Introduction
In 2020, the World Health Organization (WHO) updated
its classification of female reproductive organ tumors from the
original three-level classification of cervical intraepithelial
neoplasia (CIN1, CIN2 and CIN3) to a two-level classification:
Low-grade squamous intraepithelial lesions (LSIL/CIN1) and
high-grade squamous intraepithelial lesions (HSILs) (1). HSILs may be subdivided into HSIL
(CIN2) and HSIL (CIN3) (1),
particularly in young women (aged <30 years), because there is
evidence that the former show significantly higher regression rates
(2). Exophytic LSILs are caused by
low-risk human papillomavirus (LR-HPV) types, whereas 80–90% of
flat LSILs are attributable to high-risk HPV (HR-HPV) types
(1). The colposcopy findings for
cases of LSILs usually involve thin or translucent whitening, often
accompanied by geographic, condylomatous, raised, or papillary
changes. These changes may or may not be accompanied by fine mosaic
and/or punctation patterns (3).
HSIL is recognized as a true precancer with a higher risk of
progression than LSIL; it is usually caused by persistent HR-HPV
infection and colposcopies frequently show typical dense acetowhite
changes, with or without vascular changes (coarse mosaic and/or
punctation) (3). However, these
changes might not be observable for populations with borderline
cytologic abnormalities accompanied by small, early lesions
(4).
The relationship between the thickness of the
epithelium and the colposcopic diagnosis is controversial. It has
been previously reported that the inability to visualize certain
HSILs is associated with a thinner epithelium (5). However, it has also been suggested
that false-negative colposcopy in the presence of high-grade CIN is
likely due to the failure to detect minor or hidden lesions in the
cervical canal rather than the presence of a ‘thin’ CIN (6). In the present study, it was
investigated whether a thin HSIL is associated with a less abnormal
(i.e., thin acetowhite change) or low-grade colposcopic
impression.
Materials and methods
Study subjects
A total of 136 cases of HSIL verified by
pathological biopsy at Peking University People's Hospital between
June and October 2021 were analyzed retrospectively. The study was
approved by the Ethics Committee of Peking University People's
Hospital (IRB number: 2020PHB298-01, date of IRB approval:
30/10/2020).
Pathological analysis
Pathology slides were scanned and digitized using a
PRECICE 500B digital scanner (Beijing Una Technology Co., Ltd.).
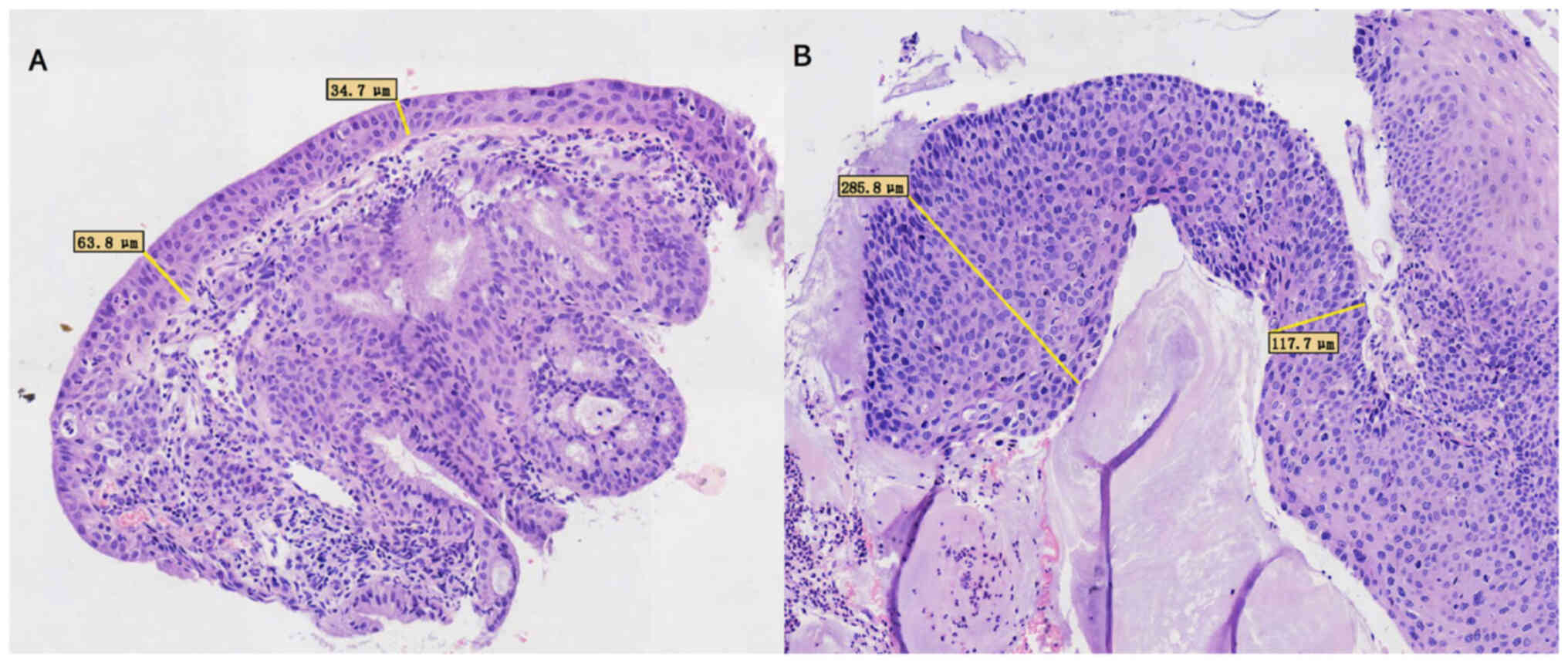
The thickness of the squamous epithelium was determined by
measuring the distance between its surface and basement membrane.
