Introduction
Targeted therapy is efficient for patients with
advanced non-small cell lung cancer (NSCLC) with related gene
mutations, highlighting the importance of actively searching for
mutations. Tyrosine receptor kinase ROS proto-oncogene 1
(ROS1) rearrangements occur in 1–2% of patients with
non-small cell lung cancer (NSCLC) (1). Crizotinib is a tyrosine kinase
inhibitor (TKI) that targets the anaplastic lymphoma kinase gene
(ALK) or ROS1 kinase domain and is considered the
standard of care for metastatic NSCLC who were positive for
ROS1 fusion gene. Although the frequency of ROS1
rearrangements in lung cancers is low, the efficient determination
of ROS1 status in patients with NSCLC is critical for
directing patient care. Here we report the case of a patient with
NSCLC harboring a rare HLA_A-ROS1 rearrangement, who showed
a clinical response to crizotinib.
Case report
A 47-year-old Japanese woman with dyspnea for 2
months was referred to our hospital. She had no relevant history
except for light smoking. Chest radiography analysis revealed a
mass in the right upper lung field. Computed tomography (CT)
analysis revealed a 7.0 cm mass in the right upper lobe with
mediastinal invasion, contralateral mediastinal lymphadenopathy and
a left adrenal gland tumor. Bronchoscopy analysis revealed a
polypoid tumor at the orifice of the right upper lobe bronchus and
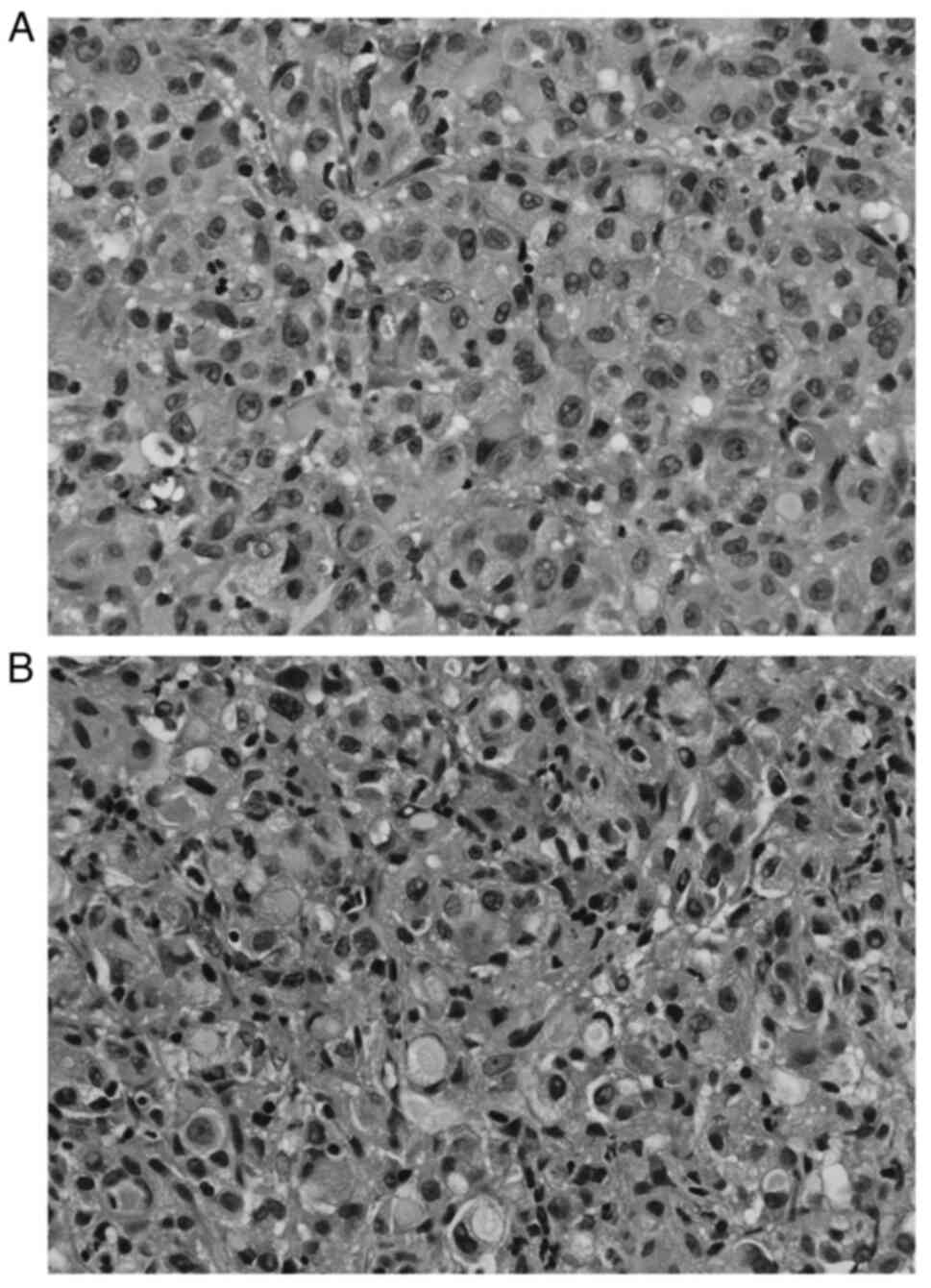
a transbronchial biopsy was performed. Pathological examination
showed primary lung adenocarcinoma with hepatoid cytology and
signet cells (Fig. 1A and B).
