Introduction
Lung cancers are some of the most common and deadly
cancers worldwide, with a poor prognosis for those who are
diagnosed (1). Non-small cell lung
cancer (NSCLC) accounts for 85% of all lung cancer cases (2), with patients exhibiting low 5-year
survival rates (2–4). While a range of treatments, including
surgery, chemotherapy, radiotherapy and immunotherapy, have
improved NSCLC patient survival outcomes, tumor recurrence and
metastasis remain common and are associated with poor prognosis
(5–7). Although targeted treatment and
immunotherapy-based interventions have made some progress in the
treatment of metastatic NSCLC, these treatments are extremely
expensive, such that they are not widely accessible to the majority
of affected patients (8,9). The use of immune checkpoint inhibitors
(ICIs) is an attractive treatment option for both patients and
clinicians. First, these compounds have a wide range of activities
(10). Second, they often induce
durable disease control. For example, nivolumab is currently
associated with a total 5-year survival rate of 34% in patients
with advanced melanoma, while its effects against other cancers are
similar. Third, ICIs usually have good toxicity characteristics
(particularly anti-PD1/PD-L1 monotherapy) (11). Although ICIs have achieved great
success in clinical practice and become a milestone in anticancer
treatment, there are still certain clinical challenges with ICI
treatments, including resistance mechanisms, individual-level
efficacy differences, immune-related adverse reactions and highly
progressive diseases (12–14). Determining reliable predictive
biomarkers of efficacy, particularly toxicity, has been a major
challenge.
The clinical efficacy of CDK4/6 inhibitors in NSCLC
depends on the development of predictive biomarkers and
biologically rational combination therapy, which may include the
addition of growth factor pathway inhibitors in patients with
signal transduction pathway mutations or the addition of ICIs in
patients with immunostimulatory tumor phenotypes. Based on these,
more basic and clinical studies are needed explore the precise
beneficiaries of CDK4/6 inhibitors in NSCLC treatment in the future
(15). It is thus vital that novel
low-cost treatments for advanced NSCLC be developed that can
reliably treat this cancer type.
Traditional Chinese medicine (TCM) has long been
practiced in China to treat human diseases for thousands of years.
It is a valuable source for drug discovery campaigns (16). The traditional Chinese medicine
Uncaria has a long history, and Uncaria rhynchophylla
(UR; Gou-Teng in Chinese), a member of the Uncaria genus of
the Rubiaceae family, is found primarily in southern China.
In the practice of traditional Chinese medicine, UR was recorded in
the famous TCM monograph Ming Yi Bie Lu and has long been used in
China and Japan to extinguish wind, pacify the liver, clear heat
and arrest convulsions (17). It is
often clinically used in the treatment of central nervous system
and cardiovascular diseases, including hypertension, epilepsy,
dizziness, convulsions, preeclampsia and tremor (17). To date, a variety of compounds have
been isolated from this medicine, including alkaloids, quinolates,
terpenes and steroids, particularly indole and oxindole alkaloids,
i.e., isorhynchophylline, rhynchophylline, corynoxeine,
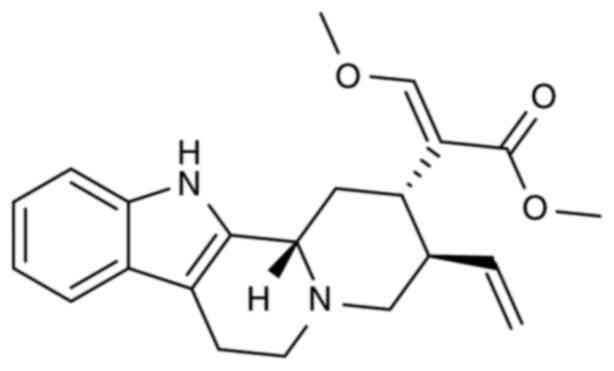
isocorynoxeine, hirsutine (HTI), hirsuteine (HTE) (PubChem ID,
169699) (Fig. 1) and geissoschizine
methyl ether (18,19). These alkaloids possess a variety of
pharmacological actions, together with a neuroprotective effect
(20–22), a vasodilatation effect (23–25), a
5-HT3 receptor binding effect (26)
and anticonvulsant effects (27).
HTI and HTE were two alkaloid monomers extracted from the
traditional Chinese medicine UR, which have pharmacological effects
including antihypertension, anti-infection and heart protection. In
terms of chemical structure, HTE has one more double bond and two
less hydrogen atoms than HTI (PubChem ID, 3037884), resulting in
the change of the chemical properties between the two substances.
In terms of chemical structure, that is why the antiproliferative
activity of HTE is not as strong as that of HTI (28). These findings indicated that
Uncaria hook alkaloids are the most important constituents
when studying HTE efficacy.
Previous studies have demonstrated that HTE could
suppress HepG2 human tumor cell migration and proliferation in a
dose-dependent manner, protecting against glutamate-induced cell
death in PC12 cells or cultured cerebellar granule cells (29–31). A
recent study by the authors showed that HTE could enhance the
inhibition of T-cell leukemia Jurkat Clone E6-1 cell proliferation
(32). However, no studies to date
have assessed the ability of HTE to suppress NSCLC NCI-H1299 cell
growth, nor have the underlying pathways governing the
pro-apoptotic and antitumor activity of this alkaloid drug been
explored in detail.
In the present study, the antiproliferative effect
of HTE and its mechanism of action in human NSCLC NCI-H1299 cells
were explored, revealing the ability of this alkaloid agent to
cause apoptosis and cell cycle arrest in these malignant cells.
Materials and methods
Reagents
HTE (ST17300105, 5 mg/dose, ≥98% pure) was obtained
from Shanghai Standard Technology Co., Ltd.
Cell lines and cell culture
Human NCI-H1299 cells were acquired from the
Institute of Biochemistry (Shanghai, China) and were cultured in
RPMI-1640 (Invitrogen; Thermo Fisher Scientific, Inc.) culture
medium at 37°C with 5% CO2 following incubation under a
humidified atmosphere. Human THLE-2 hepatocytes and normal BEAS-2B
cells were obtained from Nanjing KeyGen Biotech Co., Ltd. and
subsequently cultured in Dulbecco's modified Eagle's medium
(HyClone; Cytiva). Media were supplemented with pen/strep and 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.).
Drug treatments
HTE was suspended in DMSO and stored at −20°C at a
stock concentration of 100 mM. RPMI-1640 medium was used to dilute
the stock to 0, 10, 20 and 40 µM. The final DMSO concentration in
each working solution was kept at 0.1%. In the untreated cell
group, RPMI-1640 with 0.1% DMSO was used.
Cell counting kit-8 (CCK-8) assay
Cell proliferation was measured using a CCK-8 assay.
Briefly, NCI-H1299 cells at a density of 10,000 cells per well were
added to 96-well plates in complete F12K media, with samples being
prepared in triplicate. Next, the cells were exposed to a range of
HTE concentrations for 24, 48 and 72 h, after which 100 µl CCK-8
solution (Beyotime Institute of Biotechnology) was mixed with the
cells for another 4 h of incubation at 37°C. Then, an assay using
water-soluble tetrazolium salt was performed to assess cell
proliferation by quantifying the absorbance at 450 nm with the help
of a microplate reader (Bio-Rad Laboratories, Inc.) according to
the manufacturer's protocol. The assay protocols for THLE-2 and
BEAS-2B cells were as aforementioned, with cells plated at 4,000
cells/well and treated for 48 h with a range of HTE concentrations
prior to CCK-8 reagent addition.
Colony formation assay
To assess cell proliferation, 2–5×103
cells were added in triplicate to six-well plates. After 24 h,
cells were treated with different HTE concentrations (0, 10, 20 and
40 µM). After being cultured for 14 days, the cells were washed
twice with PBS, fixed for 15 min with 75% ethanol and stained for
20 min at room temperature with 0.1% crystal violet (Beyotime
Institute of Biotechnology), with all colonies containing ≥30 cells
being counted manually. Plate images were captured using an Epson
scanner (Seiko Epson Corporation).
Apoptosis staining
Flow cytometry was carried out to detect cell
apoptosis after labelling with Annexin V-FITC. After harvesting
using 0.25% trypsin and spinning down for 3 min at 300 × g
NCI-H1299 cells in the logarithmic phase of growth were seeded for
24 h in six-well plates at a density of 3×105
cells/well. A range of HTE concentrations (0, 10, 20 and 40 µM) was
applied to the cells for 48 h with 0.1% DMSO as the negative
control. After three washes with chilled PBS, the cells were
collected and resuspended in 500 µl of cold binding buffer.
