Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of cancer worldwide and is the second leading cause of
cancer-related death (1). As the
symptoms are usually insidious, the majority of the patients
present with advanced-stage HCC upon diagnosis. Furthermore,
certain patients possess a huge tumor with a size of ≥10 cm upon
diagnosis, resulting in a poor prognosis even after treatment
(2). For patients presenting with
resectable HCC with well-preserved liver function, hepatectomy is
preferred for treatment; however, the outcomes are compromised due
to a postoperative recurrence rate of 60–70% within 5 years
(3–5).
Hepatectomy is the preferred option for treating
huge HCC. However, the outcome following hepatectomy remains
unsatisfactory as the R0 resection rate is still <20% (6). To date, alternative choices exist for
patients in whom hepatectomy is not recommended, such as
transarterial chemoembolization (TACE), hepatic artery infusion
chemotherapy, radiofrequency ablation, and sorafenib or Lenvatinib
(7). Therefore, a proper
preoperative prognostic prediction is essential for the selection
of the optimal regimen.
The COX proportional hazard (CPH) model has been
commonly utilized in evaluating prognosis by predicting the
recurrence rate based on the prognostic factors (8–10).
However, its predictive performance is limited as it cannot model
the complicated, multidimensional, and non-linear relationships
among different prognostic variables. Therefore, there is an urgent
need to develop novel solutions for accurate prognostic prediction
even if it involves non-linear variables. Recently, additional
attention has been paid to machine learning, in which machines
mimic, recognize, and learn cognitive functions of the human mind
for performing empirical predictions (11). The random survival forest (RSF)
model is a non-parametric machine-learning strategy that can be
utilized for the prediction of survival analysis among patients
with cancer (12). RSF is superior
to CPH models as it uses non-linear functions and considers all
possible interactions between variables to improve the predictive
performance (13). Moreover, the
RSF model is superior to the conventional regression models in
predicting the prognosis of HCC (14). In the present study, the
preoperative and postoperative risk factors for the recurrence of
huge HCC were investigated. A preoperative and postoperative RSF
model for predicting the recurrence of huge HCC was constructed.
The following article/case is presented in accordance with the
STROBE reporting checklist (15).
Materials and methods
Patients and study design
The data of 1,192 consecutive patients [male, 934,
median age: 48.31±11.26 (IQR, 18–79); female: 258, median age:
49.35±12.56 (IQR, 21–89 years)] who underwent liver resection for
huge HCC at the Eastern Hepatobiliary Surgery Hospital (EHSH)
between January 2008 and December 2016 and at the Mengchao
Hepatobiliary Hospital (MHH) between January 2014 and December 2016
were retrospectively reviewed. HCC was diagnosed according to the
practice guidelines recommended by the American Association for the
Study of Liver Diseases (16). The
patients with the following conditions were included in the present
study: i) Those confirmed to have HCC by immunohistochemistry,
presenting an HCC lesion with a diameter ≥10 cm; ii) Child-Pugh A
or B liver function; iii) absence of extra-hepatic metastasis; and
iv) with R0 resection, defined as complete resection of macroscopic
tumor nodules with tumor-free margins confirmed by histological
examination. Patients with the following conditions were excluded
from the present study: i) Those who received palliative tumor
resection; ii) those who underwent preoperative anticancer
treatments; iii) those with a history of other malignancies; or iv)
those with incomplete clinical data and those lost to follow-up
within 2 months following hepatectomy.
All data in the present study were verified by three
independent researchers. To establish the RSF model, qualified
patients from the EHSH were randomly assigned to the training
cohort and the internal validation cohort with a 7:3 ratio. All
qualified patients from the MHH served as the external validation
cohort. The protocol of the present study was granted approval from
the Medical Ethics Committee of Mengchao Hepatobiliary Hospital of
Fujian Medical University (2022-027-01). Written informed consent
for participation was not required for this study in accordance
with national legislation and institutional requirements.
Preoperative assessment
All patients underwent routine preoperative
examinations, including immunology of hepatitis B virus and
hepatitis C viral infection, α-fetoprotein (AFP) concentration,
prothrombin time (PT), activated partial thromboplastin time
(APTT), fibrinogen (Fg) concentration, white blood cell count,
platelet (PLT), and liver and kidney function examination. In
addition, imaging examination was provided to each patient
including chest X-ray, abdominal ultrasound, contrast-enhanced CT
scan, and/or MRI of the abdomen.
Surgical procedures
The decision of anatomical or partial hepatectomy is
commonly based on liver function, tumor number, and location.
Specifically, anatomical hepatectomy was preferentially given to
patients with a well-preserved liver function and tumors located
within a segment, sector, and hemiliver. Partial hepatectomy was
provided to patients with poor liver function. For huge HCC, the
anterior approach was often used for hepatectomy. Specifically, the
liver was transected along the principle plane dividing the right
from the left hemiliver, with or without hepatic inflow clamping,
to the anterior of the inferior vena cava. The corresponding
hepatic pedicle, hepatic vein, and short hepatic veins were
ligated. Finally, the liver ligaments were freed to remove the
hemiliver harboring the tumor. Intraoperative liver ultrasonography
was routinely performed to ensure the complete resection of
detectable tumors, followed by pathological analysis.
End points and follow-up
The end points of the study included overall
survival (OS) and recurrence-free survival (RFS). OS was defined as
the interval between the date of surgery and the date of death or
loss to follow-up. RFS was defined as the interval between the date
of surgery and the date of recurrence. Each patient was followed up
based on the conventional program and the recurrence was confirmed
according to the criteria previously described (17).
RSF modeling process
The RSF model was applied to real data settings to
uncover highly complex interrelationships between variables; it can
also estimate the individual cumulative hazard function by
integrating the Nelson-Allen estimator in the model (18,19).
Variable Importance (VIMP) was obtained by measuring the decrease
in prediction accuracy using out-of-bag data which were not used
for building trees each time. The variables were selected by
filtering based on their VIMP. The risk index was derived from the
estimated cumulative hazard function. The Cox model involved a
continuous covariate and was utilized to evaluate the significance
of the risk index. The risk groups were generated by the 50th and
85th percentile of the risk index (20). Kaplan-Meier curves for each risk
group were plotted in each cohort.
The RSF model was constructed based on the results
of the VIMP of recurrence in the training cohort. The preoperative
and postoperative RSF model was established based on the
preoperative clinical imaging data and the clinicopathological
variables, respectively. The predictive performance of the RSF
model was measured using Harrell's concordance index (C-index), the
time-dependent areas under the receiver operating characteristic
curve (tdAUC), the prediction error curve, and the calibration plot
(21,22). Clinical usefulness was measured by
decision curve analysis (DCA) with a net benefit at a threshold of
50%. The overall performance was measured by the prediction error
curves. The cumulative recurrence between each risk group was
assessed and tested using Kaplan-Meier curves and the log-rank
test, respectively. The discrimination of the RSF model was also
compared with the AJCC TNM stage (23), Barcelona Clinic Liver Cancer (BCLC)
stage (24), and Chinese stage
(25) in each cohort.
Statistical analysis
Statistical analyses were performed using SPSS
version 22.0, R version R-4.2.3 (r-project.org/) and R studio (4.2
version, rstudio.com/). The categorical variables are presented as
the frequency and percentage, and continuous variables are
presented as the mean ± standard error. A χ2 test or
Fisher's exact test was used for the inter-group comparisons of the
categorical variables. A Student's t-test or a Mann-Whitney U test
was used for the comparison of the continuous variables.
Kaplan-Meier analysis was used to estimate the cumulative rates of
survival. The comparison of the survival curves was performed based
on a log-rank test. The continuous variables not normally
distributed were presented as the median or interquartile range and
were compared using a Mann-Whitney U test. All statistical tests
were two-tailed. P<0.05 was considered to indicate a
statistically significant difference.
Results
Participant characteristics
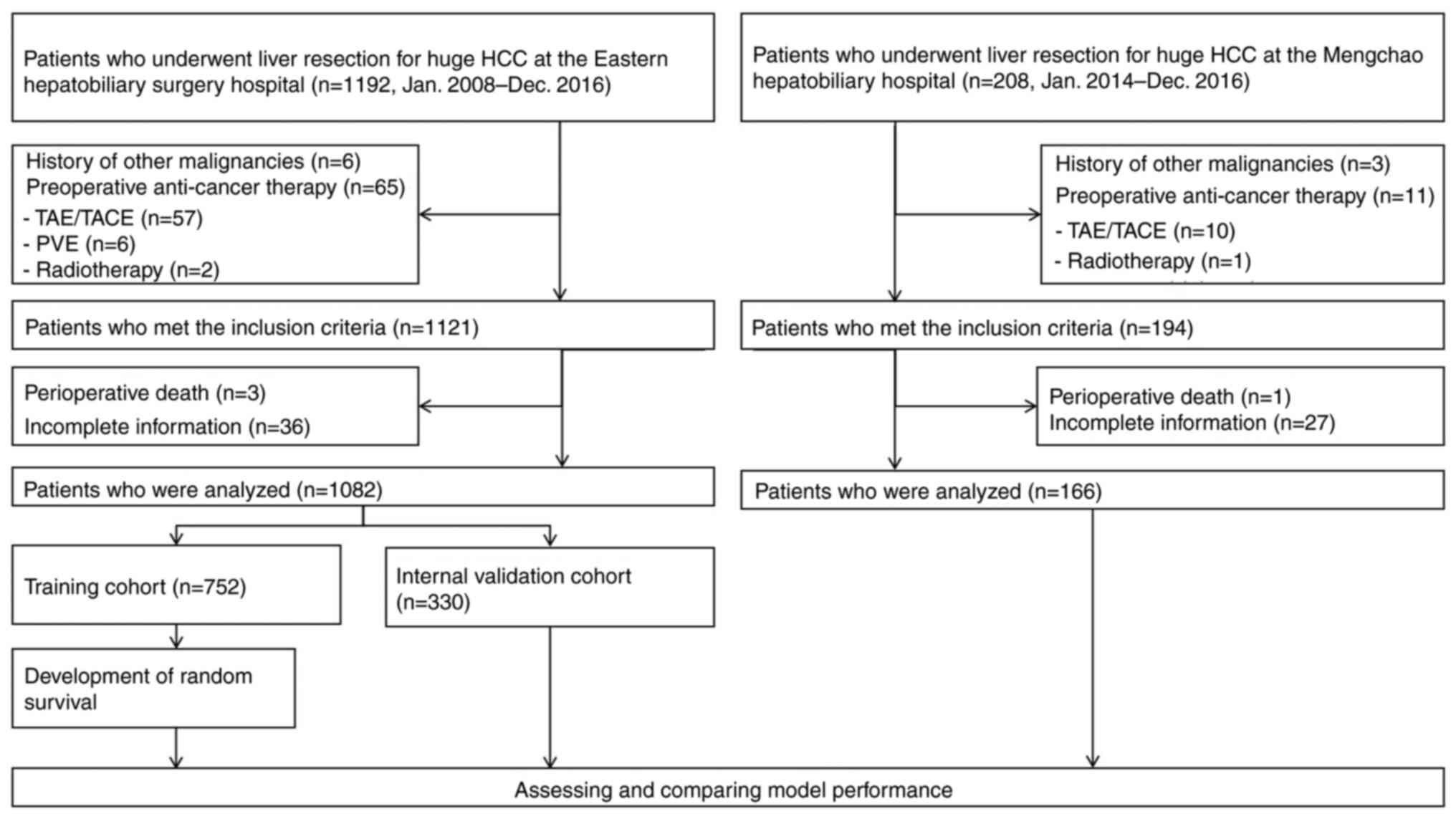
In total, 1,192 consecutive patients (male: 934;
female: 258) underwent partial hepatectomy for huge HCC at EHSH
between January 2008 and December 2016. A total of 110 patients
were excluded due to preoperative anticancer therapy (n=65),
history of other malignancies (n=6), incomplete information (n=36),
and perioperative death (n=3). Finally, 1,082 patients were
included and randomly divided into a training cohort (n=752) and a
validation cohort (n=330) based on a ratio of 7:3. For the external
validation cohort, 208 patients from MHH between January 2014 and
December 2016, and finally 166 patients met the inclusion criteria
following exclusion of the history of malignancies (n=3),
preoperative anticancer therapy (n=11), incomplete information
(n=27), and perioperative death (n=1). The flowchart of this
process is shown in Fig. 1.
