Introduction
Cervical cancer is a common malignancy of the female
reproductive system and poses a serious threat to the lives and
health of middle-aged and elderly women. The global
age-standardized incidence of cervical cancer is 13.3 per 100,000
women-years and the mortality rate is 7.2 per 100,000 women-years
(1). Squamous cell carcinoma is the
main type of cervical cancer, accounting for ~80% of cases, with
adenocarcinoma and adenosquamous carcinoma accounting for ~15% of
cases and other malignant tumors accounting for ~5% of cases, among
which sarcoma accounts for <1% of cases (2). Malignant peripheral nerve sheath
tumors (MPNSTs), also known as malignant neurilemomas, malignant
schwannomas, neurofibrosarcomas or neurogenic sarcomas, show
differentiation toward peripheral nerve sheath cells. The major
nerve trunks, including the sciatic nerve, brachial plexus and
sacral plexus, are usually the development sites, and the tumors
are associated with neurofibromatosis-1 (NF-1) in ~50% of cases
(3). Studies have reported that
MPNSTs account for only ~3% of cervical sarcomas (4), making it a rare cervical
malignancy.
The present report describes a case of MPNST of the
uterine cervix and discusses the morphological and
immunohistochemical characteristics, and the possible differential
diagnosis of MPNST and other spindle cell neoplasms.
Case report
A 36-year-old female attended the West China Second
University Hospital, Sichuan University (Chengdu, China) in
December 2022 after having a small amount of vaginal bleeding for
10 days following sexual intercourse. Vaginal ultrasonography in
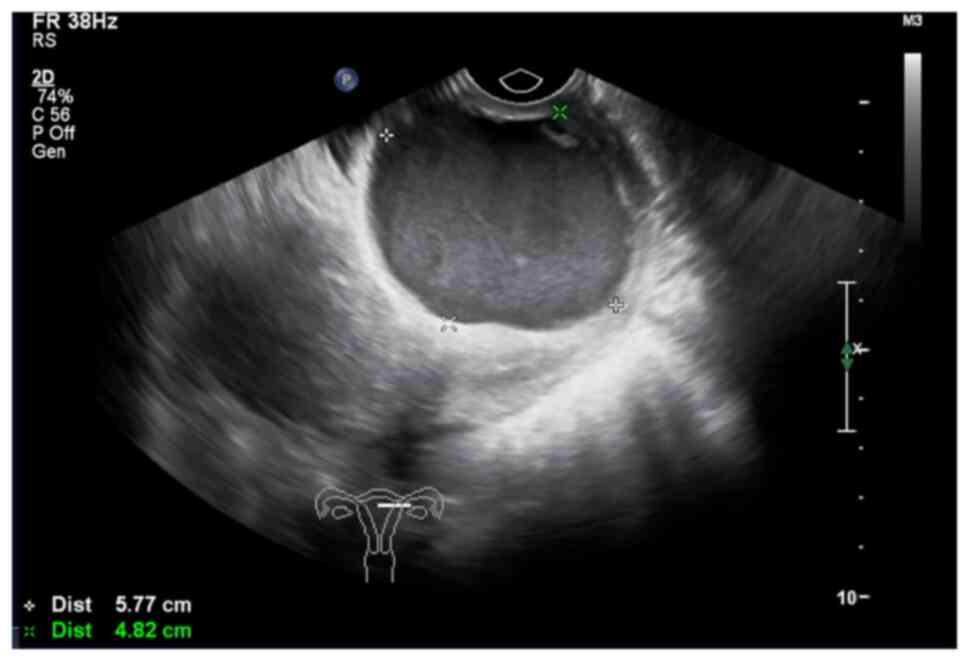
another hospital immediately prior to admission showed a cystic
mass in the cervical segment measuring ~5.8×4.8×5.3 cm (Fig. 1). Thinprep cytological tests and
human papillomavirus assessments showed no abnormalities. The
patient had no personal history of neurofibroma or a family history
of cervical cancer.
