Introduction
Lung cancer is a leading cause of cancer-related
mortality [incidence ~2.2 million; mortality ~1.8 million (18%)]
worldwide in 2020 and poses significant clinical and public health
challenges (1). Despite advances in
diagnostic techniques and therapeutic interventions, the prognosis
of lung cancer remains poor, primarily because of late-stage
diagnosis and the development of resistance to conventional
therapies (2,3). Therefore, there is an urgent need to
identify new therapeutic agents that can effectively inhibit lung
cancer progression and overcome treatment resistance (4).
Adenosine monophosphate-activated protein kinase
(AMPK) is a critical sensor of cellular energy status and plays a
vital role in the regulation of cellular metabolism, growth and
survival (5,6). AMPK is activated under conditions of
metabolic stress, such as nutrient deprivation or hypoxia, and it
functions to restore cellular energy homeostasis by promoting
catabolic processes such as glucose uptake, fatty acid oxidation
and autophagy (7–9). Activation of AMPK could also regulate
the growth of various types of cancer, including lung, colorectal,
and liver cancer, by inducing autophagy or mediating metabolic
checkpoints (10,11). Therefore, AMPK activators are
potential therapeutic candidates for cancer treatment. Inducing the
activation of the AMPK signaling cascade using pharmacological or
genetic strategies could exert potent anti-NSCLC cell activity
(12–15). Accordingly, there is a need to
develop novel AMPK activators with low toxicity and high efficiency
to induce tumor cell inhibition. Autophagy is a cellular process
that degrades and recycles unnecessary or damaged cellular
components (16,17). It plays a crucial role in cell
survival, differentiation, immune responses and cell death
(16,18). In the context of cancer, autophagy
plays a dual role, suppressing tumor growth by limiting the
availability of nutrients and energy for rapidly dividing cancer
cells or promoting tumor cell survival under stress conditions,
leading to drug resistance and tumor relapse (19,20).
Understanding the roles and mechanisms of autophagy and the related
signaling pathways in cancer cells is important for targeting
autophagy in cancer treatment.
Sakurasosaponin (S-saponin), a recently identified
saponin present in a variety of plants [leaves of Aegiceras
corniculatum, roots of Jacquinia fammea Millsp and
Primula sieboldii (P. sieboldii)], is a natural compound
that exerts anticancer effects against different cancer types, such
as breast, lung, colorectal, melanoma and prostate cancer (21,22).
However, the molecular mechanisms underlying the anti-proliferative
effects of S-saponin in lung cancer remain largely unexplored. In
the present study, the main objective was to investigate the
effects of S-saponin on the proliferation of the non-small cell
lung cancer (NSCLC) cell lines, A549 and H1299 and to elucidate the
underlying molecular mechanisms, with a focus on the roles of
autophagy and AMPK signaling pathway. The results provide new
insights into the molecular mechanisms underlying the anticancer
effects of S-saponins and support the development of
S-saponin-based therapies for NSCLC.
Materials and methods
Cell culture, antibodies and
chemicals
The human NSCLC cell lines, A549 (cat. no. CCL-185)
and H1299 (cat. no. CRL-5803) were obtained from the American Type
Culture Collection. The cells were maintained in high-glucose
Dulbecco's modified Eagle's medium (DMEM) (Lonza Group, Ltd.)
supplemented with 10% FBS (MilliporeSigma). S-saponin was kindly
provided by Dr Nam-In Baek (Department of Oriental Medicine
Biotechnology, Kyung Hee University, Yongin, Korea) (22). Antibodies against poly (adenosine
diphosphate-ribose) polymerase (1:2,000; cat. no. 9545S, PARP),
AMPK (1:3,000; cat. no. 2532S) and phosphorylated (p-)AMPK
(1:3,000; cat. no. 2535S) were purchased from Cell Signaling
Technology, Inc. Antibodies against GAPDH (1:5,000; cat. no.
sc-47724) and tubulin (1:3,000; cat. no. sc-23948) were purchased
from Santa Cruz Biotechnology, Inc.
Horseradish-peroxidase-conjugated anti-mouse (1:2,000; cat. no.
31430) and horseradish-peroxidase-conjugated anti-rabbit secondary
antibodies (1:2,000; cat. no. 31463) were purchased from Thermo
Fisher Scientific, Inc.. Annexin V and propidium iodide (PI) were
purchased from Molecular Probes; Thermo Fisher Scientific, Inc.
Anti-LC3 II (1:2,000; cat. no. NB100-2220SS) antibody was purchased
from Novus Biologicals, LLC. Compound C (cat. no. 171260) was
purchased from MilliporeSigma.
Extraction and isolation of
S-saponin
S-saponin was kindly provided by Dr Nam-In Baek
(Department of Oriental Medicine Biotechnology, Kyung Hee
University, Yongin, Korea) (22).
Briefly, the air-dried roots of P. sieboldii (200 g) were
extracted with 80% MeOH (3 L ×3), and the concentrated extract
(32.4 g) was poured in H2O (500 ml) and extracted with
EtOAc (500 ml ×3) and n-BuOH (500 ml ×3), successively. Each layer
which was concentrated in vacuo gave the EtOAc fraction
(PSE, 4.7 g), n-BuOH fraction (PSB, 12.8 g), and aqueous fraction
(PSW, 14.9 g), respectively. The PSB was subjected to a silica gel
(Kieselgel 60; Merck KGaA) column (8×15 cm) chromatography and
eluted with CHCl3-MeOH (10:1 → 5:1 → 3:1, 2l of each)
and CHCl3-MeOH-H2O (10:3:1→7:3:1→65:35:10, 2
l of each) to produce 12 fractions (PSB1 to PSB12). Fraction PSB9
(8.4 g) was applied to octadecyl silica gel (ODS; LiChroprep RP-18,
40–60 lm; Merck KGaA) column (4×15 cm) chromatography and eluted
MeOH-H2O (3:2, 3.5 l) to afford 5 fractions (PSB1-1 to
PSB-1-PBS-5) along with S-saponin at PSB-1-PBS-4 (7.1 g). The flow
rate was 10 ml/min and temperature was 25°C.
