Introduction
Gastric cancer is the fifth most common cancer
around the world; although its overall incidence has been declining
in recent years, it still ranks as the third cause of
cancer-related death (1). Clinical
treatment is radical and involves a combination of surgery and
chemical therapy (2). Effective
constituents from traditional herbal medicines, such as paclitaxel,
are often preferred for the development of chemical drugs (3). The introduction of novel bioactive
components of natural origin may be considered as a new and
reliable chemotherapeutic strategy for different types of human
cancer based on their selective molecular targets (4). Currently, much attention has been
focused on search for natural medical ingredients as novel
chemotherapeutic agents for cancer.
Luteolin, 3′,4′,5,7-tetrahydroxyflavone, is a
traditional herbal medical ingredient, belonging to the flavonoid
family (4). Luteolin is the main
constituent of Ajuga decumbens thunb, which is broadly used
for cough suppression, expectoration, and inflammation control. At
present, an increasing number of studies are being carried out
focusing on new pharmacological activities of luteolin, including
neuroprotection, antiinflammation, antioxidation, and antibacterial
action (4). It is reported that
luteolin exerts inhibitory effects on cell proliferation,
metastasis, invasion, and angiogenesis through multiple pathways
(5). Despite some progress, the
mechanism of luteolin's anticancer effects is still largely unknown
(6). Therefore, this work focused
on the inhibitory effects of luteolin on gastric cancer cells'
proliferation and explored the underlying mechanism.
Regarding carcinogenesis, mitochondria are
distinctly important organelles that provide bioenergy for cancer
cells' proliferation and metastasis and regulate the apoptosis
pathway under certain stimuli (7,8).
Apoptosis occurs through two classic pathways: intrinsic and
extrinsic (6), both of which are
closely related to mitochondria. Any alteration or interruption in
the mitochondrial membrane may activate both the intrinsic and
extrinsic apoptosis pathways-the hallmark of apoptosis (6,9).
Therefore, precisely targeting mitochondria is considered a
promising approach in cancer therapy (10). The strategy of targeting
mitochondria primarily focuses on the elevation of oxidative stress
and destabilization of the mitochondrial membrane, ultimately
inducing mitochondria-mediated apoptosis in cancer cells (7). On the outer mitochondrial membrane,
the B cell lymphoma-2 (Bcl-2) family members, such as Bcl-2 and
Bcl-2-associated X protein (Bax), are involved in regulating the
outer mitochondrial membrane's function and the apoptotic signaling
pathways. On the inner mitochondrial membrane, the mitochondrial
electron transport chain (METC) plays the central role in the
chemiosmotic theory (11). METC is
composed of complex I (NADH-coenzyme Q oxidoreductase), complex II
(succinate ubiquinone oxidoreductase), complex III (CoQ-cytochrome
c oxidoreductase), cytochrome c, complex IV (cytochrome c oxidase),
and complex V (F0F1-ATP synthase) (12). Complexes I and III, especially the
former, are considered the main sites of reactive oxygen species
(ROS) generation. METC complex V can utilize the proton potential
from other METC complexes to phosphorylate adenosine diphosphate
(ADP) to form adenosine triphosphate (ATP) (13). This is the crucial energy-generating
pathway in living cells. An interruption of the electron transfer
between METC complexes might be associated with ROS elevation.
Meanwhile, it has been reported that METC is closely associated
with cancer cell apoptosis. Xanthohumol, a polyphenol, effectively
induces apoptosis and mitochondrial superoxide generation by
inhibiting the activities of complexes I and III and by restraining
the electron transfer between them, followed by cytochrome c
release from mitochondria (14).
Due to the contribution of mitochondria to cancer
cell survival, targeting the mitochondria is a plausible and
intriguing strategy to eliminate cancer cells with relatively high
specificity (15). Therefore, this
work employed the human gastric cancer HGC-27 cell line and MKN-45
cell line, and mouse forestomach carcinoma MFC cell line to explore
the anticancer effects of luteolin and to investigate the
underlying mechanism. In addition, this study explores if luteolin
could exert the inhibitory effects on gastric cancer cells from
different species by demonstrating the different effects of
luteolin on human gastric cancer cells and mouse gastric cancer
cells. Moreover, the data from mouse gastric cancer cells will pave
the way to the research on the effects of luteolin on the immune
system in tumor-bearing mice in the future.
We aimed to identify the inhibitory effects of
luteolin on gastric cancer cells proliferation and to reveal the
vital role of mitochondria in luteolin-induced apoptosis in gastric
cancer cells. This work may provide new insights into luteolin's
anticancer effects by interfering with the mitochondrial
function.
Materials and methods
Reagents
Luteolin (purity≥98%) was purchased from
Sigma-Aldrich Company (L9283, St. Louis, MO, United States).
Luteolin was dissolved in dimethyl sulfoxide (DMSO) (D8371,
Solarbio, Beijing, China) and diluted with complete medium to the
required concentration. The final concentration of DMSO in the
working solution was less than 0.1%, which had no adverse effects
on cell viability. The other detection reagents were bought from
Sigma-Aldrich Company (St. Louis, MO, United States) and Solarbio
Science & Technology Co., Ltd (Beijing, China).
Cell culture
Human gastric cancer HGC-27 cell line
(1101HUM-PUMC000279), mouse forestomach carcinoma MFC cell line
(1101MOU-PUMC000143), and MKN-45 cell line (1101HUM-PUMC000229)
were purchased from National Infrastructure of Cell Line Resource
(Beijing, China). Cells were cultured in RPMI 1640 medium (31800,
Solarbio, Beijing, China) containing 10% fetal bovine serum
(REF10091-148, Gibco; Thermo Fisher Scientific Inc., Massachusetts,
United States) and incubated at 37°C in a 5% CO2
incubator (HF90/HF240, Heal Force, Shanghai, China). Subsequently,
the cells were treated with different concentrations of luteolin
for 24 h. RPMI 1640 complete medium containing 0.1% DMSO was used
as a control.
CCK-8 cell viability assay
The effect of luteolin on cells viability was
determined using the CCK-8 assay (16). Briefly, 100 µl of cell suspension
per well was seeded in a 96-well plate at a density of 6,000
cells/well. After incubation for 24 h at 37°C in a 5%
CO2 incubator (HF90/HF240, Heal Force, Shanghai, China),
the supernatant was discarded, and the HGC-27 and MFC cells were
treated with the different luteolin concentrations (10, 20, 30, 40,
50, 60, 70, and 80 µM in 200 µl/well) for 24 h, and MKN-45 cells
were treated for 48 h. According to the cytotoxicity assay kit
(CA1210, Solarbio, Beijing, China) protocol, 100 µl of working
solution (CCK-8: complete RPMI 1640 medium=1:10) was added to each
well. After incubation at 37°C for 60 min in the dark, the
absorbance at a wavelength of 450 nm was detected using a Thermo
3001 multi-function microplate reader (Infinite 200 PRO, Tecan
Austria GmbH, Salzburg, Austria). IC50 indicated that
the drug concentration resulted in a 50% reduction in cell
survival. Experiments were repeated more than three times. In the
following assays, gastric cancer cells were treated with 10, 40,
and 70 µM of luteolin. In line with the IC50 value of
three cell lines, 10, 40, and 70 µM of luteolin is set in the
arithmetic sequence. Under the treatment with 10 µM, 40 µM, and 70
µM luteolin, it is feasible to collect enough cells to meet
requirements for measurement accuracy, and to exhibit the
difference among various groups.
Hoechst 33258 staining
Morphological characteristics of apoptosis in cell
nuclei were detected by Hoechst 33258 staining (17). At least 2.0×105 cells
were seeded in each well of a 6-well plate. HGC-27 and MFC cells
were treated with 10, 40, and 70 µM luteolin for 24 h, and MKN-45
cells were treated for 48 h. The supernatant was discarded, and the
cells were fixed with a fixing agent (acetic acid: methyl
alcohol=1:3) for 30 min. Then, the fixing agent was discarded, and
the cells were rinsed with phosphate-buffered saline (PBS) solution
three times. Based on Hoechst 33258 stain solution (C0021,
Solarbio, Beijing, China) protocol, the cells were incubated with
500 µl of the staining working solution (Hoechst 33258: PBS=1:100)
at room temperature in the dark for 5–10 min. Inverted fluorescence
microscopy (DMI3000, Leica, Wetzlar, Germany) was employed to
record blue nuclei changes in the different groups.
Annexin V-FITC/PI double staining
assay
Quantitative analysis of the percentage of apoptosis
was performed with an Annexin-V FITC/PI apoptosis detection kit
(CA1020, Solarbio, Beijing, China) and flow cytometry (18). At least 2.5×105
cells/well were seeded in a 6-well plate. After incubation for 24 h
at 37°C in a 5% CO2 incubator, HGC-27 and MFC cells were
treated with 10, 40, and 70 µM luteolin for 24 h, and MKN-45 cells
were treated for 48 h. In accordance with the manufacturer's
instructions, the cells were collected and stained with fluorescein
isothiocyanate (Annexin-V FITC) and propidium iodide (PI) in the
dark. The fluorescence intensity was measured using a FACSCanto II
flow cytometer (Becton Dickinson and Company, Franklin Lakes,
United States), and the apoptotic rates were analyzed using the
FACSDiva software (version 6.1.3; Becton Dickinson and Company,
Franklin Lakes, United States). Experiments were repeated in HGC-27
cells (n=7), MFC cells (n=6), and MKN-45 cells (n=5).
