Introduction
It has been reported that the treatment of patients
with locally advanced non-small cell lung cancer (LA-NSCLC) with
disease recurrence after chemoradiotherapy is difficult (1–4).
Precision therapy based on different recurrence patterns are a
potential strategy for reducing disease recurrence (5). Therefore, the construction of a model
for predicting the recurrence pattern of patients with LA-NSCLC
treated with chemoradiotherapy is of great importance for precision
treatment. The recurrence patterns of patients with LA-NSCLC
undergoing chemoradiotherapy can be divided into locoregional
recurrence (LR), distant metastasis (DM) and both LR and DM (LR/DM)
(6).
The radiomic features of
fluorine-18(18F)-fluorodeoxyglucose (FDG) positron
emission tomography (PET)/computed tomography (CT) and the clinical
characteristics of patients may potentially serve a significant
role in predicting the recurrence patterns of patients with
LA-NSCLC treated with chemoradiotherapy (6–9). The
radiomic features of 18F-FDG PET/CT, including
quantitative and semi-quantitative features, commonly reflect the
spatial and metabolic heterogeneity of the tumor (10). The quantitative features refer to
the association between the grey level intensity of pixels or
voxels and their position within an image. On the other hand, the
semi-quantitative features are associated with 18F-FDG
uptake semi-quantitative values, such as metabolic tumor volume
(MTV). A previous study showed that the primary tumor volume in
patients with LA-NSCLC is significantly associated with the
recurrence of the primary tumor after chemoradiotherapy (9). Therefore, tumors with a volume of
>50 cm3 are more prone to recurrence compared with
those with a volume of ≤50 cm3. Another study
demonstrated that the total MTV of the primary tumor and regional
metastatic lymph nodes, analyzed by pre- and mid-radiotherapy
18F-FDG PET, are significantly associated with local
recurrence in patients with LA-NSCLC (11). Therefore, it has been hypothesized
that the deep learning model constructed based on the features of
CT radiomics can be used to predict the recurrence pattern of
chemoradiotherapy-treated patients with LA-NSCLC (12). It has also been suggested that the
histological subtypes can be associated with the recurrence
patterns of the aforementioned patients (5). A previous study demonstrated that
squamous cell carcinoma is more prone to LR compared with
adenocarcinoma (5). The
aforementioned studies imply that the radiomic features of
18F-FDG PET/CT combined with the clinical
characteristics of patients can exhibit considerable accuracy in
predicting the recurrence patterns of patients with LA-NSCLC
undergoing chemoradiotherapy.
In the present study, patients with LA-NSCLC who
received chemoradiotherapy were analyzed. In the training set of
patients, to predict the recurrence pattern of patients with
LA-NSCLC, logistic regression analysis was performed based on the
comprehensive quantitative values (CVs) of the radiomic features of
18F-FDG PET/CT, MTV and the clinical characteristics of
patients. Furthermore, the logistic regression analysis results
were verified in the validation set of patients.
Materials and methods
Patients
In the present study, patients with LA-NSCLC treated
with chemoradiotherapy in Shandong University Cancer Center (Jinan,
China), Henan Cancer Hospital (Zhengzhou, China), Union Hospital
Tongji Medical College Huazhong University of Science and
Technology (Wuhan, China), Zhejiang Cancer Hospital (Hangzhou,
China), Fujian Cancer Hospital (Fuzhou, China), Zhangzhou Hospital
Affiliated to Fujian Medical University (Zhangzhou, China) and The
Third Hospital Affiliated to Suzhou University (Changzhou, China)
between May 2016 and January 2020 were analyzed. The inclusion
criteria were as follows: i) Non-operative patients with LA-NSCLC
diagnosed by histology; ii) with Karnofsky performance status (KPS)
prior to therapy of ≥70; iii) treated with concurrent or sequential
chemoradiotherapy; iv) patients who underwent 18F-FDG
PET/CT scanning within two weeks prior radiotherapy; and v)
patients whose recurrence patterns were recorded. The exclusion
criteria were as follows: i) Patients with small cell lung cancer;
ii) whose 18F-FDG PET/CT images were missing; iii)
treated with radiotherapy dose of <50 Gy (equivalent effective
doses at 2 Gy per fraction); and iv) patients whose follow-up data
were missing. Based on different centers where patients were
treated, patients were randomly allocated into the training and
validation sets of patients. The current retrospective study was
approved by the Shandong University Cancer Center Medical Ethics
Committee (approval no. 201511089).
18F-FDG PET/CT imaging
All eligible patients underwent 18F-FDG
PET/CT scanning imaging (Discovery LS PET/CT system; Cytiva) within
two weeks prior to radiotherapy. Before 18F-FDG PET/CT
scan, patients fasted and rested for at least 6 h. The blood
glucose levels were <6.6 mmol/l before scanning. The patients
did not receive bladder catheterization, oral muscle relaxants or
CT contrast enhancers. Scanning started at 44–76 min following
intravenous injection of 370 MBq (10 mCi) 18F-FDG and
18F-FDG PET images were obtained from the top of the
skull to the proximal thigh. Each field of vision covered 14.5 cm
for 5 min and the thickness of each layer was 4.25 mm in the axial
direction. The peak voltage of the X-ray tube, which was used for
spiral CT scan, was 120 kV and 90 mA and the thickness of each
layer was 4.25 mm. 18F-FDG PET/CT scan images were
captured under natural breathing and were reconstructed using an
ordered subset expectation maximization algorithm.
