Introduction
Colorectal cancer (CRC) is the third-most common
cancer, accounting for 10.2% of new diagnoses as well as the
second-leading cause of cancer-related mortality, accounting for
9.2% worldwide (1,2). Although adjuvant and neo-adjuvant
chemotherapy are standard front-line approaches in support of
surgery, 5-fluorouracil (5-FU), one of the original
fluoropyrimidines, has been considered a mainstay of chemotherapy
for CRC (3,4). A recent review (5) summarized the various mechanisms
against 5-FU including the alterations in drug transport, changes
in the cell cycle, epithelial-to-mesenchymal transition (EMT), and
cancer stem cell (CSC) involvement. Certain resistance mechanisms
that are 5-FU-specific have also been ascertained in 5-FU-resistant
SNU-C5 (SNU-C5/5-FUR) CRC cells as compared with wild type SNU-C5
cells to include the upregulation of cyclooxygenase-2 derived
prostaglandin E2 (6) and
over-activation of protein kinase B (Akt) (7,8). The
differential activation of extracellular signal-regulated protein
kinase (ERK) between wild type and 5-FU-resistant CRC cells after
yeast extract treatment has been previously suggested by the
authors (8). It has also been
reported that SNU-C5/5-FUR cells are more susceptible to an aqueous
extract of Orostachys japonica A. Berger than wild type
SNU-C5 cells via the activation of mitogen-activated protein kinase
signaling pathways including ERK and p38 (9).
Although the CSC markers between cancers are not
identical (10), CSCs exhibit
common characteristics regarding the maintenance of CSC pool,
tumorigenesis, metastasis, and treatment resistance and recurrence
(11–13). As p21 attenuates Ras- and
c-Myc-dependent EMT and CSC-like gene expression in vivo
(14), it suppresses the cell cycle
and is also used as a biomarker for CSCs (15). Similarly, the Wnt/β-catenin
signaling pathway is critical for the regulation of cell
proliferation, differentiation and apoptosis during regeneration
(16). CSCs are typically
determined by the cell surface proteins [cluster of differentiation
(CD) 44, and nuclear transcription factors, octamer binding
transcription factor-4 (Oct-4), and sex determining region Y-box-2
(Sox-2)] (13,17) as well as ATP-binding cassette
super-family G member 2 (ABCG2) transporter (18). Moreover, non-CSCs can acquire a
CSC-like phenotype during EMT (19), and EMT-induced cells can form
spheres in an anchorage-independent growth environment (20).
The three-dimensional (3D) tumor spheroid formation
model has been suggested to be an essential tool for confirming
CSC-like features in vitro (21), because it resembles in vivo
solid tumors rather than the conventional, two-dimensional (2D)
monolayer culture (22). Although
various methods and conditions have been proposed, spheroids
consist of an external proliferating zone, an internal quiescent
zone, and a necrotic core (22).
During the spheroid formation process in any environment, it is
known that cells initially aggregate and then form compact
spheroids via E-cadherin (22).
E-cadherin, an epithelial cell to cell adhesion molecule, is
inversely correlated with EMT (23). Although E-cadherin is also known to
be frequently downregulated with tumorigenesis, cell adherence
between cancer cells are disturbed by addition of the soluble
fragment (80 kDa) of E-cadherin, leading to malignancy in cancers
(24,25).
Growth factors (GFs) have been used in spheroid
formation culture methods to maintain the integrity of CSCs
(17,22), which is also applied to CRC cells in
order to effectively obtain CSCs to date (26,27).
Although fetal bovine serum (FBS) supplementation has recently been
suggested as an adaptable, efficient, and cost-effective tool to
maintain pluripotency in a hepatocellular carcinoma cell (28), it has not been investigated in
acquired drug-resistant CRC cells. Therefore, it was aimed to
investigate whether spheroid formation culture depend on
supplementations is appropriate on acquired 5-FU-resistant
SNU-C5/5-FUR CRC cells as compared with wild type SNU-C5 cells.
Spheroid formation culture methods with different culture
environments supplemented with FBS and GFs, respectively, were
used. Accordingly, the feasibility of appropriate spheroid
formation culture methods was examined in each of different CRC
cell, and the differences between wild type and acquired
5-FU-resistance were revealed.
Materials and methods
Antibodies
The antibodies specific for β-catenin (1:1,000; cat.
no. sc-7199), c-Myc (9E10; 1:1,000; cat. no. sc-40), E-cadherin
(H-108; 1:1,000; cat. no. sc-7870), epithelial cell adhesion
molecule (EpCAM) (c-10; 1:1,000; cat. no. sc-25308), glyceraldehyde
3-phophage dehydrogenase (GAPDH; 1:2,000; cat. no. sc-47724),
glycogen synthase kinase-3 beta (GSK-3β; 1:1,000; cat. no.
sc-9166), Oct3/4 (C-10; 1:1,000; cat. no. sc-5279), Pan-Ras (C-4;
1:1,000; cat. no. sc-166691), Sox2 (E-4; 1:1,000; cat. no.
sc-365823) and Wilms tumor protein [WT1 (6F-H17); 1:1,000; cat. no.
sc-81619] were obtained from Santa Cruz Biotechnology, Inc. cAMP
response element-binding protein (CREB) [phosphor S133 (E113);
1:1,000; cat. no. ab32096], CD24 [M1/69] (1:1,000; cat. no.
ab64064), and CD44 (1:1,000; cat. no. ab157107) were obtained from
Abcam. ABCG2 (BCRP1, clone 5D3; cat. no. MAB4155; 1:500) and
α-smooth muscle actin (αSMA; 1:2,000; cat. no. A2547) from were
obtained MilliporeSigma. CREB (1:1,000; cat. no.
CSB-PA005947HA01HU; Cusabio Technology, LLC), fibronectin (1:2,000;
cat. no. CL54951AP; Cedarlane Laboratories), p21 (1:1,000; cat. no.
60214-1; Proteintech Group, Inc.) and p90RSK (Ab348; 1:1,000; cat.
no. 79-554; Prosci, Inc.) were obtained from the corresponding
listed company.
Cell culture
SNU-C5 (Korean Cell Line Bank; Seoul, South Korea)
and SNU-C5/5-FUR (Research Center for Resistant Cells; Chosun
University, Gwangju, South Korea) cells were cultured in RPMI-1640
medium supplemented with 10% heat-inactivated FBS, 100 U/ml
penicillin and 100 mg/ml streptomycin at 37°C (Welgene, Inc.) in a
humidified atmosphere with 5% CO2 as previously
described (8).
Spheroid formation
96-well plates were covered with
poly-2-hydroxyethylmethacylate (cat. no. P3932; Sigma-Aldrich;
Merck KGaA) to create an anchorage-independent environment. Cells
were cultured with 1% B27 supplement (cat. no. 17504-044), 20 ng/ml
epidermal GF (cat. no. PHG0311) and 20 ng/ml basic fibroblast GF
(cat. no. 13256029; all from Thermo Fisher Scientific, Inc.) in
DMEM/F12 medium (sphere/GF group) (17,21),
or in same culture media with FBS (sphere/FBS group) as previously
reported (28). Spheroid formation
was checked for morphometry on days 7, 14 and 28.
