and ≥66 days
(n=85; designated the prolonged time interval group). Differences
between groups were compared using unpaired Student's t-test,
Wilcoxon rank sum test, χ2 test or Fisher's exact test,
as applicable. Factors associated with pCR and the objective
response rate (ORR) were assessed using univariate and multivariate
logistic regression analyses. DFS and OS were evaluated using
Kaplan-Meier curves and compared using the log-rank test. The
factors associated with DFS and OS were evaluated using univariate
and multivariate Cox proportional hazards regression analyses.
P<0.05 was considered to indicate a statistically
significant result.
Results
Clinical characteristics
The mean age of all recruited patients was 61.0±7.9
years (Table I). There were 131
(81.4%) males and 30 (18.6%) females. In terms of the clinical
tumor (cT) stage, 16 (9.9%), 117 (72.7%) and 28 (17.4%) patients in
the entire cohort were classified with cT2, cT3, and cT4a stage
tumors, respectively. Patients with ESC in the cT4a stage, in which
the tumors were growing into the pericardium, pleura or diaphragm,
were resectable (19). There were
115 (71.4%) and 46 (28.6%) patients who received synchronous nCRT
and sequential nCRT, respectively. Moreover, 18 (11.2%) patients
received one cycle of chemotherapy, whereas the remaining 143
(88.8%) patients received two or more cycles of chemotherapy. The
median radiation dosage was 41.4 Gy (interquartile range, 40.0-45.0
Gy). Statistical analyses revealed that the majority of the
clinical features did not exhibit any differences when compared
between the short and prolonged time interval groups (all
P>0.05), with the exception that the proportion of females was
higher in the prolonged time interval group compared with the short
time interval group (P=0.036). The detailed clinical features of
the patients are shown in Table
I.
 | Table I.Clinical characteristics. |
Table I.
Clinical characteristics.
|
|
| Duration from nCRT
to surgery |
|
|
|---|
|
|
|
|
|
|
|---|
| Items | Patients
(N=161) | <66 days
(n=76) | ≥66 days
(n=85) |
t/χ2/Z-value | P-value |
|---|
| Demographics |
|
|
|
|
|
| Age
(years), mean ± SD | 61.0±7.9 | 61.5±8.4 | 60.6±7.4 | 0.707 | 0.481 |
| Sex, n
(%) |
|
|
| 4.379 | 0.036 |
|
Male | 131 (81.4) | 67 (88.2) | 64 (75.3) |
|
|
|
Female | 30 (18.6) | 9 (11.8) | 21 (24.7) |
|
|
| Disease
characteristics |
|
|
|
|
|
| Tumor
location, n (%) |
|
|
| 1.048 | 0.592 |
|
Upper | 22 (13.7) | 12 (15.8) | 10 (11.8) |
|
|
|
Middle | 108 (67.1) | 48 (63.2) | 60 (70.6) |
|
|
|
Lower | 31 (19.3) | 16 (21.1) | 15 (17.6) |
|
|
|
Pathological type, n (%) |
|
|
| 0.267 | 0.763a |
|
SCC | 152 (94.4) | 71 (93.4) | 81 (95.3) |
|
|
|
ADC | 9 (5.6) | 5 (6.6) | 4 (4.7) |
|
|
| Tumor
size (cm), median (IQR) | 5.0 (4.5-7.0) | 5.0 (4.5-7.0) | 5.0 (4.4-7.0) | −0.243 | 0.808 |
| cT
stage, n (%) |
|
|
| −0.800 | 0.424 |
|
cT2 | 16 (9.9) | 9 (11.8) | 7 (8.2) |
|
|
|
cT3 | 117 (72.7) | 55 (72.4) | 62 (72.9) |
|
|
|
cT4a | 28 (17.4) | 12 (15.8) | 16 (18.8) |
|
|
| cN
stage, n (%) |
|
|
| −0.082 | 0.935 |
|
cN0 | 16 (9.9) | 6 (7.9) | 10 (11.8) |
|
|
|
cN1 | 83 (51.6) | 41 (53.9) | 42 (49.4) |
|
|
|
cN2 | 51 (31.7) | 25 (32.9) | 26 (30.6) |
|
|
|
cN3 | 11 (6.8) | 4 (5.3) | 7 (8.2) |
|
|
| cM
stage, n (%) |
|
|
| 2.265 | 0.221a |
|
cM0 | 159 (98.8) | 74 (97.4) | 85 (100.0) |
|
|
|
cM1 | 2 (1.2) | 2 (2.6) | 0 (0.0) |
|
|
| cTNM
stage, n (%) |
|
|
| −1.078 | 0.281 |
|
II | 22 (13.7) | 11 (14.5) | 11 (12.9) |
|
|
|
III | 100 (62.1) | 50 (65.8) | 50 (58.8) |
|
|
|
IV | 39 (24.2) | 15 (19.7) | 24 (28.2) |
|
|
| Treatment
information |
|
|
|
|
|
| nCRT
sequence, n (%) |
|
|
| 1.318 | 0.251 |
|
Synchronous
nCRT | 115 (71.4) | 51 (67.1) | 64 (75.3) |
|
|
|
Sequential
nCRT | 46 (28.6) | 25 (32.9) | 21 (24.7) |
|
|
|
Chemotherapy cycle, n (%) |
|
|
| 0.064 | 0.801 |
|
<2 cycles | 18 (11.2) | 9 (11.8) | 9 (10.6) |
|
|
|
≥2 cycles | 143 (88.8) | 67 (88.2) | 76 (89.4) |
|
|
|
Chemotherapy regimens, n
(%) |
|
|
| 16.809 | 0.330 |
|
TP | 96 (59.6) | 39 (51.3) | 57 (67.1) |
|
|
|
TN | 14 (8.7) | 6 (7.9) | 8 (9.4) |
|
|
|
TC | 13 (8.1) | 9 (11.8) | 4 (4.7) |
|
|
|
AN | 10 (6.2) | 5 (6.6) | 5 (5.9) |
|
|
|
AC | 7 (4.3) | 3 (3.9) | 4 (4.7) |
|
|
|
FP | 4 (2.5) | 4 (5.3) | 0 (0.0) |
|
|
|
AP | 3 (1.9) | 1 (1.3) | 2 (2.4) |
|
|
|
Others | 14 (8.7) | 9 (11.8) | 5 (5.9) |
|
|
|
Radiation dose (Gy), median
(IQR) | 41.4
(40.0-45.0) | 41.4
(40.0-45.0) | 41.4
(40.0-45.5) | −0.650 | 0.516 |
|
Interruption of radiotherapy,
n (%) |
|
|
| 0.087 | 0.768 |
|
No | 87 (54.0) | 42 (55.3) | 45 (52.9) |
|
|
|
Yes | 74 (46.0) | 34 (44.7) | 40 (47.1) |
|
|
Surgery information
McKeown surgery and Ivor Lewis surgery are two main
surgical approaches for patients with resectable ESC (23,24).