Treatment
All patients were treated with concurrent or
sequential chemoradiotherapy. Intensity-modulated radiation therapy
(IMRT) was used as the type of irradiation. Radiotherapy planning
was performed using 18F-FDG PET/CT or CT scan. No
prophylactic irradiation was delivered to the lymphatic drainage
area. The gross tumor volume (GTV) included the primary tumor and
all metastatic lymph nodes [CT measurement short diameter of >10
mm or PET standardized uptake value (SUV) of >2.5], while the
clinical target volume (CTV) included GTVs exceeding 6 mm (squamous
cell carcinoma) or 8 mm (adenocarcinoma or other histological
types). The planning target volume consisted of a margin extending
outside the CTV, which was 5 and 10–15 mm in all directions of the
18F-FDG PET and CT image, respectively. Conventional
fractionated (CFRT) or late-course hyperfractionated accelerated
radiotherapy (LCHART) were used for radiotherapy fractionation.
CFRT was defined as a single 2–3 Gy fraction, once a day for five
days/week. LCHART included two phases, CFRT and hyperfractionated
accelerated radiotherapy. Hyperfractionated accelerated
radiotherapy was performed following CFRT, with fractions of 1.4
Gy, twice daily. The radiotherapy dose was prescribed to the 95%
isodose line of the respective IMRT plan, covering at least 95% of
the target volume. The GTV prescription dose was corrected to
equivalent effective doses at 2 Gy per fraction (EQD2) using the
linear quadratic model (α/β=10.0 Gy). The corrected prescription
dose was used for statistical analysis. Chemotherapy regimens
included platinum-based chemotherapy, two-drug combination
chemotherapy or single-drug chemotherapy.
Follow-up
Follow-up was performed once every 3–6 months after
treatment and included physical examination, chest CT scan and
other necessary examinations, such as craniocerebral magnetic
resonance examination, when headache occurred. Progression free
survival (PFS) was defined as the time from 18F-FDG
PET/CT scan to locoregional recurrence/distant metastasis or death.
Overall survival (OS) was defined as the time interval between
18F-FDG PET/CT scan and patient death or last follow-up.
Additionally, disease recurrence was considered as the first
disease progression recorded according to the Response Evaluation
Criteria in Solid Tumors (RECIST 1.1). Histological diagnosis was
not necessary. Recurrence patterns included LR, DM and LR/DM. LR
was characterized by the recurrence of the primary tumor and/or
regional lymph nodes, while DM was defined as metastasis outside
the primary tumor and regional lymph nodes. LR/DM is defined by
both LR and DM.
Image analysis
For the training set of patients, the regions of
interest (ROIs) were delineated using ITK-SNAP (13) or CGITA (14) software. For ITK-SNAP, the ROIs were
drawn manually by a radiation oncologist with >10 years target
delineation experience. For CGITA, a SUV value of 2.5 was selected
to delineate ROIs using an automatic threshold-based region growing
method. When the lesions were adjacent to non-lesions with high
18F-FDG uptake, such as the heart and liver, the ROIs
were drawn manually by the radiation oncologist. The primary tumors
identified by 18F-FDG PET and CT scan and drawn using
ITK-SNAP software, were defined as ROI1 and ROI2, respectively.
Subsequently, PyRadiomics software (15) was used to extract the quantitative
values of the ROI1 and ROI2 radiomic features. In addition, the
primary tumor and both the primary tumor and regional metastatic
lymph nodes displayed by 18F-FDG PET and delineated
using CGITA software, were defined as ROI3 and ROI4, respectively.
CGITA software was then used to calculate the quantitative values
of the ROI3 radiomic features and extract the MTVs of ROI3 and
ROI4, which were named MTV3 and MTV4. For the validation set of
patients, the corresponding ROIs and quantitative values of the ROI
radiomic features were also obtained as described for the training
set of patients.
Statistical analysis
All statistical analyses were carried out using SPSS
(V21.0; IBM Corp.) and MedCalc (V15.8; MedCalc software Ltd.).
P<0.05 was considered to indicate a statistically significant
difference. The 1- and 2-year recurrence rate of patients in the
training and validation sets of patients were calculated using the
following equation: Number of patients with recurrence events
within 1- or 2-year/total number of patients included in the
training or validation sets of patients. The logistic regression
equations for predicting the recurrence patterns in the training
set of patients were developed as follows: CVs were calculated
using the principal component analysis according to the following
formula: CVr-i=ai1X1′ + ai2X2′ +… +
aimXm', where r indicates 1,2,3, i indicates
1,2,…,m, aim indicates component coefficient and
Xm' indicates the Z-score standardized value of ROI
radiomic features quantitative values. In addition, the Z-score
standardized value was calculated using the following formula:
Z-score value=original value-mean value/standard deviation (SD).
CVs with eigenvalues >1 were subjected to further analysis.
Spearman's rank order correlation or association analysis were used
to calculate the correlation and association coefficients between
clinical characteristics, including CVs, MTV3, MTV4 and recurrence
patterns. Unpaired two-tailed t-test or χ2 test was
applied to evaluate whether the correlation and association
coefficients were statistically significant. Subsequently, the
features with significant correlation or association coefficients
were used to construct the logistic regression equations for
predicting recurrence patterns. The probability values of
recurrence patterns were measured using the logistic equations and
the receiver operating characteristic (ROC) curves for diagnosing
recurrence patterns were constructed based on these values. The
areas under ROC curves (AUCs), cut-off, sensitivity and specificity
values were calculated based on the maximum value of Youden index.
The logistic regression equations for predicting the recurrence
patterns were verified in the validation set of patients as
follows: The corresponding CVs in the validation set of patients
were obtained as described for the training set of patients. The
CVs and clinical characteristics were inserted into the logistic
equations. The probability values of recurrence patterns were
obtained as previously described. ROC curves were constructed based
on the predicted and actual recurrence patterns and the AUC,
sensitivity and specificity values were then calculated.