Cell viability assay
The effect of 5-FU on cell viability was evaluated
in terms of the reduction of MTT (Amresco, LLC) using a VERSAmax
microplate reader (Molecular Devices, LLC) as previously described
(10,19). Dissociated cells from 2D monolayer
and spheroid-formation cultures with trypsin-EDTA (Welgene, Inc.)
were seeded in triplicate wells in 96-well plates (2×103
cells/well), and treated with 5-FU at various concentrations as
previously described (22). The
number of viable cells was estimated for 3 days after incubation in
2D culture, and for 4 days after incubation in spheroid formation
culture methods. The effect of the drug was calculated and compared
with untreated (DMSO-treated only) cells using Microsoft Excel (MS
Office 2016).
Western blotting
Cells were incubated for 3 days in monolayer
culture, and incubated in spheroid formation culture for 4 weeks.
Cells were harvested in M-PER mammalian protein extraction reagent
(Thermo Fisher Scientific, Inc.) including 1% protease inhibitor
cocktail set III (EMD Millipore), 0.5% phosphatase inhibitor
cocktail 2 and 0.5% phosphatase inhibitor cocktail 3 (both from
Sigma-Aldrich; Merck KGaA). Protein concentration was assessed
using BCA protein assay (Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions.
The electrophoresis of protein in cell lysates on an
TGX Stain-Free FastCast™ Acrylamide Starter kit (Bio-Rad
Laboratories, Inc.) using tris/glycine buffer systems (Bio-Rad
Laboratories, Inc.) onto PVDF membranes was performed as previously
described (8,17). The membranes were first blocked at
room temperature with 5% skim milk for 1 h and then incubated with
primary antibodies overnight at 4°C. After washing, peroxidase
anti-mouse or anti-rabbit IgG antibodies (1:3,000; cat. no.
PI-2,000 and PI-1,000, Vector Laboratories, Inc.) were applied for
1 h at room temperature. Next, western lighting chemiluminescence
reagent (PerkinElmer, Inc.) was used to detect proteins. The
anti-GAPDH antibody was used as a loading control on the stripped
membranes. The bands were captured using Azure™ c300 (Azure
Biosystems, Inc.) and quantified using the AzureSpot analysis
software (version 14.2; Azure Biosystems, Inc.).
Enzyme-linked immunosorbent assay
(ELISA)
ELISA was performed according to the manufacturer's
instruction (Human E-cadherin SimpleStep ELISA Kit, cat. no.
ab233611; Abcam). Briefly, standard and spheroid formation culture
media samples were added into each well to bind E-cadherin antibody
cocktail followed by incubation of 1 h at room temperature. After
being washed with washing buffer, TMB1 substrate reagent was added
into each well and incubated for 10 min. At this point, the stop
solution was added and optical density was measured at 450 nm using
a VERSAmax microplate reader.
Immunocytochemistry
Four weeks after spheroid formation e culture, the
formed spheres were fixed for 24 h at 4°C in 4% paraformaldehyde,
and 4 µm-thick-sections were prepared for immunocytochemistry. The
sections were blocked with 10% normal horse serum (cat. no.
MP-7401; Vector) for 1 h at room temperature. Incubation with the
anti-E-cadherin antibody (1:100) was performed for overnight at
4°C. The binding was visualized using an anti-rabbit secondary
antibody (1:200; cat. no. MP-7401; Vector), and the nuclei were
counterstained with hematoxylin (cat. no. H-3404, Vector) for 1 min
at room temperature.
Statistical analysis
All data were compiled from a minimum of three
replicate experiments. Data are expressed as the mean values ± SD.
P<0.05 was considered to indicate a statistically significant
difference as determined using the Student's paired t-test or
one-way ANOVA followed by a Bonferroni post-hoc test. MS Excel 2016
was used for statistical analysis.
Results
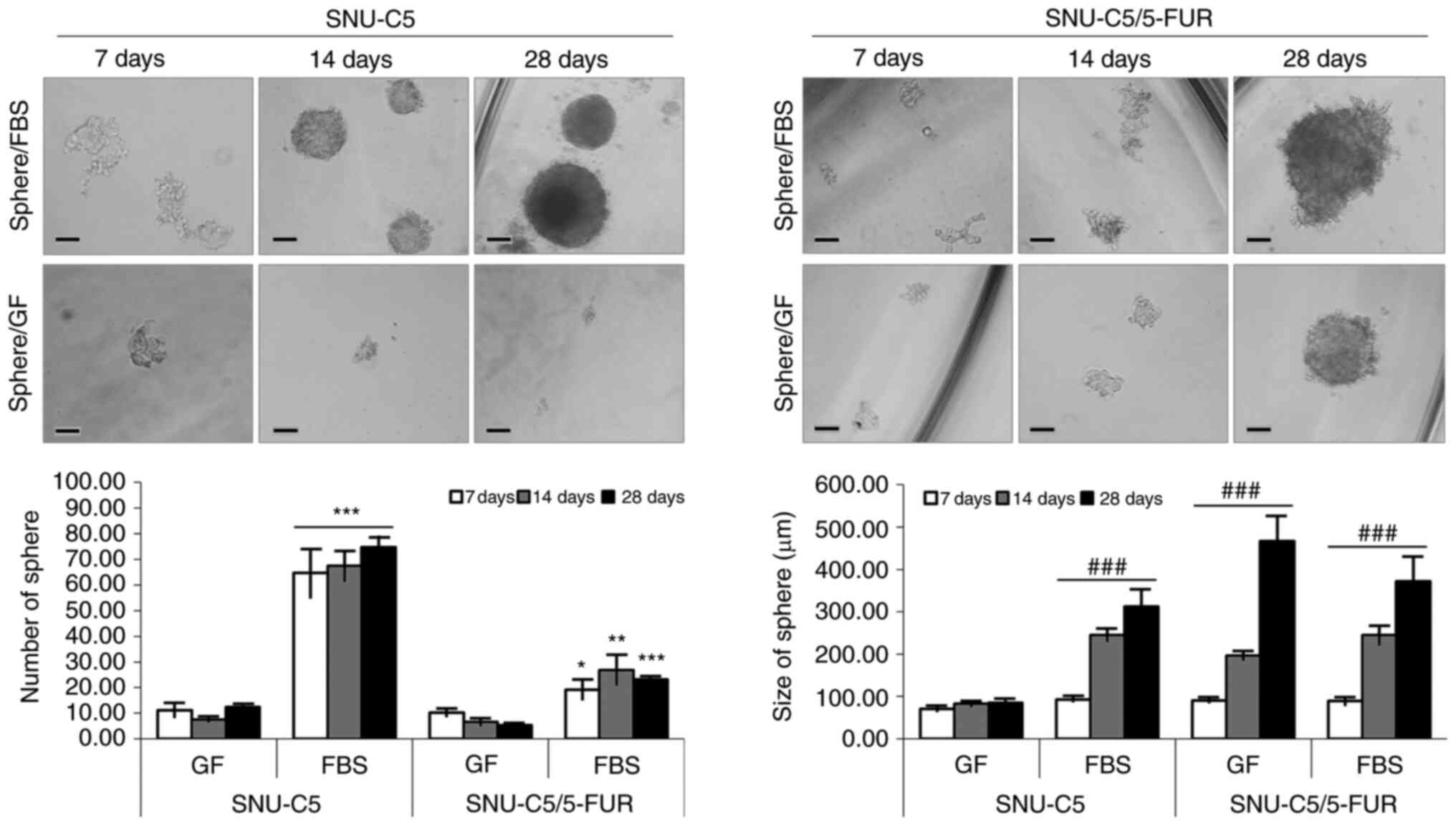
Spheroid formation in different
environments supplemented with FBS or GF
To evaluate the tumorigenic capacities of SNU-C5 and
SNU-C5/5-FUR CRC cells, the cells were cultured in an
anchorage-independent condition for 4 weeks. Both cell lines
successfully formed spheres in the FBS-supplemented environment.
