Introduction
In 2020, it was predicted that there were 1.089
million new cases of gastric cancer and 769,000 gastric
cancer-associated deaths worldwide. In addition, gastric cancer
exhibited the fifth highest incidence rate and fourth highest
mortality rate among malignant tumors (1).
At diagnosis, the majority of patients have an
advanced stage of gastric cancer and, therefore, radical surgery is
not an option. At present, patients with advanced gastric cancer
are mainly treated with a combination of chemotherapy-based
regimens. Although the overall survival time of patients with
advanced gastric cancer has increased following advances in
chemotherapeutic regimens and drugs, the median overall duration of
patient survival after chemotherapy remains at <1 year (2). The results of a phase III clinical
trial demonstrated that the use of trastuzumab, a targeted therapy
for the treatment of gastric cancer, in combination with
chemotherapy increased the survival rates of patients with human
epidermal growth factor receptor 2 (HER2)-positive advanced gastric
or gastro-esophageal cancer when compared with chemotherapy alone
(3). However, the majority of
clinical trials conducted with other targeted agents in patients
with advanced gastric cancer did not meet the primary study
endpoint (4). JACOB (5), LOGIC (6), REAL3 (7), GATSBY (8) and a number of other phase III clinical
trials all showed that targeted agents did not prolonged advanced
overall survival time in patients with gastric cancer.
Programmed cell death 1 (PD-1) is a negative
co-stimulatory transmembrane protein expressed on a variety of
immune cells that binds to PD ligand-1 (PD-L1), which is expressed
on tumor cells and inhibits antitumor immunity (9). Anti-PD-1/PD-L1 therapy blocks the
PD-1/PD-L1 pathway and promotes the innate immune response to tumor
cells (10). Notably,
anti-PD-1/PD-L1 therapy exhibits potential in gastric cancer
(11). However, the results of
previous clinical trials have demonstrated that only specific
patients may benefit from immunotherapy. Therefore, if patients
with gastric cancer are indiscriminately treated with PD-1
immunotherapy, certain patients may experience hyper-progression
and adverse side effects, including immune-related myocarditis and
encephalitis, and incur unnecessary high costs (12,13).
Therefore, the selection of patients that are likely to respond to
PD-1 immunotherapy is crucial. Moreover, it is necessary to further
investigate the specific biomarkers of gastric cancer in order to
maximize patient benefits, minimize the risk of toxicity and
achieve precision immunotherapy.
The findings of previous studies have demonstrated
that the prognostic nutritional index (PNI), advanced lung cancer
inflammation index (ALI) and albumin-globulin ratio (AGR) values
are nutritional indicators associated with albumin, globulin,
neutrophils, lymphocytes and body mass index (BMI), which reflect
the systemic nutritional levels of the patient and are associated
with the prognosis of patients with gastric cancer (14–16).
Therefore, the present study aimed to investigate the potential
associations of PNI, AGR and ALI with the efficacy of immunotherapy
and the prognosis of patients with advanced gastric cancer. In the
present study, χ2 tests were used to analyze the factors
associated with PNI, ALI and AGR, and to evaluate the association
of PNI, ALI and AGR with the objective response rate (ORR) and
disease control rate (DCR) of the patients. In addition,
differences in clinical indicators between patients in different
efficacy groups before and after treatment were analyzed using
mixed ANOVA followed by pairwise comparisons using Bonferroni post
hoc tests. Logistic regression analysis was also used to create
receiver operating characteristic (ROC) curves to analyze the
association of nutritional indicators with the effect of treatment.
Survival curves were plotted using the Kaplan-Meier method, and
differences between survival curves were examined using a log-rank
test. Furthermore, the Cox proportional risk model was used to
analyze the associations of PNI, ALI and AGR with progression-free
survival (PFS).
Materials and methods
Study population and response
assessment
A total of 273 patients with advanced gastric cancer
treated with PD-1 inhibitors, who were admitted to the Department
of Oncology of The First Hospital of Shanxi Medical University
(Taiyuan, China) from January 2020 to October 2021, were screened
for inclusion in the present study. The inclusion criteria were as
follows: i) Patients diagnosed with clinical stage IV,
HER2-negative gastric adenocarcinoma via imaging and
histopathological examination; ii) patients who received at least
three rounds of PD-1 inhibitor monotherapy, PD-1 inhibitor combined
with chemotherapy or PD-1 inhibitor combined with anti-angiogenic
agents at the First Hospital of Shanxi Medical University; and iii)
patients with complete carcinoembryonic antigen (CEA), carbohydrate
antigen19-9 (CA19-9) and a-fetoprotein (AFP) levels, PD-L1
expression level, mismatch repair (MMR) status, peripheral blood
lymphocyte, albumin, neutrophil and total protein results, and
relevant follow-up test results. Patients were excluded from the
present study according to the following criteria: i) Other types
of malignancies; ii) HER2-positive gastric adenocarcinoma; iii)
acute infections, immunodeficiency and autoimmune diseases; iv)
severe underlying diseases, affecting organs such as the heart,
lung, liver and kidney; and v) systemic hormone treatment. Among
the 273 patients that were screened, 195 patients met the
aforementioned criteria and were included in the present study. The
excluded patients comprised 27 patients with a lack of lymphocyte
subpopulation data, 23 patients with missing PD-L1 scores and MMR
test results, 6 patients who had concomitant severe underlying
diseases and 22 patients with a lack of follow-up data. Patients
included 60 female and 135 males; the mean age of the included
population was 61.44 years old, ranging from 29–84 years old. Of
these, 166 patients study underwent treatment with PD-1 inhibitors
combined with chemotherapy, 24 patients were treated with PD-1
inhibitors alone, and 5 were treated with PD-1 inhibitors with
anti-angiogenic.
Data collection
Clinicopathological characteristics of the patients
were collected, including diagnosis, biological sex, age, BMI,
history of smoking, drinking history, the presence of liver or
peritoneal metastases prior to immunotherapy, treatment regimens
and the number of treatment lines. Laboratory indices of the
patients were also collected, including CEA, CA19-9 and AFP levels,
PD-L1 expression levels, MMR status, peripheral blood lymphocyte
counts, albumin levels, neutrophil counts and total protein levels.
Blood samples were collected from the patients within the 3 days
prior to immunotherapy and after 3 cycles of immunotherapy. The
PD-1 inhibitors used included pembrolizumab, nivolumab,
tislelizumab, toripalimab, camrelizumab and sintilimab. The
chemotherapy regimens used included S-1 plus oxaliplatin,
oxaliplatin plus capecitabine, docetaxel plus S-1, folinic acid,
fluorouracil and oxaliplatin, albumin-bound paclitaxel monotherapy
and docetaxel monotherapy. The anti-angiogenic agent used was
apatinib. History of drinking was defined as drinking alcohol ≥2
per week.
The efficacy of immunotherapy and the occurrence of
liver or peritoneal metastases prior to treatment were assessed
based on computed tomography, magnetic resonance imaging or
positron emission tomography/computed tomography findings.
Short-term efficacy was classified according to the Response
Evaluation in Solid Tumors (RECIST 1.1) assessment criteria
(17) as follows: Complete response
(CR), partial response (PR), stable disease (SD) and progressive
disease (PD). Patients with a CR or PR were classified as the
responding group, and patients with SD or PD were classified as the
non-responding group. Prognosis was assessed using PFS time, which
was defined as the time from the initiation of treatment to
progression or mortality from any cause. The DCR was defined as the
ratio of the sum of CR, PR and SD cases to the total number of
cases. The ORR was defined as the ratio of the sum of CR plus PR
cases to the total number of cases. The aforementioned medical
information and imaging data were collected using the electronic
medical record system of The First Hospital of Shanxi Medical
University. The reference ranges of the tumor markers were as
follows: CEA, 0–3 µg/l; CA19-9, 0–37 U/ml; and AFP, 0–15 µg/l.
Calculation of nutrition-related
indicators
ALI, AGR and PNI values are all based on results
from peripheral blood. PNI values were calculated using the
following formula: PNI=serum albumin (g/l) + 5× total peripheral
blood lymphocytes (×109/l). The neutrophil-to-lymphocyte
ratio (NLR) was calculated and used in the calculation of ALI
values according to the following formula: ALI=BMI × albumin
(g/l)/NLR. Total serum protein levels were also obtained. AGR
values were calculated using the following formula:
AGR=albumin/(total protein-albumin). The reference ranges for each
index are as follows: Absolute neutrophil value,
1.9–6.3×109/l; absolute lymphocyte value,
1.1–3.2×109/l; albumin, 40–55 g/l; and total protein,
65–85 g/l.
