Introduction
Colorectal cancer (CRC) and pancreatic cancer (PC)
are both among the leading causes of cancer-associated deaths in
the United States (US) (1,2). CRC is the second most common cancer
diagnosed in women and the third most common cancer in men, with
population-based annual incidence rates of ~37.7 cases per 100,000
individuals in the US (1). PC is a
relatively uncommon cancer, with ~13.3 diagnoses per 100,000
individuals in the US each year (2,3);
however, it is predicted to be the second leading cause of
cancer-related mortality in the US by 2030, owing to its advanced
stage at diagnosis and resistance to chemotherapy (4).
Multiple primary neoplasms (MPNs), defined as the
presence of two or more histologically distinct neoplasms that are
not due to recurrence or metastasis in the same individual, are
grouped into two large categories, namely synchronous neoplasms
(the second primary neoplasm is diagnosed within 6 months after the
diagnosis of the first neoplasm) and metachronous neoplasms (the
second primary neoplasm is diagnosed >6 months after the
diagnosis of the first neoplasm) (5,6). Over
the past decades, MPNs have been reported quite frequently but have
rarely involved both CRC and PC (7,8). Dayer
et al (8) reported a case of
a synchronous CRC and pancreatic neuroendocrine tumor, a less
common neoplasm arising from the pancreas with a distinct molecular
profile and natural history from PDAC. Li Destri et al
(9) reported only two confirmed PC
cases (one synchronous and the other metachronous) out of 842 CRC
cases (0.24%). There are no established guidelines for the
management of MPNs, therefore making clinical decisions difficult.
The present study reports the case of a 61-year-old man presenting
with synchronous CMAC and PDAC. The study aims to report this
experience in order to shed light on potential clues regarding the
etiology of MPNs.
Case report
