Introduction
Ovarian cancer is one of the three most common malignant tumors of the female reproductive system. The mortality rate of ovarian cancer ranks first among gynecological malignancies, and the 5-year survival rate is only ~40% (1,2). Epithelial ovarian cancer is the most common histological type, accounting for ~90% of all ovarian cancers (3). Epithelial ovarian cancer cells are highly invasive, and widespread metastasis to the pelvis, abdominal cavity and retroperitoneal lymph nodes can occur in the early stage. Even if patients receive standard treatment, ~70% of the advanced patients experience recurrence, metastasis and drug resistance within 2–3 years (4), which poses a serious threat to life and health.
Transmembrane 4 L6 family member 1 (TM4SF1) is a distant relative of the four-transmembrane protein superfamily. It is highly expressed in a variety of epithelial cancer tissues, and it regulates intracellular calcium levels, tyrosine phosphorylation and protein kinase C (5,6). In addition, TM4SF1 is involved in the formation of vascular endothelial pseudopodia and angiogenesis, and plays an important role in the regulation of cell development, activation, growth and movement (7). It has been reported that the upregulation of TM4SF1 in a variety of epithelial cancer tissues is associated with a poor prognosis (8–11). Discoidin domain receptor 1 (DDR1) is a member of the DDR family of receptor tyrosine kinases, and the abnormal upregulation of DDR1 is associated with the occurrence and development of tumors. The significantly upregulated expression of DDR1 has been detected in numerous types of malignant tumors (12), including breast cancer, non-small cell lung cancer, gastric cancer and colorectal cancer, and DDR1 is regarded as an adverse prognostic factor (13–16). Similar to TM4SF1, DDR1 is critical for tumor cell survival, drug resistance, self-renewal, differentiation, adhesion and migration, and participates in the regulation of tumor progression and metastasis (17).
According to a previous study, TM4SF1 and DDR1 interact with each other and activate resting cancer cells in distant organs to cause tumor recurrence and metastasis (18). However, the relationship of the expression of TM4SF1 and DDR1 with epithelial ovarian cancer prognosis remains unclear. Therefore, the present study investigated the expression of TM4SF1 and DDR1 in epithelial ovarian cancer tissues and the association of TM4SF1 and DDR1 expression levels with the prognosis of patients.
Materials and methods
Patients and tissue samples
Patients were treated at the Department of Gynecological Oncology of Guangxi Medical University Cancer Hospital (Nanning, China) between January 2013 and June 2017. Inclusion criteria were as follows: Patients with epithelial ovarian cancer, >18 years of age, who had received cytoreductive or comprehensive staging surgery and adjuvant chemotherapy in Guangxi Medical University Cancer Hospital, and whose clinicopathological data were complete. Exclusion criteria were as follows: Patients <18 years of age, non-epithelial ovarian cancer, no debulking or staging surgery, no adjuvant chemotherapy, incomplete clinicopathological data, and patients who refused to participate in the study. A total of 94 patients with epithelial ovarian cancer were included, whose age ranged from 28 to 83 years, with a median age of 51 years according to inclusion/exclusion criteria. All patients received ovarian tumor cytoreductive or comprehensive staging surgery and adjuvant chemotherapy. In addition, 56 patients received neoadjuvant chemotherapy. All cases were confirmed by pathological diagnosis, and complete clinicopathological parameters were obtained, including age, abdominal invasion/metastasis, histopathological type, histological grade, degree of differentiation, FIGO tumor stage (19), neoadjuvant chemotherapy regimen, cancer recurrence and survival after treatment. Progression-free survival (PFS) and overall survival (OS) were defined as the time from the day of initial treatment to the day of clinical recurrence by CA125 and/or imaging such as CT, MRI or PET/CT, and to the day of death, respectively. The follow-up data were obtained by telephone calls or outpatient visits. The tissue samples of epithelial ovarian cancer were collected from January 2013 to July 2017. The present study was approved by the Ethics Committee of Guangxi Medical University Cancer Hospital (approval no. LW2023118).
Immunohistochemistry (IHC) staining
Paraffin-embedded epithelial ovarian cancer tissues were obtained from the Department of Pathology of Guangxi Medical University Cancer Hospital. The tissues were cut into 4-µm paraffin sections and heated at 60°C for 20 min. The sections were then soaked in xylene and different concentrations of alcohol (95, 70 and 50%) for dewaxing followed by soaking in distilled water for 5 min for hydration. The hydrated tissue sections were then immersed in a container containing 0.01 M citrate buffer (pH 6.0) and heated to 92–98°C for 10–15 min for antigen retrieval. A SP immunohistochemical kit (cat. no. SP-9000; ZSGB-BIO) was used for the immunohistochemical detection of TM4SF1 and DDR1 expression in epithelial ovarian cancer tissue according to the manufacturer's protocol. The tissue sections were incubated with 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity, followed by incubation with goat serum working solution for 15 min at room temperature to block the non-specific binding sites. Samples were sequentially incubated in rabbit anti-TM4SF1 (1:1,000; cat. no. ab113504; Abcam) or rabbit anti-DDR1 (1:3,000; cat. no. 5583; Cell Signaling Technology, Inc.) overnight at 4°C, biotin-labeled goat anti-mouse/rabbit IgG for 1 h at room temperature and streptavidin conjugated with horseradish peroxidase working solution for 15 min at room temperature. Finally, the tissue sections were stained with 3,3′-diaminobenzidine solution and counterstained with hematoxylin for 2 min. The immunostained tissue sections were independently observed by two experienced pathologists under a light microscope (Olympus Corporation). The staining was scored and determined to be positive or negative according to methods previously reported in the literature (20).
Gene Expression Profiling Interactive Analysis (GEPIA)
The interactive online analysis tool GEPIA (http://gepia.cancer-pku.cn/) was used to analyze the RNA sequencing expression data of 9,736 tumor tissues from The Cancer Genome Atlas and 8,587 normal tissues from the Genotype-Tissue Expression database (21). GEPIA was used to determine the expression levels of the TM4SF1 and DDR1 genes in different cancer types, and the differential expression of the TM4SF1 and DDR1 genes in ovarian cancer tissue and normal ovarian tissue was compared. Analysis of the difference in OS and disease-free survival (DFS) between low or high TM4SF1 and DDR1 expression in ovarian cancer tissue was then conducted. The hazard ratio (HR) and P-value or log-rank P-value were calculated and displayed in the graph.
Oncomine analysis
Oncomine is a large cancer microarray database and integrated data mining platform (https://www.oncomine.org), which includes 715 gene expression datasets and 86,733 cancer tissue and normal tissue samples (22,23). First, Oncomine was searched using ‘TM4SF1’ and ‘DDR1’ as keywords, and the results were displayed and set as: ‘P-value: 0.01’, ‘fold change: 1.5’ and ‘gene rank: all’ to analyze the differential expression of TM4SF1 and DDR1 in different types of tumors and the corresponding normal tissues. Then, ‘TM4SF1 and ‘DDR1’ were used as keywords, and ‘ovarian cancer’, ‘cancer vs. normal’ and ‘mRNA’ data types were used as filter conditions, and the meta-analysis performed by the Oncomine database was used to verify the differential expression of TM4SF1 and DDR1 genes in ovarian cancer tissue.
Protein-protein interaction (PPI) network
Search Tool for the Retrieval of Interacting Genes/Proteins (STRING; version 11.0) is an online search tool for the analysis of PPIs and functional protein networks, which contains confirmed and predicted direct and indirect PPI biological data (24). In the present study, TM4SF1 and DDR1 were used as query conditions, and the minimum required interaction score was set to >0.4, which is a medium level of confidence. The top 50 proteins interacting directly with TM4SF1 and DDR1 were queried according to the interaction score.
Functional and pathway enrichment analyses
Functional and pathway enrichment analyses were performed using R 3.63 software (https://www.r-project.org/). Using the R clusterProfiler package, TM4SF1, DDR1 and the top 50 filtered interacting proteins that were predicted to directly interact with TM4SF1 and DDR1 were used to perform Gene Ontology (GO) function and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses (25,26). The R enrichplot (https://bioconductor.org/packages/enrichplot/; version 1.20.0) and ggplot2 (https://CRAN.R-project.org/package=ggplot2; version 3.3.1) packages were used to visualize the enrichment analysis results. The GO functional enrichment analysis included the following three categories: Biological process, cell composition and molecular function (MF). A corrected P-value of <0.05 was considered statistically significant.