Average epithelial thickness was determined as follows:
Thickness=(thickness of the thickest part + thickness of the
thinnest part)/2 (6). A
demonstration of the measurement of average epithelial thickness is
presented in Fig. 1. A classic HSIL
has a thickness of >10 cell layers, whereas a thin HSIL is
described by the WHO as a cervix HSIL variant with a thickness of
<9 cells (7). p16 and Ki-67
immunohistochemical staining was performed when the CIN grade could
not be determined using morphology. All pathological specimens were
fixed using 4% neutral formaldehyde at room temperature for 120
min, before the sample was conventionally dehydrated (graded
alcohol series) and soaked, embedded in paraffin and sectioned (4
µm) for hematoxylin and eosin (HE) staining at room temperature for
10 min. All immunohistochemical staining was performed according to
the manufacturers' protocols. Formalin-fixed paraffin-embedded
blocks were sectioned at 4 µm each and incubated with antibodies
(37°C; 20–30 min). Immunohistochemistry was performed with the
Ventana Benchmark XT-automatic staining machine (Roche Tissue
Diagnostics). For p16INK4a detection, the CINtec
Histology Kit (cat. no. 705-4713; clone E6H4; 1:100; Roche
Diagnostics GmbH) was used following the manufacturer's protocol.
Ki-67 immunohistochemistry was performed using rat anti-human
monoclonal antibody (cat. no. EP5; clone UMAB107; 1:200; Origene
Technologies, Inc.). Secondary antibody (HRP; ready-to-use; cat.
no. ZLI-9013; Origene Technologies, Inc.) incubation was for 20–30
min at 37°C.
The number and thickness of epithelial layers were
measured by pathologists who were blinded to the HPV type,
colposcopic diagnosis or prior histologic diagnosis of
patients.
Colposcopy evaluation
Colposcopic impressions of these cases were
retrospectively analyzed. Colposcopic impressions were performed
according to the 2017 American Society for Colposcopy and Cervical
Pathology (ASSCP) standard (8).
Absence of an acetowhite abnormality was defined as ‘normal’. The
presence of thin acetowhite lesions, either on or off the
squamocolumnar junction (SCJ), indicated possible metaplasia or
LSIL and was defined as a ‘low-grade impression group’. HSIL was
indicated by the presence of dense acetowhite lesions on the SCJ,
particularly when combined with vascular changes (coarse mosaic
and/or punctation). A friable lesion with an irregular surface,
frequently accompanied by a dense white epithelium or atypical
vessels, was defined as ‘cancer’. A random biopsy was performed
when cytology indicated a high risk of HSIL [i.e., when atypical
squamous cells could not exclude HSIL (ASC-H), atypical glandular
cell (AGC) or HSIL], even if the colposcopy sample was normal.
Endocervical curettage was required when there was a type 3
transformation zone, the lesion extended to the cervical canal or
when the involvement of the cervical canal could not be excluded.
According to the colposcopic impressions, all HSILs verified by
pathological biopsy were divided into a low-grade impressions
group, which included normal, metaplasia and LSIL (n=62) and a
high-grade impressions group, which included HSIL and cancer
(n=74).
Statistical analysis
Data are presented as mean ± SD for continuous
normal distributions; 95% confidence intervals (CI) are presented
for non-normal distributions. An independent samples t-test and
Mann-Whitney U test were used to compare the mean and median values
between two groups. The differences in proportions between
classification variables were determined by Fisher's exact test.
The receiver operating characteristic curve (ROC) was used to
establish the optimal critical value of colposcopy misjudgment. All
two-tailed statistical tests were performed using SPSS software
(version 26.0, IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Overview of patient data
The median age of patients was 41.5 years and there
were no statistically significant differences between the low-grade
and high-grade colposcopy impression groups in terms of age,
cytology [≥atypical squamous cells of undetermined significance
(ASCUS)], HPV 16/18 positive rate or the location of lesions
(Table I). However, there were
significantly fewer epithelial layers (13.7±4.8 vs. 19.1±5.5) and
decreased epithelial thickness (112.0±45.1 µm vs. 153.4±49.0 µm) in
the low-grade colposcopic impression group compared with the
high-grade colposcopic impression group (Table I).
 | Table I.Clinical and pathological
characteristics of high-grade squamous intraepithelial lesions with
different colposcopic impressions. |
Table I.