A 61-year-old man presented to the local hospital
with hematochezia for half a month. The patient did not have bowel
habit changes, nausea, emesis or body weight loss during this
episode. The patient had been drinking and smoking for 20 years,
and had been taking medication for primary hypertension for 1 year.
There was no history of malignancy in the family. The patient
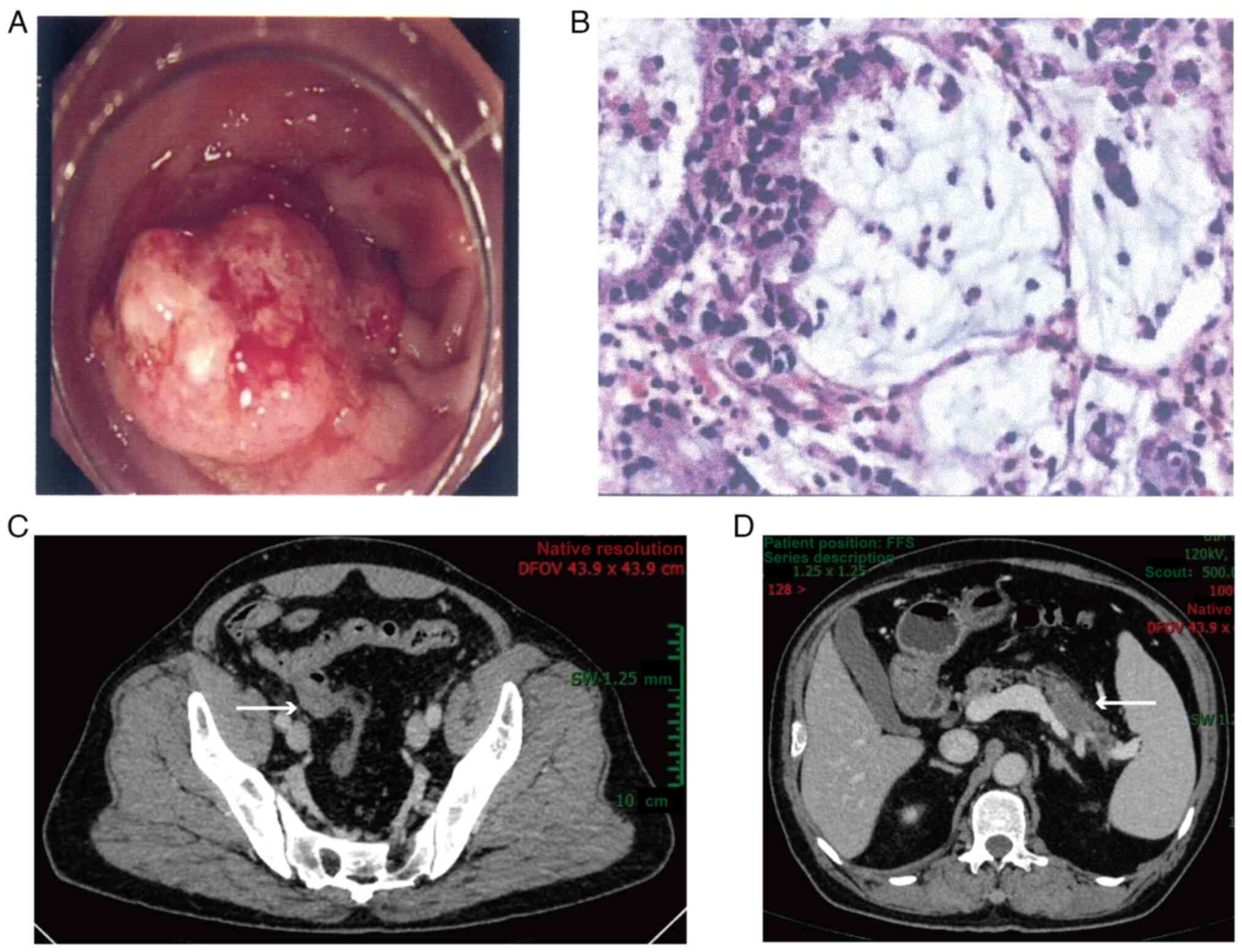
underwent a colonoscopy and was found to have an ulcerative mass of
2 cm in diameter in the sigmoid colon (Fig. 1A). Histological examination
(hematoxylin and eosin staining) of the biopsy specimens showed
abundant mucous in the extracellular space, which indicated
mucinous adenocarcinoma (Fig. 1B).
The patient was then transferred to Ren Ji Hospital (Shanghai,
China) for further diagnosis and treatment. A physical examination
revealed no specific signs of illness. Laboratory examinations
showed that tumor biomarker levels, including those for
carcinoembryonic antigen and carbohydrate antigen 19-9, were all
within the normal ranges. Consistent with the findings of the
colonoscopy, abdominal contrast-enhanced computed tomography (CT)
showed that the thickened sigmoid colon wall was unevenly
strengthened with a rough surface (Fig.
1C). Unexpectedly, a hypovascular lesion was also discovered in
the body and tail of the pancreas, which highly suggested the
occurrence of pancreatic cancer (Fig.
1D).
A multidisciplinary team of internists, surgeons,
oncologists, radiologists and pathologists diagnosed the patient
with synchronous CRC and PC after a comprehensive analysis of the
clinical findings, and proposed combined surgery for the two
tumors. Thereafter, a distal pancreaticosplenectomy and a radical
sigmoidectomy with end-to-end colorectal anastomosis were
performed. Grossly, there was an ulcerative and hard bulging mass
of 2 cm in diameter invading the serosal layer of the sigmoid
