Introduction
Breast cancer that is positive for the estrogen
receptor (ER), progesterone receptor (PR) and human epidermal
growth factor receptor-2 (HER2) is referred to as triple-positive
breast cancer (TPBC) (1). TPBC
represents 9–11% of breast cancer cases worldwide and is known for
its aggressive clinical behavior and poor prognosis (2,3). TPBC
is a heterogeneous subtype of breast cancer in terms of gene
expression profiles (4,5) and presents a therapeutic challenge, as
crosstalk between hormonal and HER2 signaling pathways leads to
tumor progression and treatment resistance (4,6,7).
Patients with HER2-positive breast cancer receive a
treatment scheme that consists of targeted therapy, mainly
trastuzumab (TTZ), plus chemotherapy, usually in the form of
anthracyclines and taxanes (8).
However, 30% of patients treated with TTZ relapse after the first
treatment scheme, and most patients with metastatic disease that
initially respond to TTZ eventually acquire resistance (9–11).
Consequently, the molecular mechanisms underlying resistance to TTZ
are under investigation. Among these mechanisms, crosstalk between
HER2 and hormonal signaling has been suggested in TPBC (7,12–14).
In addition, previous studies have demonstrated the association
between ER and resistance to TTZ (15,16).
However, little is currently known about the role of progesterone
(Pg) and the PR in TTZ resistance.
Pg and the PR are important regulators of cell
proliferation and differentiation in the mammary gland (17). In breast cancer, they control
tumorigenesis and tumor development through genes, such as CCND1,
MYC, KLF4 and STAT5, which are associated with aggressive behavior
and can predict poor outcomes in patients (5,17). PR
isoforms are associated with the expression of EGFR family members
(HER3 and HER4) and ligand activators of this family [EGF,
amphiregulin, neuregulin (NRG)3 and NRG4] (12). In addition, they have been
associated with cell proliferation and the expansion of cells with
stem cell characteristics (18). In
a previous study on TTZ-resistant TPBC cells, an increase in PR
mRNA and protein expression levels has been observed in comparison
to non-resistant cells (16).
Therefore, the aim of the present study was to determine whether
Pg, alone or in combination with estradiol (E2), interferes with
the inhibitory effect of TTZ on cell viability, in a manner
dependent on the main PR isoforms [PR isoform A (PR-A) and PR
isoform B (PR-B)].
Materials and methods
Cell lines and cell culture
The BT474 and MDAMB361 cell lines were provided by
the Mr. Salvador Jimenez-Sanchez, who oversees the panel of breast
cancer cell lines purchased from the American Type Culture
Collection at the breast cancer-institutional program of the
Biomedical Research Institute, Universidad Nacional Autónoma de
México (Mexico City, Mexico). BT474 and MDAMB361 cells were
cultured in RPMI medium (cat. no. R6504; MilliporeSigma) and
Leibovitz L-15 medium (cat. no. LVP01; Caisson Labs), respectively.
Both media contained phenol red and were supplemented with 10%
fetal bovine serum (FBS; cat. no. 26140-079; Gibco; Thermo Fisher
Scientific, Inc.), penicillin (100 U/ml)/streptomycin (100 mg/ml)
solution (cat. no. PS-B; Capricorn Scientific GmbH) and
amphotericin B (0.25 mg/ml; cat. no. 15290026; Gibco; Thermo Fisher
Scientific, Inc.). BT474 cells and MDAMB361 were incubated with 5%
CO2 and 100% air, respectively, in a humidified
environment at 37°C.
Hormonal treatment with TTZ
A total of 15,000 BT474 or MDAMB361
cells/cm2 were seeded in 48-well plates with RPMI-1640
medium (cat. no. R8755; MilliporeSigma) without phenol red
supplemented with 10% FBS stripped with activated charcoal (cat.
no. C9157; MilliporeSigma) and were allowed to adhere for 24 h at
37°C and 5% CO2. The culture medium was then replaced,
and BT474 and MDAMB361 cells were treated with an experimentally
calculated IC50 of TTZ (Fig. S1B and C): 1.6 and 1 µg/ml TTZ (lot
no. N3581B062 B20652; Roche Diagnostics), respectively, 10 nM Pg
(cat. no. P0130-25G; MilliporeSigma) and/or 100 nM Mifepristone
(RU486; cat. no. M8046-100MG; MilliporeSigma). The Pg and RU486
doses were previously proven to be non-toxic in BT474 cells
(Fig. S1D and E). For some
experiments, BT474 cells were also treated with 10 nM E2 (cat. no.
E2758-1G; MilliporeSigma), a dose obtained from a previous report
(19). DMSO at 0.0001% was used as
a vehicle for Pg, E2 and RU486. Cells were treated for 144 h, with
the culture medium and treatment changed every 48 h. Subsequently,
cell viability was determined by staining cells for 20 min at room
temperature with 0.1% crystal violet (dissolved in 10% formic acid)
and measuring the optical density using a Multiskan GO
spectrophotometer (Thermo Fisher Scientific, Inc.) at 595 nm.
Western blot analysis
BT474 cells (2×105 cells/cm2)
were treated for 144 h and were then lysed with lysis buffer [50 nM
Tris-HCl (pH 8.0), 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium
deoxycholate, 0.1% SDS, 1 mM Na3VO4, 1 mM NaF
and 1% protease inhibitor cocktail (cat. no. 11873580001;
MilliporeSigma)] and sonicated on ice at 20–25 KHz for 10 sec.
Protein concentration was determined using Protein Assay Dye
Reagent Concentrate (cat. no. 5000006; Bio-Rad Laboratories, Inc.)
and the cell lysates (30 µg) were separated by SDS-PAGE on a 9%
gel. The proteins were then transferred to a PVDF membrane, blocked
for 45 min at room temperature in Tris-buffered saline (TBS) with
0.1% Tween 20 (TBS-T) containing 5% nonfat milk, and washed with
TBS-T. Membranes were incubated with the following primary
antibodies overnight in 0.1% bovine serum albumin (cat. no. 160069;
MP Biomedicals) at 4°C: PRA/B (1:500; cat. no. sc-810; Santa Cruz
Biotechnology, Inc.), ER (1:500; cat. no. sc-8005; Santa Cruz
Biotechnology, Inc.), Akt (1:10,000; cat. no. sc-1618-R; Santa Cruz
Biotechnology, Inc.), phosphorylated (p)Akt1/2/3 (1:250; cat. no.
sc-514032; Santa Cruz Biotechnology, Inc.), CDK4 (1:500; cat. no.
sc-23896; Santa Cruz Biotechnology, Inc.), cyclin D1 (1:500; cat.
no. sc-8396; Santa Cruz Biotechnology, Inc.), p27Kip1
(1:500; cat. no. sc-1641; Santa Cruz Biotechnology, Inc.), β-actin
(1:5,000; cat. no. sc-47778; Santa Cruz Biotechnology, Inc.), pPR
ser345 (1:500; cat. no. 12783S; Cell Signaling Technology, Inc.),
HER2 (1:10,000; cat. no. 2248; Cell Signaling Technology, Inc.) or
pHER2 Y1221/1222 (1:1,000; cat. no. 2249; Cell Signaling
Technology, Inc.). Subsequently, the membranes were washed with
TBS-T and incubated with a secondary horseradish
peroxidase-conjugated anti-mouse antibody (1:20,000; cat. no.
115-035-003; Jackson ImmunoResearch Laboratories, Inc.) or
horseradish peroxidase-conjugated anti-rabbit antibody (1:20,000;
cat. no. 31460; Thermo Fisher Scientific Inc.) for 45 min at room
temperature. The chemiluminescent signal was visualized using
Supersignal™ West Pico PLUS Chemiluminescent Substrate (cat. no.
34580; Thermo Fisher Scientific, Inc.) in the Fusion FX6 XT (serial
no. 16200804; Vilber Lourmat). Densitometric analysis was performed
using ImageJ software version 1.53 (National Institutes of
Health).
PR gene silencing
According to the manufacturer's protocols of small
interfering RNA (siRNA), 15,000 BT474 cells/cm2 were
transfected for 19 h at 37°C with 1.6 µg of a pool of three
target-specific 19–25 nucleotide siRNAs targeting human PR (cat.
no. sc-270221; Santa Cruz Biotechnology, Inc.) or with 1 µg control
siRNA-A (cat. no. sc-37007; Santa Cruz Biotechnology, Inc.) using 6
µl of a commercially available siRNA transfection reagent (cat. no.
sc-29528; Santa Cruz Biotechnology, Inc.). Immediately after the
transfection period, the subsequent experiments were performed. The
sequences of the siRNAs targeting PR are shown in Table SI. siRNA-A is a scrambled sequence
that is considered proprietary information.
Data mining on clinical and expression
data
Normalized data from eight different datasets
(Table SII) were downloaded from
the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) (20) and were annotated with Biomart
(21) in the R software environment
version 4.3.1 (22). The duplicate
probes per gene were processed as follows: For Affymetrix
microarray profiles, the probe with the highest interquartile range
was selected; for the Illumina platform, the probe with the highest
value was selected; for Agilent data, the mean of all probes was
calculated. Clinical data for each gene set were retrieved from the
GEO (20). Statistical analyses
were applied to define significant differences between biological
groups using a Student's unpaired t-test in R software version
4.3.1 (22). The survival plot was
generated using the Kaplan-Meier plotter (https://kmplot.com/analysis/index.php?p=background)
which automatically performed a log-rank test on the results
produced (23).
Statistical analysis
Experimental results are presented as the mean ± SD.