In total, 145 (90.1%), 4 (2.5%) and 12 (7.5%) patients received
McKeown surgery, Ivor Lewis surgery and other surgical approaches,
respectively (Table SI). In
detail, 76 (89.4%), 1 (1.2%) and 8 (9.4%) patients in the prolonged
time interval group received the McKeown surgery, Ivor Lewis
surgery and other surgical approaches, compared with 69 (90.8%), 3
(3.9%) and 4 (5.3%) in the short time interval group, respectively.
In terms of lymphadenectomy, 145 (90.1%), 15 (9.3%) and 1 (0.6%)
patient underwent two-field, three-field and other types,
respectively. There were 77 (90.6%) and 8 (9.4%) patients in the
prolonged time interval group who received two- and three-field
lymphadenectomy, respectively, while 68 (89.5%), 7 (9.2%) and 1
(1.3) patient in the short time interval group received two-field,
three-field and other types of lymphadenectomy, respectively.
Moreover, the R0 resection rate was 93.8% for all total patients,
with rates of 97.6 and 89.5% in the prolonged and short time
interval groups, respectively. Regarding postoperative
complications, 25 (15.5%), 10 (6.2%), 5 (3.1%), 3 (1.9%) and 6
(3.7%) resectable patients in the entire cohort experienced
pulmonary infection, anastomotic leakage, anastomotic stenosis,
incision infection and other complications after surgery. In
detail, 14 (16.5%), 3 (3.5%), 3 (3.5%), 2 (2.4%) and 3 (3.5)
patients in the prolonged time interval group and 11 (14.5%), 7
(9.2%), 2 (2.6%), 1 (1.3%), and 3 (3.9) patients in the short time
interval group experienced pulmonary infection, anastomotic
leakage, anastomotic stenosis, incision infection and other
complications, respectively. No significant difference in surgical
approaches, the degree of lymphadenectomy, R0 resection rate or
postoperative complications was detected between the two groups
(all P>0.05).
Treatment response
In terms of the clinical response, 0 (0.0%), 111
(68.9%), 50 (31.1%) and 0 (0.0%) patients achieved CR, PR, SD and
PD, respectively (Table II). In
detail, a PR and SD were achieved by 63 (74.1%) and 22 (25.9%)
patients in the prolonged time interval group and by 48 (63.2%) and
28 (36.8%) patients in the short time interval group, respectively;
no patients in either group had PD. No difference in clinical
response, ORR or DCR was detected between these two groups (all
P>0.05).
 | Table II.Treatment response. |
Table II.
Treatment response.
|
|
| Duration from nCRT
to surgery |
|
|
|---|
|
|
|
|
|
|
|---|
| Items | Patients
(N=161) | <66 days
(n=76) | ≥66 days
(n=85) |
Z/χ2-value | P-value |
|---|
| Clinical
response |
|
|
|
|
|
| Overall
response, n (%) |
|
|
| 2.251 | 0.134 |
|
CR | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
|
|
PR | 111 (68.9) | 48 (63.2) | 63 (74.1) |
|
|
|
SD | 50 (31.1) | 28 (36.8) | 22 (25.9) |
|
|
|
PD | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
|
| ORR, n
(%) | 111 (68.9) | 48 (63.2) | 63 (74.1) | 2.251 | 0.134 |
| DCR, n
(%) | 161 (100.0) | 76 (100.0) | 85 (100.0) | - | - |
| Pathological
response |
|
|
|
|
|
| TRG, n
(%) |
|
|
| −3.406 | 0.001 |
|
TRG1 | 62 (38.5) | 20 (26.3) | 42 (49.4) |
|
|
|
TRG2 | 78 (48.4) | 41 (53.9) | 37 (43.5) |
|
|
|
TRG3 | 9 (5.6) | 5 (6.6) | 4 (4.7) |
|
|
|
Not assessed | 12 (7.5) | 10 (13.2) | 2 (2.4) |
|
|
| pCR, n (%) | 62 (38.5) | 20 (26.3) | 42 (49.4) | 9.039 | 0.003 |
Regarding the pathological response, there were 62
(38.5%), 78 (48.4%), 9 (5.6%) and 12 (7.5%) patients with TRG1,
TRG2, TRG3 and no assessment data, respectively. In detail, TRG1,
TRG2 and TRG3 pathological responses were achieved by 42 (49.4%),
37 (43.5%) and 4 (4.7%) patients in the prolonged time interval
group compared with 20 (26.3%), 41 (53.9%) and 5 (6.6%) patients in
the short time interval group, respectively. In addition, 2 (2.4%)
patients in the prolonged time interval group and 10 (13.2%)
patients in the short time interval group had no assessment data.