Incubation was performed for 15 min at room temperature in the dark
following mixing with 5 µl of Annexin V-FITC and 5 µl of propidium
iodide (PI). A CytoFLEX flow cytometry instrument (Beckman Coulter,
Inc.) was used to analyze the samples, and the data were processed
using FlowJo V10.0 software (Becton, Dickinson and Company). All
experimental data were acquired in triplicate.
Cell cycle analysis
Flow cytometry was performed to analyze the cell
cycle. Briefly, cells in the log phase were plated at a density of
3×105 cells/well in a six-well plate and incubated for
24 h. After incubation, the medium was exchanged for complete
medium containing a range of HTE concentrations (0, 10, 20 and 40
µM) for an additional 48 h. Cells were then harvested with 0.25%
trypsin, centrifuged at 4°C for 3 min at 300 × g washed once with
PBS, and fixed overnight at 4°C with 70% chilled ethanol. After
overnight incubation, the cells were spun down again, treated with
100 µl of RNase A (50 µg/ml), and resuspended in a water bath at
37°C for 30 min. The cells were then stained for 30 min at 4°C in
the dark with 400 µl of PI (50 µg/ml). All samples were evaluated
by using the FACScan system (BD Biosciences). Data were examined by
Cell-Quest software (BD Biosciences). All experiments were
performed three times independently.
Western blotting
Following 48 h of treatment with HTE (0, 10, 20 and
40 µM), chilled RIPA buffer (Beyotime Institute of Biotechnology)
was used for cell lysis for 30 min, after which the cell lysates
were centrifuged at 4°C for 15 min at 13,201 × g. The amounts of
protein in the supernatants were estimated by BCA assay (Beyotime
Institute of Biotechnology). Next, separation of equal amounts of
protein (40 µg) was achieved using 10–12% SDS-PAGE. After
separation, the proteins were transferred to PVDF membranes.
Membranes were blocked in 5% non-fat milk in TBS containing 0.1%
Tween-20 (TBST) for 2 h at room temperature. Overnight incubation
was then performed at 4°C with primary anti-CDK2 (cat. no. A0294;
ABclonal Biotech Co., Ltd.), anti-cyclin E (cat. no. A14225;
ABclonal Biotech Co., Ltd.), anti-Bax (cat. no. 50599-2-Ig;
Proteintech Group, Inc.), anti-Bcl-2 (cat. no. 12789-1-AP;
Proteintech Group, Inc.), anti-Apaf1 (cat. no. A0751; ABclonal
Biotech Co., Ltd.), anti-cytochrome C (cat. no. Ab133504; Abcam),
anti-cleaved caspase-9 (cat. no. AF5240; Affinity Biosciences), or
anti-cleaved caspase-3 (cat. no. Ab32042; Abcam) antibodies, all
diluted 1:1,000. The blots were then washed three times with TBST
for 45 min, followed by incubation for 2 h with HRP-conjugated goat
anti-rabbit IgG (1:5,000; cat. no. BA1054) or goat anti-mouse IgG
(1:5,000; cat. no. BA1051; both from Boster Biological Technology).
Immunoblotted proteins were analyzed with the ChemiDoc XRS imaging
system and QuantityOne software (Version 4.6.9; Bio-Rad
Laboratories, Inc.).
Reverse transcription quantitative
(RT-q) PCR analysis
Cells in the logarithmic phase were cultured in a
six-well plate at a density of 1×106 cells/well for 48
h. The cells were then treated with different doses of HTE for 48
h. Total RNA from the cultured cells was isolated using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Afterwards, cDNA synthesis was performed according to the
protocol of the QuantiTect Reverse Transcription Kit (Invitrogen;
Thermo Fisher Scientific, Inc.). qPCR was conducted on an ABI 7500
Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with TaqMan™ Multiplex Master Mix (Invitrogen;
Thermo Fisher Scientific, Inc.) with the following thermocycling
conditions: Denaturation at 95°C for 10 min and 40 cycles of 95°C
for 30 sec, annealing for 30 sec at 60°C and extension for 20 sec
at 72°C. The following primer sequences were used for the qPCR
assay: Bax forward, 5′-AAGAAGCTGAGCGAGTGTCT-3′ and reverse,
5′-GTTCTGATCAGTTCCGGCAC-3′ (236 bp); Bcl-2 forward,
5′-GCCTTCTTTGAGTTCGGTGG-3′ and reverse, 5′-GAAATCAAACAGAGGCCGCA-3′
(192 bp); and GAPDH forward, 5′-TCAAGAAGGTGGTGAAGCAGG-3′ and
reverse, 5′-TCAAAGGTGGAGGAGTGGGT-3′ (115 bp). All PCRs were
performed in triplicate. Data were analyzed using the
2−ΔΔCq method (33) with
GAPDH as the reference gene for normalization.
Statistical analysis
The mean ± standard deviations of at least three
independently conducted experiments were used to represent the data
collected in all studies. All statistical analyses were performed
using SPSS 23.0 software version (IBM Corp.). Significant
differences were performed by one-way analysis of variance,
followed by Bonferroni's test. A value of P<0.05 was considered
to indicate a statistically significant difference.
Results
HTE suppresses NSCLC NCI-H1299 cell
proliferation and colony formation
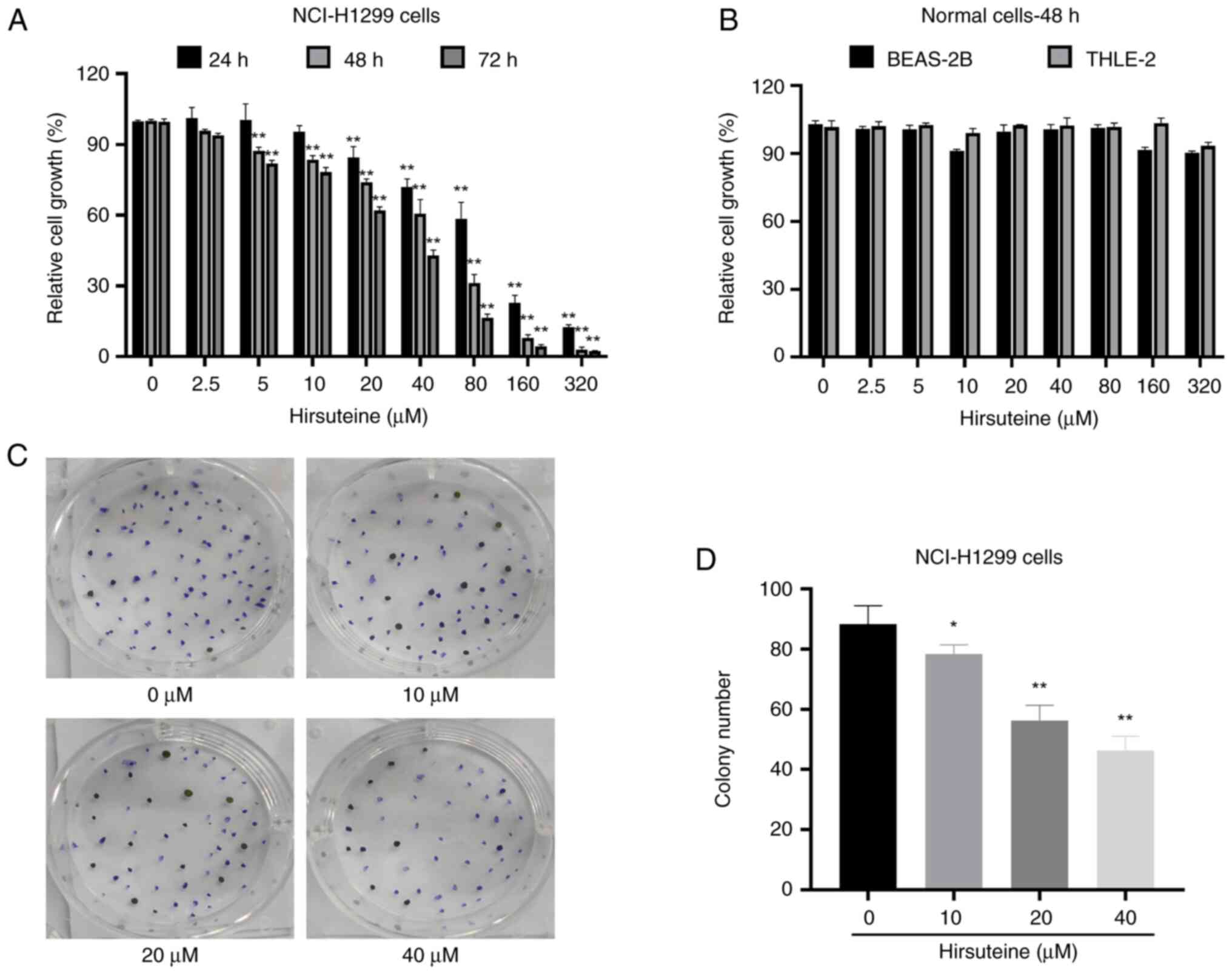
To investigate the effect of HTE on cell
proliferation, the NCI-H1299 cell line was finally selected as the
study cell line according to the pre-experimental data after the
different concentrations of HTE were applied to different types of
cancer cells (data not shown). According to the experimental
results of CCK-8, it is assumed that the HTE solution is stable
enough under the culture conditions. The cell proliferation
inhibition rate of the HTE on NCI-H1299 cells at 72 h is more
obvious than that at 48 h. Referring to previous literature, the
treatment time point for follow-up analysis was set at 48 h
(34). As revealed in Fig. 2A, the ability of HTE to inhibit the
proliferation of NCI-H1299 cells significantly increased in a time-
and concentration-dependent manner with half maximal inhibitory
concentration values of 82.81, 43.74 and 27.02 µM at these three
time points, respectively, when compared with the control group
(Fig. 2A). Next, the impact of HTE
on the proliferation of BEAS-2B and human hepatocyte THLE-2 cells
was explored using a CCK-8 assay. The results showed that the cell
viability after HTE treatment in normal cells was >80% after 48
h of treatment at doses up to and including 80 µM; thus, HTE was
considered to be non-toxic to these non-cancerous cell lines
(Fig. 2B). To expand on these
results, a colony formation assay was performed, which revealed
that HTE treatment suppressed NCI-H1299 colony formation activity
relative to control treatment (Fig. 2C
and D). Based on the results, HTE inhibited NCI-H1299 cell
proliferation and lowered NCI-H1299 colony formation activity
whilst not exerting a notable effect on healthy human cells at the
same doses.