Detection of ROS levels
The changes in ROS levels were determined using a
ROS assay kit (CA1410, Solarbio, Beijing, China) and flow cytometry
(17). After luteolin treatment for
24 h in HGC-27 cells and MFC cells, and for 48 h in MKN-45 cells,
the cells were collected and washed with PBS. The cells were
labeled with 10 µM DCFH-DA for 30 min at 37°C in the dark in
accordance with the protocol. An inverted fluorescence microscope
(DMI3000, Leica, Wetzlar, Germany) was employed to detect
morphological changes. A FACSCanto II flow cytometer (Becton
Dickinson and Company, Franklin Lakes, United States) was applied
to determine the fluorescence intensity of the stained cells, which
was analyzed using the FACSDiva software (version 6.1.3; Becton
Dickinson and Company, Franklin Lakes, United States). The
fluorescence mean values derived from the flow cytometer in P2 were
used for quantitative analysis. The data were normalized as fold
changes in comparison to the DMSO group and presented as mean ±
standard deviation. Experiments were repeated in HGC-27 cells
(n=6), MFC cells (n=7), and MKN-45 cells (n=3).
Measurement of the mitochondrial
membrane potential (MMP, ΔΨm)
MMP changes were detected in the cells using an MMP
assay kit (M8650, Solarbio, Beijing, China) and flow cytometry
(19). The cells were treated with
10, 40, and 70 µM luteolin for 24 h or 48 h. Then, the treated
cells were resuspended in a complete media and stained with a JC-1
fluorescence working solution. MMP changes were measured with the
FACSCanto II flow cytometer (Becton Dickinson and Company, Franklin
Lakes, United States), and the fluorescence intensity was analyzed
using the FACSDiva software (version 6.1.3; Becton Dickinson and
Company, Franklin Lakes, United States). The relative ratio of red
fluorescence to green fluorescence was applied for quantitative
analysis of the MMP changes. The data were normalized as fold
changes in comparison to the DMSO group. Experiments were repeated
in HGC-27 cells (n=12), MFC cells (n=11), and MKN-45 cells
(n=4).
Measurement of cellular ATP
levels
Intracellular ATP was quantified using an ATP assay
kit (S0026, Beyotime Biotechnology, Shanghai, China) (20). After treatment, at least
5×106 cells were collected in each group, followed by
the addition of 300 ul of ATP lysis buffer. After centrifugation at
12,000 × g (Thermo Scientific™ Sorvall™ Legend™ Micro 17R
centrifuge; Thermo Fisher Scientific Inc., Massachusetts, United
States) at 4°C for 5 min, the supernatant was collected to examine
the ATP content and the protein level. The cell supernatant and ATP
standard solution were diluted to the necessary concentration with
the ATP lysis buffer. Then, 20 µl of the sample or standard
solution was blended with 100 µl of a reaction working agent, which
was tested in a 96-well plate. The fluorescence was assessed using
the Thermo 3001 multi-function microplate reader (Infinite 200 PRO,
Tecan Austria GmbH, Salzburg, Austria). Each sample was measured at
least in triplicate. Experiments were repeated in HGC-27 cells
(n=5), MFC cells (n=4), and MKN-45 cells (n=4). The data were
presented as fold changes normalized to the DMSO group.
Enzyme and protein extraction and
quantification
(i) Enzyme extraction was conducted as described
below. At least 5×106 cells were trypsinized for assays
in each group. The collected cells were lysed with the
corresponding enzymes' lysis buffer and ultrasonicated on ice
(power 20%, ultrasonicate 3 s, interval 10 s, repeat 30 times). The
quantification of enzymes protein was performed by using the BCA
protein assay kit (PC0020, Solarbio, Beijing, China). Data were
obtained with a microplate reader (Infinite 200 PRO, Tecan Austria
GmbH, Salzburg, Austria) for absorbance at 562 nm. The amount of
enzyme protein was calculated in accordance with the prescribed
computational formula of the kit protocol (PC0020, Solarbio,
Beijing, China).
(ii) Protein extraction was prescribed as follows
(21). Cells were lysed with
ice-cold RIPA buffer (R0010, Solarbio, Beijing, China) containing
0.1 M PMSF (P0100, Solarbio, Beijing, China) and a protease
phosphatase inhibitor (100×) (P1261, Solarbio, Beijing, China) and
incubated on ice for at least 30 min. Then, the cell lysate was
centrifuged at 12,000 × g (Thermo Scientific™ Sorvall™ Legend™
Micro 17R centrifuge; Thermo Fisher Scientific Inc., Massachusetts,
United States) for 10 min at 4°C, and the supernatant was evaluated
using the BCA protein assay kit (PC0020, Solarbio, Beijing, China).
The absorbance was measured at 562 nm with the microplate reader
(Infinite 200 PRO, Tecan Austria GmbH, Salzburg, Austria). The
amount of protein was calculated in accordance with the prescribed
computational formula of the kit protocol (PC0020, Solarbio,
Beijing, China). The cell lysate was adjusted to 6 µg/µl with the
lysate buffer and stored at −80°C.
Analysis of
Na+/K+-ATPase and
Ca2+/Mg2+-ATPase enzyme activities
The activities of
Na+/K+-ATPase and
Ca2+/Mg2+-ATPase were assessed using the
Na+/K+-ATPase enzyme activity assay kit
(BC0065, Solarbio, Beijing China) and
Ca2+/Mg2+-ATPase enzyme activity assay kit
(BC0965, Solarbio, Beijing China), respectively. The
Na+/K+-ATPase activity was evaluated by the
concentration of inorganic phosphate (Pi) formed upon ATP
hydrolysis in accordance with a previous report (22). At least 5×106 cells in
each group were collected for enzyme extraction. An enzyme
extraction reagent was added to the reaction mixture, and the data
on Na+/K+-ATPase activity were obtained using
the microplate reader (Infinite 200 PRO, Tecan Austria GmbH,
Salzburg, Austria) at 660 nm absorbance. Experiments were repeated
in HGC-27 cells (n=9) and MFC cells (n=16). Similarly,
Ca2+/Mg2+-ATPase activity was measured by
quantifying the Pi production from the conversion of ATP into ADP
at 660 nm using the molybdenum blue spectrophotometric method
(23). According to the protocol,
one enzyme activity unit (U) was defined as µmol of inorganic
phosphate liberated per 1×104 cells per hour, which was
quantified as results for each group. The data were presented as
fold changes normalized to the DMSO group. Experiments were
repeated in HGC-27 cells (n=10) and MFC cells (n=14).
Analysis of SOD activity
To detect the activity of SOD, at least
5×106 cells in each group were collected after
treatment. Then, SOD was extracted using an extracting solution
from the SOD activity assay kit (BC0175, Solarbio, Beijing, China)
(24). SOD activity was tested
according to the protocol and assessed using absorbance at 560 nm
with the Thermo 3001 microplate reader (Infinite 200 PRO, Tecan
Austria GmbH, Salzburg, Austria). SOD activity was calculated based
on the formula from the kit protocol. Relative SOD activity was
calculated by normalizing the SOD activity in each group to that in
the DMSO group. Each sample was measured at least in triplicate.
Experiments were repeated in HGC-27 cells (n=8), MFC cells (n=6),
and MKN-45 cells (n=5).
Analysis of enzyme activities of METC
complexes I, III, and V
To detect the activities of METC complexes I, III,
and V, at least 5×106 cells were lysed in each group to
obtain METC complexes, and the activities of complexes I, III, and
V were further detected by using METC complex I, III, and V
activity assay kits (BC0515, BC3245 and BC1440, Solarbio, Beijing,
China). METC complex I activity was measured by determining the
decrease in NADH absorbance at 340 nm that leads to the reduction
of ubiquinone to ubiquinol (25).
Cells were collected by scraping, and mitochondria were isolated on
ice using the mitochondria isolation lysis of the kit. In line with
the manufacturer's instructions, 10 µl of the enzyme extraction was
added to 190 µl of the reaction mixture in a 96-well plate. Each
sample was assessed in at least three replicates. The absorbance
values at 340 nm at 10 s and 2 min were recorded with the Thermo
3001 multifunction microplate reader (Infinite 200 PRO, Tecan
Austria GmbH, Salzburg, Austria). The activity was calculated using
the extinction coefficient of 6.22 mM−1 cm−1
for NADH and expressed as nmol/min/mg protein. Experiments were
repeated in HGC-27 cells (n=15), MFC cells (n=16), and MKN-45 cells
(n=6). METC complex III activity was measured by monitoring the
reduction of cytochrome c by ubiquinol at 550 nm (25). Similarly, mitochondria were isolated
on ice using the isolation lysis reagent from the kit. In line with
the manufacturer's instructions, 20 µl of the enzyme extraction was
added to 180 µl of different reaction mixture from the testing
group and the control group in a 96-well plate. The absorbance
values at 550 nm at 10 s and 2 min were recorded with the Thermo
3001 multi-function microplate reader (Infinite 200 PRO, Tecan
Austria GmbH, Salzburg, Austria). The activity was calculated using
the extinction coefficient of 1.91×104 l
mol−1 cm−1 for cytochrome c and expressed as
nmol/min/mg protein. Experiments were repeated in HGC-27 cells
(n=6) and MFC cells (n=5). METC complex V was determined with a
mitochondrial ATP-synthase assay (25). According to the instructions, the
enzyme extraction was mixed with reagents for the quantitative
determination of phosphorus. The ATP-synthase activity was
determined as the difference between the activities obtained in the
presence and absence of oligomycin. The absorbance value at 660 nm
was recorded using the Thermo 3001 multi-function microplate reader
(Infinite 200 PRO, Tecan Austria GmbH, Salzburg, Austria), and the
results were calculated as nmol Pi/mg protein. Experiments were
repeated in HGC-27 cells (n=14), MFC cells (n=14), and MKN-45 cells
(n=4). All data were presented as fold changes normalized to the
DMSO group.