Clinical and pathological
characteristics of high-grade squamous intraepithelial lesions with
different colposcopic impressions.
| Characteristic | Low-grade impressions
(n=62) | High-grade
impressions (n=74) | P-value |
|---|
| Median age, years
(IQR) | 38.5 (33.8-53.0) | 39.5 (34.0-44.3) | 0.638 |
| High risk of cytology
(≥ASCUS), n (%) | 38 (61.3) | 51 (68.9) | 0.371 |
| HPV16 and/or HPV18
positive, n (%) | 27 (43.5) | 43 (58.1) | 0.121 |
| Layers, median (mean
± SD) | 13.7±4.8 | 19.1±5.5 | <0.001 |
| Epithelial thickness,
µm (mean ± SD) | 112.0±45.1 | 153.4±49.0 | <0.001 |
| Site of lesion, n
(%) |
|
| 0.884 |
| 6 o'clock
involved | 15 (24.2) | 17 (23.0) |
|
| 12
o'clock involved | 14 (22.6) | 13 (17.6) |
|
| Cervical
canal involved | 5 (8.1) | 7 (9.5) |
|
| Other
site | 28 (45.2) | 37 (50.0) |
|
Association between epithelial
thickness and colposcopic impressions in CIN2 and CIN3
Stratified analysis was performed to examine the
association between colposcopy impression and the thickness of the
epithelium in cases of CIN2 (n=79) and CIN3 (n=57) diagnosed by
histology (Table II). In the CIN2
group, the mean number of epithelial layers (12.8±4.2 vs. 17.8±4.2)
and epithelial thickness (105.2±41.9 µm vs. 150.3±50.0 µm) were
significantly lower in the low-grade colposcopic impression group
compared with the high-grade colposcopic impression group. However,
there were no significant differences in either epithelial
thickness or the number of epithelial layers in the CIN3 group. In
the low-grade colposcopic impression groups, CIN2 lesions had
significantly fewer epithelial layers (12.8±4.2 vs. 17.2±5.4) and
decreased epithelial thickness (105.2±41.9 µm vs. 140.4±48.6 µm)
compared with CIN3 lesions. However, in the colposcopic high-grade
impressions group, there was no significant difference in the
number of layers or epithelial thickness between CIN2 and CIN3. The
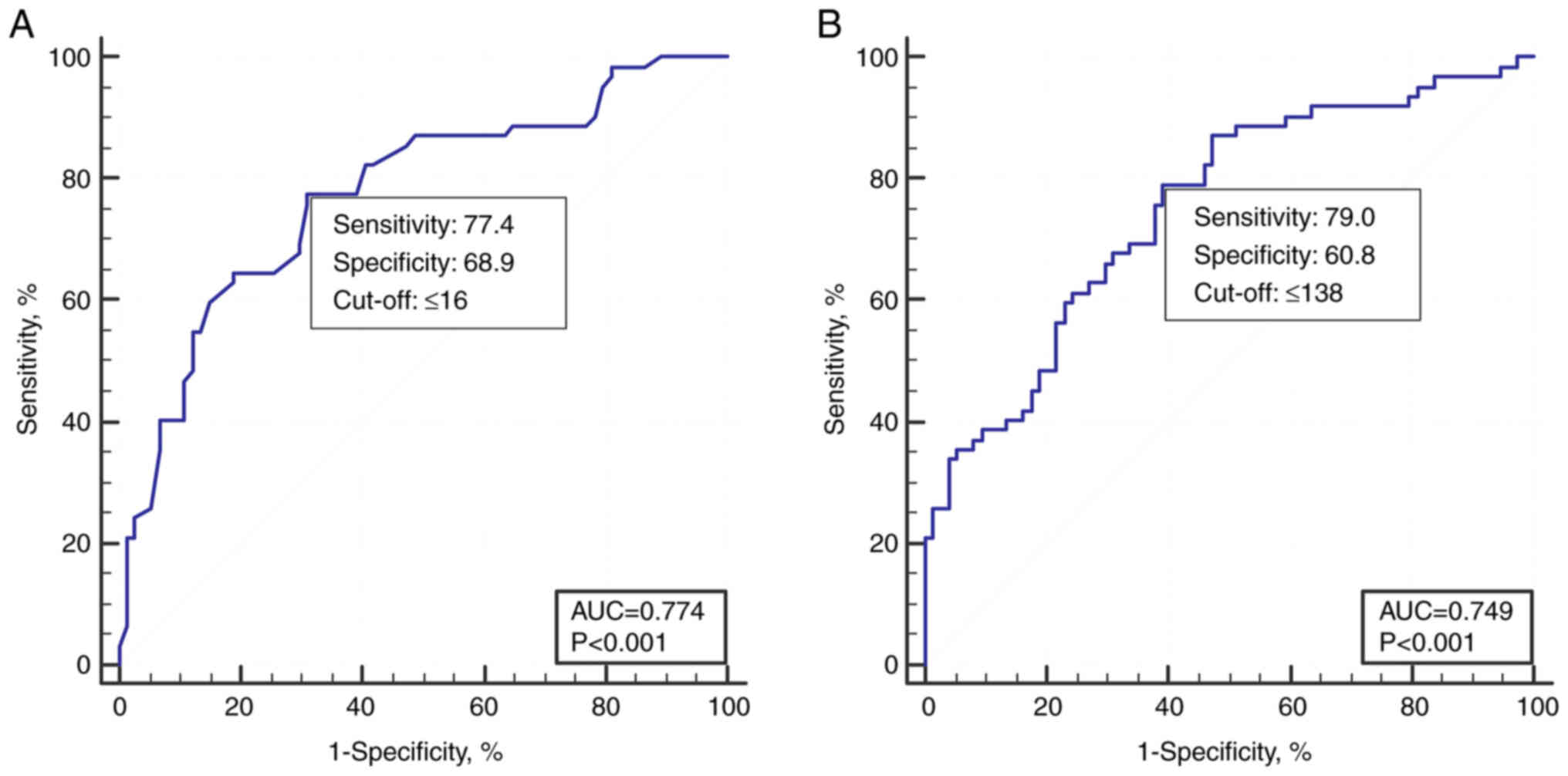
analysis for distinguishing low-grade and high-grade colposcopy
impressions by the number of epithelial layers or thickness showed
that the average number of layers at the critical threshold was 16
and the epithelial thickness was 138 µm, with a sensitivity and
specificity of 77.4-79.0 and 60.8-68.9%, and the area under the
