Introduction
Laryngeal squamous cell carcinoma (LSCC), which
originates from the epithelium of the laryngeal mucosa, is the
second most common malignant tumour in the head and neck region,
and its incidence and mortality are on the rise (1). Most patients with LSCC are diagnosed
at an advanced stage due to the occult nature of the disease. The
primary reason for the poor prognosis of patients with LSCC is the
propensity of LSCC for local invasion, cervical lymph node
metastasis and chemoresistance (2).
Despite improvement of treatment strategies, including surgery,
systemic chemotherapy and local radiotherapy, patients with LSCC
have a low survival rate (5-year survival rate was <50% between
2001 and 2004 in Switzerland) (3).
Therefore, identification of novel biomarkers for LSCC diagnosis
and investigation of effective novel therapeutic targets is
required.
Due to their covalently closed loop without 5′-3′
polyadenylation end, circular RNAs (circRNAs/circs) are more stable
than linear RNAs (4–6). Growing evidence implicates circRNAs in
progression of several cancer types, in which they act as microNA
(miRNA/miR) sponges, forming complexes with other RNAs or proteins,
regulating RNA transcription and splicing and translation into
peptides or microproteins (7–11).
circRNAs serve a variety of cell functions in LSCC progression
through different mechanisms. For example, circular RNA zinc finger
protein 609 promote LSCC progression by upregulating epidermal
growth factor receptor via sponging microRNA-134-5p (12). circ_0120175 reportedly promotes LSCC
development by upregulating solute carrier family 7 member 11
through miR-330-3p (13). Circ
coronin 1C was shown to promote LSCC progression by modulating the
let-7c-5p/PBX homeobox 3 axis (14)
In addition to acting as miRNA sponges, circRNAs also regulate LSCC
progression by forming complexes with proteins. For example,
circ-cyclin D1 promotes the proliferation of LSCC by enhancing
cyclin D1 mRNA stability by interacting with human antigen R
protein (15). circRNA microtubule
crosslinking factor 1 promotes LSCC progression by directly
recruiting complement C1q binding protein (C1QBP) and inhibiting
ubiquitin-proteasome-mediated degradation of C1QBP, thereby
increasing its expression (16). In
addition, studies have demonstrated that circRNAs serve as
biomarkers in diagnosis or prognosis of LSCC (12–15).
In a pre-clinical study, circRNAs derived from plasma cells were
screened in patients with LSCC and circ_0019201, circ_0011773 and
circ_0122790 were shown to act as potential biomarkers for the
prediction of LSCC prognosis (17).
The present study used dataset GSE117001 to identify
candidate circRNAs by identifying DEcircRNAs in the dataset. The
present study also investigated the correlation of hsa_circ 0081621
with clinicopathological characteristics, and their association
with survival of patients with LSCC.
Materials and methods
Subjects and gene information. The circRNA
expression profile (accession no. GSE117001) for LSCC was obtained
from the Gene Expression Omnibus (GEO) database (ncbi.nlm.nih.gov/geo/). It included five pairs of
matched LSCC specimens (GSM3267212, GSM3267213, GSM3267214,
GSE3267215 and GSE3267216) and normal laryngeal squamous specimens
(GSM3267217, GSM3267218, GSM3267219, GSM3267220 and GSM3267221).
The GEOquery (version 2.68.0; bioconductor.org/packages/release/bioc/html/GEOquery.html)
and limma (version 3.56.2; bioconductor.org/packages/release/bioc/html/limma.html)
packages in R software (version 3.5.0; http://www.r-project.org) were used to process the
expression matrix and differential expression analysis, as
previously described (18).
P<0.05 and |log2 fold-change (FC)|>2 were set as thresholds
to identify circRNAs that exhibited differential expression between
LSCC and laryngeal squamous specimens.
Patients and tissue specimens
A total of 77 male patients with LSCC were
recruited. The mean age of patients with LSCC was 60.7±7.9 years
(range, 43–79 years). No patients had a prior history of cancer,
chemotherapy or radiotherapy. All the fresh LSCC and corresponding
non-carcinoma tissue was collected from these patients.
Non-carcinoma tissues were resected tissues ~1 cm from the tumours
and were identified by pathology. The pathological results for all
individuals were reviewed by two experienced pathologists. The
first cohort comprised 10 patients with LSCC who underwent surgery
at The Fourth Hospital of Hebei Medical University (Shijiazhuang,
China) between June 2022 and September 2022. The first cohort was
used to explore the expression of three candidate circRNAs.
Specimens from the first cohort preserved in liquid nitrogen for
RNA extraction. The second cohort contained 67 patients untreated
LSCC who presented to The Fourth Hospital of Hebei Medical
University (Shijiazhuang, China) between January 2014 and November
2015. The second cohort were used to validate the correlation
between the expression profiles of hsa_circ_0081621 and the
clinicopathological characteristics of patients with LSCC.