Data were analyzed by one-way ANOVA with a Tukey's pairwise post
hoc test using Past4 software version 4.11 downloaded from
https://www.nhm.uio.no/english/research/resources/past/index.html.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Pg interferes with the inhibitory
effect of TTZ on viability
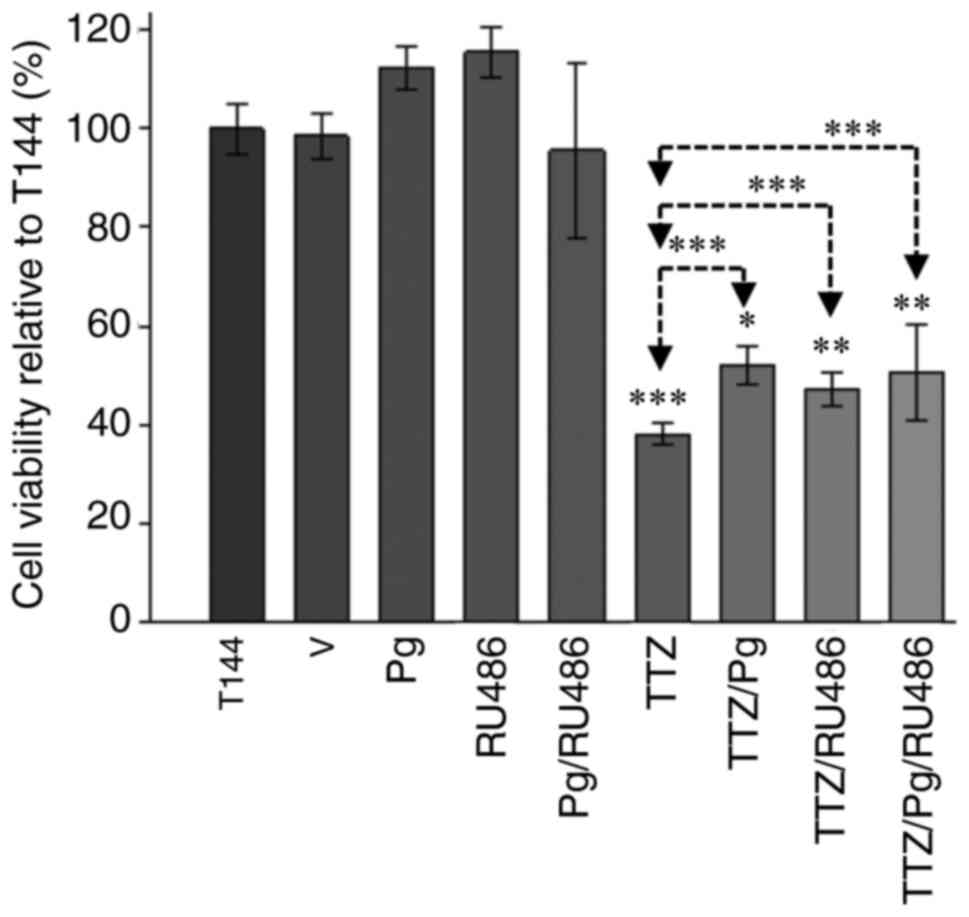
To determine if Pg interferes with the inhibitory
effect of TTZ on cell viability, BT474 cells (Fig. 1) and MDAMB361 cells (Fig. S2) were exposed to 144 h of
continuous treatment. In comparison with control cells that were
left untreated for the 144 h experiment (T144), TTZ decreased cell
viability by 61.7 and 47.7% in BT474 and MDAMB361 cells,
respectively (Figs. 1 and S2). Compared with the TTZ group, in the
TTZ/Pg-treated group, Pg reduced the inhibitory effect of TTZ on
viability by 22.5 and 26.5% in BT474 and MDAMB361 cells,
respectively (Figs. 1 and S2). Notably, when the PR antagonist RU486
was used, a Pg-like effect was observed. In comparison to TTZ
alone, the TTZ/RU486 combination reduced the inhibitory effect of
TTZ on viability by 14.9 and 65.2% in BT474 and MDAMB361 cells,
respectively (Figs. 1 and S2). Similarly, compared with TTZ alone,
treatment with TTZ/Pg/RU486 reduced the inhibitory effect of TTZ on
viability by 20.1 and 48.9% in BT474 and MDAMB361 cells,
respectively (Figs. 1 and S2). The fact that there were no
statistically significant differences in viability between the
TTZ/Pg, TTZ/RU486 and TTZ/Pg/RU486 groups suggests that RU486 did
not antagonize Pg in these experiments. Taken together these
results indicated that Pg and RU486 interfere with the inhibitory
effect of TTZ on viability. Despite similar effects being observed
on MDAMB361 cells, due to their lower expression of HER2 and PR-B
(Fig. S1A) and their low
proliferation rate, the present study focused on BT474 cells.
Pg interference in TTZ activity is
related to PR-B phosphorylation
The present study aimed to determine how Pg
interferes in the inhibitory effect of TTZ on viability. Therefore,
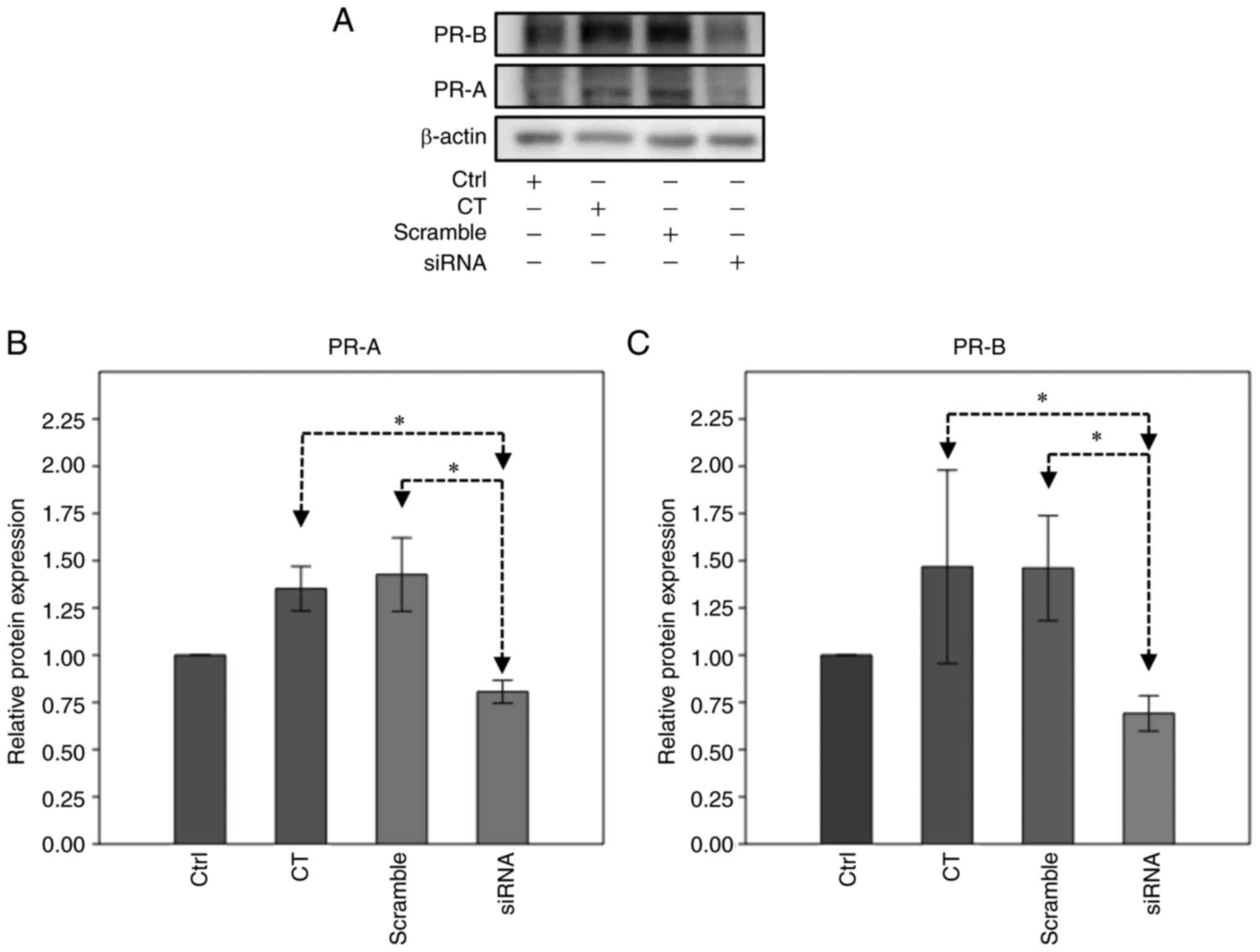
PR pathway activation was analyzed by western blotting after 144 h
of treatment (Fig. 2). The results
revealed that in BT474 cells, the basal levels of PR-B were higher
than those of PR-A (Fig. 2A).
However, neither isoform exhibited significant changes in their
relative expression levels with different treatments (Fig. 2B and C). When the phosphorylation
levels of PR isoforms were analyzed, no changes in PR-A were
observed (data not shown). On the other hand, treatment with TTZ/Pg
and TTZ/RU486 combinations induced a significant upregulation of
PR-B phosphorylation compared with that in cells treated with TTZ
alone (Fig. 2A and D).