colon. In addition, there was another gray and hard mass of 4 cm in
diameter in the body and tail of the pancreas, with surrounding
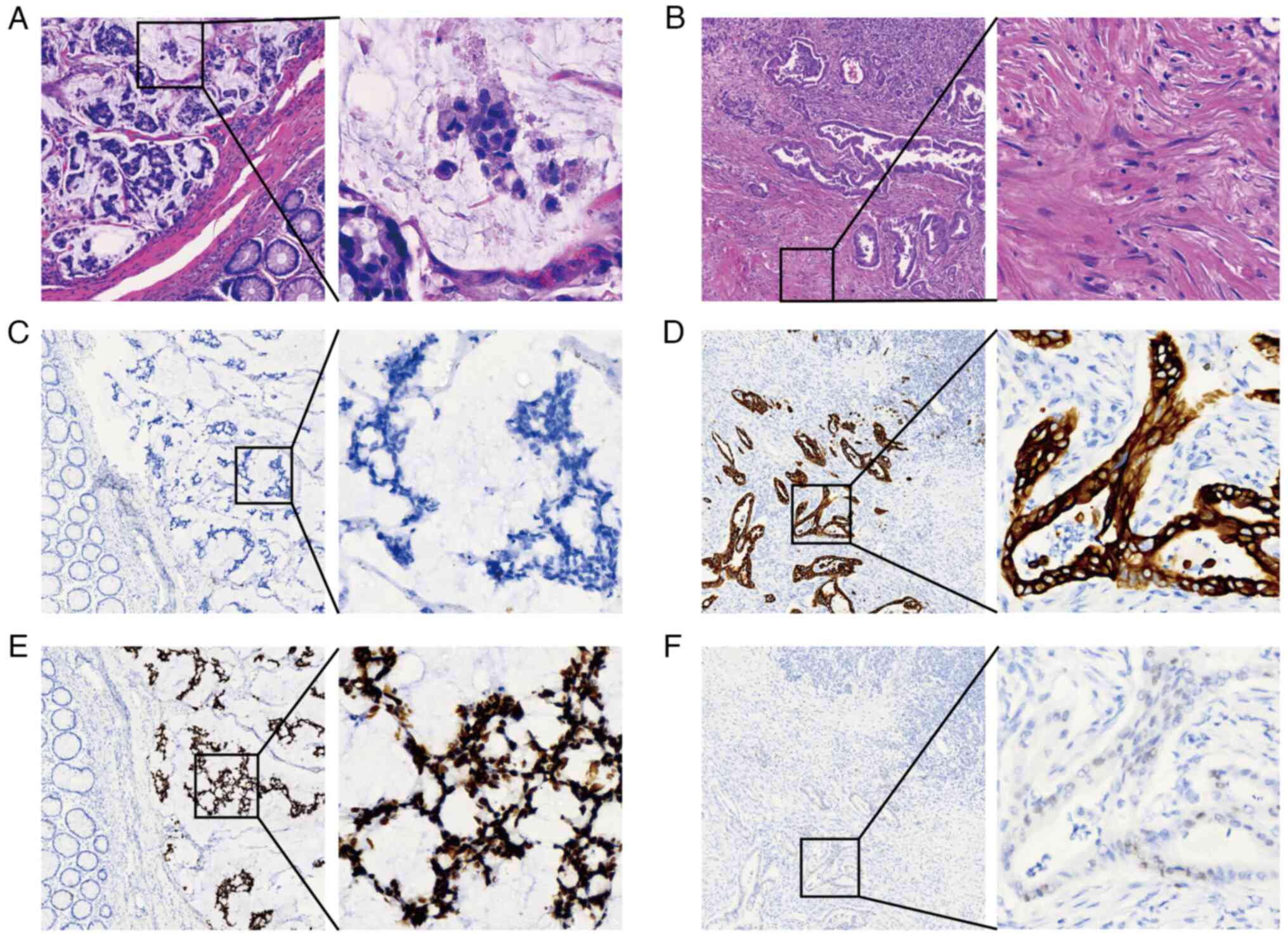
vessels dilated and congested. Microscopically, the sections of the
colon specimens showed poorly differentiated adenocarcinoma with
lymph node metastasis (3/14 lymph nodes), most of which (>50%)
consisted of a mucinous component (Fig.
2A). Angiolymphatic and perineural invasion were both positive.
The pancreatic specimen was determined to be moderately
differentiated ductal adenocarcinoma with lymph node metastasis
(1/16 lymph nodes) and angiolymphatic and perineural invasion
(Fig. 2B). Immunohistochemically,
the CRC cells were negative for CK7 (Fig. 2C), while the PC cells were positive
for CK7 (Fig. 2D), implicating a
histologically distinct origin. Additionally, in terms of the
expression level of TP53, the CRC cells exhibited a strongly
positive pattern (Fig. 2E), whereas
the PC cells demonstrated a weakly positive pattern (Fig. 2F). The mismatch repair status was
stable in both tumors according to the findings of
immunohistochemical (IHC) staining, with retained expression of DNA
mismatch repair protein Mlh1 (MLH1), DNA mismatch repair protein
Msh2 (MSH2), MSH6 and mismatch repair endonuclease PMS2 (PMS2)
proteins (Fig. S1). To uncover the
underlying genetic alterations, a comprehensive molecular analysis
by next-generation sequencing (NGS) (a panel of 520 gene hotspot
mutations closely associated with tumorigenesis and targeted
therapy) was conducted. NGS revealed a missense mutation of KRAS in
PC. With respect to CRC, a nonsense mutation of APC was discovered,
and a missense mutation of TP53 was observed, partially explaining
its high expression level as shown by IHC stain. There were no
deleterious germline mutations in the two tumors. The protocols of
hematoxylin and eosin staining, IHC staining and NGS are reported
in Data S1.