curve was 0.774 and 0.749, respectively (Fig. 2).
 | Table II.Number and thickness of epithelial
layers of high-grade squamous intraepithelial lesions with
different colposcopic impressions (mean ± SD). |
Table II.
Number and thickness of epithelial
layers of high-grade squamous intraepithelial lesions with
different colposcopic impressions (mean ± SD).
|
| Number of epithelial
layers | Thickness of
epithelium |
|---|
|
|
|
|
|---|
|
| Colposcopy
impression |
| Colposcopy
impression, µm |
|
|---|
|
|
|
|
|
|
|---|
| Variable | ≤Low grade | High grade+ | P-value | ≤Low grade | High grade+ | P-value |
|---|
| CIN2 (n=79) | 12.8±4.2 | 17.8±4.2 | <0.001 | 105.2±41.9 | 150.3±50.0 | <0.001 |
| CIN3 (n=57) | 17.2±5.4 | 19.9±6.1 | 0.166 | 140.4±48.6 | 155.4±48.8 | 0.349 |
| P-value | 0.014 | 0.665 |
| 0.004 | 0.113 |
|
Characteristics of thin HSILs
A total of 12 cases of thin HSILs were analyzed with
a median patient age of 43.2±3.4 years (range, 30–62 years)
(Table III). Cytological
impressions of 7/12 (58.3%) thin HSILs were negative, 3/12 (25.0%)
cases were low-risk (ASCUS/LSIL) and 2/12 (16.7%) cases were
high-risk (ASC-H/HSIL). A total of 5 (41.7%) thin HSILs were
positive for HPV 16/18 and the remaining 7 HSILs (58.3%) were
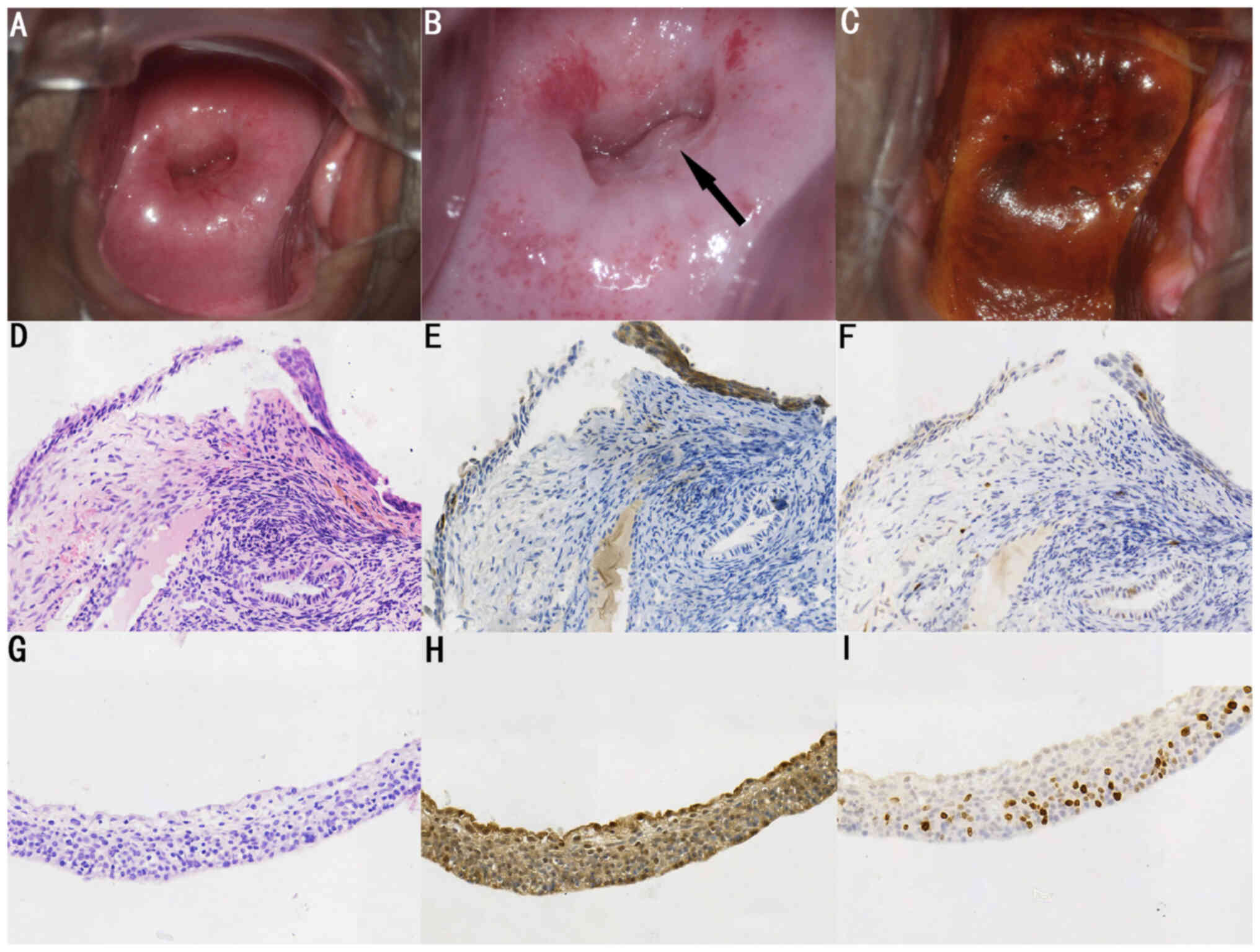
positive for other HPV genotypes. Colposcopic impressions of 11
cases were low grade and below, with 7 cases of thin acetowhite
changes located on the surface near the SCJ (one case is
demonstrated in Fig. 3); 1 case had
a lesion located outside the transformation zone; 2 cases had a
normal colposcopy, with thin HSILs identified only within the
endocervix; and 1 case had a nabothian cyst without a significant
acetic acid white area, but upon biopsy was unexpectedly found to
be CIN3. The lesion sizes of 10/12 thin HSILs under colposcopy were
≤10% of the cervical area, with isolated lesions. Examination of
hematoxylin and eosin-stained sections demonstrated that the number
of epithelial layers ranged from 5.5-9.0, epithelial thickness
ranged from 46.2-125.1 µm and the horizontal extension was between
234–833 µm. Ten of the 12 thin HSILs were CIN2. Seven of 12 thin
HSILs were located at the SCJ and 2 HSILs were located in the
endocervical columnar epithelium; this distribution was in