Moreover, 62 (38.5%) patients in the entire cohort achieved a pCR.
Comparison of the two groups revealed that the pCR rate (49.4 vs.
26.3%; P=0.003) and the TRG grade (P=0.001) were higher in the
prolonged time interval group compared with the short time interval
group.
Factors associated with pCR rate
A prolonged time interval between nCRT and surgery
was found to be associated with an improved pCR rate [odds ratio
(OR): 2.735, 95% confidence interval (95%CI): 1.407-5.315, P=0.003]
based on univariate Cox regression analyses, and independently
associated with a higher pCR rate based on a multivariate Cox
regression model (OR: 2.131, 95%CI: 1.028-4.418, P=0.042; Table III). Sex (female vs. male; OR:
5.830, 95% CI: 2.038-16.678, P=0.001) and radiation dose (≥40 vs.
<40 Gy; OR: 10.235, 95% CI: 1.120-3.552, P=0.039) were also
independently associated with an elevated pCR. The factors were
also assessed for association with the ORR as displayed in Table SII, but none of the included
factors could independently predict ORR (all P>0.05; Table SII).
 | Table III.Logistic regression analysis for
pCR. |
Table III.
Logistic regression analysis for
pCR.
|
| Univariate logistic
regression analysis | Multivariate
logistic regression analysis |
|---|
|
|
|
|
|---|
| Items | β-value | OR (95% CI) | P-value | β-value | OR (95% CI) | P-value |
|---|
| Duration from nCRT
to surgery (≥66 vs. <66 days) | 1.006 | 2.735
(1.407-5.315) | 0.003 | 0.757 | 2.131
(1.028-4.418) | 0.042 |
| Age (≥60 vs. <60
years) | −0.474 | 0.622
(0.326-1.189) | 0.151 | −0.492 | 0.611
(0.291-1.283) | 0.193 |
| Sex (female vs.
male) | 1.444 | 4.238
(1.824-9.848) | 0.001 | 1.763 | 5.830
(2.038-16.678) | 0.001 |
| Tumor location |
|
|
|
|
|
|
|
Upper | Reference |
|
| Reference |
|
|
|
Middle | −0.067 | 0.935
(0.407-2.151) | 0.874 | 0.366 | 1.442
(0.507-4.104) | 0.493 |
|
Lower | 0.531 | 1.700
(0.676-4.276) | 0.260 | 0.319 | 1.376
(0.479-3.952) | 0.554 |
| Pathological type
(SCC vs. non-SCC) | 0.825 | 2.283
(0.459-11.360) | 0.313 | 0.911 | 2.487
(0.379-16.301) | 0.342 |
| Tumor size (≥5 vs.
<5 cm) | −0.087 | 0.917
(0.453-1.854) | 0.809 | 0.044 | 1.045
(0.467-2.337) | 0.915 |
| cTNM stage (III–IV
vs. II) | −0.332 | 0.717
(0.290-1.776) | 0.473 | −0.058 | 0.944
(0.301-2.961) | 0.921 |
| nCRT sequence
(sequential nCRT vs. synchronous nCRT) | −0.493 | 0.611
(0.295-1.266) | 0.185 | −0.243 | 0.784
(0.290-2.122) | 0.632 |
| Chemotherapy cycle
(≥2 vs. <2 cycles) | 0.871 | 2.388
(0.748-7.620) | 0.141 | 1.002 | 2.724
(0.587-12.638) | 0.201 |
| Radiation dose (≥40
vs. <40 Gy) | 2.130 | 8.414
(1.066-66.417) | 0.043 | 2.326 | 10.235
(1.120-93.552) | 0.039 |
| Interruption of
radiotherapy (yes vs. no) | 0.053 | 1.055
(0.558-1.993) | 0.870 | 0.076 | 1.079
(0.511-2.278) | 0.843 |
Survival profile
The median duration of follow-up was 15.8 months
(range, 0.4-41.4 months). At the last date of follow-up, the DFS
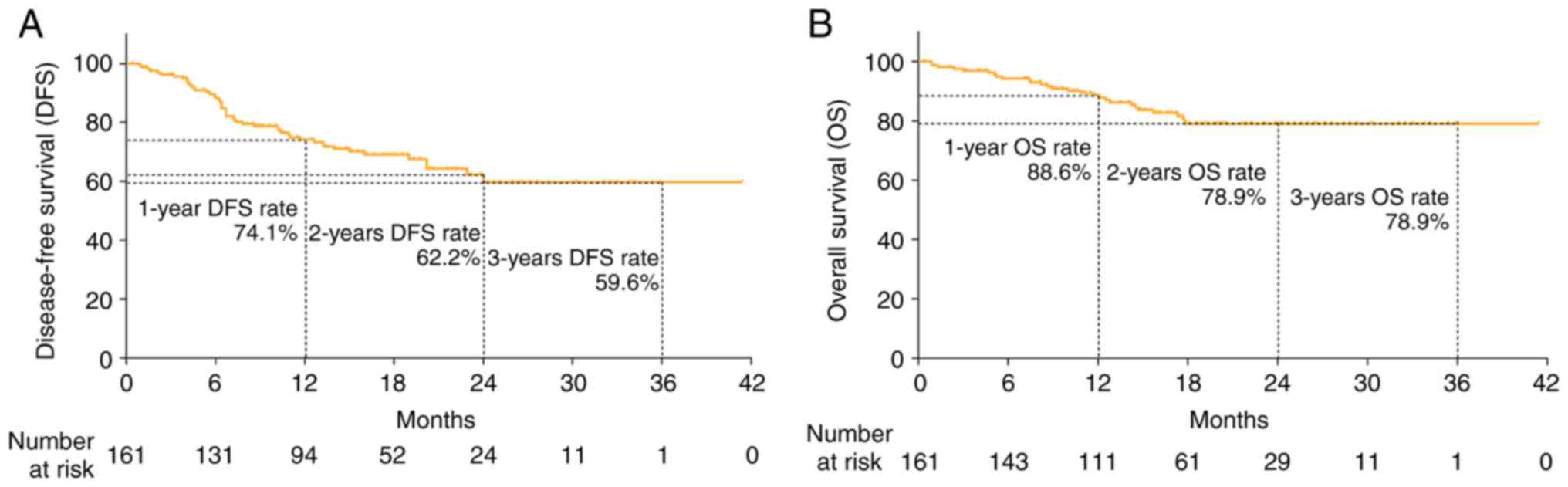
and OS rates had not attained the median value. Fig. 1A shows the DFS rate within 1 year
(74.1%), 2 years (62.2%) and 3 years (59.6%) of treatment and
Fig. 1B shows the OS rate within 1
year (88.6%), 2 years (78.9%) and 3 years (78.9%) of treatment.