Kaplan-Meier (KM) plotter database analysis
KM plotter is an online database that assesses the relationship of 54,000 genes with survival rates in 21 cancer types (27). The relationships of TM4SF1 expression, DDR1 expression and combined TM4SF1 and DDR1 expression with the OS and PFS of ovarian cancer were analyzed. The parameters were set as: Split patients by, ‘upper tertile’; follow-up threshold, ‘120 months’; array quality control, ‘exclude biased arrays’; and other parameters were the system defaults.
Statistical analysis
Statistical analysis was performed using R 3.63 software and GraphPad Prism 8.0 software (GraphPad Software; Dotmatics). The χ2 test was used to compare the differences between different clinicopathological features. To examine the prognostic significance of TM4SF1 and DDR1 in epithelial ovarian cancer patients, Kaplan-Meier survival analysis (P-value by log-rank test) was used to explore the association between the OS/PFS and TM4SF1/DDR1 expression in epithelial ovarian cancer tissue. The Cox regression model was used for the prognostic analysis of patients with epithelial ovarian cancer. The R survival (https://CRAN.R-project.org/package=survival; version 3.1–12) and survminer (https://CRAN.R-project.org/package=survminer; version 0.4.7) packages were used for data analysis and visualization. P<0.05 was considered to indicate a statistically significant result.
Results
Expression of TM4SF1 and DDR1 in cancers
The biological functions and roles of TM4SF1 and DDR1 in cancer are unclear. To investigate their roles in tumorigenesis and development, differentially expressed genes were identified in various types of cancer. The results of analyses performed using GEPIA and Oncomine showed that TM4SF1 and DDR1 were highly expressed in a variety of common malignant tumor types (Fig. 1). The analysis showed that TM4SF1 and DDR1 mRNA expression levels were significantly higher in ovarian cancer than in normal ovarian tissue (both P<0.05). A meta-analysis performed using the Oncomine database, which included 10 analyses of 7 ovarian cancer datasets, also showed that TM4SF1 and DDR1 were significantly more highly expressed in ovarian cancer than in normal ovarian tissue (TM4SF1, P=0.004; DDR1: P=3.22×10−4) as shown in Fig. 2.
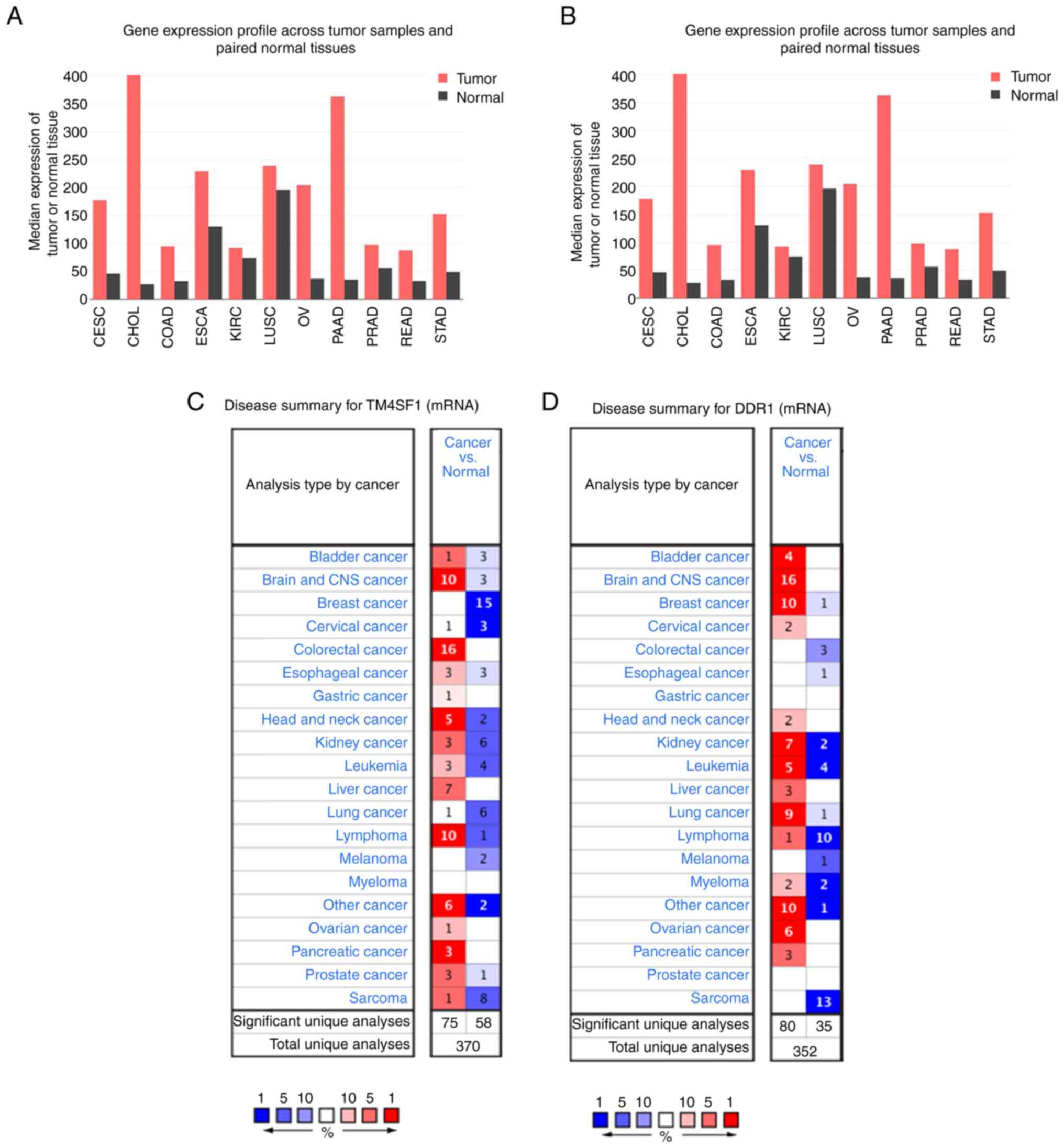 |
Figure 1.
High expression of TM4SF1 and DDR1 in a variety of tumor types. Differential expression of the (A) TM4SF1 and (B) DDR1 genes in the Gene Expression Profile Interactive Analysis database. Differential expression of the (C) TM4SF1 and (D) DDR1 gene in the Oncomine database. TM4SF1, transmembrane 4 L6 family member 1; DDR1, discoidin domain receptor 1; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; ESCA, esophageal carcinoma; KIRC, kidney renal clear cell carcinoma; LUSC, lung squamous cell carcinoma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; STAD, stomach adenocarcinoma.