According to the 7th edition of TNM staging, the patient was
classified as having stage IVB lung adenocarcinoma (T4aN3M1b).
Mutation status was negative for EGFR, ALK, BRAF, and
ROS1; the OncoGuide AmoyDx ROS1 gene fusion detection kit
was used to detect ROS1 rearrangement. Programmed death-ligand 1
expression with a tumor proportion score of 5% was confirmed by
immunohistochemistry analysis using 22C3 antibody. She was treated
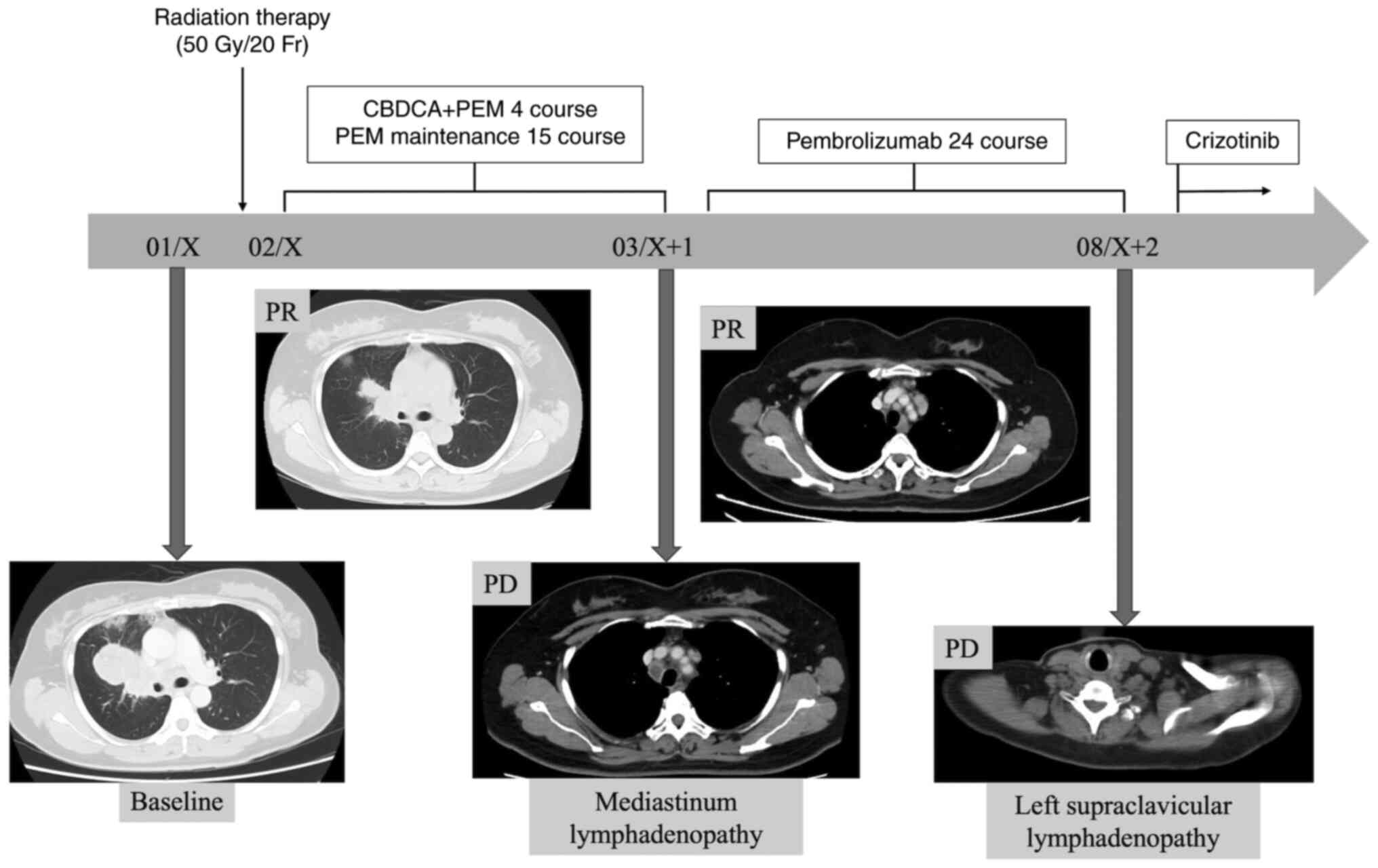
with radiation therapy for the primary lesion (50 Gy/20 Fr) as well
as chemotherapy (carboplatin plus pemetrexed), which was followed
by pemetrexed maintenance therapy. After 15 cycles of maintenance
therapy, mediastinal lymphadenopathy was newly detected and
pembrolizumab monotherapy was initiated as the second-line therapy.