Results
Patient characteristics and recurrence
patterns
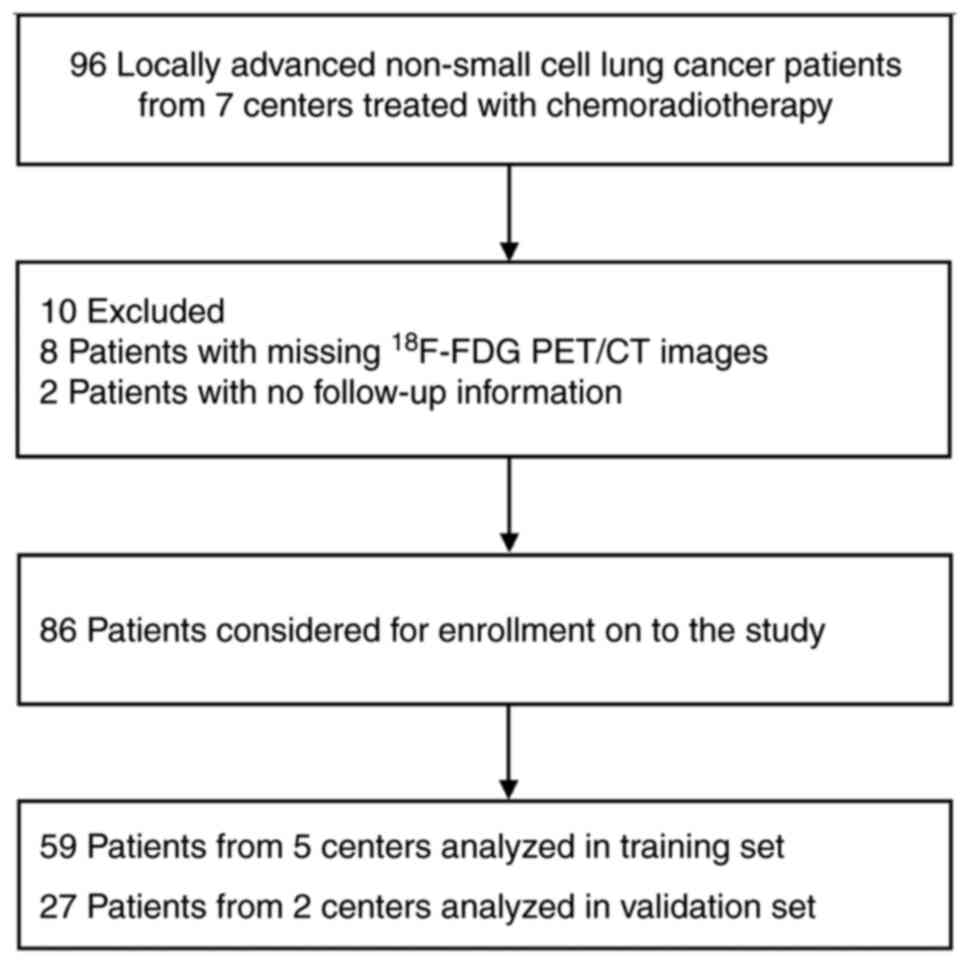
A total of 96 patients from seven centers were
enrolled between May 2016 and January 2020. Among these patients,
10 patients were excluded, eight due to lack of 18F-FDG
PET/CT and two due to missing follow-up data. Overall, 86 patients
were considered eligible for the study. The median follow-up time
was 21.5 months (range, 2.8-61.8 months), while the median PFS and
OS were 9.3 [95% confidence interval (CI), 7.7-11.0 months] and
32.0 months (95% CI, 17.6-46.4 months), respectively. A total of 59
patients from five centers were included in the training set of
patients and 27 patients from two centers in the validation set of
patients. The flow diagram illustrating the study enrollment
process is shown in Fig. 1. The
clinical characteristics of patients are listed in Table I. For the training set of patients,
the median age was 60 years (range, 39–73 years), the radiotherapy
dose was 60.00 Gy (range, 60.00-75.91 Gy), while chemotherapy
lasted for four cycles (range, 1–7 cycles). In addition, the median
follow-up time was 23.5 months (range, 3.9-41.8 months), while the
median PFS and OS were 9.4 (95% CI, 7.6-11.2 months) and 31.0
months (95% CI, 20.8-41.2 months), respectively. A total of 22
patients experienced LR, accounting for 37.3% of all cases, while
24 patients experienced DM (40.7%) and 13 patients experienced
LR/DM (22.0%). The 1- and 2-year recurrence rates were 67.8 and
88.1%, respectively. For the validation set of patients, the median
age was 58 years (range, 34–86 years), the median radiotherapy dose
was also 60.00 Gy (range, 50.00-75.25 Gy) and chemotherapy also
lasted for four cycles (range, 1–6 cycles). Additionally, the
median follow-up period was 18.9 months (range, 2.8-61.8 months),
while the median PFS and OS were 9.3 (95% CI, 4.2-14.5 months) and
40.4 months (95% CI, 30.4-50.4 months), respectively. There were 12
patients with LR (44.4%), six patients with DM (22.2%) and nine
patients with LR/DM (33.3%). Finally, the 1- and 2-year recurrence
rates were 63.0 and 88.9%, respectively.
 | Table I.Patient characteristics in the
training and validation sets of patients. |
Table I.
