In conclusion, cardiac SUVmax data
from patients with new-onset rectal cancer and distant metastasis
suggested that increased glucose uptake by tumors may reduce
glucose uptake by the heart. Cardiac SUVmax may also be related to
the occurrence of distant metastases and prognosis.
Introduction
Rectal cancer and colon cancer refer to malignant
tumors of the large intestine, which are usually treated with
surgery if found early, or with chemotherapy and surgery at later
stages (1). Thus, accurate staging
is essential in rectal cancer and colon cancer. Endoscopy is used
for staging rectal cancer and colon cancer, and computed tomography
(CT) is used for whole-body screening. Magnetic resonance imaging
(MRI) may be used to evaluate local progression, for tumor staging
and to evaluate lymph node metastasis in rectal cancer (2).
Positron emission tomography (PET)-CT is sometimes
used for whole-body screening in cases of rectal cancer and colon
cancer, and it allows PET images and CT images to be combined.
PET-CT uses 18F-fluorodeoxyglucose (18F-FDG), a radiolabeled
glucose analog, as a radiopharmaceutical. Compared with CT and MRI,
PET-CT sometimes detects additional lesions when used in the
diagnosis of new-onset rectal cancer (3). In addition, PET-CT can improve staging
in cases of lower rectal cancer (4), and has a reported sensitivity of 93.7%
in the detection of distant metastasis (5). When PET is performed before
chemoradiation therapy for rectal cancer, the response to
chemoradiation and prognosis can be predicted based on the 18F-FDG
uptake (6). Pathological remission,
in particular, can be predicted based on the 18F-FDG uptake
(7,8).
Numerous studies have shown the use of PET-CT in
rectal cancer. However, 18F-FDG is also taken up by non-tumor
tissue, including the physiological uptake of 18F-FDG by the brain
and heart. Multiple glucose transporters are present in the body,
including GLUT1, GLUT2, GLUT3, GLUT4 and GLUT5; GLUT1 transports
glucose into the tumor tissue and GLUT4 transports glucose into the
myocardial tissue (9).
Although the heart can use both glucose and fatty
acids as an energy, it mainly uses fatty acids due to the higher
amount of ATP produced; fatty acid uptake in the heart can be
detected using 123I-β-methyl-P-iodophenyl-pentadecanoic acid
(123I-BMIPP) cardiac scintigraphy. Notably, ischemia and other
events cause a rapid decline in fatty acid uptake in the heart,
which is difficult to recover from; therefore, 123I-BMIPP
scintigraphy also reveals reduced uptake at sites of ischemia. The
heart can shift to the glycolytic system and glucose metabolism
when it becomes ischemic (10). It
has been reported that 18F-FDG accumulates in the heart in cases of
unstable angina, and this uptake indicates ischemia (11). Thus, the heart and tumors may be in
competition for the metabolism of glucose. Nevertheless, to the
best of our knowledge, studies have found no association between
cardiac uptake of 18F-FDG and glucose, fatty acids, body weight or
other factors (12–14). At present, the factors affecting
cardiac 18F-FDG uptake in patients with malignant tumors remain
unknown. The glycolytic system is enhanced due to the Warburg
effect in cancer cells, and glucose metabolism is increased
(15). It may be hypothesized that
the more advanced the cancer, the less glucose can be supplied to
the heart (15). A previous study
treated patients with rectal cancer with chemoradiotherapy, and
reported a higher likelihood of metastasis and a poorer prognosis
in patients with elevated levels of GLUT1 expression following
treatment (16). From this
molecular biology research and 18F-FDG PET imaging, it was
hypothesized that cardiac 18F-FDG uptake may vary depending on the
extent of rectal cancer and colon cancer, and that this change in
uptake may affect prognosis and other factors. The present study
aimed to investigate the factors that affect the degree of cardiac
18F-FDG uptake, and the effect of this uptake on the pathology and
prognosis in patients with rectal cancer and colon cancer.
Patients and methods
Study design and participants
The present study is a single-center, case-control,
retrospective study. The study retrospectively enrolled patients
diagnosed with new-onset rectal cancer and new-onset colon cancer
(ascending, transverse, descending, sigmoid cancer) at the Iga City
General Hospital (Iga, Japan) between January 1, 2013 and March 31,
2018, who underwent an 18F-FDG PET scan for pretreatment staging.
The patients were selected using the reporting terminal system
(PSP, Japan Tokyo) and patient data were checked using the
electronic medical records. Rectal cancer and colon cancer were
diagnosed based on imaging, endoscopic and pathological findings,
and clinical course.
For the present study, it was considered that the
rectum is under the promontory of the sacrum and above the anal
canal. From this definition, rectal cancer was defined as: i)
Adenocarcinoma (pathologically diagnosed) and ii) occurring at
recto sigmoid, above peritoneal reflection and below peritoneal
reflection. Moreover, in the present study, colon cancer was
defined as: i) Adenocarcinoma (pathologically diagnosed) and ii)
occurring at the ileocecal, ascending, transverse, descending and
sigmoid colon but not the rectum. Medical imaging, endoscopic and
pathological findings, and the clinical information obtained from
the medical records (clinical diagnosis and medical reports) were
referred to for this definition.
The inclusion criteria for the present study were as
follows: i) Patients had new-onset rectal cancer and colon cancer
(pathologically diagnosed and matched clinical course) and ii)
patients underwent PET-CT for initial staging prior to therapy. The
exclusion criteria were as follows: i) Patients also diagnosed with
other types of new onset cancer (with untreated new onset other
types of cancer) and ii) patients undergoing treatment for other
types of cancer.
PET-CT evaluation
PET-CT was performed using a Discovery ST Elite
equipped with an 8-row or 16-row multi-slice CT (GE Healthcare).
The patients scheduled to undergo PET-CT fasted for 5–6 h, were
administered 18F-FDG based on their body weight, and then remained
at rest for 1 h before PET-CT imaging. PET images, CT images and
fusion images were prepared from the PET-CT scan. PETCT images were
visually assessed by a radiologist (author Yukinori Okada) with
experience the PET research (17),
a boardcertified radiologist and radiation oncologist (Japan
Radiological Society and Japanese Society for Radiation Oncology)
and a boardcertified nuclear medicine specialist (Japan Society of
Nuclear Medicine).
The images were viewed simultaneously by a
radiologist and a gastroenterologist, who visually assessed the
extent of the lesions and cardiac uptake. The radiologist defined
the regions of interest (ROIs) for the heart and the primary
lesion, and measured the maximum standard uptake value (SUVmax) on
the reading monitor. The ROI was set over the whole heart, and
included the left ventricle and the SUVmax was calculated for each
slice including the heart. The maximum was used as the cardiac
SUVmax. The 18F-FDG uptake value at the aorta inducing the
arteriosclerosis was not selected.
The presence or absence of lymph node metastasis and
distant metastasis was assessed visually by the radiologist
compared with CT images obtained in parallel, recent CT and MRI
images, and the clinical course. The radiologist defined the ROIs
on the reading monitor for the principal lesion, lymph node
metastases and distant metastases, measured and summed these areas,
and used them to calculate the tumor volumes. The iliopsoas muscle
area at the L3 level was also measured on the CT images obtained by
PET-CT.
Echocardiography
Left atrial diameter (LAD) (mm), left ventricular
end-diastolic diameter (LVDd) (mm), left ventricular end-systolic
diameter (LVDs) (mm), ejection fraction (EF) (%), E/e and E/A were
obtained from the echocardiography (iE33, Philips Healthcare)
performed at the same time as the PET-CT.
Carcinoembryonic antigen (CEA)
detection
The CEA levels were detected from a blood (200 µl of
serum) by the hospital performed simultaneously as PET-CT.
Evaluation of survival
Survival was evaluated starting from the day of the
PET-CT scan up until the final observation day on September 30,
2021. For dropouts during this period, the final observation day
was taken as the date of the last visit.
Statistical analysis
Statistical analysis was performed using EZR
software developed by the Jichi Medical University Saitama Medical
Center (Saitama, Japan) (18).
Comparisons between the two groups were made using the Mann-Whitney
U-test or Fisher's exact test. The correlation between two
variables was evaluated using the Spearman's rank correlation
coefficient. A logistic model was used for single variate analysis
and multi variate analysis. A receiver operating characteristic
(ROC) analysis was used to analyze the cutoff values. The survival
data were analyzed using the Kaplan-Meier method and log-rank test,
or Cox proportional hazards model. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patients
A total of 50 patients were selected in the present
study; 26 patients with new-onset rectal cancer and 24 patients
with new-onset colon cancer.
Rectal cancer
Participants
A total of 26 patients (14 men and 12 women) aged
72.0±10 years were enrolled in the present study. Of the 26
patients, one had previously had a myocardial infarction and been
diagnosed with gastric cancer >20 years ago. The
histopathological results of these 26 patients confirmed that they
all had tubular adenocarcinoma (one patient had combined cancer
(main tubular adenocarcinoma and partly mucinous
adenocarcinoma).
Three patients had stage I rectal cancer, eight
patients had stage II, five patients had stage III and 10 had stage
IV. One patient with stage III rectal cancer had multiple small
lung metastases that increased in size during the course of
observation; hence, the patient was characterized as having distant
metastasis. A total of 15 patients had no distant metastasis and 11
patients had distant metastasis (lung, bone, liver and peritoneal
dissemination).
The 18F-FDG dose was 231.3±44.1 MBq and the blood
glucose level was 103.1±23.7 mg/dl. The SUVmax of the primary
lesion was 18.9±8.8 and the cardiac SUVmax was 4.3±4.1. The SUVmax
values of the heart were detected at left ventricle. The tumor
volume on PET images (sum of primary lesion+lymph nodes + distant
metastases) was 74,990.1±148,317.1 cm2 (range
704–639,740 cm2), The tumor volume on PET images (only
primary lesion) was 47,616.0±93,659.0 cm2 (range
704–455,162 cm2). The tumor volume on PET images (only
lymph nodes) was 1,547.2±3,091.0 cm2 (range 0–11,331
cm2). The tumor volume on PET images (only distant
metastases) was 25,825.5±111,639.0 cm2 (range 0–56,9012
cm2). The iliopsoas muscle area at the L3 level was
1,090.9±455.6 cm2.