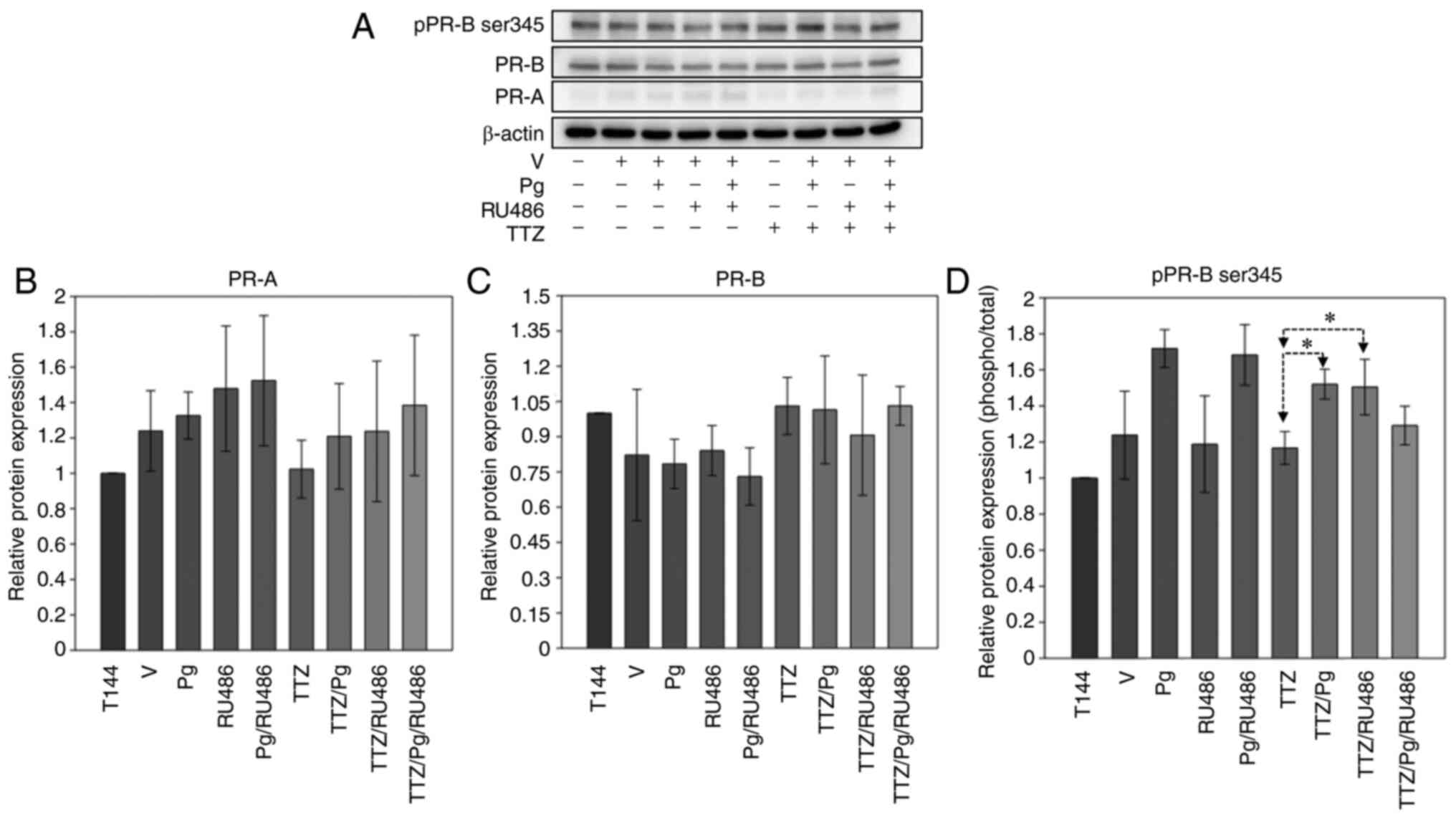 | Figure 2.Effect of Pg, RU486, TTZ and their
combinations on the expression and phosphorylation levels of PR
isoforms. (A) Representative image of the western blot analysis of
pPR-B ser345, PR-B and PR-A expression in BT474 cells treated with
Pg, RU486, TTZ alone or in combination. Densitometric analysis of
(B) PR-A, (C) PR-B and (D) pPR-B ser345. Data are presented as the
mean ± SD of three independent experiments (n=3). *P<0.05. T144,
control cells; V, vehicle (DMSO); Pg, progesterone; PR,
progesterone receptor; PR-A, PR isoform A; PR-B, PR isoform B;
pPR-B ser345, phosphorylated-PR in serine residue 345; TTZ,
trastuzumab; RU486, mifepristone. |
Pg interference in TTZ activity is
related to cell cycle-inducing proteins
After analyzing the effect of Pg on its receptor,
downstream events, which could explain the interfering effects of
Pg on TTZ activity, were assessed. Specifically, the proteins
associated with induction (CDK4 and cyclin D1) and arrest
(p27Kip1) of the cell cycle were evaluated (Fig. 3). The results showed that Pg
significantly interfered with the downregulation induced by TTZ
alone of the relative expression levels of CDK4 and cyclin D1 (CDK4
activator) (Fig. 3A-C). On the
other hand, RU486 interfered only with the downregulation of CDK4
induced by TTZ (Fig. 3A-C).
Notably, the TTZ/Pg/RU486-treated group exhibited significantly
lower expression levels of cyclin D1 in comparison with the TTZ/Pg-
and TTZ/RU486-treated groups (Fig.
3A-C). For p27Kip1, the combination of TTZ/Pg/Ru486
induced a significant downregulation in its expression, in
comparison with treatment with TTZ alone (Fig. 3A and D). Taken together, these
results suggested that Pg may interfere with the effect of TTZ on
the expression of CDK4 and cyclin D1, but not on the expression of
p27Kip1. On the other hand, it is inconclusive if RU486
modulates the effect of TTZ on the expression levels of CDK4,
cyclin D1 and p27Kip1, as TTZ/RU486 and TTZ/Pg/RU486 had
contrasting results, and the TTZ/RU486-treated group had an
unusually high SD.
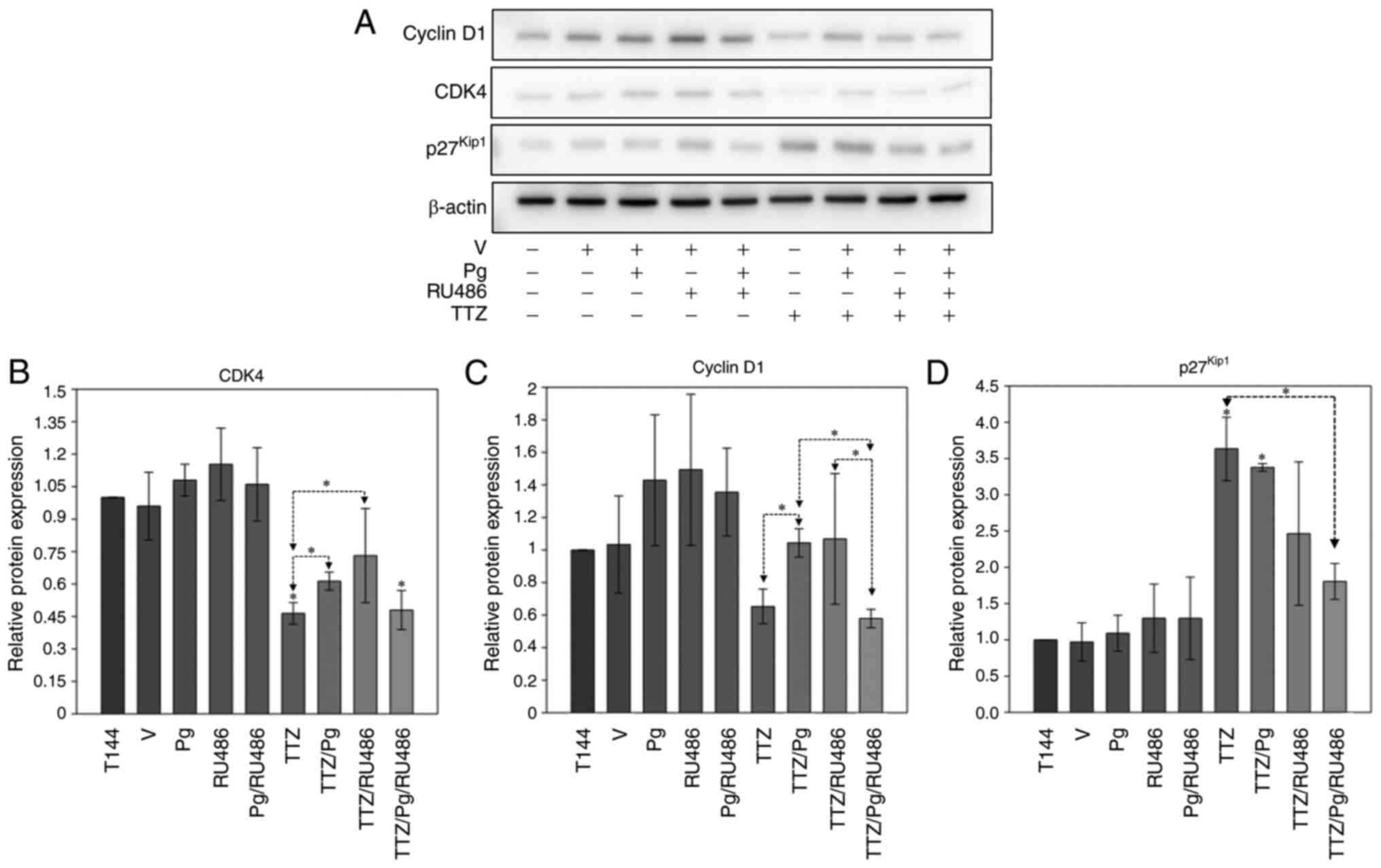 | Figure 3.Effect of Pg, RU486, TTZ and their
combinations on the expression of cyclin D1, CDK4 and
p27Kip1. (A) Representative image of the western blot
analysis of cyclin D1, CDK4 and p27Kip1 expression in
BT474 cells treated with Pg, RU486, TTZ alone or in combination.
Densitometric analysis of (B) CDK4, (C) cyclin D1 and (D)
p27Kip1. Data are presented as the mean ± SD of three
independent experiments (n=3). *P<0.05 vs. T144 or as indicated.