Patient characteristics in the
training and validation sets of patients.
|
| Training set | Validation set |
|---|
|
|
|
|
|---|
| Characteristic | Number | % | Number | % |
|---|
| Age (years) |
|
|
|
|
| ≤60 | 33 | 55.9 | 13 | 48.1 |
|
>60 | 26 | 44.1 | 14 | 51.9 |
| Sex |
|
|
|
|
| Male | 50 | 84.7 | 22 | 81.5 |
|
Female | 9 | 15.3 | 5 | 18.5 |
| Histology |
|
|
|
|
| SCC | 41 | 69.5 | 17 | 63.0 |
|
ADC | 18 | 30.5 | 8 | 29.6 |
|
Other | 0 | 0 | 2 | 7.4 |
| Stagea |
|
|
|
|
|
IIIA | 15 | 25.4 | 12 | 44.4 |
|
IIIB | 32 | 54.2 | 9 | 33.3 |
|
IIIC | 12 | 20.3 | 6 | 22.2 |
| Karnofsky
performance status scoe |
|
|
|
|
| 70 | 3 | 5.1 | 1 | 3.7 |
| 80 | 18 | 30.5 | 8 | 29.6 |
| 90 | 36 | 61.0 | 16 | 59.3 |
|
100 | 2 | 3.4 | 2 | 7.4 |
| Smoking index |
|
|
|
|
| 0 | 14 | 23.7 | 10 | 37.0 |
|
1-400 | 8 | 13.6 | 4 | 14.8 |
|
>400 | 37 | 62.7 | 12 | 44.4 |
| Treatment
model |
|
|
|
|
|
CCRT | 53 | 89.8 | 17 | 63.0 |
|
SCRT | 6 | 10.2 | 10 | 37.0 |
| Radiotherapy
doseb (Gy, GTV) |
|
|
|
|
|
≤60 | 34 | 57.6 | 12 | 44.4 |
|
>60 | 25 | 42.4 | 15 | 55.6 |
| Chemotherapy cycles
(n) |
|
|
|
|
|
1-4 | 47 | 79.7 | 22 | 81.5 |
|
>4 | 12 | 20.3 | 5 | 18.5 |
CVs and MTVs in the training set of
patients
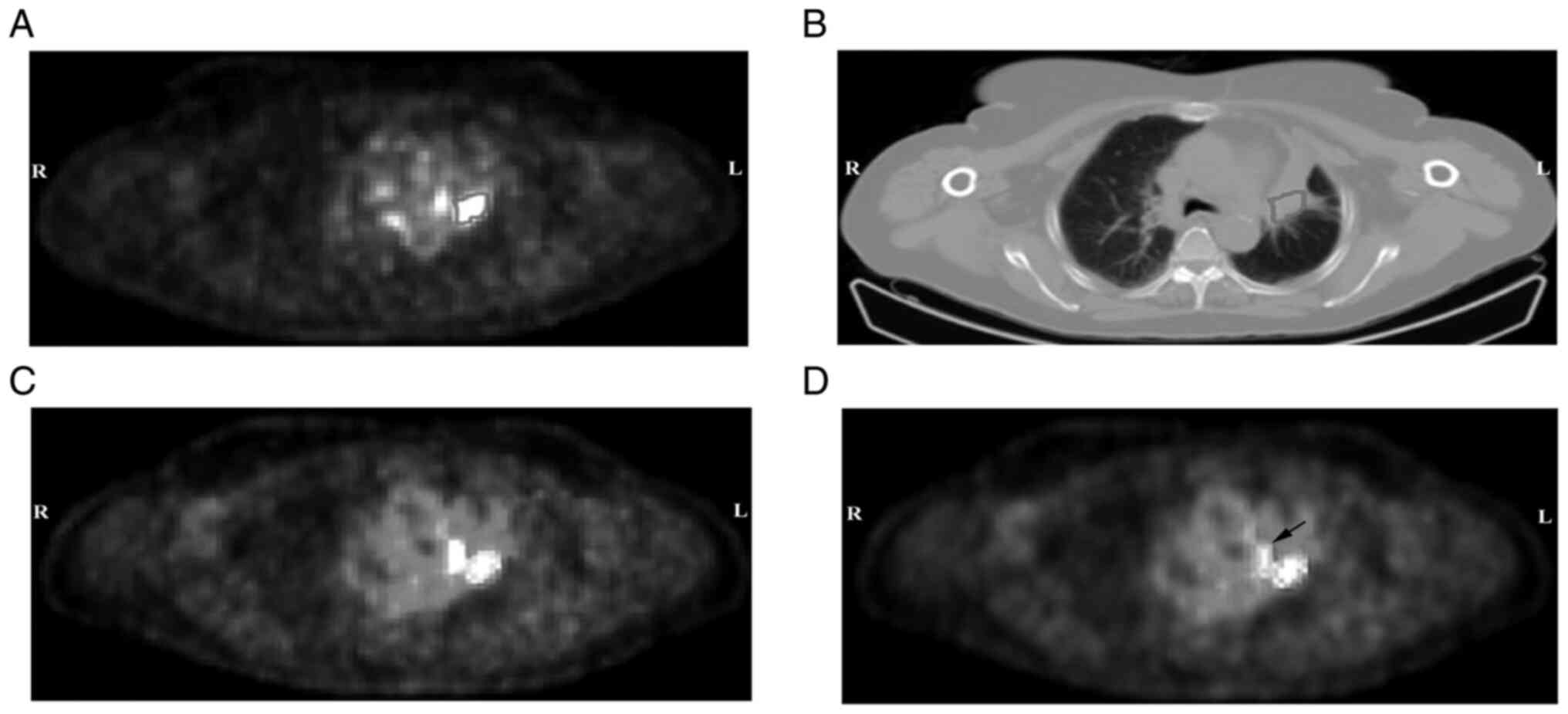
Retrospective ROI examples are shown in Fig. 2. A total of 86, 100 and 72
quantitative values of radiomic features were extracted from ROI1,
ROI2 and ROI3, respectively (Table
SI). The mean ± SD values are listed in Table SI. The CVs included 86 CV1
(CV1-1-CV1-86), 100 CV2 (CV2-1-CV2-100) and 72 CV3 (CV3-1-CV3-72).