Specimens from the second cohort were fixed at 4°C for 16 h with 4%
formaldehyde after surgery and embedded in paraffin for diagnosis
and the subsequent analysis. Patients in the second cohort were
followed up for 12–101 months. Informed consent was obtained to
participate. The study was approved by the clinical research ethics
committee of The Fourth Hospital of Hebei Medical University
(approval no. 2020ky198; Shijiazhuang, China).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to isolate total RNA from tissue
(19). GoScript™ Reverse
Transcription System (Promega Corporation) was used to prepare cDNA
from total RNA according to the manufacturer's instructions. cDNA
was used as a template for RT-qPCR using GoTaq® qPCR
Master Mix (Promega Corporation). The thermocycling conditions of
qPCR were as follows: Initial denaturation, 70°C for 5 min;
annealing, 25°C for 5 min; extension, 42°C for 60 min; and
denaturation, 70°C for 15 min. GAPDH was used for normalization.
The primer sequences were as follows: hsa_circ_0081621, forward,
5′-AATAAACTGACTGTTCGTGGCA-3′ and reverse, 5′-GCAGCGAGCGGTTCTTCT-3′;
corresponding linear RNA, forward, 5′-ATCCGACTCCCAGCCCACAAC-3′ and
reverse, 5′-TCCGTCAGCACCTCCTTCACC-3′ and GAPDH, forward,
5′-GACCACAGTCCATGCCATCAC-3′ and reverse, 5′-ACGCCTGCTTCACCACCTT-3′.
The relative expression levels were calculated using the
2−ΔΔCq method (20).
Fluorescence in situ hybridization
(FISH) and evaluation
FISH assay was performed using biotin-labelled
hsa_circ_0081621 probe (5′-CY3-GACTTCAGAATGCTTCAGACCCA-3′-CY3)
which was purchased from GenePharma Co., Ltd. FISH assay was
performed on 5 µm sections of paraffin-embedded tissue specimens.
The sections were deparaffinized in xylene and rehydrated using a
graded ethanol rinse series (50, 75, 85 and 95%). The slides were
incubated at 37°C for 30 min in 50 µl Proteinase K solution (15
ug/ml), washed with sterile distilled water. After
pre-hybridization (1X PBS/0.5% Triton X-100), cells were hybridized
in hybridization buffer (40% formamide, 10% Dextran sulfate, 1 ×
Denhardt's solution, 4 × SSC, 10 mm DDT, 1 mg/ml yeast transfer
RNA, 1 mg/ml sheared salmon sperm DNA) with biotin-labelled probes
specific to hsa_circ_0081621 at 60°C overnight. Conjugate Cy™ 5
streptavidin conjugate was used to detect the fluorescence signal
of hsa_circ_0081621 (ZyMAXTM Grade; Invitrogen; Thermo Fisher
Scientific, Inc.). Cell nuclei were stained with DAPI for 10 min at
37°C. Images were captured using a confocal microscope with a ZEISS
LSM 900 lens (Carl Zeiss AG).
Quantitative evaluation of FISH was conducted by
examining five randomly selected fields/slide using a
high-magnification light microscope (magnification, ×400). The
percentage area covered by fluorescence staining was assessed using
the following scoring system: 0, no staining; 1, 1–25%; 2, 26–50%
and 3, 51–100%. The fluorescence staining intensity was scored as
follows: 0 (no fluorescence), 1 (weak fluorescence) and 2 (mild
fluorescence) and 3 (high fluorescence). hsa_circ_0081621
expression was ranked on a scale of 0–6 based on the sum of its
intensity and area. The sample was considered to show low
expression in the 0–2 range and high expression in the 3–6 range,
with weak positive expression (3 and 4) and strong positive
expression (5 and 6).
Statistical analysis
SPSS 22.0 software (IBM Corp.) was used to perform
statistical analysis. The RT-qPCR assay repeats three times. Data
are presented as the mean ± standard deviation (SD) and compared
using Student's t-test. The data were normally distributed.