PD-L1 immunohistochemical
assessment
Immunohisto-chemical staining of the tumor tissue
was performed on the Ventata BenchMark Ultra system (Roche Tissue
Diagnostics) using monoclonal mouse anti-human PD-L1 (clone number,
22C3; 1:50; Dako; Agilent Technologies, Inc.) following the
manufacturer's instructions. The tissue specimens were fixed in 10%
neutral formalin at room temperature for 6–24 h. The fixed tissue
was placed in a tissue dehydrator overnight for dehydration and
clearing, then the tissue was paraffin-embedded and sectioned at 3
µm. Embedding protocol included transferring the tissue core into
the hole of the receptor wax block and placing it into a constant
temperature wax box at 60°C for 10–15 min and then into a
refrigerator at 4°C to cool down. Sections were incubated with
primary antibody against PD-L1 (clone number, 22C3; 1:50; Dako,
Agilent Technologies, Inc.) at 4°C overnight followed by
horseradish peroxidase-labeled goat anti-mouse secondary antibody
(Roche Diagnostic Products, Shanghai) at 37°C for 30 min and
staining with 3,3′-diaminobenzidine (DAB; Roche Diagnostics) at
room temperature for 2 min. Observation was under a light
microscope. Matching kit was applied (OptiView, Roche Diagnostics).
PD-L1-positive immunoreactive staining was localized to the cell
membrane and assessed using the combined positive score (CPS),
which is calculated as follows: CPS=total number of
immunoreactive-stained tumor cells, lymphocytes and macrophages in
the 20X objective field/total number of tumor cells in the field at
×100 magnification using a light microscope. PD-L1 CPS ≥5 was
considered as PD-L1 positive, while PD-L1 CPS <5 was considered
as PD-L1 negative.
MMR immunohistochemical
assessment
MMR staining of the tumor tissue was performed on
the Leica BOND III platform (Leica Biosystems) following the
manufacturer's instructions. The tissue specimens were fixed in 10%
neutral formalin at room temperature for 6–24 h. The fixed tissue
was placed in a tissue dehydrator overnight for dehydration and
clearing, then the tissue was paraffin-embedded and sectioned at 3
µm. Embedding protocol included transferring the tissue core into
the hole of the receptor wax block and placing it into a constant
temperature wax box at 60°C for 10–15 min and then into a
refrigerator at 4°C to cool down. The tissue specimens were fixed
in 10% neutral formalin at room temperature for 6–24 h. The fixed
tissue was placed in a tissue dehydrator overnight for dehydration
and clearing, then the tissue was paraffin-embedded and sectioned
at 3 µm. Embedding protocol included transferring the tissue core
into the hole of the receptor wax block and placing it into a
constant temperature wax box at 60°C for 10–15 min and then into a
refrigerator at 4°C to cool down. Sections were incubated with
primary antibody against MutS homolog 2 (MSH2; clone no. RED2;
1:5,000; OriGene Technologies, Inc.) and MutS homolog 6 (MSH6;
clone no. EP49; 1:100; OriGene Technologies, Inc.), MutL homolog 1
(MLH1; clone no. GM002; 1:500; Gene Tech Biotechnology Co., Ltd.)
and postmeiotic segregation increased 2 (PMS2; clone no. EP51;
1:70; Gene Tech Biotechnology Co., Ltd.) at 4°C overnight followed
by horseradish peroxidase-labeled goat anti-mouse secondary
antibody (Roche Diagnostics) at 37°C for 30 min and staining with
3,3′-diaminobenzidine (DAB; Roche Diagnostics) at room temperature
for 2 min. Staining was performed using an automatic
immunohistochemical station (BenchMark ULTRA; Roche Diagnostics).
Observation was under a light microscope. The platform kit used for
staining was BOND Polymer Refine Detection (Leica Biosystems
Newcastle Ltd.). The absence of at least one of the MMR proteins,
including MLH1, MSH2, MSH6 and PMS2, was defined as MMR deficient
(dMMR). All positive MMR protein staining results were defined as
MMR proficient (pMMR).
Statistical analysis
SPSS software (version 23.0; IBM Corp.) was used to
perform the statistical analysis. Quantitative data are expressed
as the mean ± standard deviation, and qualitative data are
expressed as the number and percentage of cases, n (%). Median
peripheral PNI, AGR and ALI values were used as the critical values
for the division of patients into high and low index groups.
Differences in the number of patients with various
clinicopathological characteristics between groups were analyzed
using χ2 tests. Differences in quantitative clinical
indicators of patients in different efficacy groups before and
after treatment were evaluated using a mixed ANOVA followed by
pairwise comparisons using Bonferroni post hoc tests. Independent
predictors of immunotherapy efficacy were determined using logistic
regression analysis. The potential value of PNI and AGR in the
prediction of short-term immunotherapy efficacy in patients with
advanced gastric cancer was determined by plotting a ROC curve and
calculating the area under the curve (AUC). Kaplan-Meier curves
were also plotted and the differences in PFS between groups were
compared using log-rank tests. Other variables were determined as
independent prognostic factors for PFS using Cox proportional risk
model analysis. P<0.05 was considered to indicate a
statistically significant result.
Results
Clinicopathological characteristics
and factors affecting the PNI, ALI and AGR values of patients
The PNI, ALI and AGR values of patients with
advanced gastric cancer prior to immunotherapy were determined, and
patients were divided into high and low groups, according to the
median value. The median PNI, ALI and AGR values were 45.50, 272.57
and 1.40, respectively. The effects of different
clinicopathological characteristics on the nutritional indices of
the patients were analyzed. The results demonstrated that low PNI
values were associated with elevated CEA levels. Moreover, low ALI
levels were associated with reduced BMI values, elevated AFP
levels, PD-L1 negative and first-line treatment while high ALI
levels were associated with normal or higher BMI values, normal AFP
levels, PD-L1 positive and second line and beyond treatment. The
results also demonstrated that the AGR levels were not
significantly associated with any of the clinical characteristics
that were analyzed. The associations between nutritional index
levels and clinicopathological characteristics in different groups
are displayed in Table I.
 | Table I.Association between clinical
characteristics and PNI, ALI and AGR values in patients with
advanced gastric cancer. |
Table I.
Association between clinical
characteristics and PNI, ALI and AGR values in patients with
advanced gastric cancer.