|
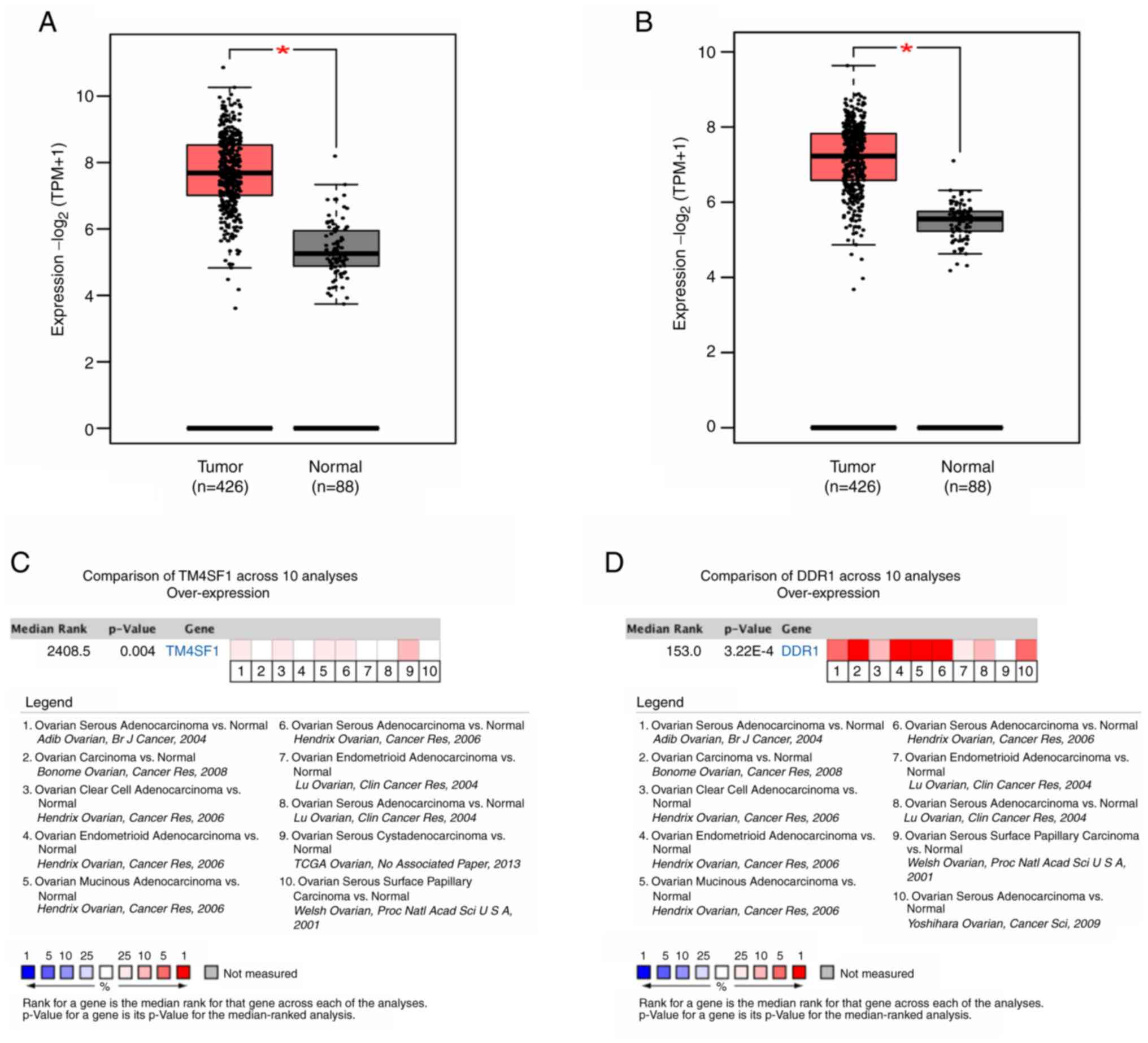 |
Figure 2.
Oncomine analysis shows the high expression of TM4SF1 and DDR1 in ovarian cancer tissue. Expression of (A) TM4SF1 and (B) DDR1 in ovarian cancer compared with normal ovarian tissue. The mRNA expression of (C) TM4SF1 and (D) DDR1 in ovarian cancer and normal tissue was compared in 10 analyses. Red indicates upregulation. *P<0.05. TM4SF1, transmembrane 4 L6 family member 1; DDR1, discoidin domain receptor 1; TPM, transcripts per million.
|
Enrichment analysis of TM4SF1- and DDR1-interacting proteins
The top 50 proteins that directly interact with TM4SF1 and DDR1 were queried using the STRING online tool, and 28 and 25 proteins were found to interact with TM4SF1 and DDR1, respectively. Among these interacting proteins, both CYSTM1 and NT5M interacted with TM4SF1 and DDR1. In addition, TM4SF1 interacted with DDR1 (Fig. 3).
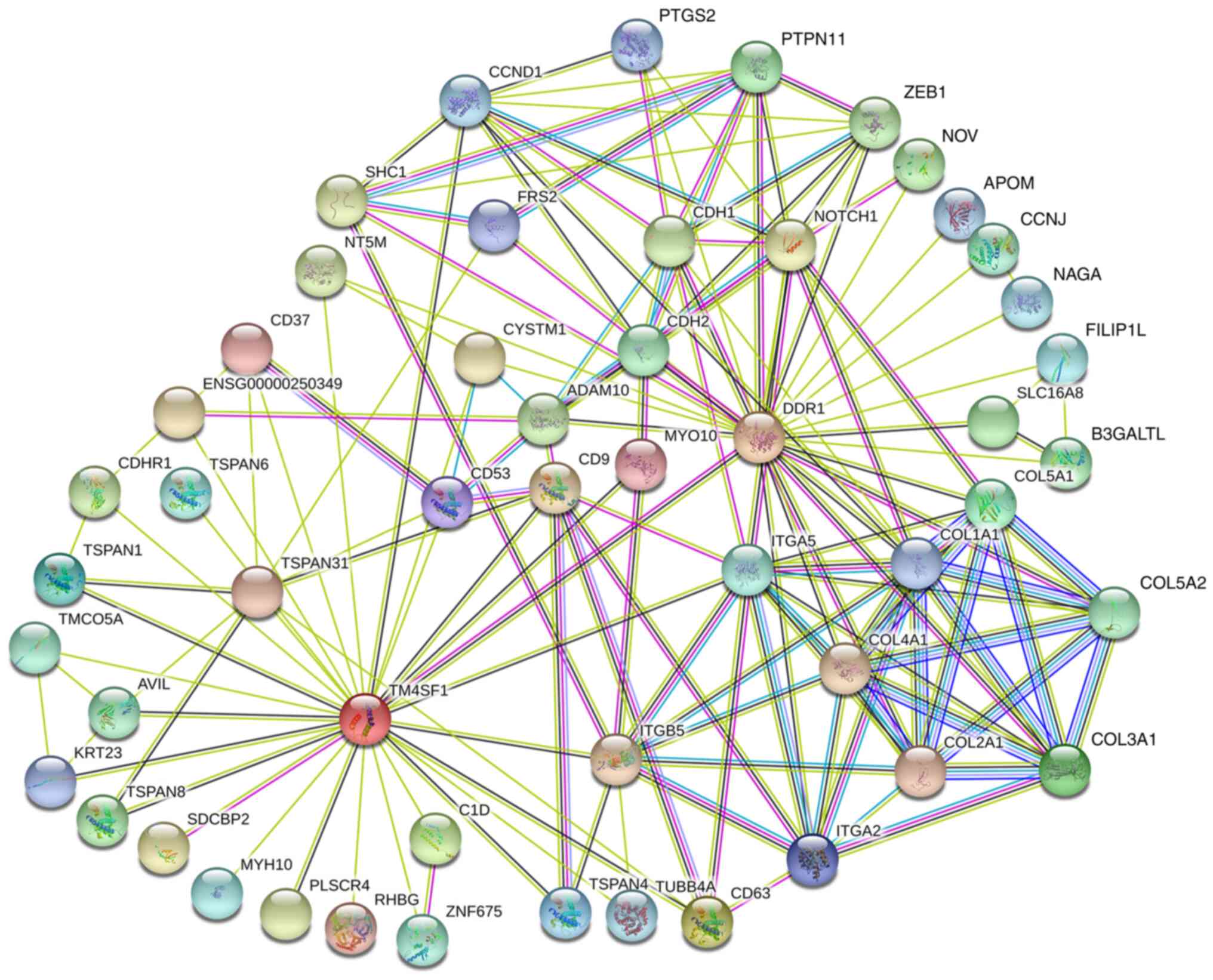 |
Figure 3.
Protein-protein interaction network of TM4SF1, DDR1 and their interacting proteins. TM4SF1, transmembrane 4 L6 family member 1; DDR1, discoidin domain receptor 1.
|
GO enrichment analysis of the interacting proteins showed that they were mainly enriched in terms associated with the extracellular matrix (ECM), collagen, integrin and their complexes as well as adhesion sites, the endoplasmic reticulum and other intracellular and extracellular components. GO enrichment analysis also indicated that these interacting proteins had the MFs of binding with integrin, cell adhesion molecules, growth factors, proteoglycans, neurotrophic factor receptors and γ-catenin. The GO analysis also indicated that the interacting proteins were involved in cell-matrix adhesion and the response of cells to stimulation by amino acids, which suggests that their biological functions are mediated via the activation of integrin and collagen-mediated signaling pathways (Fig. 4A; Table SI). KEGG enrichment analysis showed that these interacting proteins were mainly enriched in the terms ‘focal adhesion’, ‘ECM-receptor interaction’, ‘regulation of actin cytoskeleton’, ‘microRNAs in cancer’, ‘proteoglycans in cancer’, ‘PI3K/Akt signaling pathway’, ‘neurotrophin signaling pathway’ and a variety of tumor-related pathways, such as ‘small cell lung cancer’, ‘chronic myeloid leukemia’, ‘thyroid cancer’ and ‘bladder cancer’ (Fig. 4B; Table SII).