χ2 test were used to evaluate the potential association
between the expression of hsa_circ_0081621 and various
clinicopathological factors. Survival analysis was performed using
Kaplan-Meier analysis with log-rank test. The Cox regression model
was used for univariate and multivariate analysis of overall
survival and prognostic factors. The statistical analyses were
conducted using two-sided tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification of differentially
expressed circRNAs
R3.5.0 limma package (P<0.05 and
|log2FC|>2) was used to analyse GEO dataset
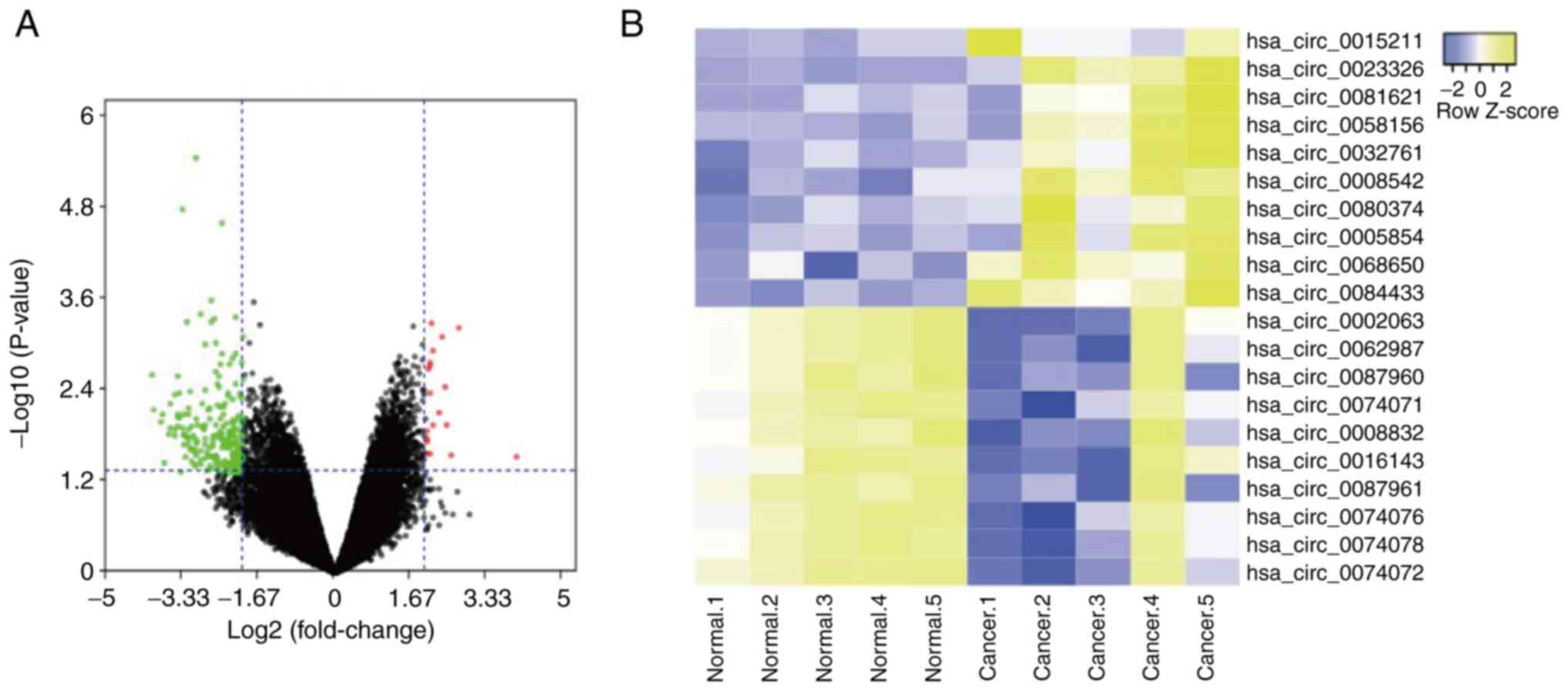
GSE117001. A total of 19 up- and 226 downregulated circRNAs in LSCC
tissue were identified. The three most significantly upregulated
circRNAs in LSCC tissue were hsa_circ_0015211, hsa_circ_0023326 and
hsa_circ_0081621 (Fig. 1A and B).
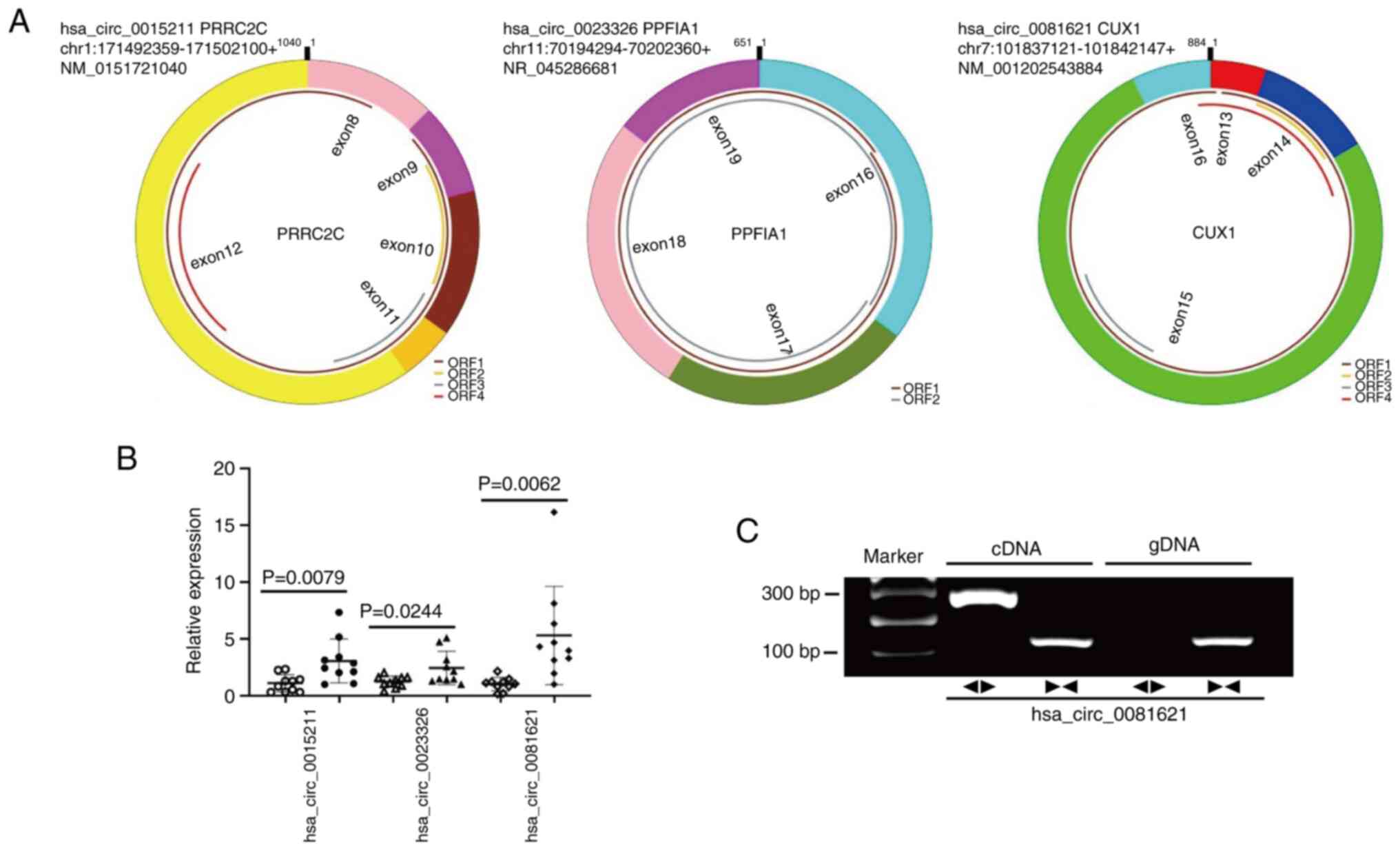
Fig. 2A shows the basic structure
of these circRNAs, which are derived from exonic regions of their
respective parent genes. According to qPCR results in 10 paired
fresh LSCC tissues and the corresponding non-carcinoma tissue (the
first cohort), all aforementioned circRNAs were found to be
upregulated in LSCC tissue; hsa_circ_0081621 exhibited the highest
expression levels in LSCC tissue (Fig.
2B). Agarose gel electrophoresis demonstrated the presence of
hsa_circ_0081621 in LSCC (Fig.
2C).
hsa_circ_0081621 is highly expressed
in LSCC specimens
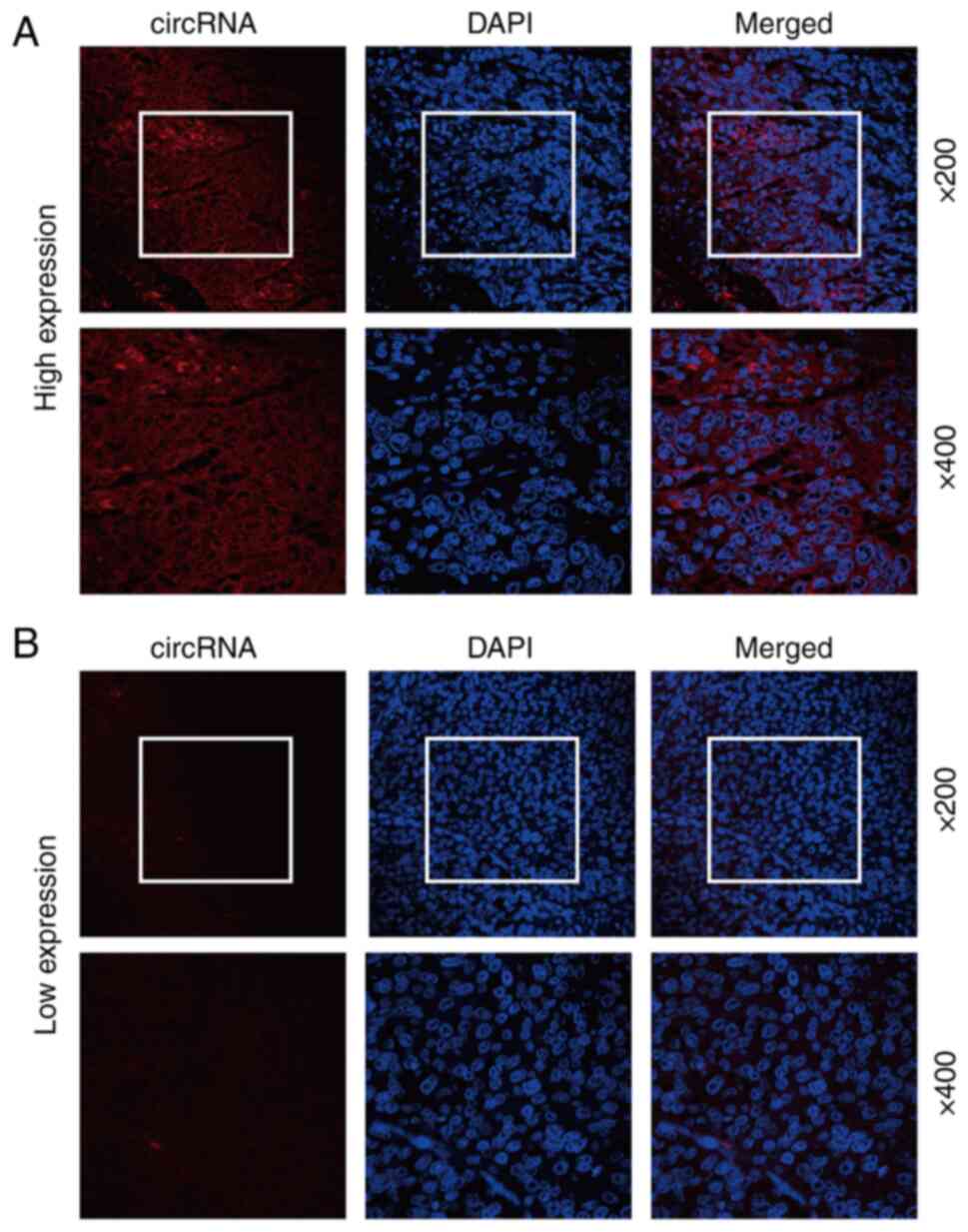
To explore the expression expression of
hsa_circ_0081621 in LSCC, a biotin-labelled hsa_circ_0081621 probe,
which can recognize the junction site of hsa_circ_0081621, was used
to stain 67 LSCC specimens (the second cohort). Representative