Western blot
After the denaturation of total protein, 60 µg cell
lysate from each group were separated by SDS-PAGE (a 6% spacer gel
and a 10% separating gel) and transferred to a PVDF membrane
(ISEQ00010, Millipore Sigma) with 200 mA constant current (Biorad
Powerpac Basic 164-5050, Bio-Rad Laboratories Inc, California,
United States) on ice for 2 h (17). The membranes were then blocked with
5% milk in Tris-buffered saline with Tween 20 (TBST) solution for 2
h and washed with TBST for 5 min for three times. Then, the blocked
membranes were incubated in a primary antibody solution at 4°C
overnight. The primary antibodies in this work were: β-actin mouse
monoclonal antibody (cat. no. TA-09; 1:2,000; OriGene Technologies
Inc., Beijing, China), Bcl-2 rabbit monoclonal antibody (cat. no.
ab182858; 1:1,000; Abcam, Cambridge, UK), and Bax rabbit monoclonal
antibody (cat. no. ab182733; 1:1,000; Abcam, Cambridge, UK). The
membranes were rinsed with TBST for 10 min three times and probed
with the secondary antibody (peroxidase-conjugated goat anti-mouse
IgG (H+L) (cat. no. ZB-2305; 1:50,000; OriGene Technologies Inc.,
Beijing, China) and goat anti-rabbit IgG (H&L) (cat. no.
ab6721; 1:20,000; Abcam, Cambridge, UK)) at room temperature for 50
min. After rinsing with the TBST solution, the membranes were
detected with the ECL chemiluminescence kit (WF326284, Thermo
Fisher Scientific Inc., Massachusetts, United States), and
visualized with the gel imaging analysis system (BioSpectrum 510
Imaging System Motorized Platform). Scanning grey analysis was
performed using the Photoshop CC 2019 software (Adobe Systems Inc.,
California, United States). The grayscale value of each band was
normalized to its corresponding β-actin. All data were presented as
fold changes normalized to the DMSO group, and used to plot
histograms. Experiments were repeated in HGC-27 cells (n=6), MFC
cells (n=5), and MKN-45 cells (n=5).
Statistical analysis
The experiments were carried out at least three
times. Data of enzymes activities were analyzed in accordance with
the prescribed calculation formula on the kit protocol. The data
were normalized as fold changes to DMSO group and presented as
means ± standard deviation. The analyses were performed using the
SPSS 21.0 software package (version 21.0, SPSS Inc, Chicago, United
States) and drew by the GraphPad Prism 6.0 software (version 6.0,
GraphPad Software Inc., San Diego, United States). Statistical
difference was calculated by ANOVA followed by Tukey's post hoc
test. P-values less than 0.05 were considered statistically
significant.
Results
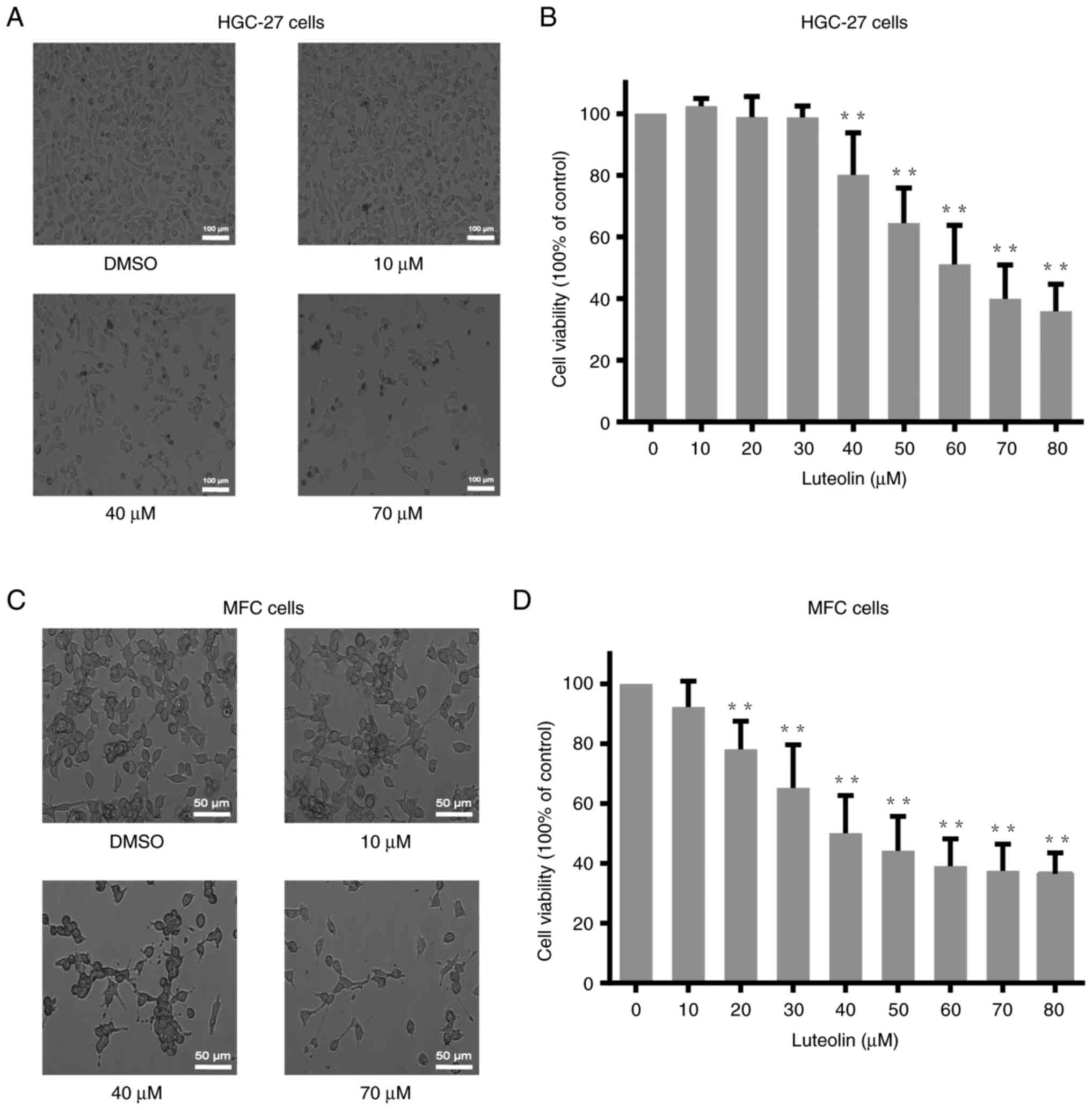
Luteolin decreased gastric cancer
cells viability
After treatments with different doses of luteolin
(0, 10, 20, 30, 40, 50, 60, 70, and 80 µM) for 24 h or 48 h, the
viability of HGC-27, MFC, and MKN-45 cells were analyzed using the
CCK-8 assay. The viability of HGC-27 and MFC cells decreased in a
dose-dependent manner (Fig. 1B and
D), and the viability of MKN-45 cells also reduced in a
dose-dependent manner (Fig. S1B).
According to the cell viability analysis, the cell viability curve
is created. Based on the curve, the IC50 value of
luteolin for HGC-27 and MFC cells was approximately 60 and 40 µM,
respectively. And the IC50 for MKN-45 at 48 h was about
50 µM. The morphological changes were observed under a light
microscope, illustrating a notably reduced cell number (Figs. 1A, C, and S1A). Moreover, there are several dark
speckles with lower refractive index in 40 and 70 µM, which are
different with the cells in DMSO group. Due to the luteolin
treatment, the cell morphology become irregular.