The baseline clinicopathological features of the
participants are listed in Table I.
No statistical differences were noted in the baseline
clinicopathological characteristics between the training and the
internal validation cohorts (Table
I). In contrast to these observations, several
clinicopathological features did differ amongst the training,
internal, and external cohorts, including PT, APTT, Fg, tumor
capsular, microvascular invasion (MVI), and Edmondson-Steiner grade
(P<0.01).
 | Table I.Clinicopathologic features. |
Table I.
Clinicopathologic features.
| Variable | Training cohort,
n=752 | Internal cohort,
n=330 | External cohort,
n=164 |
P-valued |
P-valuee |
|---|
| Age, Mean (SD),
years | 47.9 (11.4) | 49.2 (10.9) | 48.9 (11.4) | 0.066 | 0.282 |
| Sex, n (%) |
|
|
| 0.783 | 0.446 |
|
Female | 102 (13.6%) | 42 (12.7%) | 26 (15.9%) |
|
|
|
Male | 650 (86.4%) | 288 (87.3%) | 138 (84.1%) |
|
|
| Etiology, n
(%) |
|
|
| 0.237 | 0.292 |
|
HBV | 564 (75.0%) | 263 (79.7%) | 133 (81.1%) |
|
|
|
HCV | 2 (0.3%) | 1 (0.3%) | 1 (0.6%) |
|
|
|
Others | 186 (24.7%) | 66 (20.0%) | 30 (18.3%) |
|
|
| ALB, Mean (SD),
g/l | 40.7 (3.92) | 40.3 (3.47) | 38.4 (3.94) | 0.08 | 0.003b |
| Mean TBIL (SD),
µmol/l | 14.7 (11.6) | 13.9 (5.58) | 15.4 (7.0) | 0.126 | 0.141 |
| AST, Mean (SD),
U/l | 50.0 (23.3) | 49.8 (22.2) | 60.1(48.3) | 0.922 | 0.009b |
| PT (s), Mean
(SD) | 12.4 (4.38) | 12.3 (1.43) | 13.1 (1.28) | 0.662 |
<0.001c |
| APTT (s), Mean
(SD) | 27.9 (4.26) | 27.5 (3.95) | 33.5 (6.66) | 0.122 |
<0.001c |
| Fg, (mg/dl), Mean
(SD) | 3.07 (0.923) | 3.03 (0.903) | 3.59 (1.02) | 0.529 |
<0.001c |
| AFP (ng/ml), Median
(IQR) | 920
(21.9-1210) | 531
(21.6-1,210) | 761
(43.3-1,210) | 0.313 | 0.157 |
| Neutrophil Count,
Mean (SD), ×109/l | 3.85 (1.55) | 3.68 (1.36) | 4.16 (1.71) | 0.074 | 0.012a |
| Lymphocyte Count,
Mean (SD), ×109/l | 1.51 (0.536) | 1.55 (0.57) | 1.48 (0.483) | 0.331 | 0.277 |
| Mean platelets
(SD), ×109/l | 197 (84.8) | 200 (82.4) | 209 (92.9) | 0.558 | 0.141 |
| PLR, Mean (SD) | 139. 76 (69.1) | 143.54 (74.48) | 150.98 (74.97) | 0.622 | 0.162 |
| PNLR, Mean
(SD) | 564.14
(426.08) | 545.50
(416.96) | 645.14
(424.74) | 0.483 | 0.016a |
| Tumor number, n
(%) |
|
|
| 0.362 | 0.157 |
| 1 | 547 (72.7%) | 249 (75.5%) | 120 (73.2%) |
|
|
| 2 | 96 (12.8%) | 38 (11.5%) | 25 (15.2%) |
|
|
| 3 | 31 (4.1%) | 7 (2.1%) | 9 (5.5%) |
|
|
| ≥4 | 78 (10.4%) | 36 (10.9%) | 10 (6.1%) |
|
|
| Mean tumor size
(SD), cm | 13.34 (2.65) | 13.6 (2.69) | 13.27 (2.58) | 0.152 | 0.462 |
| Tumor
numberf, n (%) |
|
|
| 0.415 | 0.121 |
| 1 | 547 (72.7%) | 249 (75.5%) | 120 (73.2%) |
|
|
| 2 | 100 (13.3%) | 40 (12.1%) | 25 (15.2%) |
|
|
| 3 | 27 (3.6%) | 6 (1.8%) | 9 (5.5%) |
|
|
| ≥4 | 78 (10.4%) | 35 (10.6%) | 10 (6.1%) |
|
|
| Tumor
sizef, Mean (SD),
cm | 13.28 (2.57) | 13.46 (2.52) | 13.2 (2.48) | 0.282 | 0.526 |
| Satellite nodules,
n (%) |
|
|
| 0.0554 | 0.08 |
|
Present | 416 (55.3%) | 161 (48.8%) | 100 (61.0%) |
|
|
|
Absent | 336 (44.7%) | 169 (51.2%) | 64 (39.0%) |
|
|
| Tumor capsule, n
(%) |
|
|
| 0.253 |
<0.001c |
|
Complete | 214 (28.5%) | 106 (32.1%) | 20 (12.2%) |
|
|
|
Incomplete | 538 (71.5%) | 224 (67.9%) | 144 (87.8%) |
|
|
| Cirrhosis, n
(%) | 539 (71.7%) | 238 (72.1%) | 109 (66.5%) | 0.939 | 0.188 |
| Macrovascular
invasion, n (%) | 114 (15.2%) | 45 (13.6%) | 31 (18.9%) | 0.577 | 0.2 |
| Macrovascular
invasionf, n (%) | 110 (14.6%) | 43 (13.0%) | 30 (18.3%) | 0.549 | 0.2 |
| Microvascular
invasion, n (%) | 345 (45.9%) | 154 (46.7%) | 125 (76.2%) | 0.862 |
<0.001c |
| Edmondson-Steiner
classification, n (%) |
|
|
| 0.937 |
<0.001c |
|
I–II | 34 (4.5%) | 16 (4.8%) | 31 (18.9%) |
|
|
|
III–IV | 718 (95.5%) | 314 (95.2%) | 133 (81.1%) |
|
|
| Intraoperative
blood transfusion, n (%) |
|
|
| 0.993 | 0.812 |
|
Yes | 175 (23.3%) | 76 (23.0%) | 40 (24.4%) |
|
|
| No | 577 (76.7%) | 254 (77.0%) | 124 (75.6%) |
|
|
| BCLC staging
system, n (%) |
|
|
| 0.787 | 0.181 |
| A | 470 (62.5%) | 225 (68.2%) | 99 (60.4%) |
|
|
| B | 168 (22.3%) | 73 (22.1%) | 34 (20.7%) |
|
|
| C | 114 (15.2%) | 32 (9.7%) | 31 (18.9%) |
|
|
| AJCC staging
system8th, n (%) |
|
|
| 0.559 |
<0.001c |
| Ib | 312 (41.5%) | 133 (40.3%) | 37 (22.6%) |
|
|
| II | 158 (21.0%) | 92 (27.9%) | 68 (41.5%) |
|
|
|
IIIa | 168 (22.3%) | 73 (22.1%) | 28 (17.1%) |
|
|
|
IIIb | 114 (15.2%) | 32 (9.7%) | 31 (18.9%) |
|
|
| Chinese staging
system, n (%) |
|
|
| 0.077 | 0.027a |
| Ib | 470 (62.5%) | 225 (68.2%) | 99 (60.4%) |
|
|
|
IIa | 99 (13.2%) | 39 (11.8%) | 27 (16.5%) |
|
|
|
IIb | 69 (9.2%) | 34 (10.3%) | 7 (4.3%) |
|
|
|
IIIa | 114 (15.2%) | 32 (9.7%) | 31 (18.9%) |
|
|
Prognosis
The study was censored on December 31, 2021, for the
training and internal validation cohorts. The median follow-up
period was 31.41 (range, 2–143) months and 25.94 (range, 2–97)
months in the training and internal validation cohorts. The 1-, 3-,
and 5-year OS rates in the training cohort were 84, 59.1, and
45.8%, and the 1-, 3-, and 5-year RFS rates were 69.2, 44.1, and
23.7%, respectively (Table II). In
the internal validation cohort, the median survival was 41.6 months
(range, 2–97) months. The 1-, 3-, and 5-year OS rates in the
internal validation cohort were 80.78, 55.4, and 43.55%, while the
1-, 3-, and 5-year RFS rates were 69.3, 45.2, and 29.7%,
respectively. The last follow-up for patients in the external
validation cohort was on December 31, 2021. The median survival of
these patients was 25.06 (range, 2–84) months. In the external
validation cohort, the 1-, 3-, and 5-year OS rates were 78.75,
52.38, and 38.26%, respectively; in the same cohorts, the 1-, 3-,
and 5-year RFS rates were 60.5, 39.8, and 29.2%, respectively. No
significant differences were noted among these three cohorts in the
1-, 3-, and 5-year OS and RFS rates following liver resection
(P=0.117 and 0.052, respectively).
 | Table II.Prognosis of training cohort,
internal validation cohort, and external validation cohort. |
Table II.