After completing all preoperative examinations, such
as computed tomography imaging and relevant blood tests, the
patient underwent a total abdominal hysterectomy and bilateral
salpingectomy, as well as a pelvic lymph node dissection when
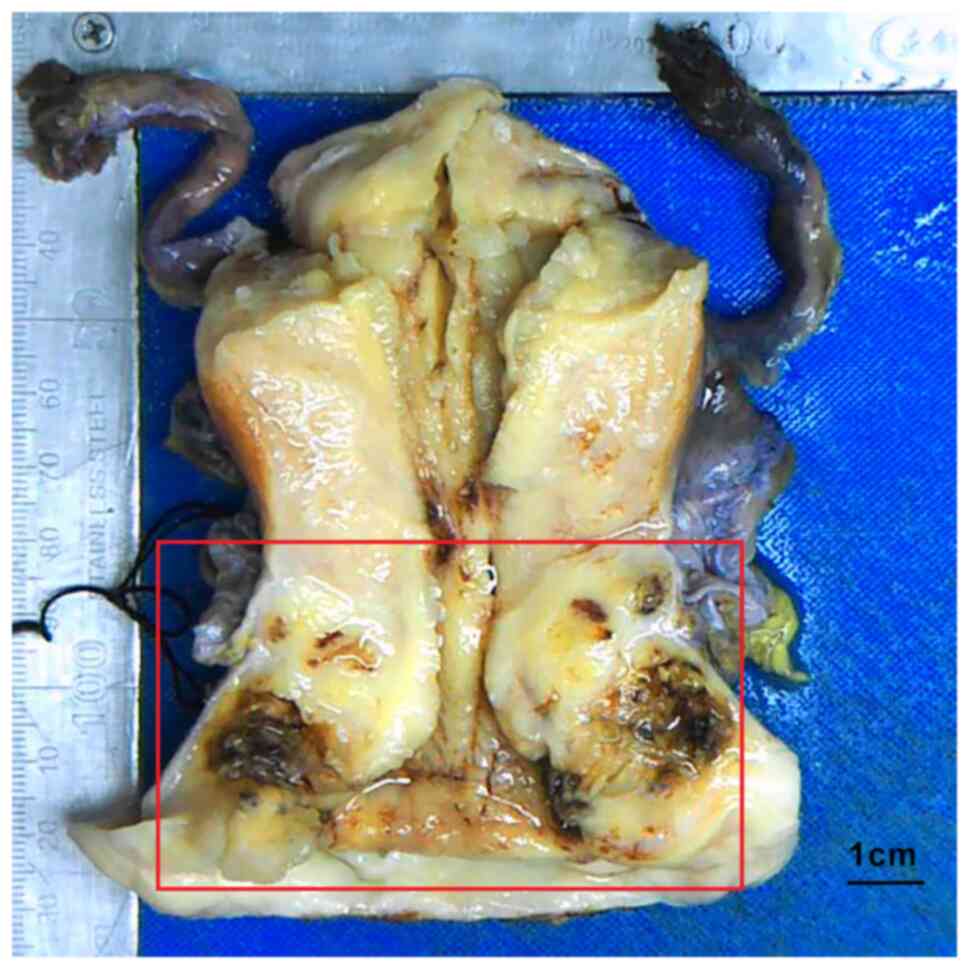
malignancy was suspected during surgery. The mass was observed in
the external cervical os and the cervical canal, with a size of
~4×3.5×2 cm, and the mass section was grayish-yellow. Bleeding and
necrosis could be seen in certain areas, infiltrating the whole
layer of the cervical interstitium and part of the uterine muscle
wall, and the mass was found to involve the vaginal vault and
vaginal wall (Fig. 2). The
endometrium was slightly thickened and there was no obvious mass in
the bilateral adnexa. Extensive sampling and sectioning of the
cervical mass, myometrium and endometrium were performed for
histopathological examination. The tissue was fixed with 4% neutral
formalin (for 24 h at 25°C) and embedded in paraffin, and then 4-µm
sections were prepared that were subjected to hematoxylin and eosin
(H&E) staining (for 8 h at 25°C).
Microscopically (Olympus BX43), the tumor was
composed mainly of tightly packed spindle cells with dense cell
proliferation. The cell boundaries were not clear and the tumor was
arranged in a cross bundle. The tumor showed invasive growth,
invading the whole layer of the cervical interstitium, the vaginal
fornix and its disjunction, and the uterine muscle wall. The
cytoplasm was sparse, the nuclei were oval, elongated and
moderately atypical, and some cells had small eosinophilic
nucleoli. Mitotic activity was high, with ≤30 mitoses per 10
high-power field, and bleeding and necrosis were also observed. The
tumor did not destroy the squamous epithelium, endocervical glands
or endometrial glands (Fig. 3). In
addition, myxoid and edematous areas of low cell density were also
observed, alternating with areas of dense cells. No tumor was found
in the vaginal stump, bilateral parametrial tissues, bilateral
pelvic lateral wall surgical margins or bilateral fallopian tubes.
All negative margins were >3 cm away from the tumor.