accordance with that from the colposcopy impression.
 | Table III.Clinical and pathological
characteristics of thin high-grade squamous intraepithelial
lesions. |
Table III.
Clinical and pathological
characteristics of thin high-grade squamous intraepithelial
lesions.
| Patient no. | Patient age at
diagnosis, years | Cytology | HPV genotype | Colposcopy
impression | Lesion size, %
cervical area | Location of thin
HSIL | Diagnosis | Number of epithelial
layers | Epithelial thickness,
µm | Horizontal diameter
of thin HSIL, µm |
|---|
| 1 | 50 | NILM | HPV 52 | NILM | 0 | Endocervix | CIN2 | 7.0 | 90.7 | 673 |
| 2 | 34 | HSIL | HPV 33 | LSIL | 10 | 11° | CIN2 | 7.5 | 74.5 | 833 |
| 3 | 36 | NILM | HPV 16 | LSIL | 0 | 6° | CIN2 | 5.5 | 125.1 | 459 |
| 4 | 62 | LSIL | HPV 51,56 | LSIL | 5 | 9° | CIN2 | 7.5 | 110. 0 | 529 |
| 5 | 41 | NILM | HPV 53,31 | NILM | 0 | Endocervix | CIN2 | 8.0 | 68.5 | 685 |
| 6 | 60 | ASC-H | HPV 52 | Nabothian | 5 | Nabothian | CIN3 | 6.5 | 84.2 | 385 |
|
|
|
|
| cyst |
| cyst |
|
|
|
|
| 7 | 34 | NILM | HPV 42,58 | LSIL | 10 | 6° | CIN2 | 9.0 | 53.1 | 234 |
| 8 | 59 | ASCUS | HPV 16,18 | LSIL | 10 | 12° | CIN2 | 7.0 | 75.4 | 327 |
| 9 | 41 | NILM | HPV 33 | HSIL | 20 | 6° | CIN3 | 7.0 | 71.2 | 692 |
| 10 | 40 | NILM | HPV 16,68 | LSIL | 10 | 6° | CIN2 | 8.0 | 69.5 | 612 |
| 11 | 31 | NILM | HPV 16 | LSIL | 10 | 12° | CIN2 | 8.0 | 68.3 | 368 |
| 12 | 30 | LSIL | HPV 16 | LSIL | 20 | 1,5,7° | CIN2 | 8.5 | 46.2 | 634 |
Discussion
Previous studies (5,6) are
conflicting on whether false negative colposcopy is associated with
epithelial thickness. This controversy was addressed in the present
study, by investigating the relationship between HSILs and the
underdiagnosis of CIN by colposcopy. The findings of the present
study suggested that the underestimation of colposcopy for
high-grade lesions was associated with the number of epithelial
layers and thickness. In particular, HSILs with a low-grade
colposcopic impression were found to have a thinner epithelium with
fewer layers compared with those with a high-grade colposcopic
impression, particularly those classified as CIN2. In 12 women with
thin HSILs, 91.6% received a colposcopic impression of low grade or
below, which further indicated that HSIL was easily missed by
colposcopy in these patients.