SNU-C5/5-FUR cells only formed spheres in the GF-supplemented
environment. The mean size of each cell was significantly increased
with the passage of time (P<0.001) (Fig. 1 and Table I).
 | Table I.Number and size (µm) of spheres in
GF- and FBS-supplemented environments of SNU-C5 and SNU-C5/5-FUR
cells. |
Table I.
Number and size (µm) of spheres in
GF- and FBS-supplemented environments of SNU-C5 and SNU-C5/5-FUR
cells.
|
| SNU-C5 | SNU-C5/5-FUR |
|---|
|
|
|
|
|---|
| Variables | Sphere/GF | Sphere/FBS | P-value | Sphere/GF | Sphere/FBS | P-value |
|---|
| No. |
|
|
|
|
|
|
|
D7 | 11.08±2.57 | 64.67±9.35 | <0.001 | 10.25±1.27 | 19.25±3.72 | <0.0309 |
|
D14 | 7.46±0.77 | 67.46±5.77 | <0.001 | 6.58±1.36 | 26.88±5.74 | <0.0069 |
|
D28 | 12.39±1.21 | 74.78±4.00 | <0.001 | 5.28±0.35 | 23.17±0.52 | <0.001 |
| Size (µm) |
|
|
|
|
|
|
|
D7 | 71.25±5.12 | 93.09±3.16 | - | 90.30±3.13 | 89.00±8.33 | - |
|
D14 | 82.59±4.72 |
245.36±15.08a | <0.001 |
197.04±10.24a |
245.06±22.03a | <0.001 |
|
D28 | 85.50±5.04 |
312.34±41.14a | <0.001 |
467.19±59.78a |
371.57±55.88a | <0.001 |
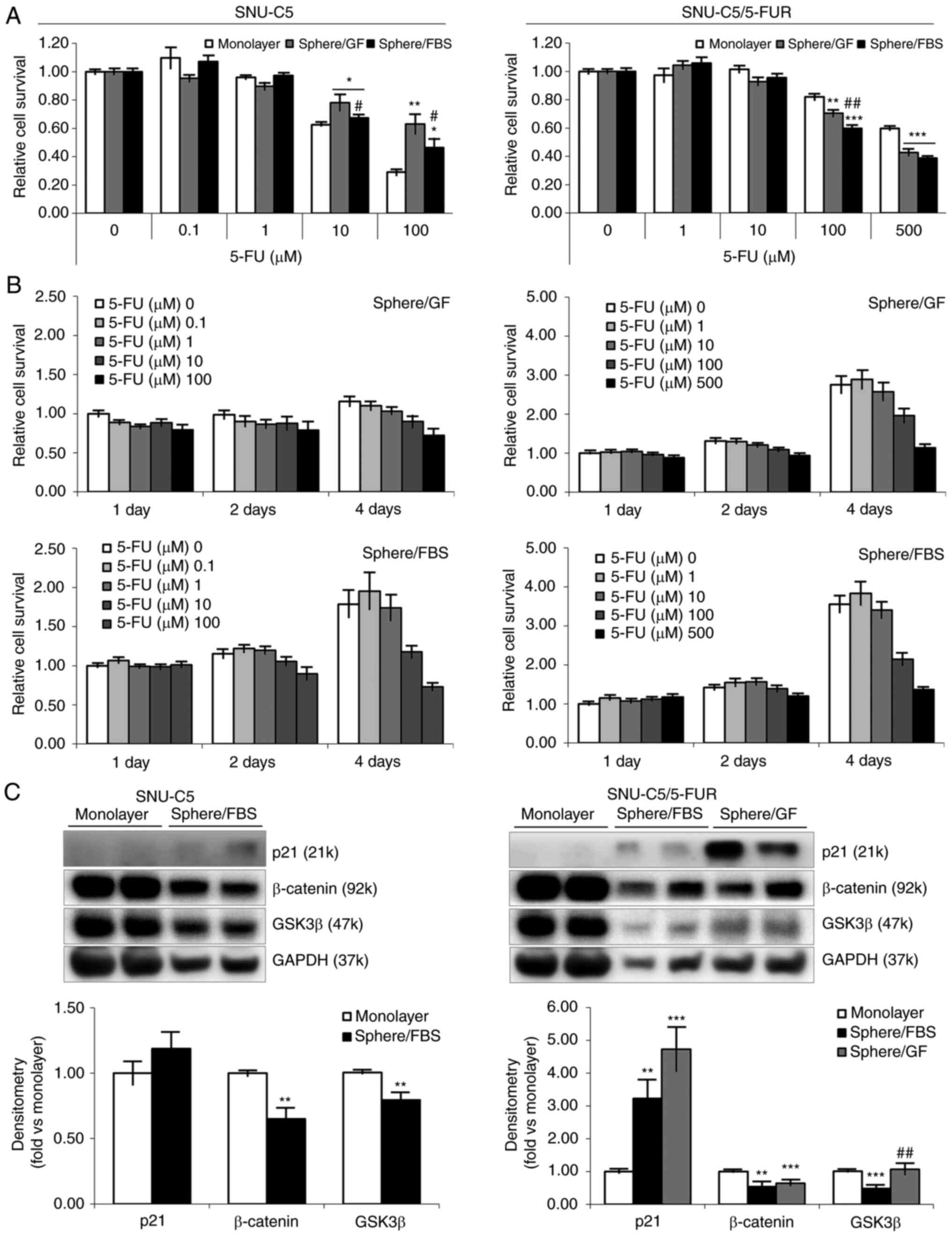
Cell viability and proliferation of
spheroid formation in different environments supplemented with FBS
or GF
To investigate the proliferation and acquisition of
drug resistance in spheroid formation, the effect of an anticancer
drug (5-FU) on cell viability was assessed using the MTT assay.
Spheroid-formed cells from SNU-C5 cells revealed higher cell
viability to 5-FU at 10 (P=0.0174 in sphere/GF; P=0.0421 in
sphere/FBS) and 100 µM (P=0.0030 in sphere/GF; P=0.0162 in
sphere/FBS) compared with the cells from monolayer culture. The
difference in cell viability between environments (sphere/GF vs.
sphere/FBS) was statistically significant (P=0.0421/10 µM;
P=0.0455/100 µM). Resistance-acquired SNU-C5/5-FUR cells did not
show any change to 5-FU with spheroid formation (Fig. 2A). Sphere-formed cells demonstrated
significantly slower proliferation than those in monolayer, wherein
sphere-formed cells reached the same level at 4 days of incubation
when original cells reached proliferation level at 3 days of
incubation (8). In addition,
sphere-formed cells in GF-supplemented environment showed
relatively slower proliferation than those in FBS-supplemented
environment (Fig. 2B).