|
|
| PNI |
|
| ALI |
|
| AGR |
|
|
|---|
| Clinical
characteristic |
|
|
|
|
|
|
|
|
|
|
|---|
| n | <45.5 | ≥45.5 | χ2 | P-value | <272.57 | ≥272.57 | χ2 | P-value | <1.40 | ≥1.40 | χ2 | P-value |
|---|
| Total | 195 | 96 | 99 |
|
| 98 | 97 |
|
| 100 | 95 |
|
|
| Sex |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Female | 60 | 30 (31.3) | 30 (30.3) | 0.021 | 0.886 | 28 (28.6) | 32 (33.0) | 0.447 | 0.504 | 28 (28.0) | 32 (33.7) | 0.739 | 0.390 |
|
Male | 135 | 66 (68.8) | 69 (69.7) |
|
| 70 (71.4) | 65 (67.0) |
|
| 72 (72.0) | 63 (66.3) |
|
|
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
<65 | 120 | 60 (62.5) | 60 (60.6) | 0.074 | 0.786 | 66 (67.3) | 54 (55.7) | 2.808 | 0.094 | 59 (59.0) | 61 (64.2) | 0.559 | 0.455 |
|
≥65 | 75 | 36 (37.5) | 39 (39.4) |
|
| 32 (32.7) | 43 (44.3) |
|
| 41 (41.0) | 34 (35.8) |
|
|
| BMI,
kg/m2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
<18.5 | 59 | 29 (30.2) | 30 (30.3) | <0.001 | 0.989 | 40 (40.8) | 19 (19.6) | 10.411 | 0.001 | 33 (33.0) | 26 (27.4) | 0.732 | 0.392 |
|
≥18.5 | 136 | 67 (69.8) | 69 (69.7) |
|
| 58 (59.2) | 78 (80.4) |
|
| 67 (67.0) | 69 (72.6) |
|
|
| Drinking
history |
|
|
|
|
|
|
|
|
|
|
|
|
|
| No | 159 | 77 (80.2) | 82 (82.8) | 0.222 | 0.637 | 79 (80.6) | 80 (82.5) | 0.112 | 0.728 | 83 (83.0) | 76 (80.0) | 0.291 | 0.589 |
|
Yes | 36 | 19 (19.8) | 17 (17.2) |
|
| 19 (19.4) | 17 (17.5) |
|
| 17 (17.0) | 19 (20.0) |
|
|
| Smoking
history |
|
|
|
|
|
|
|
|
|
|
|
|
|
| No | 126 | 63 (65.6) | 63 (63.6) | 0.084 | 0.772 | 63 (64.3) | 63 (64.9) | 0.009 | 0.923 | 65 (65.0) | 61 (64.2) | 0.013 | 0.908 |
|
Yes | 69 | 33 (34.4) | 36 (36.4) |
|
| 35 (35.7) | 34 (35.1) |
|
| 35 (35.00) | 34 (35.8) |
|
|
| Liver
metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
|
| No | 112 | 52 (54.2) | 60 (60.6) | 0.827 | 0.363 | 51 (52.0) | 61 (62.9) | 2.346 | 0.126 | 58 (58.0) | 54 (56.8) | 0.027 | 0.870 |
|
Yes | 83 | 44 (45.8) | 39 (39.4) |
|
| 47 (48.0) | 36 (37.1) |
|
| 42 (42.0) | 41 (43.2) |
|
|
| Peritoneal
metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
|
| No | 152 | 72 (75.0) | 80 (80.8) | 0.957 | 0.328 | 73 (74.5) | 79 (81.4) | 1.371 | 0.242 | 77 (77.0) | 75 (78.9) | 0.107 | 0.743 |
|
Yes | 43 | 24 (25.0) | 19 (19.2) |
|
| 25 (25.5) | 18 (18.6) |
|
| 23 (23.0) | 20 (21.1) |
|
|
| PD-L1 expression,
CPS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
<5 | 105 | 54 (56.3) | 51 (51.5) | 0.440 | 0.507 | 62 (63.3) | 43 (44.3) | 7.033 | 0.008 | 60 (60.0) | 45 (47.4) | 3.128 | 0.077 |
| ≥5 | 90 | 42 (43.8) | 48 (48.5) |
|
| 36 (36.7) | 54 (55.7) |
|
| 40 (40.0) | 50 (52.6) |
|
|
| MMR status |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
pMMR | 178 | 88 (91.7) | 90 (90.9) | 0.035 | 0.851 | 92 (93.9) | 86 (88.7) | 1.668 | 0.197 | 91 (91.0) | 87 (91.6) | 0.021 | 0.886 |
|
dMMR | 17 | 8 (8.3) | 9 (9.1) |
|
| 6 (6.1) | 11 (11.3) |
|
| 9 (9.0) | 8 (8.4) |
|
|
| Treatment line |
|
|
|
|
|
|
|
|
|
|
|
|
|
| First
line | 130 | 67 (69.8) | 63 (63.6) | 0.831 | 0.362 | 73 (74.5) | 57 (58.8) | 5.426 | 0.020 | 67 (67.0) | 63 (66.3) | 0.010 | 0.919 |
| Second
line and beyond | 65 | 29 (30.2) | 36 (36.4) |
|
| 25 (25.5) | 40 (41.2) |
|
| 33 (33.0) | 32 (33.7) |
|
|
| CEA, µg/l |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
<3 | 83 | 34 (35.4) | 49 (49.5) | 3.951 | 0.047 | 35 (35.7) | 48 (49.5) | 3.781 | 0.052 | 38 (38.0) | 45 (47.4) | 1.749 | 0.186 |
| ≥3 | 112 | 62 (64.6) | 50 (50.5) |
|
| 63 (64.3) | 49 (50.5) |
|
| 62 (62.0) | 50 (52.6) |
|
|
| CA-199, U/ml |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
<37 | 121 | 53 (55.2) | 68 (68.7) | 3.760 | 0.052 | 61 (62.2) | 60 (61.9) | 0.003 | 0.955 | 62 (62.0) | 59 (62.1) | <0.001 | 0.988 |
|
≥37 | 74 | 43 (44.8) | 31 (31.3) |
|
| 37 (37.8) | 37 (38.1) |
|
| 38 (38.0) | 36 (37.9) |
|
|
| AFP, µg/l |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
<15 | 168 | 78 (81.2) | 90 (90.9) | 3.812 | 0.051 | 77 (78.6) | 91 (93.8) | 9.495 | 0.002 | 85 (85.0) | 83 (87.4) | 0.229 | 0.632 |
|
≥15 | 27 | 18 (18.8) | 9 (9.1) |
|
| 21 (21.4) | 6 (6.2) |
|
| 15 (15.0) | 12 (12.6) |
|
|
Changes in nutritional indicators and
tumor markers in patients before and after treatment
The 195 patients with advanced gastric cancer in the
present study had all undergone immunotherapy, and based on the
assessment of immunotherapy efficacy after three cycles, the
patients were divided into a responding group (n=68; CR, 3; PR, 65)
and a non-responding group (n=127; SD, 74; PD, 53). The proportion
of patients in the responding group was 34.9%. Comparison of the
two groups revealed that patients in the responding group had
higher PNI and AGR values than those in the non-responding group,
both before and after treatment, but had lower CEA and CA19-9
levels than the non-responding group after treatment. The other
indicators examined, namely ALI and AFP, exhibited no differences
between the two groups both before and after treatment. In a
comparison of pre- and post-treatment results, no significant
changes were observed in PNI, ALI, AGR, CEA, CA19-9 and AFP in the
responding group after treatment compared with the pre-treatment
period. In the non-responding group, PNI and AGR values were
decreased, CEA values were increased, and ALI, CA19-9 and AFP
values were not significantly changed following treatment, compared
with the respective values in the pre-treatment period. Comparisons
between PNI, ALI, AGR, CEA, CA19-9 and AFP values before and after
immunotherapy in the responding and non-responding groups are
displayed in Table II.
 | Table II.Comparison of PNI, ALI, AGR, CEA,
CA19-9 and AFP values before and after immunotherapy in responding
and non-responding groups. |
Table II.
Comparison of PNI, ALI, AGR, CEA,
CA19-9 and AFP values before and after immunotherapy in responding
and non-responding groups.
|
|
|
|
| Between time
points | Between groups | Interaction |
|---|
| Variable | Responding
group | Non-responding
group | P-value |
|
|
|
|---|
| F-value | P-value | F-value | P-value | F-value | P-value |
|---|
| PNI |
|
|
|
|
|
|
|
|
|
| Before
immunotherapy | 48.08±5.95 | 43.81±5.93 | <0.001 | 11.879 | 0.001 | 40.874 | <0.001 | 1.933 | 0.166 |
| After
immunotherapy | 47.05±5.89 | 41.38±6.61 | <0.001 |
|
|
|
|
|
|
|
P-value | 0.204 | <0.001 |
|
|
|
|
|
|
|
| ALI |
|
|
|
|
|
|
|
|
|
| Before
immunotherapy | 384.08±271.45 | 355.78±300.63 | 0.518 | 0.781 | 0.378 | 0.616 | 0.434 | 0.001 | 0.981 |
| After
immunotherapy | 363.03±237.36 | 335.86±287.83 | 0.506 |
|
|
|
|
|
|
|
P-value | 0.574 | 0.468 |
|
|
|
|
|
|
|
| AGR |
|
|
|
|
|
|
|
|
|
| Before
immunotherapy | 1.50±0.22 | 1.31±0.30 | <0.001 | 14.561 | <0.001 | 45.239 | <0.001 | 8.592 | 0.043 |
| After
immunotherapy | 1.48±0.29 | 1.18±0.28 | <0.001 |
|
|
|
|
|
|
|
P-value | 0.584 | <0.001 |
|
|
|
|
|
|
|
| CEA |
|
|
|
|
|
|
|
|
|
| Before
immunotherapy | 28.71±51.06 | 69.48±407.03 | 0.412 | 0.942 | 0.333 | 2.771 | 0.098 | 3.941 | 0.049 |
| After
immunotherapy | 16.62±24.01 | 104.68±234.65 | 0.002 |
|
|
|
|
|
|
|
P-value | 0.530 | 0.013 |
|
|
|
|
|
|
|
| CA19-9 |
|
|
|
|
|
|
|
|
|
| Before
immunotherapy | 122.66±323.63 | 133.10±282.47 | 0.816 | 0.023 | 0.879 | 3.830 | 0.052 | 4.336 | 0.039 |
| After
immunotherapy | 27.03±28.55 | 215.70±680.91 | 0.024 |
|
|
|
|
|
|
|
P-value | 0.168 | 0.104 |
|
|
|
|
|
|
|
| AFP |
|
|
|
|
|
|
|
|
|
| Before
immunotherapy | 41.43±156.59 | 221.78±1066.02 | 0.168 | 1.266 | 0.262 | 1.930 | 0.166 | 1.092 | 0.297 |
| After
immunotherapy | 48.98±345.97 | 425.67±2331.69 | 0.187 |
|
|
|
|
|
|
|
P-value | 0.960 | 0.068 |
|
|
|
|
|
|
|
Analysis of treatment efficacy in
different groups
Analysis of treatment efficacy in patients in the
low and high PNI, ALI and AGR groups according to RECIST 1.1
criteria is displayed in Table
III. The ORR and DCR of patients in the low PNI group were 24.0
and 64.6%, respectively, compared with 45.5 and 80.8%,
respectively, in the high PNI group. The results revealed that the
ORR and DCR of patients in the low PNI group were significantly
lower than those in the high PNI group. The ORR and DCR of patients
in the low ALI group were 31.6 and 69.4%, respectively, while those
in the high ALI group were 38.1 and 76.3%, respectively. Notably,
these rates were not found to be statistically significant between
the two groups. The ORR and DCR of patients in the low AGR group
were 23.0 and 60.0%, respectively, compared with 47.4 and 86.3%,
respectively, in the high AGR group. The ORR and DCR of patients in
the low AGR group were significantly lower than those in the high
AGR group.