At 6 weeks after surgery, the patient started to
receive a combination of 5-fluorouracil (2,400 mg/m2 of
body surface area), leucovorin (400 mg/m2), irinotecan
(150 mg/m2) and oxaliplatin (85 mg/m2)
(modified FOLFIRINOX regimen) every 2 weeks and completed 12
cycles, without serious adverse events documented. The patient was
followed up every third month after surgery and had recovered well
and was free of cancer recurrence at the 1-year follow-up.
Discussion
Emerging evidence suggests that the increasing
occurrence of MPNs is affected by a myriad of factors, including
the late effects of cancer therapy, genetic predisposition,
behavioral and lifestyle factors, environmental determinants (such
as viral infection and occupation), host effects (such as age, sex,
immune function and hormones) and combinations of these factors
(such as gene-environment interactions) (10). On the basis of the major etiological
factors, Travis et al (11)
categorized MPNs into three distinct groups, including
treatment-associated MPNs, syndromic MPNs and those with shared
etiology.
Despite the fact that great advances in cancer
therapy, such as chemotherapy, immunotherapy and targeted therapy,
have markedly improved the overall survival time of patients with
cancer, they have significantly increased the risk of MPNs for
cancer survivors, partially due to acquired somatic gene mutations
and chromosomal abnormalities (12). For instance, the use of alkylating
agents and DNA-topoisomerase inhibitors has increased the risk of
secondary leukemia (13). However,
in the present case, the patient had not previously undergone
anticancer therapy, therefore excluding the possibility of
treatment-associated MPNs.
Individuals with germline mutations in cancer
predisposition genes are prone to various benign or malignant
tumors; therefore, they may be eligible for high-risk screening and
prevention strategies. To date, >100 of these genes have been
identified, such as TP53, APC, BRCA1, BRCA2 and PMS2 (14,15).
Heterozygous germline mutations occur in most cases (16). The most common cancer syndrome
identified in patients with CRC is hereditary nonpolyposis CRC,
also termed Lynch syndrome, which is characterized by a germline
mutation of a mismatch repair gene, including MLH1, MSH2, PMS2 and
MSH6, or a germline deletion of EpCAM (17). In this study, as shown by the
results of IHC, tumor cells in both CMAC and PDAC stably expressed
MLH1, MSH2, PMS2 and MSH6, therefore excluding the possibility of
Lynch syndrome. Additionally, no pathogenic germline mutations were
detected by NGS, indicating that the two tumors may arise from a
sporadic occurrence.
It is acknowledged that progressive accumulation of
somatic gene mutations resulting from replication errors or DNA
damage throughout life could result in sporadic cancer (18). In the present case, somatic
mutations of oncogenes were observed in both cancer types. A
somatic mutation of KRAS, an essential gene controlling MAPK
signaling, is the most common oncogenic alteration in PDAC,
occurring in ~90% of cases (19),
including the present case. This mutation facilitates PDAC cell
survival by protecting against inflammation-associated senescence
and promoting autophagy, micropinocytosis and stress granule
formation (20). KRAS mutation is
also frequently observed in CRC and non-small cell lung cancer
(NSCLC), and serves as a promising target for cancer therapy
(21–24). TP53 is an essential tumor suppressor
gene that can sense DNA damage and then arrest the cell cycle,
acting as the so-called ‘guardian of the genome’. A somatic
mutation of TP53 can be found in various cancer types, including
CRC, PDAC, gastric cancer (GC), NSCLC, lymphoma and leukemia
(25–27). This mutation is closely associated
with colorectal adenoma-carcinoma transition and confers a poor
prognosis (28). Compared with
mutations in the aforementioned two genes, the somatic mutation of
APC predominantly occurs in CRC (1). Through the regulation of β-catenin
levels and localization, APC functions as a tumor suppressor, and
inactivation attributable to somatic mutation occurs in 70–80% of
sporadic CRC cases (29).