During the follow-up period, 27 (16.8%) total deaths
were recorded among which 14 (16.5%) cases occurred in the
prolonged time interval group and 13 (17.1%) cases occurred in the
short time interval group; no difference in death rate was detected
between these two groups (P=0.914; Table SIII). The cause of death was
cancer, pulmonary infection and esophageal-tracheal fistulate in 25
(92.6%), 1 (3.7%) and 1 (3.7%) patients, respectively, and no
significant difference in the cause of death was detected between
the prolonged and short time interval groups (P=0.367).
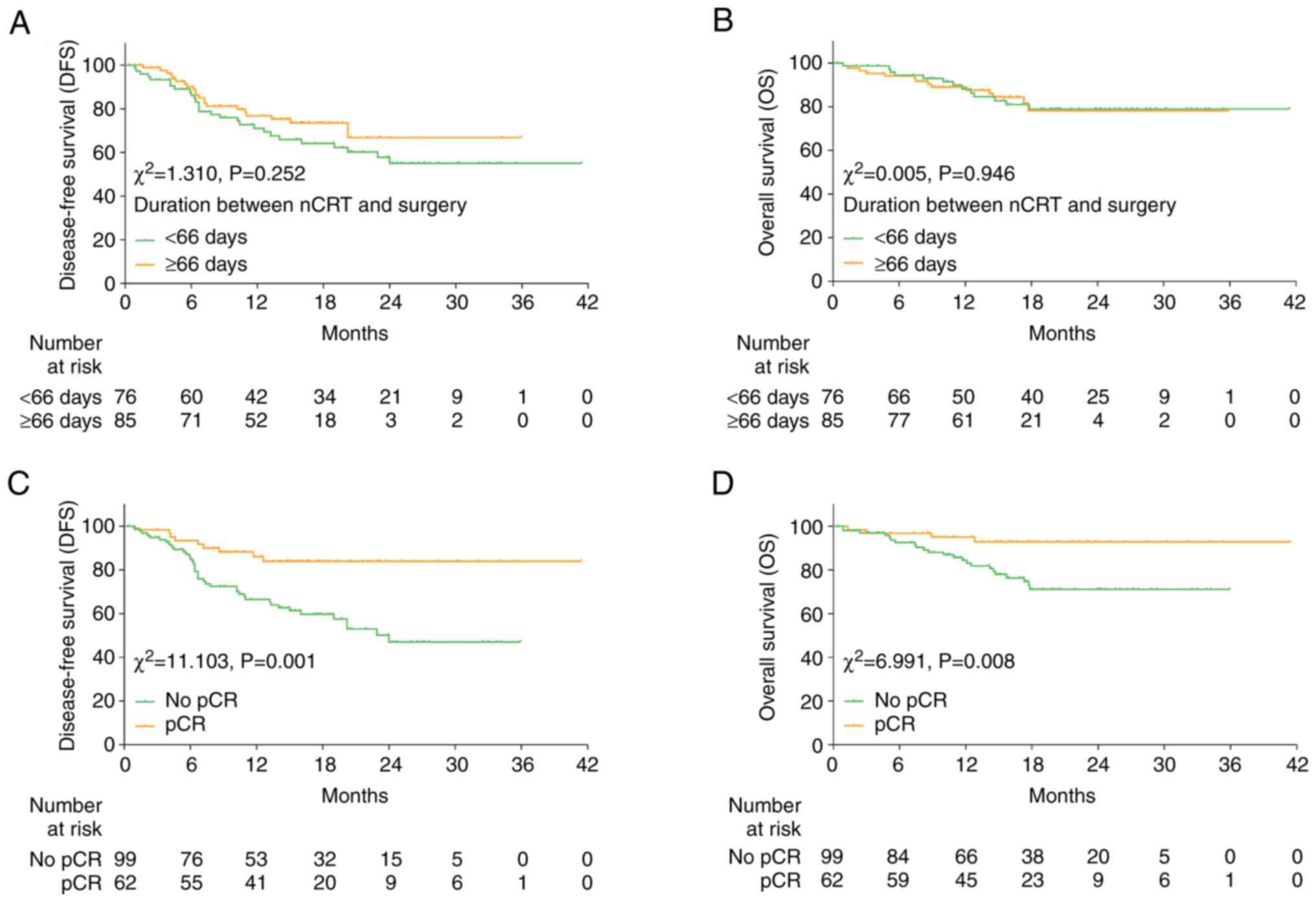
Kaplan-Meier analyses revealed that DFS (P=0.252)
and OS (P=0.946) did not differ between the prolonged and short
time interval duration groups (Fig. 2A
and B). However, DFS (P=0.001; Fig.