 | Table III.Analysis of treatment efficacy in
patients according to PNI, ALI and AGR values. |
Table III.
Analysis of treatment efficacy in
patients according to PNI, ALI and AGR values.
| Variable | n | CR | PR | SD | PD | ORR | DCR |
|---|
| PNI |
|
|
|
|
|
|
|
|
Low | 96 | 1 (1.0) | 22 (22.9) | 39 (40.6) | 34 (35.4) | 23 (24.0) | 62 (64.6) |
|
High | 99 | 2 (2.0) | 43 (43.4) | 35 (35.4) | 19 (19.2) | 45 (45.5) | 80 (80.8) |
|
χ2 |
|
|
|
|
| 9.916 | 6.482 |
|
P-value |
|
|
|
|
| 0.002 | 0.011 |
| ALI |
|
|
|
|
|
|
|
|
Low | 98 | 0 (0.0) | 31 (31.6) | 37 (37.8) | 30 (30.6) | 31 (31.6) | 68 (69.4) |
|
High | 97 | 3 (3.1) | 34 (35.1) | 37 (38.1) | 23 (23.7) | 37 (38.1) | 74 (76.3) |
|
χ2 |
|
|
|
|
| 0.910 | 1.173 |
|
P-value |
|
|
|
|
| 0.340 | 0.279 |
| AGR |
|
|
|
|
|
|
|
|
Low | 100 | 0 (0.0) | 23 (23.0) | 37 (37.0) | 40 (40.0) | 23 (23.0) | 60 (60.0) |
|
High | 95 | 3 (3.2) | 42 (44.2) | 37 (38.9) | 13 (13.7) | 45 (47.4) | 82 (86.3) |
|
χ2 |
|
|
|
|
| 12.738 | 17.046 |
|
P-value |
|
|
|
|
| <0.001 | <0.001 |
Association between baseline
nutritional indicators and short-term patient outcomes
The aforementioned analysis of PNI, ALI and AGR
values prior to treatment between the 68 patients in the responding
group and the 127 patients in the non-responding group highlighted
that the PNI and AGR values were higher in the responding group
than in the non-responding group, while no significant difference
in ALI values was detected between the responding and
non-responding groups. Differences in PD-L1 expression, MMR status,
sex, age, BMI, smoking history, alcohol consumption and liver or
peritoneal metastasis were compared between the responding and
non-responding groups using χ2 tests (Table IV). The results reveal that the
proportion of dMMR- and PD-L1-positive patients in the responding
group was higher than that in the non-responding group, and the
remaining indices did not differ between the two groups. The
inclusion of PNI, AGR, PD-L1 and MMR status as variables in a
logistic regression model demonstrated that PNI [OR, 0.890; 95%
confidence interval (CI), 0.830–0.955; P=0.001], AGR (OR, 0.109;
95% CI, 0.027–0.444; P=0.002), PD-L1 levels (OR, 0.150; 95% CI,
0.071–0.317; P<0.001) and MMR status (OR, 0.212; 95% CI,
0.064–0.708; P=0.012) were independent predictors of the short-term
efficacy of immunotherapy in patients with advanced gastric cancer.
In addition, when ROC curves for PNI and AGR values in patients
prior to treatment were plotted, the AUCs were 0.699 (P<0.001)
and 0.696 (P<0.001), respectively (Fig. 1), which highlights that these
factors are independent predictors of treatment efficacy. Notably,
the cut-off value was 47.18 for PNI (sensitivity, 0.603;
specificity, 0.764) and 1.29 for AGR (sensitivity, 0.853;
specificity, 0.496).
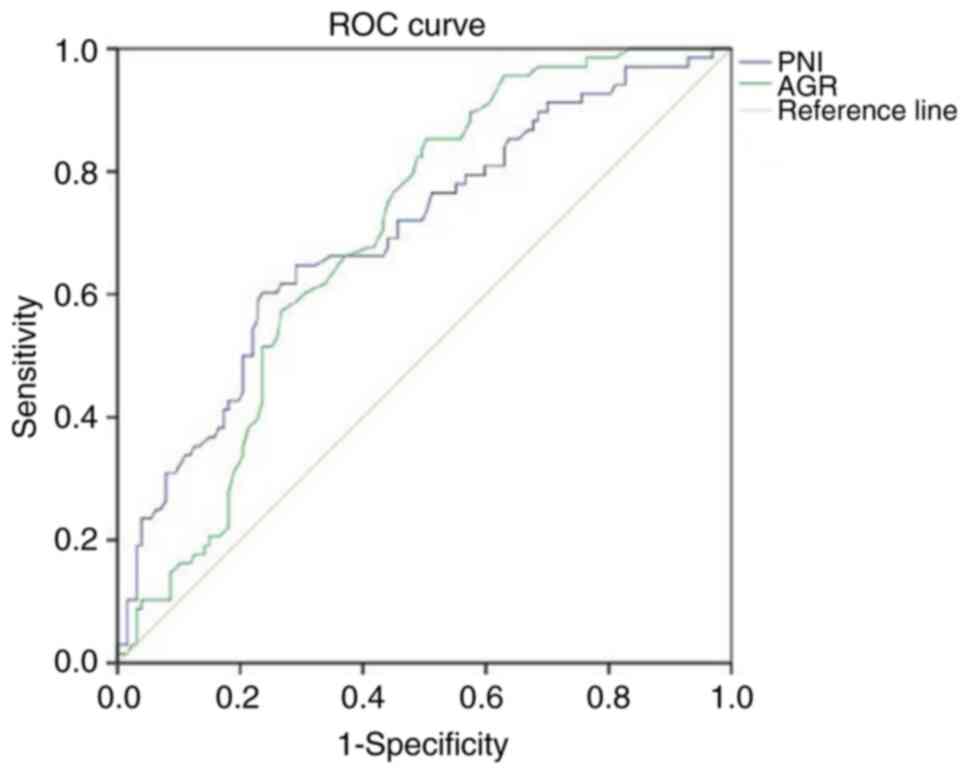 | Figure 1.ROC curves of PNI and AGR values
prior to immunotherapy. PNI: area under the curve, 0.699;
P<0.001; cut-off value, 47.18; sensitivity, 0.603; specificity,
0.764. AGR: area under the curve, 0.696; P<0.001; cut-off value,
1.29; sensitivity, 0.853; specificity, 0.496. Diagonal segments in
the curves are produced by ties. ROC, receiver operating
characteristic; PNI, prognostic nutritional index; AGR,
albumin-globulin ratio. |
 | Table IV.Comparison of PD-L1 expression, MMR
status, sex, age, BMI, smoking history, alcohol consumption, liver
and peritoneal metastasis between responding and non-responding
patients. |
Table IV.