Previous reports of MPNs involving CRC or PDAC
highlight genetic alteration as a potential factor fostering cancer
initiation and progression (30–34).
Hirata et al (32) reported
a case of Lynch syndrome. The patient, who had a history of rectal
and urinary bladder cancer, developed a liposarcoma in the left
thigh. Genetic tests of a blood sample revealed a pathogenic
germline mutation of MSH2 (32). In
another case, an 86-year-old man was diagnosed with synchronous CRC
and B-cell chronic lymphocytic leukemia. Somatic mutations of KRAS
and BRAF were determined in the CRC specimen (34). In another patient with metachronous
CRC and breast cancer, genetic tests demonstrated constitutional
hypermethylation of the MLH1 promoter in both of the cancer
specimens, while no germline or somatic mutations were found,
suggesting an essential role of epigenetic mutation in
tumorigenesis (31). Consistent
with the present case, a somatic mutation of KRAS was discovered in
a previous case involving PDAC (33). Furthermore, in a 56-year-old man
with metachronous PDAC and hereditary diffuse GC, a germline
mutation of CDH1 was identified in the GC. In terms of PDAC,
somatic mutations of KRAS and TP53 were determined, but no CDH1
mutation was found, indicating a histologically distinct origin of
the two tumors (33).
The role of lifestyle factors, such as tobacco use,
excessive alcohol intake and obesity, emerges as an area of great
interest with regard to MPNs. Tobacco products lead to millions of
cancer-associated deaths per year worldwide and are one of the
major causes of MPNs. Tobacco delivers a plethora of carcinogens,
such as polycyclic aromatic hydrocarbons and volatile organic
compounds, which are responsible for the somatic mutations observed
in cancer predisposition genes (35). Alcohol intake is another
acknowledged risk factor for cancer. Multiple mechanisms are
responsible for its oncogenic effect, including the genotoxic
effect of acetaldehyde (the primary metabolite of alcohol),
increased estrogen levels, the production of reactive oxygen
species and nitrogen species, and changes in folate metabolism
(36). Notably, Prabhu et al
(37) discovered a positive
synergistic effect of alcohol and tobacco use on the risk of
esophageal squamous cell carcinoma. In the present case, the
patient had a 20-year history of drinking and smoking, therefore
placing himself at an elevated risk of various tumors. There is
therefore a pressing need to improve people's lifestyles, for
example, encouraging them to control weight and alcohol
consumption, and quit smoking, which may contribute to the
prevention of MPNs.
Adenocarcinoma is the most common form of CRC,
occurring in ~85% of patients with CRC, whereas only 10–15% of
patients with CRC are diagnosed with mucinous adenocarcinoma, which
is characterized by abundant extracellular mucin comprising at
least 50% of the tumor volume (38). Compared with the non-mucinous
subtype, mucinous colorectal adenocarcinoma is more frequently
diagnosed at an advanced stage (39). In addition, it has an aberrant
metastatic pattern and it often metastasizes to more than one site,
especially to extrahepatic regions, such as distant lymph nodes and
the peritoneum, which predicts a poor prognosis (38). In the present case, no distant
metastases were found, with only regional lymph node metastases,
suggesting localized advanced disease.
Due to the lack of specific symptoms at an early
stage, ~90% of patients with PC are diagnosed at an advanced stage,
with systematic metastasis observed in >50% of cases, therefore
leading to a high mortality rate (40). Regarding the diagnostic modalities,
abdominal CT angiography exhibits a sensitivity ranging from 76 to
96% in the detection of PC, which is helpful in the assessment of
vascular anatomy and stage of disease (41). Endoscopic ultrasound-guided
fine-needle aspiration (EUS-FNA) is recognized as an adjunctive
tool for tissue acquisition to confirm the histologic diagnosis and
facilitate molecular analysis (2).
In the present case, it would have been more rational to have
conducted EUS-FNA before the surgery; however, the patient declined
due to the potential complications, such as infection, hemorrhage
and pancreatitis.