Echocardiography was performed on 24 patients,
giving a LAD (mm) of 37.4±6.1, LVDd (mm) of 44.4±5.0, LVDs (mm) of
30.1±4.6, EF (%) of 65.6±7.6, E/e of 8.3±3.0 and E/A of 1.2±1.8.
CEA was measured as 844.9±3,128.1 ng/ml in 24 patients. The results
are shown in Table I.
 | Table I.Characteristics of patients with
rectal cancer. |
Table I.
Characteristics of patients with
rectal cancer.
| Characteristic | Value (mean ±
SD) |
|---|
| Number of
patients | 26 |
| Age, years | 72±10 |
| Staging |
|
| I | 3 |
| II | 8 |
|
III | 5 |
| IV | 10 |
| Therapy |
|
| Surgery
(no distant metastasis) | 15 |
|
Chemotherapy (distant
metastasis) | 6 |
| Surgery
at primary site (distant metastasis) | 1 |
| Surgery
at primary site + liver radiofrequency ablation (distant
metastasis) | 1 |
| Surgery
at the primary site + moved to a different institute | 1 |
|
Supportive care | 2 |
| Pathology |
|
| Tubular
adenocarcinoma | 26a |
| Distant
metastasis |
|
|
Absent | 15 |
|
Present | 11 |
| PET- CT (mean ±
SD) |
|
| 18F-FDG
dose, MBq | 231.3±44.1 |
| Blood
glucose, mg/dl | 103.1±23.7 |
| Primary
lesion SUVmax | 18.9±8.8 |
| Cardiac
SUVmax | 4.3±4.1 |
| Tumor
volume on PET images, cm2 |
74,990.1±148317.1 |
| L3
iliopsoas muscle area, cm2 | 1,090.9±455.6 |
| Echocardiography
(mean ± SD) |
|
| LAD,
mm | 37.4±6.1 |
| LVDd,
mm | 44.4±5.0 |
| LVDs,
mm | 30.1±4.6 |
| EF,
% | 65.6±7.6 |
|
E/e | 8.3±3.0 |
|
E/A | 1.2±1.8 |
| Carcinoembryonic
antigen (mean ± SD) |
|
| ng/ml | 844.9±3,128.1 |
Absence/presence of distant metastasis
and investigation of various factors
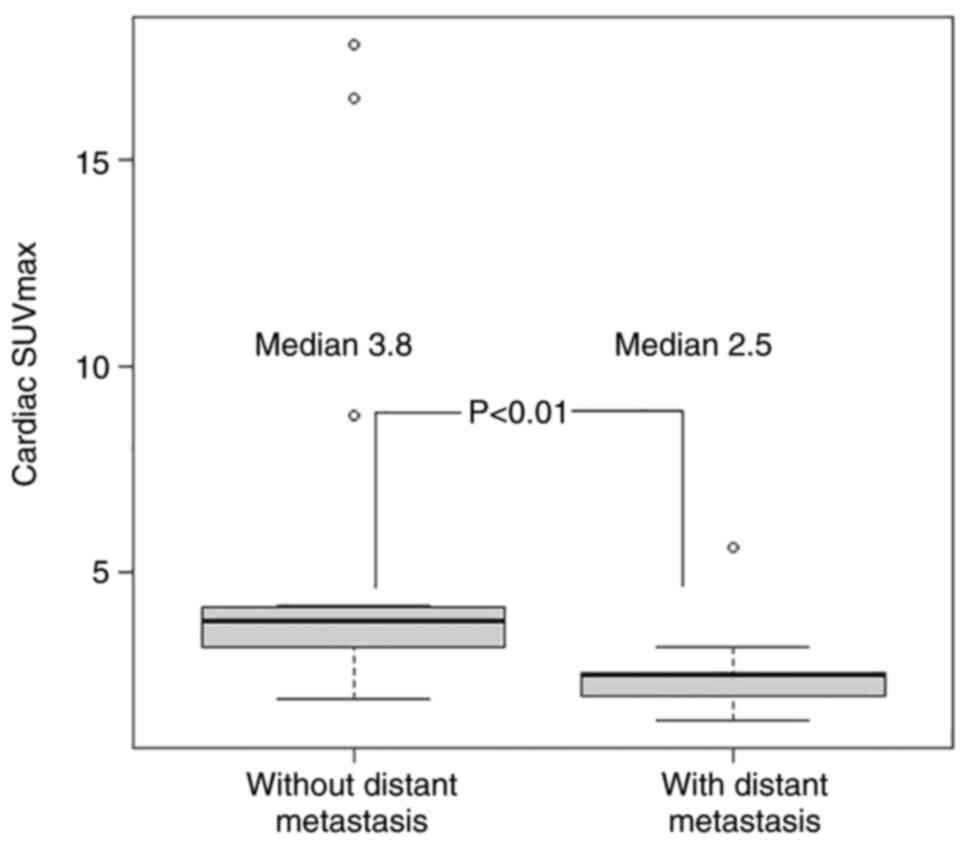
The median cardiac SUVmax was 3.8 and 2.5 in
patients with no distant metastasis and distant metastasis,
respectively, revealing a statistically significant difference
(P<0.01; Fig. 1).
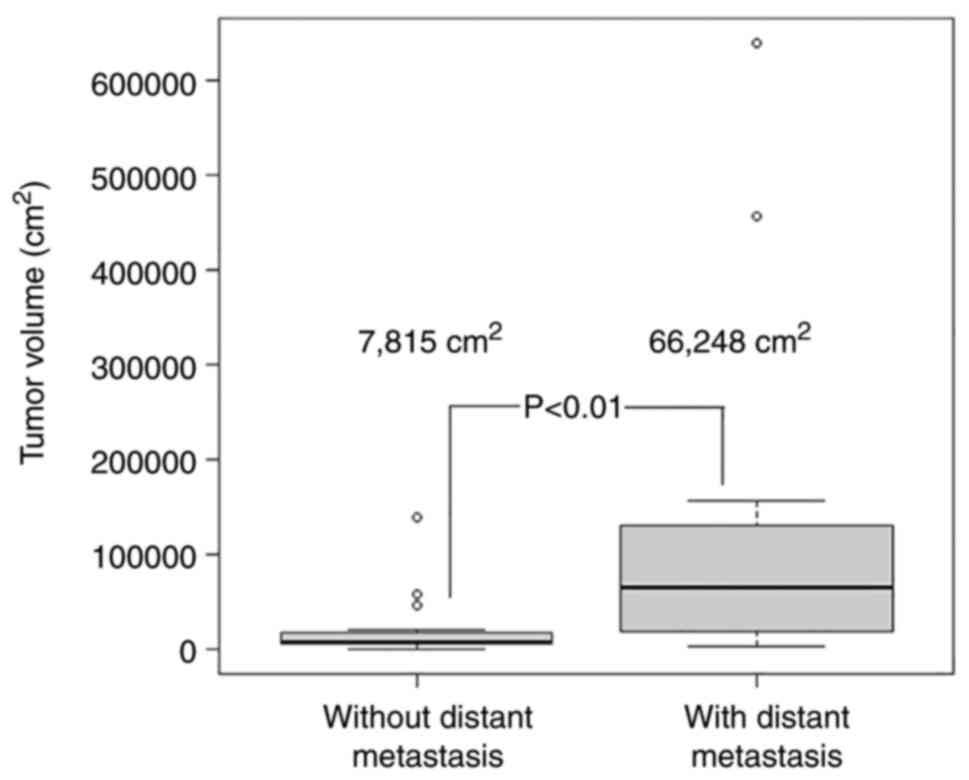
The median total tumor volume was 7,815
cm2 and was 66,248 cm2 in patients with no
distant metastasis and distant metastasis, respectively, revealing
a statistically significant difference (P<0.01; Fig. 2). However, two patients had lung
metastases with small tumor volumes that proved difficult to
measure.
The median CEA was 3.3 and 79.3 ng/ml in patients
with no distant metastasis and distant metastasis, respectively,
revealing a statistically significant difference (P<0.01). No
other factors revealed a statistically significant difference. The
results are shown in Table II.
 | Table II.Presence/absence of distant
metastasis and investigated factors in rectal cancer. |
Table II.
Presence/absence of distant
metastasis and investigated factors in rectal cancer.