Prognosis of training cohort,
internal validation cohort, and external validation cohort.
| Survival | Cohort | 1-year | 3-years | 5-years | P-value |
|---|
| Overall
survival |
|
|
|
| 0.117 |
|
| Training set | 0.8400 | 0.5910 | 0.4580 |
|
|
| Internal
validation | 0.8078 | 0.5540 | 0.4355 |
|
|
| External
validation | 0.7875 | 0.5238 | 0.3826 |
|
| Recurrence-free
survival |
|
|
|
| 0.052 |
|
| Training set | 0.6920 | 0.4410 | 0.2370 |
|
|
| Internal
validation | 0.6930 | 0.4520 | 0.2970 |
|
|
| External
validation | 0.6050 | 0.3980 | 0.2920 |
|
Preoperative and postoperative RSF
models for predicting recurrence
The common variables used for preoperative and
postoperative analyses were the demographic data, including age,
sex, HCC family history, preoperative serological data, imaging
data, and platelet-to-lymphocyte ratio (PLR) as listed in Table I. The preoperative modeling was
based on the MRI findings, while the postoperative modeling was
conducted based on the pathological results. Finally, the
preoperative RSF model was constructed using 16 features. The
prediction error rate was low and stable during the process of
constructing 1,000 survival trees (Fig.
2A); subsequently, the VIMP for all the features used for
growing the trees was generated. The top 10 variables were
macrovascular invasion (MaVI), satellite nodules, tumor number,
age, AFP, aspartate aminotransferase, fibrinogen, platelet
neutrophil/lymphocyte ratio (PNLR), PLR, and PT. Postoperatively,
the RSF model was constructed using 21 features and the top 10
variables were MaVI, satellite nodules, tumor number, age, AFP,
fibrinogen, aspartate aminotransferase, PNLR, PLR, and PT (Fig. 2B).
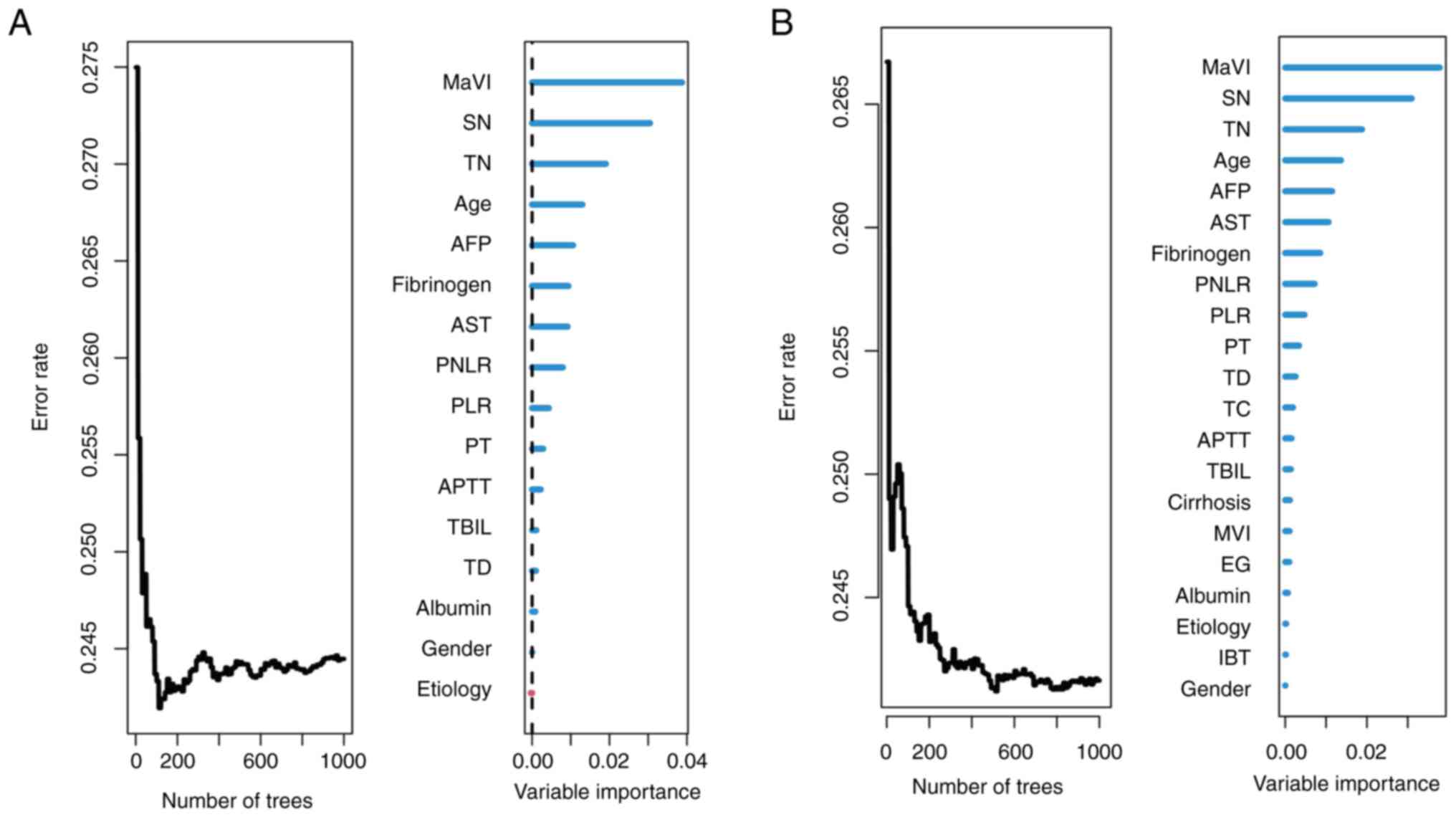 | Figure 2.Construction of the preoperative RSF
model and postoperative RSF model for prediction of recurrence in
the training cohort. (A) preoperative RSF model, (B) postoperative
RSF model. RSF, random survival forests; SN, satellite nodules;
AST, aspartate aminotransferase; AFP, α-fetoprotein; TN, tumor
number; PNLR, platelet-neutrophil-lymphocyte ratio; MaVI,
macrovascular invasion; TD, tumor diameter; PT, prothrombin time;
PLR, platelet-to-lymphocyte ratio; APTT, activated partial
thromboplastin time; HCVAb, hepatitis C virus antibody; HBsAg,
hepatitis B virus surface antigen; EG, Edmondson-Steiner
classification; TC, tumor capsule; IBT, intraoperative blood
transfusion; ALT, aspartate aminotransferase; MVI, microvascular
invasion; AFP, α fetoprotein; ALB, albumin; PLT, platelet count;
TBIL, total bilirubin. |
Efficiency of the preoperative and
postoperative RSF model in recurrence prediction
Both preoperative and postoperative RSF models of
the training, internal, and external validation cohorts could
feasibly predict the recurrence of HCC following surgery. The
C-index of the preoperative RSF model in the training, internal
validation, and external validation cohorts was 0.766 (95% CI:
0.749-0.785), 0.745 (95% CI: 0.726-0.764), and 0.731 (95% CI:
0.713-0.749), respectively. The Gönen & Heller's K values of
the preoperative RSF model were 0.699 (95% CI: 0.681-0.717), 0.695
(95% CI: 0.666-0.724), and 0.683 (95% CI: 0.638-0.728),
respectively. The recurrence prediction of the preoperative RSF
model in the three cohorts was significantly improved compared with
that obtained based on the 8th edition of the AJCC TNM stage
(23), BCLC stage (24), and Chinese stage (25) (Table
III).
 | Table III.Prognostic performance of the pre-
and postoperative random survival forests model. |
Table III.
Prognostic performance of the pre-
and postoperative random survival forests model.
| Measure of
discrimination | Cohort | RSF-preoperative
(SE) | RSF-postoperative
(SE) | BCLC (SE) | AJCC TNM (SE) | Chinese staging
system (SE) |
|---|
| Harrell's
c-index | Training set | 0.766 (0.097) | 0.775 (0.097) | 0.665 (0.097) | 0.663 (0.097) | 0.671 (0.097) |
|
| Internal
validation | 0.745 (0.095) | 0.746 (0.095) | 0.634 (0.095) | 0.645 (0.095) | 0.639 (0.095) |
|
| External
validation | 0.731 (0.094) | 0.758 (0.095) | 0.632 (0.094) | 0.574 (0.094) | 0.631 (0.094) |
| tdAUC (5
years) | Training set | 0.784 (0.028) | 0.793 (0.028) | 0.671 (0.027) | 0.676 (0.029) | 0.677 (0.027) |
|
| Internal
validation | 0.774 (0.041) | 0.778 (0.041) | 0.634 (0.042) | 0.671 (0.047) | 0.631 (0.045) |
|
| External
validation | 0.740 (0.065) | 0.774 (0.062) | 0.651 (0.056) | 0.624 (0.061) | 0.649 (0.056) |
| Gönen &
Heller's K | Training set | 0.699 (0.009) | 0.704 (0.008) | 0.622 (0.008) | 0.643 (0.011) | 0.624 (0.008) |
|
| Internal
validation | 0.695 (0.015) | 0.693 (0.015) | 0.606 (0.014) | 0.639 (0.017) | 0.606 (0.014) |
|
| External
validation | 0.683 (0.023) | 0.696 (0.021) | 0.603 (0.021) | 0.588(0.027) | 0.595(0.021) |
| Royston-Sauerbrei's
R2 | Training set | 0.184 | 0.205 | 0.067 | 0.047 | 0.061 |
|
| Internal
validation | 0.218 | 0.266 | 0.103 | 0.034 | 0.028 |
|
| External
validation | 0.251 | 0.256 | 0.071 | 0.112 | 0.102 |
| Time-dependent
Brier (5 years) | Training set | 0.189 | 0.184 | 0.228 | 0.228 | 0.226 |
|
| Internal
validation | 0.192 | 0.185 | 0.221 | 0.231 | 0.230 |
|
| External
validation | 0.186 | 0.179 | 0.221 | 0.217 | 0.217 |
For the postoperative RSF model, the C-index values
were 0.775 (95% CI: 0.756-0.794) in the training cohort, 0.746 (95%
CI: 0.727-0.765) in the internal validation cohort, and 0.758 (95%
CI: 0.739-0.777) in the external validation cohort, respectively.
The Gönen & Heller's K values of the postoperative RSF model
were 0.704 (95% CI: 0.688-0.719), 0.693 (95% CI: 0.664-0.722), and
0.696 (95% CI: 0.655-0.737), respectively. The recurrence
prediction of the postoperative RSF model was improved compared
with that of the BCLC stage, the 8th edition of the AJCC stage, and
the Chinese staging systems, together with the time-dependent Brier
score and R2 (Table III).