Cell proliferation assay
Cell viability was determined using a Cell Counting
Kit-8 (CCK-8) assay kit (Dojindo Laboratories, Inc.) according to
the manufacturer's instructions. Briefly, A549 and H1299 cells were
seeded in 96-well plates at a density of 5×103. After 24
h, the cells were treated with S-saponin at the doses or times
indicated in the figures. CCK-8 solution (10/100 µl medium) was
added to each well and the plate was incubated for 1 h in a
CO2 incubator at 37°C. The absorbance of each well was
measured at 450 nm using a microplate reader (Molecular Devices,
LLC).
Clonogenic assay
The clonogenic assay was performed as previously
described (22). Briefly, A549 and
H1299 cells were seeded at equal densities in six-well plates
(1×103 cells/well) for 24 h and then treated with
S-saponin at doses of 0, 1, 2.5, 5, 7.5 and 10 µg/ml. The treated
cells were cultured at 37°C and 5% CO2 for 7 days, and
the colonies (≥50 cells) were fixed with 20% methanol for 10 min at
room temperature and stained with 0.01% crystal violet for 10 min
and counted using ImageJ (version 1.54f; National Institutes of
Health).
Protein isolation and western
blotting
Protein isolation and western blotting were
performed as previously described (22). Briefly, A549 and H1299 cells were
treated with S-saponin at the aforementioned doses and time
intervals; subsequently, they were lysed in lysis buffer A [20 mM
HEPES (pH 7.5), 150 mM NaCl, 1 mM EDTA, 2 mM EGTA, 1% Triton X-100,
10% glycerol and protease inhibitor cocktail Set II (Sigma-Aldrich;
Merck KGaA)], and centrifuged at 14,000 × g at 4°C for 10 min. Cell
lysates were collected after centrifugation and protein
concentrations were determined using the Bradford method with
Bio-Rad Protein Assay Kit II (Bio-Rad Laboratories, Inc.). The
lysates (30 µg/lane) were separated through 10–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The samples
were transferred onto nitrocellulose membrane (Bio-Rad
Laboratories, Inc.). After blocking with 5% milk in TBST (in 50 mM
Tris, 200 mM NaCl, 0.2% Tween 20), the membrane was incubated in
the first antibody solution diluted in 10% milk at 4°C overnight.
Subsequently, membranes were incubated with either
horseradish-peroxidase-conjugated anti-mouse or anti rabbit
secondary antibody for 1 h at room temperature. Proteins bands were
visualized using ECL Western blotting reagents (GE-Life Science,
Piscataway, NJ, USA). The results from western blot analysis were
quantified using ImageJ (version 1.54f; National Institutes of
Health).
Annexin V/PI staining
A549 and H1299 cells (3×105 cells/well)
were treated with S-saponin for 24 h. Cell death was measured
through fluorescence-activated cell sorting (FACS) using an Annexin
V-fluorescein isothiocyanate/propidium iodide (Annexin V-FITC/PI)
staining kit (cat. no. 556570; BD Biosciences). Fluorescence was
measured using a fluorescence-activated cell sorting (FACS) Calibur
(BD Biosciences), and the data were analyzed using CellQuest
software (BD FACSDiva version 9.2; BD Biosciences).
Immunofluorescence analysis
For LC3-II puncta detection, A549 and H1299 cells
were transfected with GFP-LC3 vector (kindly provided by Dr
Seung-Yong Yoon, Department of Brain Science, University of Ulsan
College of Medicine, Seoul, Korea) and treated with or without
S-saponin at 37°C for 12 h. Cells were fixed with 4%
paraformaldehyde in PBS at room temperature for 10 min. The cells
were counterstained with Hoechst 33342 (cat. no. 62249, 1:1,000,
Thermo Fisher Scientific) for 1 min at room temperature for nuclear
staining. The coverslips containing the cells were then mounted
with AquaMount (Lerner Laboratories; Thermo Fisher Scientific,
Inc.) containing 0.01% 1,4-diazobicy clo(2,2,2)octane. Fluorescent
images were obtained using a Leica confocal laser scanning
microscope (Leica Microsystems GmbH).
Small-interfering (si)RNA and plasmid
transfection
For AMPK knockdown, a siRNA against AMPKα
(5′-AUGAUGUCAGAUGGUGAAUUU-3′; Bioneer Corporation) was constructed,
exhibiting a specific knockdown efficiency of >90%. A549 and
H1299 cells were transfected with the aforementioned specific siRNA
or with a non-targeting siRNA (5′-UUCUUCGAACGUGUCACGU-3′; Bioneer
Corporation) at a final concentration of 50 nmol/l for 72 h using
Lipofectamine® RNAimax (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocols.
Statistical analysis
All the data in the present study are expressed as
the mean ± standard deviation obtained from the results of three
independent experiments. Statistical difference between multiple
groups was analyzed using one-way analysis of variance (ANOVA)
followed by Bonferroni's correction, using SigmaPlot 12.0 software
(2013, Systat Software, Inc.). P<0.05 was considered to indicate
a statistically significant difference.