| Factor | No metastasis | Metastasis | P-value |
|---|
| Age, years | 72 | 67 | 0.09 |
| Sex
male/female | 6/9 | 8/3 | 0.13 |
| 18F-FDG, MBq | 216.9 | 229.1 | 0.63 |
| Glucose, mg/dl | 99 | 98 | 0.47 |
| Primary lesion
SUVmax | 22.2 | 16.3 | 0.10 |
| Cardiac SUVmax | 3.8 | 2.5 | <0.01 |
| Total tumor volume
on PET images | 7,815 | 66,248 | <0.01 |
| L3 iliopsoas muscle
area | 1,105 | 1,069 | 0.90 |
| LAD | 38.0 | 36.1 | 0.73 |
| LVDd | 47.5 | 47.8 | 0.78 |
| LVDs | 29.0 | 28.7 | 0.69 |
| EF | 67.5 | 66.2 | 0.39 |
| E/e | 8.5 | 7.6 | 0.18 |
| E/A | 0.8 | 0.8 | 0.66 |
| CEA | 3.3 | 79.3 | <0.01 |
Correlation between tumor volume and
cardiac SUVmax
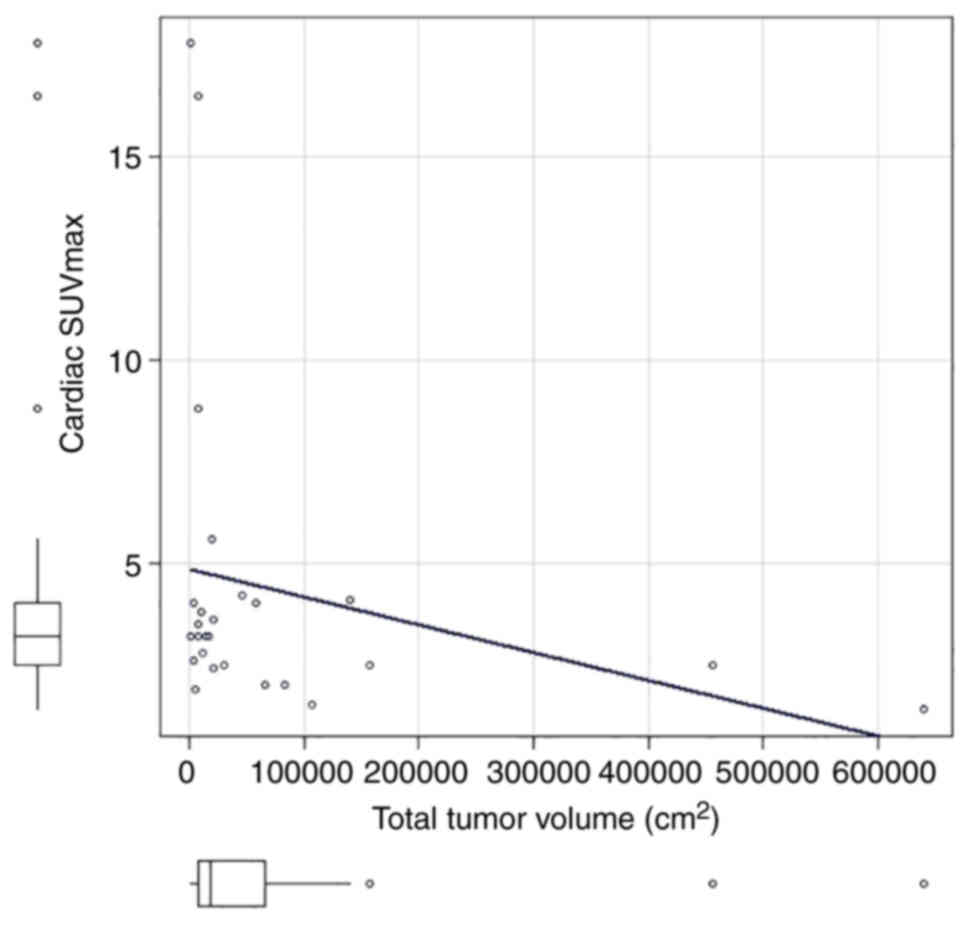
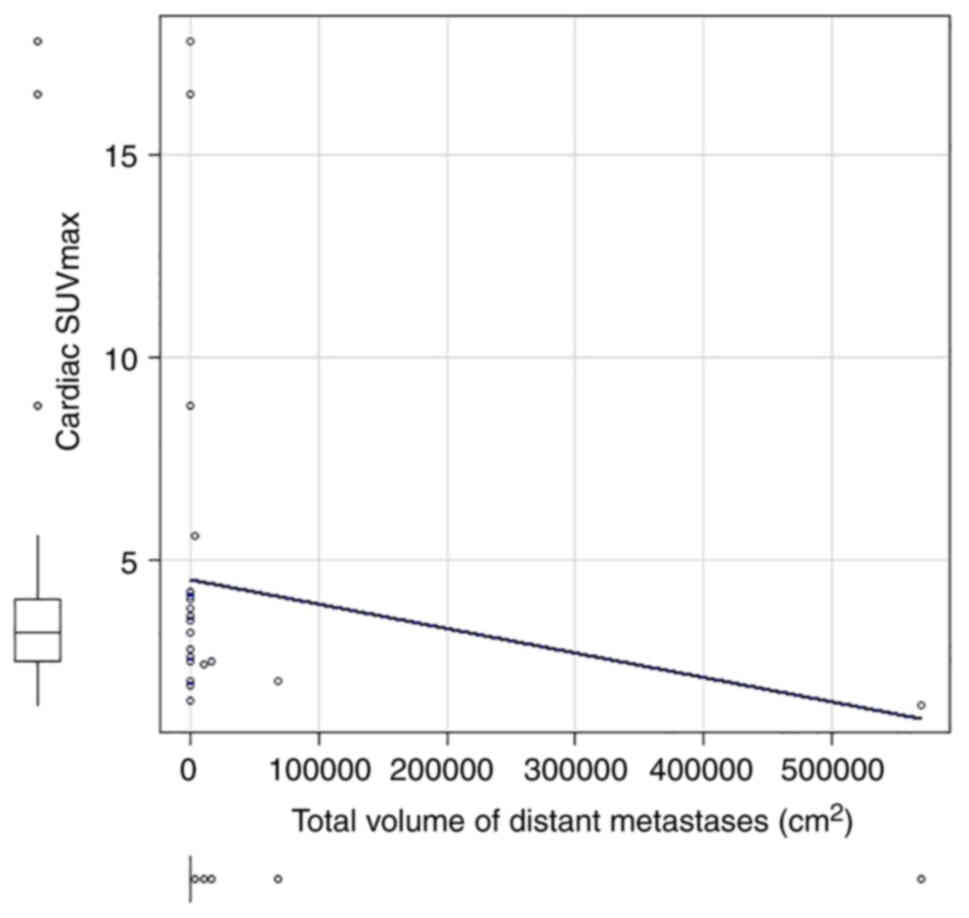
The correlation coefficient between cardiac SUVmax
and total tumor volume (sum of primary lesion tumor volume + lymph
nodes tumor volume + distant metastases tumor volume) was
statistically significant (correlation coefficient=−0.42, P=0.03;
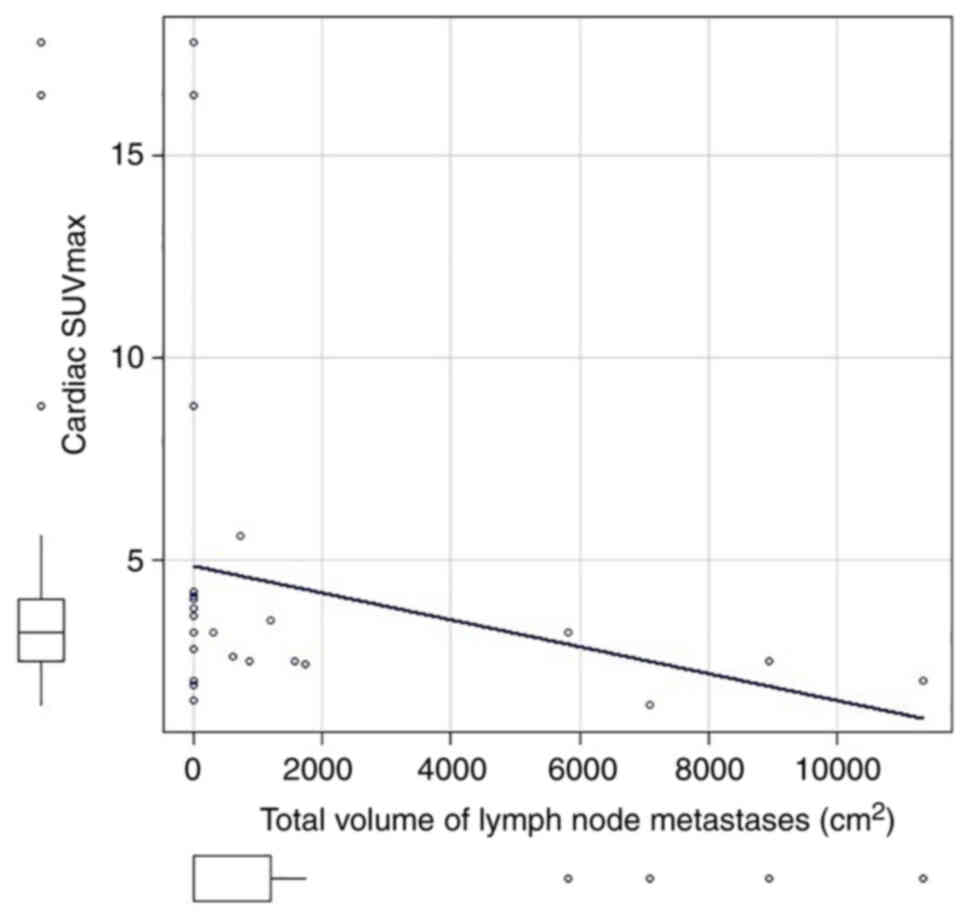
Fig. 3). There was a statistically
significant correlation between the total volume of lymph node
metastases (0 in patients with no lymph node metastasis) and
cardiac SUVmax (correlation coefficient=−0.46, P=0.02; Fig. 4), and between the total volume of
distant metastases (0 in patients with no distant metastasis) and
cardiac SUVmax (correlation coefficient=−0.6, P<0.01; Fig. 5).