In the present case, the patient complained of
hematochezia for half a month, which is a common sign of CRC. The
subsequent colonoscopy and hematoxylin and eosin staining of the
biopsy specimens revealed the diagnosis of mucinous adenocarcinoma
in the sigmoid colon. The findings of the abdominal
contrast-enhanced CT showed an unevenly strengthened mass in the
sigmoid colon, further validating the diagnosis of CMAC, the origin
of which was unknown. Another hypodense lesion in the pancreas was
also identified in the CT scan. It has been established that
primary PC typically appears as hypodense relative to the
pancreatic parenchyma owing to high interstitial pressures within
the tumor tissue (42), which is in
line with the present case. However, metastatic pancreatic lesions
arising from CRC appeared hyperdense in a previous case, indicating
that the possibility of metastatic PC was relatively low (43). The most likely method of cancer
metastasis from the pancreas to the sigmoid colon is through the
blood vessels. However, the vein of the pancreas leads to the liver
via the portal vein. Hence, the possibility of solitary colonic
metastasis without liver metastasis is extremely low. Based on the
aforementioned facts, in the present study, it was assumed that the
patient had synchronous colonic and pancreatic cancer, and the
postoperative pathological tests corroborated this hypothesis.
As there are no established guidelines for MPNs
involving both CMAC and PDAC, treatment protocols should be
tailored to the specific condition of the patient, including
functional status, tumor stage, gene mutation and economic status.
In the present study, after a comprehensive analysis of the
preoperative CT scan, the tumors were both determined to be
resectable and a combined surgical resection was performed followed
by adjuvant chemotherapy with curative intent. According to results
of the multicenter PRODIGE-24 trial, the modified FOLFIRINOX
regimen is recommended as the first-line adjuvant chemotherapy for
patients with resected PDAC who exhibit excellent performance
status (Eastern Cooperative Oncology Group Score, 0–1) (44,45).
Fluoropyrimidine and oxaliplatin are the cornerstones of adjuvant
chemotherapy for patients with CRC in stage III and a subset of
those in stage II. The therapeutic role of irinotecan in metastatic
CRC has been widely acknowledged (1). Therefore, in the present study, the
modified FOLFIRINOX regimen was chosen as the adjuvant
chemotherapy, due to it ability to exert cytotoxic effects on both
cancer cell types. CMAC has been reported with impaired responses
to current chemotherapies, as the surrounding mucous may function
as a physical barrier to the drugs (39). Nevertheless, the current patient
responded well and has not shown any sign of recurrence or
metastasis up to the time of this report.
In conclusion, the present study reports a rare case
of synchronous CMAC and PDAC in a 61-year-old man without a family
history of malignancy. Radical surgery was undertaken to remove the
two tumors concurrently, followed by adjuvant chemotherapy, which
proved to be effective. As demonstrated by the NGS results, no
deleterious germline mutations were found, whereas critical somatic
mutations were discovered in both tumors. In addition, unhealthy
lifestyle factors were identified in the patient, suggesting that
the disease may be a result of a complex interaction between genes
and the environment. Overall, it is of great importance to identify
additional primary malignancies in clinical practice, as they could
largely influence the planned therapeutic schedule, and thereby the
long-term outcomes of patients.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was funded by The Natural Science Foundation of
Shanghai (grant no. 22ZR1438800).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request. Data sequences from NGS are not available due to the use
of proprietary computational algorithms and to protect patient
anonymity.
Authors' contributions
XQ, ZZ and CZ conceived and designed the study. BN,
YS, YZ, XX and HC acquired and interpreted the clinical data. XQ,
BN and YS drafted and revised the manuscript. ZZ and CZ confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the case report and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wood LD, Canto MI, Jaffee EM and Simeone
DM: Pancreatic cancer: Pathogenesis, screening, diagnosis, and
treatment. Gastroenterology. 163:386–402.e1. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park W, Chawla A and O'Reilly EM:
Pancreatic cancer: A review. JAMA. 326:851–862. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhai C, Cai Y, Lou F, Liu Z, Xie J, Zhou
X, Wang Z, Fang Y, Pan H and Han W: Multiple primary malignant
tumors-a clinical analysis of 15,321 patients with malignancies at
a single center in China. J Cancer. 9:2795–2801. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warren S: Multiple primary malignant
tumors. A survey of the literature and a statistical study. Am J
Cancer. 16:1358–1414. 1932.
|
|
7
|
Sakellakis M, Peroukides S, Iconomou G,
Boumpoucheropoulos S and Kalofonos H: Multiple primary
malignancies: A report of two cases. Chin J Cancer Res. 26:215–218.