T144, control cells; V, vehicle (DMSO); Pg, progesterone; TTZ,
trastuzumab; RU486, mifepristone. |
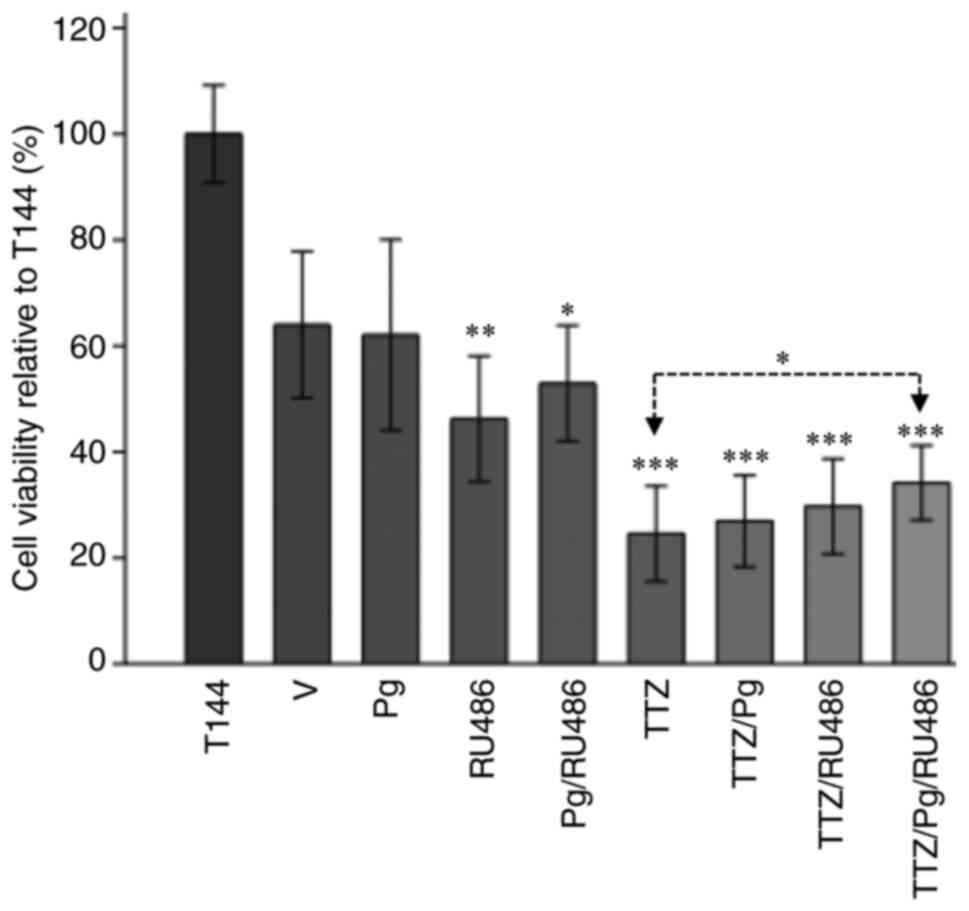
PR silencing suppresses Pg
interference in TTZ activity
To confirm that the interfering effects of Pg on the
inhibitory effect of TTZ on viability were PR-dependent, PR gene
silencing was performed (Fig. 4).
Using this strategy, PR-A and PR-B expression levels were silenced
by 20 and 31%, respectively, compared with in untransfected cells
(Fig. 4B and C).
Pg treatment of PR-silenced cells did not interfere
with the inhibitory effect of TTZ on viability (Fig. 5). Notably, RU486 significantly
increased cell viability by 12.7% only in the TTZ/Pg/RU486
combination treatment group compared with TTZ alone (Fig. 5). Taken together, these findings
indicated that Pg and RU486-induced interference with TTZ activity
is PR pathway-dependent.
Pg-induced interference with TTZ
activity does not reactivate the HER2/Akt signaling pathway
To determine whether the effects of Pg on the
inhibitory effect of TTZ on viability were due to HER2/Akt
reactivation, the changes in the expression and phosphorylation
state of these proteins were analyzed after 144 h of treatment
(Fig. 6). The combination of TTZ
with Pg or RU486 did not alter HER2 or Akt protein expression
levels or phosphorylation status, compared with treatment with TTZ
alone (Fig. 6A-E). However, when
both Pg and RU486 were combined with TTZ, an increase in pHER2 and
in the expression levels of Akt occurred in comparison with
treatment with TTZ alone (Fig. 6A, C
and D); despite this, Akt phosphorylation remained unchanged
(Fig. 6A and E). These results
suggested that the interfering effects of Pg on the inhibitory
effect of TTZ on viability were not due to reactivation of the
HER2/Akt pathway.
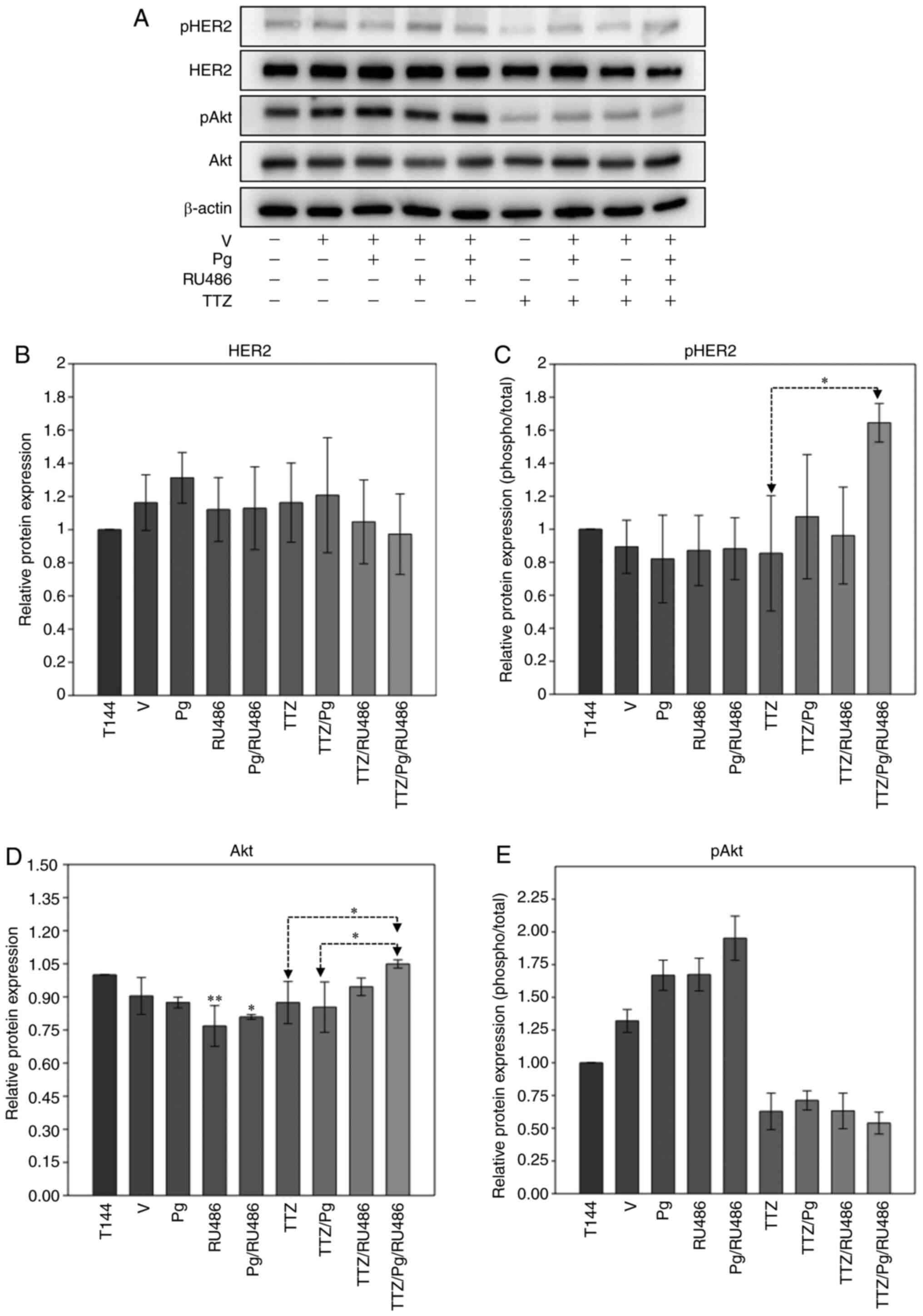 | Figure 6.Effect of Pg, RU486, TTZ and their
combinations on the expression and phosphorylation levels of HER2
and Akt in BT474 cells. (A) Representative western blot analysis of
the protein expression levels of pHER2, HER2, pAkt and Akt in BT474
cells treated with Pg, RU486, TTZ alone or in combination.