HTE induces cell cycle arrest
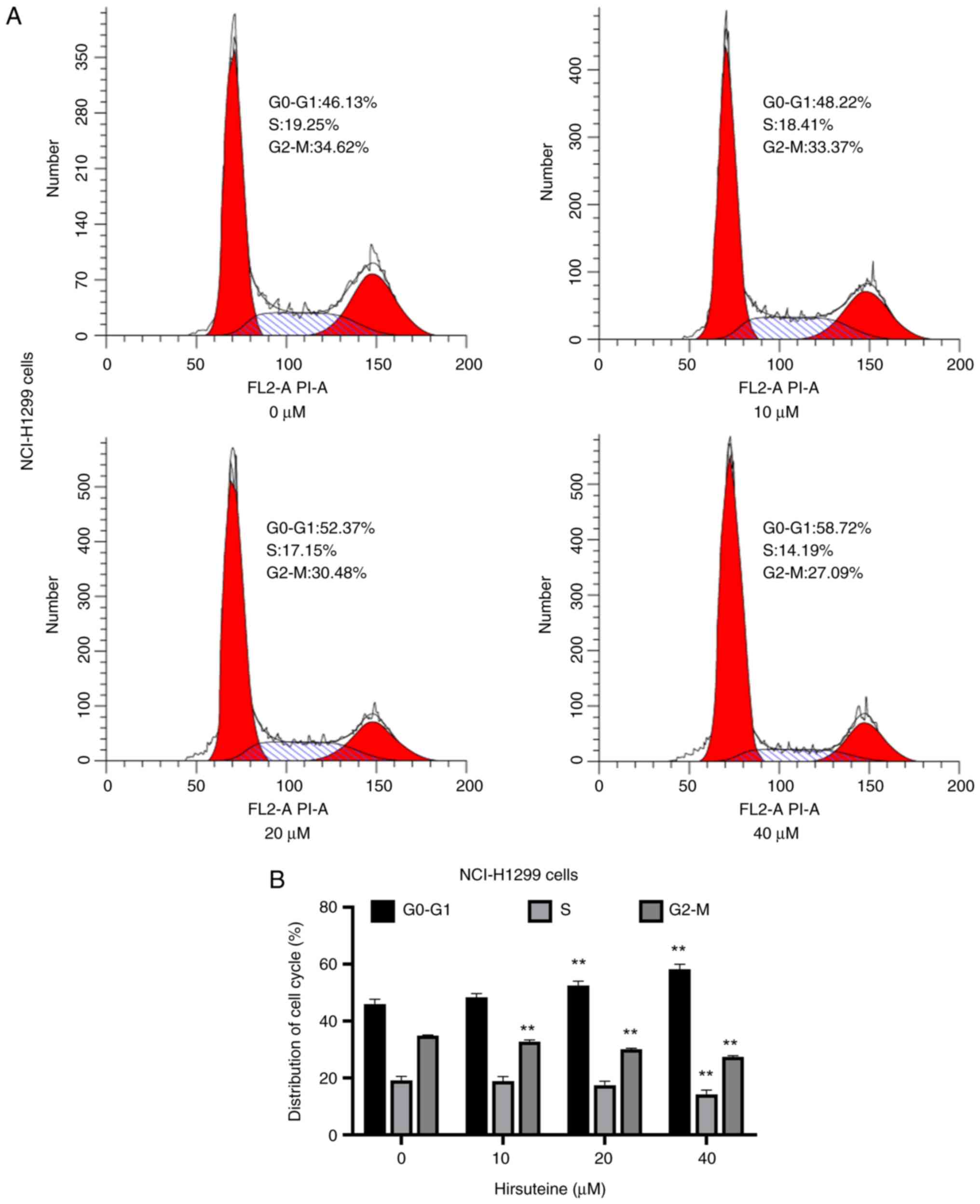
Following treatment with several HTE doses for 48 h,
the effect of HTE on NCI-H1299 cell cycle progression was
investigated. In the diagram of the cell cycle, the red portion on
the left represents G0-G1 phase, the curve marked in the middle
represents S phase, and the red portion on the right indicates G2-M
phase. Flow cytometric analysis revealed that HTE treatment (0, 10,
20 and 40 µM) was associated with an increase in the frequency of
NCI-H1299 cells in G0/G1 phase. A significant increase in the
proportion of NCI-H1299 cells in G0-G1 phase was observed in the
HTE treatment groups (20 and 40 µM) (Fig. 3).