images of high and low fluorescence staining of hsa_circ_0081621 in
LSCC samples are shown in Fig. 3.
Overall, 40 of 67 (59.7%) LSCC specimens exhibited high
hsa_circ_0081621 and 27 of 67 (40.3%) LSCC specimens exhibited low
hsa_circ_0081621 expression. In most specimens, hsa_circ_0081621
expression was primarily observed in the cytoplasm (Fig. 3).
Hsa_circ_0081621 expression is
associated with clinicopathological factors indicating poor
prognosis of LSCC
To investigate the potential effect of
hsa_circ_0081621 in LSCC progression, the association between
hsa_circ_0081621 expression and clinicopathological factors of
patients with LSCC was evaluated. hsa_circ_0081621 expression was
not associated with patient age, smoking status, pathological
differentiation or T stage (Table
I). hsa_circ_0081621 high expression was more frequent in LSCC
samples with lymph node metastasis (11/13; 84.6%) than in those
without (29/54; 53.7%). hsa_circ_0081621 high expression was found
in 18 out of 22 (81.8%) LSCC specimens with clinical stages III and
IV, which was higher than expression in specimens with clinical
stages I and II (22/45; 48.9%; P<0.05). These results suggested
that high expression of hsa_circ_0081621 may be indicative of a
poor prognosis in patients with LSCC.
 | Table I.Association between the expression of
hsa_circ_0081621 and clinical pathological features in patients
with laryngeal squamous cell carcinoma. |
Table I.
Association between the expression of
hsa_circ_0081621 and clinical pathological features in patients
with laryngeal squamous cell carcinoma.
|
|
| hsa_circ_0081621 |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | n | Low | High | χ2 | P-value |
|---|