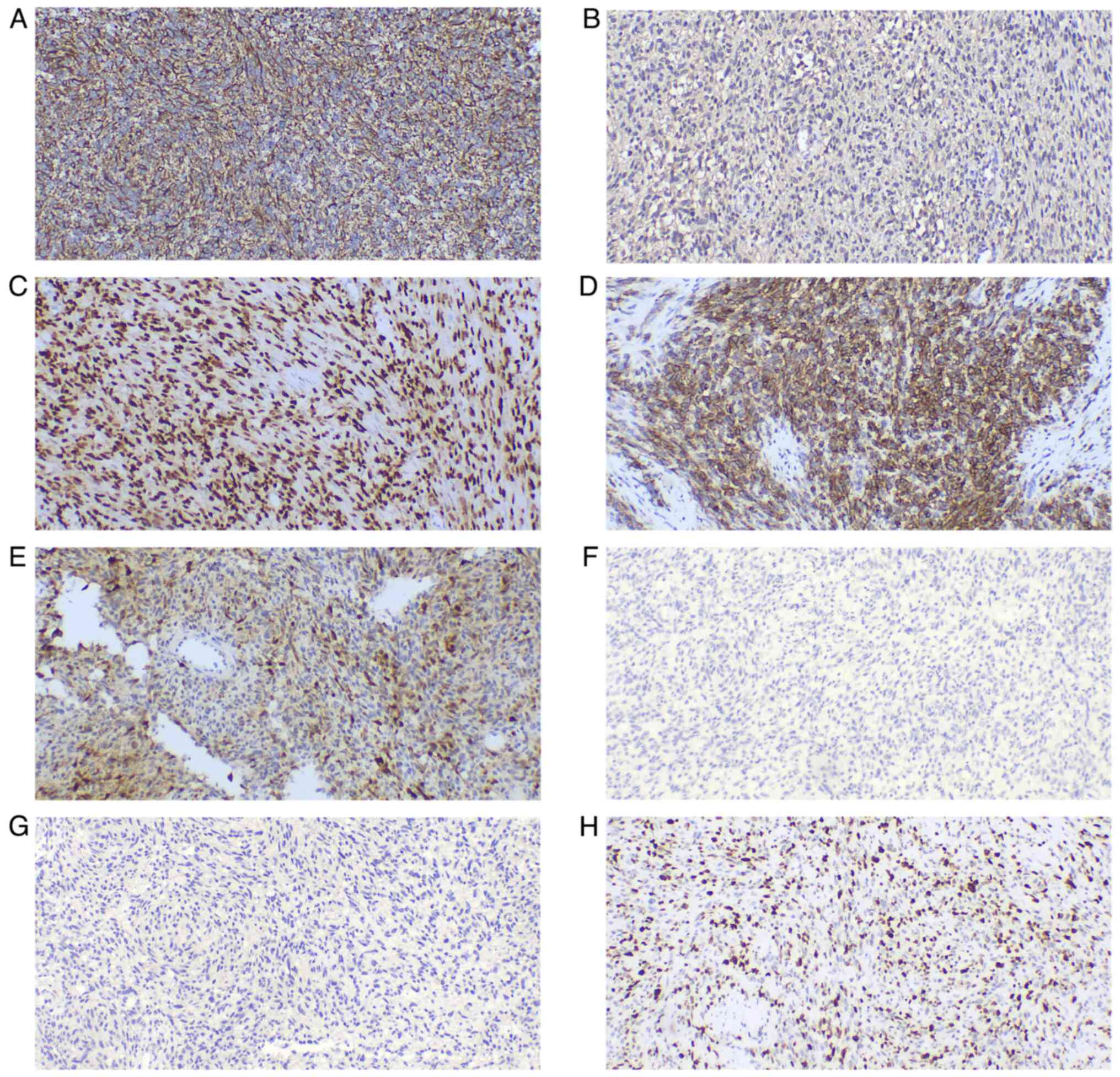
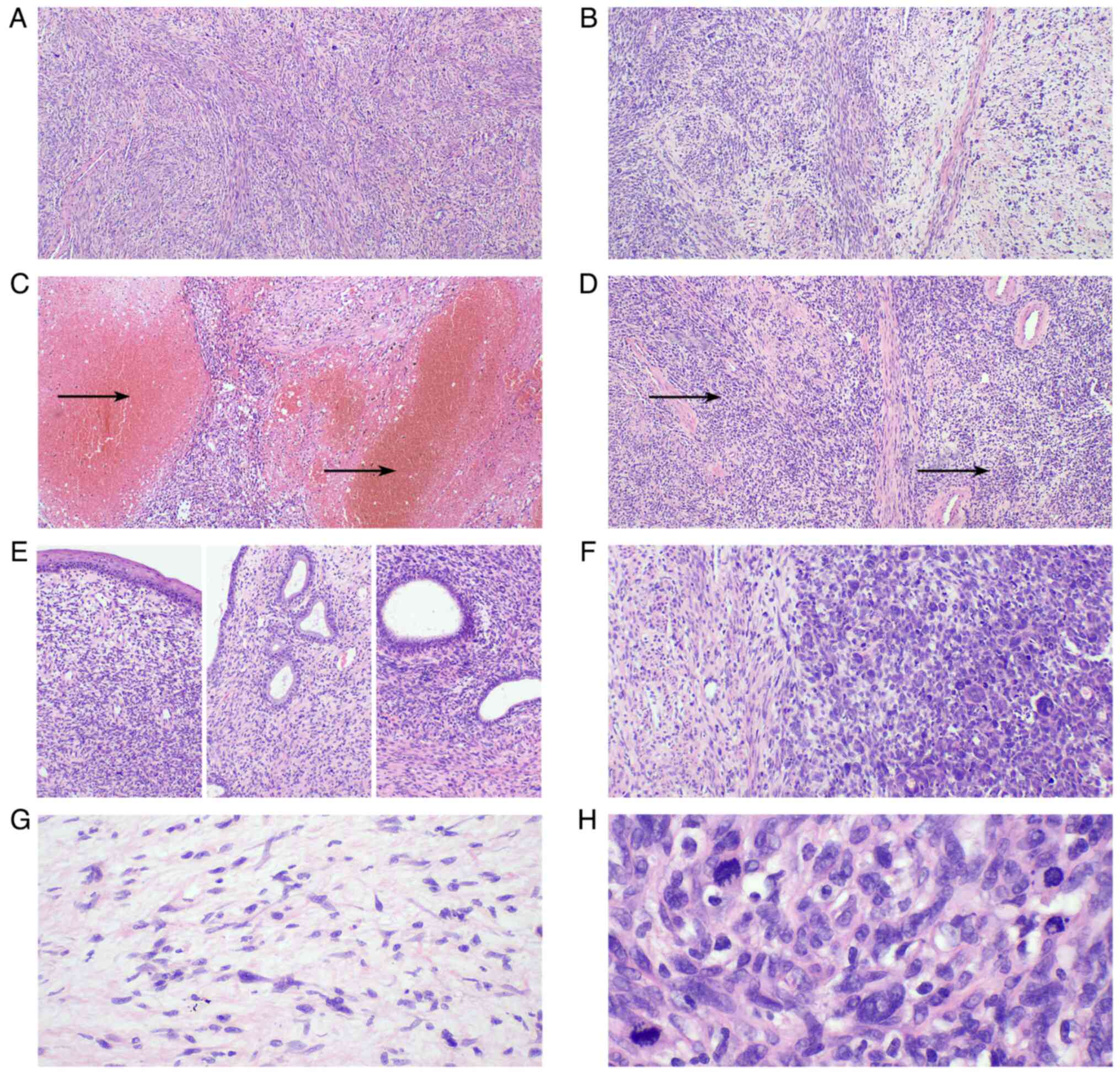 | Figure 3.Morphological characteristics of the
cervical malignant peripheral nerve sheath tumor. (A) The tumor was
composed mainly of spindle cells (magnification, ×40). (B) Certain
areas exhibited obvious cell density and loose areas
(magnification, ×40). (C) Bleeding and necrosis were present in
certain areas, as indicated by the arrows (magnification, ×40). (D)
The tumor contained a number of thick-walled blood vessels and the
tumor cells formed a plexiform structure around the blood vessels,
as indicated by the arrows (magnification, ×40). (E) The tumor did
not involve the squamous epithelium, endocervical glands or
endometrial glands (magnification, ×100). (F) The tumor cells in
some areas were epithelioid, with numerous multinuclear giant cells
observed (magnification, ×100). (G) Comma-like Schwann cells can be
seen in the loose area of cells (magnification, ×200). (H) Numerous
mitotic figures can be seen at a high magnification (magnification,
×400). |
Section preparation for immunohistochemical staining
was the same as that for H&E staining. All immunohistochemical
staining was performed with an automated staining system Bond III
(Leica Biosystems Newcastle Ltd.) based on the EnVision method. All
primary antibodies with working liquid were from Fuzhou Maixin
Biotech Co., Ltd., and were incubated with the sections for 15 min
at room temperature. The BOND polymer Refine Detection kit (cat.