HTE decreases the levels of cyclin and
CDK in NCI-H1299 cells
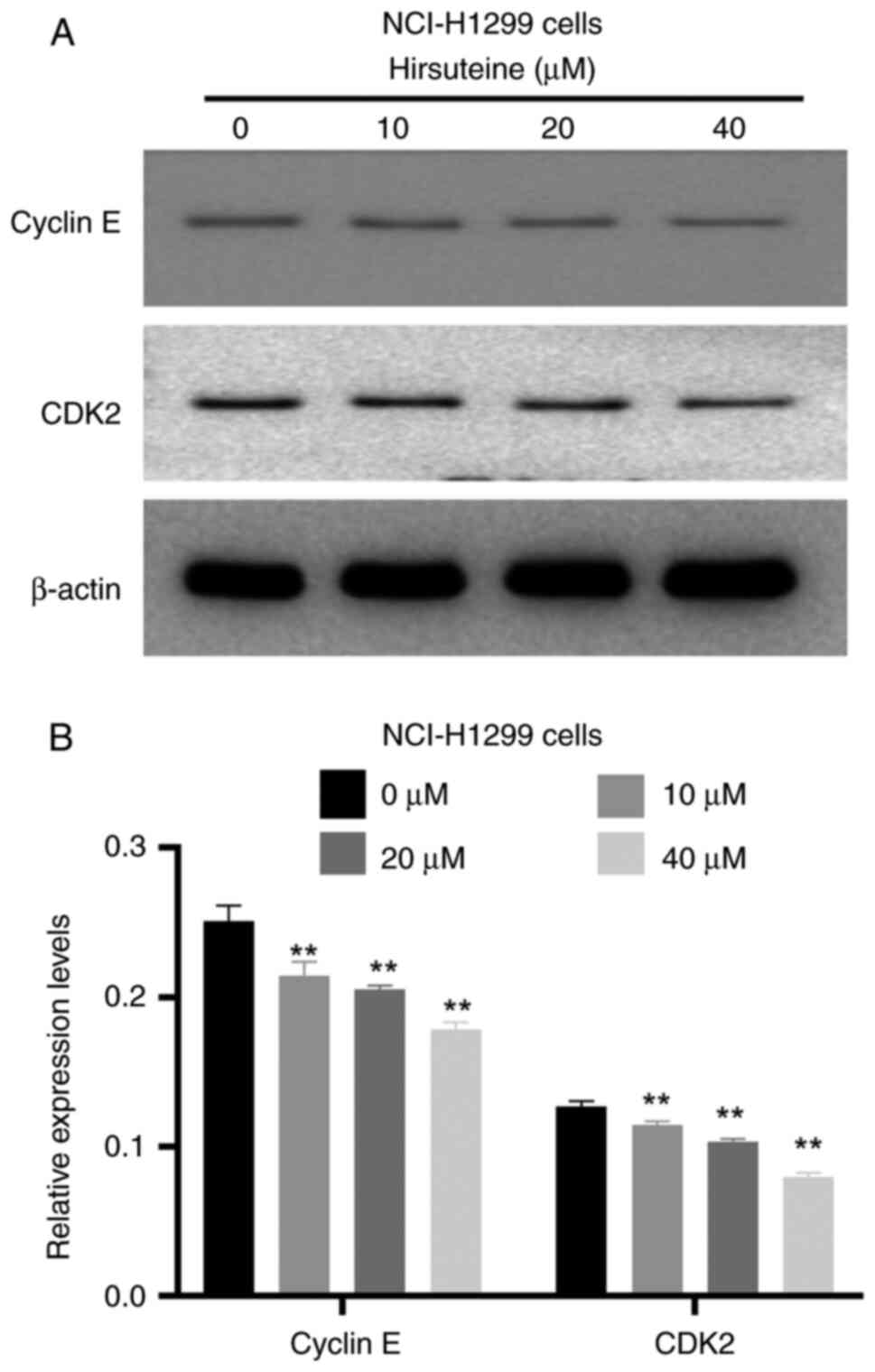
To investigate the mechanism by which the cell cycle
is arrested in NCI-H1299 cells, the expression of cell
cycle-related proteins was next determined via western blotting in
HTE-treated cells. Significant reductions in the CDK2 and cyclin-E
expression levels were observed in NCI-H1299 cells treated with HTE
(0, 10, 20 and 40 µM) (Fig. 4A and
B), suggesting that HTE may be responsible for G0-G1 phase
arrest.
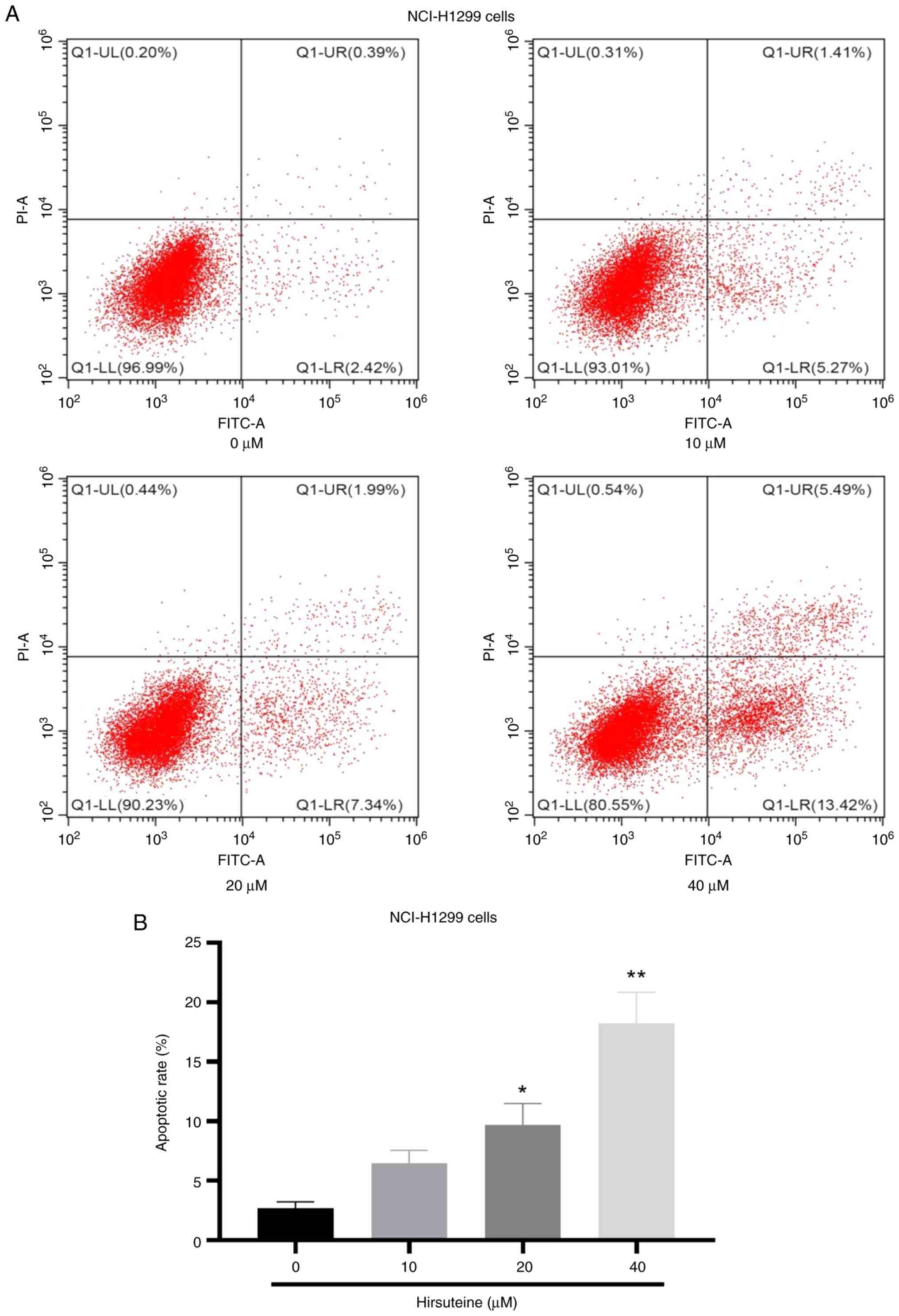
HTE induces NCI-H1299 cell apoptotic
death
As indicated in Fig.
5, the ability of HTE to induce NCI-H1299 cell apoptosis was
next assessed via flow cytometry, which confirmed the increase in
NCI-H1299 cell apoptosis frequency after treatment with HTE.