2C) and OS (P=0.008; Fig. 2D)
were both found to be significantly longer in patients who achieved
pCR compared with patients who did not achieve pCR. Further
subgroup analyses stratified patients based on TRG and the results
demonstrated that neither DFS nor OS differed between the prolonged
and short time interval group in patients with TRG1 (both
P>0.05; Fig. S1A and B); in
patients with TRG2 (both P>0.05; Fig. S1C and D) or in patients with TRG3
(both P>0.05; Fig. S1E and
F).
Factors associated with DFS and
OS
Univariate and multivariate Cox regression analyses
revealed that the time interval between the completion of nCRT and
surgery failed to enable DFS (Table
IV) or OS (Table V) to be
estimated (all P>0.05). Furthermore, only pCR achievement [yes
vs. no; hazard ratio (HR): 0.295, 95%CI: 0.135-0.641, P=0.002]
served as an independent factor for prolonged DFS. In addition, pCR
(yes vs. no; HR: 0.228, 95%CI: 0.070-0.748, P=0.015) was
independently associated with longer OS. TRG grade was not included
in the regression analyses because it is a well-known confounding
factor associated with other tumor features, including cT stage and
lymphovascular invasion (25–27);
therefore, TRG grade served as a compounding factor in the
regression model.
 | Table IV.Cox proportional hazards regression
analysis for disease-free survival. |
Table IV.
Cox proportional hazards regression
analysis for disease-free survival.
|
| Univariate Cox
regression analysis | Multivariate Cox
regression analysis |
|---|
|
|
|
|
|---|
| Items | β-value | HR (95% CI) | P-value | β-value | HR (95% CI) | P-value |
|---|
| Duration from nCRT
to surgery (≥66 vs. <66 days) | −0.334 | 0.716
(0.403-1.272) | 0.255 | −0.157 | 0.855
(0.471-1.552) | 0.607 |
| Age (≥60 vs. <60
years) | −0.162 | 0.850
(0.483-1.498) | 0.574 | −0.307 | 0.735
(0.411-1.316) | 0.301 |
| Sex (female vs.
male) | −0.280 | 0.756
(0.339-1.683) | 0.493 | 0.007 | 1.007
(0.410-2.474) | 0.987 |
| Tumor location |
|
|
|
|
|
|
|
Upper | Reference |
|
| Reference |
|
|
|
Middle | −0.233 | 0.792
(0.366-1.716) | 0.555 | −0.346 | 0.707
(0.270-1.855) | 0.481 |
|
Lower | 0.304 | 1.355
(0.625-2.936) | 0.442 | 0.428 | 1.533
(0.656-3.587) | 0.324 |
| Pathological type
(SCC vs. non-SCC) | −0.214 | 0.807
(0.250-2.601) | 0.720 | −0.252 | 0.778
(0.179-3.370) | 0.737 |
| Tumor size (≥5 vs.
<5 cm) | 0.153 | 1.165
(0.607-2.236) | 0.647 | 0.186 | 1.205
(0.610-2.379) | 0.592 |
| cTNM stage (III–IV
vs. II) | 0.658 | 1.931
(0.694-5.375) | 0.208 | 0.533 | 1.704
(0.576-5.042) | 0.336 |
| nCRT sequence
(sequential nCRT vs. synchronous nCRT) | 0.106 | 1.112
(0.606-2.042) | 0.732 | 0.053 | 1.054
(0.494-2.248) | 0.891 |
| Chemotherapy cycle
(≥2 vs. <2 cycles) | −0.058 | 0.944
(0.401-2.218) | 0.894 | 0.162 | 1.176
(0.415-3.330) | 0.760 |
| Radiation dose (≥40
vs. <40 Gy) | 0.153 | 1.165
(0.417-3.254) | 0.770 | 0.455 | 1.576
(0.543-4.578) | 0.403 |
| Interruption of
radiotherapy (yes vs. no) | −0.158 | 0.854
(0.486-1.501) | 0.584 | −0.112 | 0.894
(0.492-1.623) | 0.712 |
| pCR (yes vs.
no) | −1.163 | 0.313
(0.152-0.645) | 0.002 | −1.222 | 0.295
(0.135-0.641) | 0.002 |
 | Table V.Cox proportional hazards regression
analysis for overall survival. |
Table V.
Cox proportional hazards regression
analysis for overall survival.
|
| Univariable Cox
regression analysis | Multivariable Cox
regression analysis |
|---|
|
|
|
|
|---|
| Items | β-value | HR (95% CI) | P-value | β-value | HR (95% CI) | P-value |
|---|
| Duration from nCRT
to surgery (≥66 vs. <66 days) | 0.026 | 1.027
(0.481-2.192) | 0.946 | 0.226 | 1.254
(0.562-2.795) | 0.581 |
| Age (≥60 vs. <60
years) | −0.071 | 0.931
(0.432-2.007) | 0.856 | −0.564 | 0.569
(0.217-1.489) | 0.251 |
| Sex (female vs.
male) | 0.079 | 1.082
(0.409-2.862) | 0.873 | 0.454 | 1.575
(0.497-4.993) | 0.440 |
| Tumor location |
|
|
|
|
|
|
|
Upper | Reference |
|
| Reference |
|
|
|
Middle | −0.118 | 0.889
(0.330-2.395) | 0.816 | −0.697 | 0.498
(0.110-2.265) | 0.367 |
|
Lower | 0.257 | 1.293
(0.436-3.832) | 0.643 | 0.368 | 1.445
(0.450-4.644) | 0.536 |
| Pathological type
(SCC vs. non-SCC) | −0.758 | 0.469
(0.141-1.557) | 0.216 | −1.384 | 0.251
(0.038-1.643) | 0.149 |
| Tumor size (≥5 vs.