no. DS9800) from Leica Biosystems, using Bond III, including 3–4%
hydrogen peroxide as the peroxide block, was used for 5 min at room
temperature, and then anti-rabbit poly-HRP IgG (<25 µg/ml; from
DS9800 kit) was incubated with the sections for 8 min at room
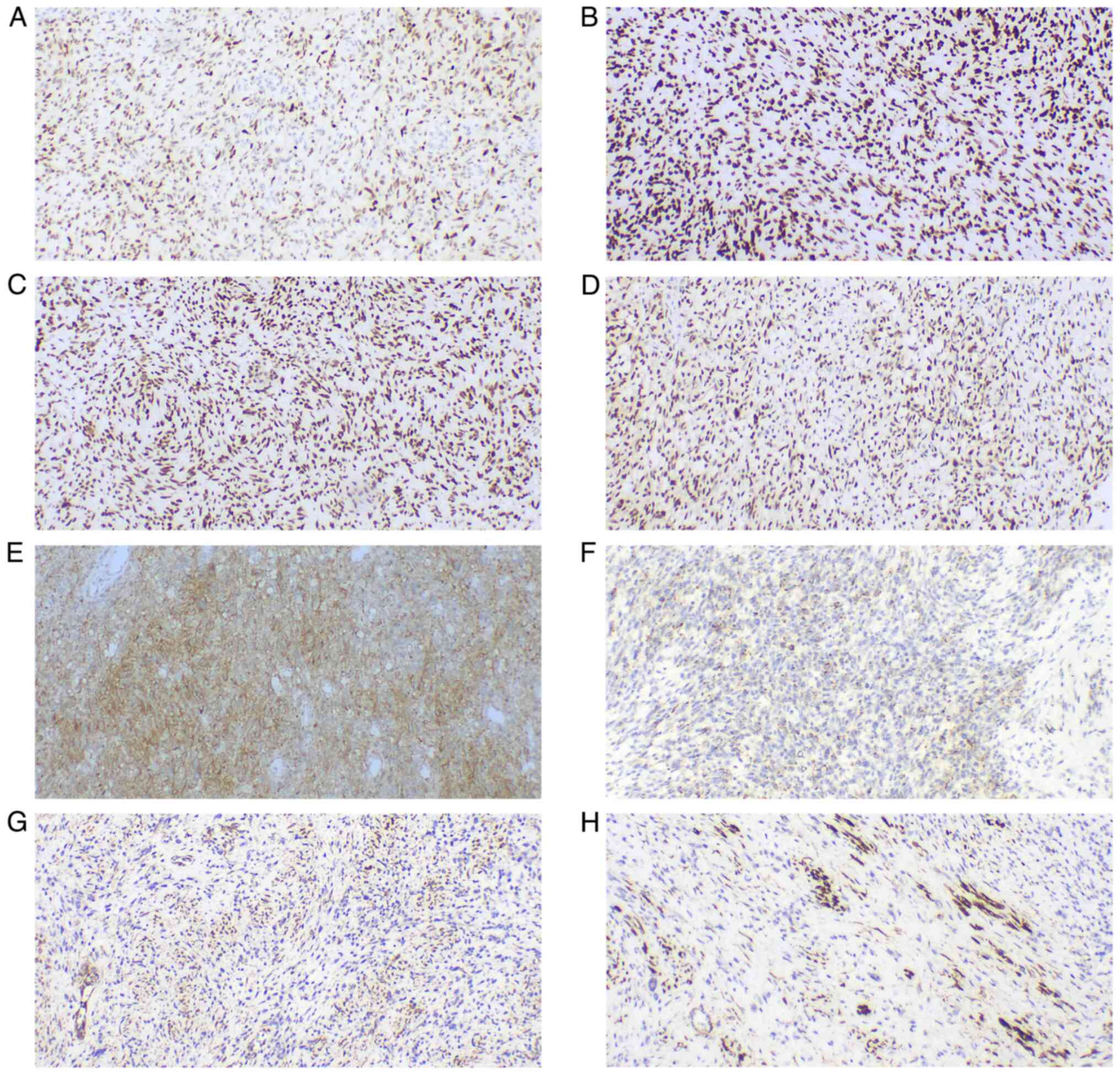
temperature. All immunohistochemical staining was observed using an
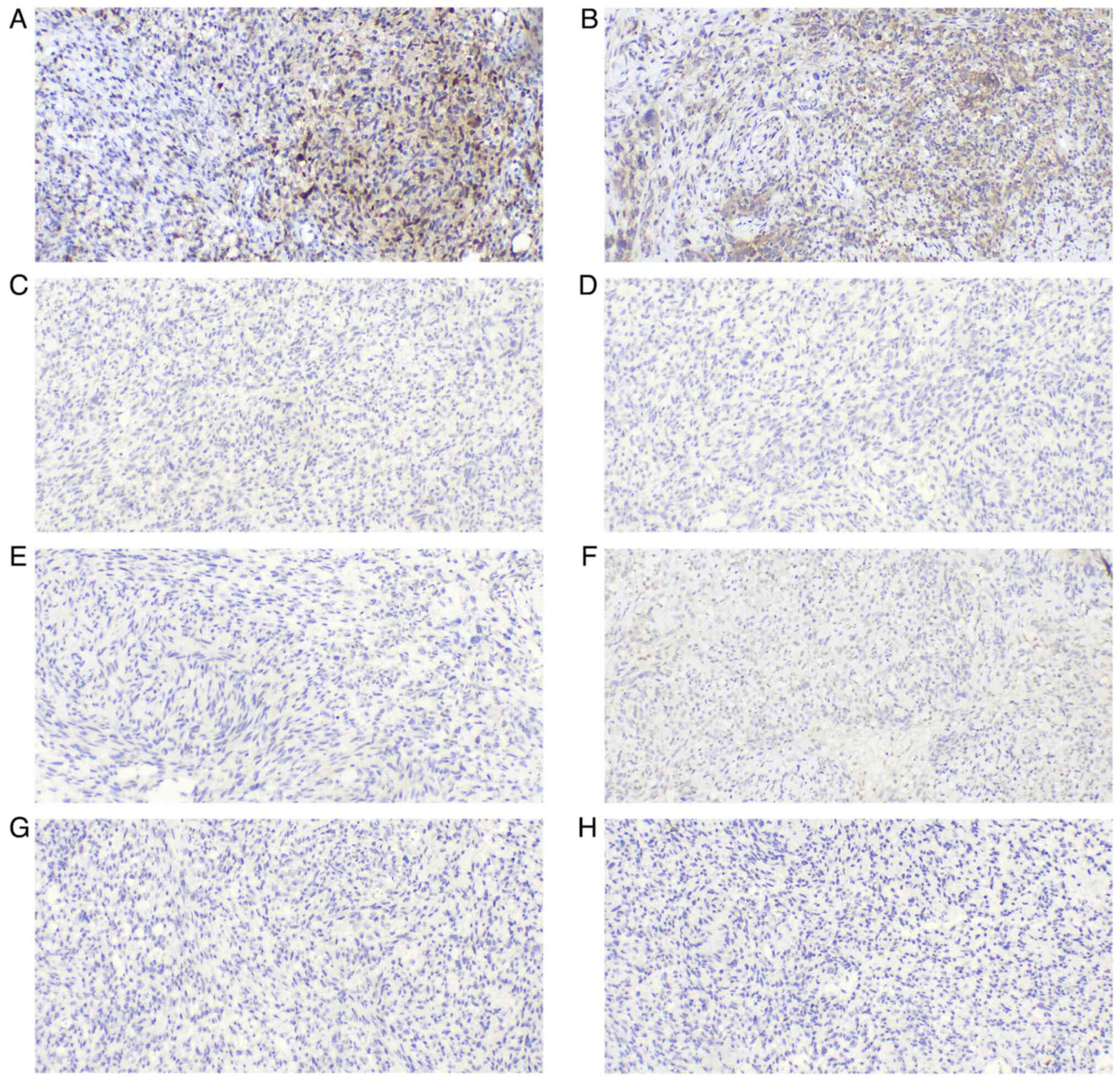
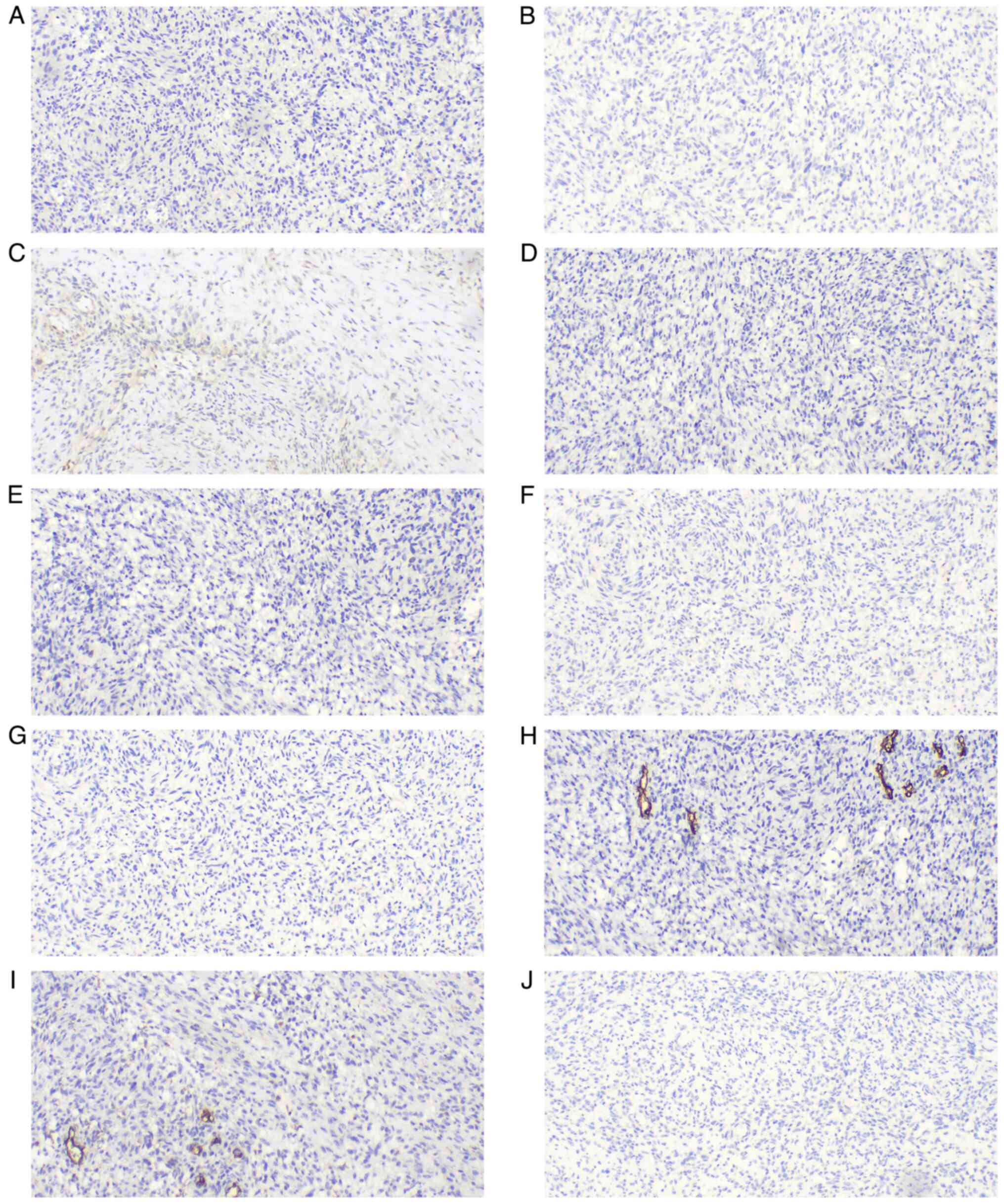
Olympus BX43 microscope. The neoplastic cells were strongly and
diffusely immunoreactive for vimentin (cat. no. MAB-0735), S-100
(cat. no. Kit-0007), sex determining region Y-box 10 (SOX-10; cat.
no. RMA-1058), CD56 (cat. no. MAB-0743), protein gene product 9.5
(PGP9.5; cat. no. RAB-0076), p53 (cat. no. MAB-0674),
brahma-related gene 1 (BRG-1; cat. no. RMA-1063), integrase