Factors related to the occurrence of
distant metastasis
Analyzing cardiac SUVmax as a continuous variable
showed a significant association between the occurrence of distant
metastasis and cardiac SUVmax [Odds ratio (OR): 0.30, 95%
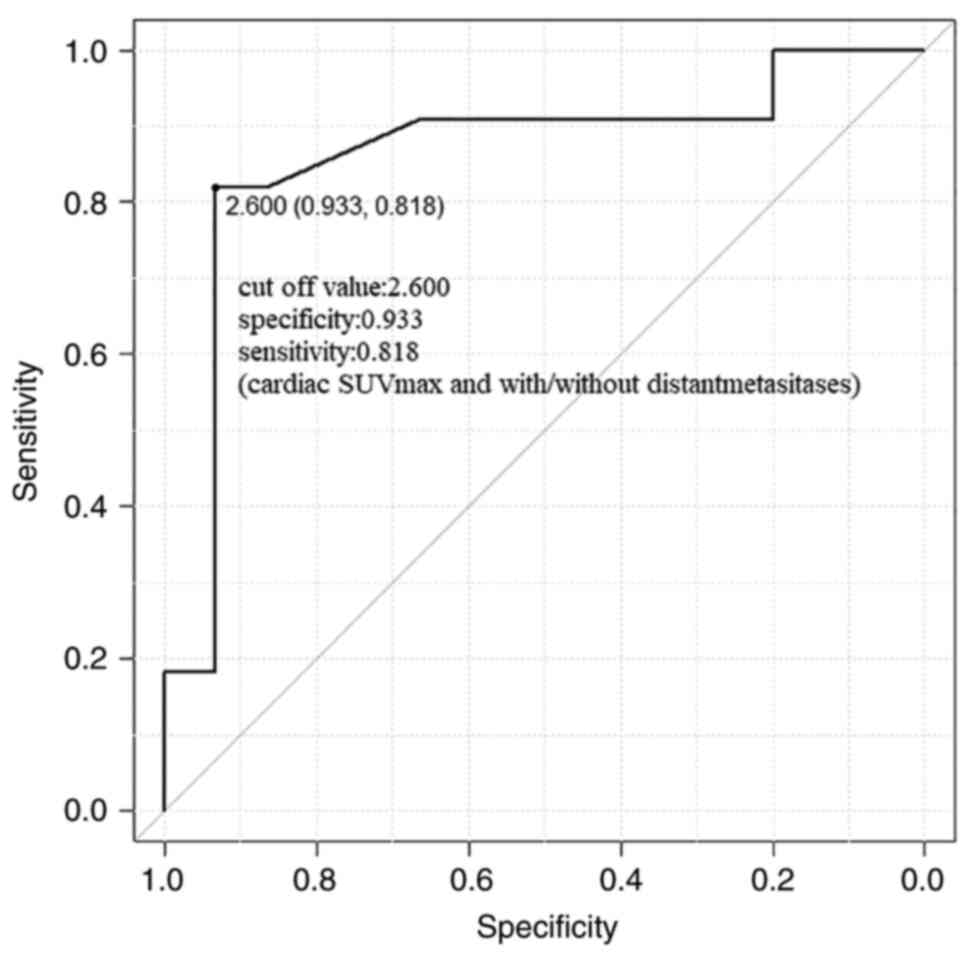
confidence interval (CI): 0.09-0.98, P=0.045] (Table III). ROC analysis showed that a
cardiac SUVmax of 2.6 had the best an area under the curve (AUC)
for predicting the presence of distant metastasis.
 | Table III.Factors related to the occurrence of
distant metastasis in rectal cancer. |
Table III.
Factors related to the occurrence of
distant metastasis in rectal cancer.
| Factor | Odds ratio | 95% CI | P-value |
|---|
| Age | 0.90 | 0.84–1.01 | 0.09 |
| Sex | 4.00 | 0.74–21.5 | 0.11 |
| Primary lesion
SUVmax | 0.89 | 0.78–1.00 | 0.049 |
| Cardiac SUVmax | 0.30 | 0.09–0.98 | 0.045 |
| Total tumor volume
on PET images | 1.00 | 1.00–1.00 | 0.10 |
| L3 iliopsoas muscle
area | 1.00 | 0.99–1.00 | 0.69 |
| LAD | 0.97 | 0.85–1.13 | 0.75 |
| LVDd | 1.05 | 0.88–1.25 | 0.59 |
| LVDs | 1.13 | 0.92–1.39 | 0.23 |
| EF | 0.89 | 0.77–10.0 | 0.16 |
| E/e | 0.79 | 0.55–1.15 | 0.23 |
| E/A | 0.18 | 0.00–9.13 | 0.39 |
| CEA | 1.04 | 1.00–1.08 | 0.051 |
By using the cut off value 2.6 of cardiac SUVmax,
the AUC value was 0.86, specificity 0.933, sensitivity 0.818 and
95% CI: 0.70-1.00 for predicting the presence of distant metastasis
(Fig. 6). Analyzing primary tumor
SUVmax as a continuous variable showed a significant association
between the occurrence of distant metastasis and primary tumor
SUVmax (HR: 0.89, 95% CI: 0.78-1.00, P=0.049). ROC analysis showed
that a primary tumor SUVmax of 21 had an AUC of 0.82 for
determining the presence of distant metastasis (specificity 0.533,
sensitivity 1.000; 95% CI: 0.46-1.00). No other factor produced a
statistically significant result (Table III).
Investigation of overall survival
The median observation time was 56 months, and nine
patients died during observation. Four patients were lost from
follow up.
Two of the 15 patients without distant metastasis
and seven of the 11 patients with distant metastasis died during
the observation period. Two patients with distant metastases
received only best supportive care (BSC) and died ~1 month after
staging. A total of 24 patients received standard therapy (surgery
and/or chemotherapy). All patients without distant metastases
underwent surgery for primary tumor. Six patients with distant
metastases received first line Folinic acid, Fluorouracil and
Oxaliplatin (FOLFOX) treatment. One patient with distant metastases
underwent surgery at the primary site, another underwent surgery at
the primary site and radiofrequency ablation (RFA) for liver
metastasis and another underwent surgery at the primary site before
moving to a different hospital.
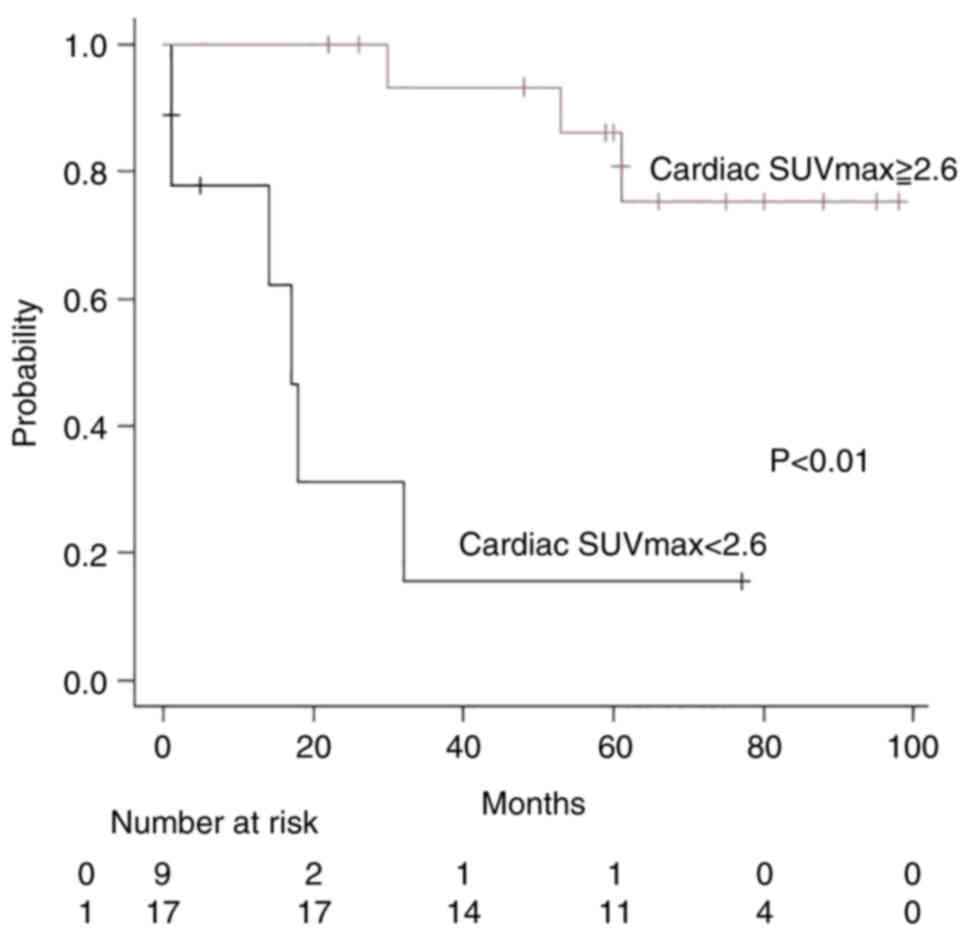
Analyzing the association between overall survival
(including the two patients that received BSC) and cardiac SUVmax
(cutoff: 2.6) showed 95% CI: 0.01-0.45, HR: 0.06, and
P<0.01(Table IV). The
Kaplan-Meier method and log-rank test showed that there was a
statistically significant difference in overall survival between
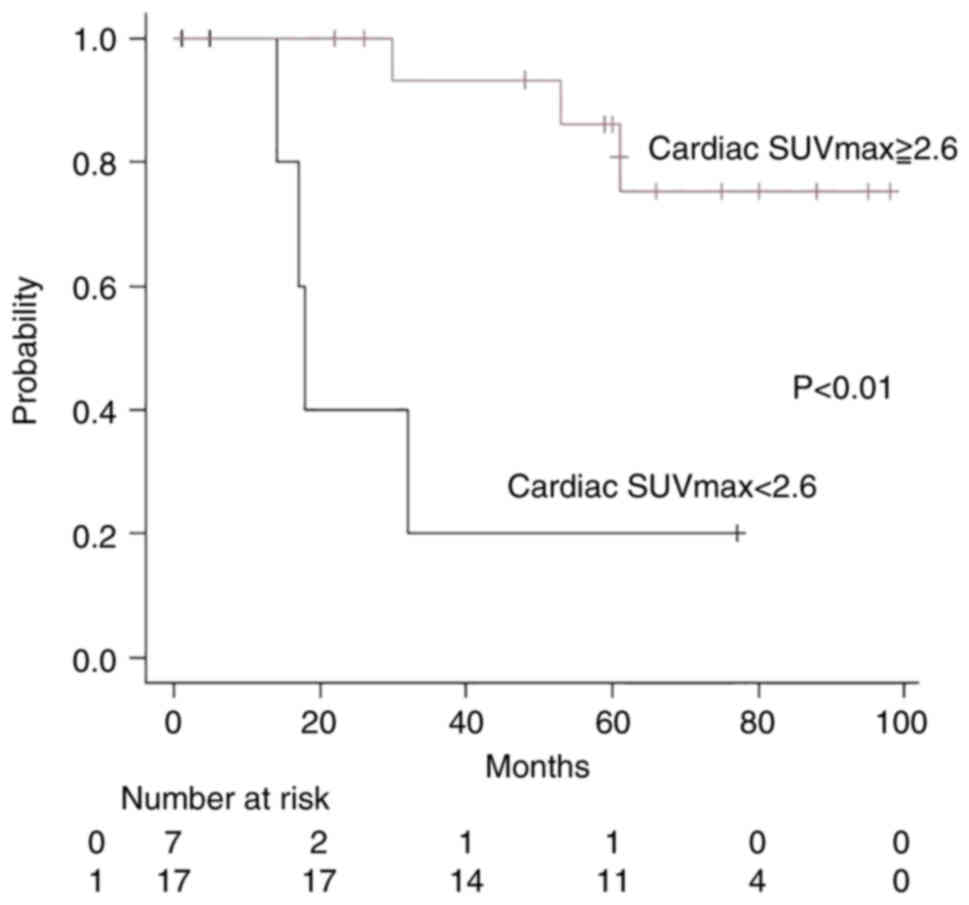
the cardiac SUVmax ≥2.6 group (n=17; three patients died; median
follow-up time, 60 months) and the cardiac SUVmax <2.6 group
(n=9; six patients died; median follow-up time, 14 months). The
SUVmax ≥2.6 group did not achieve median survival time (MST),
whereas in the SUVmax <2.6 group the MST was 17 months
(P<0.01; Fig. 7). Analyzing the
association between overall survival (including the two patients
that received BSC) and distant metastasis showed 95% CI:
1.72-116.4, HR: 14.1, and P<0.01 (Table IV). The Kaplan-Meier method and
log-rank test showed that there was a statistically significant
difference in overall survival between the group without distant
metastasis (n=15; two patients died; median follow up time, 60
months) and the group with distant metastasis (n=11; seven patients
died; median follow up time, 18 months). The group without distant
metastasis did not achieve MST, whereas in the group with distant
metastasis the MST was 32 months (P<0.01; data not shown).