<5 cm) | 0.451 | 1.569
(0.594-4.147) | 0.364 | 0.525 | 1.690
(0.626-4.561) | 0.300 |
| cTNM stage (III–IV
vs. II) | 0.231 | 1.260
(0.379-4.187) | 0.706 | −0.174 | 0.840
(0.227-3.116) | 0.795 |
| nCRT sequence
(sequential nCRT vs. synchronous nCRT) | 0.078 | 1.081
(0.473-2.470) | 0.853 | −0.087 | 0.916
(0.291-2.890) | 0.882 |
| Chemotherapy cycle
(≥2 vs. <2 cycles) | −0.536 | 0.585
(0.222-1.545) | 0.279 | −0.582 | 0.559
(0.145-2.153) | 0.398 |
| Radiation dose (≥40
vs. <40 Gy) | 0.900 | 2.460
(0.333-18.145) | 0.377 | 1.156 | 3.179
(0.409-24.714) | 0.269 |
| Interruption of
radiotherapy (yes vs. no) | 0.023 | 1.023
(0.481-2.178) | 0.953 | 0.064 | 1.066
(0.468-2.426) | 0.879 |
| pCR (yes vs.
no) | −1.332 | 0.264
(0.091-0.763) | 0.014 | −1.478 | 0.228
(0.070-0.748) | 0.015 |
Discussion
The effect of the time interval between nCRT and
surgery on the pCR rate of patients with ESC is unclear. For
example, a time interval of >13 weeks was found to be associated
with an increased likelihood of a prolonged pCR rate in patients
with ESC or gastroesophageal junction cancer (GEJC) in one study
(16). Interestingly, another study
divided the time interval between nCRT and surgery into five
different quantiles, specifically 15–37, 38–45, 46–53, 54–64 and
65–90 days, and discovered that the time interval was positively
correlated with the pCR rate of patients with ESC (17). However, a different study observed
no association of a prolonged time interval of 7–8 weeks with
improved pCR rates in patients with ESC and GEJC (15). Comparing these studies reveals that
the findings were inconsistent, and the time intervals being
investigated were also quite different. To identify the optimal
time interval, the median value of the time interval between nCRT
and surgery was found to be 66 days in the present study.
Subsequently, a series of analyses were conducted to compare the
response profiles of patients with ESC with time intervals of ≥66
and <66 days. Based on these analyses, it was observed that a
prolonged time interval was associated with a higher pCR rate in
patients with ESC; furthermore, the time interval was independently
associated with an elevated pCR rate in patients with ESC. These
findings are in line with those of previous studies (15,17).
We hypothesize that there may be a delay, or lag phase, after the
receipt of nCRT before the patients with ESC benefit fully from the
antitumor effects of the radiotherapy, and therefore an extended
period is necessary, which accounts for a longer time interval
being associated with improved pCR rates in patients with ESC.
Aside from the pCR rate, the effect of the time
interval between nCRT and surgery on the survival profile of
patients with ESC is also of great interest. However, the findings
from previous studies in this regard are also inconsistent. For
example, one study reported that neither RFS nor OS differed among
patients with esophageal squamous cell carcinoma cancer according
to whether they experienced shorter or longer time intervals
between nCRT and surgery (28).
Furthermore, patients with ESC or GEJC with a prolonged time
interval of ≥50 days achieved a similar OS to those patients with
time interval of <50 days (15).
By contrast, another study suggested that a time interval >100
days was associated with reduced OS in patients with esophageal
adenocarcinoma (18). In the
present study, it was observed that in patients with ESC there was
no association between the time interval from nCRT to surgery and
the survival profile, whereas DFS and OS were prolonged in patients
with ESC who reached pCR compared with those who did not. These
findings are also in line with previous studies (15,28).
The possible explanations for these observations are as follows: i)
The follow-up period was relatively short in the present study,
since neither DFS nor OS reached the median follow-up date, and few
cases of disease relapse or mortality occurred in patients with
ESC, which led to low statistical power and no significant
differences in DFS or OS for patients with ESC between the
prolonged or short time interval groups; and ii) numerous factors
are capable of affecting the survival of patients with ESC,
including the heterogeneous properties of ESC, postoperative
surveillance and management, and consequently, the time interval
may have little effect on ESC survival (29–31).
The present study also identified that sex (female
vs. male) and radiation dose (≥40 vs. <40 Gy) were independently
associated with improved pCR rates in patients with ESC. These
findings regarding sex and radiation dose may be explained as
follows: i) Women are subjected to lower levels of androgenic
hormones such as testosterone, dihydrotestosterone and
androstenedione, compared with men, which may afford them some
protection from carcinogenesis mediated via downstream androgen
receptor signaling, thereby leading to improved pCR rates in female
patients with ESC (32); and ii)
high radiation doses may exert stronger antitumor effects compared
with low radiation doses by directly causing genetic damage in
cancer cells and indirectly promoting the immune response in the
local tumor microenvironment, thereby leading to improved pCR rates
in patients with ESC (33).
Moreover, achieving a pCR served as an independent factor for
longer DFS and OS in patients with ESC, since this indicated that
no residual tumor cells remained in the patient following the
surgical removal of the tumor, which would be favorable to the
survival of the patient.
Although it is recommended that the surgery is
performed 6–8 weeks after the completion of nCRT in patients with
resectable ESC, the time interval between nCRT and surgery differs
in the existing literature. Several studies observed a prolonged
time interval of >8 weeks between nCRT and surgery for patients
with resectable ESC, the reasons for which may be older age, more
morbidities, advanced cancer stage or overloaded surgical schedules
(16,17,34).