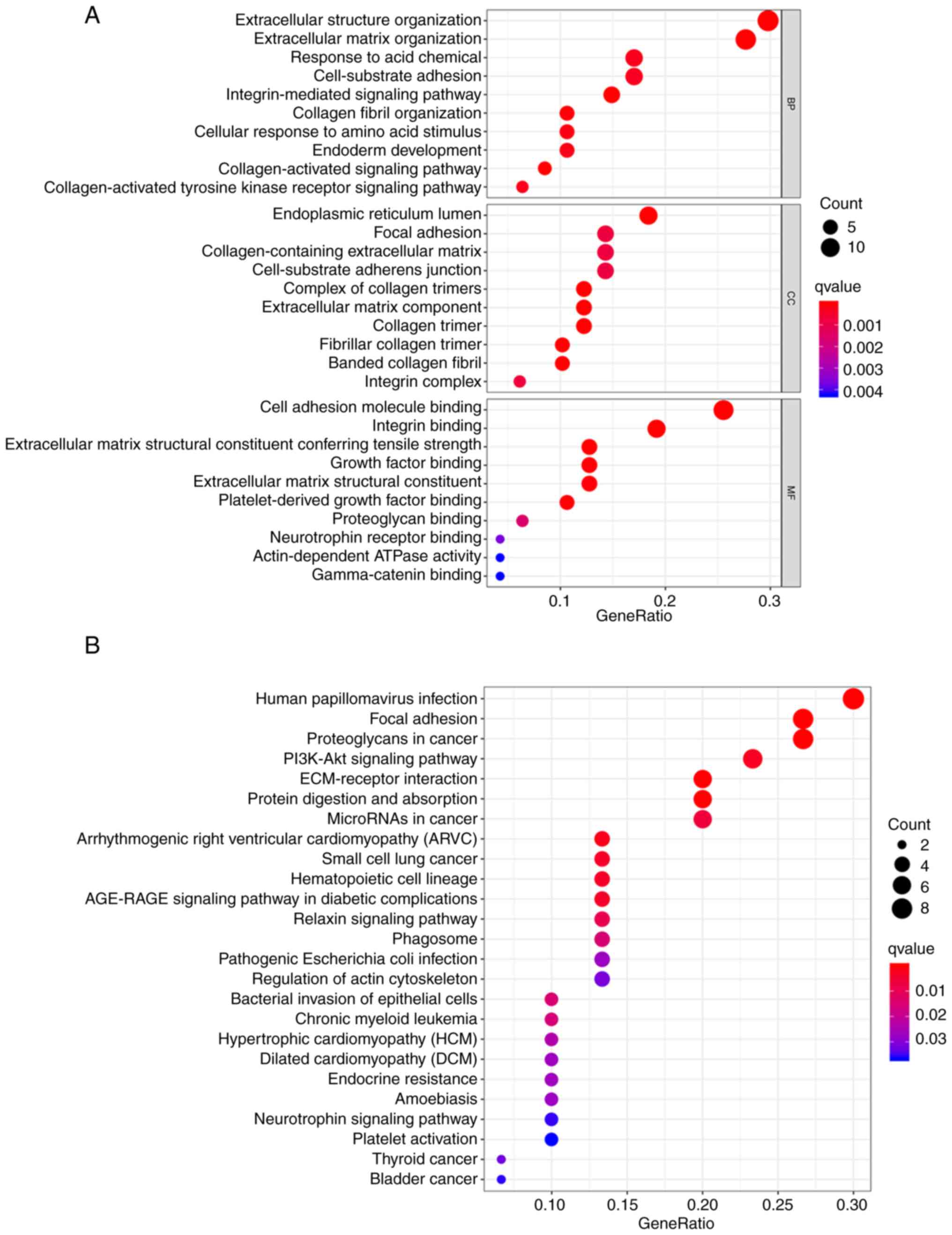 |
Figure 4.
GO and KEGG enrichment analyses of proteins interacting with TM4SF1 and DDR1. (A) GO and (B) KEGG pathways found to be enriched with TM4SF1- and DDR1-interacting proteins. GO, Gene Ontology; KEGG, Kyoto Encyclopaedia of Genes and Genomes; TM4SF1, transmembrane 4 L6 family member 1; DDR1, discoidin domain receptor 1; BP, biological process; CC, cellular constituent; MF, molecular function.
|
Expression of TM4SF1 and DDR1 proteins in epithelial ovarian cancer and their association with clinicopathologic features
The IHC results of the 94 patients with epithelial ovarian cancer showed that 46 patients (48.94%) were positive for TM4SF1 protein, and 52 patients (55.32%) were positive for DDR1 protein. In addition, 26 patients (27.66%) were positive for both TM4SF1 and DDR1 proteins. Representative staining images of TM4SF1 and DDR1 proteins in epithelial ovarian cancer tissues are shown in Fig. 5. Analysis using χ2 tests showed that only the positive expression of DDR1 protein differed significantly according to the histological grade, FIGO stage and presence or absence of intraperitoneal metastases (all P<0.05; Table I).
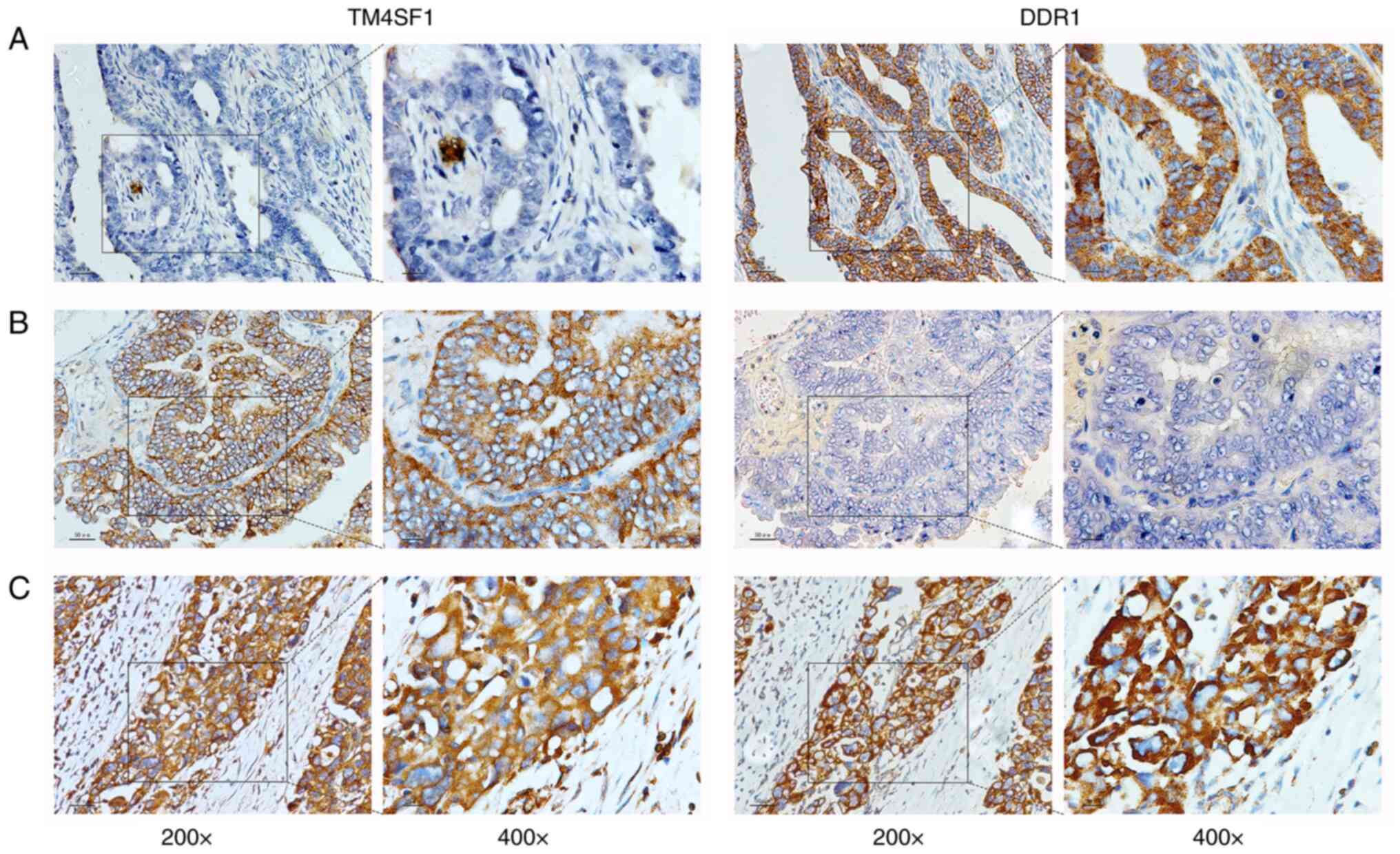 |
Figure 5.