The mean ± SD values of MTV3 and MTV4 were 88.97±114.79 and
118.39±136.91 cm3, respectively. Among these CVs, nine
CV1, 10 CV2 and nine CV3 were selected for further analysis. These
CVs and their eigenvalues are shown in Table SII.
Recurrence pattern prediction in the
training set of patients
Analysis revealed that histological subtypes CV2-5,
CV2-7 and CV3-4 were significantly associated with recurrence
patterns (Table II). No
significant association was obtained between age, sex, clinical
stage, KPS, smoking index, treatment model, radiotherapy dose,
chemotherapy cycle, MTV3, MTV4 and the other 25 CVs and recurrence
patterns (data not shown). The logistic regression equations for
predicting recurrence patterns are listed in Table III. The logistic equations
identified histological subtype and two CVs, namely CV2-5 and
CV3-4, as significant independent variables. The ROC curves used
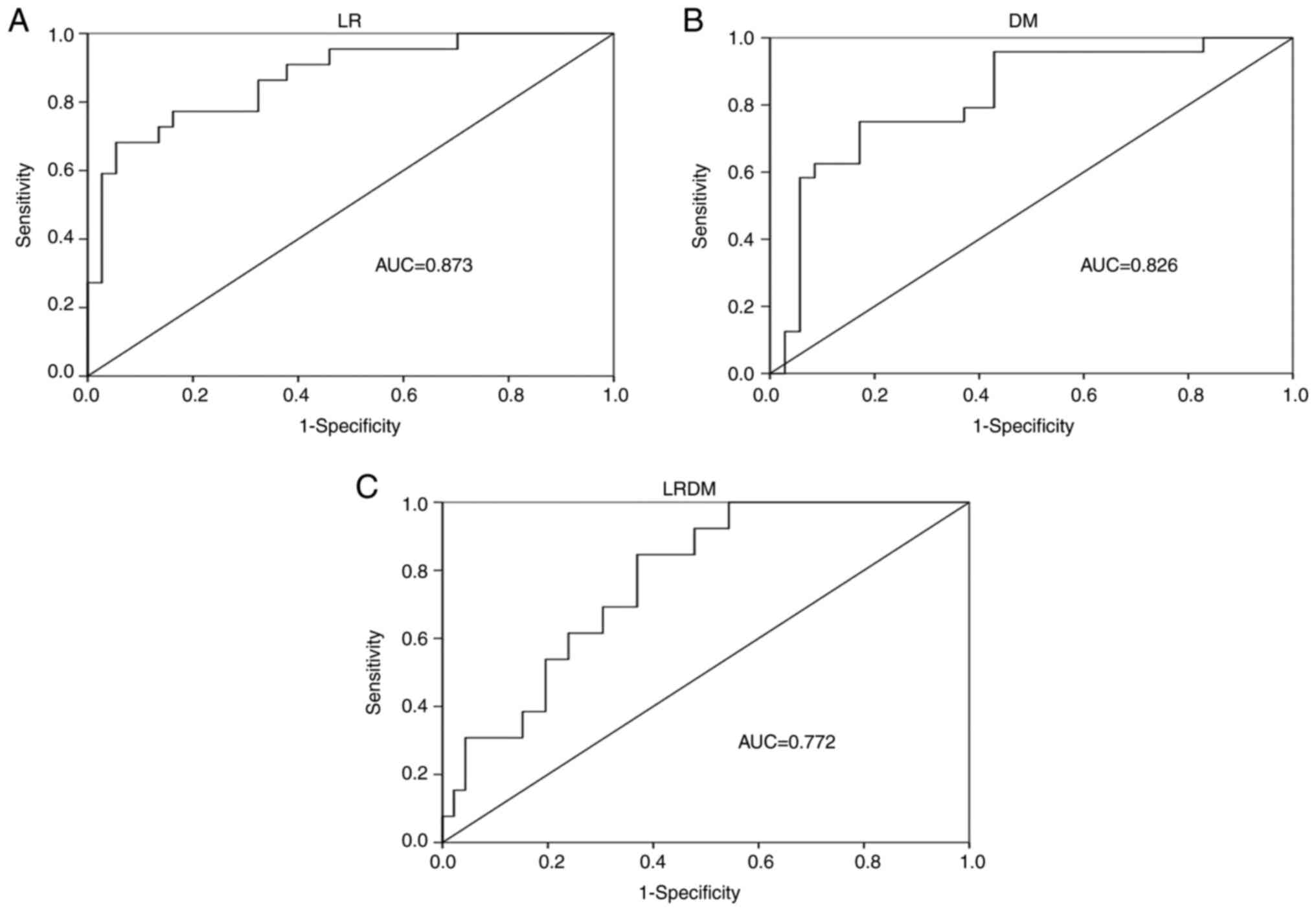
for diagnosing recurrence patterns are presented in Fig. 3A-C. For diagnosing LR, the AUC,
cut-off, sensitivity and specificity values were 0.873, 0.560,
0.682 and 0.946, respectively. In addition, the AUC, cut-off,
sensitivity and specificity values for diagnosing DM were 0.826,
0.370, 0.750 and 0.829, respectively, while the corresponding
values for diagnosing LR/DM were 0.772, 0.170, 0.846 and 0.609,
respectively.
 | Table II.Correlation and association
coefficients between histology type, CVs and recurrence patterns in
the training set of patients. |
Table II.