interactor 1 (INI-1; cat. no. MAB-0696), friend leukemia virus
integration 1 (FLI-1; cat. no. MAB-0649) and CD99 (cat. no.
MAB-1012), with focal reactivity for CD57 (cat. no. MAB-0257),
smooth muscle actin (SMA; cat. no. MAB-0890), calponin (cat. no.
MAB-0712), cyclin D1 (cat. no. RMA-0541) and anaplastic lymphoma
kinase (ALK, cat. no. RMA-1032) (all ready-to-use). The tumor cells
were negative for human melanoma black 45 (HMB-45; cat. no.
MAB-0098), melanoma antigen (Melan-A; cat. no. MAB-0275), glial
fibrillary acidic protein (GFAP; cat. no. MAB-0769), epidermal
growth factor receptor (EGFR; cat. no. RMA-0804), signal transducer
and activator of transcription 6 (STAT6; cat. no. RMA-0845),
epithelial membrane antigen (EMA; cat. no. Kit-0011),
cytokeratin-pan (CK-pan; cat. no. RAB-0050), desmin (cat. no.
MAB-0766), caldesmon (cat. no. MAB-0643), CD10 (cat. no. MAB-0668),
B-cell lymphoma 6-corepressor (BCOR; cat. no. MAB-0879), myogenin
(cat. no. MAB-0866), myoblast determination protein 1 (MyoD1; cat.
no. MAB-0822), estrogen receptor (ER; cat. no. Kit-0012),
progesterone receptor (PR; cat. no. Kit-0013), CD34 (cat. no.
Kit-0004), CD31 (cat. no. MAB-0720) and tyrosine receptor kinase
(TRK; cat. no. RMA-1072) (all ready-to-use). The Ki67 (cat. no.
MAB-0672; ready-to-use) proliferation index was the proportion of
positive tumor cells counted, and the average of the four
high-power fields was calculated from the selected hot spot. Due to
the uneven distribution of positive tumor cells, the Ki67
proliferation index was 20–50%. All immunohistochemical results are
shown in Fig. 4, Fig. 5, Fig.
6, Fig. 7.