2014.PubMed/NCBI
|
|
8
|
Dayer N, Fasquelle F, Salati E and
Dietrich G: Multiple primary malignancies: Synchronous lymphoma,
pancreatic neuroendocrine tumour and colorectal cancer. BMJ Case
Rep. 14:e2419382021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Destri G, Giarrizzo A, Bellavia N,
Milazzotto R, Frattalone ME, Scilletta B and Di Cataldo A:
Synchronous double cancers of the colon and the pancreas: A case
report. Eur Rev Med Pharmacol Sci. 18:28–31. 2014.PubMed/NCBI
|
|
10
|
Travis LB, Demark Wahnefried W, Allan JM,
Wood ME and Ng AK: Aetiology, genetics and prevention of secondary
neoplasms in adult cancer survivors. Nat Rev Clin Oncol.
10:289–301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Travis LB, Rabkin CS, Brown LM, Allan JM,
Alter BP, Ambrosone CB, Begg CB, Caporaso N, Chanock S, DeMichele
A, et al: Cancer survivorship-genetic susceptibility and second
primary cancers: Research strategies and recommendations. J Natl
Cancer Inst. 98:15–25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wood ME, Vogel V, Ng A, Foxhall L, Goodwin
P and Travis LB: Second malignant neoplasms: Assessment and
strategies for risk reduction. J Clin Oncol. 30:3734–3745. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leone G, Pagano L, Ben-Yehuda D and Voso
MT: Therapy-related leukemia and myelodysplasia: Susceptibility and
incidence. Haematologica. 92:1389–1398. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Walsh MF, Wu G, Edmonson MN,
Gruber TA, Easton J, Hedges D, Ma X, Zhou X, Yergeau DA, et al:
Germline mutations in predisposition genes in pediatric cancer. N
Engl J Med. 373:2336–2346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rahman N: Realizing the promise of cancer
predisposition genes. Nature. 505:302–308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carbone M, Arron ST, Beutler B, Bononi A,
Cavenee W, Cleaver JE, Croce CM, D'Andrea A, Foulkes WD, Gaudino G,
et al: Tumour predisposition and cancer syndromes as models to
study gene-environment interactions. Nat Rev Cancer. 20:533–549.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sinicrope FA: Lynch syndrome-associated
colorectal cancer. N Engl J Med. 379:764–773. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martincorena I and Campbell PJ: Somatic
mutation in cancer and normal cells. Science. 349:1483–1489. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cancer Genome Atlas Research Network.
Electronic address: andrew_aguirre@dfci.harvard.edu, . Cancer
Genome Atlas Research Network: Integrated genomic characterization
of pancreatic ductal adenocarcinoma. Cancer Cell. 32:185–203.e13.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hayashi A, Hong J and Iacobuzio-Donahue
CA: The pancreatic cancer genome revisited. Nat Rev Gastroenterol
Hepatol. 18:469–481. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
JCO Flashback: Predictive value of KRAS
mutations on the outcomes of panitumumab monotherapy in colorectal
cancer. J Clin Oncol. 41:32772023. View Article : Google Scholar
|
|
22
|
Dy GK, Govindan R, Velcheti V, Falchook
GS, Italiano A, Wolf J, Sacher AG, Takahashi T, Ramalingam SS,
Dooms C, et al: Long-term outcomes and molecular correlates of
sotorasib efficacy in patients with pretreated KRAS G12C-mutated
non-small-cell lung cancer: 2-year analysis of CodeBreaK 100. J
Clin Oncol. 41:3311–3317. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van de Haar J, Ma X, Ooft SN, van der Helm
PW, Hoes LR, Mainardi S, Pinato DJ, Sun K, Salvatore L, Tortora G,
et al: Codon-specific KRAS mutations predict survival benefit of
trifluridine/tipiracil in metastatic colorectal cancer. Nat Med.