Comparison of PD-L1 expression, MMR
status, sex, age, BMI, smoking history, alcohol consumption, liver
and peritoneal metastasis between responding and non-responding
patients.
| Variable | n | Responding
group | Non-responding
group | χ2 | P-value |
|---|
| Sex |
|
|
|
|
|
|
Female | 60 | 25 (36.8) | 35 (27.6) | 1.762 | 0.184 |
|
Male | 135 | 43 (63.2) | 92 (72.4) |
|
|
| Age, years |
|
|
|
|
|
|
<65 | 120 | 45 (66.2) | 75 (59.1) | 0.949 | 0.330 |
|
≥65 | 75 | 23 (33.8) | 52 (40.9) |
|
|
| BMI,
kg/m2 |
|
|
|
|
|
|
<18.5 | 59 | 23 (33.8) | 36 (28.3) | 0.630 | 0.428 |
|
≥18.5 | 136 | 45 (66.2) | 91 (71.7) |
|
|
| Drinking
history |
|
|
|
|
|
| No | 159 | 58 (85.3) | 101 (79.5) | 0.978 | 0.323 |
|
Yes | 36 | 10 (14.7) | 26 (20.5) |
|
|
| Smoking
history |
|
|
|
|
|
| No | 126 | 46 (67.6) | 80 (63.0) | 0.420 | 0.517 |
|
Yes | 69 | 22 (32.4) | 47 (37.0) |
|
|
| Liver
metastasis |
|
|
|
|
|
| No | 112 | 37 (54.4) | 75 (59.1) | 0.391 | 0.531 |
|
Yes | 83 | 31 (45.6) | 52 (40.9) |
|
|
| Peritoneal
metastasis |
|
|
|
|
|
| No | 152 | 57 (83.8) | 95 (74.8) | 2.096 | 0.148 |
|
Yes | 43 | 11 (16.2) | 32 (25.2) |
|
|
| PD-L1 expression,
CPS |
|
|
|
|
|
|
<5 | 105 | 19 (27.9) | 86 (67.7) | 28.193 | <0.001 |
| ≥5 | 90 | 49 (72.1) | 41 (32.3) |
|
|
| MMR status |
|
|
|
|
|
|
pMMR | 178 | 57 (83.8) | 121 (95.3) | 7.299 | 0.007 |
|
dMMR | 17 | 11 (16.2) | 6 (4.7) |
|
|
Association between pre-treatment
nutrition-associated indicators and the PFS of patients
The median PFS time of the patients with advanced
gastric cancer was 4.0 months in the present study. Results of the
univariate analysis demonstrated no significant association of age,
sex, BMI, smoking history, alcohol consumption, liver metastasis or
peritoneal metastasis with PFS. Notably, PD-L1 level, MMR status,
and PNI, ALI and AGR values exhibited an association with PFS.
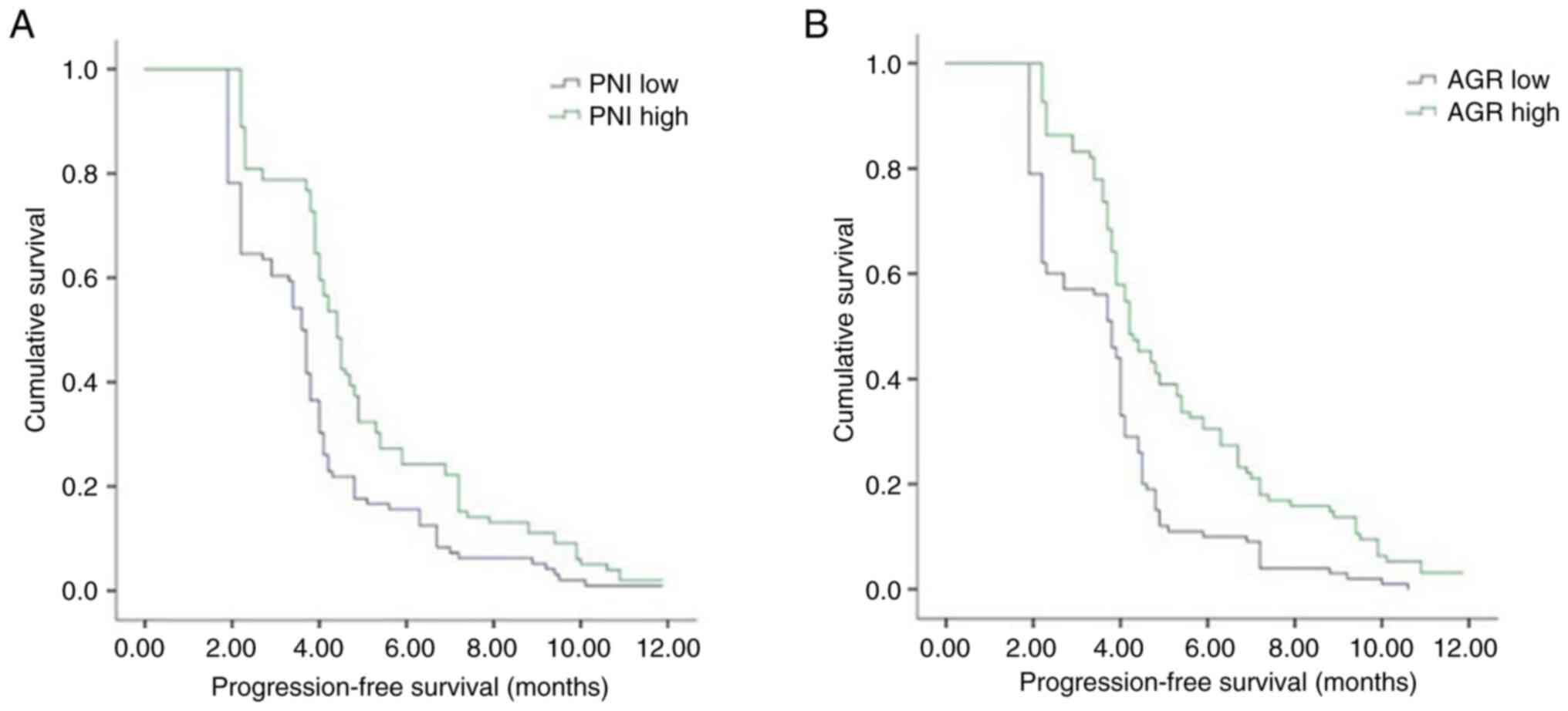
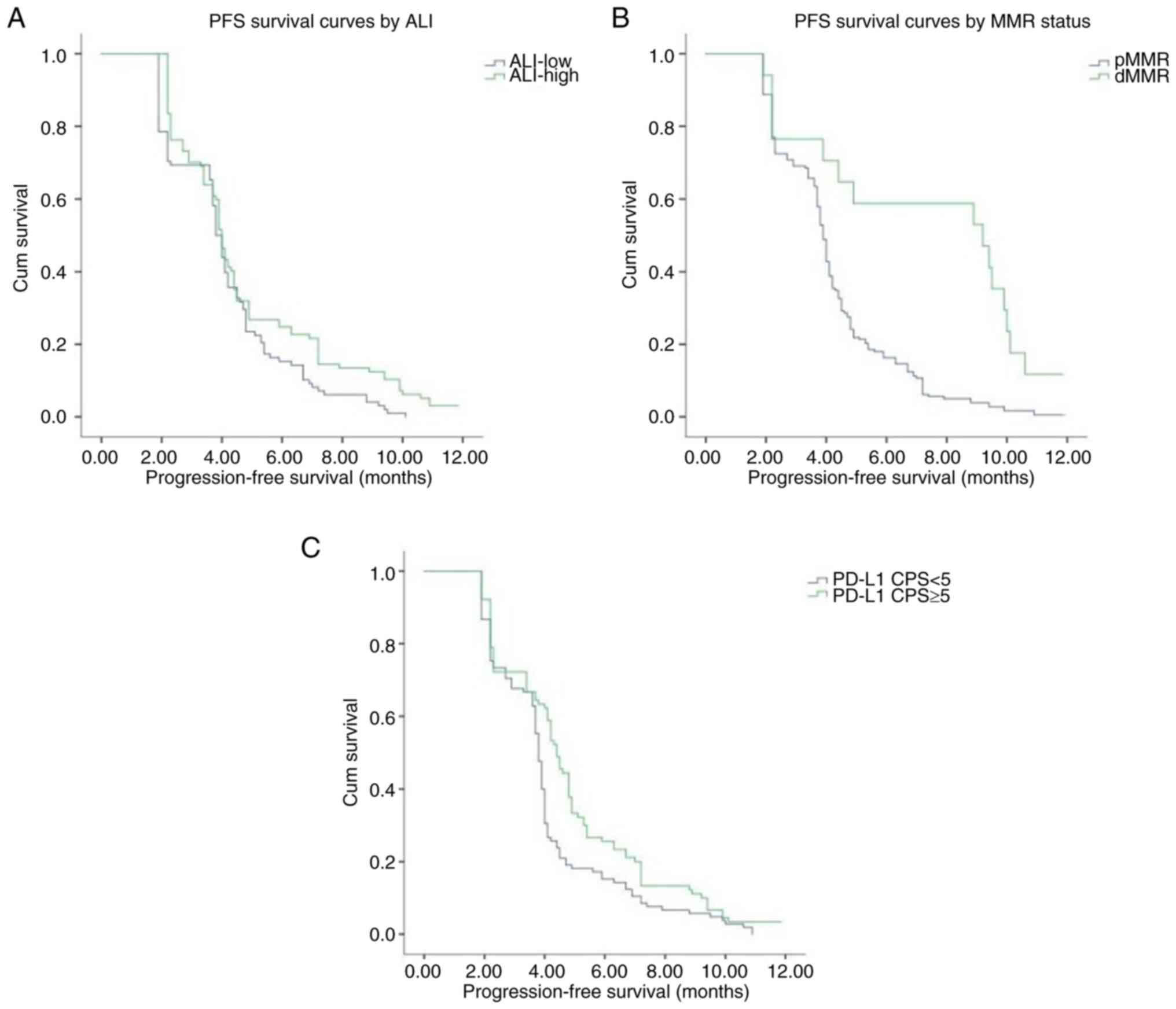
Moreover, patients in the PD-L1-positive, dMMR, high PNI, high ALI
and high AGR groups exhibited significantly longer PFS times than
patients in the PD-L1-negative, pMMR, low PNI, low ALI and low AGR
groups, respectively. The median PFS durations in the patients in
the pMMR, low PNI, low ALI and low AGR groups were 3.9, 3.6, 3.8
and 3.8 months, respectively, while the median PFS durations in
patients in the dMMR, high PNI, high ALI and high AGR groups were
9.2, 4.4, 4.0 and 4.2 months, respectively. The results of the Cox
proportional risk model analysis revealed that PNI [hazard ratio
(HR), 0.949; 95% CI, 0.924–0.975; P<0.001], AGR (HR, 0.516; 95%
CI, 0.318–0.836; P=0.007), MMR status (HR, 0.337; 95% CI,
0.191–0.597; P<0.001) and PD-L1 levels (HR, 0:685; 95% CI,
0.509–0.921; P=0.012) were independent prognostic factors for PFS.
Kaplan-Meier curves of PFS also demonstrated that patients in the
high PNI and AGR groups exhibited a prolonged PFS time compared
with patients in the low PNI and AGR groups (Fig. 2). Kaplan-Meier curves also showed
that patients with high ALI, dMMR and PD-L1 positive had higher PFS
times compared with those with low ALI, pMMR and PD-L1 negative
(Fig. 3). The differences in PFS
between patients grouped according to various characteristics as
analyzed using univariate analysis are displayed in Table V.
 | Table V.Univariate analysis of the
association between baseline variables and PFS in 195 patients with
gastric cancer. |
Table V.
Univariate analysis of the
association between baseline variables and PFS in 195 patients with
gastric cancer.
| Variable | PFS time (95% CI),
months | Log-rank | P-value |
|---|
| Age, years |
| 0.141 | 0.707 |
|
<65 | 4.00
(3.82–4.18) |
|
|
|
≥65 | 4.00
(3.78–4.2) |
|
|
| Sex |
| 2.620 | 0.106 |
|
Female | 4.10
(3.68–4.52) |
|
|
|
Male | 3.90
(3.73–4.07) |
|
|
| BMI,
kg/m2 |
| 0.075 | 0.784 |
|
<18.5 | 3.90
(3.60–4.20) |
|
|
|
≥18.5 | 4.00
(3.85–4.15) |
|
|
| Smoking
history |