| Age, years |
|
|
| 0.298 | 0.585 |
|
<60 | 30 | 11 | 19 |
|
|
| ≥60 | 37 | 16 | 21 |
|
|
| Smoking status |
|
|
| 0.155 | 0.694 |
|
Non-smoker | 14 | 5 | 9 |
|
|
|
Smoker | 53 | 22 | 31 |
|
|
| Pathological
differentiation |
|
|
| 2.223 | 0.136 |
| I | 16 | 9 | 7 |
|
|
| II and
III | 51 | 18 | 33 |
|
|
| T stage |
|
|
| 3.159 | 0.075 |
| 1 and
2 | 41 | 20 | 21 |
|
|
| 3 and
4 | 26 | 7 | 19 |
|
|
| Lymph node
metastasis |
|
|
| 4.161 | 0.041 |
| No | 54 | 25 | 29 |
|
|
|
Yes | 13 | 2 | 11 |
|
|
| Clinical stage |
|
|
| 6.660 | 0.010 |
| I and
II | 45 | 23 | 22 |
|
|
| III and
IV | 22 | 4 | 18 |
|
|
Hsa_circ_0081621 expression is
associated with poor survival of patients with LSCC
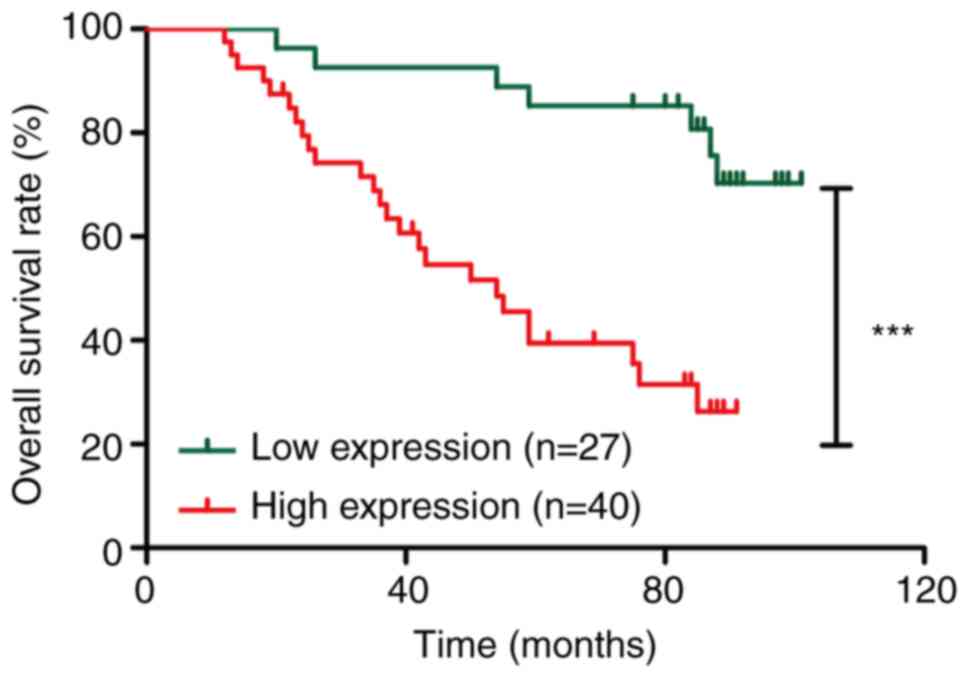
To evaluate the prognostic significance of
hsa_circ_0081621 expression in patients with LSCC, the association
between expression levels of hsa_circ_0081621 and the 5-year
overall survival of patients with LSCC was investigated (second
cohort). Survival data based on Kaplan-Meier analysis showed that
patients with high hsa_circ_0081621 expression had a significantly
shorter overall survival than patients with low hsa_circ_0081621
expression (χ2=15.03; Fig.
4). Furthermore, the present study analysed the association
between hsa_circ_0081621 and various clinicopathological factors
and overall survival of patients with LSCC. Overall survival of
patients with LSCC with high T stage, lymph node metastasis or high
hsa_circ_0081621 expression was shorter than that of patients with
low T stage, non-lymph node metastasis or low hsa_circ_0081621
expression (Table II).
 | Table II.Univariate and multivariate analyses
of prognostic factors in laryngeal squamous cell carcinoma. |
Table II.