Staining of TM4SF1 and DDR1 protein in ovarian cancer tissue. (A) TM4SF1 protein is negative and DDR1 protein is positive. (B) TM4SF1 protein is positive, and DDR1 protein is negative. (C) TM4SF1 and DDR1 protein are both positive. TM4SF1, transmembrane 4 L6 family member 1; DDR1, discoidin domain receptor 1.
|
 |
Table I.
Expression of TM4SF1 and DDR1 in patients with ovarian cancer and different clinicopathologic features.
|
Table I.
Expression of TM4SF1 and DDR1 in patients with ovarian cancer and different clinicopathologic features.
| Parameter |
N |
TM4SF1(+), n (%) |
P-value |
DDR1(+), n (%) |
P-value |
TM4SF1(+) + DDR1(+), n (%) |
P-value |
| Age, years |
|
|
|
|
|
|
|
| ≤50 |
46 |
27 (58.70) |
0.064 |
26 (56.52) |
0.818 |
16 (34.78) |
0.131 |
| >50 |
48 |
19 (39.58) |
|
26 (50.00) |
|
10 (20.83) |
|
| Histological grade |
|
|
|
|
|
|
|
| G1-2 |
20 |
11 (55.00) |
0.541 |
6 (30.00) |
0.010 |
5 (25.00) |
0.764 |
| G3 |
74 |
35 (47.30) |
|
46 (62.16) |
|
21 (28.66) |
|
| FIGO stage |
|
|
|
|
|
|
|
| I–II |
12 |
5 (41.67) |
0.590 |
2 (16.67) |
0.004 |
2 (16.67) |
0.571a |
| III–IV |
82 |
41 (50.00) |
|
50 (60.98) |
|
24 (29.37) |
|
| Histopathological type |
|
|
|
|
|
|
|
| Serous carcinoma |
82 |
37 (45.12) |
0.053 |
47 (57.32) |
0.308 |
22 (26.83) |
0.901a |
| Non-serous carcinoma |
12 |
9 (75.00) |
|
5 (41.67) |
|
4 (33.33) |
|
| Abdominal invasion |
|
|
|
|
|
|
|
| Yes |
63 |
33 (52.38) |
0.341 |
42 (66.67) |
0.002 |
20 (31.75) |
0.207 |
| No |
31 |
13 (41.94) |
|
10 (32.26) |
|
6 (19.35) |
|
| Neoadjuvant chemotherapy |
|
|
|
|
|
|
|
| Yes |
56 |
25 (44.62) |
0.312 |
32 (57.14) |
0.666 |
14 (25.00) |
0.484 |
| No |
38 |
21 (55.26) |
|
20 (52.63) |
|
12 (31.58) |
|
| Lymphatic metastasisb |
|
|
|
|
|
|
|
| Yes |
25 |
13 (52.00) |
0.453 |
15 (60.00) |
0.113 |
7 (28.00) |
0.282 |
| No |
31 |
13 (52.00) |
|
12 (38.70) |
|
5 (16.13) |
|
Relationship of TM4SF1 and DDR1 expression with the prognosis of ovarian cancer
Bioinformatics database analysis showed that patients with ovarian cancer and high expression of TM4SF1 had significantly lower DFS or PFS than those with low TM4SF1 expression (DFS: HR=1.3, P=0.047, n=424, Fig. 6A; PFS: HR=1.17, P=0.019, n=1,435, Fig. 6E). However, the expression of DDR1 was not found have a significant association with patient DFS or PFS, and neither TM4SF1 nor DDR1 expression had a significant association with patient OS (Fig. 6B-D and F-H). However, multigene analysis performed using KM plotter showed that higher expression of TM4SF1 and DDR1 coexpression was significantly associated with a shorter PFS in patients with ovarian cancer (HR=1.15, P=0.039, n=1,435, Fig. 6I) but not with OS (Fig. 6J).
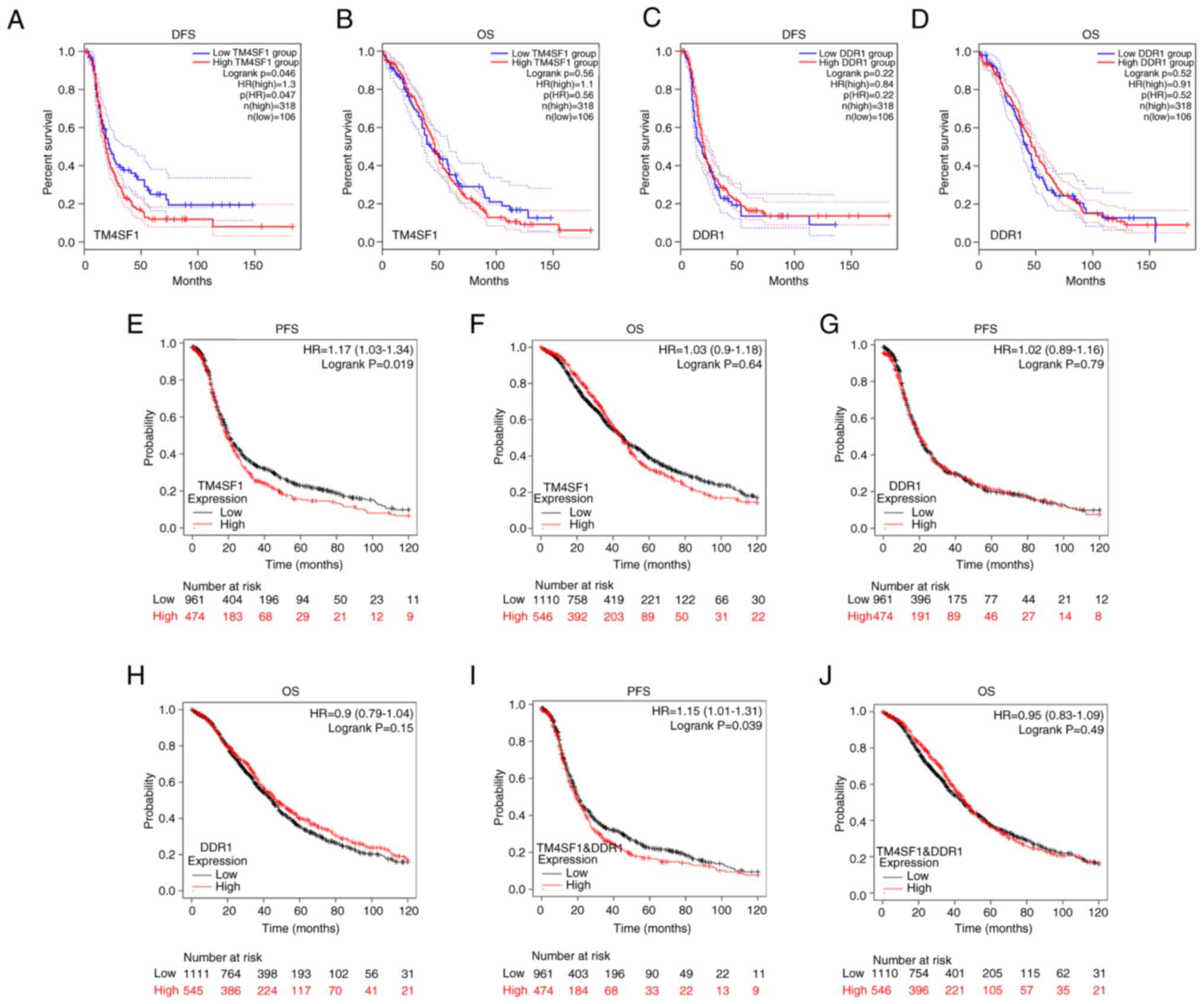 |
Figure 6.