In the present study, the median time interval between nCRT and
surgery was 66 days in patients with resectable ESC, which is
longer than recommended. One possible reason for this is that
patients with resectable ESC may require an extended period of time
to recover from nCRT due to poor performance status, nutrient
status or chronic comorbidities, which is in line with previous
studies (16,17).
The present study applied contrast-enhanced CT
rather than positron emission tomography (PET)/CT for the
assessment of the treatment response in patients with resectable
ESC. The reasons for this were as follows: i) Contrast-enhanced CT
for the initial workup exhibited more sensitivity and a
well-differentiated cT stage compared with PET/CT for patients with
T1-T3 tumors (35); ii) PET/CT was
unnecessary for patients with the absence of distant metastasis;
and iii) the cost of PET/CT is not covered by health insurance in
China, so patients are required to pay for this expensive
examination fees themselves. Moreover, PET/CT usually requires
multiple assessments during the whole perioperative period, which
may be a considerable financial burden on patients.
The present study only enrolled patients with
resectable ESC; therefore, preoperative chemoradiotherapy was
applied in a neoadjuvant setting. Patients with unresectable ESC
may be offered curative surgery following treatment with definitive
chemotherapy or chemoradiotherapy preoperatively, which is known as
conversion surgery. However, none of the patients included in the
current study received conversion surgery. The reason for this was
that conversion surgery is only available for a small proportion of
patients, and most patients with unresectable ESC experienced rapid
progression and distant metastasis. Therefore, the current study
did not include any patients with conversion surgery, but this
could be addressed in future studies.
The present study has certain limitations. First,
the follow-up period was relatively short since most patients were
not local residents and some of them transferred to local hospitals
after discharge, which increased the difficulty of regular
follow-up for these patients. Therefore, further studies with a
longer follow-up period are required to address this issue.
Moreover, further studies could explore the relationship between
time interval and the change in the standardized uptake value
obtained using PET/CT in resectable ESC patients. Also, a larger
sample size is necessary to enable more subgroup analyses to be
conducted, with the aim of identifying the optimal time interval
for patients with ESC with different causes of death.
In conclusion, the present study shows that a
prolonged time interval between nCRT and surgery is associated with
a higher pCR rate, although it is not possible to estimate the DFS
or OS in patients with ESC from the time interval. Based on these
findings, the optimal time interval for balancing the benefit of
pathological response with prognosis remains uncertain. This may
serve as an interesting topic for clinicians and prompt them to
perform more research into this topic.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YML designed and supervised the study. JQL and XXZ
conceived the study. XJZ, YX and ZYD participated in the
experiments and data collection. YH, YY and LQC performed the data
analysis, and wrote the manuscript. JW and YL contributed to the
analysis of the results and revised the manuscript. YML and JQL
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of West China Hospital, Sichuan University (Chengdu,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang FL and Yu SJ: Esophageal cancer:
Risk factors, genetic association, and treatment. Asian J Surg.
41:210–215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vaghjiani RG and Molena D: Surgical
management of esophageal cancer. Chin Clin Oncol. 6:472017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou N, Rajaram R and Hofstetter WL:
Management of locally advanced esophageal cancer. Surg Oncol Clin N
Am. 29:631–646. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Imazeki H and Kato K: Development of
chemotherapeutics for unresectable advanced esophageal cancer.
Expert Rev Anticancer Ther. 20:1083–1092. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kelly RJ: Emerging multimodality
approaches to treat localized esophageal cancer. J Natl Compr Canc
Netw. 17:1009–1014. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Borggreve AS, Kingma BF, Domrachev SA,
Koshkin MA, Ruurda JP, van Hillegersberg R, Takeda FR and Goense L:
Surgical treatment of esophageal cancer in the era of multimodality
management. Ann N Y Acad Sci. 1434:192–209. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kakeji Y, Oshikiri T, Takiguchi G, Kanaji
S, Matsuda T, Nakamura T and snf Suzuki S: Multimodality approaches
to control esophageal cancer: Development of chemoradiotherapy,
chemotherapy, and immunotherapy. Esophagus. 18:25–32. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morimoto H, Fujiwara Y, Lee S, Amano K,
Hosono M, Miki Y and Osugi H: Treatment results of neoadjuvant
chemoradiotherapy followed by radical esophagectomy in patients
with initially inoperable thoracic esophageal cancer. Jpn J Radiol.
36:23–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eyck BM, van der Wilk BJ, Lagarde SM,
Wijnhoven BPL, Valkema R, Spaander MCW, Nuyttens JJME, van der
Gaast A and van Lanschot JJB: Neoadjuvant chemoradiotherapy for
resectable oesophageal cancer. Best Pract Res Clin Gastroenterol.
36–37. 37–44. 2018.PubMed/NCBI
|
|
11
|
Faiz Z, Kats-Ugurlu G, Mul VEM, Karrenbeld
A, Burgerhof HGM, Plukker JTM and Muijs CT: Locoregional residual
esophageal cancer after neo-adjuvant chemoradiotherapy and surgery
regarding anatomic site and radiation target fields: A
histopathologic evaluation study. Ann Surg. 275:e759–e765. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sasaki K, Uchikado Y, Omoto I, Arigami T,
Osako Y, Noda M, Okumura H, Maemura K, Higashi R, Yoshiura T and
Natsugoe S: Neoadjuvant chemoradiotherapy with docetaxel,
cisplatin, and 5-fluorouracil (DCF-RT) for locally advanced
esophageal squamous cell carcinoma. Cancer Chemother Pharmacol.