| 0.258 | 0.612 |
| No | 4.00
(3.79–4.21) |
|
|
|
Yes | 3.90
(3.63–4.17) |
|
|
| Drinking
history |
| 0.110 | 0.740 |
| No | 4.00
(3.82–4.18) |
|
|
|
Yes | 3.90
(3.55–4.25) |
|
|
| Liver
metastasis |
| 0.036 | 0.850 |
| No | 3.90
(3.68–4.12) |
|
|
|
Yes | 4.00
(3.80–4.20) |
|
|
| Peritoneal
metastasis |
| 0.011 | 0.915 |
| No | 3.90
(3.72–4.08) |
|
|
|
Yes | 4.00
(3.79–4.21) |
|
|
| PD-L1 expression,
CPS |
| 7.220 | 0.007 |
|
<5 | 3.80
(3.68–3.93) |
|
|
| ≥5 | 4.40
(4.04–4.76) |
|
|
| MMR status |
| 16.475 | <0.001 |
|
pMMR | 3.90
(3.76–4.05) |
|
|
|
dMMR | 9.20
(3.15–15.25) |
|
|
| PNI |
| 15.936 | <0.001 |
|
Low | 3.60
(3.37–3.83) |
|
|
|
High | 4.40
(4.12–4.68) |
|
|
| ALI |
| 5.201 | 0.023 |
|
Low | 3.80
(3.59–4.01) |
|
|
|
High | 4.00
(3.82–4.18) |
|
|
| AGR |
| 18.915 | <0.001 |
|
Low | 3.80
(3.39–4.21) |
|
|
|
High | 4.20
(3.65–4.75) |
|
|
Discussion
Following the success of immunotherapy trials in
numerous solid tumors (18),
immunotherapy and combination therapies are included in the
National Comprehensive Cancer Network (NCCN) guidelines as the
standard of care for advanced gastric cancer (19). In various authoritative guidelines,
including the NCCN and Chinese Society of Clinical Oncology
guidelines, anti-PD-1 drugs have become the standard palliative
immunotherapy regimen for gastric cancer, while anti-PD-L1 drugs
have not (19,20); therefore, anti-PD-1 therapy rather
than anti-PD-L1 therapy was selected for evaluation in the present
study. However, the benefits of immunotherapy are limited to
certain populations of patients; thus, further investigations of
potential biomarkers are required to predict patient prognosis and
maximize patient benefits. Malignant tumors are characterized by
the high consumption of nutrients, and the development of tumors is
often accompanied by a decline in nutritional levels. Furthermore,
as nutritional levels decrease, treatment tolerance and survival
rates also decrease (21). As
gastric cancer affects the digestive system, the nutritional intake
and absorption in patients with gastric cancer may be worse than
those of other malignancies. Notably, if PNI, ALI and AGR values
were found to be independent predictors of gastric cancer, this
would exhibit advantages compared with the conventional use of
PD-L1 score and MMR status. For example, PNI, ALI and AGR are
determined via the analysis of peripheral blood; therefore, they
are less invasive to investigate and are inexpensive for
patients.
The results of the present study demonstrated that a
low PNI was associated with higher than normal CEA levels.
Moreover, a low ALI was associated with a low BMI, elevated AFP
levels, PD-L1 negative and first-line treatment. These factors are
associated with deterioration in the nutritional status of patients
with a higher tumor load following disease progression. Moreover,
when the patients in the responding and non-responding groups were
compared, the patients in the responding group had higher PNI and
AGR values than those in the non-responding group both before and
after treatment, but had lower CA19-9 and CEA levels than those in
the non-responding group after treatment. In a comparison of before
and after treatment results in the non-responding group, PNI and
AGR levels were decreased and CEA values were increased following
treatment compared with those prior to treatment. These results
further indicate that the control of tumor progression and
reduction of tumor load may alleviate issues with eating and
nutrition absorption, and improve the nutritional status of
patients to a certain extent.
PNI is a marker used to assess inflammation and
nutritional status, which is based on serum lymphocyte count and
albumin levels. PNI was initially used to assess the risks
associated with gastrointestinal surgery (22), and is also widely used in the
prognostic assessment of a variety of malignancies (23–25).