Relationship of TM4SF1 and DDR1 expression with OS, DFS and PFS in patients with ovarian cancer determined using GEPIA and KM plotter analyses. GEPIA results of (A) DFS and (B) OS according to TM4SF1 expression and (C) DFS and (D) OS according to DDR1 expression. KM plotter results of (E) PFS and (F) OS according to TM4SF1 expression; (G) PFS and (H) OS according to DDR1 expression; and (I) PFS and (J) OS according to coexpression of TM4SF1 and DDR1. TM4SF1, transmembrane 4 L6 family member 1; DDR1, discoidin domain receptor 1; OS, overall survival; DFS, disease-free survival; PFS, progression-free survival; GEPIA, Gene Expression Profile Interactive Analysis; KM, Kaplan-Meier.
|
Analysis of the primary clinical data showed that the 94 patients with epithelial ovarian cancer had a median follow-up of 33 months, and detected no significant difference in the median OS between TM4SF1-positive and TM4SF1-negative patients (29 vs. 47 months). By contrast, the median OS of DDR1-positive patients was significantly shorter than that of DDR1-negative patients (31 vs. >73 months, P<0.05). The median OS of patients with TM4SF1 and DDR1 coexpression was significantly shorter than that of patients lacking TM4SF1 and DDR1 coexpression (21 vs. 49 months, P<0.05). In addition, the median PFS of TM4SF1-positive patients was significantly shorter than that of TM4SF1-negative patients (18 vs. 26 months, P<0.05). The median PFS of patients with TM4SF1 and DDR1 coexpression was significantly shorter than that of patients lacking TM4SF1 and DDR1 coexpression (12 vs. 26 months, P<0.05), while the expression of DDR1 alone was not associated with the median PFS (Fig. 7). Thus, the TM4SF1 and DDR1 coexpression indicated that patients with epithelial ovarian cancer had shorter PFS and OS.
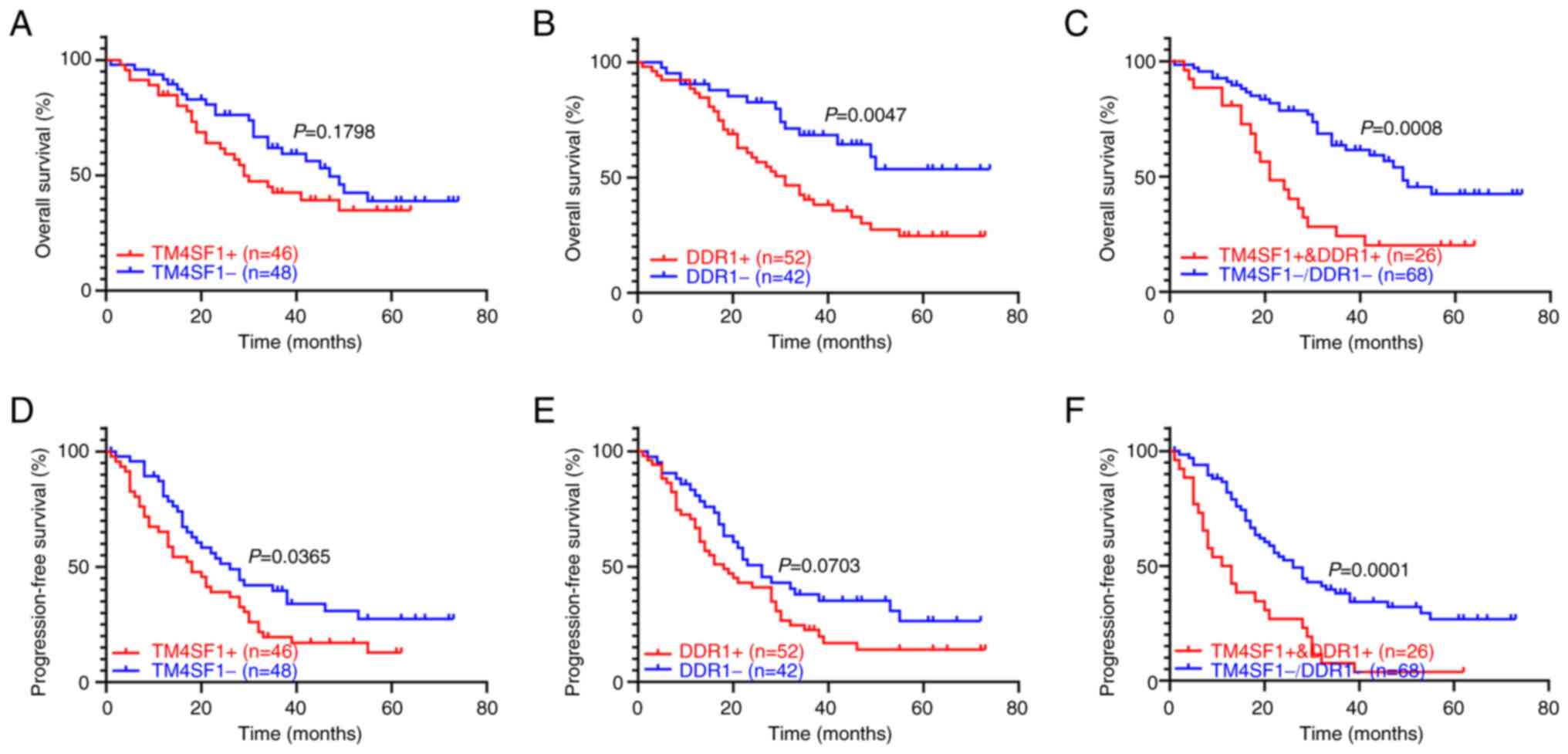 |
Figure 7.
Association of TM4SF1 and DDR1 protein expression with the prognosis of patients with ovarian cancer. Overall survival curves of patients with or without (A) TM4SF1, (B) DDR1 and (C) TM4SF1 + DDR1 protein expression. Progression-free survival curves of patients with or without (D) TM4SF1, (E) DDR1 and (F) TM4SF1 + DDR1 protein expression. TM4SF1, transmembrane 4 L6 family member 1; DDR1, discoidin domain receptor 1.
|
Analysis of prognostic factors in epithelial ovarian cancer
Regarding the clinicopathological factors of the patients, univariate analysis showed that FIGO stage, abdominal invasion, DDR1 expression and coexpression of DDR1 and TM4SF1 were factors affecting the OS of patients with epithelial ovarian cancer, while FIGO stage, TM4SF1 expression and coexpression of TM4SF1 and DDR1 were factors affecting the PFS of epithelial ovarian cancer patients (Table II).
 |
Table II.
Univariate analysis of the association of clinicopathological factors with OS and PFS in ovarian cancer.
|
Table II.
Univariate analysis of the association of clinicopathological factors with OS and PFS in ovarian cancer.
| |
OS |
PFS |
| |
|
|
| Parameter |
HR |
95% CI |
P-value |
HR |
95% CI |
P-value |
| Age |
0.877 |
0.506–1.520 |
0.641 |
0.866 |
0.541–1.385 |
0.548 |
| Histological grade |
1.146 |
0.573–2.292 |
0.700 |
1.387 |
0.742–2.592 |
0.305 |
| FIGO stage |
6.860 |
1.650–28.520 |
0.008 |
2.999 |
1.287–6.990 |
0.011 |
| Histopathological type |
0.877 |
0.374–2.057 |
0.763 |
1.574 |
0.680–3.640 |
0.289 |
| Abdominal invasion |
2.253 |
1.193–4.257 |
0.012 |
1.631 |
0.981–2.711 |
0.059 |
| Neoadjuvant chemotherapy |
1.750 |
0.953–3.214 |
0.071 |
1.565 |
0.946–2.589 |
0.082 |
| Lymph node excision |
0.617 |
0.355–1.072 |
0.087 |
0.628 |
0.390–1.010 |
0.055 |
| TM4SF1 |
1.454 |
0.838–2.525 |
0.183 |
1.646 |
1.026–2.642 |
0.039 |
| DDR1 |
2.324 |
1.271–4.249 |
0.006 |
1.555 |
0.962–2.513 |
0.072 |
| TM4SF1 + DDR1 |
2.559 |
1.450–4.517 |
0.001 |
2.572 |
1.566–4.224 |
<0.001 |
Cox multivariate analysis showed that TM4SF1 and DDR1 coexpression was the only independent risk factor affecting OS and PFS in the patients with epithelial ovarian cancer (Fig. 8), suggesting that the expression of TM4SF1 and DDR1 may be synergistically involved in the development of epithelial ovarian cancer.