Results
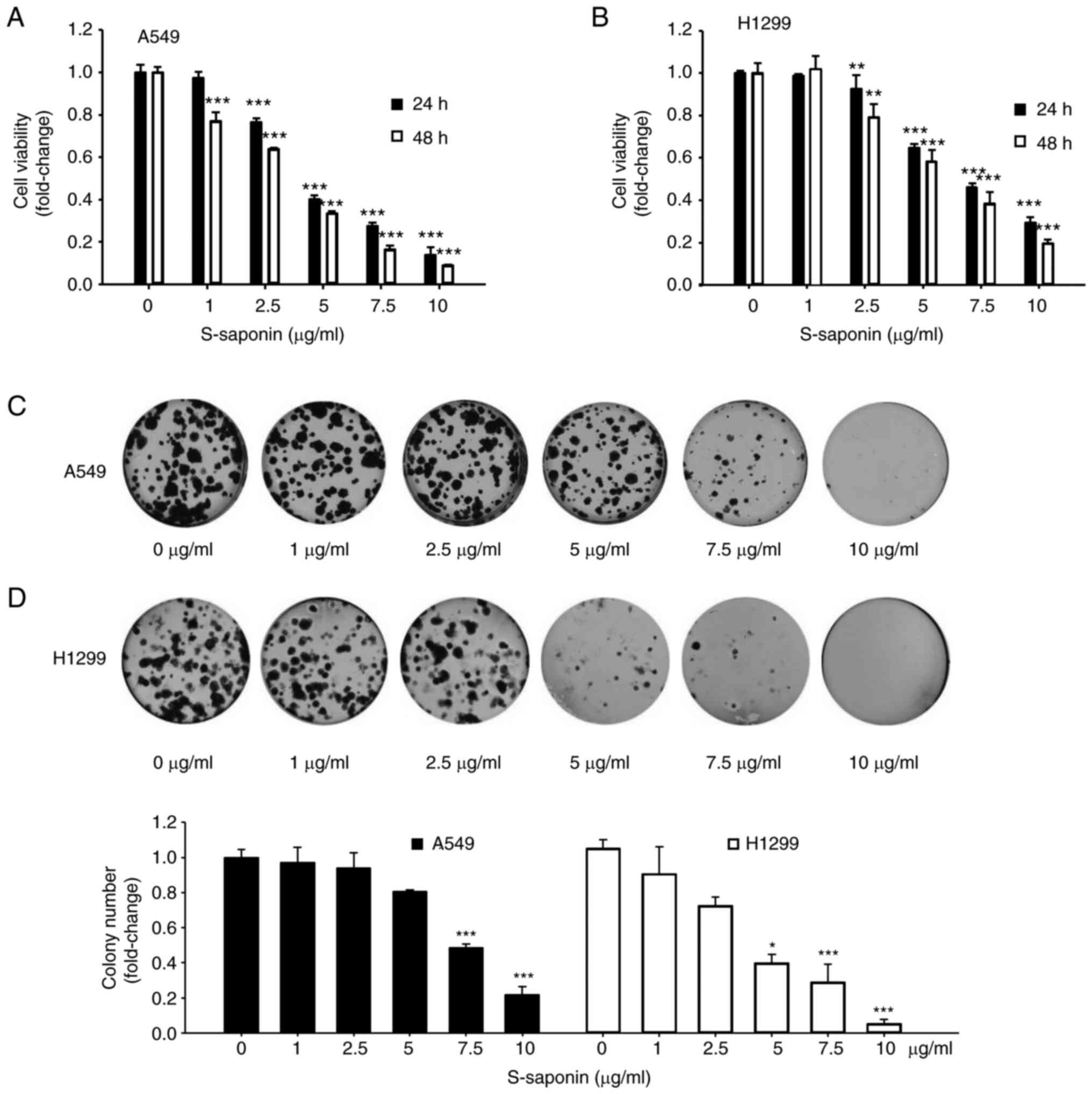
S-saponin inhibits cell proliferation
of NSCLC cells
To verify the anticancer effects of S-saponin on
NSCLC cells, its effects were first examined on NSCLC cell
proliferation. A549 and H1299 cells were incubated in growth medium
containing various concentrations of S-saponin for 24 or 48 h, and
their proliferation was determined using a CCK-8 cell viability
assay. S-saponin treatment significantly inhibited the
proliferation of A549 and H1299 cells in dose- and time-dependent
manners (Fig. 1A and B). In
addition, the clonogenic assay demonstrated that treatment with
S-saponin markedly suppressed colony formation in the treated A549
(Fig. 1C) and H1299 (Fig. 1D) cells compared with that in the
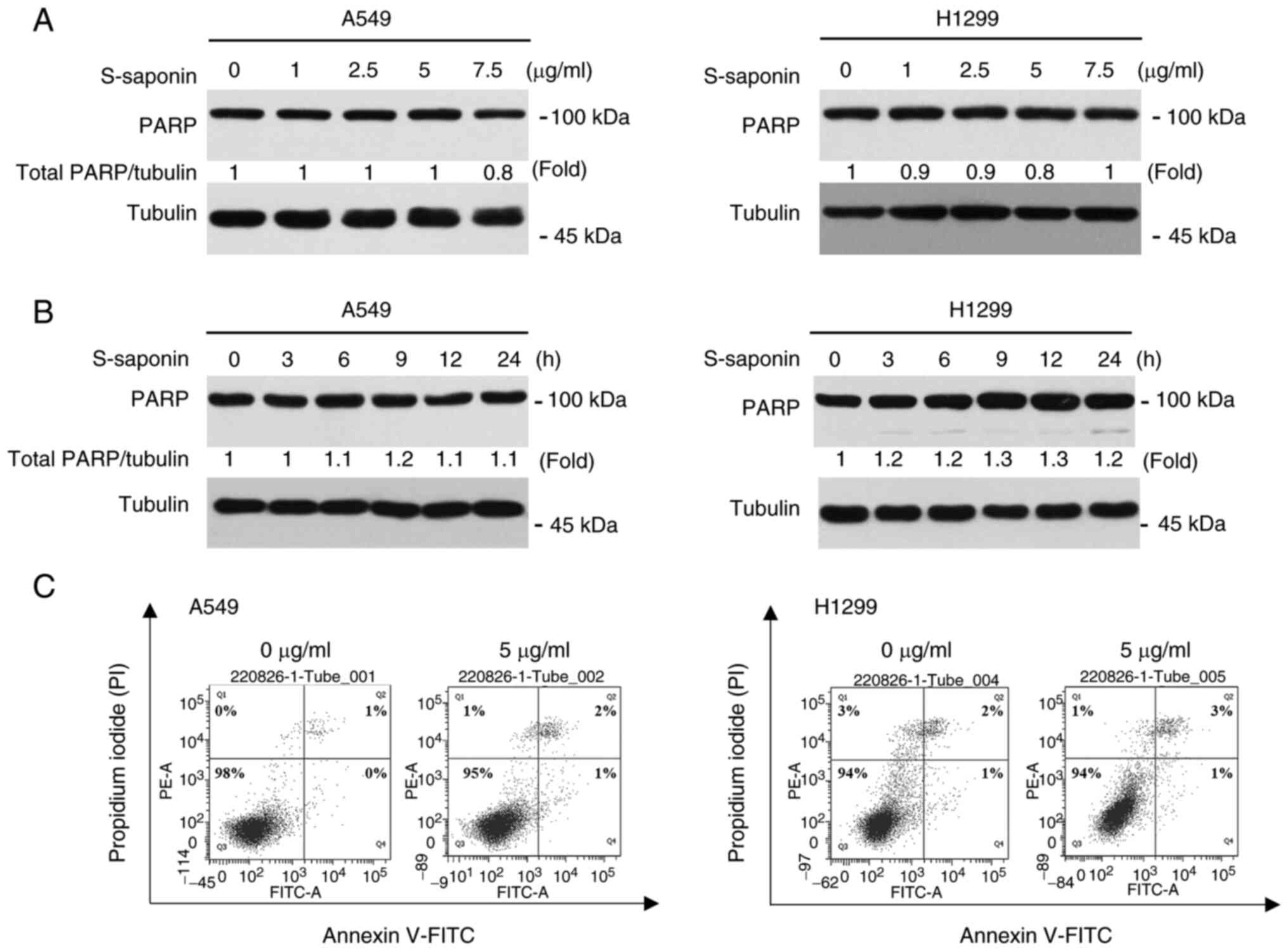
control cells. Next, it was examined whether apoptosis was involved
in the effects of S-saponin on cell proliferation. Western blotting
and Annexin V/PI double staining were used to assess the apoptotic
rate in A549 and H1299 cells. PARP cleavage was assessed through
western blotting to determine the status of apoptosis in
S-saponin-treated A549 and H1299 cells in a time- and
dose-dependent manner (Fig. 2A and
B). S-saponin did not affect PARP cleavage. A549 and H1299
cells treated with S-saponin were subjected to Annexin V/PI double
staining; S-saponin did not induce significant cell death (Fig. 2C). Therefore, it was concluded that
the inhibitory effect of S-saponin on cell proliferation does not
involve apoptosis.