83:581–587. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu
Z, Mao W, Xiang J, Han Y, Chen Z, et al: Neoadjuvant
chemoradiotherapy followed by surgery versus surgery alone for
locally advanced squamous cell carcinoma of the esophagus
(NEOCRTEC5010): A phase III multicenter, randomized, open-label
clinical trial. J Clin Oncol. 36:2796–2803. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin Q, Xu H, Liu J, Zhang C, Xu L, Di X,
Zhang X and Sun X: Does timing of esophagectomy following
neoadjuvant chemoradiation affect outcomes? A meta-analysis. Int J
Surg. 59:11–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Klevebro F, Nilsson K, Lindblad M, Ekman
S, Johansson J, Lundell L, Ndegwa N, Hedberg J and Nilsson M:
Association between time interval from neoadjuvant
chemoradiotherapy to surgery and complete histological tumor
response in esophageal and gastroesophageal junction cancer: A
national cohort study. Dis Esophagus. 33:doz0782020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van der Werf LR, Dikken JL, van der Willik
EM, van Berge Henegouwen MI and Nieuwenhuijzen GAP: Time interval
between neoadjuvant chemoradiotherapy and surgery for oesophageal
or junctional cancer: A nationwide study. Eur J Cancer. 91:76–85.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Azab B, Amundson JR, Picado O, Ripat C,
Macedo FI, Franceschi D, Livingstone AS and Yakoub D: Impact of
chemoradiation-to-surgery interval on pathological complete
response and short- and long-term overall survival in esophageal
cancer patients. Ann Surg Oncol. 26:861–868. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raman V, Jawitz OK, Voigt SL, Yang CJ,
Wang H, Harpole DH and D'Amico TA: Effect of time to surgery on
outcomes in stage I esophageal adenocarcinoma. J Thorac Cardiovasc
Surg. 159:1626–1635.e1. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, et
al: Esophageal and esophagogastric junction cancers, version
2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr
Canc Netw. 17:855–883. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Al-Batran SE, Hofheinz RD, Pauligk C, Kopp
HG, Haag GM, Luley KB, Meiler J, Homann N, Lorenzen S, Schmalenberg
H, et al: Histopathological regression after neoadjuvant docetaxel,
oxaliplatin, fluorouracil, and leucovorin versus epirubicin,
cisplatin, and fluorouracil or capecitabine in patients with
resectable gastric or gastro-oesophageal junction adenocarcinoma
(FLOT4-AIO): Results from the phase 2 part of a multicentre,
open-label, randomised phase 2/3 trial. Lancet Oncol. 17:1697–1708.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D'Amico TA: Mckeown esophagogastrectomy. J
Thorac Dis. 6 (Suppl 3):S322–S324. 2014.PubMed/NCBI
|
|
24
|
Jung MK, Schmidt T, Chon SH, Chevallay M,
Berlth F, Akiyama J, Gutschow CA and Mönig SP: Current surgical
treatment standards for esophageal and esophagogastric junction
cancer. Ann N Y Acad Sci. 1482:77–84. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ryu HS, Lee JL, Kim CW, Yoon YS, Park IJ,
Lim SB, Yu CS, Kim JH and Kim JC: Correlative significance of tumor
regression grade and ypT category in patients undergoing
preoperative chemoradiotherapy for locally advanced rectal cancer.
Clin Colorectal Cancer. 21:212–219. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee HS, Choi DH, Park HC, Park W, Yu JI
and Chung K: Correlation between tumor regression grade and rectal
volume in neoadjuvant concurrent chemoradiotherapy for rectal
cancer. Radiat Oncol J. 34:186–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chao YK, Chang CB, Chuang WY, Wen YW,
Chang HK, Tseng CK, Yeh CJ and Liu YH: Correlation between tumor
regression grade and clinicopathological parameters in patients
with squamous cell carcinoma of the esophagus Who received
neoadjuvant chemoradiotherapy. Medicine (Baltimore). 94:e14072015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Furukawa T, Hamai Y, Hihara J, Emi M,
Yamakita I, Ibuki Y, Kurokawa T and Okada M: Impact of interval
between neoadjuvant chemoradiation and surgery upon morbidity and
survival of patients with squamous cell carcinoma of thoracic
esophagus. Anticancer Res. 38:5239–5245. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen K, Yu S, Zhang Z, Zhang Y, Li W, Kang
M and Lin Y: Investigation and analysis of influencing factors of
early activity compliance of patients after minimally invasive
esophagectomy. Ann Palliat Med. 10:12657–12663. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie SH, Santoni G, Malberg K, Lagergren P
and Lagergren J: Prediction model of long-term survival after
esophageal cancer surgery. Ann Surg. 273:933–939. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Turgeman I and Ben-Aharon I: Evolving
treatment paradigms in esophageal cancer. Ann Transl Med.
9:9032021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sukocheva OA, Li B, Due SL, Hussey DJ and
Watson DI: Androgens and esophageal cancer: What do we know? World
J Gastroenterol. 21:6146–6156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jarosz-Biej M, Smolarczyk R, Cichoń T and
Kułach N: Tumor microenvironment as a ‘game changer’ in cancer
radiotherapy. Int J Mol Sci. 20:32122019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haisley KR, Laird AE, Nabavizadeh N,
Gatter KM, Holland JM, Vaccaro GM, Thomas CR Jr, Schipper PH,
Hunter JG and Dolan JP: Association of intervals between
neoadjuvant chemoradiation and surgical resection with pathologic
complete response and survival in patients with esophageal cancer.
JAMA Surg. 151:e1627432016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tirumani H, Rosenthal MH, Tirumani SH,
Shinagare AB, Krajewski KM and Ramaiya NH: Esophageal carcinoma:
Current concepts in the role of imaging in staging and management.
Can Assoc Radiol J. 66:130–139. 2015. View Article : Google Scholar : PubMed/NCBI
|