Densitometric analysis of (B) HER2, (C) pHER2, (D) Akt and (E)
pAkt. Data are presented as the mean ± SD of three independent
experiments (n=3). *P<0.05 and **P<0.01 vs. T144 or as
indicated. T144, control cells; V, vehicle (DMSO); Pg,
progesterone; TTZ, trastuzumab; HER2, human epidermal growth factor
receptor-2; pHER2, phosphorylated HER2; pAkt, phosphorylated Akt;
RU486, mifepristone. |
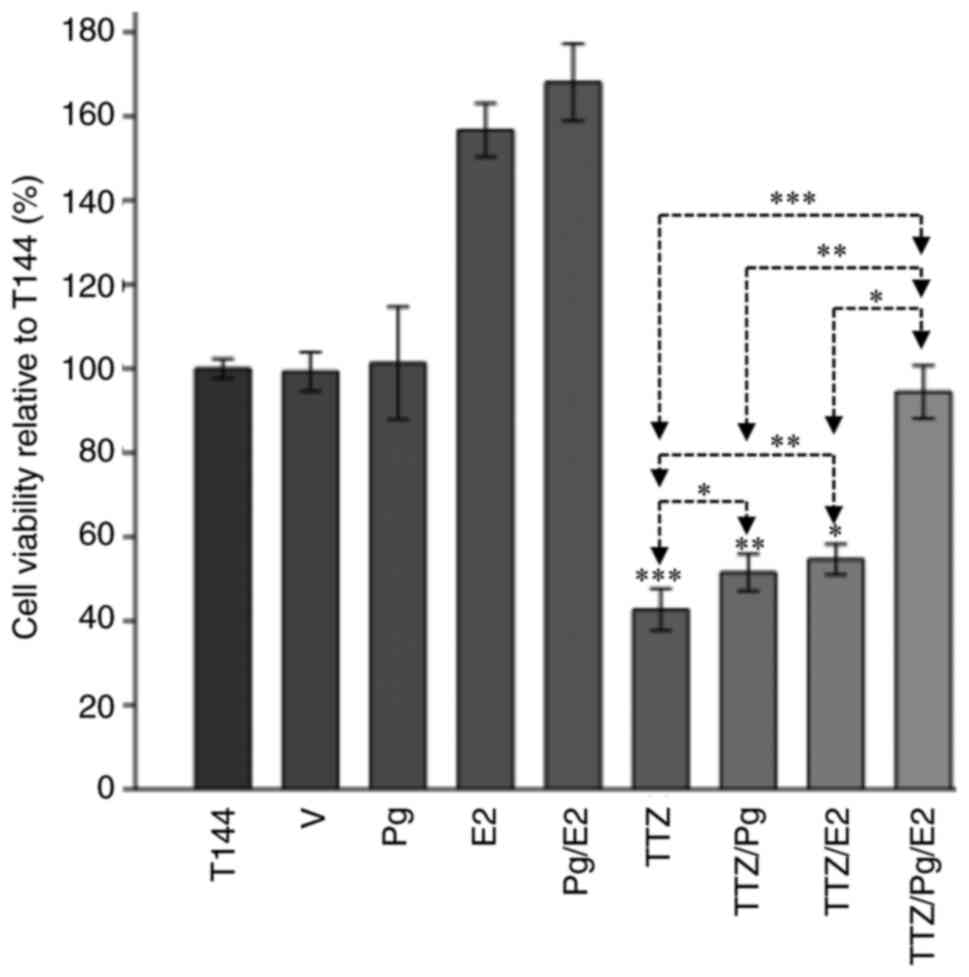
E2 potentiates the interfering effects
of Pg on the inhibitory effect of TTZ on viability
In hormone-dependent breast cancer, a close
relationship between Pg and E2 has been observed (1,24). For
this reason, an assay to investigate whether the combination of
both hormones interfered in the inhibitory effect of TTZ on
viability was performed (Fig. 7).
Compared with TTZ alone, when E2 was combined with TTZ, a
significant interference in the inhibitory effects of TTZ on
viability was observed (20.8%), although the interference was like
that achieved with TTZ/Pg treatment (14%). Notably, the combination
of TTZ/Pg/E2 had a greater effect on cell viability, which was
statistically different to the effects of TTZ/Pg and TTZ/E2. Taken
together, these results indicated that an interaction between Pg
and E2 potentiates their interference in the inhibitory effect of
TTZ on viability.
Effect of E2, Pg, TTZ and their
combinations on the expression and phosphorylation status of PR
isoforms
To investigate how E2 and Pg interact to interfere
with TTZ activity, whether E2 modifies the expression and
phosphorylation status of PR isoforms in a similar manner to Pg was
evaluated (Fig. 8). In comparison
to TTZ and TTZ/Pg treatment, E2 in combination with TTZ
significantly upregulated the protein expression levels of PR-A
(Fig. 8A and B). Notably, E2
induced the presence of an additional band that had a lower
molecular weight than PR-B (Fig.
8A). The additional PR-B band was upregulated when E2 was
combined with TTZ but was downregulated when Pg was added to the
TTZ/E2 combination. The significance of this additional band is
currently unknown and could be explored in future studies.
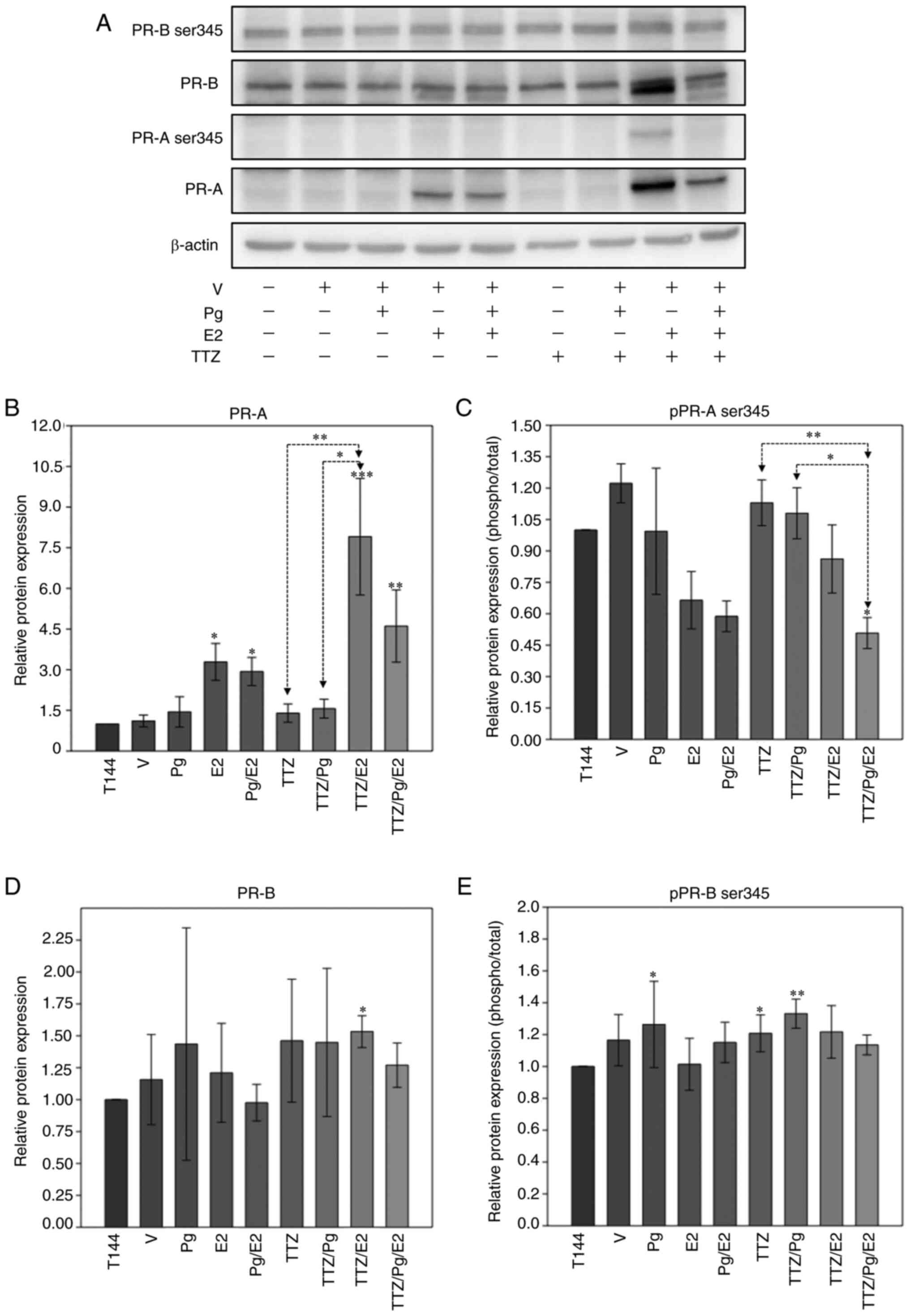 | Figure 8.Effect of Pg, E2, TTZ and their
combinations on the expression and phosphorylation levels of PR
isoforms. (A) Representative western blot analysis of the protein
expression levels of pPR-B ser345, PR-B, pPR-A ser345 and PR-A in
BT474 cells treated with Pg, E2, TTZ alone or in combination.
Densitometric analysis of (B) PR-A, (C) pPR-A ser345, (D) PR-B and
(E) pPR-B ser345. Data are presented as the mean ± SD of three
independent experiments (n=3). *P<0.05, **P<0.01 and
***P<0.001 vs. T144 or as indicated. T144, control cells; V,
vehicle (DMSO); Pg, progesterone; TTZ, trastuzumab; E2, estradiol;
PR, progesterone receptor; PR-A, PR isoform A; PR-B, PR isoform B;
pPR-A/B ser345, phosphorylated-PRA/B in serine residue 345. |
Regarding the phosphorylated status of the PR
isoforms, PR-A phosphorylation at ser345 was significantly
downregulated by the combination of TTZ/Pg/E2 compared with
treatments with TTZ and TTZ/Pg (Fig. 8A
and C). No statistically significant changes were observed in
the expression or phosphorylation levels of PR-B in response to any
of the treatments (Fig. 8D and
E).
Association between TTZ treatment
response and the expression profile of hormone receptors, and their
downstream genes in patients with TPBC
To define the possible coordinated and unique
activity of PR, ER, cyclin D1, CDK4 and p27Kip1,
clinical data from patients (ER+ and PR+,
HER2+) treated with neoadjuvant or adjuvant TTZ, as a
monotherapy or in conjunction with chemotherapy or hormone therapy,
were analyzed. The expression profiles of these genes in different
clinical groups according to the response to treatment or presence
of any clinical event were observed (Figs. 9 and S3). In one of the datasets (Fig. 9A), in patients with a partial
response to therapy, a significant enrichment of PGR (gene that
codes for PR) expression and joint ESR1 (gene that codes for
ER)/PGR expression, but a non-significant upward trend in the
expression of ESR1 and CCND1 (gene that codes for cyclin D1), was
determined. Additionally, in these patients, the expression of CDK4
was not significantly enriched and CDKN1B (gene that codes for
p27Kip1) was not significantly decreased (Fig. 9A). Nevertheless, in the rest of the
datasets (Fig. S3), like the one
depicted in Fig. 9B, the enrichment
of PR expression in partial responders to treatment was not
significant, and the significance of the changes in expression of
the rest of the genes of interest differed between each database.
Notably, throughout the clinical cohorts in which there was no
optimal therapeutic response, i.e., in patients with partial
response or residual disease after TTZ treatment, there was a
higher frequency of TPBC tumors compared with other immunochemical
subtypes (Fig. 9C). Moreover, when
looking at overall survival in patients with HER2+ and
ER+ tumors, PR expression may have an impact on clinical
outcome as depicted in the Kaplan-Meier plot, where tumors
overexpressing the PR transcript exhibited a trend towards a lower
overall survival compared with tumors with low PR expression
(Fig. 9D).