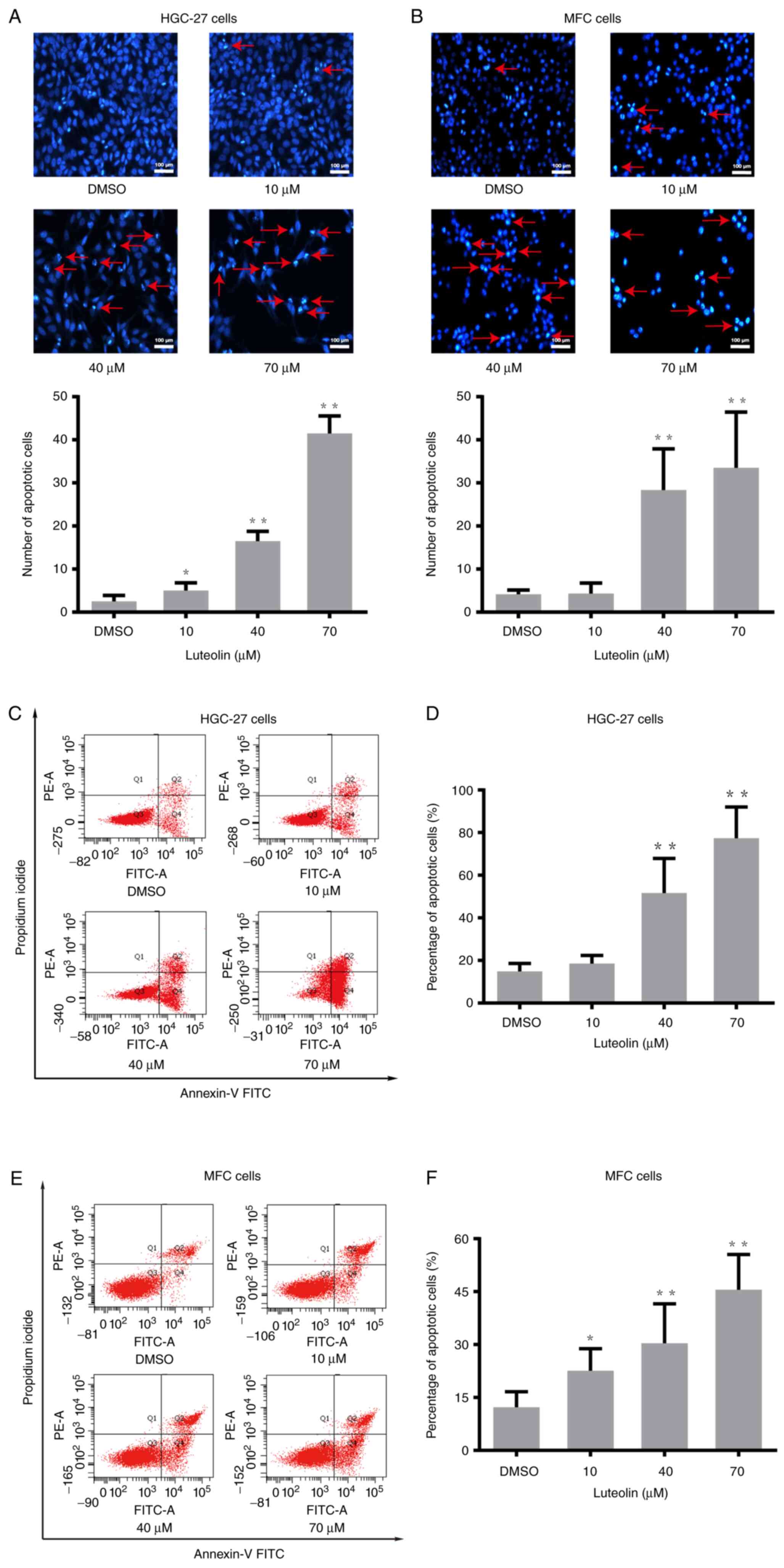
To explore whether apoptosis was involved in the
reduction of cell number induced by luteolin, morphology assessment
and flow cytometry were used to detect apoptosis. HGC-27 and MFC
cells were treated with luteolin (10, 40, and 70 µM) for 24 h, and
MKN-45 cells were treated for 48 h. Morphological features of
luteolin-induced apoptosis were detected by Hoechst 33258 staining
on a fluorescence inversion microscope system. As shown in Figs. 2A, B, and S2A, apparent morphological features, such
as karyopyknosis, nucleosome and chromosome condensation, were
observed in the luteolin groups, while fewer apoptotic
characteristics were found in the DMSO group. HGC-27, MFC, and
MKN-45 cells stained with Annexin-V FITC and PI were quantified by
flow cytometry. The percentage of apoptotic cells increased in a
concentration-dependent manner in HGC-27 cells (Fig. 2C and D), MFC cells (Fig. 2E and F), and MKN-45 cells (Fig. S2B and S2C). These results showed that apoptosis
might be largely involved in the inhibition of HGC-27, MFC, and
MKN-45 cells' proliferation induced by luteolin.
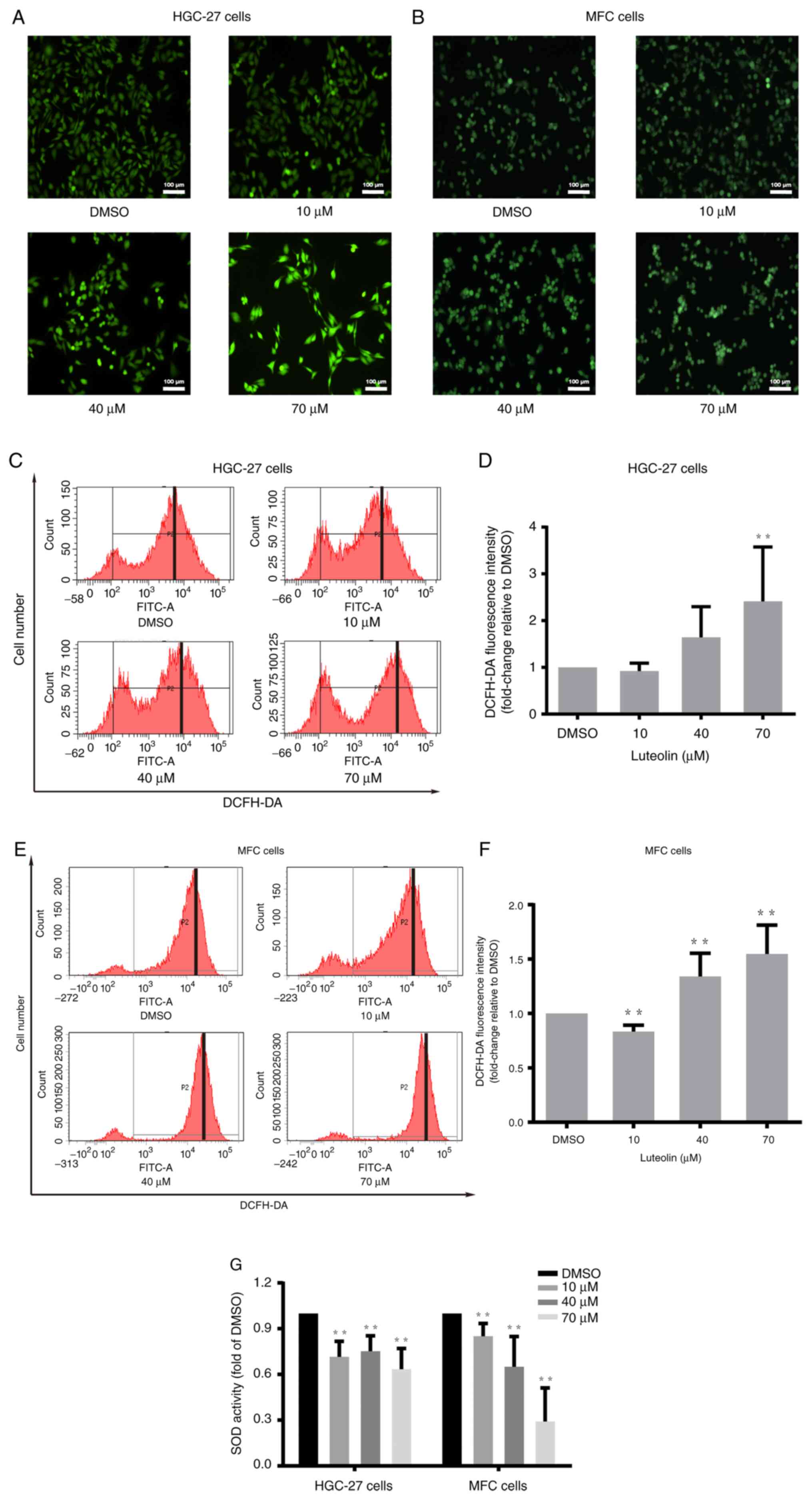
Luteolin influenced ROS accumulation
in gastric cancer cells
Oxidative stress plays a crucial role in cancer
pathophysiology (4). ROS can cause
the apoptosis of cancer cells via oxidative stress (26). Luteolin, as a flavonoid, regulates
the cellular redox state. DCFH-DA staining and flow cytometry were
performed to illustrate luteolin-induced ROS accumulation in
HGC-27, MFC and MKN-45 cells. HGC-27 and MFC cells were treated
with luteolin (10, 40, and 70 µM) for 24 h, and MKN-45 cells were
treated for 48 h. There was an obvious increase in green
fluorescence in the luteolin groups (Figs. 3A, B, and S3A). Moreover, the peak moved to the
righter with the increase of the luteolin dose in HGC-27 cells
(Fig. 3C), MFC cells (Fig. 3E), and MKN-45 (Fig. S3B). The data suggested that ROS
significantly increased after exposure of HGC-27 cells (Fig. 3D) and MFC cells (Fig. 3F) to luteolin. It was also observed
that ROS remarkably increased in the high dose of luteolin in
MKN-45 cells (Fig. S3C). In
addition, an SOD activity test suggested that luteolin could induce
SOD activity reduction, especially in the high dose of luteolin
groups in HGC-27 and MFC cells (Fig.