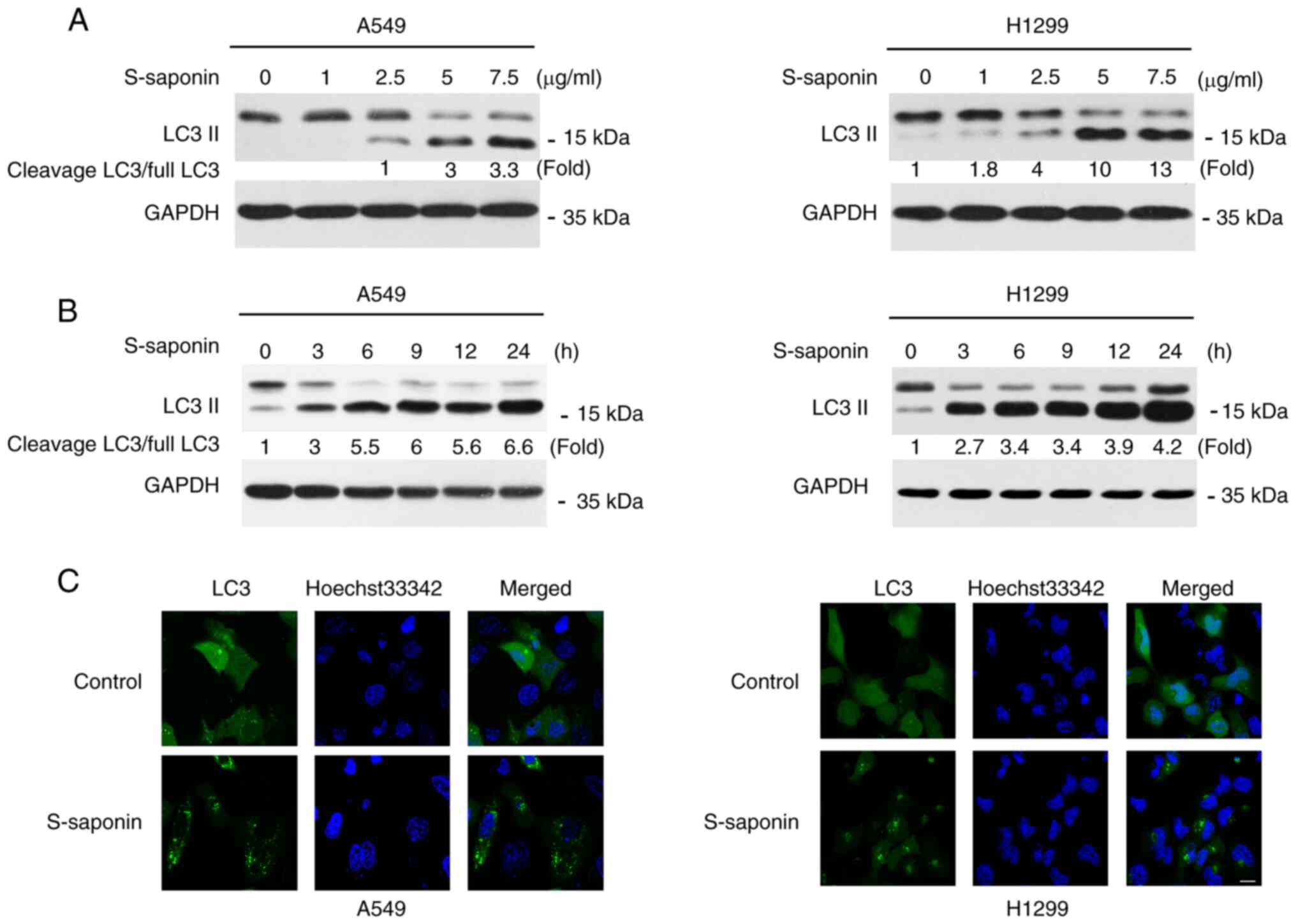
S-saponin induces autophagy in NSCLC
cells
S-saponin was extensively investigated in order to
understand if autophagy activation is involved in the
anti-proliferative effect of S-saponin in A549 and H1299 cells. The
conversion of LC3 I to LC3 II and its subsequent translocation to
autophagic vacuoles during autophagy induction is a hallmark of
mammalian autophagy (23). A549 and
H1299 cells were treated with different doses of S-saponin for
different time periods, and the cell lysates were prepared and
subjected to western blotting. Dose- and time-dependent increases
in LC3 II levels following S-saponin treatment were observed in
both cell lines (Fig. 3A and B). To
further confirm S-saponin-induced autophagy, A549 and H1299 cells
were transfected with a green fluorescent protein (GFP)-LC3 vector
for 12 h followed by treatment with or without 5 µg/ml S-saponin.