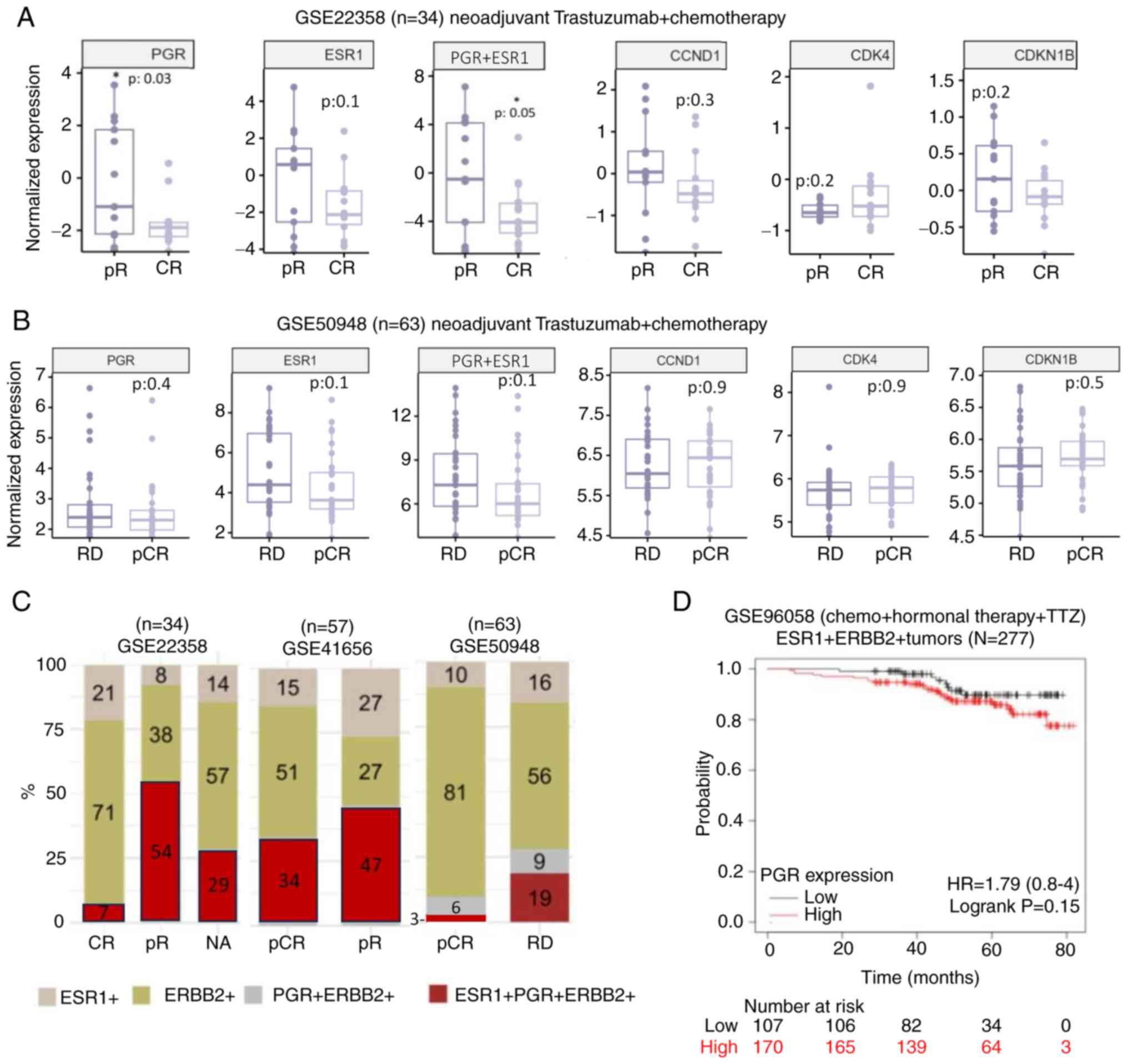 | Figure 9.Association between TTZ treatment
response and the expression profile of PGR, ESR1 and their
downstream genes in patients with TPBC. Normalized mRNA expression
levels of PGR, ESR1, CCND1, CDK4 and CDKN1B in (A) patients with
TPBC and pR or CR to therapy from the GSE22358 dataset (37) or in (B) patients with TPBC and RD or
pCR from the GSE50948 dataset (38). (C) Percentage of patients from the
GSE22358, GSE50948 and GSE41656 (39) datasets with varied responses to
therapy for which their tumors express ESR1, PGR, ERBB2 or a
combination of these genes. (D) Survival curve of patients from the
GSE96058 dataset (40) with
ER+/HER2+ breast cancer, low or high levels
of PGR and following a treatment scheme that included TTZ.
*P<0.05 vs. CR. pR, partial response; CR, complete response;
PGR, progesterone receptor; ESR1, estrogen receptor 1; CCND1,
cyclin D1; CDKN1B, p27Kip1; RD, residual disease; pCR,
pathological complete response; ERBB2, human epidermal growth
factor receptor-2; TPBC, triple-positive breast cancer; HR, hazard
ratio. |
Discussion
TTZ is the primary treatment for HER2-positive
breast cancer (1); however, this
therapy does not differentiate between breast cancer subtypes with
HER2 positivity, which may explain the benefits and/or failures of
HER2 therapies. In TPBC, it has been suggested that the interaction
between hormonal receptors and HER2 may explain the decrease in
efficacy of anti-HER2 therapy (25,26).
It has been demonstrated that Pg and its receptor are responsible
for tumor heterogeneity, conferring plasticity, proliferative
ability, and progression (27,28).
In TPBC cell lines, treatment with anti-HER2 therapies, such as
TTZ, has been found to increase PR expression (16,29).
Notably, a report on patients treated with lapatinib (another
anti-HER2 therapy) confirmed that an increase in PR expression is
associated with HER2 inhibition (29).
The aim of the present study was to provide further
information on how Pg, the PR, RU486 and E2 interfere with the
inhibitory effect of TTZ on viability. The findings of the present
study support those of a previous report where Pg was shown to
interfere with TTZ activity in a 3D system (30). However, the present data also showed
that the inhibitory effect of TTZ on viability was poorly
interfered with by Pg. The main limitation of the present study is
that a single cell line was used for most of the experiments.
During the preliminary phase of the project, two human TPBC cell
lines were used, BT474 and MDAMB361. The effect of TTZ, Pg and
their combination on the viability of MDAMB361 cells was like that
observed in BT474 cells. Notably, MDAMB361 cells had an increased
response to RU486 when compared with BT474 cells. However, MDAMB361
cells had a low proliferation rate and expression of HER2 and PR-B
(Fig. S1A) compared to BT474
cells. Therefore, the subsequent experiments used BT474 cells.
In the present study, a known antagonist of the PR,
RU486, induced interference with the inhibitory effect of TTZ on
viability. Previous reports on BT474 cells used RU486 at a
concentration of 1 µM to block the action of Pg (31,32)
and treated cells 1 h before the addition of Pg (33). In the present study, 100 nM RU486
was used to treat cells 1 h before Pg. The dose of RU486 was chosen
because it was not toxic to BT474 cells. However, it is unclear if,
in the present study, RU486 stabilized the agonist or antagonist
structures of the PR, coupled to coactivator or corepressor
proteins, respectively. To corroborate this, future studies would
need to show, for example, that RU486 in the conditions of the
present study could inhibit the expression of a reporter gene under
the control of Pg response elements, as we did in a previous
publication (34). In other words,
RU486 in the present study may be at a sub-optimal dose to be able
to antagonize the effects of Pg. Moreover, even though the RU486
used is of the same brand as previous reports (32–34),
the present lot number, and thus the biological activity, may be
different. Based on the present counterintuitive results with
RU486, for future experiments other PR antagonists could be used,
such as Proellex, which inhibits PR-B biosynthesis (35).
Pg binding to the PR is associated with
transcriptional activation. Individual overexpression of PR
isoforms regulates the differential expression of genes, as
follows: PR-A regulates the expression of genes related to cell
stemness, whereas PR-B is related to the regulation of genes
associated with cell proliferation (CCND1, MYC) (5,18). In
the present study, in the absence of E2, Pg treatment induced an
increase in PR-B phosphorylation and in the expression of cell
cycle-inducing proteins, CDK4 and cyclin D1. This effect occurred
even if Pg was combined with TTZ, but not in the TTZ/Pg/RU486
combination. Nevertheless, the latter triple combination
downregulated p27Kip1 protein levels, which is possibly
linked to RU486. p27Kip1 downregulation has previously
been associated with TTZ resistance (36). Therefore, p27Kip1 may be
involved in the mechanism by which RU486 interferes in the
inhibitory effect of TTZ on viability.
It has been noted that Pg promotes the expression of
growth factors that induce reactivation of the HER2/HER3-dependent
signaling pathway (30). However,
these data were obtained with short-term Pg exposures and without
TTZ. The present results, at an extended exposure time (144 h),
excluded the possibility that Pg interference in TTZ's inhibitory
effect on viability was caused by reactivation of the HER2/Akt
signaling pathway or alternative growth factors pathways where Akt
is involved.
Under standard cell culture conditions, it has been
reported that nuclear receptors are activated; therefore, to
analyze PR activation, elimination of all possible unknown ligands
(using charcoal-stripped FBS) or the agonistic effect of phenol red
is required. Only under these conditions can it be certain that
activation of PR was due to the Pg added. By contrast to Pg, RU486
binds to several different nuclear receptors, such as the
glucocorticoid receptor (32). The
present study also attempted to describe the relevance of the
observed phenomena when estrogens are present. The results
confirmed those shown in previous reports (16,32)
that, in TPBC, E2 functions as an escape and/or survival mechanism
against TTZ. However, the present results also demonstrated that
the interference effect of E2 is like that of Pg. Notably, the
effect of E2 was enhanced when it was combined with Pg.