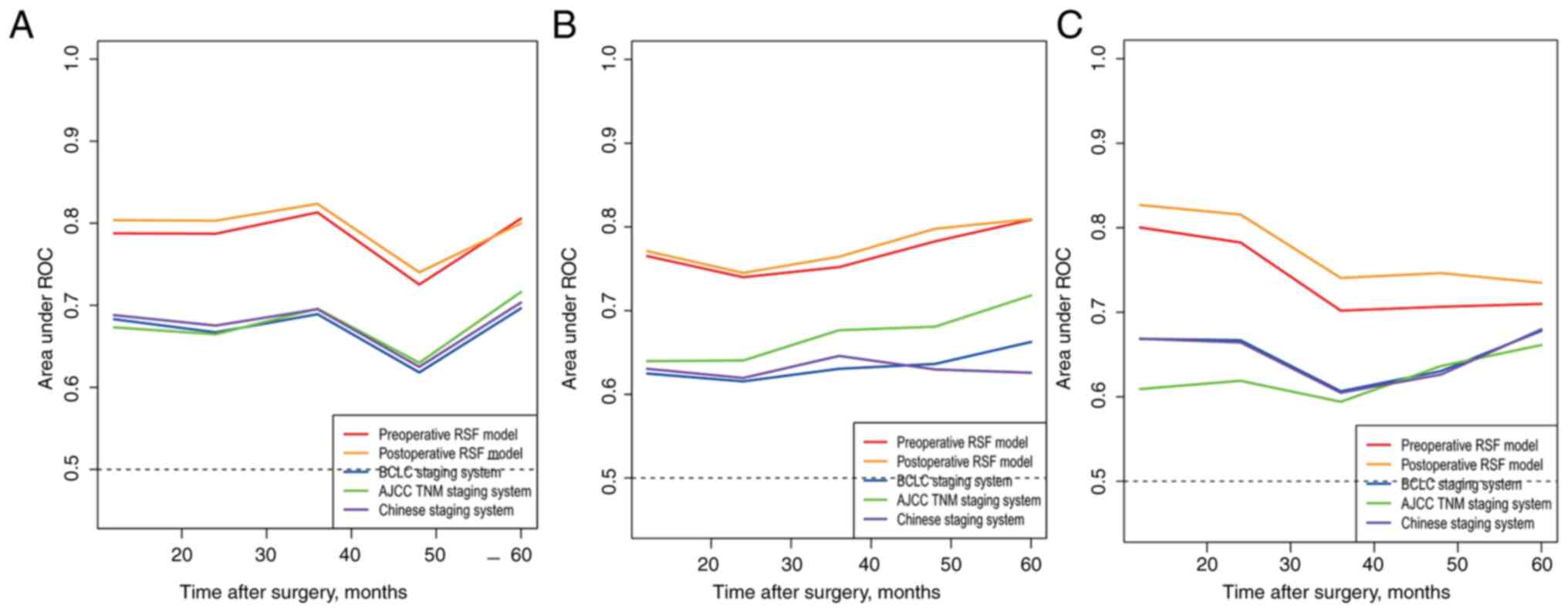
Time-dependent receiver operating characteristic
curve analysis was also performed to assess the discriminative
efficiency of the RSF model. For the preoperative RSF model, the
median time dependent AUCs of the RSF model were 0.784 (95% CI:
0.725-0.813) in the training cohort, 0.778 (95% CI: 0.745-0.809) in
the internal validation cohort, and 0.74 (95% CI: 0.702-0.801) in
the external validation. For the postoperative RSF model, the
corresponding tdAUC was 0.793 (95% CI: 0.742-0.825) in the training
cohort, 0.777 (95% CI: 0.745-0.813) in the internal validation
cohort, and 0.774 (95% CI: 0.732-0.828) in the external validation
cohort, respectively. Both models had higher tdAUCs than those of
the BCLC, AJCC, and Chinese staging systems (Fig. 3A and C).
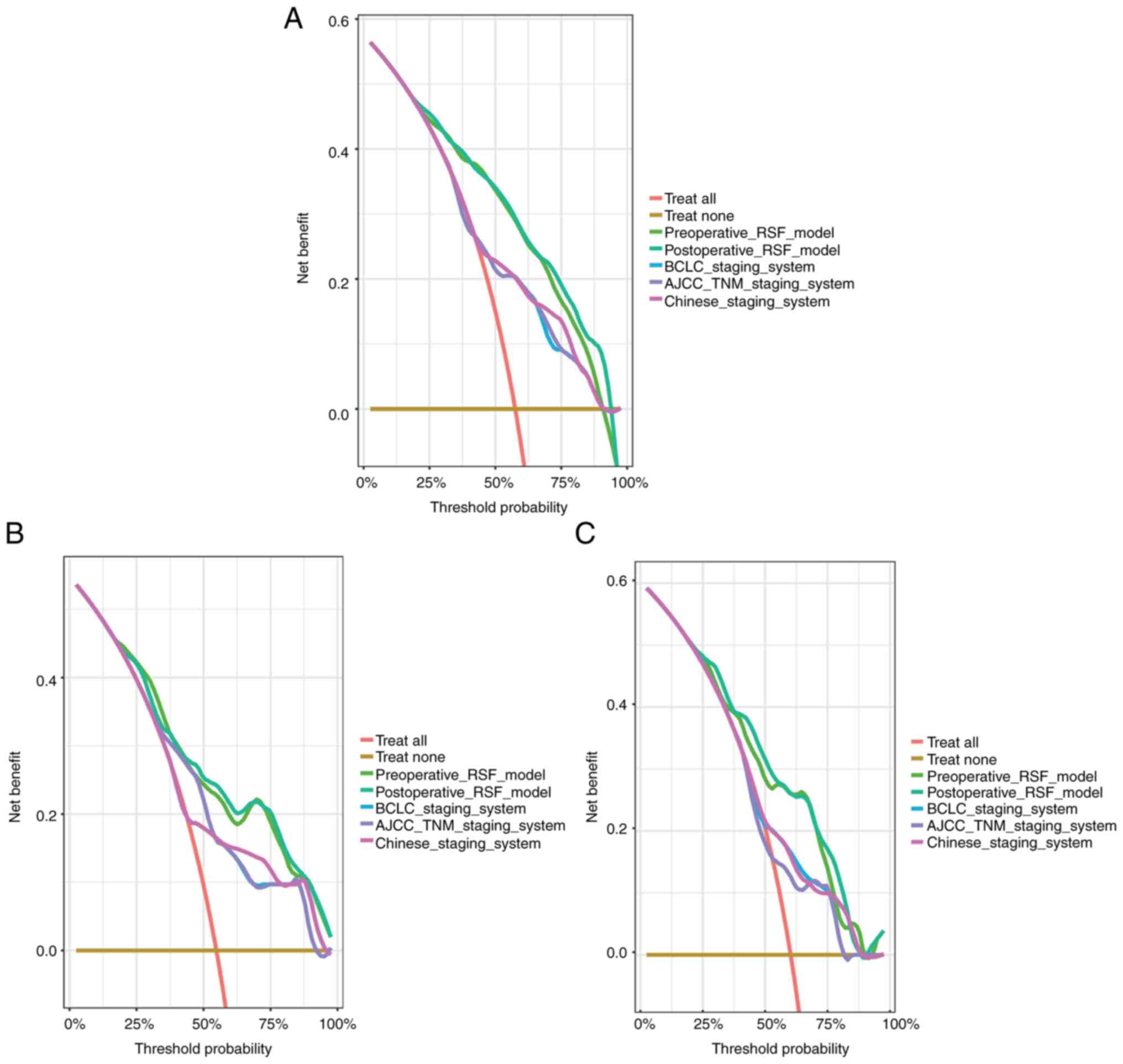
DCA was used to compare the predictive performance
of the preoperative and postoperative RSF models with BCLC, AJCC,
and Chinese staging system-based models in the three cohorts. The
net benefit of the RSF models was superior to that of the other
models as revealed by DCA (Fig.
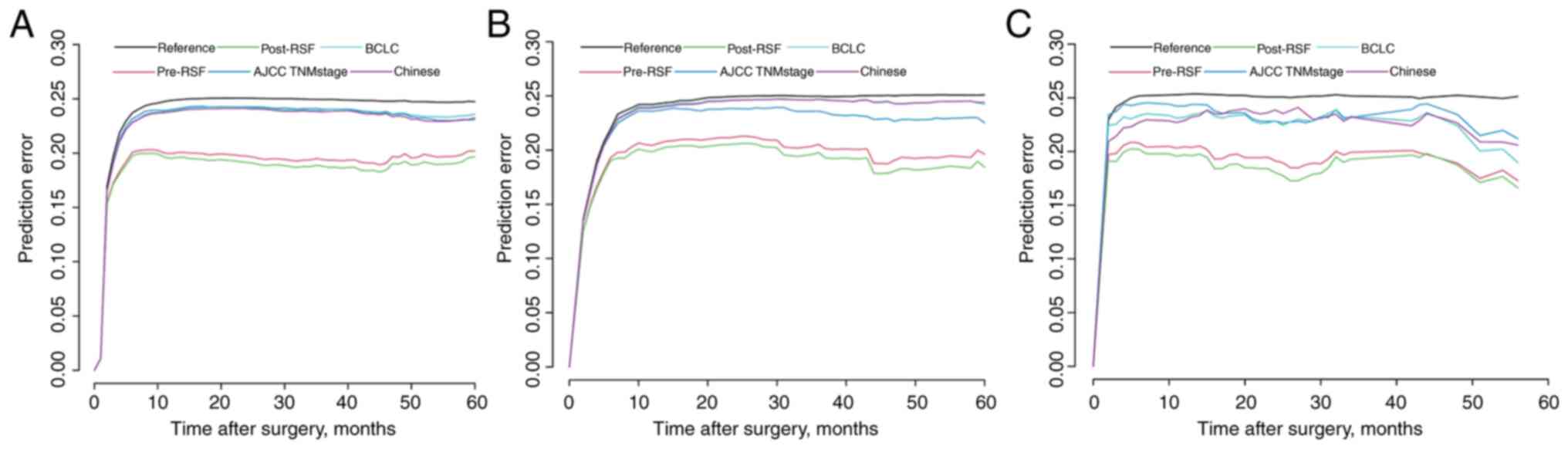
4A-C). The prediction error curve analysis was used to assess
the overall predictive performance of the RSF models. The RSF
models had a lower prediction error rate than those of the
conventional staging systems (Fig. 5A
and C). An optimal consistency was noted between the
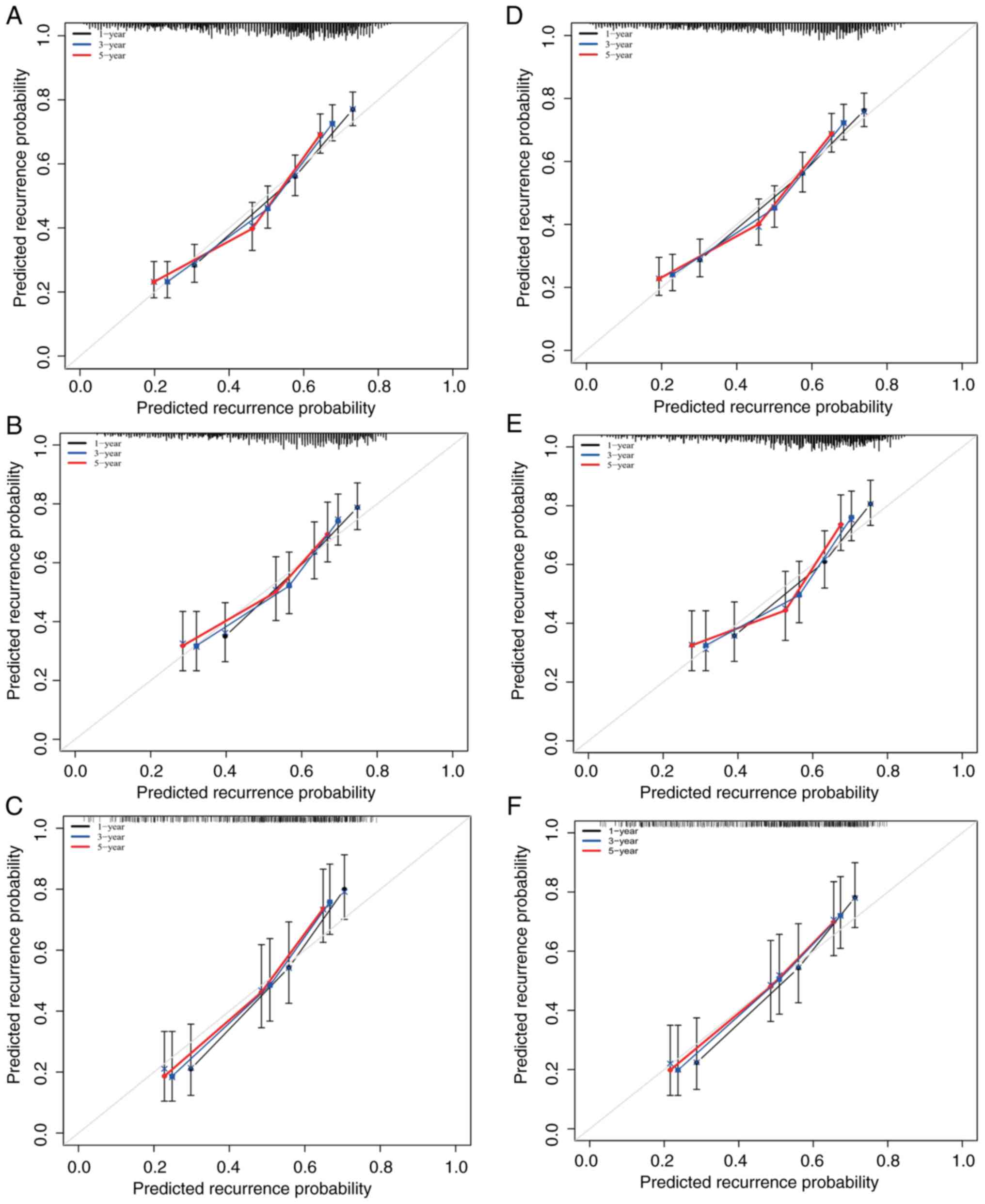
calibration plots of the preoperative and postoperative RSF models.