After 12 h, the distribution of LC3 was observed using a
fluorescence microscope. S-saponin treatment significantly induced
the formation of GFP-LC3 puncta in both A549 and H460 cells
(Fig. 3C).
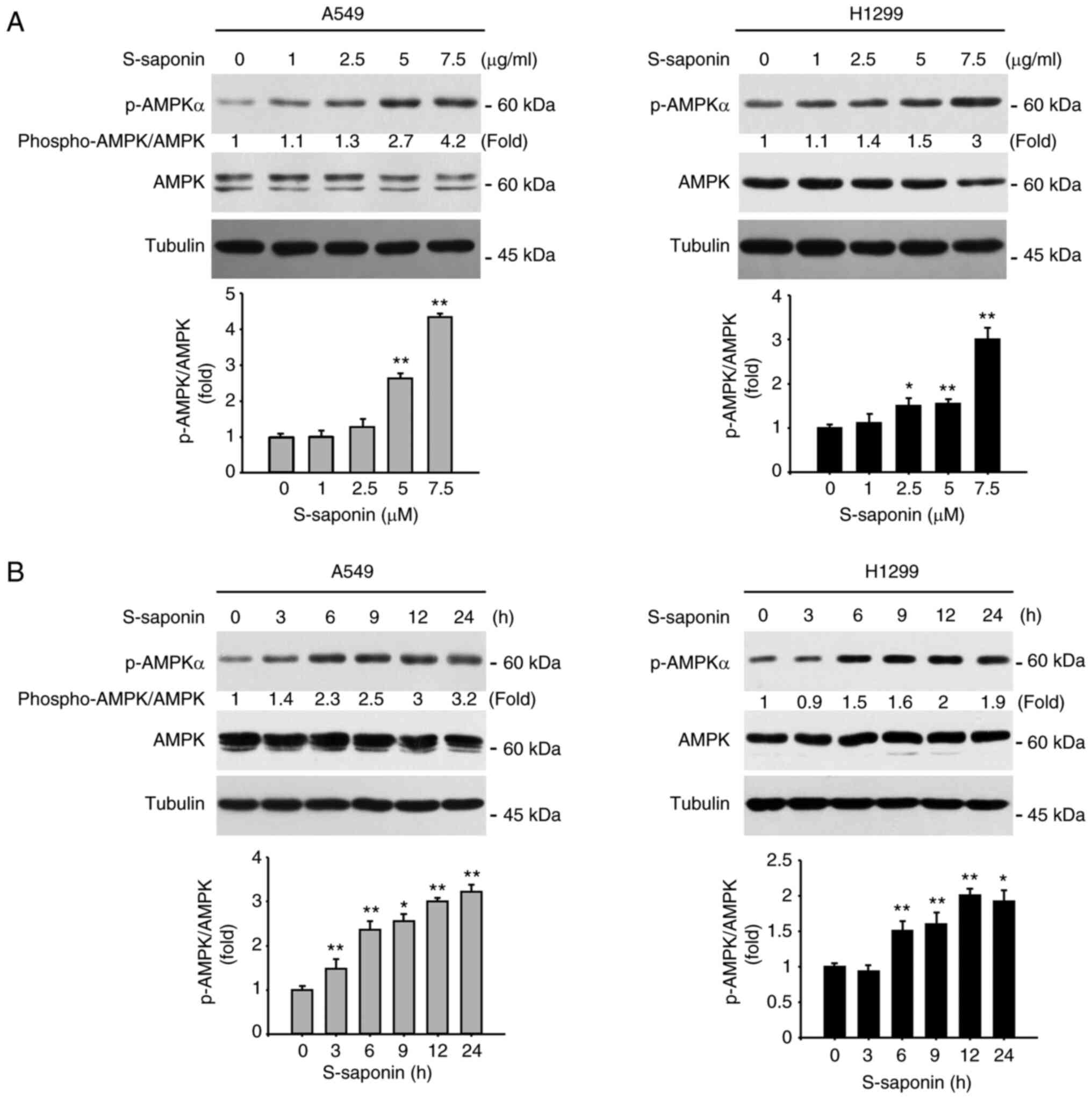
S-saponin influenced the AMPK
phosphorylation pathway in NSCLC cells
AMPK is a sensor of cellular energy status and is
activated under high intracellular AMP conditions, such as hypoxia
or nutrient deprivation, inducing autophagy (9). To examine whether S-saponin
phosphorylated AMPK in A549 and H1299 cells, the activation of
AMPKα by S-saponin in the NSCLC cells was determined. A549 and
H1299 cells were treated with different doses of S-saponin for
different time periods, and the cell lysates were analyzed using
western blotting. P-AMPKα was upregulated by S-saponin in a dose-
and time-dependent manner (Fig. 4A and
B).