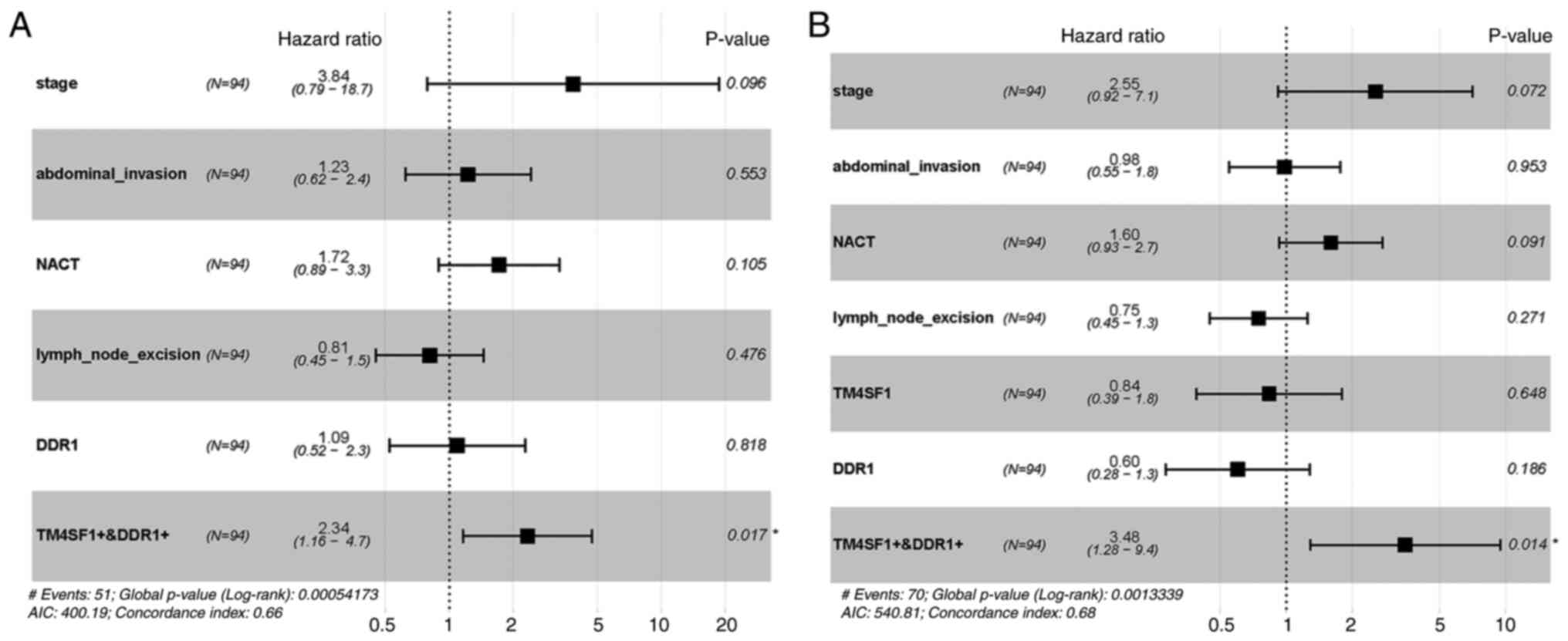 |
Figure 8.
Forest plot of the association of TM4SF1 and DDR1 coexpression with prognosis in ovarian cancer. Forest maps of (A) overall survival and (B) progression-free survival in patients with ovarian cancer according to TM4SF1 and DDR1 coexpression. TM4SF1, transmembrane 4 L6 family member 1; DDR1, discoidin domain receptor 1; NACT, neoadjuvant chemotherapy; AIC, Akaike information criterion.
|
Discussion
Ovarian cancer is a highly malignant gynecological tumor. Despite the development of treatment methods, the prognosis of patients is far from ideal. The pathogenesis of ovarian cancer is not fully understood, and few effective therapeutic targets are available. TM4SF1 and DDR1 proteins have been reported to be expressed in a variety of cancer tissues and are associated with poor prognosis in patients (8–11,17), and database analysis has yielded similar results. However, the role of TM4SF1 and DDR1 in the occurrence, development and prognosis of epithelial ovarian cancer remains unclear.
Antitumor immunity induced by TM4SF1 has been shown to inhibit the growth and migration of TM4SF1-positive cancer cells in vitro and in vivo (28). In addition, a previous study found that the positive rate of TM4SF1 protein expression in epithelial ovarian cancer tissues, particularly metastatic lymph nodes, was significantly higher than that in benign ovarian tumors and normal ovarian tissues, and the expression status of TM4SF1 in epithelial ovarian cancer was significantly associated with FIGO staging and tissue differentiation (29). Similarly, the present study also found that the positive rate of TM4SF1 protein in epithelial ovarian cancer tissues was higher than that in normal ovarian tissue, although the rate was slightly lower than that previously reported. This difference may be due to certain cases having received neoadjuvant chemotherapy and the prolonged storage of some specimens as well as different experimental conditions, sample sizes and operators. With regard to the effect of TM4SF1 on the prognosis of patients, the PFS of TM4SF1-positive patients was significantly shorter than that of TM4SF1-negative patients but TM4SF1 expression status did not affect the OS of patients. The present study found that the positive expression of TM4SF1 was not an independent risk factor for PFS and OS in epithelial ovarian cancer. In view of the difference in the positive rate of TM4SF1 in the present study compared to previous studies, the effect of neoadjuvant chemotherapy on the tissue expression of TM4SF1 requires further research.
The effects of DDR1 on the biological functions, disease progression and prognosis of different types of tumor cells vary, but there is evidence to show that DDR1 is involved in prometastatic and prosurvival signals (30). Although DDR1 was not found to be an independent risk factor affecting the prognosis of epithelial ovarian cancer in the present study, univariate analysis showed that it was significantly associated with OS. The study found that the positive rate of DDR1 in epithelial ovarian cancer tissues was 55.32%, while the positive expression rate in patients with advanced stage (stages III–IV), low differentiation (or high histological grade) and abdominal metastasis was significantly higher than that in patients with early stage (stages I–II), well-differentiated tissues and no abdominal metastasis. Advanced stage and high-grade cancer are poor prognostic factors for epithelial ovarian cancer. DDR1 was highly expressed in the sample types with these factors in the present study, suggesting that DDR1 upregulation is positively associated with the severity of the disease and that high pathological grade affects the OS of patients. These findings are consistent with previous results reported by Quan et al (31), implying that DDR1 might play a role in promoting the survival and metastasis of epithelial ovarian cancer cells.
The immunohistochemical staining of epithelial ovarian cancer tissue sections showed that TM4SF1 and DDR1 proteins were mainly distributed in the cell membrane and that both proteins were expressed in some cases. STRING analysis indicated that there is an interaction between TM4SF1 and DDR. Moreover, the coexpression of M4SF1 and DDR1 was found to be significantly associated with PFS and OS in patients with epithelial ovarian cancer and also serve as an independent poor prognostic factor. Therefore, TM4SF1 and DDR1 may play important biological functions through interaction. Gao et al (18) found that TM4SF1 interacts with DDR1 in breast cancer cells and that collagen I promotes their interaction and the reactivation of dormant metastatic breast cancer cells in multiple organs, leading to breast cancer recurrence and multiple organ metastasis. Due to the limitations of immunostaining for performing colocalization analysis, whether TM4SF1 and DDR1 proteins are colocalized and interact with each other in epithelial ovarian cancer cells and whether a mutual regulatory mechanism between TM4SF1 and DDR exists requires further study.
The present study evaluated the relationship between TM4SF1 and DDR1 expression alone and in combination with the prognosis of patients with epithelial ovarian cancer. The OS and PFS results in the primary dataset are consistent with the results of online databases in general. However, it is worth noting some differences in the OS of DDR1 alone or the combined analysis of TM4SF1 and DDR1 in different datasets. Regarding the prognostic factors for OS and DFS, the results based on GEPIA and KM analysis were not as strong as some of those obtained from the primary patient dataset. We hypothesize that the possible reasons for these differences are as follows: Firstly, there may have been some bias in the statistical analysis, such as admission bias and information bias. In the primary patient dataset, the prognostic data of epithelial ovarian cancer were obtained from a single center and hospital, with a high proportion of advanced cases, a relatively high positive rate of TM4SF1 and DDR1, and a worse prognosis for patients. By contrast, the results based on GEPIA and KM data are from a wide range of sources, and the classification or inclusion criteria might not be completely consistent for different data. In addition, as aforementioned, some cases that had received neoadjuvant chemotherapy and some old specimens were included in the 94 cases of epithelial ovarian cancer. The lack of cases and inadequate follow-up time are likely to have affected the accuracy of the results. Furthermore, differences in experimental conditions may unavoidably contribute to diagnostic bias or detection bias.