In addition, the probabilities of 1-, 3-, and 5-year recurrence in
the training and validation cohorts were also consistent (Fig. 6).
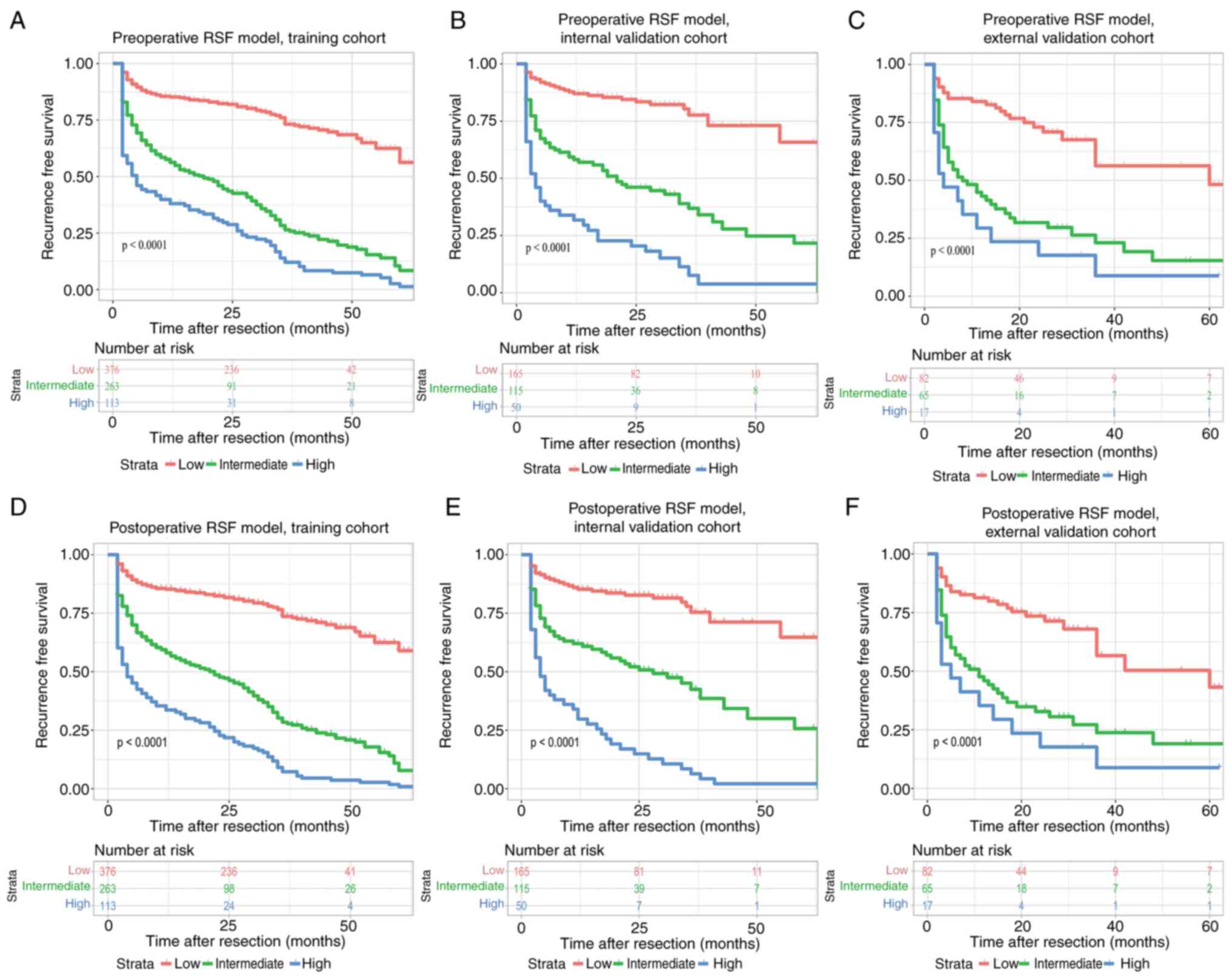
Risk stratification based on the RSF
score
For the risk stratification, the patients were
stratified into three subgroups including low-risk,
intermediate-risk, and high-risk groups based on the cut-off points
that corresponded to the 50th (29.377) and 85th (58.741) percentile
of the risk index in the training cohort of the preoperative and
postoperative RSF models (50th percentile: 29.692; 85th percentile:
59.183). The model exhibited optimal discriminative ability for
recurrence in the presence of apparent distinction from the
recurrence curves of the subgroups based on Kaplan-Meier analysis
(Fig. 7). To facilitate the
clinical application, two web-based prediction tools (https://preoperative-prediction.shinyapps.io/pre-operative_predict/;
and http://preoperative-prediction.shinyapps.io/post-operative_predict/)
were developed for clinicians to use the RSF model (Figs. S1 and S2). This tool can output the risk index,
risk groups, and the recurrence-free probability at 3, 6, 9, 12,
18, 24, 36, 48, and 60 months.