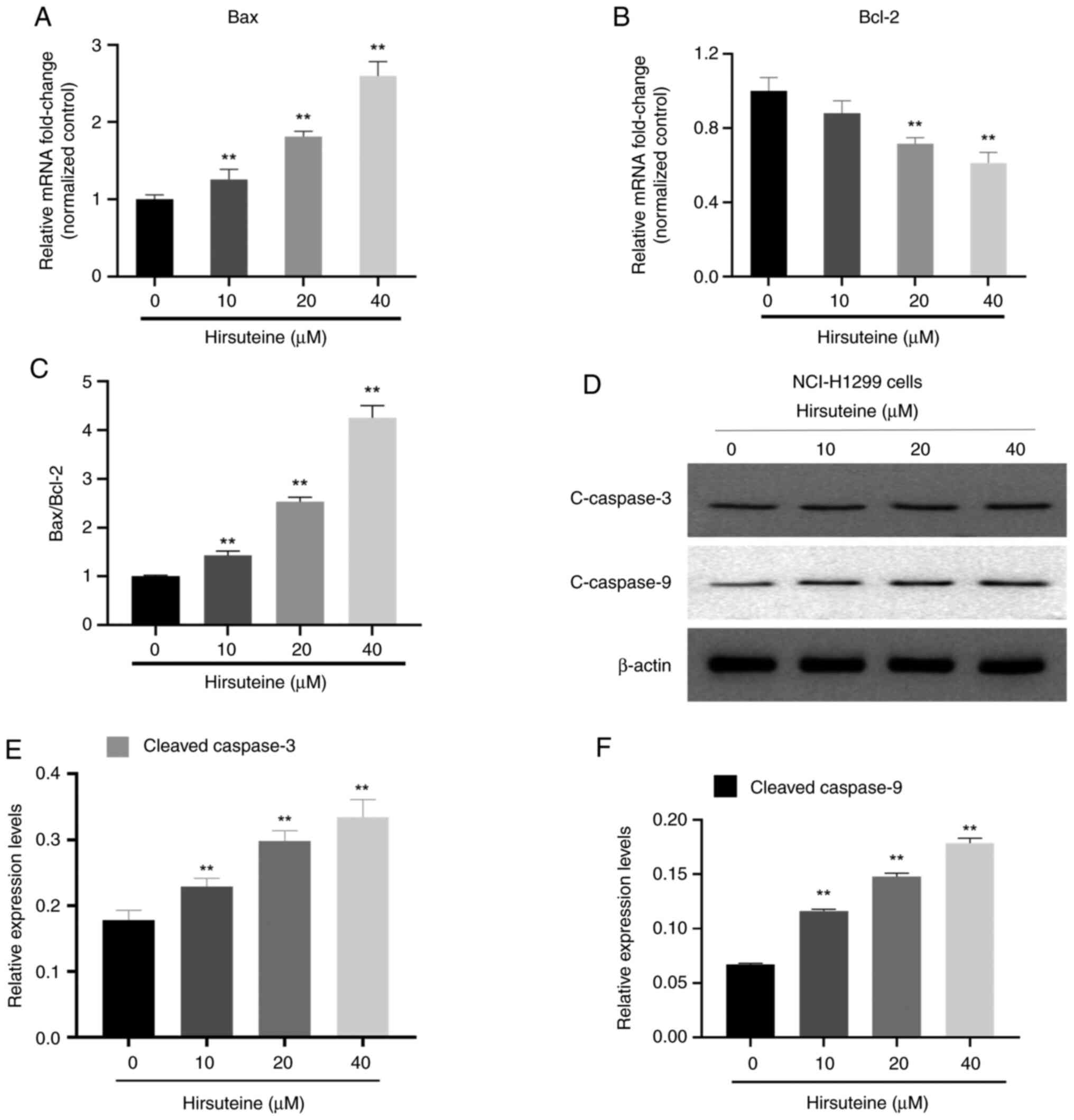
HTE treatment alters the expression of
apoptosis-related genes and proteins
To explore the mechanisms underlying HTE-induced
apoptosis in these NSCLC cells, the cellular Bax and Bcl-2 levels
were assessed following 48 h of treatment with this drug through
RT-qPCR and western blot analyses. In the RT-qPCR experiments, the
Bax mRNA levels increased in NCI-H1299 cells whereas the expression
levels of Bcl-2 decreased following treatment with HTE. These
effects were significant at HTE concentrations of 0, 10, 20 and 40
µM compared with untreated cells (P<0.05 or P<0.01) (Fig. 6A and B). HTE may decrease Bcl-2 mRNA
levels while increasing the mRNA content of Bax, resulting in a
higher pro-apoptotic vs. anti-apoptotic protein ratio (Fig. 6C). The expression levels of cleaved
caspase-3 and cleaved caspase-9 were analyzed using western blot
ting to assess antiproliferative activity of HTE-induced NCI-H1299
cells, and the results indicated that the expression levels of
cleaved caspase-3/9 increased following treatment with HTE
(Fig. 6D-F). These results
demonstrated that HTE could cause death in NCI-H1299 cells via a
caspase-mediated mechanism.
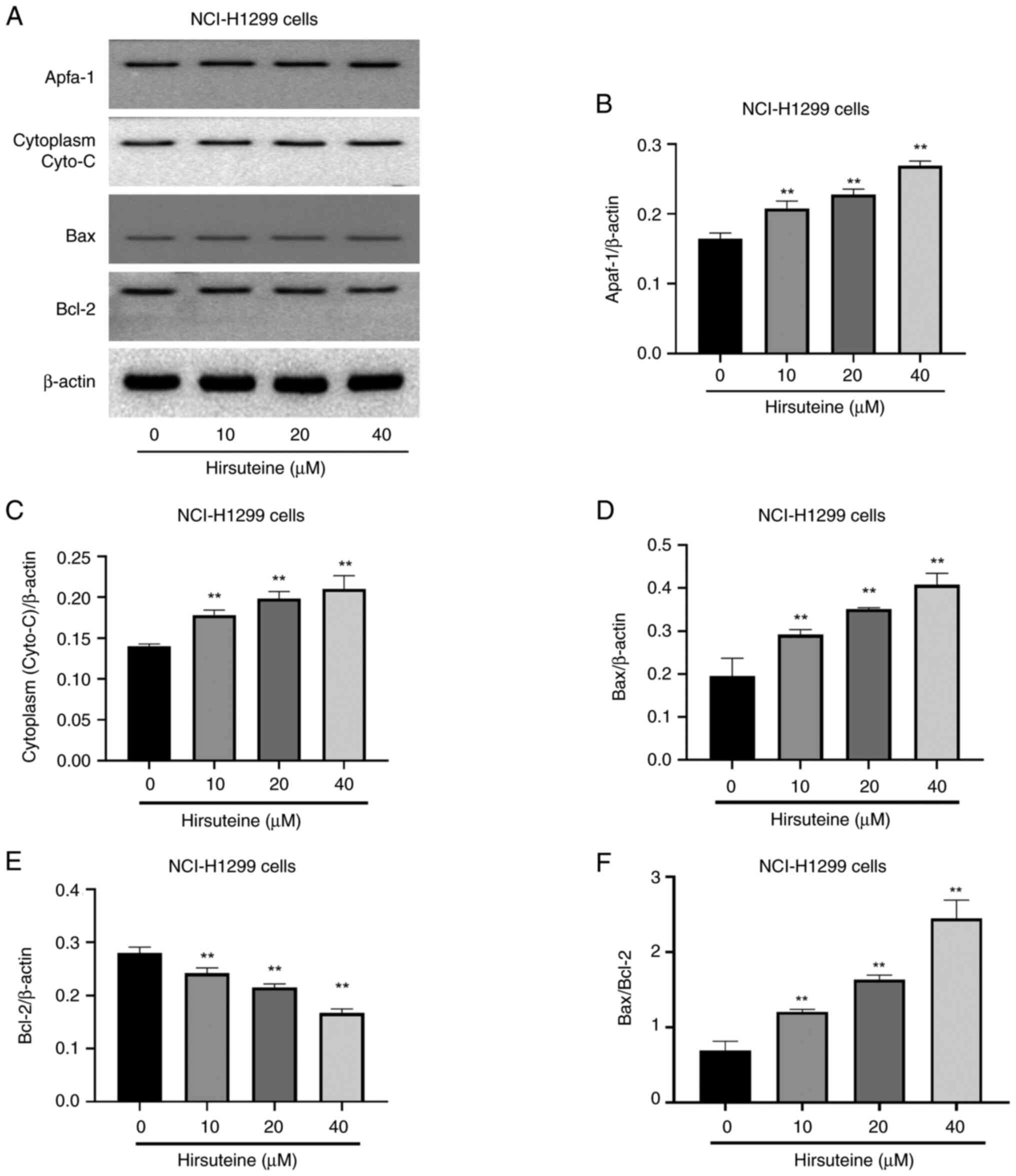
HTE induces NCI-H1299 cell apoptotic
death via the Bcl-2/Bax signaling pathway
Moreover, it was aimed to further understand the
underlying mechanisms of HTE-induced apoptosis in NCI-H1299 cells.
Western blotting was performed to determine the expression levels
of the pro-apoptotic proteins and anti-apoptotic proteins Bax and
Bcl-2, as well as cytoplasmic cytochrome c (Cyto-C) and Apaf1 in
vitro. HTE treatment resulted in Bcl-2 downregulation and
increased cytoplasmic Cyto-C, Bax and Apaf1 levels, causing the
Bax/Bcl-2 ratio to be unbalanced (Fig.