The presence of acetowhite changes is an important
evaluation indicator used in determining a colposcopic impression
(8). Low-grade lesions or
metaplasia present as thin/translucent acetowhite lesions, whereas
high-grade lesions demonstrate dense/thick acetowhite changes. Due
to the subjectivity of colposcopy, high-grade lesions can be under-
or over-diagnosed according to the acetowhite appearance. The
diagnostic accuracy differed greatly when the colposcopy result was
based on a CIN2+ impression or when the colposcopist speculated
that there was some disease present (DP). The sensitivities were
68.5 and 95.7% and specificities were 75.9 and 34.2% for CIN2+
impression and DP, respectively (9); thus, the wide range of published
diagnostic accuracy figures could be due to the use of two
different methods to evaluate colposcopy findings.
The relationship between epithelial thickness and
colposcopy impression was first reported by Yang et al
(5), who found that the epithelial
thickness of the cervical quadrants of patients with CIN2/CIN3 with
a normal colposcopic impression (184 µm) was less than that from
patients with low, high or cancer colposcopic impressions (321 µm).
They concluded that colposcopists cannot see certain CIN2/CIN3
lesions associated with the thickness of the epithelium. However,
Ghosh et al (6) reported
that CIN3 lesions with high-grade abnormalities were slightly
thicker than those without a visible colposcopic abnormality.
Dysplasia thickness was positively correlated with the CIN grade
but was reported to be unassociated with colposcopic appearance,
which led to the conclusion that false-negative colposcopy with
high-grade CIN was possibly caused by the failure to detect small
or predominantly endocervical lesions rather than a ‘thin’ CIN
(6).
In the present study, the epithelial thickness of
HSILs with low-grade colposcopic impressions (112.0±45.1 µm) was
significantly thinner than that of lesions with high-grade
colposcopic impressions (153.4±49.0 µm), which supported the
finding that a low-grade colposcopic impression was associated with
a thin CIN. High-grade lesions were the most likely to be
underdiagnosed using colposcopy when there were <16 layers and
the thickness of the epithelium was <138 µm. This was similar to
Yang et al (5) who reported
that colposcopy sensitivity for CIN2/CIN3 was only 31.3% when the
epithelial thickness was <139 µm. Therefore, the findings from
the present study provide additional evidence for the ASSCP
Evidence-Based Consensus recommendation to perform multiple
biopsies targeting all acetowhitening regions, regardless of the
presence of metaplasia or higher levels of abnormality (8).
Yang et al (5) reported that for all colposcopic
impressions [normal, low-grade or high-grade (not including
cervical cancer)], the epithelial thickness of lesions
histologically determined to be a high grade (CIN2/3) was lower
than that of those determined to be CIN1 and normal. Similar
results were also reported by Wang et al (10). One possible explanation is that
HSILs are primarily composed of enlarged nuclear atypia cells,
which are smaller than cells in LSILs, so the epithelium of HSILs
is thinner than that of LSILs. However, Ghosh et al
(6) reported that the dysplasia
thickness increased with CIN grade, but that there was no
correlation between the total epithelium thickness and the
neoplasia severity [CIN1 (271.9 µm) > CIN3 (218.5 µm) >
normal histopathology (212.8 µm) > CIN2 (191.4 µm)]. Although
LSILs confirmed by pathological biopsy were not included in the
present study, the thicknesses of CIN2 and CIN3 lesions were
compared, which indicated that the epithelia of CIN3 (152.2±48.7
µm) were thicker than those of CIN2 (121.8±49.8 µm), which was
consistent with the results of Ghosh et al (6).