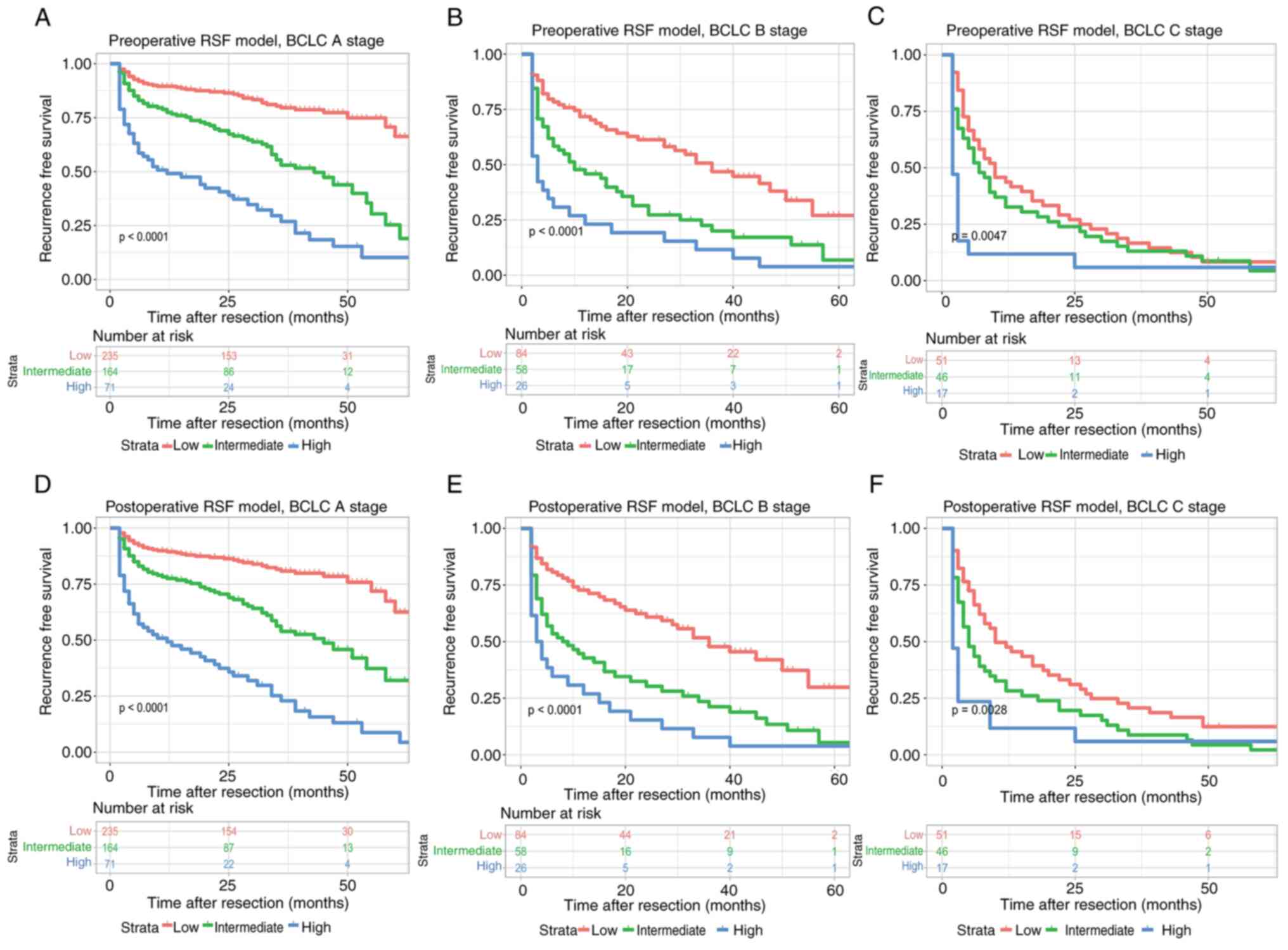
As shown in Fig. 8,
both preoperative and postoperative RSF models could be used to
re-stratify the patients with different recurrence risks at BCLC
stage A (P<0.0001), BCLC stage B (P<0.0001), and BCLC stage C
(P=0.0047, preoperative RSF model; P=0.0028, postoperative RSF
model), respectively. In addition, both preoperative and
postoperative RSF models could be used to distinguish the patients
at the AJCC stage IB (P<0.0001), stage II (P<0.0001), stage
IIIA (P<0.0001), and stage IIIB (P=0.015, preoperative RSF
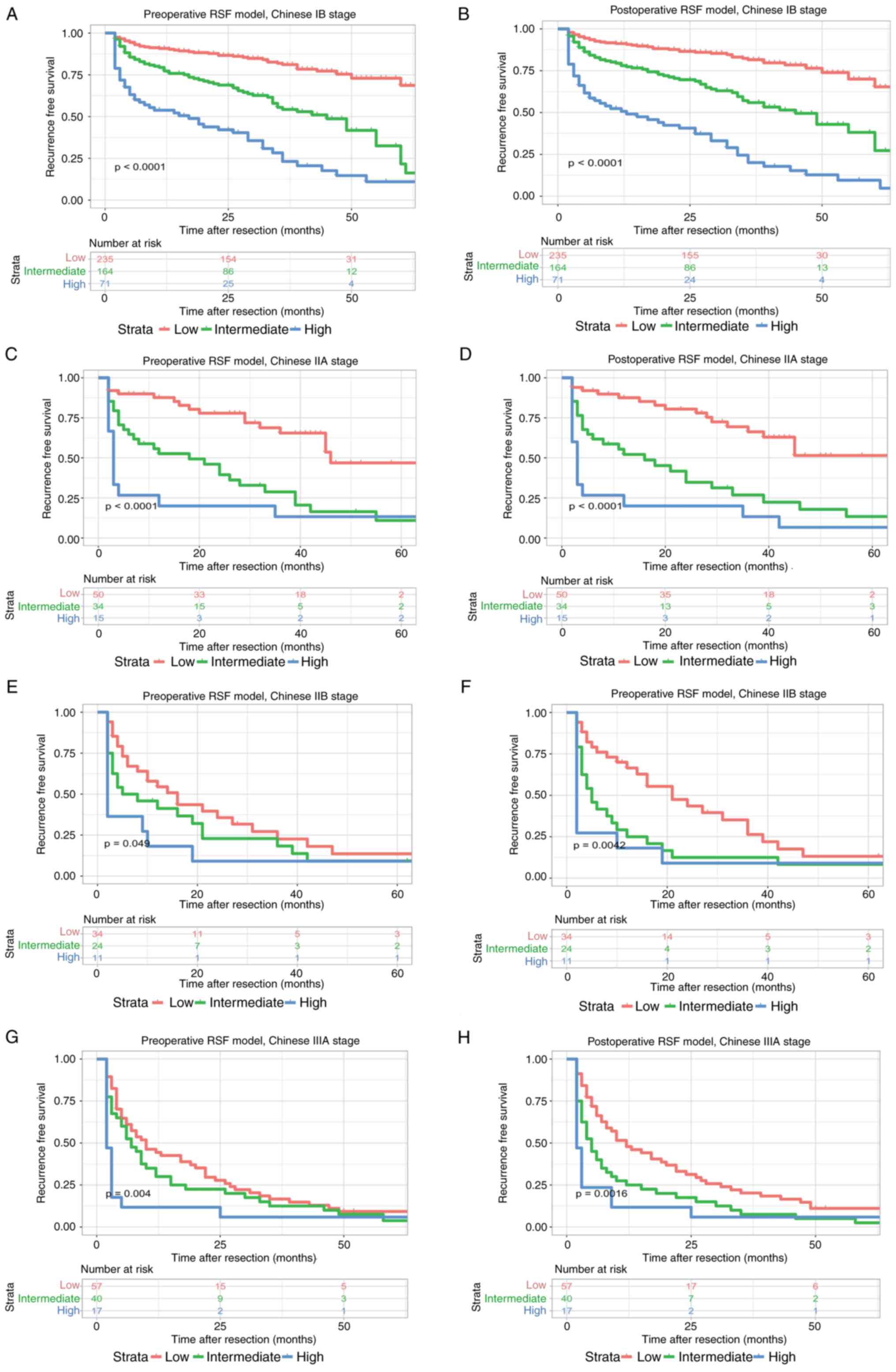
model; P=0.0039, postoperative RSF model), respectively (Fig. 9). Moreover, the preoperative and
postoperative RSF models were effective in distinguishing the
patients at stage IB of the Chinese staging system (P<0.0001),
IIA (P<0.0001), IIB (P=0.049, preoperative RSF model; P=0.0042,
postoperative RSF model), and IIIA (P=0.004, preoperative RSF
model; P=0.0016, postoperative RSF model), respectively (Fig. 10).
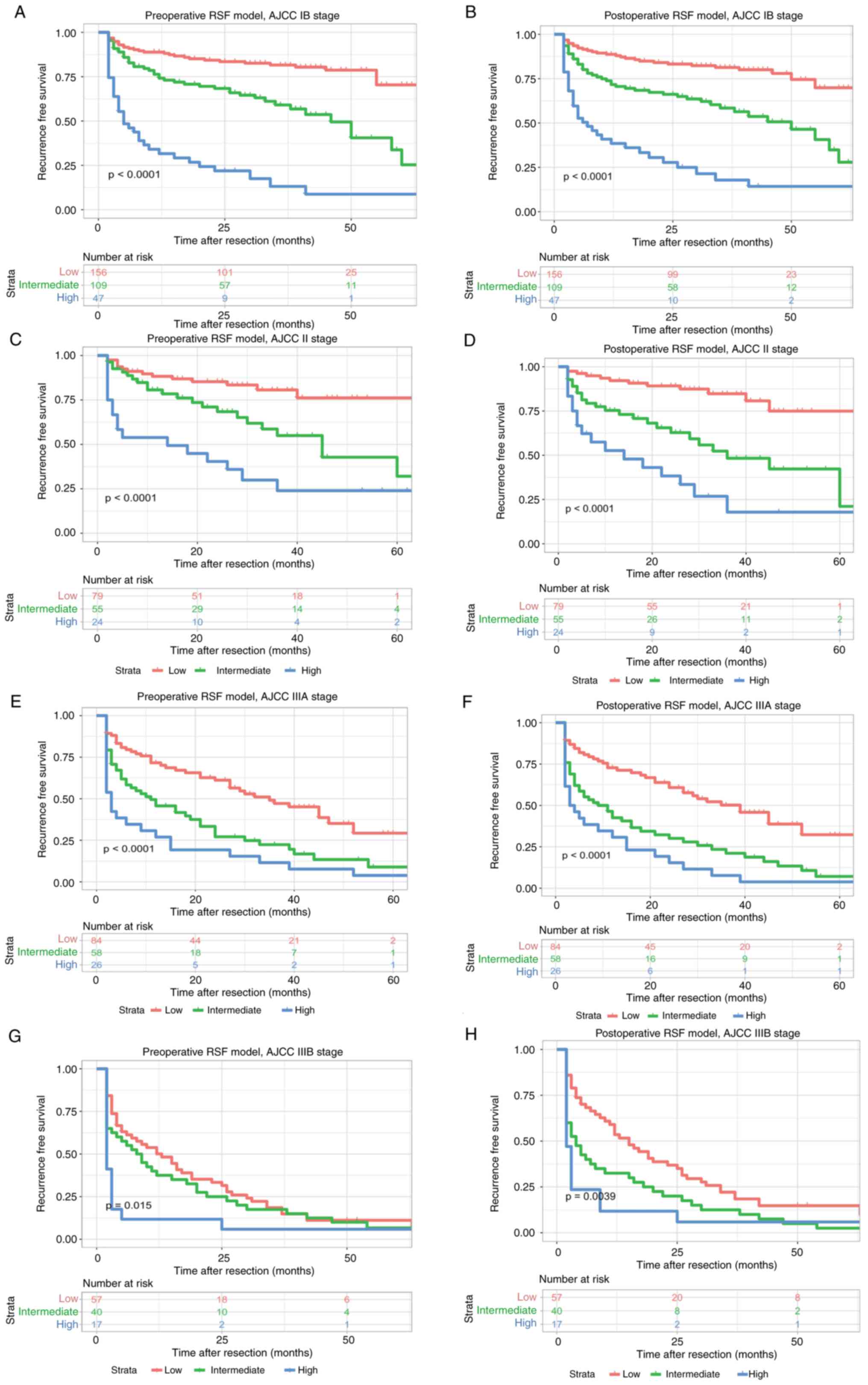 | Figure 9.Kaplan-Meier plots for recurrence
free survival rate of risk subgroups defined by the RSF model
scores in different AJCC8th stage. (A) preoperative RSF model,
AJCC8th IB stage; (B) postoperative RSF model, AJCC8th IB stage;
(C) preoperative RSF model, AJCC8th II stage; (D) postoperative RSF
model, AJCC8th II stage; (E) preoperative RSF model, AJCC8th IIIA
stage; (F) postoperative RSF model, AJCC8th IIIA stage; (G)
preoperative RSF model, AJCC8th IIIB stage; and (H) postoperative
RSF model, AJCC8th IIIB stage. RSF, random survival forests; AJCC,
American Joint Committee on Cancer; RSF, random survival forests;
American Joint Committee on Cancer. |
Discussion
To date, a lack of reliable methods for the
prediction of postoperative prognosis has been reported among
patients with HCC (26). As a type
of model superior to the CPH model, machine learning models offer a
novel methodology based on their non-linear functions and can be
used to improve the predictive efficiency for HCC as it considers
all possible interactions between variables (27). In the present study, two RSF models
with optimal preoperative and postoperative prediction for
long-term prognosis following hepatectomy were constructed, and
their predictive efficiency was evaluated using internal and
external validations. The preoperative model contributed to the
selection of treatment regimens for patients with huge HCC, while
the postoperative model offered a more accurate and individualized
prediction of prognosis following surgery.