7A-F), and the high-dose HTE group demonstrated a high level of
expression in comparison with the control group (P<0.05 or
P<0.01). By contrast, expression was lower in the low-dose HTE
group than in the high-dose HTE group, indicating that HTE induces
apoptosis in NCI-H1299 cells in a dose-dependent fashion. All of
these data demonstrated that HTE activates the Bcl-2/Bax signaling
pathway, causing apoptosis in NCI-H1299 cells.
Discussion
NSCLC is one of the principal causes of
cancer-related morbidity and mortality (2). There has been increasing interest in
finding natural compounds that can act as preventive and
therapeutic agents for cancer treatment. UR is a well-known
traditional Chinese herbal medicine that is used for the treatment
of a variety of malignancies as well as other disorders, including
hypertension and Parkinson's disease (34–37).
HTE is a natural compound from the bark of UR, and few studies to
date have explored the antiproliferative properties of this novel
drug. In the present study, it was found that HTE treatment was
sufficient to inhibit NCI-H1299 cells from proliferating as
determined by CCK-8 and colony formation experiments, and
subsequent Annexin V/PI staining demonstrated that this treatment
may stimulate cell apoptosis and cell cycle arrest in these NSCLC
cells. HTE was also able to modify the cell cycle by affecting
apoptosis-related protein levels and upstream regulatory signaling
pathways.
Cell cycle dysregulation is a hallmark of cancer.
Under normal physiological conditions, the cell cycle exhibits
tight multilayer regulation (38),
carefully controlling the progression of the cell through G1 phase
before entering into DNA synthesis, S phase, during which DNA is
replicated, and the subsequent G2 phase. Cyclin-dependent kinases
(CDKs) and their associated cyclins form complexes with one another
to govern cell cycle regulation, and they are highly evolutionarily
conserved in eukaryotic species (39–41),
with substantial cyclin and CDK gene expansion and
subfunctionalization observed across species (42–44).
Adverse events related to ICIs are particularly challenging to
control with adjuvant therapies because delayed (occasionally
severe) toxicity may cause permanent injury to patients who may
have had their tumorigenic disease cured by surgery (13). The expression and activation of cell
cycle mediators is deranged, particularly within the CDK-cyclin-RB
pathways, and is involved in malignant transformation and tumor
progression in lung cancer (45).
In >90% of lung cancers, the cell cycle occurs as dysregulation,
which causes the disorder of cell cycle mediators in expression
and/or activation, particularly within the CDK-cyclin-RB pathways,
and is closely related to malignant transformation and tumor
progression, which destroys the cell proliferation mechanism
controlling the growth of advanced NSCLC (45–47).
However, the clinical trial results of evaluating single-dose CDK
inhibitors in NSCLC and SCLC patients were disappointing. In
addition, cell cycle regulation involves complex interactions of
multiple signal pathways and mediators. Therefore, although
targeted CDK family members may display preclinical activity in
selective tumor models, they may not be sufficient to produce
meaningful responses in more complex clinical environments
(45). CDK inhibitors have
favorable antitumor effects, but their lack of accuracy, side
effects to biological targets and limitations of the therapeutic
population lead to unsatisfactory therapeutic results. Therefore,
embedding the CDK pathway in lung cancer treatment remains
challenging (45). The current
western blotting data indicated that HTE treatment reduces CDK2 and
cyclin E levels in HTE-treated NCI-H1299 cells, causing cell cycle
arrest in G0/G1 phase and an increase in the relative population of
cells' G1 phase.
Apoptosis is an essential cellular process in which
cells are constrained to self-destruction (48). Two classical activation pathways are
involved in the initiation and regulation of apoptosis (49). The intrinsic apoptotic pathway is
activated by stress-related intracellular signals, including the
mitochondrial intermembrane protein Cyto-C, which initiates signal
transduction cascades and eventually activates caspase-3. The
intrinsic apoptotic pathway is regulated by a family of Bcl-2
proteins and has been broadly grouped into two key regulatory
categories, including pro-apoptotic proteins (Bax, Bak and Bok/MTD)
and anti-apoptotic proteins (including Bcl-2) (50). Numerous cancers exhibit
overexpression of Bcl-2, which influences the onset, progression
and chemoresistance of these tumors (51–53).
Overexpression of Bax, by contrast, can induce apoptotic death
(54). A reduction in mitochondrial
membrane potential (MMP) occurs due to a drop in the relative
anti-apoptotic to pro-apoptotic Bcl-2 family protein ratio and
leads to apoptosis (55). HTE
treatment in the present study resulted in significant
downregulation of Bax expression, whereas Bax levels were
significantly elevated upon treatment. The results showed that the
ratio of Bax/Bcl-2 expression exhibited an increasing tendency with
increasing HTE concentration, and this ratio increased
significantly when the cells were stimulated by 10 and 25 µmol/l
HTE. These findings complemented the prior findings of decreased
cell viability and increased apoptosis, suggesting that HTE may
impact NCI-H1299 cells by modulating the Bcl-2 family-dependent
intrinsic apoptotic pathway. Notably, the expression levels of the
Bcl-2 family members, and particularly the ratio of pro-apoptotic
Bax/anti-apoptotic Bcl-2, are widely used to indicate an intrinsic
apoptotic mechanism (56).
The initiation of apoptotic signaling cascades
within cells can drive increased mitochondrial permeability
(57). Bax is widely distributed on
the outer mitochondrial membrane to control MMP and thereby
influences the release of Cyto-C and other drivers of apoptotic
death (58). The released activated
cytochrome can, in turn, bind to Apaf1 and thereby activate
caspase-9 (59,60), subsequently stimulating caspase-3
and caspase-7 to initiate apoptotic cascade signaling pathways
(57). The expression of these
apoptosis-related proteins was investigated in NCI-H1299 cells, and
HTE administration was identified to cause dose-dependent apoptosis
in these NSCLC cells. HTE was also linked to increases in Apaf1,
Bax, cytoplasmic Cyto-C, cleaved-caspase-3 and −9 levels, as well
as the downregulation of Bcl-2 expression. These data suggested
that HTE can thus initiate the mitochondrial-mediated signaling
pathway of apoptosis in NCI-H1299 cells by modulating the relative
Bcl-2-related protein levels.
Caspases are important in the pro-apoptotic process.
Caspase-8 is involved in extrinsic (mitochondrial) apoptosis
pathways, whereas caspase-9 is involved in intrinsic
(mitochondrial) apoptosis pathways (61). The increase in the ratio of
Bax/Bcl-2 promotes mitochondrial dysfunction, the release of some
apoptotic factors, and caspase-9 activation. Then, active caspase-9
cleaves the downstream apoptosis effector caspase-3 (62). When Cyto-C is transferred to the
cytoplasm from the mitochondria, it forms a large multiprotein
complex with Apaf1, procaspase-9 and Cyto-C, and activation of the
intrinsic mitochondria-mediated pathway occurs. In the current
investigation, HTE significantly increased the activity of Bax,
caspase-3, and caspase-9 in NCI-H1299 cells. This indicated that
the mitochondria-mediated apoptosis pathway was activated, and that
subsequent apoptosis was induced by the caspase-dependent and
Cyto-C-mediated pathways. In addition, HTE-induced apoptosis is
also involved the delivery of the flavoprotein apoptosis-inducing
factor (AIF) from the mitochondria to the cytoplasm through a
subsequent dose-dependent shift in the nucleus. The expulsion of
AIF from mitochondria to the nucleus, while translocating from the
cytosol, interacts with DNA, thus functioning through a
caspase-independent pathway. Similar findings have been previously
reported, stating that mitochondria-mediated pathways are important
in A. adenophora-induced apoptosis and cell death (63–65).
HTE may also cause cell death and apoptosis as a result of
mitochondria-mediated pathway stimulation.
The present study demonstrated that HTE could
inhibit NCI-H1299 cell proliferation by inducing apoptosis through
the Bax/Bcl-2 signaling pathway and induce G0/G1 phase arrest
through cyclin E- and CDK2-mediated mechanisms. The current
findings offer a theoretical basis to understand the underlying
mechanism of the antiproliferative effect of HTE and show that this
compound can be used as an effective natural medicine in the
treatment and prevention of human NSCLC.
Ultrahigh-Performance Liquid Chromatography-Mass
Spectrometry (UPLC-MS) was used for the detection of HTI and HTE in
tissues to help understand in an improved way the pharmacological
mechanisms of HTI and HTE (28,66).
However, certain limitations to the present study should be noted.