Correlation and association
coefficients between histology type, CVs and recurrence patterns in
the training set of patients.
|
| Recurrence
patternsa |
|---|
|
|
|
|---|
| Feature | Correlation
coefficient | P-value |
|---|
| Histology
typeb | 0.449c | 0.001 |
| CV2-5 | 0.259d | 0.048 |
| CV2-7 | −0.282d | 0.031 |
| CV3-4 | −0.365d | 0.004 |
 | Table III.Logistic regression equations for
predicting recurrence patterns in the training set of
patientsa. |
Table III.
Logistic regression equations for
predicting recurrence patterns in the training set of
patientsa.
|
|
|
|
|
| 95% Confidence
interval |
|---|
|
|
|
|
|
|
|
|---|
| Predicted
recurrence pattern | Feature | Coefficient | P-value | Hazard ratio | Lower | Upper |
|---|
| DM | Histology
typeb | −2.768 | 0.006 | 0.063 | 0.009 | 0.447 |
|
| CV3-4 | −1.280 | 0.008 | 0.278 | 0.108 | 0.716 |
|
| Constant | 2.353 | 0.012 | - | - | - |
| LR/DM | CV2-5 | 0.933 | 0.041 | 2.542 | 1.037 | 6.232 |
|
| CV3-4 | −1.580 | 0.003 | 0.206 | 0.072 | 0.591 |
Efficiency of the prediction model in
the validation set
A total of 27 patients were allocated to the
validation set of patients, including 25 patients with squamous
cell carcinoma/adenocarcinoma and two patients with other
histological subtypes. However, the recurrence pattern prediction
model constructed in the training set of patients included only
patients with squamous cell carcinoma/adenocarcinoma. Therefore, 25
patients with squamous cell carcinoma/adenocarcinoma were used to
verify the recurrence pattern prediction model, while the two
patients with the other histological subtypes were excluded.
Representative examples for ROI2 and ROI3 are shown in Fig. 2. The corresponding mean ± SD values
are listed in Table SIII, while
the values of CV2-5 and CV3-4 are shown in Table SIV. Furthermore, the ROC curves for
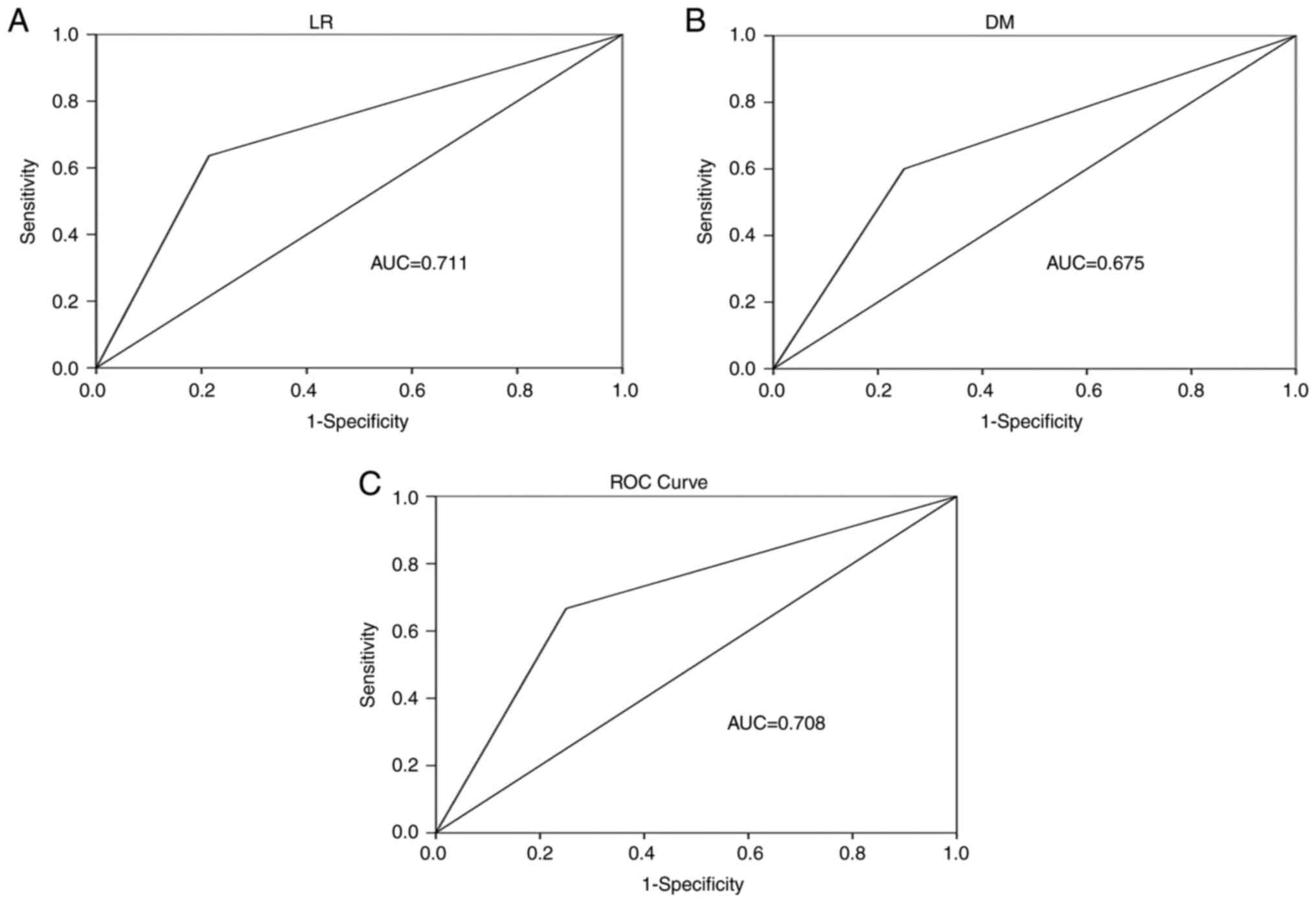
diagnosing recurrence patterns are presented in Fig. 4A-C. Therefore, the AUC, sensitivity
and specificity values for diagnosing LR were 0.711, 0.636 and
0.786, respectively. The AUC, sensitivity and specificity values
for diagnosing DM and LR/DM were 0.675, 0.600 and 0.750 and 0.708,
0.667 and 0.750, respectively.