Univariate and multivariate analyses
of prognostic factors in laryngeal squamous cell carcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | P-value | 95% CI | HR | P-value | 95% CI |
|---|
| Expression of
hsa_circ_0081621, high vs. low | 4.749 | <0.001 | 2.01–11.222 | 3.934 | 0.002 | 1.627–9.512 |
| Age, <60 vs. ≥60
years | 1.300 | 0.466 | 0.642–2.634 |
|
|
|
| Smoking status,
non-smoker vs. smoker | 1.224 | 0.656 | 0.504–2.974 |
|
|
|
| Histological grade,
I vs. II and III | 1.116 | 0.788 | 0.501–2.486 |
|
|
|
| T stage, 1 and 2
vs. 3 and 4 | 3.259 | 0.001 | 1.603–6.627 | 2.402 | 0.019 | 1.155–4.996 |
| Lymph node
metastasis, no vs. yes | 3.168 | 0.002 | 1.513–6.634 | 2.173 | 0.047 | 1.010–4.678 |
| Clinical stage, I
and II vs. III and IV | 4.716 | <0.001 | 2.318–9.595 |
|
|
|
The prognostic significance of hsa_circ_0081621
expression was assessed in multivariate analyses. Multivariate Cox
regression analysis of the association between factors, including
patient age, smoking status, histological grade, T stage, lymph
node metastasis and hsa_circ_0081621 expression, and overall
survival was performed. T stage [hazard ratio (HR), 2.402; 95% CI,
1.155–4.996], lymph node metastasis (HR, 2.173; 95% CI,
1.010–4.678) and hsa_circ_0081621 expression (HR, 3.934; 95% CI,
1.627–9.512) were independent prognostic factors for patients with
LSCC (Table II).
Discussion
LSCC is one of the most common types of cancer of
the head and neck and has a low 5-year survival rate (2,3).
Furthermore, most patients with LSCC are diagnosed at an advanced
stage, which limits treatment options (2,3).
Therefore, the identification of novel prognostic biomarkers and
molecular targets for therapy is required.