During the treatment, as the Oncomine CDx Target Test (Oncomine
DxTT) was newly covered by insurance as a multigene panel assay for
NSCLC in Japan, the residual specimen was subjected to Oncomine
DxTT and HLA_A-ROS1 rearrangement was detected. After 21
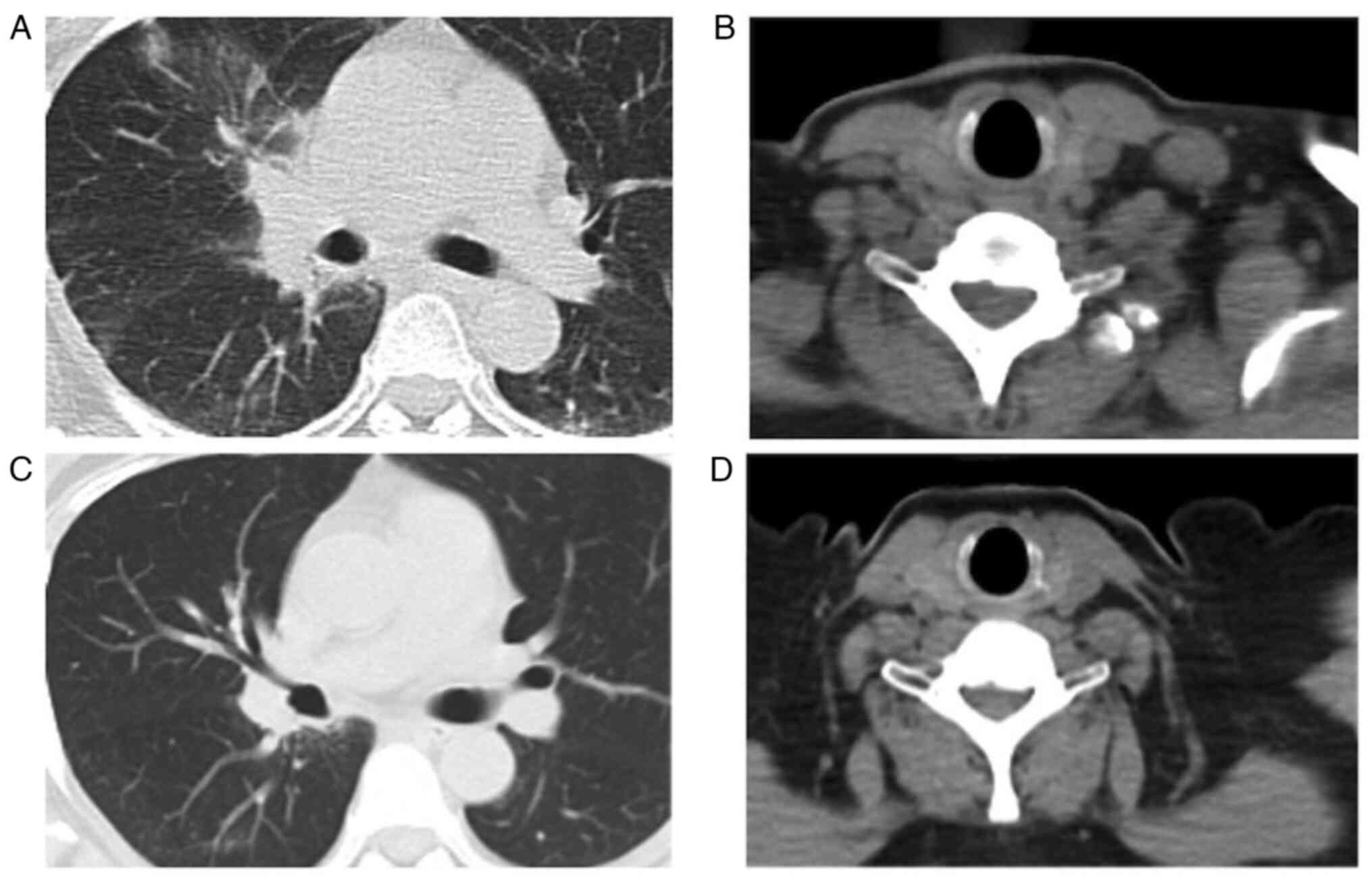
cycles of pembrolizumab monotherapy (Fig. 2), the CT scan showed left
supraclavicular lymphadenopathy (Fig. 3
A and B), and the patient was administered crizotinib therapy
as the third-line therapy. She achieved a partial response as
defined by the Response Evaluation Criteria in Solid Tumors version
1.1. Currently, cancer has been stable for over 16 months and
patient follow up is ongoing (Fig. 3C
and D).
Discussion
This is the first case report of a patient with lung
adenocarcinoma harboring an HLA_A-ROS1 rearrangement with a
clinical course and response to crizotinib. To our knowledge, there
is only one case report of HLA_A-ROS1 rearrangement in
melanoma (2). HLA_A-ROS1
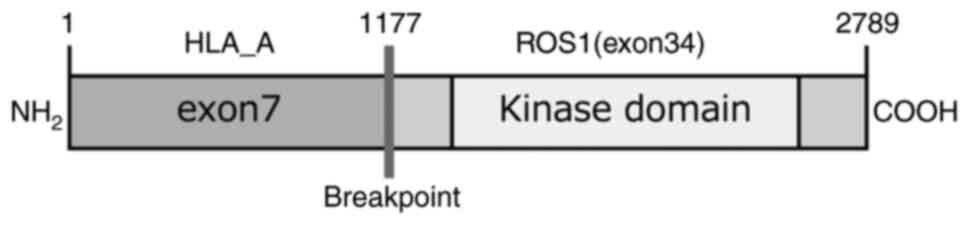
rearrangement was generated by the fusion of exon 34 of ROS1
located on the long arm of chromosome 6 with exon 7 of HLA_A
located on the short arm of chromosome 6 (Fig. 4). ROS1-rearranged NSCLC was
majorly observed in young females with no or light smoking history
(3). Currently, more than 34
ROS1 fusion partner genes have been reported in NSCLC,
including CD74, SDC4, EZR, and SLC34A2 (4). In Japan we previously often used the
OncoGuide AmoyDx ROS1 gene fusion detection kit to detect