 | Table IV.Factors related to overall survival
in 26 patients with rectal cancer (including two patients treated
with best supportive care). |
Table IV.
Factors related to overall survival
in 26 patients with rectal cancer (including two patients treated
with best supportive care).
| Factor | Hazard ratio | 95% CI | P-value |
|---|
| Age | 1.00 | 0.94–1.07 | 0.96 |
| Sex | 1.05 | 0.25–4.43 | 0.95 |
| Primary lesion
SUVmax | 0.98 | 0.90–1.06 | 0.63 |
| Cardiac SUVmax | 0.45 | 0.17–1.17 | 0.10 |
| Cardiac SUVmax |
|
|
|
| (2.6 cutoff) | 0.06 | 0.01–0.45 | <0.01 |
| CEA | 1.00 | 1.00–1.00 | 0.01 |
| Total tumor volume
on PET images | 1.00 | 1.00–1.00 | <0.01 |
| L3 iliopsoas muscle
area | 0.99 | 0.99–1.01 | 0.38 |
| Presence/absence of
distant metastasis | 14.10 | 1.72–116.40 | <0.01 |
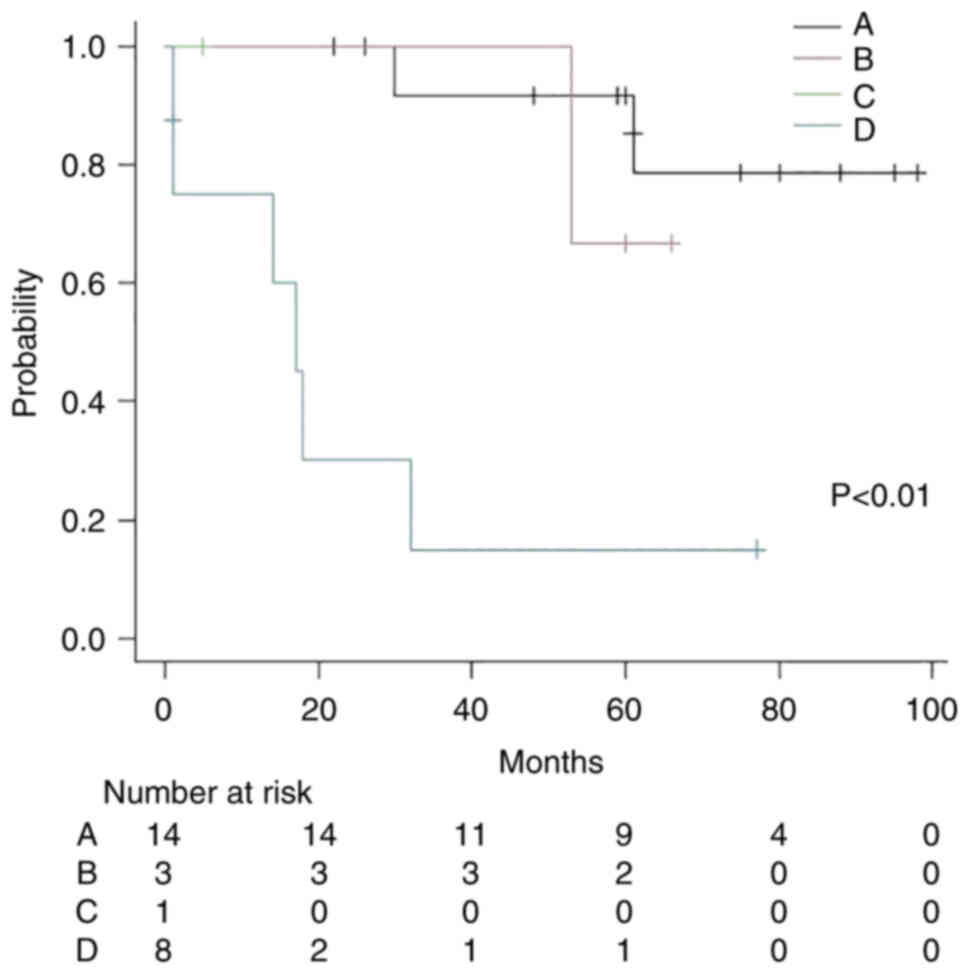
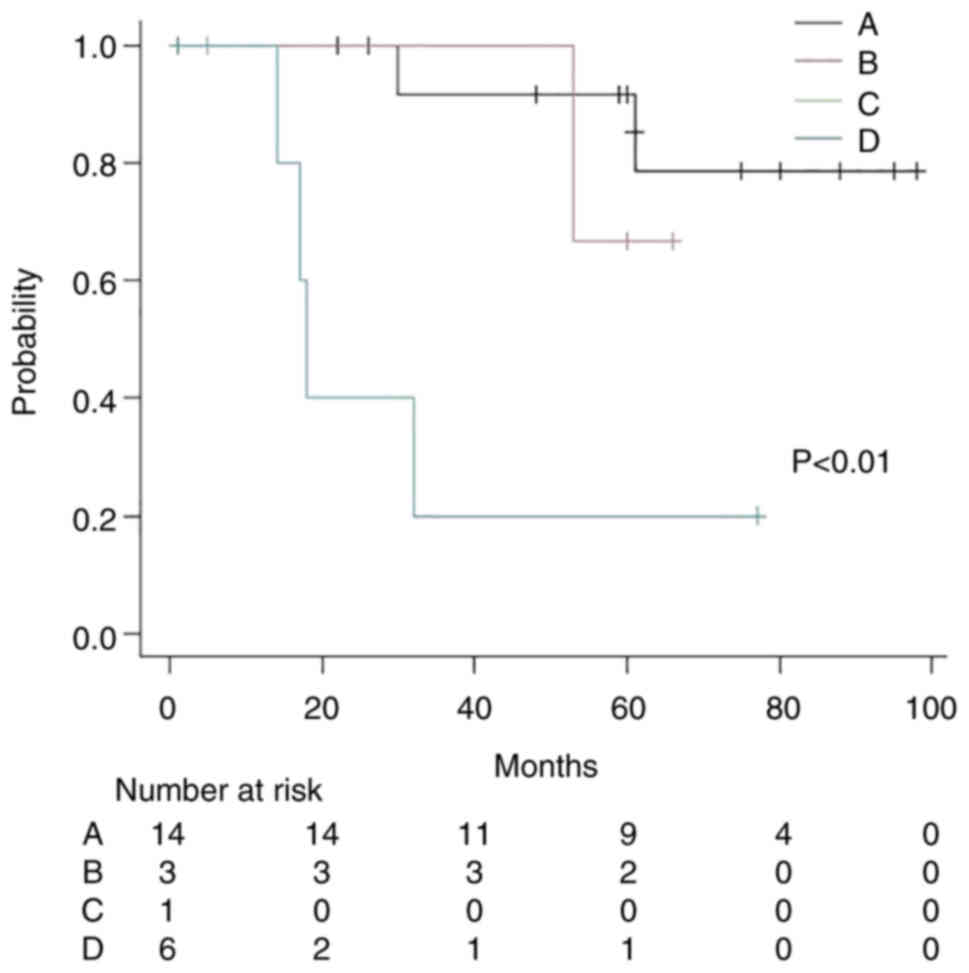
Subsequently, the patients were classified into four
groups: Group A (cardiac SUVmax ≥2.6+ without distant metastasis;
n=14; two patients died; median follow-up time, 60 months), group B
(cardiac SUVmax ≥2.6+ with distant metastasis; n=3; one patient
died; median follow-up time, 60 months), group C (cardiac SUVmax
<2.6+ without distant metastasis; n=1; median follow-up time, 5
months) and group D (cardiac SUVmax <2.6+ with distant
metastasis; n=8; six patients died; median follow-up time, 15.5
months). The Kaplan-Meier method and log-rank test showed that
there was a statistically significant difference between the four
groups; groups A, B and C did not achieve MST, whereas group D had
an MST of 17 months (P<0.01; Fig.
8).