Due to the covalently closed circular RNA molecules,
circRNAs are unaffected by RNA exonuclease and are more stable than
linear RNAs. In addition, circRNAs have tissue specificity, and
participate in multiple physiopathological processes. Therefore,
circRNAs may serve as biomarkers for diagnosis and prognosis of
various types of disease (21). For
example, based on a machine learning classification model,
hsa_circ_0005505, circ erb-b2 receptor tyrosine kinase 2 and circ
carbohydrate sulfotransferase 12 have been identified as potential
diagnostic biomarkers for intracerebral haemorrhage (22). circ meningioma expressed antigen 5
has been identified as a novel premetastatic factor and latent
biomarker in osteosarcoma by analysing the expression profile of
circRNAs (23). Through
high-throughput RNA sequencing, hsa_circRNA_0101388 and
hsa_circRNA_0022426 have potential predictive value for malignant
transformation of human colorectal inflammation into colorectal
cancer (24). In addition, circRNAs
are stably enriched in exosomes and show a unique circular
structure, high stability, conservation and tissue specificity,
thereby exhibiting great potential as tumor biomarkers and
anti-tumor targets (25). For
example, through analysis of the urinary exosomal circRNA profile,
hsa_circ_0001250 derived from urinary extracellular vesicles has
been detected as a potential biomarker for idiopathic membranous
nephropathy (26).
In the present study, GEO dataset GSE117001 was
analysed to explore differentially expressed circRNAs in LSCC; 19
upregulated and 226 downregulated circRNAs were identified in LSCC
tissue. Through analysis of the top three circRNAs by RT-qPCR in 10
paired fresh LSCC and corresponding non-carcinoma tissues, the
highest expression of hsa_circ_0081621 was found in LSCC tissues.
Due to the difficulty of preparing RNA from paraffin-embedded
sections, FISH staining was used to analyse hsa_circ_0081621
expression in 67 LSCC samples. The present results revealed high
hsa_circ_0081621 expression in 59.7% of patients with LSCC. These
results suggested that hsa_circ_0081621 may be associated with the
developmental process of LSCC.
Here, high hsa_circ_0081621 expression was
associated with lymph node metastasis and high clinical stage. In
survival analysis, the overall survival rate of patients with LSCC
with high hsa_circ_0081621 expression, as determined by log-rank
analysis, was markedly lower than that of patients with low
hsa_circ_0081621 expression. In addition, hsa_circ_0081621
expression was an independent prognostic factor for patients with
LSCC.
Numerous circRNAs have been identified as diagnostic
or prognostic biotargets for patients with LSCC. For example, as an
oncogenic RNA, circ coronin 1C can serve as a novel target for LSCC
treatment and may act as a diagnostic and prognostic marker for
LSCC detection (14). Circ
bifunctional apoptosis regulator is associated with poor prognosis
and accelerated LSCC progression (27). circ_0067934 is an oncogene promoting
LSCC and may be a viable prognostic biomarker and target for
diagnosis and treatment (28). Zhao
et al (29) reported that
circ ATP binding cassette subfamily B member 10 promotes LSCC
progression and may act as a possible target in LSCC therapy. In
addition, circRNA_103862 promotes LSCC proliferation by targeting
the miR-493-5p/golgi membrane protein 1 axis and might serve as a
potential prognosis marker and therapy target for LSCC (30). The present study demonstrated that
hsa_circ_0081621 may be a prognostic marker for patients with
LSCC.
The present study has limitations. First, only
GSE117001 was analysed. Secondly, the biological functions of
hsa_circ_0081621 in LSCC were not investigated and should be
validated by in vitro cytology experiments and in
vivo animal experiments.
Through bioinformatics analysis and retrospective
study, the present results revealed that high hsa_circ_0081621
expression was associated with poor prognosis of patients with
LSCC, and hsa_circ_0081621 may be a target for LSCC treatment.
Further research is needed to determine the role of
hsa_circ_0081621 in LSCC progression.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Financial Supporting
Program of Hebei Province (grant no. 202030701070004).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML and SL designed the study. ML, RZ, XS, YZ, HC and
YX performed experiments and data analysis. ML, RZ and SL confirm
the authenticity of all the raw data. ML and RZ interpreted the
data and wrote the manuscript. ML revised the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients. The study was approved by the clinical research ethics
committee of The Fourth Hospital of Hebei Medical University
(approval no. 2020ky198; Shijiazhuang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bradford CR, Ferlito A, Devaney KO,
Mäkitie AA and Rinaldo A: Prognostic factors in laryngeal squamous
cell carcinoma. Laryngoscope Investig Otolaryngol. 5:74–81. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anschuetz L, Shelan M, Dematte M, Schubert
AD, Giger R and Elicin O: Long-term functional outcome after
laryngeal cancer treatment. Radiat Oncol. 14:1012019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lasda E and Parker R: Circular RNAs:
Diversity of form and function. RNA. 20:1829–1842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao W, Guo H, Niu M, Zheng X, Zhang Y, Xue
X, Bo Y, Guan X, Li Z, Guo Y, et al: circPARD3 drives malignant
progression and chemoresistance of laryngeal squamous cell
carcinoma by inhibiting autophagy through the PRKCI-Akt-mTOR
pathway. Mol Cancer. 19:1662020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meng L, Liu S, Liu F and Sang M, Ju Y, Fan
X, Gu L, Li Z, Geng C and Sang M: ZEB1-mediated transcriptional
upregulation of circWWC3 promotes breast cancer progression through
activating ras signaling pathway. Mol Ther Nucleic Acids.