When CIN2 and CIN3 were analyzed separately, it was
found that CIN2 was more likely to have false negative colposcopy
impressions compared with CIN3. The number of epithelial layers and
thickness of CIN2 were significantly lower for low-grade
colposcopic impressions than for high-grade impressions, but there
was no significant difference observed for CIN3. In addition,
epithelial thickness for CIN2 (105.2±41.9 µm) was lower than that
for CIN3 (140.4±48.6 µm) in the low-grade colposcopic impressions
group, which indicated that CIN2 is susceptible to misdiagnosis.
However, patients with CIN3 frequently presented with typical
thick/dense acetowhite lesions, which were often accompanied by
coarse mosaic patterns or punctation and were therefore difficult
to overlook.
It has been reported that the formation of HSILs
occurs via two pathways (11).
Classic HSILs typically arise from LSILs after HR-HPV infection of
mature stratified metaplastic squamous epithelium (MSE). HSILs can
also develop from early MSE (11),
these lesions (defined as thin HSILs) occur in non-stratified or
very thin immature squamous epithelia, which explains why certain
HSILs develop without an anteceding LSIL (11–13).
The frequency of thin HSILs has been reported in only a few
studies. Reich and Regauer reported that out of 25 conization
specimens, 76% contained both thin HSILs and classic HSILs, 16%
contained only thin HSILs and 4% contained only classic-type HSILs,
and 1 (4%) contained thin HSIL and LSIL (14). The present study retrospectively
analyzed 136 cases of HSILs diagnosed within a 4-month period and
demonstrated that 8.8% of these cases were thin HSILs only and
41.2% were classic HSILs; among the classic HSILs, 54.8% were
accompanied by thin HSILs, the majority of which occurred
multifocally of the cervix. This indicates that thin HSILs combined
with classical HSILs are frequent, but because the classical HSILs
are more obvious, pathologists may concentrate primarily on their
morphology. Thin HSILs alone are relatively rare and have a higher
risk of being missed.
In previous research, 65% of HSILs were reported to
be located on the SCJ, 19% were located in the endocervical
columnar epithelium and 16% were found in both locations (15). Similar to the geographic
distribution reported by Regauer et al (15), in the present study 7/12 (58.3%) of
thin HSILs were located near the SCJ and 2/12 (16.7%) were located
within the endocervical epithelium. Of the patients with thin
HSILs, 83.3% were CIN2, 83.3% had lesions covering less than 10% of
the cervical area and 91.6% had low-grade colposcopic impressions.
Although thin HSILs are unequivocally diagnostic when they are
between 5–9 cell layers thick, lesions with <5 cell layers often
resemble immature metaplastic squamous epithelium (14,15).
In the present study, the number of epithelial layers ranged from
5.5 to 9.0, with a maximum epithelium thickness of 125.1 µm, which
was less than the cut-off of 138 µm, where HSIL can be easily
missed by colposcopy. The challenges in colposcopic detection may
be explained by the tiny dimensions of thin HSILs. Although thin
HSIL may occasionally be confused with immature metaplasia with
mild atypia or atypical immature metaplasia, immunohistochemically
p16INK4a overexpression can be used as a marker to make
an unequivocal diagnosis of thin HSIL (14,16).
Regarding the association with HPV, it was
previously reported that 74% of thin HSILs have HR-HPV infection,
among which HPV 16 was the dominant subtype, present in as many as
37% of lesions and was followed by HPV 53 (15,17).
In the present study, 58.3% of thin HSILs had negative cytology and
58.3% had non-HPV 16/18 HR-HPV subtypes; these HSILs likely develop
less aggressive behavior when compared with classic HSILs (15).
The strength of the present study is that it
explored the confusion surrounding the relationship between
underdiagnosed colposcopic impressions and epithelial thickness.
The limitations of this study were that colposcopy is somewhat
subjective even though the colposcopists involved had an average of
10 years colposcopy experience and had received thorough colposcopy
training including colposcopy operations and terminology. In a
future study, colposcopic images should be reviewed and the true
causes of discrepancies evaluated through a blinded review.
Finally, the accuracy of colposcopic assessment may also be
affected by additional variables, such as age, the type of
transformation zone, microbiota dysbiosis and postmenopausal
atrophy, which could also inevitably affect the accuracy of
colposcopic assessment. These factors should also be stratified and
analyzed in future studies with larger samples.
Underdiagnosis of CIN by colposcopic impressions is
likely associated with thin HSIL, particularly CIN2. Thin HSILs
usually present as small to minute lesions and lack the classic
HSIL characteristic colposcopic impression, which may explain why
this type of lesion is underdiagnosed by colposcopy. To prevent the
underdiagnosis of thin HSILs, it is necessary to highlight the need
for biopsy in regions with acetowhitening, even if the colposcopy
impression might be metaplasia or LSIL.
Acknowledgements
Not applicable.