AMPK is critical for
autophagy-mediated cell proliferation of S-saponin-treated NSCLC
cells
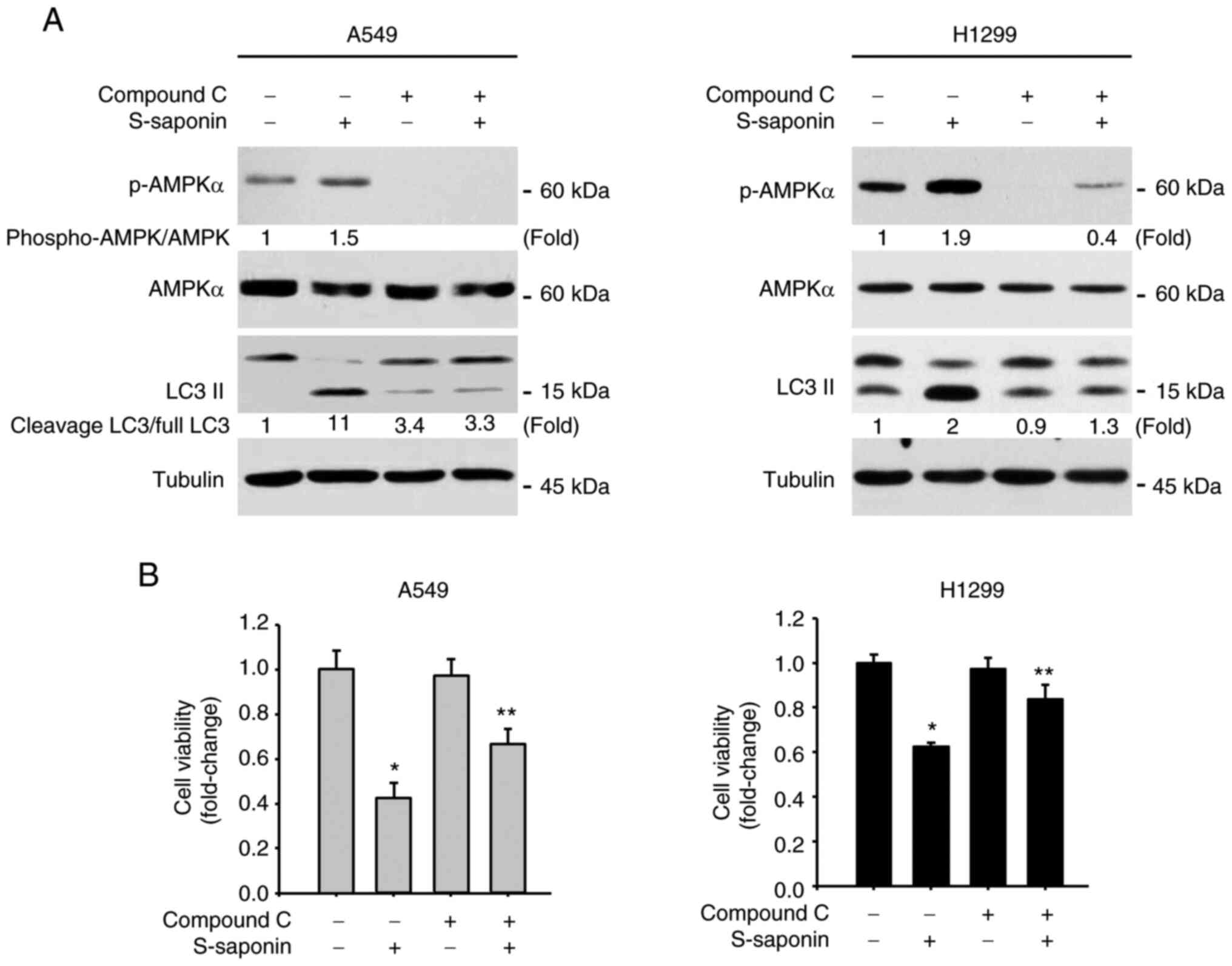
To further prove that S-saponin activates the AMPK
signaling pathway, A549 and H1299 cells were incubated with 20 µM
Compound C to block the AMPK signaling pathway before treatment
with S-saponin. Western blotting results showed that Compound C
inhibited AMPK activation by S-saponin and abolished the increase
in LC3 II protein levels in S-saponin-treated cells (Fig. 5A). In addition, when A549 and H1299
cells were treated with 5 µg/ml S-saponin in the presence or
absence of 20 µM Compound C for 18 h, the decrease in the
proliferation of A549 and H1299 cells induced by S-saponin was
significantly rescued (Fig. 5B). To
illustrate the essential role of the AMPK signaling pathway in the
autophagy-inducing effect of S-saponin, siRNA-mediated knockdown of
AMPKα was utilized, an indispensable catalytic subunit of AMPK, to
block the AMPK pathway. siRNA-AMPKα-transfected cells displayed a
reduction in AMPKα expression, which blocked the upregulation of
LC3 II by S-saponin (Fig. 6A). In
addition, the clonogenic assay demonstrated that the colony numbers
were significantly rescued in the siRNA-AMPKα-transfected A549 and
H1299 cells following S-saponin treatment (Fig. 6B).
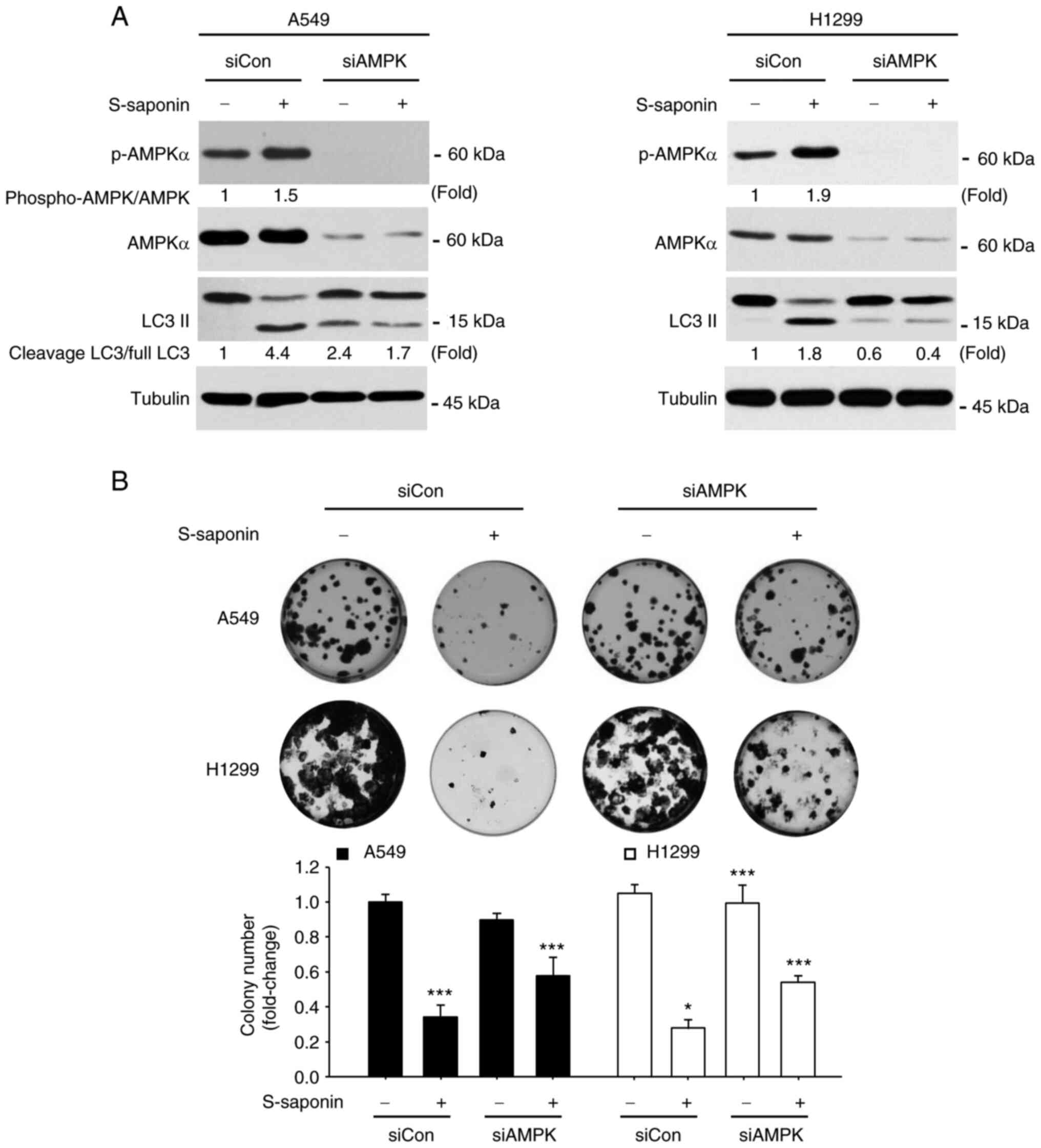 | Figure 6.Knockdown of AMPK attenuates
S-saponin-induced autophagy and cell proliferation inhibition. (A)
A549 and H1299 cells were transfected with control siRNA or
siRNA-AMPKα and treated with or without 5 µg/ml S-saponin. The
cells were subjected to western blotting using p-AMPKα, AMPKα, and
LC3 II antibodies. Tubulin was used as the loading control. The
experiment was independently performed thrice, quantified through
the ImageJ software and normalized with AMPK or full LC3 band. (B)
A549 and H1299 cells were transfected with control siRNA or
siRNA-AMPK and treated with or without 5 µg/ml S-saponin. Following
the treatment with S-saponin, the media was changed once after 24
h, and then once every 2 days; the cells were fixed after 4 days of
observation and counted following the staining with crystal violet.
Representative images of colonies are shown, and quantitative data
represent the mean ± SD of three independent experiments.
*P<0.05 and ***P<0.001 compared with control group. AMPK,
adenosine monophosphate-activated protein kinase; S-saponin,
sakurasosaponin; si, small interfering; p-, phosphorylated. |
Discussion
Lung cancer is the leading cause of cancer-related
deaths worldwide, with NSCLC accounting for ~85% of all lung cancer
cases (24). Despite advancements
in diagnostic and treatment modalities, the overall survival rate
of lung cancer patients remains low, emphasizing the need for new
therapeutic strategies (2). In this
context, the present study contributes to the growing body of
research investigating the potential of natural compounds, such as
S-saponin, in the treatment of NSCLC.
Natural products have long been considered rich
sources of bioactive compounds with potential therapeutic
applications in various diseases, including cancer (25–27).
One such compound is saponin, a bioactive component found in
various plants (28). Saponin
possesses multiple pharmacological properties, including
anti-inflammatory, anti-fibrotic, antioxidant and anticancer
effects (29,30). The anticancer potential of saponins
have been demonstrated in various types of cancers, including