To delineate the characteristics of spheroid-formed
cells, the levels of p21, β-catenin and GSK3β were first measured
to confirm the feasible mechanisms of slower proliferation
(Fig. 2C and Table II). Compared with monolayer
culture, p21 was increased in both CRC cells. Whereas β-catenin was
found to be decreased in both CRC cells, GSK3β was decreased in
FBS-supplemented environments and sustained in GF-supplemented
environment (P=0.0046 between environments).
 | Table II.Densitometric results of western
blotting on SNU-C5 and SNU-C5/5-FUR cells. |
Table II.
Densitometric results of western
blotting on SNU-C5 and SNU-C5/5-FUR cells.
|
| SNU-C5 | SNU-C5/5-FUR |
|---|
|
|
|
|
|---|
| Variables | Sphere/FBS | P-value | Sphere/FBS | P-value | Sphere/GF | P-value |
|---|
| Proliferation |
|
|
|
|
|
|
|
p21 | 1.19±0.13 | 0.1248 | 3.22±0.57 | 0.0015 | 4.73±0.67 | <0.001 |
|
β-catenin | 0.65±0.09 | 0.0013 | 0.54±0.14 | 0.0043 | 0.64±0.07 | 0.0014 |
|
GSK3β | 0.80±0.06 | 0.0032 | 0.48±0.06 | <0.001 | 1.06±0.16 | 0.3696 |
| Stemness |
|
|
|
|
|
|
|
pan-Ras | 1.38±0.04 | 0.0065 | 0.71±0.07 | 0.0011 | 1.24±0.06 | 0.0013 |
|
Oct3/4 | 0.90±0.04 | 0.0948 | 0.61±0.07 | <0.001 | 1.09±0.08 | 0.1597 |
|
Sox2 | 0.64±0.08 | 0.0019 | 0.49±0.03 | <0.001 | 0.61±0.05 | <0.001 |
|
WT1 | 0.88±0.05 | 0.0158 | 0.50±0.05 | <0.001 | 1.35±0.08 | 0.0005 |
| Drug
resistance |
|
|
|
|
|
|
|
ABCG2 | 0.64±0.05 | <0.001 | 0.85±0.10 | 0.0982 | 0.72±0.12 | 0.0284 |
|
P90RSK | 0.80±0.08 | 0.0370 | 0.97±0.21 | 0.4514 | 1.39±0.05 | <0.001 |
|
pCREB | 0.96±0.17 | 0.4190 | 1.33±0.16 | 0.0473 | 0.92±0.10 | 0.2358 |
|
CREB | 0.82±0.05 | 0.0073 | 0.67±0.08 | <0.001 | 0.81±0.14 | 0.0800 |
| Surface marker |
|
|
|
|
|
|
|
CD44 | 0.21±0.01 | <0.001 | 0.16±0.03 | <0.001 | 0.60±0.07 | <0.001 |
|
CD24 | 0.69±0.03 | <0.001 | 0.73±0.07 | 0.0075 | 0.94±0.08 | 0.2360 |
| EMT |
|
|
|
|
|
|
|
Fibronectin | 4.90±0.73 | <0.001 | 11.57±2.15 | <0.001 | 0.67±0.10 | 0.0094 |
|
EpCAM | 0.33±0.08 | <0.001 | 0.11±0.03 | <0.001 | 0.20±0.08 | <0.001 |
|
αSMA | 0.71±0.03 | 0.0095 | 0.51±0.06 | <0.001 | 1.01±0.15 | 0.4869 |
|
E-cadherin |
|
|
|
|
|
|
|
135-120 kDa | 0.88±0.12 | 0.1727 | 0.55±0.13 | 0.0073 | 0.83±0.10 | 0.0873 |
|
80 kDa | 3.80±0.70 | 0.0013 | 4.52±0.96 | 0.0054 | 0.70±0.10 | 0.0550 |
Cellular and molecular markers of
spheroid formation in different environment supplemented with FBS
or GF
CSC markers were assessed in the spheroid-formed
cells to reveal the stemness (Fig.
3A and Table II). Compared
with monolayer culture, pan-Ras was significantly increased while
other markers were decreased in SNU-C5 cells. In the case of
SNU-C5/5-FUR cells, pan-Ras and WT1 were increased in sphere/GF
environment but decreased in sphere/FBS environment, and
considerable differences were observed between supplementations
(P<0.001/each). Oct3/4 and Sox2 were decreased or sustained.
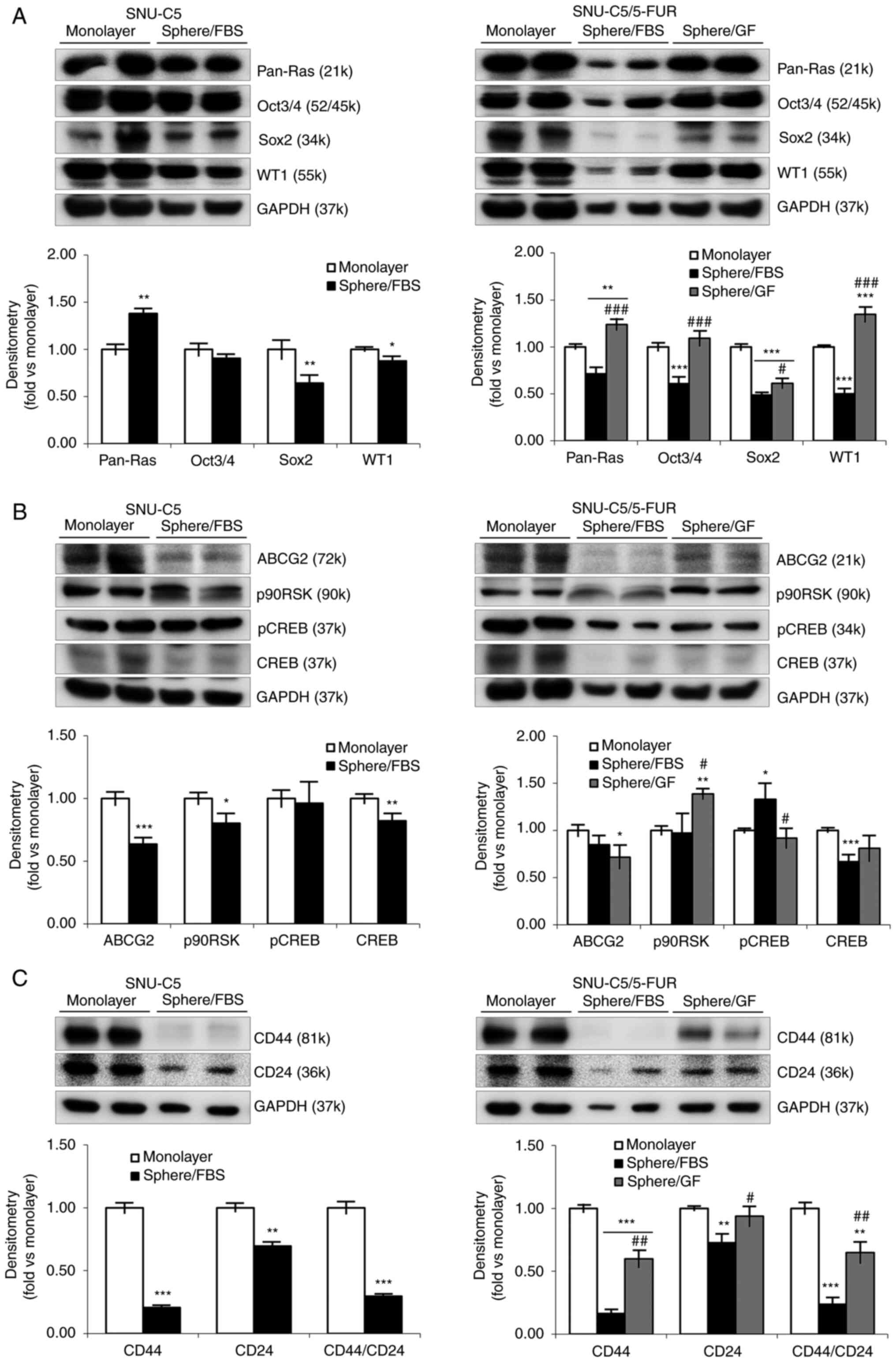 | Figure 3.Cellular and molecular markers for
CSCs and drug resistance on spheroid formation of colorectal cancer
cells in different environments. (A) Expression levels of CSC
markers in monolayer and spheroid formation cultures in SNU-C5 and
SNU-C5/5-FUR cells were detected by immunoblotting. Immunoblotting
analysis was performed for pan-Ras, Oct3/4, Sox2 and WT1, while
GAPDH was used for a loading control. Band density was analyzed by