The results of a previous study demonstrated a significant
association of PNI with disease-free and overall survival following
radical gastric cancer surgery (26). Moreover, PNI has been demonstrated
to be an independent predictor of prognosis in non-small cell lung
cancer (27), melanoma (28) and uroepithelial carcinoma (29). The results of the present study
suggest that ineffective treatment may lead to a reduction in PNI
in patients with advanced gastric cancer. These results indicate
that effective immunotherapy may, to some extent, alleviate
deterioration of the nutritional status and clinical symptoms of
patients, such as difficulty in eating. The ORR and DCR were higher
in the high PNI group compared with those in the low PNI group. In
addition, PNI was found to be an independent predictor of the
short-term efficacy of immunotherapy and prognosis of patients with
advanced gastric cancer. This is consistent with previous results
in uroepithelial carcinoma, in which patients with a high PNI
exhibited significantly higher ORR and DCR than those with a low
PNI (29).
The ALI is a combined indicator of nutrition and
inflammation. The results of a previous study demonstrated that the
prognostic ability of ALI was superior to that of other
inflammatory or nutrition-based indices in a cohort of patients
with various types and all stages of lung cancer (30). Notably, ALI has been shown to
exhibit prognostic significance in numerous types of cancer,
including bile duct cancer (31),
head and neck cancer (32), and
colorectal cancer (33). In
addition, ALI has been demonstrated to be an independent predictor
of prognosis in patients undergoing radical surgery for gastric
cancer (34). The results of the
univariate analysis in the present study revealed a longer PFS time
in the high ALI group compared with that in the low ALI group.
However, multivariate analysis revealed no statistically
significant association of ALI with short-term patient outcomes and
prognosis. A previous study on advanced non-small cell lung cancer
demonstrated that ALI is a prognostic and predictive biomarker for
patients treated with PD-L1 inhibitors alone but not in combination
with chemotherapy (35). Notably,
the majority of patients in the present study underwent treatment
with PD-1 inhibitors combined with chemotherapy, and fewer patients
were treated with PD-1 inhibitors alone. Due to the retrospective
nature of the present study, further investigations are required to
clarify the correlation of ALI with the efficacy of immunotherapy
and the prognosis of patients with advanced gastric cancer. In
addition, further stratification of the patients and additional ALI
cut-off values may be required.
Albumin levels are key nutritional indicators that
may also reflect the levels of inflammation in patients (36). Globulin is a cortisol-binding
protein associated with immunity and inflammation levels in
patients (37,38). Research has focused on the clinical
application of AGR as a prognostic marker for tumors, and as a
serological indicator of nutritional status and systemic
inflammation. Previous studies have provided results indicating
that AGR may be an independent prognostic indicator in patients
with cancer cachexia (39), and
that AGR exhibits prognostic significance in metastatic prostate
(40), rectal (41), gastric (15), cervical (42) and nasopharyngeal cancer (43). Furthermore, Liu et al
(44) demonstrated that AGR affects
the PD-1/PD-L1 pathway in breast cancer, with implications for
immunotherapy. Notably, the present study indicated that treatment
progression may contribute to a reduction in AGR, and that patients
with a high AGR exhibited higher ORR and DCR than those with a low
AGR. In addition, it demonstrated that AGR may be an independent
predictor of both the short-term efficacy of immunotherapy and
prognosis in patients with advanced gastric cancer. These results
are consistent with those of previous studies conducted in multiple
tumor types (36). However, the
cut-off value of AGR varies among studies, which may be due to
differences in sample size and detection instruments (45). Thus, further investigations into the
use of AGR as a biomarker of immunotherapy efficacy in patients
with advanced gastric cancer are required, with increased sample
sizes and improved standardization of detection instruments.
The present study exhibits numerous limitations. For
example, the overall survival rates of patients were not
investigated, and further investigations are required to determine
the predictive ability of PNI, ALI and AGR in the efficacy of
immunotherapy and the prognosis of patients with advanced gastric
cancer. In addition, patients treated with immunotherapeutic
monotherapy, immunotherapy combined with chemotherapy and
immunotherapy combined with anti-angiogenic agents were included in
the present study. Thus, further subgroup analyses are required to
evaluate the nutritional status of patients following different
treatment regimens. Further subgroup analyses are also required to
verify the predictive ability of nutrition-associated indicators
for different treatment regimens. Moreover, the present study
included a widely varied patient population from a single
institution, including patients with different lines of therapy and
different combinations of immunotherapy, the majority of whom were
Asian individuals. Thus, further studies with larger sample sizes
are required.
In conclusion, the present study indicates that
tumor progression may lead to a decline in the nutritional levels
of patients, and that effective immunotherapy may alleviate the
deterioration of nutritional status in patients, to some
extent.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW was responsible for data curation. XL and HD were
responsible for investigations and for selecting resources. XW and
JJ were responsible for the methodology. HD and JJ supervised the
study. XW was responsible for writing the original draft of the
manuscript, and XW, XL, HD and JJ were responsible for reviewing
and editing the manuscript. XW and JJ confirm the authenticity of
all the raw data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
Due to the retrospective nature of the present
study, patients were contacted via telephone to provide informed
consent and telephone recordings were obtained. Ethics approval was
obtained from the Ethics Committee of The First Hospital of Shanxi
Medical University (approval no. KYLL-2023-012).
Patient consent for publication
Not applicable.
Authors' information
ORCID of Dr Xinyan Wang: 0000-0001-9816-8773. ORCID
of Dr Junmei Jia: 0000-0003-1132-7191.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ricci AD, Rizzo A and Brandi G: DNA damage
response alterations in gastric cancer: Knocking down a new wall.
Future Oncol. 17:865–868. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ricci AD, Rizzo A, Rojas Llimpe FL, Di
Fabio F, De Biase D and Rihawi K: Novel HER2-directed treatments in
advanced gastric carcinoma: AnotHER paradigm shift? Cancers
(Basel). 13:16642021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tabernero J, Hoff PM, Shen L, Ohtsu A,
Shah MA, Cheng K, Song C, Wu H, Eng-Wong J, Kim K and Kang YK:
Pertuzumab plus trastuzumab and chemotherapy for HER2-positive
metastatic gastric or gastro-oesophageal junction cancer (JACOB):
Final analysis of a double-blind, randomised, placebo-controlled
phase 3 study. Lancet Oncol. 19:1372–1384. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hecht JR, Bang YJ, Qin SK, Chung HC, Xu
JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero A, et al:
Lapatinib in combination with capecitabine plus oxaliplatin in
human epidermal growth factor receptor 2-positive advanced or
metastatic gastric, esophageal, or gastroesophageal adenocarcinoma:
TRIO-013/LOGiC-A randomized phase III Trial. J Clin Oncol.
34:443–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Waddell T, Chau I, Cunningham D, Gonzalez
D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G,
Wadsley J, et al: Epirubicin, oxaliplatin, and capecitabine with or
without panitumumab for patients with previously untreated advanced
oesophagogastric cancer (REAL3): A randomised, open-label phase 3
trial. Lancet Oncol. 14:481–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thuss-Patience PC, Shah MA, Ohtsu A, Van
Cutsem E, Ajani JA, Castro H, Mansoor W, Chung HC, Bodoky G,
Shitara K, et al: Trastuzumab emtansine versus taxane use for
previously treated HER2-positive locally advanced or metastatic
gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An
international randomised, open-label, adaptive, phase 2/3 study.
Lancet Oncol. 18:640–653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rihawi K, Ricci AD, Rizzo A, Brocchi S,
Marasco G, Pastore LV, Llimpe FLR, Golfieri R and Renzulli M:
Tumor-associated macrophages and inflammatory microenvironment in
gastric cancer: Novel translational implications. Int J Mol Sci.
22:38052021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y,
Wu W, Han L and Wang S: The role of PD-1/PD-L1 and application of
immune-checkpoint inhibitors in human cancers. Front Immunol.