Numerous compounds were screened with different types of cells in
the pre-experiment including HTI and HTE. According to the results
of the preliminary experiment CCK-8, the anti-proliferation effect
of HTI on cells is stronger than that of HTE. As HTI has been
previously investigated before, the activity of HTE was researched
(34). The difference between the
anti-proliferative effect of HTI and HTE appears in the
pre-experimental data (data not shown), as a reference for the
preliminary research of this project. In the future study by the
authors, the data of the pre-experiment shall be included in the
manuscript. Furthermore, the antitumor effects of HTE were not
examined in a lung-xenografted model in nude mice. Moreover,
another weakness was that only one cell line was used in the
present study. The NCI-H1299 cell line was finally selected as the
study cell line according to the pre-experimental data (data not
shown) after the different concentrations of HTE were applied to
different types of cancer cells. Using additional cell lines such
as A549 is recommended in future studies. The stability of the HTE
is only based on the results of CCK-8, and no specific test has
been carried out on the stability. In addition, UPLC-MS technology
will be used to detect the concentration of HTE in patient lung
tissues and in vivo experiments will be increased in future
research by the authors. In conclusion, the results of the present
study revealed that HTE inhibits cell proliferation in NCI-H1299
cells and induces apoptosis via a mitochondrial-mediated signaling
pathway. The current study provides evidence for the development of
HTE as an anti-lung drug.
In summary, the present data revealed that HTE can
suppress NCI-H1299 cell proliferation by regulating the proteins
CDK2 and cyclin E, inducing their apoptotic death via the
mitochondrial signaling pathway. The present study not only
highlights a promising new treatment for human NSCLC but also
offers a sound theoretical foundation for the proliferative
activity of this deadly disease.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY and BY made substantial contributions to
conception and design of the research. XY analyzed the data,
performed the experiments, prepared the figures and reviewed or
authored drafts of the manuscript. HQ, YL and BD collected cell
samples for qPCR and made substantial contributions to acquisition
of data, or analysis and interpretation of data. YP made
substantial contributions to analysis and interpretation of data.
BY made substantial contributions to the revision of the manuscript
and gave final approval of the manuscript to be published. XY and
BY confirm the authenticity of all the raw data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL and Jemal A: Lung
cancer statistics. Adv Exp Med Biol. 893:1–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duma N, Santana-Davila R and Molina JR:
Non-small cell lung cancer: Epidemiology, screening, diagnosis, and
treatment. Mayo Clin Proc. 94:1623–1640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brueckl WM, Achenbach HJ, Ficker JH and
Schuette W: Erlotinib treatment after platinum-based therapy in
elderly patients with non-small-cell lung cancer in routine
clinical practice-results from the ElderTac study. BMC Cancer.
18:3332018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirsch FR, Sequist LV, Gore I, Mooradian
M, Simon G, Croft EF, DeVincenzo D, Munley J, Stein D, Freivogel K,
et al: Long-term safety and survival with gefitinib in select
patients with advanced non-small cell lung cancer: Results from the
US IRESSA clinical access program (ICAP). Cancer. 124:2407–2414.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ciuleanu T, Stelmakh L, Cicenas S,
Miliauskas S, Grigorescu AC, Hillenbach C, Johannsdottir HK,
Klughammer B and Gonzalez EE: Efficacy and safety of erlotinib
versus chemotherapy in second-line treatment of patients with
advanced, non-small-cell lung cancer with poor prognosis (TITAN): A
randomised multicentre, open-label, phase 3 study. Lancet Oncol.
13:300–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olszewski AJ, Ali S and Witherby SM:
Disparate survival trends in histologic subtypes of metastatic
non-small cell lung cancer: A population-based analysis. Am J
Cancer Res. 5:2229–2240. 2015.PubMed/NCBI
|
|
7
|
Chang JS, Chen LT, Shan YS, Lin SF, Hsiao
SY, Tsai CR, Yu SJ and Tsai HJ: Comprehensive analysis of the
incidence and survival patterns of lung cancer by histologies,
including rare subtypes, in the era of molecular medicine and
targeted therapy: A nation-wide cancer registry-based study from
Taiwan. Medicine (Baltimore). 94:e9692015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jonna S and Subramaniam DS: Molecular
diagnostics and targeted therapies in non-small cell lung cancer
(NSCLC): An update. Discov Med. 27:167–170. 2019.PubMed/NCBI
|
|
9
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wolchok JD: PD-1 blockers. Cell.
162:9372015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnson DB, Sullivan RJ and Menzies AM:
Immune checkpoint inhibitors in challenging populations. Cancer.
123:1904–1911. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma K, Jin Q, Wang M, Li X and Zhang Y:
Research progress and clinical application of predictive biomarker
for immune checkpoint inhibitors. Expert Rev Mol Diagn. 19:517–529.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Miguel M and Calvo E: Clinical
challenges of immune checkpoint inhibitors. Cancer Cell.
38:326–333. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bando Y, Furukawa J, Terakawa T, Harada K,
Hinata N, Nakano Y and Fujisawa M: Treatment outcomes of molecular
targeted therapy following nivolumab in metastatic renal cell
carcinoma. Jpn J Clin Oncol. 51:1313–1318. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Xu D, Zhou Y, Zhu Z and Yang X:
Mechanisms and implications of CDK4/6 inhibitors for the treatment
of NSCLC. Front Oncol. 11:6760412021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koehn FE and Carter GT: The evolving role
of natural products in drug discovery. Nat Rev Drug Discov.
4:206–220. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Horie S, Yano S, Aimi N, Sakai S and
Watanabe K: Effects of hirsutine, an antihypertensive indole
alkaloid from Uncaria rhynchophylla, on intracellular
calcium in rat thoracic aorta. Life Sci. 50:491–498. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ndagijimana A, Wang X, Pan G, Zhang F,
Feng H and Olaleye O: A review on indole alkaloids isolated from
Uncaria rhynchophylla and their pharmacological studies.
Fitoterapia. 86:35–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakazawa T, Banba K, Hata K, Nihei Y,
Hoshikawa A and Ohsawa K: Metabolites of hirsuteine and hirsutine,
the major indole alkaloids of Uncaria rhynchophylla, in
rats. Biol Pharm Bull. 29:1671–1677. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xian YF, Lin ZX, Mao QQ, Hu Z, Zhao M, Che
CT and Ip SP: Bioassay-guided isolation of neuroprotective
compounds from Uncaria rhynchophylla against
beta-amyloid-induced neurotoxicity. Evid Based Complement Alternat
Med. 2012:8026252012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lan YL, Zhou JJ, Liu J, Huo XK, Wang YL,
Liang JH, Zhao JC, Sun CP, Yu ZL, Fang LL, et al: Uncaria
rhynchophylla ameliorates Parkinson's disease by inhibiting
HSP90 expression: Insights from quantitative proteomics. Cell
Physiol Biochem. 47:1453–1464. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu CH, Lin YW, Tang NY, Liu HJ and Hsieh
CL: Neuroprotective effect of Uncaria rhynchophylla in
kainic acid-induced epileptic seizures by modulating hippocampal
mossy fiber sprouting, neuron survival, astrocyte proliferation,
and S100B expression. Evid Based Complement Alternat Med.
2012:1947902012.PubMed/NCBI
|
|
23
|
Ozaki Y: Pharmacological studies of indole
alkaloids obtained from domestic plants, Uncaria
rhynchophylla Miq. and Amsonia elliptica Roem. et Schult. Nihon
Yakurigaku Zasshi. 94:17–26. 1989.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozaki Y: Vasodilative effects of indole
alkaloids obtained from domestic plants, Uncaria
rhynchophylla Miq. and Amsonia elliptica Roem. et Schult. Nihon
Yakurigaku Zasshi. 95:47–54. 1990.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuzurihara M, Ikarashi Y, Goto K,
Sakakibara I, Hayakawa T and Sasaki H: Geissoschizine methyl ether,
an indole alkaloid extracted from Uncariae Ramulus et Uncus, is a
potent vasorelaxant of isolated rat aorta. Eur J Pharmacol.