breast, gastric and colorectal cancers (31). However, the effects of S-saponin, a
newly identified saponin from the root of P. sieboldii, on
NSCLC cells and the underlying molecular mechanisms have not been
fully elucidated.
S-saponin inhibited the proliferation of A549 and
H1299 NSCLC cells in a dose- and time-dependent manner. This effect
on cell proliferation was not mediated by apoptosis, as evidenced
by the lack of PARP cleavage and Annexin V/PI double staining.
Instead, S-saponin induces autophagy in NSCLC cells, as
demonstrated by the dose- and time-dependent increases in LC3 II
levels and GFP-LC3 puncta formation. These results are consistent
with those of previous studies, highlighting the role of autophagy
in the anticancer effects of various natural compounds (32).
Blocking the AMPK signaling pathway, either by using
the AMPK inhibitor Compound C or siRNA-mediated knockdown of AMPKα,
significantly rescued the inhibition of cell proliferation and
induction of autophagy by S-saponin. These results emphasize the
critical role of AMPK activation in the autophagy-mediated
antiproliferative effects of S-saponin in NSCLC cells. The results
of the present study are in accordance with a study by Liu et
al (33), which reported that
gitogenin, a saponin isolated from Tribulus longipetalus,
induces autophagy in lung cancer cells via the AMPK signaling
pathway. Similarly, a study by Xiang et al (34) demonstrated that Paris saponin VII, a
bioactive constituent extracted from Trillum tschonoskii
Maxim., inhibits the growth of NSCLC cells by inducing autophagy
via the AMPK/mTOR pathway (34).
These studies, together with results that were revealed in the
present study, provide strong evidence for the involvement of
AMPK-mediated autophagy in the anticancer effects of saponins in
NSCLC cells.
In conclusion, the present study provides new
insights into the molecular mechanisms underlying the anticancer
effects of S-saponin in NSCLC cells, showing that S-saponin
inhibits cell proliferation through the induction of autophagy via
the activation of the AMPK signaling pathway. These findings
contribute to the understanding of the potential therapeutic
applications of S-saponin in NSCLC treatment and pave the way for
future research on its efficacy in combination therapies and in
in vivo models.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Research Program
through the National Research Foundation of Korea (NRF) funded by
MSIT (grant nos. RS-2023-00207868 and RS-2023-00247255) and the
Ministry of Education (grant no. NRF-2020R1F1A1072646).
Availability of data and materials
All data generated and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS, CL and SJ designed the present study. YS, CL,
JL, JK, YK and PL performed experiments. YS, CL and SJ analyzed the
data. YS and SJ wrote the manuscript. YS, JL, JK, YK, PL and SJ
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, DeCamp M,
et al: NCCN guidelines® insights: Non-small cell lung
cancer, version 2.2023. J Natl Compr Canc Netw. 21:340–350. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs from 1981 to 2014. J Nat Prod. 79:629–661.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trefts E and Shaw RJ: AMPK: restoring
metabolic homeostasis over space and time. Mol Cell. 81:3677–3690.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herzig S and Shaw RJ: AMPK: Guardian of
metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol.