AzureSpot analysis software, and results are expressed as the mean
± SD (n=3). (B) Expression levels of drug efflux markers in
monolayer and spheroid formation cultures in SNU-C5 and
SNU-C5/5-FUR cells were detected by immunoblotting. Immunoblotting
analysis was performed for ABCG2, p90RSK, pCREB, and CREB, while
GAPDH was used for a loading control. Band density was analyzed by
AzureSpot analysis software, and results are expressed as the mean
± SD (n=3). (C) Expression levels of cell surface markers in
monolayer and spheroid formation cultures in SNU-C5 and
SNU-C5/5-FUR cells were detected by immunoblotting. Immunoblotting
analysis was performed for CD44 and CD24, while GAPDH was used for
a loading control. Band density was analyzed by AzureSpot analysis
software, and results are expressed as the mean ± SD (n=3).
*P<0.05, **P<0.01 and ***P<0.001 vs. monolayer;
#P<0.05, ##P<0.01 and
###P<0.001 vs. sphere/FBS. CSCs, cancer stem cells;
FBS, fetal bovine serum; GF, growth factor; p-, phosphorylated. |
Next, drug resistance-related markers were assessed
in the spheroid-formed cells (Fig.
3B and Table II). Compared
with monolayer culture, ABCG2, p90RSK, phosphorylated (p)-CREB and
CREB were all decreased or unchanged in SNU-C5 cells. Regarding
SNU-C5/5-FUR cells, p90RSK was increased in GF-supplemented
environment and pCREB was increased in FBS-supplemented
environment. The other markers were decreased or unchanged in
sphere-forming cells.
Surface markers related to stemness and/or EMT were
also assessed in the spheroid-formed cells (Fig. 3C and Table II). Compared with monolayer
culture, epithelial CD24 and mesenchymal CD44 were decreased in
spheres in both CRC cells. The ratio between CD44/CD24, which is
useful data for searching CSCs, was significantly decreased in
sphere/FBS (0.30±0.02-fold in SNU-C5 cells, 0.24±0.06-fold in
SNU-C5/5-FUR cells; P<0.001/each) and in sphere/GF (0.65±0.08
fold; P=0.0048). There was a significant difference between
supplementations (P=0.0013) in SNU-C5/5-FUR cells.
Differential expression of EMT markers
of spheroid formation in different environment supplemented with
FBS or GF
As the spheroid formation culture method inhibits
the adhesion of cells to the base of culture dish, the EMT markers
were assessed (Fig. 4A and Table II). Compared with the monolayer
culture, fibronectin was significantly increased in
FBS-supplemented environment in both CRC cells but decreased in
GF-supplemented environment in SNU-C5/5-FUR cells (P<0.001
between environments). EpCAM and αSMA were not changed in the
spheroid formation culture. Compared with monolayer culture,
135–120 kDa E-cadherin was decreased in all spheroid formation
conditions that were examined in this experiment. Soluble
E-cadherin (80 kDa) was significantly increased in the
FBS-supplemented environment in both CRC cells, but sustained in
the GF-supplemented environment of SNU-C5/5-FUR cells (Fig. 4B and Table II). The ratio of soluble to 135–120
kDa E-cadherin was significantly increased in FBS-supplemented
environment in SNU-C5 (2.50±0.49-fold; P=0.0062) and in
SNU-C5/5-FUR (4.64±0.42-fold; P<0.001) cells. The ratio was
sustained in sphere/GF (1.06±0.08-fold), while there was a
significant difference between supplementations (P<0.001) in
SNU-C5/5-FUR cells.