444:183–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakamura Y, Ishida Y, Kondo M and Shimada
S: Yokukansan contains compounds that antagonize the
5-HT3 receptor. Phytomedicine. 43:120–125. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mimaki Y, Toshimizu N, Yamada K and
Sashida Y: Anti-convulsion effects of choto-san and chotoko
(Uncariae Uncis cam Ramlus) in mice, and identification of the
active principles. Yakugaku Zasshi. 117:1011–1021. 1997.(In
Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang M, Guo J, Wang Z, Zhang G, Yu H,
Chang R and Chen A: Simultaneous separation and determination of
hirsutine and hirsuteine by cyclodextrin-modified micellar
electrokinetic capillary chromatography. Phytochem Anal.
31:112–118. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang BY, Zeng Y, Li YJ, Huang XJ, Hu N,
Yao N, Chen MF, Yang ZG, Chen ZS, Zhang DM and Zeng CQ:
Uncaria alkaloids reverse ABCB1-mediated cancer multidrug
resistance. Int J Oncol. 51:257–268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimada Y, Goto H, Itoh T, Sakakibara I,
Kubo M, Sasaki H and Terasawa SK: Evaluation of the protective
effects of alkaloids isolated from the hooks and stems of
Uncaria sinensis on glutamate-induced neuronal death in
cultured cerebellar granule cells from rats. J Pharm Pharmacol.
51:715–722. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kawakami Z, Kanno H, Ikarashi Y and Kase
Y: Yokukansan, a kampo medicine, protects against glutamate
cytotoxicity due to oxidative stress in PC12 cells. J
Ethnopharmacol. 134:74–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meng J, Su R, Wang L, Yuan B and Li L:
Inhibitory effect and mechanism of action (MOA) of hirsutine on the
proliferation of T-cell leukemia Jurkat clone E6-1 cells. PeerJ.
9:e106922021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang R, Li G, Zhang Q, Tang Q, Huang J,
Hu C, Liu Y, Wang Q, Liu W, Gao N and Zhou S: Hirsutine induces
mPTP-dependent apoptosis through ROCK1/PTEN/PI3K/GSK3β pathway in
human lung cancer cells. Cell Death Dis. 9:5982018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen XX, Leung GP, Zhang ZJ, Xiao JB, Lao
LX, Feng F, Mak JC, Wang Y, Sze SC and Zhang KY: Proanthocyanidins
from Uncaria rhynchophylla induced apoptosis in MDA-MB-231
breast cancer cells while enhancing cytotoxic effects of
5-fluorouracil. Food Chem Toxicol. 107:248–260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee JS, Kim J, Kim BY, Lee HS, Ahn JS and
Chang YS: Inhibition of phospholipase cgamma1 and cancer cell
proliferation by triterpene esters from Uncaria
rhynchophylla. J Nat Prod. 63:753–756. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shim JS, Kim HG, Ju MS, Choi JG, Jeong SY
and Oh MS: Effects of the hook of Uncaria rhynchophylla on
neurotoxicity in the 6-hydroxydopamine model of Parkinson's
disease. J Ethnopharmacol. 126:361–365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Ji P, Liu J, Broaddus RR, Xue F
and Zhang W: Centrosome-associated regulators of the G(2)/M
checkpoint as targets for cancer therapy. Mol Cancer. 8:82009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Endicott JA and Noble ME: Structural
principles in cell-cycle control: Beyond the CDKs. Structure.
6:535–541. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Coudreuse D and Nurse P: Driving the cell
cycle with a minimal CDK control network. Nature. 468:1074–1079.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Harashima H, Dissmeyer N and Schnittger A:
Cell cycle control across the eukaryotic kingdom. Trends Cell Biol.
23:345–356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cao L, Chen F, Yang X, Xu W, Xie J and Yu
L: Phylogenetic analysis of CDK and cyclin proteins in premetazoan
lineages. BMC Evol Biol. 14:102014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Z: Regulation of the cell division
cycle in trypanosoma brucei. Eukaryot Cell. 11:1180–1190. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gutierrez C: The arabidopsis cell division
cycle. Arabidopsis Book. 7:e01202009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qin A, Reddy HG, Weinberg FD and
Kalemkerian GP: Cyclin-dependent kinase inhibitors for the
treatment of lung cancer. Expert Opin Pharmacother. 21:941–952.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Otto T and Sicinski P: Cell cycle proteins
as promising targets in cancer therapy. Nat Rev Cancer. 17:93–115.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
No authors listed. Milestones in cell
division. Nat Cell Biol. 3:E2652001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fulda S, Gorman AM, Hori O and Samali A:
Cellular stress responses: Cell survival and cell death. Int J Cell
Biol. 2010:2140742010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Long S, Wilson M, Bengtén E, Clem LW,
Miller NW and Chinchar VG: Identification and characterization of a
FasL-like protein and cDNAs encoding the channel catfish
death-inducing signaling complex. Immunogenetics. 56:518–530. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Haldar S, Negrini M, Monne M, Sabbioni S
and Croce CM: Down-regulation of bcl-2 by p53 in breast cancer
cells. Cancer Res. 54:2095–2097. 1994.PubMed/NCBI
|
|
52
|
Hwang KT, Han W, Kim J, Moon HG, Oh S,
Song YS, Kim YA, Chang MS and Noh DY: Prognostic influence of BCL2
on molecular subtypes of breast cancer. J Breast Cancer. 20:54–64.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jamous A and Salah Z: WW-domain containing
protein roles in breast tumorigenesis. Front Oncol. 8:5802018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jensen K, WuWong DJ, Wong S, Matsuyama M
and Matsuyama S: Pharmacological inhibition of Bax-induced cell
death: Bax-inhibiting peptides and small compounds inhibiting Bax.
Exp Biol Med (Maywood). 244:621–329. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nemec KN and Khaled AR: Therapeutic
modulation of apoptosis: Targeting the BCL-2 family at the
interface of the mitochondrial membrane. Yonsei Med J. 49:689–697.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chresta CM, Masters JR and Hickman JA:
Hypersensitivity of human testicular tumors to etoposide-induced
apoptosis is associated with functional p53 and a high Bax: Bcl-2
ratio. Cancer Res. 56:1834–1841. 1996.PubMed/NCBI
|
|
57
|
Strasser A, Cory S and Adams JM:
Deciphering the rules of programmed cell death to improve therapy
of cancer and other diseases. EMBO J. 30:3667–3683. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jiang X and Wang X: Cytochrome C-mediated
apoptosis. Annu Rev Biochem. 73:87–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Eskes R, Antonsson B, Osen-Sand A,
Montessuit S, Richter C, Sadoul R, Mazzei G, Nichols A and Martinou
JC: Bax-induced cytochrome C release from mitochondria is
independent of the permeability transition pore but highly
dependent on Mg2+ ions. J Cell Biol. 143:217–224. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lee CH, Shih YL, Lee MH, Au MK, Chen YL,
Lu HF and Chung JG: Bufalin induces apoptosis of human osteosarcoma
U-2 OS cells through endoplasmic reticulum stress, caspase- and
mitochondria-dependent signaling pathways. Molecules. 22:4372017.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Monie TP and Bryant CE: Caspase-8
functions as a key mediator of inflammation and pro-IL-1β
processing via both canonical and non-canonical pathways. Immunol
Rev. 265:181–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Vaculova A and Zhivotovsky B: Caspases:
Determination of their activities in apoptotic cells. Methods
Enzymol. 442:157–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
He Y, Chen W, Hu Y, Luo B, Wu L, Qiao Y,
Mo Q, Xu R, Zhou Y, Ren Z, et al: E. adenophorum induces cell cycle
and apoptosis of renal cells through mitochondrial pathway and
caspase activation in saanen goat. PLoS One. 10:e01385042015.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
He Y, Mo Q, Hu Y, Chen W, Luo B, Wu L,
Qiao Y, Xu R, Zhou Y, Zuo Z, et al: E. adenophorum induces cell
cycle arrest and apoptosis of splenocytes through the mitochondrial
pathway and caspase activation in saanen goats. Sci Rep.
5:159672015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
He Y, Mo Q, Luo B, Qiao Y, Xu R, Zuo Z,
Deng J, Nong X, Peng G, He W, et al: Induction of apoptosis and
autophagy via mitochondria- and PI3K/Akt/mTOR-mediated pathways by
E. adenophorum in hepatocytes of saanen goat. Oncotarget.
7:54537–54548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhou Q, Ma J and Chen L: Tissue
distribution of hirsutine and hirsuteine in mice by
ultrahigh-performance liquid chromatography-mass spectrometry. J
Anal Methods Chem. 2020:72043152020. View Article : Google Scholar : PubMed/NCBI
|