Analyzing the association between overall survival
(excluding the two patients that received BSC) and cardiac SUVmax
(cutoff: 2.6) showed 95% CI: 0.02-0.53, HR: 0.12 and P<0.01
(Table V). The Kaplan-Meier method
and log-rank test showed that there was a statistically significant
difference between the cardiac SUVmax ≥2.6 group (n=17; three
patients died; median follow-up time, 60 months) and the cardiac
SUVmax <2.6 group (n=7; four patients died; median follow-up
time, 17 months). The SUVmax ≥2.6 group did not achieve MST,
whereas the MST in the SUVmax <2.6 group was 17 months
(P<0.01; Fig. 9). Analyzing the
association between overall survival (excluding the two patients
that received BSC) and distant metastasis showed 95% CI: 1.13-30.5,
HR: 5.88 and P=0.03 (Table V). The
Kaplan-Meier method and log-rank test showed that there was a
statistically significant difference between the group without
distant metastasis (n=15; two patients died; median follow-up time,
60 months) and the group with distant metastasis (n=9; five
patients died; median follow-up time, 32 months). The group without
distant metastasis did not achieve MST, whereas the group with
distant metastasis had an MST of 42.5 months (P<0.01; data not
shown).
 | Table V.Factors related to overall survival
in 24 patients with rectal cancer (excluding two patients that
received best supportive care). |
Table V.
Factors related to overall survival
in 24 patients with rectal cancer (excluding two patients that
received best supportive care).
| Factor | Hazard ratio | 95% CI | P-value |
|---|
| Age | 0.98 | 0.91–1.06 | 0.66 |
| Sex | 0.81 | 0.18–3.66 | 0.79 |
| Primary lesion
SUVmax | 0.97 | 0.88–1.06 | 0.45 |
| Cardiac SUVmax | 0.68 | 0.32–1.48 | 0.33 |
| Cardiac SUVmax |
|
|
|
| (2.6 cutoff) | 0.12 | 0.02–0.53 | <0.01 |
| CEA | 1.01 | 0.99–1.03 | 0.23 |
| Total tumor volume
on PET images | 1.00 | 1.00–1.00 | 0.13 |
| L3 iliopsoas muscle
area | 1.00 | 1.00–1.00 | 0.96 |
| Presence/absence of
distant metastasis | 5.88 | 1.13–30.5 | 0.03 |
The patients (exclude two BSC patients) were then
classified into the following four groups: Group A (cardiac SUVmax
≥2.6+ without distant metastasis; n=14; two patients died; median
follow-up time, 60 months), group B (cardiac SUVmax ≥2.6+ with
distant metastasis, n=3; one patient died; median follow-up time,
60 months), group C (cardiac SUVmax <2.6+ without distant
metastasis; n=1; median follow-up time, 5 months) and group D
(cardiac SUVmax <2.6+ with distant metastasis; n=6; four
patients died; median follow-up time, 17.5 months). The
Kaplan-Meier method and log-rank test showed that there was a
statistically significant difference between the four groups;
groups A, B and C did not achieve MST; group D had an MST of 17
months (P<0.01; Fig. 10).
Colon cancer
All patients
A total of 24 patients had colon cancer in a region
other than the rectum. The 24 patients included 16 men and eight
women with a mean age of 72.5±14.0 years. Distant metastasis was
absent in 14 patients and present in 10 patients. Colon cancer was
located in the ileocecal region in three patients, the ascending
colon in five patients, the transverse colon in one patient, the
descending colon in two patients and the sigmoid colon in 13
patients. Histopathological results were as follows: 22 cases of
tubular adenocarcinoma, one case of tubular adenocarcinoma + poorly
differentiated adenocarcinoma combined, and one case of poorly
differentiated adenocarcinoma. Double colon/rectum cancer was
detected in three patients (two patients in the sigmoid colon and
rectum and one patient in the ascending colon and appendiceal
mucinous adenocarcinoma).
All of the 14 patients without distant metastasis
underwent primary tumor resection and four patients received
adjuvant chemotherapy (two patients received capecitabine and four
tegafur-uracil (UFT). Of the 10 patients with distant metastasis,
one patient received FOLFOX6 + bevacizumab, one patient received
modified FOLFOX, five patients underwent surgery at the primary
site + FOLFOX6, one patient underwent surgery at the primary site +
capecitabine, one patient underwent surgery at the primary site +
RFA (liver metastasis) + 5-fluorouracil + Bevacizumab, and one
patient received BSC.
The 18F-FDG dose was 237.3±43.0 MBq, and the blood
glucose level was 97.1±15.5 mg/dl. The SUVmax of the primary lesion
was 18.7±6.0 and the cardiac SUVmax was 4.5±4.4. The iliopsoas
muscle area at the L3 level was 1,279.6±539.6 cm2.
Echocardiography was performed on 22 patients,
giving a LAD (mm) of 37.7±5.0, LVDd (mm) of 47.1±4.3, LVDs (mm) of
30.4±3.8, EF (%) of 63.6±10.0, E/e of 9.8±3.4 and E/A of
1.0±0.4.
The median L3 iliopsoas muscle area was 1,042 and
1,410 cm2 in patients with no distant metastasis and
with distant metastasis, respectively; this difference was
statistically significant (P=0.04). The median E/e was 10.8 and 7.3
in patients with no distant metastasis and distant metastasis,
respectively, revealing a statistically significant difference
(P=0.01). When patients with colon cancer in regions other than the
rectum were grouped based on the presence or absence of distant
metastasis, no statistically significant difference was found in
terms of age, sex, 18F-FDG dose, blood glucose level, primary
lesion SUVmax, cardiac SUVmax, LAD, LVDd and EF. The distant
metastasis group was compared with the non-distant metastasis group
and the results for age, sex, 18F-FDG dose, glucose, Primary lesion
SUVmax, Cardiac SUVmax, L3 iliopsoas muscle area, LAD, LVDd, LVDs,
EF, E/A and E/e are shown in Table
VI.
 | Table VI.Presence/absence of distant
metastasis and investigated factors in 24 patients with colon
cancer |
Table VI.
Presence/absence of distant
metastasis and investigated factors in 24 patients with colon
cancer
| Factor | No metastasis | Metastasis | P-value |
|---|
| Age | 75.5 | 65.0 | 0.11 |
| Sex | Men:8 |
|
|
| Women:6 | Men:8 |
|
|
| Women:2 | 0.39 |
|
|
| 18F-FDG, MBq | 227.0 | 242.5 | 0.48 |
| Glucose, mg/dl | 96 | 90 | 0.77 |
| Primary lesion
SUVmax | 19.5 | 18.0 | 0.58 |
| Cardiac SUVmax | 2.9 | 2.8 | 0.36 |
| L3 iliopsoas muscle
area | 1,042 | 1,410 | 0.04 |
| LAD | 37.0 | 37.5 | 0.79 |
| LVDd | 47 | 48 | 0.57 |
| LVDs | 30 | 31 | 0.35 |
| EF | 66 | 62 | 0.13 |
| E/A | 0.9 | 0.8 | 0.47 |
| E/e | 10.8 | 7.3 | 0.01 |
Analysis excluding the three patients
with double colon or rectum cancer
A total of 21 patients had colon cancer in a region
other than the rectum. Colon cancer was located in the ileocecal
region in three patients, the ascending colon in four patients, the
transverse colon in one patient, the descending colon in two
patients and the sigmoid colon in 11 patients. The 21 patients
included 13 men and 8 women with a mean age of 69.9±14.9 years.
Distant metastasis was absent in 12 patients and present in nine
patients. Histopathological results were as follows: 19 cases of
tubular adenocarcinoma and two cases of poorly differentiated
adenocarcinoma.
The 18F-FDG dose was 238.3±43.0 MBq, and the blood
glucose level was 97.3±16.5 mg/dl. The SUVmax of the primary lesion
was 18.5±6.4 and the cardiac SUVmax was 4.7±4.7. The iliopsoas
muscle area at the L3 level was 1,274.4±536.6 cm2.
Echocardiography was performed on 19 patients,
giving a LAD (mm) of 37.3±5.1, LVDd (mm) of 46.6±4.1, LVDs (mm) of
30.2±4.0, EF (%) of 63.4±10.9, and E/A of 1.0±0.5.
The median L3 iliopsoas muscle area was 1,042.5 and
1,455 cm2 in patients with no distant metastasis and
with distant metastasis, respectively, revealing a statistically
significant difference (P=0.01). The median E/e was 10.7 and 7.1 in
patients with no distant metastasis and with distant metastasis,
respectively, revealing a statistically significant difference
(P=0.04). When patients with colon cancer in regions other than the
rectum were grouped based on the presence or absence of distant
metastasis, no statistically significant difference was found in
terms of age, sex, 18F-FDG dose, blood glucose level, primary
lesion SUVmax, cardiac SUVmax, LAD, LVDd and EF. The distant
metastasis group was compared with the non-distant metastasis group
and the results for age, sex, 18F-FDG dose, glucose, Primary lesion
SUVmax, Cardiac SUVmax, L3 iliopsoas muscle area, LAD, LVDd, LVDs,
EF, E/A, E/e are shown in Table
VII.
 | Table VII.Presence/absence of distant
metastasis and investigated factors in 21 patients with colon
cancer (ascending, transverse, descending, sigmoid cancer)
excluding three patients with double colon/rectal cancer. |
Table VII.