Funding
This work was supported by The National Key Research and
Development Program of China (grant nos. 2021YFC2701200 and
2021YFC2701202) and the Research and Development Fund of Peking
University People's Hospital (grant no. RDL2020-02).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML conceptualized and designed the study and was a
major contributor to writing the manuscript. XZ participated in
pathological review and assessment. QZ participated in pathological
section collection and pathological assessment. YZ, CZ, and JL
participated in colposcopic image evaluation and review. DS
organized sample collection and participated in pathological review
and assessment. HT was responsible for statistical analysis and
data interpretation. LW was involved in the study design,
interpretation of data, revising the manuscript critically for
important intellectual content, reviewing the manuscript and
providing advice during the study. All authors read and approved
the final version of the manuscript. ML and XZ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Board or Ethics Committee of Peking University People's Hospital
Ethics Committee (IRB number, 2020PHB298-01; date of IRB approval,
30/10/2020).
Patient consent for publication
Written consent was obtained from the patient in
respect to publishing images in Fig.
3.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Waxman AG, Chelmow D, Darragh TM, Lawson H
and Moscicki AB: Revised terminology for cervical histopathology
and its implications for management of high-grade squamous
intraepithelial lesions of the cervix. Obstet Gynecol.
120:1465–1471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tainio K, Athanasiou A, Tikkinen KAO,
Aaltonen R, Hernándes JC, Glazer-Livson S, Jakobsson M, Joronen K,
Kiviharju M, Louvanto K, et al: Clinical course of untreated
cervical intraepithelial neoplasia grade 2 under active
surveillance: Systematic review and meta-analysis. BMJ.
360:k4992018. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reid R and Scalzi P: Genital warts and
cervical cancer. VII. An improved colposcopic index for
differentiating benign papillomaviral infections from high-grade
cervical intraepithelial neoplasia. Am J Obstet Gynecol.
153:611–618. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferris DG and Litaker MS; ALTS Group, :
Prediction of cervical histologic results using an abbreviated Reid
Colposcopic Index during ALTS. Am J Obstet Gynecol. 194:704–710.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang B, Pretorius RG, Belinson JL, Zhang
X, Burchette R and Qiao YL: False negative colposcopy is associated
with thinner cervical intraepithelial neoplasia 2 and 3. Gynecol
Oncol. 110:32–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghosh I, Mittal S, Banerjee D, Chowdhury N
and Basu P: Study of correlation of cervical epithelial thickness
with the grade of colposcopic abnormality. Int J Gynecol Pathol.
35:269–274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stoler M, Bergeron C, Colgan TJ, Ferenczy
AS, Herrington CS and Kim KR: Squamous Cell Tumours and Precursors.
Kurman RJ, Carcangiu ML, Herrington CS and Young RH: WHO
Classification of Tumours of Female Reproductive Organs. IARC;
Lyon: pp. pp171812014
|
|
8
|
Wentzensen N, Massad LS, Mayeaux EJ Jr,
Khan MJ, Waxman AG, Einstein MH, Conageski C, Schiffman MH, Gold
MA, Apgar BS, et al: Evidence-Based consensus recommendations for
colposcopy practice for cervical cancer prevention in the United
States. J Low Genit Tract Dis. 21:216–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brown BH and Tidy JA: The diagnostic
accuracy of colposcopy-A review of research methodology and impact
on the outcomes of quality assurance. Eur J Obstet Gynecol Reprod
Biol. 240:182–186. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang AC, Wang LQ, Li J, Li MX, Tu LL,
Zhang YX and Liu AJ: Artificial intelligence aided measurement of
cervical squamous epithelial thickness and its correlation with
cervical precancerous lesions. Zhonghua Bing Li Xue Za Zhi.
50:339–343. 2021.(In Chinese). PubMed/NCBI
|
|
11
|
Reich O and Regauer S: Two major pathways
for development of high-grade squamous intraepithelial lesions of
the cervix. Am J Surg Pathol. 38:1579–1580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Regauer S, Reich O and Kashofer K:
Cervical precancers originate from infected proliferating reserve
cells: A Comparative histologic and genetic study of thin and thick
high-grade squamous intraepithelial lesions. Am J Surg Pathol.
46:519–527. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Woodman CB, Collins SI and Young LS: The
natural history of cervical HPV infection: Unresolved issues. Nat
Rev Cancer. 7:11–22. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reich O and Regauer S: Thin HSIL of the
cervix: Detecting a variant of high-grade squamous intraepithelial
lesions with a p16INK4a antibody. Int J Gynecol Pathol. 36:71–75.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Regauer S, Reich O and Kashofer K: Thin
variant of high-grade squamous intraepithelial lesion-relationship
with high-risk and possibly carcinogenic human papilloma virus
subtypes and somatic cancer gene mutations. Histopathology.
75:405–412. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Regauer S and Reich O: CK17 and p16
expression patterns distinguish (atypical) immature squamous
metaplasia from high-grade cervical intraepithelial neoplasia (CIN
III). Histopathology. 50:629–635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barzon L, Militello V, Pagni S, Franchin
E, Dal Bello F, Mengoli C and Palu G: Distribution of human
papillomavirus types in the anogenital tract of females and males.
J Med Virol. 82:1424–1430. 2010. View Article : Google Scholar : PubMed/NCBI
|