22:124–137. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gu L, Sang Y, Nan X, Zheng Y, Liu F, Meng
L, Sang M and Shan B: circCYP24A1 facilitates esophageal squamous
cell carcinoma progression through binding PKM2 to regulate
NF-κB-induced CCL5 secretion. Mol Cancer. 21:2172022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zang J, Lu D and Xu A: The interaction of
circRNAs and RNA binding proteins: An important part of circRNA
maintenance and function. J Neurosci Res. 98:87–97. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu CX and Chen LL: Circular RNAs:
Characterization, cellular roles, and applications. Cell.
185:2016–2034. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin X, Wang J, Shan C, Jia Q, Bian Y and
Zhang H: Circular RNA ZNF609 promotes laryngeal squamous cell
carcinoma progression by upregulating epidermal growth factor
receptor via sponging microRNA-134-5p. Bioengineered. 13:6929–6941.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan D and Zhu Y: Circ_0120175 promotes
laryngeal squamous cell carcinoma development through up-regulating
SLC7A11 by sponging miR-330-3p. J Mol Histol. 53:159–171. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y, Zhang Y, Zheng X, Dai F, Lu Y, Dai
L, Niu M, Guo H, Li W, Xue X, et al: Circular RNA circCORO1C
promotes laryngeal squamous cell carcinoma progression by
modulating the let-7c-5p/PBX3 axis. Mol Cancer. 19:992020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zang Y, Li J, Wan B and Tai Y: circRNA
circ-CCND1 promotes the proliferation of laryngeal squamous cell
carcinoma through elevating CCND1 expression via interacting with
HuR and miR-646. J Cell Mol Med. 24:2423–2433. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Z, Sun A, Yan A, Yao J, Huang H, Gao
Z, Han T, Gu J, Li N, Wu H, et al: Circular RNA MTCL1 promotes
advanced laryngeal squamous cell carcinoma progression by
inhibiting C1QBP ubiquitin degradation and mediating beta-catenin
activation. Mol Cancer. 21:922022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han J, Lin Q and Dong C: Plasma cell-free
circRNAs panel act as fingerprint predicts the occurrence of
laryngeal squamous cell carcinoma. Aging (Albany NY).
13:17328–17336. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: Limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chomczynski P and Sacchi N: The
single-step method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction: Twenty-something years
on. Nat Protoc. 1:581–585. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Loganathan T and Doss C GP: Non-coding
RNAs in human health and disease: Potential function as biomarkers
and therapeutic targets. Funct Integr Genomics. 23:332023.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bai C, Hao X, Zhou L, Sun Y, Song L, Wang
F, Yang L, Liu J and Chen J: Machine learning-based identification
of the novel circRNAs circERBB2 and circCHST12 as potential
biomarkers of intracerebral hemorrhage. Front Neurosci.
16:10025902022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu W, Long Q, Zhang W, Zeng D, Hu B, Liu
S and Li C: Circular RNA expression profile identifies circMGEA5 as
a novel metastasis-promoting factor and potential biomarker in
osteosarcoma. J Biochem Mol Toxicol. 37:e232862022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu L, Liu Y, Zhang G, Xu Y, Hu D, Ji G and
Xu H: The circRNA expression profile of colorectal inflammatory
cancer transformation revealed potential predictive biomarkers.
Aging (Albany NY). 14:9280–9299. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang D, Li R, Jiang J, Qian H and Xu W:
Exosomal circRNAs: Novel biomarkers and therapeutic targets for
gastrointestinal tumors. Biomed Pharmacother. 157:1140532023.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Q, Xu M, Zhang Z, Yin M, Zhang Y and
Liu F: Urinary exosomal hsa_circ_0001250 as a novel diagnostic
biomarker of idiopathic membranous nephropathy. J Transl Med.
20:6072022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gong H, Wu W, Fang C and He D: CircBFAR
correlates with poor prognosis and promotes laryngeal squamous cell
cancer progression through miR-31-5p/COL5A1 axis. Laryngoscope
Investig Otolaryngol. 7:1951–1962. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chu YL: Circ_0067934 correlates with poor
prognosisand promotes laryngeal squamous cell cancer progression by
sponging miR-1324. Eur Rev Med Pharmacol Sci. 24:4320–4327.
2020.PubMed/NCBI
|
|
29
|
Zhao J, Li XD, Wang M, Song LN and Zhao
MJ: Circular RNA ABCB10 contributes to laryngeal squamous cell
carcinoma (LSCC) progression by modulating the miR-588/CXCR4 axis.
Aging (Albany NY). 13:14078–14087. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Wu T, Wang P, Yang L, Li Q, Wang
J, Zhao R, Zhang J, Liu M, Cao J, et al: Circular RNA 103862
promotes proliferation and invasion of laryngeal squamous cell
carcinoma cells through the miR-493-5p/GOLM1 axis. Front Oncol.
10:10642020. View Article : Google Scholar : PubMed/NCBI
|