Presence/absence of distant
metastasis and investigated factors in 21 patients with colon
cancer (ascending, transverse, descending, sigmoid cancer)
excluding three patients with double colon/rectal cancer.
| Factor | No metastasis | Metastasis | P-value |
|---|
| Age | 75.6 | 65.0 | 0.13 |
| Sex
male/female | 6/6 | 6/2 | 0.36 |
| 18F-FDG, Bq | 96 | 89 | 1.00 |
| Glucose, mg/dl | 224.8 | 253.9 | 0.24 |
| Primary lesion
SUVmax | 19.6 | 17.0 | 0.59 |
| Cardiac SUVmax | 3.05 | 2.70 | 0.15 |
| L3 iliopsoas muscle
area | 1,042.5 | 1,455.0 | 0.01 |
| LAD | 37.0 | 37.5 | 0.77 |
| LVDd | 47 | 47 | 0.36 |
| LVDs | 28.0 | 30.5 | 0.23 |
| EF | 66.0 | 61.0 | 0.17 |
| E/A | 1.1 | 0.8 | 0.17 |
| E/e | 10.7 | 7.1 | 0.04 |
Discussion
The present study aimed to investigate the factors
affecting 18F-FDG uptake in the heart in patients with new-onset
rectal cancer and new-onset colon cancer. In new-onset rectal
cancer, compared with the patients with distant metastasis, the
patients without distant metastasis had a i) statistically higher
cardiac SUVmax and ii) statistically smaller tumor volume. A
statistically significant association was found between the
occurrence of distant metastasis and cardiac SUVmax; however, no
statistically significant association was found between the
occurrence of distant metastasis and tumor volume. The correlation
coefficient between cardiac SUVmax and tumor volume was also
statistically significant. The results revealed that cardiac SUVmax
decreased with an increasing volume of lymph node metastases and an
increasing volume of distant metastases. Thus, the present study
indicated the probability that heart metabolic mechanism is
affected by cancer burden, especially distant metastasis. A high
GLUT1 expression following chemoradiotherapy in rectal cancer has
been associated with a large number of distant metastases and a
poor prognosis (16). In addition,
although not related to rectal cancer, a previous study showed that
the prognosis of neuroblastoma may be improved by GLUT1 inhibitors
(19). It may be hypothesized that
as cancer cells and tumor volume increase, the demand of glucose by
cancer cells expands, thus the glucose supply in the body becomes
smaller and less available for use by the heart, thus heart
metabolism changes from using glucose to fatty acids. This may be
the mechanism underlying the change in cardiac SUVmax. Diabetes
mellitus has also been associated with reduced glucose uptake by
the heart (20). However, in the
present study, the blood glucose levels were normal in all
patients, and since the blood glucose levels were strictly managed
and averaged ~102 mg/dl before the examination, the possibility of
the blood glucose levels affecting the findings appears to be low.
In addition, 18F-FDG uptake by the heart could potentially be
affected by a history of angina pectoris or myocardial infarction.
However, the presence or absence of distant metastasis had no
significant effect on the echocardiography findings; therefore, the
uptake was unlikely to be affected by cardiac function or other
causes originating in the heart. The cardiac uptake in cases of
malignant lymphoma has also been weakly correlated with free fatty
acids in the blood (21).
Furthermore, cardiac uptake has been shown to be stronger in cases
of Hodgkin lymphoma than in cases of non-Hodgkin lymphoma, where
the involvement of tumor-related factors has been reported as a
possible cause (22). Accordingly,
cardiac uptake may be affected by the tumor or associated
factors.
In new-onset rectal cancer, distant metastasis and
cardiac SUVmax were considered as prognostic factors of overall
survival. Cardiac 18F-FDG accumulation is not used for staging but
cardiac 18F-FDG accumulation and cardiac SUVmax may be indicators
for disease characteristics, especially glucose demand by cancer.
In the present study, cardiac SUVmax and distant metastasis were
considered prognostic factors. Although only three patients, the
patients with cardiac SUVmax ≥2.6 and with distant metastasis had a
longer prognosis compared with patients with cardiac SUVmax <2.6
and with distant metastasis. When excluding the two patients
treated with BSC, cardiac SUVmax (cutoff 2.6) was more
significantly associated with survival (Table V). Although the sample size was
limited, cardiac SUVmax may be a prognostic factor at new onset
rectal cancer.
Notably, analysis of patients with new-onset colon
cancer found no statistically significant difference in cardiac
SUVmax or other factors between the patients with and without
distant metastasis, suggesting that the pathophysiology of rectal
cancer differs from that of colon cancer. The reason for
differences between new-onset rectal cancer and new-onset colon
cancer regarding the association between cardiac SUVmax and distant
metastases are unclear.
It has been reported that patients with advanced
rectal cancer have a poor prognosis due to cachexia (23), raising the potential involvement of
inflammatory substances, such as cytokines. Furthermore, rectal
cancer with high GLUT1 expression treated with chemoradiotherapy
was associated with a poor progress (16). It was hypothesized that rectal
cancer has a specific metabolic change, such as strong demand of
glucose with distant metastasis, especially the correlation between
the GLUT-1 expression and tumor volume. This glucose metabolic
change induces this cardiac 18F-FDG accumulation change correlated
to the distant metastasis and tumor volume. The reason may be that
the disease uniformity (ascending, transverse, descending, sigmoid)
and GLUT 1,4 uniformity may cause these results. However no studies
were on this were or any studies on the correlation between GLUT1
and GLUT 4 with cancer and in vivo.
The present study considered that 18F-FDG PET-CT can
evaluate GLUT1 expression pattern at tumor and whole-body organs
such as brain, heart and muscle, which express GULT 1, 2, 3 and 4.
PET-CT has semi quantitative indicators such as SUV. Semi
quantitative evaluation of GLUT expression is possible by using
PET-CT and was used PET-CT in the present study.
There are reports on GLUT-1 expression in cancer
(16,19). Moreover, GLUT1 expression is not
only detected in rectal cancer but also in neuroblastoma (16,19).
PET-CT data from other types of gastroenterological cancer were
checked; however, the sample size was small, especially for
esophageal carcinoma, gastric carcinoma, pancreas carcinoma, bile
duct carcinoma and hepatocellular carcinoma. In addition, the
frequency of 18F-FDG PET-CT use for hepatocellular carcinoma
staging is rare, due to the low uptake of 18F-FDG. However, we
reported the research about rectal cancer and colon cancer from our
institute before (24–28), and investigated the rectum and colon
cancer in this PET-CT study.
Commonly, overall survival rate and 5-year survival
rate are used as indicators of survival, whereas the present study
used MST. As the median follow-up time was 56 months (4 years and 6
months) in rectum cancer, the 5-year survival rate could not be
evaluated. In addition, some patients were lost during the
follow-up, thus making it difficult to evaluate the true overall
survival rate. Moreover, a long time is necessary to calculate
overall survival rate. The present study is retrospective in
design; therefore, the timing for follow-up examination (such as
blood test, CT, MRI, PET) was not constant. This variation may
induce bias when assessing progression-free survival rate and
disease-free survival rate. Thus, MST was used as an indicator of
survival.
The limitations of the present study include the
retrospective study design and the enrollment of a limited number
of individuals from a single study site. In addition, cytokines,
other humoral factors and fatty acid were not evaluated. There was
difficulty in the evaluation about the percentage of colon and
rectum cancer patients received PET-CT in all colon and rectum
cancer patients Moreover, the cardiac 18F-FDG accumulation in other
types of cancer was not assessed. Furthermore, the treatment
strategy was heterogeneous and the probability of treatment bias
which affect the overall survival rate cannot be excluded. The
present study also did not evaluate the 18F-FDG accumulation in the
brain. Initially, we aimed to evaluate brain 18F-FDG accumulation
by visual evaluation; however, visual evaluation is subjective and
cannot indicate minor changes. 18F-FDG metabolism in the brain in
each segment may change by physiological changes caused by
non-small lung cancer (NSCLC) (29). In this previous retrospective study
(29), they compare the brain
18F-FDG uptake pattern between normal group and cancer group by
statistical parametric mapping 8 (29), Increased 18F-FDG uptake was detected
at the insula, putamen, pallidum, thalamus, hippocampus and
amygdala, whereas decreased 18F-FDG uptake was detected at the
inferior parietal lobule, left superior parietal lobule and left
fusiform gyrus in the NSCLC patient group (29). This may be induced by lung-cancer
related visceral sympathetic activation and a decrease in dorsal
attention network function (29).
This mechanism is still hypothetical but this research is
important. Paradoxically, there is difficulty setting a suitable
ROI in the brain (stable area) by PET-CT. In addition, 18F-FDG
accumulation in the brain is affected by other factors, such as
aging and dementia. Three-dimensional stereotactic surface
projection (3D-SSP; Nihon Medi-Physics Co., Ltd.) is used for brain
18F-FDG accumulation. 3D-SSP uses Z score, which is an index
showing the difference in patient data from the standard deviation
in a database of normal subjects (30). In Lewy body disease,
N-isopropyl-(123I)-p-iodoamphetamine (cerebral blood) accumulation
at frontal lobe is decreased in visual hallucination patients
(31). As the present study did not
evaluate the 18F-FDG accumulation in the brain, this should be
evaluated in future studies, perhaps using a larger group of
patients with different types of cancer and prospective multicenter
study design
In conclusion, cardiac SUVmax data in patients with
rectal cancer and distant metastasis suggested that increased
glucose uptake by tumors may reduce glucose uptake by the heart.
Cardiac SUVmax may be related to the occurrence of distant
metastases and could influence the prognosis of rectal cancer.
Acknowledgements
The authors would like to thank Dr Kouta Kato, Dr
Masatoshi Ikeda, Dr Sae Moroto, Dr Shigeyuki Yoshiyama, Dr Ryo
Uratani, Dr Takeshi Yokoe and Dr Bunsuke Hara for support for the
present study in patient clinical practice. The authors would also
like to thank Dr Takashi Iwata and Dr Motoyoshi Tanaka for support
for the present study in patient clinical chemotherapy. The authors
would finally like to thank Dr Chikao Miki for support for the
present study in patient clinical practice and the research system
arrangement as a hospital director. All are from Iga City General
Hospital.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KS and YuO initially designed the present study (KS,
gastroenterology; YuO, radiology), collected data, analyzed data,
performed visual evaluation of PET-CT and wrote the manuscript. KS
and YuO confirm the authenticity of all the raw data. YA, RyoY, ST,
RyuY, TMi, HF, TMo, MH, KH and HS designed and advised the present
study based on clinical practice (gastroenterology), interpreted
data, checked and revised the manuscript. NT contributed experience
of PET-CT, designed and advised the present study on radiology,
interpreted data and checked and revised the manuscript. SS and KT
reported on related previous research (GLUT-1), designed and
advised the present study on molecular biology (GLUT-1), checked
the manuscript, interpreted data and revised the manuscript. YoO
checked and corrected the initial design, interpreted data and
checked and revised the manuscript. YT, KO and MN designed the
present study, interpreted data and checked and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was reviewed and approved by the Ethical
Review Board of the Iga City General Hospital (approved in February
2022; approval no. 785). Study opt-out was implemented through the
hospital website.
Patient consent for publication
Not applicable.