Discussion
It has been previously reported that the prognosis
of patients with LA-NSCLC treated with chemoradiotherapy is poor,
as the majority of these patients may experience disease recurrence
after chemoradiotherapy (8,16–18).
The present study showed that the 1 year recurrence rate was 67.8
and 63.0% in the training and validation sets of patients,
respectively. A randomized controlled study (RTOG0617) demonstrated
that the 1 year recurrence rate of patients with LA-NSCLC treated
with 60 Gy conventional radiotherapy combined with chemotherapy is
49.2% (16). Additionally, a
previous retrospective study demonstrated that the total recurrence
rate for patients with LA-NSCLC who underwent chemoradiotherapy is
65.9% (17). Furthermore, another
retrospective study showed that the 1 year recurrence rate of
LA-NSCLC is 74% (19). The
aforementioned results indicate that the majority of patients with
LA-NSCLC can experience disease recurrence after chemoradiotherapy.
Therefore, precision treatment should be considered to reduce
disease recurrence.
The current study also demonstrated that CV2-5 and
CV3-4 combined with histological subtype could predict the
recurrence patterns of chemoradiotherapy-treated patients with
LA-NSCLC. CV2-5 and CV3-4 were the linear combination and were
composed of the radiomic feature quantitative values from the
primary tumors imaged using 18F-FDG PET/CT. Therefore,
CV2-5 and CV3-4 could reflect the spatial and metabolic
heterogeneity of the primary tumors. The higher the CV2-5 value,
the higher the probability for LR/DM. However, a lower CV2-5 value
was associated with a higher probability for LR. Additionally, a
greater CV3-4 value was associated with an increased risk for LR,
while the lower value was associated with a greater probability for
DM and LR/DM. The results also demonstrated that patients with
squamous cell carcinoma were prone to LR, while those with
adenocarcinoma were prone to DM. The aforementioned findings
indicated that the recurrence patterns were different for patients
with LA-NSCLC treated with chemoradiotherapy and were associated
with CV2-5, CV3-4 and histological subtype. Therefore, to reduce
the disease recurrence rate, different treatment approaches should
be considered according to different CV2-5, CV3-4 and histological
subtypes.
Furthermore, the present study demonstrated that the
CV2-5 of the pre-radiotherapy CT scan radiomic features was also
markedly associated with the recurrence patterns of
chemoradiotherapy-treated patients with LA-NSCLC. In addition,
CV2-5 combined with CV3-4 and histological subtype could also
predict the recurrence patterns of the aforementioned patients. A
previous retrospective study reported that the deep learning model
constructed by radiomic features of pre- and post-treatment CT
scans can also predict the recurrence patterns of patients with
LA-NSCLC (12). Consistent with the
results of the current study, previous research also indicated that
patients with squamous cell carcinoma are more prone to LR compared
with those suffering from adenocarcinoma (5). These findings suggest that enhancing
local and systemic treatment to reduce LR and DM in patients with
squamous cell carcinoma and adenocarcinoma could be the appropriate
approach for patients with LA-NSCLC undergoing
chemoradiotherapy.
Previous studies showed that the MTVs of the primary
tumor and regional metastatic lymph nodes displayed by
18F-FDG PET pre- and mid-radiotherapy are significantly
associated with the recurrence patterns of patients with LA-NSCLC
who receive chemoradiotherapy (11). The AUC values of pre- and
mid-radiotherapy MTVs for predicting LR are 0.71 and 0.76,
respectively (11). A previous
study demonstrated that patients with LA-NSCLC and a tumor volume
>50 cm3 are more prone to primary tumor recurrence
after chemoradiotherapy compared with those with a tumor volume ≤50
cm3 (9). These two
retrospective studies suggested that tumor volume could be
significantly associated with local recurrence in patients with
LA-NSCLC treated with chemoradiotherapy. In addition, the results
of the present study demonstrated that neither the MTV of the
primary tumor (MTV3) nor the MTV of the primary tumor combined with
regional metastatic lymph nodes (MTV4) were notably associated with
the recurrence patterns of chemoradiotherapy-treated patients with
LA-NSCLC. However, further studies are needed to verify whether
MTVs in mid-radiotherapy could be significantly associated with the
recurrence patterns of these patients.
The present retrospective study has some
limitations, including the loss of particular clinical follow-up
data and patient selective bias. Therefore, further research with a
larger sample size of patients is needed to verify the results of
the current study.