The present study has mainly focused on clinical studies. In subsequent studies, an increase in the number of cases and extension of the follow-up time is necessary. In addition, the relevant regulatory mechanisms of TM4SF1 and DDR1 in epithelial ovarian cancer cells requires further research in vitro and in vivo.
In conclusion, TM4SF1 and DDR1 proteins were found to be coexpressed in some epithelial ovarian cancer tissues, and may be adverse prognostic factors for patients with epithelial ovarian cancer. Also, there may be an interaction or mutual regulatory mechanism between TM4SF1 and DDR1.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 82060476), the Guangxi Natural Science Foundation (grant no. 2017GXNSFDA198009), the Guangxi Zhuang Autonomous Region Key Clinical Specialty Construction Project (grant no. 2018-39), the Guangxi Medical High-level Backbone Personnel Training ‘139’ Project (grant no. 2018-22) and the Special Fund of the 17th Guangxi New Century ‘Ten, Hundred, Thousand’ Talent Project (grant no. 2014210).
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to their containing information that could compromise the privacy of research participants but are available from the corresponding author on reasonable request.
Authors' contributions
ZH and HY were responsible for study conception and carried out the literature research, clinical study, data analysis, experimental operations, manuscript writing and editing. ZY was responsible for study design, experimental guidance and manuscript modification. ZH, HY and ZY confirm the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
All procedures were approved by the Ethics Committee of Guangxi Medical University Cancer Hospital and were performed in keeping with the standards set out in the Declaration of Helsinki and Laboratory Guidelines of Research in China. All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
GEPIA
|
Gene Expression Profiling Interactive Analysis
|
|
GO
|
Gene Ontology
|
|
STRING
|
Search Tool for the Retrieval of Interacting Genes/Proteins
|
|
KEGG
|
Kyoto Encyclopedia of Genes and Genomes
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
TM4SF1
|
transmembrane 4 L6 family member 1
|
|
DDR1
|
discoidin domain receptor 1
|
|
DFS
|
disease-free survival
|
|
KM
|
Kaplan-Meier
|
|
ECM
|
extracellular matrix
|
|
PPI
|
protein-protein interaction
|
References
|
1
|
Jelovac D and Armstrong DK: Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, Amso NN, Apostolidou S, Benjamin E, Cruickshank D, et al: Ovarian cancer screening and mortality in the UK collaborative trial of ovarian cancer screening (UKCTOCS): A randomised controlled trial. Lancet. 387:945–956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cho KR and Shih IeM: Ovarian cancer. Annu Rev Pathol. 4:287–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bookman MA: Standard treatment in advanced ovarian cancer in 2005: The state of the art. Int J Gynecol Cancer. 15 (Suppl 3):S212–S220. 2005. View Article : Google Scholar
|
|
5
|
Stipp CS, Kolesnikova TV and Hemler ME: Functional domains in tetraspanin proteins. Trends Biochem Sci. 28:106–112. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sciuto TE, Merley A, Lin CI, Richardson D, Liu Y, Li D, Dvorak AM, Dvorak HF and Jaminet SC: Intracellular distribution of TM4SF1 and internalization of TM4SF1-antibody complex in vascular endothelial cells. Biochem Biophys Res Commun. 465:338–343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hemler ME: Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 19:397–422. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xing P, Dong H, Liu Q, Zhao T, Yao F, Xu Y, Chen B, Zheng X, Wu Y, Jin F and Li J: Upregulation of transmembrane 4 L6 family member 1 predicts poor prognosis in invasive breast cancer: A STROBE-compliant article. Medicine (Baltimore). 96:e94762017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park YR, Lee ST, Kim SL, Liu YC, Lee MR, Shin JH, Seo SY, Kim SH, Kim IH, Lee SO and Kim SW: MicroRNA-9 suppresses cell migration and invasion through downregulation of TM4SF1 in colorectal cancer. Int J Oncol. 48:2135–2143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao R, Wang G, Qian K, Chen L, Ju L, Qian G, Wu CL, Dan HC, Jiang W, Wu M, et al: TM4SF1 regulates apoptosis, cell cycle and ROS metabolism via the PPARү-SIRT1 feedback loop in human bladder cancer cells. Cancer Lett. 414:278–293. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao J, Yang JC, Ramachandran V, Arumugam T, Deng DF, Li ZS, Xu LM and Logsdon CD: TM4SF1 regulates pancreatic cancer migration and invasion in vitro and in vivo. Cell Physiol Biochem. 39:740–750. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vogel W, Gish GD, Alves F and Pawson T: The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1:13–23. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peretti M, Badaoui M, Girault A, Van Gulick L, Mabille MP, Tebbakha R, Sevestre H, Morjani H and Ouadid-Ahidouch H: Original association of ion transporters mediates the ECM-induced breast cancer cell survival: Kv10.1-Orai1-SPCA2 partnership. Sci Rep. 9:11752019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang SH, Baek HA, Lee HJ, Park HS, Jang KY, Kang MJ, Lee DG, Lee YC, Moon WS and Chung MJ: Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung carcinomas. Oncol Rep. 24:311–319. 2010.PubMed/NCBI
|
|
15
|
Hu Y, Liu J, Jiang B, Chen J, Fu Z, Bai F, Jiang J and Tang Z: MiR-199a-5p loss up-regulated DDR1 aggravated colorectal cancer by activating epithelial-to-mesenchymal transition related signaling. Dig Dis Sci. 59:2163–2172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin H, Ham IH, Oh HJ, Bae CA, Lee D, Kim YB, Son SY, Chwae YJ, Han SU, Brekken RA and Hur H: Inhibition of discoidin domain receptor 1 prevents stroma-induced peritoneal metastasis in gastric carcinoma. Mol Cancer Res. 16:1590–1600. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gadiya M and Chakraborty G: Signaling by discoidin domain receptor 1 in cancer metastasis. Cell Adh Migr. 12:315–323. 2018.PubMed/NCBI
|
|
18
|
Gao H, Chakraborty G, Zhang Z, Akalay I, Gadiya M, Gao Y, Sinha S, Hu J, Jiang C, Akram M, et al: Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell. 166:47–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prat J: Ovarian, fallopian tube and peritoneal cancer staging: Rationale and explanation of new FIGO staging 2013. Best Pract Res Clin Obstet Gynaecol. 29:858–869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stierer M, Rosen H, Weber R, Hanak H, Spona J and Tuchler H: Immunohistochemical and biochemical measurement of estrogen and progesterone receptors in primary breast cancer. Correlation of histopathology and prognostic factors. Ann Surg. 218:13–21. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang Z, Li C, Kang B, Gao G, Li C and Zhang Z: GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45((W1)): W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM: Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression. Proc Natl Acad Sci USA. 101:9309–9314. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ, Kincead-Beal C, Kulkarni P, et al: Oncomine 3.0: Genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 9:166–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, et al: STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47((D1)): D607–D613. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu G, Wang LG, Han Y and He QY: ClusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qiu Y, Pu C, Li Y and Qi B: Construction of a circRNA-miRNA-mRNA network based on competitive endogenous RNA reveals the function of circRNAs in osteosarcoma. Cancer Cell Int. 20:482020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gyorffy B, Lanczky A and Szallasi Z: Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr Relat Cancer. 19:197–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin SI, Huang MH, Chang YW, Chen IH, Roffler S, Chen BM, Sher YP and Liu SJ: Chimeric peptide containing both B and T cells epitope of tumor-associated antigen L6 enhances anti-tumor effects in HLA-A2 transgenic mice. Cancer Lett. 377:126–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao C, Yao H, Liu H, Feng Y and Yang Z: TM4SF1 is a potential target for anti-invasion and metastasis in ovarian cancer. BMC Cancer. 19:2372019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yeh YC, Lin HH and Tang MJ: Dichotomy of the function of DDR1 in cells and disease progression. Biochim Biophys Acta Mol Cell Res. 1866:1184732019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Quan J, Yahata T, Adachi S, Yoshihara K and Tanaka K: Identification of receptor tyrosine kinase, discoidin domain receptor 1 (DDR1), as a potential biomarker for serous ovarian cancer. Int J Mol Sci. 12:971–982. 2011. View Article : Google Scholar : PubMed/NCBI
|






