3G) and in MKN-45 cells (Fig.
S3D). Thus, it was inferred that ROS played a significant role
in luteolin-induced apoptosis in HGC-27, MFC, and MKN-45 cells and
that the increase in ROS was related to the decreased activity of
antioxidative enzymes, especially SOD.
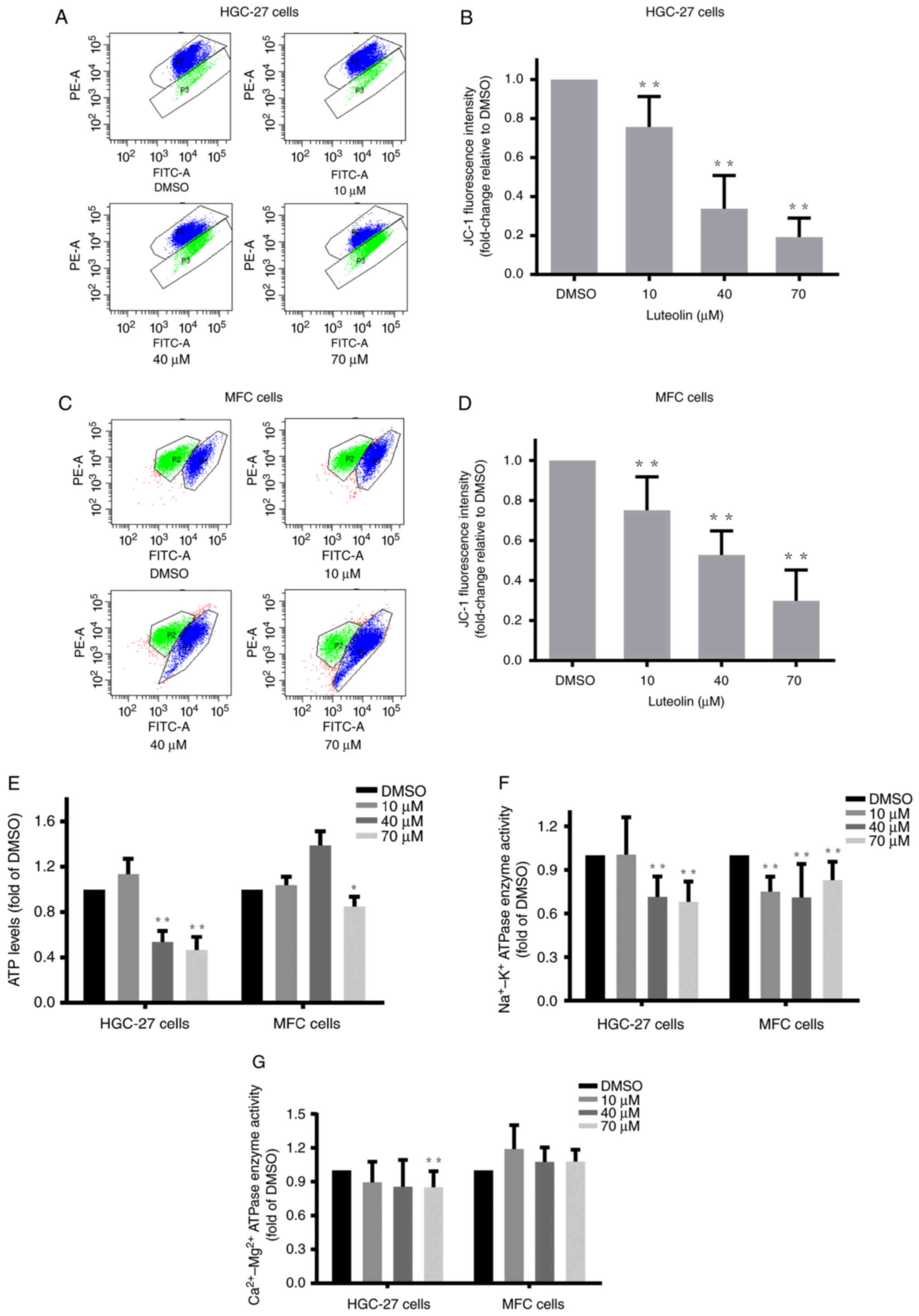
Luteolin impaired the mitochondrial
potential, ATP generation and enzyme activities in gastric cancer
cells
Intracellular ROS overaccumulation is one of the
characteristics of mitochondrial dysfunction (27). Mitochondrial dysfunction directly
leads to apoptosis (28). Here, we
explored the effects of luteolin on main mitochondrial functions,
such as mitochondrial membrane potential, energy metabolism, and
key protein expression levels, in gastric cancer cells.
Luteolin-treated HGC-27, MFC, and MKN-45 cells were stained with
JC-1 and analyzed using flow cytometry. The flow cytometry results
revealed that the ratio of red/green fluorescence dramatically
decreased in the luteolin groups (Figs.
4A, C, and S4A), indicating
that luteolin effectively decreased the mitochondrial membrane
potential of HGC-27 cells (Fig.
4B), MFC cells (Fig. 4D), and
MKN-45 cells (Fig. S4B). Next, an
ATP content assay was carried out to examine the effects of
luteolin on mitochondrial energy metabolism. ATP content decreased,
especially in the high-dose groups (Figs. 4E and S4C). These results demonstrated that
luteolin could remarkably reduce ATP levels, suggesting that
luteolin produced deleterious effects on mitochondrial energy
generation in HGC-27, MFC, and MKN-45 cells. Following the decrease
in ATP contents, luteolin effectively downregulated plasma
membrane-bound Na+/K+-ATPase (Fig. 4F) and
Ca2+/Mg2+-ATPase (Fig. 4G) enzyme activities, especially in
the high-dose groups in HGC-27 cells, indicating that luteolin
impaired the membrane permeability.
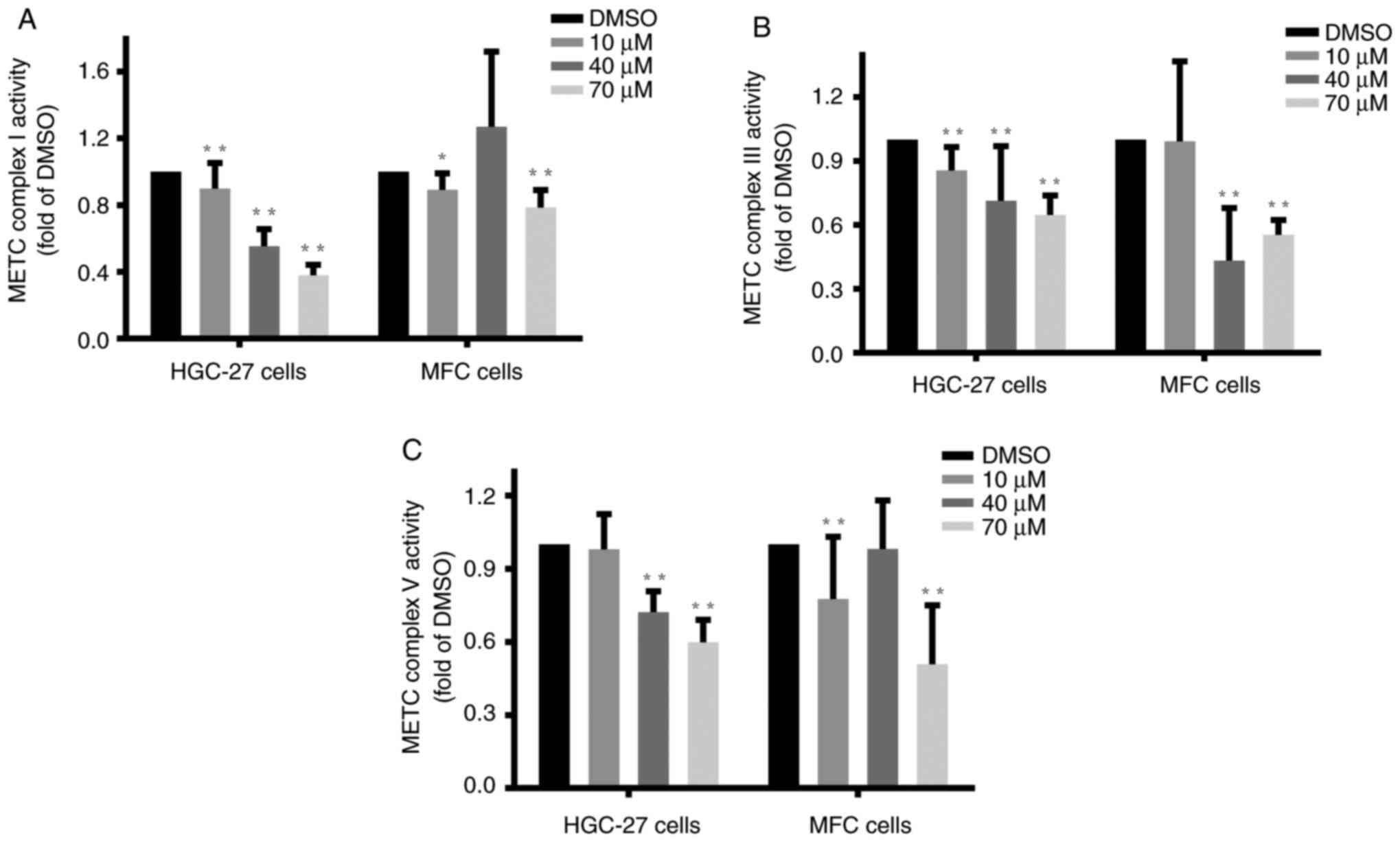
Luteolin inhibited the activities of
key enzymes of the METC in gastric cancer cells
The METC complexes, located at the inner
mitochondrial membrane, are coupled with respiratory electron
transfer and ATP synthesis (29).