Additionally, E2 can induce an increase in PR-A and a low-molecular
weight variant of PR-B, as PR is a gene regulated by E2 and its
receptor (1,24). These findings corroborated
previously reported data where TTZ treatment increased PR levels
(16). Despite these increases in
the protein levels of the PR isoforms, the Pg/E2 combination
reduced the phosphorylation of PR-A, but not of PR-B. This suggests
that PR-A phosphorylation may act as a negative regulator in the
interference of TTZ activity.
Finally, the significance of PR expression in the
resistance to TTZ was assessed using a data mining analysis of
several cohorts of patients with TPBC that were subjected to a
treatment scheme that included TTZ (Fig. 9). The plots showed that PR
expression tended to be higher in patients that had reduced overall
survival, partial pathological response, or a recurrence event.
Nevertheless, this trend only achieved statistical significance in
one of the datasets. A limitation of this data mining analysis was
that each dataset had a limited number of patients with TPBC. In
the future, the transcriptome of more patients with TPBC could
become available and the analysis may be repeated. In addition,
better results could be obtained by analyzing the phosphorylation
status of PR-B in patients with TPBC. The reasoning being that
in vitro results showed that PR-B phosphorylation was
associated with Pg interference with TTZ. However, the necessary
phospho-proteomic data needed to be able to perform such data
mining analysis could not be found. Alternatively, future studies
could analyze the levels of Pg levels in the metabolome of patients
with TPBC, to see if they are associated with response to TTZ
treatment. Notably, the present study revealed that patients with
TPBC tended to have a partial response to treatment more often than
other breast cancer subtypes. Even if patients with triple-negative
breast cancer do not appear in this analysis, future research could
be directed at identifying the reason behind this, which could be
related to the effect of Pg, but not necessarily through its
canonical genomic pathway, as PR expression did not seem to be
robustly enriched in non-responders to treatment.
In conclusion, the inhibitory effect of TTZ on
viability in BT474 TPBC cells may be interfered with by Pg and E2,
and to a greater extent by their combination (Fig. 10). Pg, in the absence of E2, may
promote interference in TTZ activity by reactivating cell
proliferation and promoting PR-B phosphorylation (Fig. 10B), and when E2 is present, by
inducing PR-A dephosphorylation (Fig.
10C). Pg-dependent reactivation of cell proliferation did not
induce the HER2/Akt signaling pathway, suggesting an alternate
pathway dependent on Pg and the PR. In addition, the PR antagonist
RU486 did not produce the expected effect in the present study.
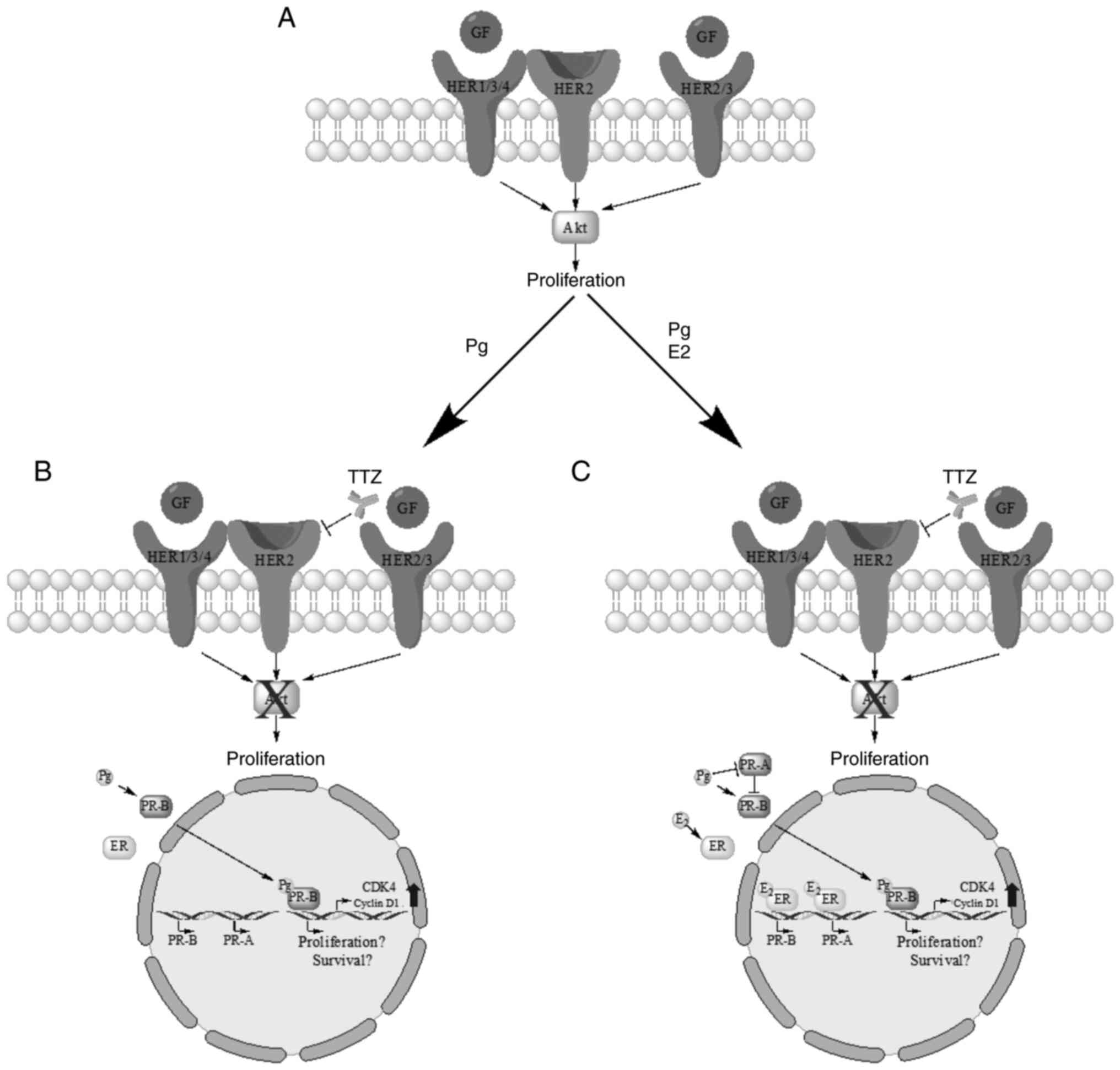 | Figure 10.Schematic diagram of the findings on
the effect of Pg, E2, TTZ and their combinations on BT474 cells.
(A) HER2/Akt-dependent proliferation pathway in tumor cells. (B)
Interference of TTZ activity by Pg is associated with PR-B
phosphorylation and induction of CDK4 and cyclin D1. (C)
Combinatorial effect of Pg and E2 on TTZ activity is associated
with PR-A dephosphorylation. GF, growth factor; Pg, progesterone;
TTZ, trastuzumab; E2, estradiol; HER2, human epidermal growth
factor receptor-2; PR, progesterone receptor; PR-A, PR isoform A;
PR-B, PR isoform B. |
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The present work constitutes a partial fulfilment by
JALM of the requirements to obtain a PhD in the ‘Programa de
Doctorado en Ciencias Biológicas, Universidad Nacional Autónoma de
México’. The authors would like to thank Mr. Salvador
Ramírez-Jiménez (Programa de Investigación de Cáncer de Mama/Banco
de Células del Instituto de Investigaciones Biomédicas, Universidad
Nacional Autónoma de México) for providing the BT474 and MDAMB361
cell lines.
Funding
This work was supported by institutional funds provided to AZD
by the Instituto Nacional de Ciencias Médicas y Nutrición Salvador
Zubirán and the Instituto de Investigaciones Biomédicas de la
Universidad Nacional Autónoma de México. JALM received a PhD grant
(grant no. 703562) from the Consejo Nacional de Humanidades
Ciencias y Tecnologías.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The GEO datasets analysed in this study are available in
the links provided in Table
SII.
Authors' contributions
AZD conceptualized the project and procured the
necessary funds and infrastructure for it. JALM and AZD designed
the project. JALM performed the in vitro experiments with
aid from JLVG and AAPV. JALM and AZD confirm the authenticity of
all the raw data. JALM performed the statistical analysis of the
in vitro data. SLRC conceived and designed the part of the
study related to clinical and transcriptomic data; in addition they
curated, analysed and aided in the interpretation of such data.
AZD, ICA, ML, MMV, NJJH and ALDR supervised the study, and
substantially contributed to the conception and design of the
experiments. JALM and AJCQ interpreted the data and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Use of artificial intelligence tools
During the preparation of this work, AI tools
(ChatGPT-4 through the Microsoft Bing chat interface) were used to
improve the readability and language of the manuscript, and
subsequently, the authors revised and edited the content produced
by the AI tools as necessary, taking full responsibility for the
ultimate content of the present manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lv H, Yan M and Jiang Z: Recent advances
in the treatment of hormone receptor-positive/human epidermal
growth factor 2-positive advanced breast cancer. Ther Adv Med
Oncol. 13:175883592110133262021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parise CA and Caggiano V: Differences in
clinicopatholgic characteristics and risk of mortality between the
triple positive and ER+/PR+/HER2-breast cancer subtypes. Cancer
Causes Control. 30:417–424. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vici P, Pizzuti L, Natoli C, Gamucci T, Di
Lauro L, Barba M, Sergi D, Botti C, Michelotti A, Moscetti L, et
al: Triple positive breast cancer: A distinct subtype? Cancer Treat
Rev. 41:69–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tran B and Bedard PL: Luminal-B breast
cancer and novel therapeutic targets. Breast Cancer Res.