To the best of our knowledge, this is the first
study to validate a machine-learning model for predicting the
recurrence of patients with huge HCC treated with curative
resection. The data indicated that the RSF model was superior to
the traditional staging models in model discrimination, clinical
usefulness, and overall prediction efficiency. The primary
advantage of the RSF model was attributed to the involved variables
based on the non-linear risk function, without CPH-related
assumptions. The preoperative and postoperative RSF models also
indicated that the recurrence of huge HCC was primarily associated
with tumor characteristics, such as MaVI, satellite nodules, tumor
number, age, and higher AFP. According to previous studies,
patients with MaVI may exhibit a decrease in liver function
reserve, which serves as a high-risk factor for the recurrence of
huge HCC (28,29), along with the satellite nodule
(30). The satellite nodule was an
independent risk factor for recurrence within 1 year following
surgery (31). Studies have shown
that the tumor number correlated with the recurrence of huge HCC
(32,33). Xia et al (34) also reported that age was an
independent risk factor for HCC early recurrence within 1 year of
curative hepatectomy. Finally, a recent study indicated that a
higher AFP level was also associated with a high 5-year recurrence
rate of HCC (35). These studies
further confirmed the association between tumor characteristics and
recurrence prediction by the RSF modeling.
The preoperative and postoperative RSF models of the
current study were able to re-stratify patients in the same
traditional staging system stages and may therefore play a
supplementary role to the traditional staging system. The
preoperative RSF model, with a significantly improved predictive
performance than the BCLC staging system, may serve as an
additional tool for surgeons to identify high-risk patients prior
to operation. It is important to note that the preoperative model
can provide an important basis for the selection of the treatment
regimen. The prognostic discrimination of the RSF model was able to
accurately stratify patients into three prognostic subgroups as
shown in Fig. 7. In clinical
practice, TACE, sorafenib, or other alternative options are usually
recommended to the patients with a poor RFS score prior to surgery
and to those with poor tolerance to hepatectomy (36). Furthermore, the down-staging
procedures may also be considered based on arterial
chemoembolization, portal vein embolization (37), or even the associated liver
partition with portal vein ligation for staged hepatectomy
(38).
To date, there are still several follow-up
procedures, which suggest that HCC patients present with a high
possibility of recurrence (24).
The postoperative RSF model contributes to the design of the
follow-up procedures by surgeons, such as reduced interval for the
follow-up and more high-end imaging tests, as well as the
utilization of adjuvant therapy for those with a high risk of
recurrence (39). For the
predictive efficiency of the models, the tdAUC and C-index of the
postoperative RSF model were higher than those of the preoperative
model; the prediction error curve and DCA also indicated that the
postoperative model was more effective compared with that of the
preoperative model. Additionally, the postoperative model may be
superior to the preoperative model as it may include pathological
variables, such as liver cirrhosis, tumor capsule,
Edmondson-Steiner classification, MIV, as well as intraoperative
blood transfusion. Patients with a huge HCC without tumor capsule
were not likely to show a clear resection margin, leading to
increased operative risks and a poorer prognosis following
hepatectomy. Previously, a tumor size of ≥10 cm with no tumor
capsule was shown to be an independent prognostic factor for poor
OS and RFS after hepatectomy (40).
Therefore, a complete tumor capsule for a huge HCC was an important
factor for surgical safety and optimal long-term survival.
Furthermore, an intact tumor capsule was considered a protective
factor for recurrence, especially for those with huge tumors as it
may inhibit local and vascular invasion (41,42).
It has been well acknowledged that liver cirrhosis is related to
the pathogenesis of HCC (43). In
line with the previous data (44),
the results of the present study confirmed that MVI was an
independent risk factor for recurrence in huge HCC. Previously,
Edmondson-Steiner grade had been reported as an independent
predictive factor for recurrence (45). Wang et al (46) indicated that intraoperative
transfusion of allogeneic blood was associated with a poorer
clinical prognosis in patients with huge HCC who underwent radical
hepatectomy. When the preoperative and postoperative models were
used simultaneously, the preoperative model should be selected in
the presence of indication of a poor prognosis for the preoperative
model and the optimal prognosis based on the postoperative model,
as the cost of shortening the follow-up interval was considerably
lower than that of the cost of HCC recurrence. In the future, more
prospective studies are required to distinguish the model with
improved predictive power.
In addition, two inflammatory indices were included
in the present study. According to VIMP analysis, PNLR and PLR were
two important risk factors for the recurrence of huge HCC.
According to previous studies, PNLR or PLR can be used as clinical
indicators of the host inflammatory response and immune status,
while an increasing PNLR or PLR is a strong predictor of poor
survival in certain types of malignancies (47–50).
It has been reported that a higher PNLR and PLR were correlated
with a poorer prognosis among patients with HCC (48). Given that PNLR and PLR consist of a
serum neutrophil count, a lymphocyte count, and a platelet count, a
change in PNLR and PLR could be viewed as a relative increase in
the number of neutrophils and platelet count, or a relative
decrease in the number of lymphocytes. It has been demonstrated
that neutrophils may promote tumor growth by increasing vascular
endothelial growth factor, which serves as an important factor in
promoting tumor angiogenesis (51).
By contrast, several experimental studies have confirmed the
relationship between the presence of malignancy and thrombocytosis
(52–54). Following an increase in their
number, the platelets can secrete several types of growth factors,
which may stimulate the growth and proliferation of the tumor.
Lastly, serving as one of the most important components of
antitumor immunity, the reduction of the lymphocyte number was
suggestive of abnormal immune mechanisms and a decline in antitumor
immunity, which subsequently contributed to tumor invasion and
metastasis (55). PNLR and PLR may
reflect the tumor inflammation and immune status in the body; once
this dynamic balance is interrupted, the tumor inflammatory
response and antitumor inflammatory response will be destroyed. On
this basis, normal immune function is impaired, thereby promoting
metastasis and invasion of malignancies. It may help to explain why
PNLR and PLR can be used to evaluate the probability of recurrence
among patients with HCC. In the future, further studies are
required to illustrate the mechanism underlying the association
between PNLR, PLR, and the recurrence of huge HCC.
The present study has certain limitations. Firstly,
this was a retrospective study involving a relatively small sample
size, which may lead to unavoidable selection bias. Secondly, other
variables that may be associated with the prognosis of patients
with HCC (such as postoperative adjunctive therapies) were not
evaluated in the present study. Thirdly, the model established was
primarily based on two Chinese institutions of patients with HCC in
hepatitis B virus-endemic areas. Additional cohorts from different
geographic locations are required to validate our model to extend
the results to patients with HCC of various etiologies. Finally,
the present study was generated using data from patients who
underwent radical resection, which may not be applicable for
patients receiving other therapies, such as TACE and sorafenib. In
the future, additional prospective studies involving long-term
follow-up are essential to extend the feasibility of the
established model.
In summary, the present study developed preoperative
and postoperative RSF models based on machine learning algorithms
for predicting the risk of huge HCC following resection. These two
RSF models have accurate predictive ability and can play a
supplementary role to the traditional staging system. Patients
classified as high risk for recurrence based on the preoperative
RSF model were recommended to receive non-surgical based treatment,
or down-staging procedures followed by surgery, while those with
low or moderate risks of recurrence on the preoperative model were
recommended to receive surgery. It was suggested that patients with
high risk of recurrence based on the postoperative model should
receive postoperative adjuvant therapy (such as TACE), while
follow-up and monitoring were suggested for those with low and
moderate risks.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science Foundation of
Fujian Province (grant no. 2021J011283); and the Fuzhou Science and
Technology Innovation Platform project (grant no. 2021-P-055).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL and JL conceived the study. QZ, GF and TH,
curated the data. QZ, GF, TH and GW performed the analysis. QZ, GF
and TH confirm the authenticity of all the raw data. QZ, HL and JL
wrote and reviewed the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study protocol used in the present study was
approved by the Medical Ethics Committee of Mengchao Hepatobiliary
Hospital of Fujian Medical University (approval no. 2022-027-01).
Written informed consent for participation was not required for
this study in accordance with the national legislation and the
institutional requirements.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim JM, Joh JW, Yi NJ, Choi GS, Kim K, Lee
KW and Suh KS: Predicting hepatocellular carcinoma recurrence
beyond milan criteria after liver resection for solitary
hepatocellular carcinoma. J Gastrointest Surg. 24:2219–2227. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakagawa S, Wei L, Song WM, Higashi T,
Ghoshal S, Kim RS, Bian CB, Yamada S, Sun X, Venkatesh A, et al:
Molecular liver cancer prevention in cirrhosis by organ
transcriptome analysis and lysophosphatidic acid pathway
inhibition. Cancer Cell. 30:879–890. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang H, Liu F, Wen N, Li B and Wei Y:
Patterns, timing, and predictors of recurrence after laparoscopic
liver resection for hepatocellular carcinoma: Results from a
high-volume HPB center. Surg Endosc. 36:1215–1223. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ruiz E, Pineau P, Flores C, Fernández R,
Cano L, Cerapio JP, Casavilca-Zambrano S, Berrospi F, Chávez I,
Roche B and Bertani S: A preoperative nomogram for predicting
long-term survival after resection of large hepatocellular
carcinoma (>10 cm). HPB (Oxford). 24:192–201. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hong SK, Lee KW, Hong SY, Suh S, Hong K,
Han ES, Lee JM, Choi Y, Yi NJ and Suh KS: Efficacy of liver
resection for single large hepatocellular carcinoma in child-pugh a
cirrhosis: Analysis of a nationwide cancer registry database. Front
Oncol. 11:6746032021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhong NB, Lv GM and Chen ZH: Stereotactic
body radiotherapy combined with transarterial chemoembolization for
huge (≥10 cm) hepatocellular carcinomas: A clinical study. Mol Clin
Oncol. 2:839–844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bai Y, Wu J, Zeng Y, Chen J, Wang S, Chen
S, Qiu F, Zhou S, You S, Tian Y, et al: Nomogram for predicting
long-term survival after synchronous resection for hepatocellular
carcinoma and inferior vena cava tumor thrombosis: A multicenter
retrospective study. J Oncol. 2020:32640792020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chan AWH, Zhong J, Berhane S, Toyoda H,
Cucchetti A, Shi K, Tada T, Chong CCN, Xiang BD, Li LQ, et al:
Development of pre and post-operative models to predict early
recurrence of hepatocellular carcinoma after surgical resection. J
Hepatol. 69:1284–1293. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mao S, Yu X, Shan Y, Fan R, Wu S and Lu C:
Albumin-Bilirubin (ALBI) and monocyte to lymphocyte ratio
(MLR)-based nomogram model to predict tumor recurrence of
AFP-negative hepatocellular carcinoma. J Hepatocell Carcinoma.