Complexes I and III are closely associated with ROS generation,
whereas complex V is primarily responsible for ATP production.
Complexes I, III, and V play critical roles in cancer cell
apoptosis (30). HGC-27, MFC, and
MKN-45 cells at the logarithmic phase were treated with luteolin as
mentioned previously, and specific assay kits were employed to
examine the changes in the activities of complexes I (31), III, and V (25). The enzymes activities of complex I
(Figs. 5A and S5A), complex III (Fig. 5B), and complex V (Figs. 5C and S5B) significantly decreased compared with
the DMSO group, indicating that luteolin exerted inhibitory effects
on the activities of METC complexes I, III, and V, especially in
the high dose of luteolin groups in HGC-27, MFC, and MKN-45 cells.
These findings suggested that luteolin could exert inhibitory
effects on METC complex activities in HGC-27 and MFC cells in a
dose-dependent manner. And high dose of luteolin could effectively
inhibit METC complexes I and V activities in MKN-45 cells.
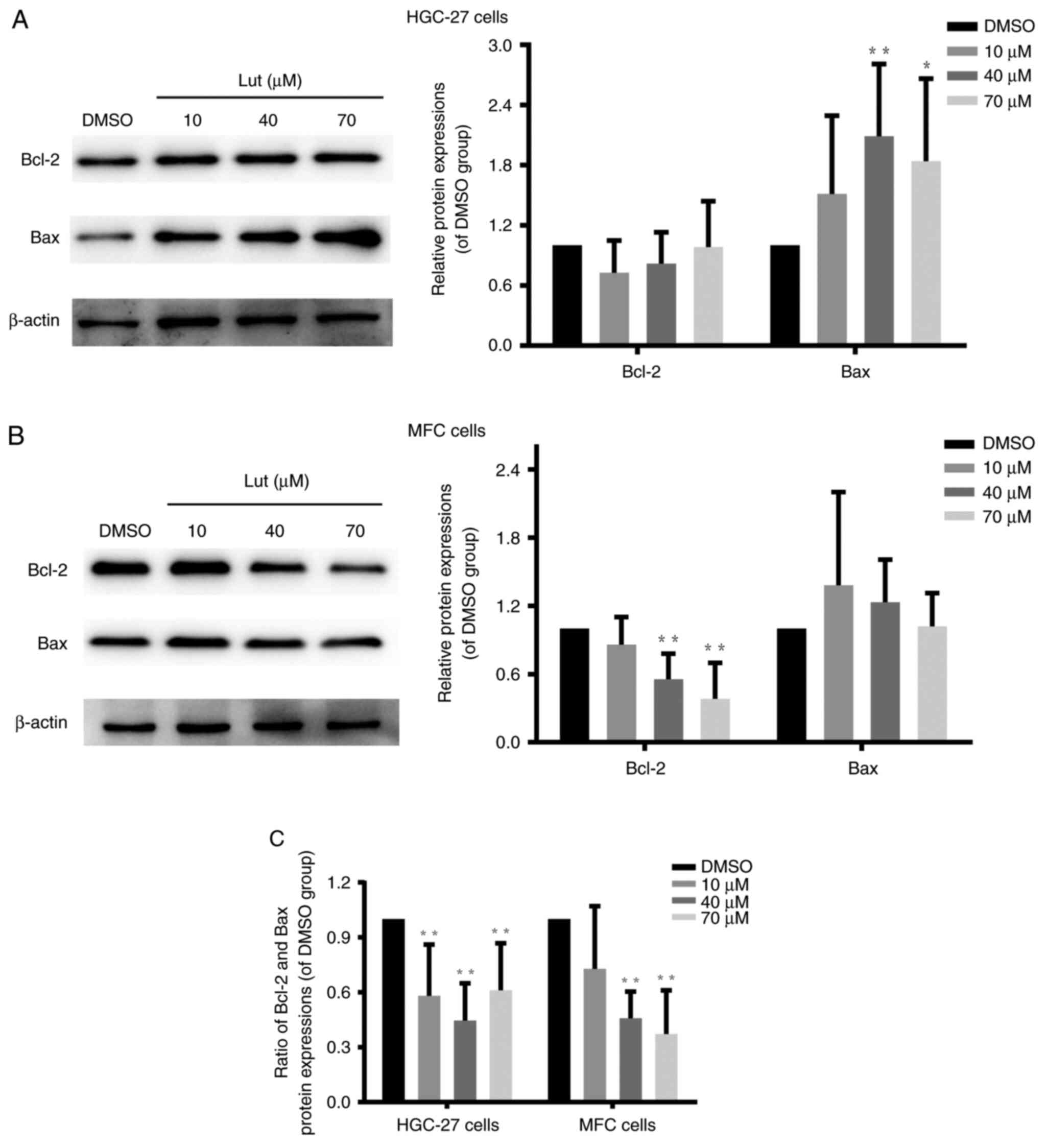
Luteolin unbalanced Bcl-2 and Bax
protein expression in gastric cancer cells
Bcl-2 family members are involved in modulating the
mitochondrial permeability transition (32) and regulating the integrity and
function of the outer mitochondrial membrane (33). Bcl-2 and Bax are core regulators of
the intrinsic pathway of apoptosis in response to stress stimuli
(33). Further, they play important
roles in regulating the permeabilization of the outer mitochondrial
membrane (34). Western blot
findings demonstrated that luteolin significantly decreased the
ratio between Bcl-2 and Bax in HGC-27, MFC, and MKN-45 cells
(Figs. 6C and S6B) by downregulating Bcl-2 expression
(Figs. 6B and S6A) and/or upregulating Bax expression
(Figs. 6A and S6A). Thus, these results verified that
luteolin exerted destructive effects on mitochondrial function in
HGC-27, MFC, and MKN-45 cells by unbalancing the expression of
Bcl-2 and Bax proteins.
Discussion
Chemical compounds in natural extracts may
reportedly have potential as therapeutic agents for gastric cancer
(35). Luteolin is a natural
ingredient found in a variety of fruits, vegetables, and herbs. The
cost of luteolin has been greatly reduced, as it can now be
extracted from peanut shells, which is economical for mass
production and clinical practice (36). Therefore, several studies have been
conducted to reveal many biological effects of luteolin, including
anti-inflammatory (37),
antioxidative (38), analgesic
(39), and anticancer activities
(4). Our study demonstrated that
luteolin exerted significant inhibitory effects on the
proliferation of HGC-27, MFC, and MKN-45 gastric cancer cells.
Luteolin treatment-induced distinct morphological and biochemical
features associated with apoptosis in HGC-27, MFC, and MKN-45
cells, such as chromatin condensation, membrane surface blebbing,
and formation of apoptotic bodies. Thus, apoptosis is the main
mechanism of luteolin-induced inhibition of cell proliferation. We
also found that luteolin could effectively interfere with the redox
state of gastric cancer cells. Namely, with an increased dose of
luteolin, rising ROS levels were observed in HGC-27 and MFC cells.
And high dose of luteolin could induce ROS increase in MKN-45
cells. It is indicated that the effect of luteolin on stimulating
ROS increase is various in different cell lines. As the most
significant signaling molecules, ROS participate in regulating many
biological processes, such as redox state changes and apoptosis.
Low ROS levels are necessary to maintain cellular signaling
processes (40). In contrast, a
sudden and substantial increase in ROS levels commits cells to
apoptosis (28). Presumably,
luteolin could induce gastric cancer cells apoptosis by elevating
ROS levels. Luteolin's properties are probably predetermined by its
chemical structure, which includes four phenolic hydroxyl groups in
C5 and C7 of the benzene A ring and C3′ and C4′ of the benzene B
ring. Luteolin's antioxidant activity has been attributed to the
ortho-dihydroxy structure of its B-ring and the 2,3-double bond in
conjugation with the 4-oxo function of the C ring (4,41).