Competing interests
Iga City and the Iga City General Hospital have
established endowed chairs with the Department of Gastroenterology
and Hepatology at the Kansai Medical University and with the
Departments of Gastroenterology and Pediatric Surgery at Mie
University. This study was conducted as a research project with
Department of Gastroenterology and Hepatology at the Kansai Medical
University, Departments of Gastroenterology and Pediatric Surgery
at Mie University, and Iga city General Hospital at Iga city
General Hospital. YO received a lecture fee (not related this
research) and is an officer of EIIS (Expert Imaging and
Interventional Support) which charges for the imaging reading at
Iga city General Hospital (no reward as an officer). However, the
authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
SUVmax
|
maximum standard uptake value
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
PET-CT
|
positron emission tomography-CT
|
|
18F-FDG
|
18F-fluorodeoxyglucose
|
|
GLUT
|
glucose transporter
|
|
123I-BMIPP
|
123I-β-methyl-P-iodophenyl-pentadecanoic acid
|
|
LVDd
|
left ventricular end-diastolic
diameter
|
|
LVDs
|
left ventricular end-systolic
diameter
|
|
EF
|
ejection fraction
|
|
E/e
|
maximum value of early diastolic
filling velocity/maximum value of mitral annulus velocity
|
|
E/A
|
maximum value of early diastolic
filling velocity/maximum value of atrial filling wave velocity
|
|
CEA
|
carcinoembryonic antigen
|
|
AUC
|
area under the curve
|
|
ROC
|
receiver operating characteristic
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
MST
|
mean survival time
|
|
BSC
|
best supportive care
|
|
SD
|
standard deviation
|
References
|
1
|
Watanabe T, Muro K, Ajioka Y, Hashiguchi
Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S,
et al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2016 for the treatment of colorectal cancer. Int J Clin
Oncol. 23:1–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Horvat N, Carlos Tavares Rocha C, Clemente
Oliveira B, Petkovska I and Gollub MJ: MRI of rectal cancer: Tumor
staging, imaging techniques, and management. Radiographics.
39:367–387. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eglinton T, Luck A, Bartholomeusz D,
Varghese R and Lawrence M: Positron-emission tomography⁄computed
tomography (PET⁄CT) in the initial staging of primary rectal
cancer. Colectal Dis. 12:667–673. 2010. View Article : Google Scholar
|
|
4
|
Gearhart SL, Frassica D, Rosen R, Choti M,
Schulick R and Wahl R: Improved staging with pretreatment positron
emission tomography/computed tomography in low rectal cancer. Ann
Surg Oncol. 13:397–404. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nahas CS, Akhurst T, Yeung H, Leibold T,
Riedel E, Markowitz AJ, Minsky BD, Paty PB, Weiser MR, Temple LK,
et al: Positron emission tomography detection of distant metastatic
or synchronous disease in patients with locally advanced rectal
cancer receiving preoperative chemoradiation. Ann Surg Oncol.
15:704–711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bang JI, Ha S, Kang SB, Lee KW, Lee HS,
Kim JS, Oh HK, Lee HY and Kim SE: Prediction of neoadjuvant
radiation chemotherapy response and survival using pretreatment
[18F]FDG PET/CT scans in locally advanced rectal cancer. Eur J Nucl
Med Mol Imaging. 43:422–431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shanmugan S, Arrangoiz R, Nitzkorski JR,
Yu JQ, Li T, Cooper H, Konski A, Farma JM and Sigurdson ER:
Predicting pathological response to neoadjuvant chemoradiotherapy
in locally advanced rectal cancer using 18FDG-PET/CT. Ann Surg
Oncol. 19:2178–2185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krug B, Crott R, de Cannière L, D'Hondt L
and Vander Borght T: A systematic review of the predictive value of
18F-fluoro 2-deoxyglucose positron emission tomography on survival
in locally advanced rectal cancer after neoadjuvant chemoradiation.
Colorectal Dis. 15:e627–e633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tahara N, Tahara A, Honda A, Nitta Y,
Kodama Y and Fukumoto Y: Cardiac FDG-PET examinations. Heart.
45:1220–1228. 2013.(Article In Japanese).
|
|
10
|
Yoshinaga K, Naya M, Shiga T, Suzuki E and
Tamaki N: Ischaemic memory imaging using metabolic
radiopharmaceuticals: Overview of clinical settings and ongoing
investigations. Eur J Nucl Med Mol Imaging. 41:384–393. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dou KF, Xie BQ, Gao XJ, Li Y, Yang YJ, He
ZX and Yang MF: Use of resting myocardial 18F-FDG imaging in the
detection of unstable angina. Nucl Med Commun. 36:999–1006. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Groot M, Meeuwis AP, Kok PJ, Corstens
FH and Oyen WJ: Influence of blood glucose level, age and fasting
period on non-pathological FDG uptake in heart and gut. Eur J Nucl
Med Mol Imaging. 32:98–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nose H, Otsuka H, Otomi Y, Terazawa K,
Takao S, Iwamoto S, Iwase T, Yamada H, Sata M and Harada M: The
physiological uptake pattern of 18F-FDG in the left ventricular
myocardium of patients without heart disease. J Med Invest.
61:53–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inglese E, Leva L, Matheoud R, Sacchetti
G, Secco C, Gandolfo P, Brambilla M and Sambuceti G: Spatial and
temporal heterogeneity of regional myocardial uptake in patients
without heart disease under fasting conditions on repeated
whole-body 18F-FDG PET/CT. J Nucl Med. 48:1662–1669. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saigusa S, Toiyama Y, Tanaka K, Okugawa Y,
Fujikawa H, Matsushita K, Uchida K, Inoue Y and Kusunoki M:
Prognostic significance of glucose transporter-1 (GLUT1) gene
expression in rectal cancer after preoperative chemoradiotherapy.
Surg Today. 42:460–469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okumura N, Okada Y, Kumai K, Hosokawa T,
Oonuma J, Takata Y and Ito M: The changes in the 18F FDG
metabolism in the muscles by the use of cuboid support insoles.
Indian J Nucl Med. 37:178–185. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsushita K, Uchida K, Saigusa S, Ide S,
Hashimoto K, Koike Y, Otake K, Inoue M, Tanaka K and Kusunoki M:
Glycolysis inhibitors as a potential therapeutic option to treat
aggressive neuroblastoma expressing GLUT1. J Pediatr Surg.
47:1323–1330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu L, Qiu C, Wang X, Xu M, Shao X and Wang
Y: The association between diabetes mellitus and reduction in
myocardial glucose uptake: A population-based 18F-FDG
PET/CT study. BMC Cardiovasc Disord. 18:2032018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nuutinen J, Minn H, Bergman J, Haaparanta
M, Ruotasalainen U, Laine H and Knuuti J: Uncoupling of fatty acid
and glucose metabolism in malignant lymphoma: A PET study. Br J
Cancer. 80:513–518. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heckmann MB, Totakhel B, Finke D, Anker
MS, Müller-Tidow C, Haberkorn U, Katus HA and Lehmann LH: Evidence
for a cardiac metabolic switch in patients with Hodgkin's lymphoma.
ESC Hear Fail. 6:824–829. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi MH, Oh SN, Lee IK, Oh ST and Won DD:
Sarcopenia is negatively associated with long-term outcomes in
locally advanced rectal cancer. J Cachexia Sarcopenia Muscle.
9:53–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okugawa Y, Toiyama Y, Yamaoto A, Shigemori
T, Ide S, Kitajima T, Fujikawa H, Yasuda Y, Hito J, Yoshiyama S, et
al: Lymphocyte-C-reactive protein ratio as promising new marker for
predicting surgical and oncological outcomes in colorectal cancer.
Ann Surg. 272:342–351. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shirai Y, Morita S, Iwata T, Nakai H,
Yoshikawa M, Yoshida K, Iwamoto H, Miyaji K, Okugawa Y, Miki C and
Tanaka K: Anti-inflammatory and nutritional improvement effects of
dietary supplementation combined with fish oil in patients with
epithelial cancer. Oncol Lett. 24:3062022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Okugawa Y, Shirai Y, Toiyama Y, Saigusa S,
Hishida A, Yokoe T, Tanaka K, Tanaka M, Yasuda Y, Fujikawa H, et
al: Clinical burden of modified glasgow prognostic scale in
colorectal cancer. Clinical burden of modified glasgow prognostic
scale in colorectal cancer. Anticancer Res. 38:1599–1610.
2018.PubMed/NCBI
|
|
27
|
Okugawa Y, Shirai Y, Nodono H, Matutani F,
Ito M, Hishida A, Morimoto Y, Nishikawa R, Yokoe T, Tanaka K, et
al: Objective predictive score as a feasible biomarker for
short-term survival in terminalIy Ill patients with cancer. Anti
Cancer Res. 37:267–275. 2017.
|
|
28
|
Hishida A, Yamada H, Ando Y, Okugawa Y,
Shiozawa M, Miyagi Y, Daigo Y, Toiyama Y, Shidai Y, Tanaka K, et
al: Investigation of miRNA expression profiles using cohort samples
reveals potential early detectability of colorectal cancers by
serum miR-26a-5p before clinical diagnosis. Oncol Lett. 23:872022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang W, Ning N, Li X, Niu G, Bai L and
Guo Y: Changes of brain glucose metabolism in the pretreatment
patients with non-small cell lung cancer: A retrospective PET/CT
study. PLoS One. 11:e01613252016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fällmar D, Lilia J, Danfors T, Kilander L,
Iyer V, Lubberink M, Larsson EM and Sörensen M: Z-score maps from
low-dose 18F-FDG PET of the brain in neurodegenerative dementia. Am
J Nucl Med Mol Imaging. 8:239–246. 2018.PubMed/NCBI
|
|
31
|
Okada Y, Shiraishi M, Hori K, Yamaguchi K
and Hasegawa Y: Relationship between cerebral blood flow reduction
patterns on scintigraphy and nonmotor symptoms in new-onset Lewy
body disease. Nucl Med Rec Cent East Eur. 25:18–24. 2022.
View Article : Google Scholar : PubMed/NCBI
|