ROS1 rearrangements including those in major partner genes
(5,6); however, this single gene testing was
unable to detect HLA_A-ROS1 rearrangement. Pathologically,
ALK- and ROS1-rearranged lung adenocarcinomas often
show a solid growth pattern and possess hepatoid cells and signet
ring cells (7). These pathological
features are the indicators of ALK and ROS1
rearrangement status in NSCLC. Hence, an additional multigene panel
assay should be considered for patients suspected of carrying
driver oncogenes based on patient background and pathological
patterns even if single gene tests were negative.
Crizotinib is a potent TKI for ALK, ROS1, and
mesenchymal-epithelial transition mutation-positive NSCLC. A
previous study showed that crizotinib was associated with an
overall response rate of 72% and a median progression-free survival
of 19.3 months in advanced ROS1-rearranged NSCLC (8). The efficacy of crizotinib varies
depending on ROS1 fusion partner (9). A previous study showed that the
patients carrying non-CD74-ROS1 fusions could get better
prognostic outcomes than those with CD74-ROS1 fusions by
crizotinib treatment (10). Hicks
et al reported that a patient with lung adenocarcinoma
harboring a rare ZCCHC8-ROS1 fusion who responded to
crizotinib showed progression-free survival of 7 months and total
duration of treatment of 15 months (11). The dramatic response to crizotinib
therapy in this case may be a reference for the treatment and
prognosis for NSCLC with the same fusion partner.