The ΔΨm collapse has been associated with
apoptosis-related mitochondrial fragmentation, which is considered
an irreversible point in the death signaling cascade (42). In this study, luteolin was shown to
damage ΔΨm as reflected by the decreased ratio of red/green
fluorescence found in all luteolin groups of HGC-27, MFC, and
MKN-45 cells. Several reports have shown that the sudden ΔΨ
decrease with a corresponding rise in ROS generation may be
attributed to mitochondrial permeability transition pore (mPTP)
induction (43). mPTP is a
transmembrane channel formed at the contact sites between the inner
and outer mitochondrial membranes (44). There is evidence that brief mPTP
openings are critical to maintaining healthy mitochondrial
homeostasis. However, when ROS accumulation exceeds the threshold,
it leads to longer mPTP openings, resulting in an ROS burst release
(43). This regenerative cycle of
mitochondrial ROS formation and release was defined as ROS-induced
ROS release (RIRR) (43). Longer
mPTP openings possibly play a physiological role in
luteolin-induced gastric cancer apoptosis. The mPTP formation leads
to the mitochondrial permeability transition (MPT), which was
modulated by Bcl-2 family members (32). The Bcl-2 family proteins, including
antiapoptotic (Bcl-2) and proapoptotic (Bax) members, can form ion
channels when incorporated into synthetic lipid bilayers (33). mPTP is regulated by proapoptoic and
antiapoptotic Bcl-2 family proteins, such as Bax and Bcl-2, through
the composition of the voltage-dependent anion channel (VDAC)
(45). Moreover, members of the
Bcl-2 family, such as Bcl-2 and Bax, are involved in regulating the
integrity and function of the outer mitochondrial membrane
(33). Unbalanced expressions of
Bcl-2 and Bax proteins is involved in the apoptosis induced by
luteolin in cancer cells (46). Our
data demonstrated that luteolin remarkably decreased the ratio of
Bcl-2 and Bax by downregulating Bcl-2 protein expression and/or
upregulating Bax protein expression in three gastric cancer cell
lines. Therefore, we inferred that luteolin might induce longer
mPTP formation and destroy the outer mitochondrial membrane by
unbalancing Bcl-2 and Bax protein expression in HGC-27, MFC, and
MKN-45 cells, leading to apoptosis.
Luteolin induced ΔΨm decrease and ROS increase,
resulting in disrupting the proton-motive force. Then, the
integrity of the inner mitochondrial membrane is impaired, and the
oxidative phosphorylation is uncoupled (47). METCs are located on the inner
mitochondrial membrane, where complexes I, III, and IV are proton
pumps, while CoQ and cytochrome c are electron carriers (13). METC is the central player in the
chemiosmotic theory, in which the proton circuit across the inner
mitochondrial membrane actuates the oxidative phosphorylation,
coupling substrate oxidation and adenosine 5′-diphosphate (ADP)
phosphorylation (29). Substrate
oxidation releases electrons to cofactors, such as nicotinamide
adenine dinucleotide (NADH) or 1,5-dihydroflavin adenine
dinucleotide (FADH2). These electrons pass through electron
carriers of METC complexes with increasing oxidation potentials,
ultimately reducing molecular oxygen to water (48). Electrons carried by NADH are
transferred to the flavin mononucleotide (IF) site in
complex I, where they are normally passed down the chain of Fe-S
centers to the ubiquinone-binding site (IQ). At both the
IF and IQ sites, electrons react with
O2, forming superoxide (O2•−)
within the mitochondrial matrix (13,49).
In complex III, QH2 binds to the QO site, and
electrons are transferred in the Q-cycle and react directly with
oxygen to form superoxide that is released to both sides of the
inner mitochondrial membrane (50).
In our study, luteolin downregulated the activities
of complexes I and III in HGC-27 and MFC cells, resulting in
luteolin-induced ROS generation and mitochondrial dysfunction. And
high dose of luteolin could exert more effective inhibition on
complex I in MKN-45 cells. This might have occurred, because
luteolin inhibited enzyme activities by binding to the
quinone-binding site of the complexes, backing up electrons in the
chain of Fe-S clusters and leading to rapid ROS generation
(51). In addition to luteolin,
plant-derived chemicals with anticancer activity directed against
METC complexes include resveratrol (52), xanthohumol (14), and deguelin (53). As inhibition of METC complexes is
associated with increased ROS production, targeting METC complexes
to increase ROS production is a plausible and intriguing strategy
to eliminate cancer cells with relatively high specificity
(28). Moreover, the ATP level was
reduced in the high dose of luteolin groups. In line with the ATP
content, METC complex V activity was remarkably reduced by
luteolin. ATP is mainly derived from the METC complex V, which can
convert the proton gradient created by METC complexes I–IV into ATP
by phosphorylating ADP (28).
Therefore, decreased METC complex V activity induced by luteolin is
the leading cause of ATP reduction, which can impair mitochondrial
function. ATP downregulation also reduces ATP-dependent
Na+/K+-ATPase and
Ca2+/Mg2+-ATPase enzyme activities,
destroying cellular membrane permeability and promoting the
apoptosis. This suggests that luteolin may impair mitochondrial
function and membrane ionic equilibrium by increasing ROS and
decreasing ATP synthesis, where METC downregulation is
indispensable.
In addition, it is reported that luteolin could
induce cell cycle arrest and apoptosis through extrinsic and
intrinsic signaling pathways in MCF-7 breast cancer cell (46). The cells from different tissues have
different reaction to luteolin treatment. And different tissues
trigger different signaling pathway induced by luteolin. We tested
the ROS change, ATP content, and mitochondrial electron transport
chain complexes activities, which are the indicators of
mitochondrial function. Our study found that luteolin could
downregulate the activities of mitochondrial electron transport
chain complexes, which are distinctively important for cell
survival, cell carcinogenesis, and cell energy supply. So we
propose that luteolin induces the apoptosis by interfering the
cellular energy metabolism of the mitochondria in gastric cancer
cells.
This study was conducted in vitro to
demonstrate the anti-gastric cancer effects of luteolin and to
reveal the underlying mechanism. Luteolin unbalanced ROS levels and
ATP generation by destroying the mitochondrial membrane potential
and downregulating the enzyme activities of METC complexes (mainly
complexes I, III, and V). Luteolin also impaired mitochondrial
integrity and function by unbalancing the protein expression of
Bcl-2 family members (Bcl-2 and Bax), eventually inducing apoptosis
of gastric cancer cells. Therefore, the intrinsic apoptosis pathway
was involved in luteolin's anti-gastric cancer effects, and
mitochondria were the main target in luteolin-induced gastric
cancer apoptosis.
Supplementary Material
Supporting Data
Acknowledgements
This study was conducted in the laboratory of the
Taishan Scholars Construction Engineering of Shandong Province and
the Yantai High-End Talent Introduction Plan ‘Double Hundred’,
(Yantai, China).
Funding
This study was supported by the National Natural Science
Foundation of China (grant no. 31471338), the Key Research and
Development Program of Shandong Province of China (grant no.
2019GSF108214) and the Natural Science Foundation of Shandong
Province (grant no. ZR2016HB51).
Availablity of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JM conceived the work and wrote the manuscript. JM
carried out the experiments and analyzed the data. ZP provided the
assistance on the experiments. XC and XZ performed the data
analysis and provided technical support. HD and WH offered guidance
and support, and assisted in the acquisition of data. QZ and XT
designed the experiments and reviewed the manuscript. All authors
read and approved the final manuscript. JM and QZ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Bax
|
Bcl-2-associated X
|
|
Bcl-2
|
B cell lymphoma-2
|
|
HGC-27
|
human gastric cancer HGC-27 cell
line
|
|
METC
|
mitochondrial electron transport
chain
|
|
MFC
|
mouse forestomach carcinoma cell
line
|
|
MMP
|
mitochondrial membrane potential
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Xiao S and Zhou L: Gastric cancer:
Metabolic and metabolomics perspectives (Review). Int J Oncol.
51:5–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song Z, Wu Y, Yang J, Yang D and Fang X:
Progress in the treatment of advanced gastric cancer. Tumour Biol.
39:10104283177146262017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Falah M, Rayan M and Rayan A: A novel
paclitaxel conjugate with higher efficiency and lower toxicity: A
new drug candidate for cancer treatment. Int J Mol Sci.
20:49652019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Imran M, Rauf A, Abu-Izneid T, Nadeem M,
Shariati MA, Khan IA, Imran A, Orhanh IE, Rizwan M, Atif M, et al:
Luteolin, a flavonoid, as an anticancer agent: A review.
Biomedicine Pharmacotherapy. 112:1086122019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tesio AY and Robledo SN: Analytical
determinations of luteolin. Biofactors. 47:141–164. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tuorkey MJ: Molecular targets of luteolin
in cancer. Eur J Cancer Prev. 25:65–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mani S, Swargiary G and Singh KK: Natural
agents targeting mitochondria in cancer. Int J Mol Sci.
21:69922020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gong G, Jiao Y, Pan Q, Tang H, An Y, Yuan
A, Wang K, Huang C, Dai W, Lu W, et al: Antitumor effect and
toxicity of an albumin-paclitaxel nanocarrier system constructed
via controllable alkali-induced conformational changes. ACS
Biomater Sci Eng. 5:1895–1906. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu X, Lai Y and Hua ZC: Apoptosis and
apoptotic body: Disease message and therapeutic target potentials.
Biosci Rep. 39:BSR201809922019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y, He PY, Zhang Y and Li N: Natural
products targeting the mitochondria in cancers. Molecules.
26:922020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kalpage HA, Wan J, Morse PT, Zurek MP,
Turner AA, Khobeir A, Yazdi N, Hakim L, Liu J, Vaishnav A, et al:
Cytochrome c phosphorylation: Control of mitochondrial electron
transport chain flux and apoptosis. Int J Biochem Cell Biol.
121:1057042020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He C, Jiang S, Jin H, Chen S, Lin G, Yao
H, Wang X, Mi P, Ji Z, Lin Y, et al: Mitochondrial electron
transport chain identified as a novel molecular target of SPIO
nanoparticles mediated cancer-specific cytotoxicity. Biomaterials.