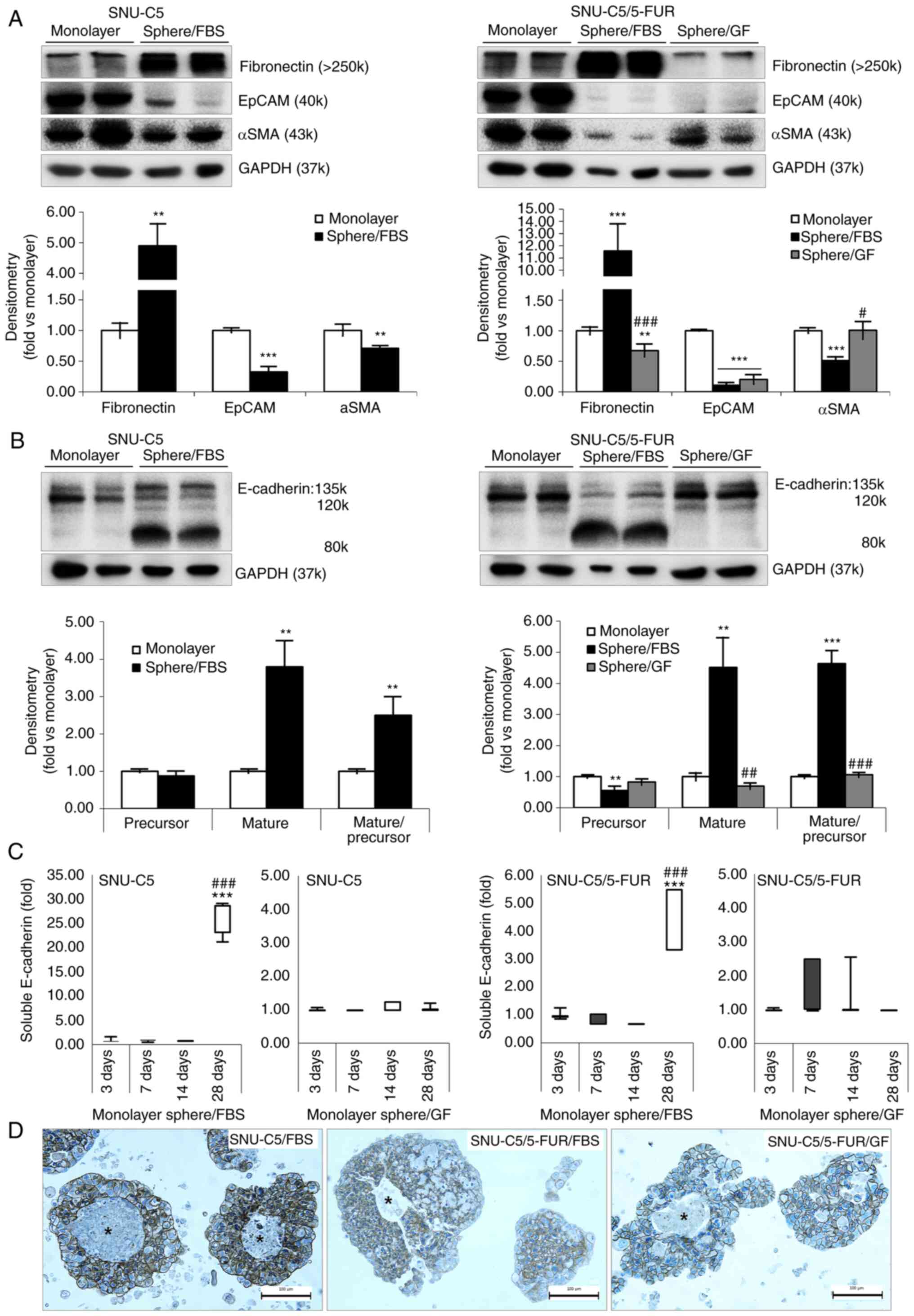 | Figure 4.Markers for EMT on spheroid formation
of colorectal cancer cells in different environments. (A)
Expression levels of EMT markers in monolayer and spheroid
formation cultures in SNU-C5 and SNU-C5/5-FUR cells were detected
by immunoblotting. Immunoblotting analysis was performed for
fibronectin, EpCAM and αSMA, while GAPDH was used as a loading
control. Band density was analyzed by AzureSpot analysis software,
and results are expressed as the mean ± SD (n=3). (B) Expression of
E-cadherin in monolayer and spheroid formation cultures in SNU-C5
and SNU-C5/5-FUR cells was detected by immunoblotting.
Immunoblotting analysis was performed for E-cadherin, which was
subdivided into 135 −120 kDa and soluble 80 kDa fractions, while
GAPDH was used as a loading control. Band density was analyzed by
AzureSpot analysis software, and results are expressed as the mean
± SD (n=3). (C) Enzyme-linked immunosorbent assay of E-cadherin on
culture media in SNU-C5 and SNU-C5/5-FUR cells. Data are presented
as the mean ± SD (n=3). (D) Immunocytochemical staining of
E-cadherin on spheroid formation in SNU-C5 and SNU-C5/5-FUR cells.
Asterisks indicate areas that were neither immunostained with
E-cadherin, nor stained with hematoxylin (Scale bar, 100 µm).
**P<0.01 and ***P<0.001 vs. monolayer; #P<0.05,
##P<0.01 and ###P<0.001 vs. sphere/FBS.
EMT, epithelial-to-mesenchymal transition; FBS, fetal bovine serum;
GF, growth factor; EpCAM, epithelial cell adhesion molecule. |
The concentration of E-cadherin in cultured media of
SNU-C5 and SNU-C5/5-FUR cells in 2D monolayer, sphere/FBS, and
sphere/GF group were estimated by ELISA (Fig. 4C). The ratio of soluble E-cadherin
was found to be significantly increased in 28 days of incubation in
sphere/FBS compared with 2D monolayer (3 days of incubation) and
with 7 days of incubation in sphere/FBS environment in SNU-C5 cells
(25.47±1.98-fold vs. monolayer, P<0.001), and in SNU-C5/5-FUR
cells (4.48±0.50-fold vs monolayer, P<0.001), respectively.
However, the ratio of E-cadherin was not changed until 28 days
after incubation in GF-supplemented environments in both CRC
cells.
The morphological features were evaluated by the
expression of E-cadherin (Fig. 4D).
E-cadherin was immunostained on the cell membrane of
spheroid-formed cells in both CRC cells with GF and FBS
supplementations. Some central area was neither immunostained with
E-cadherin, nor stained with hematoxylin for nuclei.
Discussion
The efficiency of spheroid formation is known to
differ between cell lines, even within the same tumor type
(22), which was also observed in a
recent study by the authors (17).
It was found that the spheroid formations of both CRC cell lines
were induced in an improved manner when supplemented with FBS
compared with GFs, while SNU-C5/5-FUR cells only formed spheres
supplemented with GFs. Sphere-formed cells showed 5-FU resistance
in SNU-C5 cells irrespective of the supplementations used as
previously suggested (22).
However, SNU-C5/5-FUR cells did not show any further changes
against 5-FU in spheroid formation culture. Sphere-formed cells
showed slower cell proliferation than cells from monolayer culture,
which coincided with an increased level of p21 and a decreased
level of β-catenin as previously reported (19,21,22).
However, cellular and molecular markers for CSCs, drug resistance,
and EMT were not significantly changed between 2D and 3D culture
conditions although the cells could acquire CSC-like phenotype via
EMT to form spheres (19,20). As a result, spheroid formation
culture methods are not appropriate to study CSCs or drug
resistance in acquired 5-FU-resistant CRC cells, at least in
long-term maintenance condition. Notably, spheroid formation
supplemented with FBS environment showed significantly increased
level of soluble E-cadherin.
GFs were used in spheroid formation culture methods
(17,22,26,27).
The suitability of spheroid formation culture methods has been
investigated, and the results of such studies have revealed that
cancer cells showed differential efficiency of spheroid formation,
where the efficiency differed depending on cell lines even for the
same tumor type (22). SNU-C5/5-FUR
cells showed variable morphology of spheroids in different
environments, wherein complete spheroids were formed in
GF-supplemented environment with smaller numbers than those formed
in FBS-supplemented environment. Although the spheroids in
GF-supplemented environment did not acquire further drug
resistance, they exhibited increased CSC markers compared with
monolayer or FBS-supplemented spheroids. Accordingly,
drug-resistance-acquired cells were not suitable for spheroid
formation culture methods to investigate CSCs depending on drug
resistance, at least in SNU-C5/5-FUR cells. As FBS supplementation
was suggested for the in vitro cultivation of CSCs (28), spheroid formation was easily induced
and maintained for up to 4 weeks in FBS-supplemented environment in
both CRC cells. The spheres showed acquired drug resistance, slow
proliferation, and increased level of pan-Ras. However, further
changes on cellular and molecular markers of CSCs, drug resistance,
surface protein, and EMT were not observed. Therefore,
FBS-supplemented environment was not considered to be such an
effective tool for CSCs in CRC cells, at least in SNU-C5/5-FUR
cells and in long-term maintenance.