8:1355–1365. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Camacho DM, Collins KM, Powers RK,
Costello JC and Collins JJ: Next-generation machine learning for
biological networks. Cell. 173:1581–1592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang S and Summers RM: Machine learning
and radiology. Med Image Anal. 16:933–951. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rajkomar A, Dean J and Kohane I: Machine
learning in medicine. N Engl J Med. 380:1347–1358. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang Y, Chen H, Zeng Y, Liu Z, Ma H and
Liu J: Development and validation of a machine learning prognostic
model for hepatocellular carcinoma recurrence after surgical
resection. Front Oncol. 10:5937412020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Knottnerus A and Tugwell P: STROBE-a
checklist to strengthen the reporting of observational studies in
epidemiology. J Clin Epidemiol. 61:3232008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marrero JA, Kulik LM, Sirlin CB, Zhu AX,
Finn RS, Abecassis MM, Roberts LR and Heimbach JK: Diagnosis,
staging, and management of hepatocellular carcinoma: 2018 practice
guidance by the american association for the study of liver
diseases. Hepatology. 68:723–750. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang P, Qiu J, Li J, Wu D, Wan X, Lau WY,
Yuan Y and Shen F: Nomograms for pre- and postoperative prediction
of long-term survival for patients who underwent hepatectomy for
multiple hepatocellular carcinomas. Ann Surg. 263:778–786. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taylor JM: Random survival forests. J
Thorac Oncol. 6:1974–1975. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishwaran H, Kogalur UB, Blackstone EH and
Lauer MSJTaoas: Random survival forests. The Annals of Applied
Statistics. 2:841–860. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Royston P and Altman DG: External
validation of a Cox prognostic model: Principles and methods. BMC
Med Res Methodol. 13:332013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mogensen UB, Ishwaran H and Gerds TA:
Evaluating random forests for survival analysis using prediction
error curves. J Stat Softw. 50:1–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang XP, Wang K, Gao YZ, Wei XB, Lu CD,
Chai ZT, Zhen ZJ, Li J, Yang DH, Zhou D, et al: Prognostic model
for identifying candidates for hepatectomy among patients with
hepatocellular carcinoma and hepatic vein invasion. Br J Surg.
107:865–877. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chun YS, Pawlik TM and Vauthey JN: 8th
edition of the AJCC cancer staging manual: Pancreas and
hepatobiliary cancers. Ann Surg Oncol. 25:845–847. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
European Association for the Study of the
Liver. Electronic address, . simpleeasloffice@easloffice.eu;
European Association for the Study of the Liver: EASL clinical
practice guidelines: Management of hepatocellular carcinoma. J
Hepatol. 69:182–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiu G, Jin Z, Chen X and Huang J:
Interpretation of guidelines for the diagnosis and treatment of
primary liver cancer (2019 edition) in China. Glob Health Med.
2:306–311. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Xia Y, Li J, Wu D, Wan X, Wang K, Wu
M, Liu J, Lau WY and Shen F: Prognostic nomograms for pre- and
postoperative predictions of long-term survival for patients who
underwent liver resection for huge hepatocellular carcinoma. J Am
Coll Surg. 221:962–974.e964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moncada-Torres A, van Maaren MC, Hendriks
MP, Siesling S and Geleijnse G: Explainable machine learning can
outperform Cox regression predictions and provide insights in
breast cancer survival. Sci Rep. 11:69682021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang Q, Xie QS, Chen JM, Shan SL, Xie K,
Geng XP and Liu FB: Long-term outcomes after hepatectomy of huge
hepatocellular carcinoma: A single-center experience in China.
Hepatobiliary Pancreat Dis Int. 18:532–537. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li M, Zhao Y, Liu X, Zhang S, Jiang Y and
Yang Z: Early risk warning system for distant metastasis of
hepatitis B virus-associated hepatocellular carcinoma with portal
vein tumor thrombus. Oncol Lett. 19:3249–3257. 2020.PubMed/NCBI
|
|
30
|
Wang L, Liu Z, Liu X, Zeng Y and Liu J:
The hepatectomy efficacy of huge hepatocellular carcinoma and its
risk factors: A meta analysis. Medicine (Baltimore). 96:e92262017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shin S, Kim TS, Lee JW, Ahn KS, Kim YH and
Kang KJ: Is the anatomical resection necessary for single
hepatocellular carcinoma smaller than 3 cm?: Single-center
experience of liver resection for a small HCC. Ann Hepatobiliary
Pancreat Surg. 22:326–334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang XH, Liu QB, Xiang CL, Mao XH, Yang B,
Li Q, Zhou QF, Li SQ, Zhou ZG and Chen MS: Multi-institutional
validation of novel models for predicting the prognosis of patients
with huge hepatocellular carcinoma. Int J Cancer. 149:127–138.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dai T, Deng M, Ye L, Lin G, Liu R, Deng Y,
Li R, Liu W, Li H, Yang Y, et al: Nomograms based on
clinicopathological factors and inflammatory indicators for
prediction of early and late recurrence of hepatocellular carcinoma
after surgical resection for patients with chronic hepatitis B. Ann
Transl Med. 9:122021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xia W, Peng T, Guan R, Zhou Y, Zeng C, Lin
Y, Wu Z and Tan H: Development of a novel prognostic nomogram for
the early recurrence of liver cancer after curative hepatectomy.
Ann Transl Med. 9:15412021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ding HF, Zhang XF, Bagante F, Ratti F,
Marques HP, Soubrane O, Lam V, Poultsides GA, Popescu I,
Alexandrescu S, et al: Prediction of tumor recurrence by
α-fetoprotein model after curative resection for hepatocellular
carcinoma. Eur J Surg Oncol. 47:660–666. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li H, Li S, Geng J, Zhao S, Tan K, Yang Z,
Feng D and Liu L: Efficacy evaluation of the combination therapy of
sorafenib and transarterial chemoembolization for unresectable HCC:
A systematic review and meta-analysis of comparative studies. Ann
Transl Med. 8:5402020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Terasawa M, Allard MA, Golse N, Cunha AS,
Cherqui D, Adam R, Saiura A and Vibert E: Sequential transcatheter
arterial chemoembolization and portal vein embolization versus
portal vein embolization alone before major hepatectomy for
patients with large hepatocellular carcinoma: An intent-to-treat
analysis. Surgery. 167:425–431. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Torres OJ, Vasques RR, Silva TH,
Castelo-Branco ME and Torres CC: The ALPPS procedure for
hepatocellular carcinoma larger than 10 centimeters. Int J Surg
Case Rep. 26:113–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma L, Deng K, Zhang C, Li H, Luo Y, Yang
Y, Li C, Li X, Geng Z and Xie C: nomograms for predicting
hepatocellular carcinoma recurrence and overall postoperative
patient survival. Front Oncol. 12:8435892022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang W, Zhang ZW, Zhang BX, Huang ZY,
Zhang WG, Liang HF and Chen XP: Outcomes and prognostic factors of
spontaneously ruptured hepatocellular carcinoma. J Gastrointest
Surg. 23:1788–1800. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Arnaoutakis DJ, Mavros MN, Shen F,
Alexandrescu S, Firoozmand A, Popescu I, Weiss M, Wolfgang CL,
Choti MA and Pawlik TM: Recurrence patterns and prognostic factors
in patients with hepatocellular carcinoma in noncirrhotic liver: A
multi-institutional analysis. Ann Surg Oncol. 21:147–154. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee JI, Lee JW, Kim YS, Choi YA, Jeon YS
and Cho SG: Analysis of survival in very early hepatocellular
carcinoma after resection. J Clin Gastroenterol. 45:366–371. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang CH, Yuan X, Li JF, Xie YF, Zhang AZ,
Wang XL, Yang L, Liu CX, Liang WH, Pang LJ, et al:
Bioinformatics-based screening of key genes for transformation of
liver cirrhosis to hepatocellular carcinoma. J Transl Med.
18:402020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zheng J, Shen S, Jiang L, Yan L, Yang J,
Li B, Wen T, Wang W and Xu M: Outcomes of anterior approach major
hepatectomy with diaphragmatic resection for single huge right lobe
HCC with diaphragmatic invasion. Medicine (Baltimore).
97:e121942018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou L, Rui JA, Zhou WX, Wang SB, Chen SG
and Qu Q: Edmondson-Steiner grade: A crucial predictor of
recurrence and survival in hepatocellular carcinoma without
microvascular invasio. Pathol Res Pract. 213:824–830. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang JC, Hou JY, Chen JC, Xiang CL, Mao
XH, Yang B, Li Q, Liu QB, Chen JB, Ye ZW, et al: Development and
validation of prognostic nomograms for single large and huge
hepatocellular carcinoma after curative resection. Eur J Cancer.
155:85–96. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Caram LJ, Calderon F, Masino E, Ardiles V,
Mauro E, Haddad L, Pekolj J, Vicens J, Gadano A, de Santibañes E
and de Santibañes M: Do changes in inflammatory markers predict
hepatocellular carcinoma recurrence and survival after liver
transplantation? Ann Hepatobiliary Pancreat Surg. 26:40–46. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen K, Zhan MX, Hu BS, Li Y, He X, Fu SR,
Xin YJ and Lu LG: Combination of the neutrophil to lymphocyte ratio
and the platelet to lymphocyte ratio as a useful predictor for
recurrence following radiofrequency ablation of hepatocellular
carcinoma. Oncol Lett. 15:315–323. 2018.PubMed/NCBI
|
|
49
|
Ismael MN, Forde J, Milla E, Khan W and
Cabrera R: Utility of inflammatory markers in predicting
hepatocellular carcinoma survival after liver transplantation.
Biomed Res Int. 2019:72840402019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lin S, Hu S, Ran Y and Wu F:
Neutrophil-to-lymphocyte ratio predicts prognosis of patients with
hepatocellular carcinoma: A systematic review and meta-analysis.
Transl Cancer Res. 10:1667–1678. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kusumanto YH, Dam WA, Hospers GA, Meijer C
and Mulder NH: Platelets and granulocytes, in particular the
neutrophils, form important compartments for circulating vascular
endothelial growth factor. Angiogenesis. 6:283–287. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
An S, Shim H, Kim K, Kim B, Bang HJ, Do H,
Lee HR and Kim Y: Pretreatment inflammatory markers predicting
treatment outcomes in colorectal cancer. Ann Coloproctol.
38:97–108. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lin MS, Gao MJ, Zhang DL, Li XY, Huang JX
and Yu H: Prognostic significance of preoperative
platelet-lymphocyte ratio in a Chinese cohort patient with
colorectal cancer. Int J Clin Exp Pathol. 10:8686–8694.
2017.PubMed/NCBI
|
|
54
|
Yoshida A, Sarian LO, Marangoni MJ,
Firmano IC and Derchain SF: diagnostic value of the
neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and
thrombocytosis in the preoperative investigation of ovarian masses.
Rev Bras Ginecol Obstet. 42:397–403. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kitayama J, Yasuda K, Kawai K, Sunami E
and Nagawa H: Circulating lymphocyte number has a positive
association with tumor response in neoadjuvant chemoradiotherapy
for advanced rectal cancer. Radiat Oncol. 5:472010. View Article : Google Scholar : PubMed/NCBI
|