In conclusion, this report describes a case of
advanced lung adenocarcinoma harboring an HLA_A-ROS1
rearrangement with a dramatic response to crizotinib therapy. The
information presented in our report highlights to oncologists and
pulmonologists the importance of accurate multigene panel assays in
detecting driver oncogenes for treating patients with NSCLC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RK analyzed and interpreted the patient lung cancer
data and majorly contributed to writing the manuscript. MN
interpreted the data and assisted in the preparation of the
manuscript. HK, SH, MS and YH coordinated the clinics, performed
the treatment, participated in the follow-up of the patients,
obtained specimens and acquired data. MK, NT and HM made
substantial contributions to conception and design and edited the
manuscript. All authors read and approved the final manuscript. RK
and MN confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Written informed consent was provided by the
patient.
Patient consent for publication
The patient provided written informed consent for
the publication of the data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALK
|
anaplastic lymphoma kinase gene
|
|
BRAF
|
V-Raf murine sarcoma viral oncogene
homolog B
|
|
EGFR
|
epidermal growth factor receptor
|
|
CT
|
computed tomography
|
|
NSCLC
|
non-small cell lung cancer
|
|
Oncomine DxTT
|
Oncomine CDx Target Test
|
|
RET
|
rearranged during transfection
|
|
ROS1
|
tyrosine receptor kinase ROS
proto-oncogene 1
|
|
TKI
|
tyrosine kinase inhibitor
|
References
|
1
|
Rikova K, Guo A, Zeng Q, Possemato A, Yu
J, Haack H, Nardone J, Lee K, Reeves C, Li Y, et al: Global survey
of phosphotyrosine signaling identifies oncogenic kinases in lung
cancer. Cell. 131:1190–1203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wiesner T, He J, Yelensky R, Esteve-Puig
R, Botton T, Yeh I, Lipson D, Otto G, Brennan K, Murali R, et al:
Kinase fusion are frequent in Splitz tumors and spitzoid melanomas.
Nat Commun. 5:31162014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shaw AT, Ou SHI, Bang YJ, Camidge DR,
Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa
DB, et al: Crizotinib in ROS1-rearranged non-small-cell lung
cancer. N Engl J Med. 371:1963–1971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gendarme S, Bylicki O, Chouaid C and
Guisier F: ROS-1 fusions in non-small-cell lung cancer:
Evidence to date. Curr Oncol. 29:641–658. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu J, Lin Y, He X, Yang H, He P, Fu X, Li
G and Gu X: Comparison of detection methods and follow-up study on
the tyrosine kinase inhibitors therapy in non-small cell lung
cancer patients with ROS1 fusion rearrangement. BMC Cancer.
16:5992016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ke W, Xu Z and Xue L: ROS1 gene
rearrangement and clinicopathological characteristics in Chinese
NSCLC patients. Int J Clin Exp Pathol. 9:5594–5599. 2016.
|
|
7
|
Zhao J, Zheng J, Kong M and Zhou J, Ding W
and Zhou J: Advanced lung adenocarcinomas with ROS1-rearrangement
frequently show hepatoid cell. Oncotarget. 7:74162–74170. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shaw AT, Riety GJ, S, Bang YJ, Kim DW,
Camidge DR, Solomon BJ, Varella-Garcia M, Iafrate AJ, Shapiro GI,
Usari T, et al: Crizotinib in ROS1-rearranged advanced
non-small-cell lung cancer (NSCLC): Updated results, including
overall survival, from PROFILE 1001. Ann Oncol. 30:1121–1126. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Zhang X, Zhang R, Xu Q, Yang H,
Lizaso A, Xu C, Liu J, Wang W, Ou SHI, et al: Clinical and
molecular factors that impact the efficacy of first-line crizotinib
in ROS1-rearranged non-small-cell lung cancer: A large multicenter
retrospective study. BMC Med. 19:2062021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Shen L, Ding D, Huang J, Zhang J,
Chen Z and Lu S: Efficacy of crizotinib among different types of
ROS1 fusion partners in patients with ROS1-rearranged non-small
cell lung cancer. J Thorac Oncol. 13:987–995. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hicks JK, Boyle T, Albacker LA, Madison R,
Frampton G and Creelan BC: Clinical activity of crizotinib in lung
adenocarcinoma harboring a rare ZCCHC8-ROS1 fusion. J Thorac Oncol.
13:e148–e150. 2018. View Article : Google Scholar : PubMed/NCBI
|