19:121–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weikel KA, Ruderman NB and Cacicedo JM:
Unraveling the actions of AMP-activated protein kinase in metabolic
diseases: Systemic to molecular insights. Metabolism. 65:634–645.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hardie DG: AMPK: Positive and negative
regulation, and its role in whole-body energy homeostasis. Curr
Opin Cell Biol. 33:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Saud SM, Young MR, Chen G and Hua B:
Targeting AMPK for cancer prevention and treatment. Oncotarget.
6:7365–7378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu CC, Peng D, Cai Z and Lin HK: AMPK
signaling and its targeting in cancer progression and treatment.
Semin Cancer Biol. 85:52–68. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ashrafizadeh M, Mirzaei S, Hushmandi K,
Rahmanian V, Zabolian A, Raei M, Farahani MV, Goharrizi MASB, Khan
H, Zarrabi A and Samarghandian S: Therapeutic potential of AMPK
signaling targeting in lung cancer: Advances, challenges and future
prospects. Life Sci. 278:1196492021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia YC, Zha JH, Sang YH, Yin H, Xu GQ,
Zhen J, Zhang Y and Yu BT: AMPK activation by ASP4132 inhibits
non-small cell lung cancer cell growth. Cell Death Dis. 12:3652021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu T, Li M, Zhao M, Huang Y, Bi G, Liang
J, Chen Z, Zheng Y, Xi J, Lin Z, et al: by regulating AMPK
Metformin inhibits human non-small cell lung cancer-CEBPB-PDL1
signaling pathway. Cancer Immunol Immunother. 71:1733–1746. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patra KC, Weerasekara VK and Bardeesy N:
AMPK-mediated lysosome biogenesis in lung cancer growth. Cell
Metab. 29:238–240. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ichimiya T, Yamakawa T, Hirano T, Yokoyama
Y, Hayashi Y, Hirayama D, Wagatsuma K, Itoi T and Nakase H:
Autophagy and autophagy-related diseases: A review. Int J Mol Sci.
21:98742020. View Article : Google Scholar
|
|
17
|
Cao W, Li J, Yang K and Cao D: An overview
of autophagy: Mechanism, regulation and research progress. Bull
Cancer. 108:304–322. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khandia R, Dadar M, Munjal A, Dhama K,
Karthik K, Tiwari R, Yatoo MI, Iqbal HMN, Singh KP, Joshi SK and
Chaicumpa W: A comprehensive review of autophagy and its various
roles in infectious, non-infectious, and lifestyle diseases:
Current knowledge and prospects for disease prevention, novel drug
design, and therapy. Cells. 8:6742019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yun CW and Lee SH: The roles of autophagy
in cancer. Int J Mol Sci. 19:34662018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lim SM, Mohamad Hanif EA and Chin SF: Is
targeting autophagy mechanism in cancer a good approach? The
possible double-edge sword effect. Cell Biosci. 11:562021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vinh LB, Nguyet NTM, Yang SY, Kim JH,
Thanh NV, Cuong NX, Nam NH, Minh CV, Hwang I and Kim YH: Cytotoxic
triterpene saponins from the mangrove Aegiceras
corniculatum. Nat Prod Res. 33:628–634. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song IS, Jeong YJ, Kim J, Seo KH, Baek NI,
Kim Y, Kim CS and Jang SW: Pharmacological inhibition of androgen
receptor expression induces cell death in prostate cancer cells.
Cell Mol Life Sci. 77:4663–4673. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dikic I and Elazar Z: Mechanism and
medical implications of mammalian autophagy. Nat Rev Mol Cell Biol.
19:349–364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Atanasov AG, Zotchev SB and Dirsch VM;
International Natural Product Sciences Taskforce; Supuran CT, :
Natural products in drug discovery: Advances and opportunities. Nat
Rev Drug Discov. 20:200–216. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seca AML and Pinto DGA: Plant secondary
metabolites as anticancer agents: Successes in clinical trials and
therapeutic application. Int J Mol Sci. 19:2632018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dehelean CA, Marcovici I, Soica C, Mioc M,
Coricovac D, Iurciuc S, Cretu OM and Pinzaru I: Plant-derived
anticancer compounds as new perspectives in drug discovery and
alternative therapy. Molecules. 26:11092021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Podolak I, Galanty A and Sobolewska D:
Saponins as cytotoxic agents: A review. Phytochem Rev. 9:425–474.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bildziukevich U, Wimmerova M and Wimmer Z:
Saponins of selected triterpenoids as potential therapeutic agents:
A review. Pharmaceuticals (Basel). 16:3862023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee H, Jeong H, Lee CM, Shin JA, Jang SW,
Lee JH, Park SY, Kim JY and Tchah H: Antifibrotic effects of
sakuraso-saponin in primary cultured pterygium fibroblasts in
comparison with mitomycin C. Invest Ophthalmol Vis Sci.
60:4784–4791. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu M, Sun Y, Bai H, Wang Y, Yang B, Wang
Q and Kuang H: Effects of saponins from Chinese herbal medicines on
signal transduction pathways in cancer: A review. Front Pharmacol.
14:11599852023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie Q, Chen Y, Tan H, Liu B, Zheng LL and
Mu Y: Targeting autophagy with natural compounds in cancer: A
renewed perspective from molecular mechanisms to targeted therapy.
Front Pharmacol. 12:7481492021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu T, Li Y, Sun J, Tian G and Shi Z:
Gitogenin suppresses lung cancer progression by inducing apoptosis
and autophagy initiation through the activation of AMPK signaling.
Int Immunopharmacol. 111:1088062022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiang YC, Shen J, Si Y, Liu XW, Zhang L,
Wen J, Zhang T, Yu QQ, Lu JF, Xiang K and Liu Y: Paris saponin VII,
a direct activator of AMPK, induces autophagy and exhibits
therapeutic potential in non-small-cell lung cancer. Chin J Nat
Med. 19:195–204. 2021.PubMed/NCBI
|