13:9644422022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Viscardi G, Tralongo AC, Massari F,
Lambertini M, Mollica V, Rizzo A, Comito F, Di Liello R, Alfieri S,
Imbimbo M, et al: Comparative assessment of early mortality risk
upon immune checkpoint inhibitors alone or in combination with
other agents across solid malignancies: A systematic review and
meta-analysis. Eur J Cancer. 177:175–185. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guidi A, Violati M, Blasi M, Ferrari E,
Luciani A, Codecà C and Ferrari D: Autoimmune-related encephalitis
during treatment with nivolumab for advanced head and neck cancer:
A case report. Tumori. 106:NP23–NP28. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ding P, Guo H, Sun C, Yang P, Kim NH, Tian
Y, Liu Y, Liu P, Li Y and Zhao Q: Combined systemic
immune-inflammatory index (SII) and prognostic nutritional index
(PNI) predicts chemotherapy response and prognosis in locally
advanced gastric cancer patients receiving neoadjuvant chemotherapy
with PD-1 antibody sintilimab and XELOX: A prospective study. BMC
Gastroenterol. 22:1212022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Zhu JY, Zhou LN, Tang M, Chen MB
and Tao M: Predicting the prognosis of gastric cancer by
albumin/globulin ratio and the prognostic nutritional index. Nutr
Cancer. 72:635–644. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin C, Toiyama Y, Okugawa Y, Omura Y,
Kusunoki Y, Kusunoki K, Imaoka Y, Yasuda H, Ohi M and Kusunoki M:
Clinical significance of advanced lung cancer inflammation index, a
nutritional and inflammation index, in gastric cancer patients
after surgical resection: A propensity score matching analysis.
Clin Nutr. 40:1130–1136. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: Guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yi M, Zheng X, Niu M, Zhu S, Ge H and Wu
K: Combination strategies with PD-1/PD-L1 blockade: Current
advances and future directions. Mol Cancer. 21:282022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 20:167–192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang FH, Zhang XT, Li YF, Tang L, Qu XJ,
Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, et al: The Chinese society
of clinical oncology (CSCO): Clinical guidelines for the diagnosis
and treatment of gastric cancer, 2021. Cancer Commun (Lond).
41:747–795. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bossi P, Delrio P, Mascheroni A and
Zanetti M: The spectrum of malnutrition/cachexia/sarcopenia in
oncology according to different cancer types and settings: A
narrative review. Nutrients. 13:19802021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Onodera T, Goseki N and Kosaki G:
Prognostic nutritional index in gastrointestinal surgery of
malnourished cancer patients. Nihon Geka Gakkai Zasshi.
85:1001–1005. 1984.(In Japanese). PubMed/NCBI
|
|
23
|
Okadome K, Baba Y, Yagi T, Kiyozumi Y,
Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M and Baba
H: Prognostic nutritional index, tumor-infiltrating lymphocytes,
and prognosis in patients with esophageal cancer. Ann Surg.
271:693–700. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang D, Hu X, Xiao L, Long G, Yao L, Wang
Z and Zhou L: Prognostic nutritional index and systemic
immune-inflammation index predict the prognosis of patients with
HCC. J Gastrointest Surg. 25:421–427. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen F, Ma Y, Guo W and Li F: Prognostic
value of geriatric nutritional risk index for patients with
non-small cell lung cancer: A systematic review and meta-analysis.
Lung. 200:661–669. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nogueiro J, Santos-Sousa H, Pereira A,
Devezas V, Fernandes C, Sousa F, Fonseca T, Barbosa E and Barbosa
JA: The impact of the prognostic nutritional index (PNI) in gastric
cancer. Langenbecks Arch Surg. 407:2703–2714. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsubara T, Takamori S, Haratake N,
Toyozawa R, Miura N, Shimokawa M, Yamaguchi M, Seto T and
Takenoyama M: The impact of immune-inflammation-nutritional
parameters on the prognosis of non-small cell lung cancer patients
treated with atezolizumab. J Thorac Dis. 12:1520–1528. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guven DC, Aktepe OH, Taban H, Aktas BY,
Guner G, Yildirim HC, Sahin TK, Aksun MS, Dizdar O, Aksoy S, et al:
Lower prognostic nutritional index is associated with poorer
survival in patients receiving immune checkpoint inhibitors.
Biomark Med. 15:1123–1130. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ishiyama Y, Kondo T, Nemoto Y, Kobari Y,
Ishihara H, Tachibana H, Yoshida K, Hashimoto Y, Takagi T, Iizuka J
and Tanabe K: Predictive impact of prognostic nutritional index on
pembrolizumab for metastatic urothelial carcinoma resistant to
platinum-based chemotherapy. Anticancer Res. 41:1607–1614. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song M, Zhang Q, Song C, Liu T, Zhang X,
Ruan G, Tang M, Xie H, Zhang H, Ge Y, et al: The advanced lung
cancer inflammation index is the optimal inflammatory biomarker of
overall survival in patients with lung cancer. J Cachexia
Sarcopenia Muscle. 13:2504–2514. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu H, Ding F, Lin M, Shi Z, Mei Z, Chen S,
Jiang C, Qiu H, Zheng Z, Chen Y and Zhao P: Use of the advanced
lung cancer inflammation index as a prognostic indicator for
patients with cholangiocarcinoma. Front Surg. 9:8017672022.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gaudioso P, Borsetto D, Tirelli G,
Tofanelli M, Cragnolini F, Menegaldo A, Fabbris C, Molteni G,
Marchioni D, Nicolai P, et al: Advanced lung cancer inflammation
index and its prognostic value in HPV-negative head and neck
squamous cell carcinoma: A multicentre study. Support Care Cancer.
29:4683–4691. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pian G, Hong SY and Oh SY: Prognostic
value of advanced lung cancer inflammation index in patients with
colorectal cancer liver metastases undergoing surgery. Tumori.
108:56–62. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X, Wang D, Sun T, Li W and Dang C:
Advanced lung cancer inflammation index (ALI) predicts prognosis of
patients with gastric cancer after surgical resection. BMC Cancer.
22:6842022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mountzios G, Samantas E, Senghas K, Zervas
E, Krisam J, Samitas K, Bozorgmehr F, Kuon J, Agelaki S, Baka S, et
al: Association of the advanced lung cancer inflammation index
(ALI) with immune checkpoint inhibitor efficacy in patients with
advanced non-small-cell lung cancer. ESMO Open. 6:1002542021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guven DC, Aktepe OH, Aksun MS, Sahin TK,
Kavgaci G, Ucgul E, Cakir IY, Yildirim HC, Guner G, Akin S, et al:
The association between albumin-globulin ratio (AGR) and survival
in patients treated with immune checkpoint inhibitors. Cancer
Biomark. 34:189–199. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hill LA, Bodnar TS, Weinberg J and Hammond
GL: Corticosteroid-binding globulin is a biomarker of inflammation
onset and severity in female rats. J Endocrinol. 230:215–225. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bae YJ and Kratzsch J:
Corticosteroid-binding globulin: Modulating mechanisms of
bioavailability of cortisol and its clinical implications. Best
Pract Res Clin Endocrinol Metab. 29:761–772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xie HL, Zhang Q, Ruan GT, Ge YZ, Hu CL,
Song MM, Song CH, Zhang X, Zhang XW, Li XR, et al: Evaluation and
validation of the prognostic value of serum albumin to globulin
ratio in patients with cancer cachexia: Results from a large
multicenter collaboration. Front Oncol. 11:7077052021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Salciccia S, Frisenda M, Bevilacqua G,
Viscuso P, Casale P, De Berardinis E, Di Pierro GB, Cattarino S,
Giorgino G, Rosati D, et al: Prognostic value of albumin to
globulin ratio in non-metastatic and metastatic prostate cancer
patients: A meta-analysis and systematic review. Int J Mol Sci.
23:115012022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rong G, Liu S, Xi C, Wang C, Deng J and
Qin T: Correlation between preoperative serum albumin-globulin
ratio and prognosis of patients undergoing low rectal cancer
surgery. Clin Lab. 68:2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oymak E, Guler OC and Onal C: Prognostic
significance of albumin and globulin levels in cervical cancer
patients treated with chemoradiotherapy. Int J Gynecol Cancer.
33:19–25. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gundog M and Basaran H: Pretreatment low
prognostic nutritional index and low albumin-globulin ratio are
predictive for overall survival in nasopharyngeal cancer. Eur Arch
Otorhinolaryngol. 276:3221–3230. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu C, Wang W, Meng X, Sun B, Cong Y, Liu
J, Wang Q, Liu G and Wu S: Albumin/globulin ratio is negatively
correlated with PD-1 and CD25 mRNA levels in breast cancer
patients. Onco Targets Ther. 11:2131–2139. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lv GY, An L, Sun XD, Hu YL and Sun DW:
Pretreatment albumin to globulin ratio can serve as a prognostic
marker in human cancers: A meta-analysis. Clin Chim Acta.
476:81–91. 2018. View Article : Google Scholar : PubMed/NCBI
|