29:605–614. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mullard A: The KRAS crowd targets its next
cancer mutations. Nat Rev Drug Discov. 22:167–171. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karlsson K, Przybilla MJ, Kotler E, Khan
A, Xu H, Karagyozova K, Sockell A, Wong WH, Liu K, Mah A, et al:
Deterministic evolution and stringent selection during
preneoplasia. Nature. 618:383–393. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Frankell AM, Dietzen M, Al Bakir M, Lim
EL, Karasaki T, Ward S, Veeriah S, Colliver E, Huebner A, Bunkum A,
et al: The evolution of lung cancer and impact of subclonal
selection in TRACERx. Nature. 616:525–533. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen R, Fu D, Dong L, Zhang MC, Shi Q, Shi
ZY, Cheng S, Wang L, Xu PP and Zhao WL: Simplified algorithm for
genetic subtyping in diffuse large B-cell lymphoma. Signal
Transduct Target Ther. 8:1452023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iacopetta B: TP53 mutation in colorectal
cancer. Hum Mutat. 21:271–276. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fearon ER: Molecular genetics of
colorectal cancer. Ann Rev Pathol. 6:479–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aaquist T, Dembic M, Thomassen M, de
Stricker K, Bertelsen M, Christensen LG, Mortensen MB and Detlefsen
S: Synchronous detection of pancreatic adenocarcinoma and
paraganglioma in a Whipple resection specimen. Pathology Res Pract.
226:1535902021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brandler J, Wu TT and Sweetser S: Young
onset breast and colon cancer. Gastroenterology. 152:e12–e13. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hirata K, Kanemitsu S, Nakayama Y, Nagata
N, Itoh H, Ohnishi H, Ishikawa H and Furukawa Y: A novel germline
mutation of MSH2 in a hereditary nonpolyposis colorectal cancer
patient with liposarcoma. Am J Gastroenterol. 101:193–196. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ottenhof NA, de Wilde RF, Morsink FH, de
Leng WW, Ausems MG, Morreau H, van Hillegersberg R, Offerhaus GJ
and Milne AN: Pancreatic ductal adenocarcinoma in hereditary
diffuse gastric cancer. A case report. Hum Pathol. 43:457–461.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Silvestris N, Zito FA, Fiore MG, Simone G,
Tommasi S, Izzi G, Guarini A and Colucci G: Synchronous
presentation of B-cell chronic lymphocytic leukemia/small-cell
lymphoma and colon adenocarcinoma within the same mesenteric lymph
nodes and a single liver metastasis. J Clin Oncol. 29:e11–e13.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hecht SS and Hatsukami DK: Smokeless
tobacco and cigarette smoking: Chemical mechanisms and cancer
prevention. Nat Rev Cancer. 22:143–155. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boffetta P and Hashibe M: Alcohol and
cancer. Lancet Oncol. 7:149–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Prabhu A, Obi KO and Rubenstein JH: The
synergistic effects of alcohol and tobacco consumption on the risk
of esophageal squamous cell carcinoma: A meta-analysis. Am J
Gastroenterol. 109:822–827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hugen N, Brown G, Glynne-Jones R, de Wilt
JH and Nagtegaal ID: Advances in the care of patients with mucinous
colorectal cancer. Nat Rev Clin Oncol. 13:361–369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luo C, Cen S, Ding G and Wu W: Mucinous
colorectal adenocarcinoma: Clinical pathology and treatment
options. Cancer Commun (Lond). 39:132019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chu LC, Goggins MG and Fishman EK:
Diagnosis and detection of pancreatic cancer. Cancer J. 23:333–342.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mizrahi JD, Surana R, Valle JW and Shroff
RT: Pancreatic cancer. Lancet. 395:2008–2020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Su L and Wernberg J: Synchronous distal
pancreatic metastatic lesion arising from colonic adenocarcinoma:
Case report and literature review. Clin Med Res. 12:166–170. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Conroy T, Castan F, Lopez A, Turpin A, Ben
Abdelghani M, Wei AC, Mitry E, Biagi JJ, Evesque L, Artru P, et al:
Five-year outcomes of FOLFIRINOX vs. gemcitabine as adjuvant
therapy for pancreatic cancer: A randomized clinical trial. JAMA
Oncol. 8:1571–1578. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Conroy T, Hammel P, Hebbar M, Ben
Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi
JJ, et al: FOLFIRINOX or gemcitabine as adjuvant therapy for
pancreatic cancer. N Engl J Med. 379:2395–2406. 2018. View Article : Google Scholar : PubMed/NCBI
|