13:2212011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Knutson TP and Lange CA: Tracking
progesterone receptor-mediated actions in breast cancer. Pharmacol
Ther. 142:114–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Untch M, Gelber RD, Jackisch C, Procter M,
Baselga J, Bell R, Cameron D, Bari M, Smith I, Leyland-Jones B, et
al: Estimating the magnitude of trastuzumab effects within patient
subgroups in the HERA trial. Ann Oncol. 19:1090–1096. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schettini F, Buono G, Cardalesi C,
Desideri I, De Placido S and Del Mastro L: Hormone receptor/human
epidermal growth factor receptor 2-positive breast cancer: Where we
are now and where we are going. Cancer Treat Rev. 46:20–26. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dahabreh IJ, Linardou H, Siannis F,
Fountzilas G and Murray S: Trastuzumab in the adjuvant treatment of
early-stage breast cancer: A systematic review and meta-analysis of
randomized controlled trials. Oncologist. 13:620–630. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wilken JA and Maihle NJ: Primary
trastuzumab resistance: New tricks for an old drug. Ann N Y Acad
Sci. 1210:53–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang S, Huang WC, Li P, Guo H, Poh SB,
Brady SW, Xiong Y, Tseng LM, Li SH, Ding Z, et al: Combating
trastuzumab resistance by targeting SRC, a common node downstream
of multiple resistance pathways. Nat Med. 17:461–469. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zazo S, González-Alonso P, Martín-Aparicio
E, Chamizo C, Cristóbal I, Arpí O, Rovira A, Albanell J, Eroles P,
Lluch A, et al: Generation, characterization, and maintenance of
trastuzumab-resistant HER2+ breast cancer cell lines. Am J Cancer
Res. 6:2661–2678. 2016.PubMed/NCBI
|
|
12
|
Lindet C, Révillion F, Lhotellier V,
Hornez L, Peyrat JP and Bonneterre J: Relationships between
progesterone receptor isoforms and the HER/ErbB receptors and
ligands network in 299 primary breast cancers. Int J Biol Markers.
27:e111–e117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baselga J, Bradbury I, Eidtmann H, Di
Cosimo S, de Azambuja E, Aura C, Gómez H, Dinh P, Fauria K, Van
Dooren V, et al: Lapatinib with trastuzumab for HER2-positive early
breast cancer (NeoALTTO): A randomised, open-label, multicentre,
phase 3 trial. Lancet. 379:633–640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kolarova I, Vanasek J, Odrazka K, Melichar
B, Ryska A, Petera J, Vosmik M and Dolezel M: Therapeutic
significance of hormone receptor positivity in patients with HER-2
positive breast cancer. Biomed Pap Med Fac Univ Palacky Olomouc
Czech Repub. 163:285–292. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Collins DC, Cocchiglia S, Tibbitts P,
Solon G, Bane FT, McBryan J, Treumann A, Eustace A, Hennessy B,
Hill AD and Young LS: Growth factor receptor/steroid receptor cross
talk in trastuzumab-treated breast cancer. Oncogene. 34:525–530.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang YC, Morrison G, Gillihan R, Guo J,
Ward RM, Fu X, Botero MF, Healy NA, Hilsenbeck SG, Phillips GL, et
al: Different mechanisms for resistance to trastuzumab versus
lapatinib in HER2-positive breast cancers-role of estrogen receptor
and HER2 reactivation. Breast Cancer Res. 13:R1212011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cenciarini ME and Proietti CJ: Molecular
mechanisms underlying progesterone receptor action in breast
cancer: Insights into cell proliferation and stem cell regulation.
Steroids. 152:1085032019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Truong TH, Dwyer AR, Diep CH, Hu H, Hagen
KM and Lange CA: Phosphorylated progesterone receptor isoforms
mediate opposing stem cell and proliferative breast cancer cell
fates. Endocrinology. 160:430–446. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cervantes-Badillo MG, Paredes-Villa A,
Gómez-Romero V, Cervantes-Roldán R, Arias-Romero LE, Villamar-Cruz
O, González-Montiel M, Barrios-García T, Cabrera-Quintero AJ,
Rodríguez-Gómez G, et al: IFI27/ISG12 downregulates estrogen
receptor α transactivation by facilitating its interaction with
CRM1/XPO1 in breast cancer cells. Front Endocrinol (Lausanne).
11:5683752020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets-update. Nucleic Acids Res. 41:D991–D995. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Durinck S, Spellman PT, Birney E and Huber
W: Mapping identifiers for the integration of genomic datasets with
the R/Bioconductor package biomaRt. Nat Protoc. 4:1184–1191. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
R Core Team, . R: A language and
environment for statistical computing. R Foundation for Statistical
Computing; Vienna, Austria: 2023
|
|
23
|
Győrffy B: Survival analysis across the
entire transcriptome identifies biomarkers with the highest
prognostic power in breast cancer. Comput Struct Biotechnol J.
19:4101–4109. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arpino G, Wiechmann L, Osborne CK and
Schiff R: Crosstalk between the estrogen receptor and the HER
tyrosine kinase receptor family: Molecular mechanism and clinical
implications for endocrine therapy resistance. Endocr Rev.
29:217–233. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dieci MV and Guarneri V: Should
triple-positive breast cancer be recognized as a distinct subtype?
Expert Rev Anticancer Ther. 20:1011–1014. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schedin TB, Borges VF and Shagisultanova
E: Overcoming therapeutic resistance of triple positive breast
cancer with CDK4/6 inhibition. Int J Breast Cancer.
2018:78350952018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sathyamoorthy N and Lange CA: Progesterone
and breast cancer: an NCI workshop report. Horm Cancer. 11:1–12.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hernández-Hernández OT and Camacho-Arroyo
I: Regulation of gene expression by progesterone in cancer cells:
Effects on cyclin D1, EGFR and VEGF. Mini Rev Med Chem. 13:635–642.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia W, Bacus S, Hegde P, Husain I, Strum
J, Liu L, Paulazzo G, Lyass L, Trusk P, Hill J, et al: A model of
acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor
and a therapeutic strategy to prevent its onset in breast cancer.
Proc Natl Acad Sci USA. 103:7795–7800. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kitowska K, Kowalska A, Mieszkowska M,
Piasecka D, Skladanowski AC, Romanska HM and Sadej R: Progesterone
impairs Herceptin effect on breast cancer cells. Oncol Lett.
15:1817–1822. 2018.PubMed/NCBI
|
|
31
|
Hyder SM, Liang Y, Wu J and Welbern V:
Regulation of thrombospondin-1 by natural and synthetic progestins
in human breast cancer cells. Endocr Relat Cancer. 16:809–817.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang Y, Wu J, Stancel GM and Hyder SM:
p53-dependent inhibition of progestin-induced VEGF expression in
human breast cancer cells. J Steroid Biochem Mol Biol. 93:173–182.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luo LY, Grass L and Diamandis EP: Steroid
hormone regulation of the human kallikrein 10 (KLK10) gene in
cancer cell lines and functional characterization of the KLK10 gene
promoter. Clin Chim Acta. 337:115–126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mitre-Aguilar IB, Barrios-Garcia T,
Ruiz-Lopez VM, Cabrera-Quintero AJ, Mejia-Dominguez NR,
Ventura-Gallegos JL, Moreno-Mitre D, Aranda-Gutierrez A,
Mejia-Rangel J, Escalona-Guzman AR, et al: Glucocorticoid-dependent
expression of IAP participates in the protection against
TNF-mediated cytotoxicity in MCF7 cells. BMC Cancer. 19:3562019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gupta A, Mehta R, Alimirah F, Peng X,
Murillo G, Wiehle R and Mehta RG: Efficacy and mechanism of action
of Proellex, an antiprogestin in aromatase overexpressing and
Letrozole resistant T47D breast cancer cells. J Steroid Biochem Mol
Biol. 133:30–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nahta R, Takahashi T, Ueno NT, Hung MC and
Esteva FJ: P27(kip1) down-regulation is associated with trastuzumab
resistance in breast cancer cells. Cancer Res. 64:3981–3986. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Glück S, Ross JS, Royce M, McKenna EF Jr,
Perou CM, Avisar E and Wu L: TP53 genomics predict higher clinical
and pathologic tumor response in operable early-stage breast cancer
treated with docetaxel-capecitabine ± trastuzumab. Breast Cancer
Res Treat. 132:781–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Prat A, Bianchini G, Thomas M, Belousov A,
Cheang MCU, Koehler A, Gómez P, Semiglazov V, Eiermann W, Tjulandin
S, et al: Research-based PAM50 subtype predictor identifies higher
responses and improved survival outcomes in HER2-positive breast
cancer in the NOAH study. Clin Cancer Res. 20:511–521. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
de Ronde JJ, Rigaill G, Rottenberg S,
Rodenhuis S and Wessels LFA: Identifying subgroup markers in
heterogeneous populations. Nucleic Acids Res. 41:e2002013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brueffer C, Vallon-Christersson J, Grabau
D, Ehinger A, Häkkinen J, Hegardt C, Malina J, Chen Y, Bendahl PO,
Manjer J, et al: Clinical value of RNA sequencing-based classifiers
for prediction of the five conventional breast cancer biomarkers: A
report from the population-based multicenter sweden cancerome
analysis network-breast initiative. JCO Precis Oncol.
2:PO.17.00135. 2018.PubMed/NCBI
|
|
41
|
Triulzi T, De Cecco L, Sandri M, Prat A,
Giussani M, Paolini B, Carcangiu ML, Canevari S, Bottini A, Balsari
A, et al: Whole-transcriptome analysis links trastuzumab
sensitivity of breast tumors to both HER2 dependence and immune
cell infiltration. Oncotarget. 6:28173–28182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Triulzi T, Regondi V, De Cecco L,
Cappelletti MR, Di Modica M, Paolini B, Lollini PL, Di Cosimo S,
Sfondrini L, Generali D and Tagliabue E: Early immune modulation by
single-agent trastuzumab as a marker of trastuzumab benefit. Br J
Cancer. 119:1487–1494. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Castagnoli L, Iezzi M, Ghedini GC,
Ciravolo V, Marzano G, Lamolinara A, Zappasodi R, Gasparini P,
Campiglio M, Amici A, et al: Activated d16HER2 homodimers and SRC
kinase mediate optimal efficacy for trastuzumab. Cancer Res.
74:6248–6259. 2014. View Article : Google Scholar : PubMed/NCBI
|