During the spheroid formation process in any
environment, it is known that cells initially aggregate and then
form compact spheroids via a high level of E-cadherin (22). However, expression of E-cadherin was
reported as ‘decrease’ in CSC, as induced by spheroid formation
culture methods, of the colon (29,30),
breast (31,32), liver (33), pancreas (34), and oral and prostate (35) cancers with variable vendors in
short-term maintenance. Previous studies (28,29,31–33)
reported fractions with 120 kDa, not soluble (80 kDa), E-cadherin.
The decrease in E-cadherin in CSC was also supported by the
findings of confocal imaging (36)
and immunohistochemistry (31,32).
However, there have also been controversial studies regarding the
expression of E-cadherin in spheroid formation. Transforming
GF-beta-induced EMT decreases the expression of E-cadherin in
spheroid formation (33,34). E-cadherin enhances CSC-like
properties and induces mesenchymal features in colon cancer
(37). The overexpression of
E-cadherin compromises the EMT-like properties of spheroid
formation (32). Spheroid formation
in breast cancers depends on the expression of E-cadherin (21). Moreover, Morata-Tarifa et al
(38) suggested that
trypsin-resistant subpopulation showed increased expression of
E-cadherin, but trypsin-sensitive subpopulation (EMT-like)
represented CSC-like colon cancer cells.
Previous studies have indicated that decreased
expression of E-cadherin in cancers may be re-interpreted as the
decrease of 120 kDa E-cadherin irrespective of vendors. In the
present study, 120 kDa E-cadherin was significantly decreased only
in the sphere/FBS of SNU-C5/5-FUR cells. In contrast to
expectations, the soluble E-cadherin was significantly increased in
the sphere/FBS of both CRC cells as revealed using western blotting
and ELISA, which means the essential component of spheroid
formation culture was the soluble E-cadherin in FBS-supplemented
environment. As the increase in soluble E-cadherin was observed in
28 days after incubation in both CRC cells, it may promote growth
and proliferation of spheroids rather than act as an CSCs marker or
drug resistance. These findings are confirmed by previous studies
that E-cadherin could be a target for spheroid formation or the
formation of CSC-like cells in breast cancer (39), and soluble E-cadherin could be an
oncogene like EGF when it induces EMT in 3D (24,25,40) as
well as in 2D (24) culture
environments. Nevertheless, there has been controversy about the
mechanism of soluble E-cadherin. The soluble E-cadherin influences
invasion via activation of matrix metalloproteinase (MMP), and thus
contributes to skin carcinogenesis (24). Exogenous soluble E-cadherin promotes
migration and invasion of non-small-cell lung cancer cells, but
silencing MMP9 suppresses soluble E-cadherin expression (41). Although ABCG2 could also modulate
the expression of E-cadherin in lung cancer (42), ABCG2 did not significantly change in
the present study.
Nonetheless, the present study did not reveal the
feasible mechanisms of soluble E-cadherin on spheroid formation. As
the increase in soluble E-cadherin was observed in long-term
maintenance of spheroids, the application of exogenous E-cadherin
in spheroid formation culture should be performed to reveal whether
soluble E-cadherin is a result or a cause of spheroid formation. If
soluble E-cadherin act as an oncogene as it was hypothesized, the
possibility of replacement to EGF would be explored based on serial
dilution as performed in a recent study (26). Based on these experiments, following
signaling pathways of soluble E-cadherin should be further
investigated under the more detailed strategy.
In conclusion, spheroid formation culture methods
are not appropriate to study CSCs or drug resistance in acquired
5-FU-resistant CRC cells as compared with wild type cells, at least
in long-term maintenance condition. Spheroids were easily formed in
FBS-supplemented environment via adaptation to
anchorage-independent condition, which are related to soluble
E-cadherin. These results suggested that soluble E-cadherin could
act like an oncogene to grow the spheroids supplemented with FBS,
at least in the long-term maintenance condition.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korea government
(MSIT; grant no. 2021R1F1A1063023).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IYC and SPY conceived and designed the present
study, performed the experiments for data acquisition and analysis
and interpreted the experimental results. IYC and SPY confirm the
authenticity of all the raw data. IYC wrote the original
manuscript. SPY revised the manuscript. Both authors read and
approved the final manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kwakman JJ and Punt CJ: Oral drugs in the
treatment of metastatic colorectal cancer. Expert Opin
Pharmacother. 17:1351–1361. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stintzing S: Recent advances in
understanding colorectal cancer. F1000Res. 7:F10002018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Azwar S, Seow HF, Abdullah M, Jabar MF and
Mohtarrudin N: Recent updates on mechanisms of resistance to
5-fluorouracil and reversal strategies in colon cancer treatment.
Biology (Basel). 10:8542021.PubMed/NCBI
|
|
6
|
Choi CH, Lee TB, Lee YA, Choi S and Kim
KJ: Up-regulation of cyclooxygenase-2-derived prostaglandin E(2) in
colon cancer cells resistant to 5-fluorouracil. J Korean Surg Soc.
81:115–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim EJ, Kang GJ, Kang JI, Boo HJ, Hyun JW,
Koh YS, Chang WY, Kim YR, Kwon JM, Maeng YH, et al: Over-activation
of AKT signaling leading to 5-Fluorouracil resistance in
SNU-C5/5-FU cells. Oncotarget. 9:19911–19928. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moon D, Kang HK, Kim J and Yoon SP: Yeast
extract induces apoptosis and cell cycle arrest via activating p38
signal pathway in colorectal cancer cells. Ann Clin Lab Sci.
50:31–44. 2020.PubMed/NCBI
|
|
9
|
Kim JW, Kim SH, Mariappan R, Moon D, Kim J
and Yoon SP: Anti-cancer effects of the aqueous extract of
Orostachys japonica A. Berger on 5-fluorouracil-resistant
colorectal cancer via MAPK signalling pathways in vitro and in
vivo. J Ethnopharmacol. 280:1144122021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Corrò C and Moch H: Biomarker discovery
for renal cancer stem cells. J Pathol Clin Res. 4:3–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nguyen LV, Vanner R, Dirks P and Eaves CJ:
Cancer stem cells: An evolving concept. Nat Rev Cancer. 12:133–143.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murphy AJ, Pierce J, de Caestecker C,
Ayers GD, Zhao A, Krebs JR, Saito-Diaz VK, Lee E, Perantoni AO, de
Caestecker MP and Lovvorn HN III: CITED1 confers stemness to Wilms
tumor and enhances tumorigenic responses when enriched in the
nucleus. Oncotarget. 5:386–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Telang NT: Stem cell models for breast and
colon cancer: Experimental approach for drug discovery. Int J Mol
Sci. 23:92232022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu M, Casimiro MC, Wang C, Shirley LA,
Jiao X, Katiyar S, Ju X, Li Z, Yu Z, Zhou J, et al: p21CIP1
attenuates Ras- and c-Myc-dependent breast tumor epithelial
mesenchymal transition and cancer stem cell-like gene expression in
vivo. Proc Natl Acad Sci USA. 106:19035–19039. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao BD, Zhao YJ, Jia XY, Wu J, Wang YG
and Huang F: Multifaceted p21 in carcinogenesis, stemness of tumor
and tumor therapy. World J Stem Cells. 12:481–487. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Telang N: Drug-resistant stem cells: Novel
approach for colon cancer therapy. Int J Mol Sci. 23:25192022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang I, Ohn T, Moon D, Maeng YH, Jang BG
and Yoon SP: SNU-333 cells as an appropriate cell line for the
orthotopic renal cell carcinoma model. Technol Cancer Res Treat.