In conclusion, the present study showed that spatial
and metabolic heterogeneity quantitative values from the primary
tumor combined with histological subtype could predict the
recurrence patterns of patients with LA-NSCLC treated with
chemoradiotherapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was partially funded by the Natural Science
Foundation of China (grant nos. NSFC81872475 and NSFC82073345) and
the Jinan Clinical Medicine Science and Technology Innovation Plan
(grant no. 202019060).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY conceived and designed the experiments and
supervised the project. WL, XQ, HG, SZ, XS, JL, WC and WG performed
the experiments. WL and XQ analyzed the data. WL wrote the
manuscript. All authors read and approved the final version of the
manuscript. SY and WL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
This retrospective study was approved by the
Shandong University Cancer Center Medical Ethics Committee
(approval no. 201511089). Due to the retrospective nature of the
study, the Medical Ethics Committee waived the requirement for
obtaining informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gelb AF, Tashkin DP, Epstein JD, Szeftel A
and Fairshter R: Physiologic characteristics of malignant
unilateral main-stem bronchial obstruction. Diagnosis and Nd-YAG
laser treatment. Am Rev Respir Dis. 138:1382–1385. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim EY, Chapman TR, Ryu S, Chang EL,
Galanopoulos N, Jones J, Kubicky CD, Lee CP, The BS, Traughber BJ,
et al: ACR Appropriateness Criteria® nonspine bone
metastases. J Palliat Med. 18:11–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chow E, Harris K, Fan G, Tsao M and Sze
WM: Palliative radiotherapy trials for bone metastases: A
systematic review. J Clin Oncol. 25:1423–1436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Howell DD, James JL, Hartsell WF,
Suntharalingam M, Machtay M, Suh JH, Demas WF, Sandler HM, Kachnic
LA and Berk LB: Single-fraction radiotherapy versus multifraction
radiotherapy for palliation of painful vertebral bone
metastasesequivalent efficacy, less toxicity, more convenient: A
subset analysis of Radiation Therapy Oncology Group trial 97-14.
Cancer. 119:888–896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ito H, Matsuo Y, Ohtsu S, Nishimura T,
Terada Y, Sakamoto T and Mizowaki T: Impact of histology on
patterns of failure and clinical outcomes in patients treated with
definitive chemoradiotherapy for locally advanced non-small cell
lung cancer. Int J Clin Oncol. 25:274–281. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lou F, Sima CS, Rusch VW, Jones DR and
Huang J: Differences in patterns of recurrence in early stage
versus locally advanced non-small cell lung cancer. Ann Thorac
Surg. 98:1755–1760; discussion 1760-1. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lacoppidan T, Vogelius IR, Pøhl M, Strange
M, Persson GF and Nygård L: An investigative expansion of a
competing risk model for first failure site in locally advanced
non-small cell lung cancer. Acta Oncol. 58:1386–1392. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jouglar E, Isnardi V, Goulon D,
Ségura-Ferlay C, Ayadi M, Dupuy C, Douillard JY, Mahé MA and Claude
L: Patterns of locoregional failure in locally advanced non-small
cell lung cancer treated with definitive conformal radiotherapy:
Results from the Gating 2006 trial. Radiother Oncol. 126:291–299.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abe T, Kobayashi N, Aoshika T, Ryuno Y,
Saito S, Igari M, Hirai R, Kumazaki YU, Miura YU, Kaira K, et al:
Pattern of local failure and its risk factors of locally advanced
non-small cell lung cancer treated with concurrent
chemo-radiotherapy. Anticancer Res. 40:3513–3517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chicklore S, Goh V, Siddique M, Roy A,
Marsden PK and Cook GJ: Quantifying tumour heterogeneity in 18F-FDG
PET/CT imaging by texture analysis. Eur J Nucl Med Mol Imaging.
40:133–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Binkley MS, Koenig JL, Kashyap M, Xiang M,
Liu Y, Sodji Q, Maxim PG, Diehn M, Loo BW Jr and Gensheimer MF:
Predicting per-lesion local recurrence in locally advanced
non-small cell lung cancer following definitive radiation therapy
using pre- and mid-treatment metabolic tumor volume. Radiat Oncol.
15:1142020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Hosny A, Zeleznik R, Parmar C,
Coroller T, Franco I, Mak RH and Aerts HJWL: Deep learning predicts
lung cancer treatment response from serial medical imaging. Clin
Cancer Res. 25:3266–3275. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yushkevich PA, Piven J, Hazlett HC, Smith
RG, Ho S, Gee JC and Gerig G: User-guided 3D active contour
segmentation of anatomical structures: Significantly improved
efficiency and reliability. Neuroimage. 31:1116–1128. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang YH, Lin CY, Shih MJ, Wang HM, Ho TY,
Liao CT and Yen TC: Development and evaluation of an opensource
software package ‘CGITA’ for quantifying tumor heterogeneity with
molecular images. Biomed Res Int. 2014:2485052014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Griethuysen JJM, Fedorov A, Parmar C,
Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC,
Pieper S and Aerts HJWL: Computational radiomics system to decode
the radiographic phenotype. Cancer Res. 77:e104–e107. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bradley JD, Paulus R, Komaki R, Masters G,
Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A,
et al: Standard-dose versus high-dose conformal radiotherapy with
concurrent and consolidation carboplatin plus paclitaxel with or
without cetuximab for patients with stage IIIA or IIIB
nonsmall-cell lung cancer (RTOG 0617): A randomised, two-by-two
factorial phase 3 study. Lancet Oncol. 16:187–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kaderbhaï CG, Coudert B, Bertaut A, Adnet
J, Favier L, Lagrange A, Peignaux-Casasnovas K, Mettey L, Tharin Z,
Foucher P and Martin E: Outcomes of concurrent radiotherapy with
weekly docetaxel and platinum-based chemotherapy in stage III
non-small-cell lung cancer. Cancer Radiother. 24:279–287. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spina R, Chu SY, Chatfield M, Chen J, Tin
MM and Boyer M: Outcomes of chemoradiation for patients with
locally advanced non-small-cell lung cancer. Intern Med J.
43:790–797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grass GD, Naghavi AO, Abuodeh YA, Perez BA
and Dilling TJ: Analysis of relapse patterns after definitive
chemoradiotherapy in locally advanced non-small-cell lung cancer
patients. Clin Lung Cancer. 20:e1–e7. 2019. View Article : Google Scholar : PubMed/NCBI
|