83:102–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao RZ, Jiang S, Zhang L and Yu ZB:
Mitochondrial electron transport chain, ROS generation and
uncoupling (Review). Int J Mol Med. 44:3–15. 2019.PubMed/NCBI
|
|
14
|
Zhang B, Chu W, Wei P, Liu Y and Wei T:
Xanthohumol induces generation of reactive oxygen species and
triggers apoptosis through inhibition of mitochondrial electron
transfer chain complex I. Free Radic Biol Med. 89:486–497. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Q, Wang L, Liu J, Cao WL, Pan Q and
Li M: Targeting the complex I and III of mitochondrial electron
transport chain as a potentially viable option in liver cancer
management. Cell Death Discovery. 7:2932021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan Y, Zhai Y, Chen J, Xu X and Wang H:
Kaempferol ameliorates oxygen-glucose
deprivation/reoxygenation-induced neuronal ferroptosis by
activating Nrf2/SLC7A11/GPX4 axis. Biomolecules. 11:9232021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Pan Z, Cheng XL, Zhang K, Zhang X,
Qin Y, Fan J, Yan T, Han T, Shiu KK, et al: A red-light-activated
sulfonamide porphycene for highly efficient photodynamic therapy
against hypoxic tumor. Eur J Med Chem. 209:1128672021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu T, Linghu K, Huang S, Battino M,
Georgiev MI, Zengin G, Li D, Deng Y, Wang YT and Cao H: Flaxseed
extract induces apoptosis in human breast cancer MCF-7 cells. Food
Chem Toxicol. 127:188–196. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Qin Y, Pan Z, Li M, Liu X, Chen
X, Qu G, Zhou L, Xu M, Zheng Q and Li D: Cannabidiol induces cell
cycle arrest and cell apoptosis in human gastric cancer SGC-7901
cells. Biomolecules. 9:3022019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lei L, Zhu Y, Gao W, Du X, Zhang M, Peng
Z, Fu S, Li X, Zhe W, Li X and Liu G: Alpha-lipoic acid attenuates
endoplasmic reticulum stress-induced insulin resistance by
improving mitochondrial function in HepG2 cells. Cell Signal.
28:1441–1450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan Z, Luo Y, Xia Y, Zhang X, Qin Y, Liu
W, Li M, Liu X, Zheng Q and Li D: Cinobufagin induces cell cycle
arrest at the S phase and promotes apoptosis in nasopharyngeal
carcinoma cells. Biomed Pharmacother. 122:1097632020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dergousova EA, Petrushanko IY, Klimanova
EA, Mitkevich VA, Ziganshin RH, Lopina OD and Makarov AA:
Enhancement of Na,K-ATPase activity as a result of removal of redox
modifications from cysteine residues of the a1 subunit: The effect
of reducing agents. Mol Biol (Mosk). 52:247–250. 2018.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin J, Zhao HS, Xiang LR, Xia J, Wang LL,
Li XN, Li JL and Zhang Y: Lycopene protects against
atrazine-induced hepatic ionic homeostasis disturbance by
modulating ion-transporting ATPases. J Nutr Biochem. 27:249–256.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Y, Li J, Wei C, He Y, Cao Y, Zhang Y,
Sun W, Qiao B and He J: Amelioration of nonalcoholic fatty liver
disease by swertiamarin in fructose-fed mice. Phytomedicine.
59:1527822019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
OuYang Q, Tao N and Zhang M: A damaged
oxidative phosphorylation mechanism is involved in the antifungal
activity of citral against Penicillium digitatum. Front
Microbiol. 9:2392018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanikoglu A, Ozben H, Hanikoglu F and
Ozben T: Hybrid compounds & oxidative stress induced apoptosis
in cancer therapy. Curr Med Chem. 27:2118–2132. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Y, Karakhanova S, Hartwig W, D'Haese
JG, Philippov PP, Werner J and Bazhin AV: Mitochondria and
mitochondrial ROS in cancer: Novel targets for anticancer therapy.
J Cell Physiol. 231:2570–2581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu Y, Dean AE, Horikoshi N, Heer C, Spitz
DR and Gius D: Emerging evidence for targeting mitochondrial
metabolic dysfunction in cancer therapy. J Clin Invest.
128:3682–3691. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo R, Gu J, Zong S, Wu M and Yang M:
Structure and mechanism of mitochondrial electron transport chain.
Biomed J. 41:9–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo Y, Ma J and Lu W: The significance of
mitochondrial dysfunction in cancer. Int J Mol Sci. 21:55982020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu H, Zhang W, Zhao Y, Shu X, Wang W,
Wang D, Yang Y, He Z, Wang X and Ying Y: GSK3β-mediated tau
hyperphosphorylation triggers diabetic retinal neurodegeneration by
disrupting synaptic and mitochondrial functions. Mol Neurodegener.
13:622018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Means RE and Katz SG: Balancing life and
death: BCL-2 family members at diverse ER-mitochondrial contact
sites. FEBS J. 289:7075–7112. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Edlich F: BCL-2 proteins and apoptosis:
Recent insights and unknowns. Biochem Biophys Res Commun.
500:26–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peña-Blanco A and García-Sáez AJ: Bax, bak
and beyond-mitochondrial performance in apoptosis. FEBS J.
285:416–431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee YK, Bae K, Yoo HS and Cho SH: Benefit
of adjuvant traditional herbal medicine with chemotherapy for
resectable gastric cancer. Integr Cancer Ther. 17:619–627. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sheng S, Zhang L and Chen G: Determination
of 5,7-dihydroxychromone and luteolin in peanut hulls by capillary
electrophoresis with a multiwall carbon nanotube/poly(ethylene
terephthalate) composite electrode. Food Chem. 145:555–561. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang XF, Zhang JL, Huang DP, Huang AS,
Huang HT, Liu Q, Liu XH and Liao HL: A network pharmacology
strategy to investigate the anti-inflammatory mechanism of luteolin
combined with in vitro transcriptomics and proteomics. Int
Immunopharmacol. 86:1067272020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gendrisch F, Esser PR, Schempp CM and
Wölfle U: Luteolin as a modulator of skin aging and inflammation.
BioFactors. 47:170–180. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hashemzaei M, Abdollahzadeh M, Iranshahi
M, Golmakani E, Rezaee R and Tabrizian K: Effects of luteolin and
luteolin-morphine co-administration on acute and chronic pain and
sciatic nerve ligated-induced neuropathy in mice. J Complement
Integr Med. 14:2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fruehauf JP and Meyskens FL Jr: Reactive
oxygen species: A breath of life or death? Clin Cancer Res.
13:789–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zheng YZ, Chen DF, Deng G, Guo R and Fu
ZM: The surrounding environments on the structure and antioxidative
activity of luteolin. J Mol Model. 24:1492018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lu J, Wu L, Wang X, Zhu J, Du J and Shen
B: Detection of mitochondria membrane potential to study CLIC4
knockdown-induced HN4 cell apoptosis in vitro. J Vis Exp.
563172018.PubMed/NCBI
|
|
43
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bauer TM and Murphy E: Role of
mitochondrial calcium and the permeability transition pore in
regulating cell death. Circ Res. 126:280–293. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dudko HV, Urban VA, Davidovskii AI and
Veresov VG: Structure-based modeling of turnover of Bcl-2 family
proteins bound to voltage-dependent anion channel 2 (VDAC2):
Implications for the mechanisms of proapoptotic activation of bak
and bax in vivo. Comput Biol Chem. 85:1072032020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Park SH, Ham S, Kwon TH, Kim MS, Lee DH,
Kang JW, Oh SR and Yoon DY: Luteolin induces cell cycle arrest and
apoptosis through extrinsic and intrinsic signaling pathways in
MCF-7 breast cancer cells. J Environ Pathol Toxicol Oncol.
33:219–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Beutner G, Alavian KN, Jonas EA and Porter
GA Jr: The mitochondrial permeability transition pore and ATP
synthase. Handb Exp Pharmacol. 240:21–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fernandez-Vizarra E and Zeviani M:
Mitochondrial disorders of the OXPHOS system. FEBS Lett.
595:1062–1106. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cogliati S, Lorenzi I, Rigoni G, Caicci F
and Soriano ME: Regulation of mitochondrial electron transport
chain assembly. J Mol Biol. 430:4849–4873. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Affourtit C, Wong HS and Brand MD:
Measurement of proton leak in isolated mitochondria. Methods Mol
Biol. 1782:157–170. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Larosa V and Remacle C: Insights into the
respiratory chain and oxidative stress. Bioscience Reports.
38:BSR201714922018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
de Oliveira MR, Nabavi SF, Manayi A,
Daglia M, Hajheydari Z and Nabavi SM: Resveratrol and the
mitochondria: From triggering the intrinsic apoptotic pathway to
inducing mitochondrial biogenesis, a mechanistic view. Biochim
Biophys Acta. 1860:727–745. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Preston S, Korhonen PK, Mouchiroud L,
Cornaglia M, McGee SL, Young ND, Davis RA, Crawford S, Nowell C,
Ansell BRE, et al: Deguelin exerts potent nematocidal activity via
the mitochondrial respiratory chain. FASEB J. 31:4515–4532. 2017.
View Article : Google Scholar : PubMed/NCBI
|