20:153303382110384872021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim JB, Hwang SE and Yoon SP:
Dexamethasone reduces side population fraction through
downregulation of ABCG2 transporter in MCF-7 breast cancer cells.
Mol Med Rep. 16:453–458. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibue T and Weinberg RA: EMT, CSCs, and
drug resistance: The mechanistic link and clinical implications.
Nat Rev Clin Oncol. 14:611–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singla M, Kumar A, Bal A, Sarkar S and
Bhattacharyya S: Epithelial to mesenchymal transition induces stem
cell like phenotype in renal cell carcinoma cells. Cancer Cell Int.
18:572018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iglesias JM, Beloqui I, Garcia-Garcia F,
Leis O, Vazquez-Martin A, Eguiara A, Cufi S, Pavon A, Menendez JA,
Dopazo J and Martin AG: Mammosphere formation in breast carcinoma
cell lines depends upon expression of E-cadherin. PLoS One.
8:e772812013. View Article : Google Scholar
|
|
22
|
Han SJ, Kwon S and Kim KS: Challenges of
applying multicellular tumor spheroids in preclinical phase. Cancer
Cell Int. 21:1522021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brouxhon SM, Kyrkanides S, Teng X, Athar
M, Ghazizadeh S, Simon M, O'Banion MK and Ma L: Soluble E-cadherin:
A critical oncogene modulating receptor tyrosine kinases, MAPK and
PI3K/Akt/mTOR signaling. Oncogene. 33:225–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu QP, Kuang JY, Yang QK, Bian XW and Yu
SC: Beyond a tumor suppressor: Soluble E-cadherin promotes the
progression of cancer. Int J Cancer. 138:2804–2815. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou G, Lv X, Zhong X, Ying W, Li W, Feng
Y, Xia Q, Li J, Jian S and Leng Z: Suspension culture strategies to
enrich colon cancer stem cells. Oncol Lett. 25:1162023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gheytanchi E, Naseri M, Karimi-Busheri F,
Atyabi F, Mirsharif ES, Bozorgmehr M, Ghods R and Madjd Z:
Morphological and molecular characteristics of spheroid formation
in HT-29 and Caco-2 colorectal cancer cell lines. Cancer Cell Int.
21:2042021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Min SO, Lee SW, Bak SY and Kim KS: Ideal
sphere-forming culture conditions to maintain pluripotency in a
hepatocellular carcinoma cell lines. Cancer Cell Int. 15:952015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han XY, Wei B, Fang JF, Zhang S, Zhang FC,
Zhang HB, Lan TY, Lu HQ and Wei HB: Epithelial-mesenchymal
transition associates with maintenance of stemness in
spheroid-derived stem-like colon cancer cells. PLoS One.
8:e733412013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Z, Bu X, Chen H, Wang Q and Sha W:
Bmi-1 promotes the invasion and migration of colon cancer stem
cells through the downregulation of E-cadherin. Int J Mol Med.
38:1199–1207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Klopp AH, Lacerda L, Gupta A, Debeb BG,
Solley T, Li L, Spaeth E, Xu W, Zhang X, Lewis MT, et al:
Mesenchymal stem cells promote mammosphere formation and decrease
E-cadherin in normal and malignant breast cells. PLoS One.
5:e121802010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang T, Yang Z, Zhu Q, Wu Y, Sun K,
Alahdal M, Zhang Y, Xing Y, Shen Y, Xia T, et al: Up-regulation of
miR-210 induced by a hypoxic microenvironment promotes breast
cancer stem cells metastasis, proliferation, and self-renewal by
targeting E-cadherin. FASEB J. 6:fj201801013R2018.PubMed/NCBI
|
|
33
|
Park NR, Cha JH, Jang JW, Bae SH, Jang B,
Kim JH, Hur W, Choi JY and Yoon SK: Synergistic effects of CD44 and
TGF-β1 through AKT/GSK-3β/β-catenin signaling during
epithelial-mesenchymal transition in liver cancer cells. Biochem
Biophys Res Commun. 477:568–574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Izumiya M, Kabashima A, Higuchi H,
Igarashi T, Sakai G, Iizuka H, Nakamura S, Adachi M, Hamamoto Y,
Funakoshi S, et al: Chemoresistance is associated with cancer stem
cell-like properties and epithelial-to-mesenchymal transition in
pancreatic cancer cells. Anticancer Res. 32:3847–3853.
2012.PubMed/NCBI
|
|
35
|
Ohnishi Y, Yasui H, Kakudo K and Nozaki M:
Lapatinib-resistant cancer cells possessing epithelial cancer stem
cell properties develop sensitivity during sphere formation by
activation of the ErbB/AKT/cyclin D2 pathway. Oncol Rep.
36:3058–3064. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Acikgoz E, Guven U, Duzagac F, Uslu R,
Kara M, Soner BC and Oktem G: Enhanced G2/M arrest, caspase related
apoptosis and reduced E-cadherin dependent intercellular adhesion
by trabectedin in prostate cancer stem cells. PLoS One.
10:e01410902015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qian Y, Wu X, Yokoyama Y, Okuzaki D,
Taguchi M, Hirose H, Wang J, Hata T, Inoue A, Hiraki M, et al:
E-cadherin-fc chimera protein matrix enhances cancer stem-like
properties and induces mesenchymal features in colon cancer cells.
Cancer Sci. 110:3520–3532. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morata-Tarifa C, Jiménez G, García MA,
Entrena JM, Griñán-Lisón C, Aguilera M, Picon-Ruiz M and Marchal
JA: Low adherent cancer cell subpopulations are enriched in
tumorigenic and metastatic epithelial-to-mesenchymal
transition-induced cancer stem-like cells. Sci Rep. 6:187722016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang S, Cai M, Zheng Y, Zhou L, Wang Q
and Chen L: miR-888 in MCF-7 side population sphere cells directly
targets E-cadherin. J Genet Genomics. 41:35–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Patil PU, D'Ambrosio J, Inge LJ, Mason RW
and Rajasekaran AK: Carcinoma cells induce lumen filling and EMT in
epithelial cells through soluble E-cadherin-mediated activation of
EGFR. J Cell Sci. 128:4366–4379. 2015.PubMed/NCBI
|
|
41
|
Deng X, Chen C, Wu F, Qiu L, Ke Q, Sun R,
Duan Q, Luo M and Luo Z: Curcumin inhibits the migration and
invasion of non-small-cell lung cancer cells through
radiation-induced suppression of epithelial-mesenchymal transition
and soluble E-cadherin expression. Technol Cancer Res Treat.
19:15330338209474852020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liang SC, Yang CY, Tseng JY, Wang HL, Tung
CY, Liu HW, Chen CY, Yeh YC, Chou TY, Yang MH, et al: ABCG2
localizes to the nucleus and modulates CDH1 expression in lung
cancer cells. Neoplasia. 17:265–278. 2015. View Article : Google Scholar : PubMed/NCBI
|