Based on the results of histomorphology and
immunohistochemistry, the patient was diagnosed with MPNST after a
review by two senior pathologists. After that, next-generation
sequencing (NGS) was performed by Precision Scientific (Beijing)
Co., Ltd. All gene exons were detected for the DNA of germ lines
and tumor tissues (the tumor cell content should be >10%), and
the NGS was performed using the NovaSeq 6000 Sequencing System
(Illumina Inc.) with the Oncobuster kit (Precision Scientific,
Ltd.). The results showed that no pathogenic or potentially
pathogenic germline mutations were present in the patient. The only
somatic mutations detected with potential clinical significance
were those in tumor protein 53 (c.422G>A p.C141Y) and erb-B2
receptor tyrosine kinase 2 (c.2329G>T p.V777L). The molecular
changes in this case were not specific to MPNST or typical of it,
which made the clinical follow-up treatment difficult. The patient
received one dose of ifosfamide (IFO; 1.5 g ivgtt, days 1–3) +
etoposide (VP16; 120 mg ivgtt, days 1–3) chemotherapy, followed by
12 radiotherapy sessions and then cisplatin (DDP; 40 mg ivgtt, days
1–3) concurrent chemotherapy. At 8 months post-surgery, the general
condition of the patient was good. Positron emission
tomography-computed tomography was performed at 3-month intervals,
with no tumor recurrence or metastasis detected.
Discussion
MPNSTs rarely occur in the soft tissue, especially
in the uterine cervix. These tumors are more common in middle-aged
women presenting with abnormal vaginal bleeding and have a poor
prognosis due to the highly aggressive nature of the tumors.
Currently, only 17 cases of cervical MPNSTs have been reported in
the literature (2,5–16), and
the clinicopathological characteristics of these cervical MPNSTs
are presented in Table I, together
with those of the current case.
 | Table I.Clinicopathological characteristics of
all cervical malignant peripheral nerve sheath tumors reported in
the literature. |
Table I.
Clinicopathological characteristics of
all cervical malignant peripheral nerve sheath tumors reported in
the literature.
| First author/s,
year | Age, years | Symptom | Tumor size, cm | Mitotic figures | Immunohistochemistry
results | Neurofibro-matosis-1
diagnosis | Pregnancy
history | (Refs.) |
|---|
| Sloan, 1988 | 47 | Vaginal bleeding | 4×3×2 | Numerous mitoses |
S-100− | NA | G3P2 | (5) |
| Junge et al,
1989 | 45 | Vaginal bleeding | 1.2×1×1 | Scattered
mitotic | S-100+
(focal), vimentin+, |
|
|
|
|
|
|
|
| figures | desmin and
CK− | NA | G?P2 | (6) |
| Keel et al,
1998 | 25 | Found a cervical | 1.3 | Easily found | S-100+,
vimentin+, | NA | NA | (7) |
|
|
| polyp |
| mitotic figures | desmin−,
SMA−, CK− and |
|
|
|
|
|
|
|
|
|
HMB45− |
|
|
|
|
| 65 | Vaginal bleeding | 4.4×2.5×1.4 | Easily found
mitotic | S-100+,
SMA−, CK− and | NA | NA |
|
|
|
|
|
| figures |
HMB45− |
|
|
|
|
| 73 | Vaginal bleeding | 5×5 | Easily found
mitotic | S-100+,
vimentin+, | NA | NA |
|
|
|
|
|
| figures | desmin−,
SMA−, CK− |
|
|
|
|
|
|
|
|
| and
HMB45− |
|
|
|
| Lallas et
al, 1999 | 51 | Vaginal
bleeding | 3×3 | 10 per 10 HPF | S-100−,
vimentin+ and | NA | G3P2 | (8) |
|
|
|
|
|
|
SMA− |
|
|
|
| Bernstein et
al, 1999 | 65 | Postcoital
bleeding | 4.4×2.5×1.4 | 6-10 per 10
HPF | S-100+,
CK− and HMB45− | No | G1P1 | (9) |
| Di Giovannantonio
et al, | 27 | Vaginal
bleeding | NA | High mitotic
rate | S-100+,
vimentin+, | NA | NA | (10) |
| 2005 |
|
|
|
| desmin−,
CK− and HMB45− |
|
|
|
| Rodriguez et
al, 2006 | 22 | Postcoital
bleeding | 3 | Average 2–3
per | S-100+,
CD10−, desmin−, | NA | G?P0 | (11) |
|
|
|
|
| 10 HPF, ≤6 per | SMA−,
HMB45− and |
|
|
|
|
|
|
|
| 10 HPF |
Melan-A− |
|
|
|
| Kim et al,
2009 | 50 | Vaginal
bleeding | 6×3.5×2 | Abundant
mitotic | S-100+
(focal), desmin−, | NA | G4P4 | (12) |
|
|
|
|
| figures | SMA−,
HMB45+ (focal) |
|
|
|
|
|
|
|
|
| and
Melan-A− |
|
|
|
| Mills et al,
2011 | 32 | Found a
cervical | 2 | 2 per 10 HPF | S-100+
(focal), vimentin+, | No | NA | (13) |
|
|
| polyp |
|
| CD34+
(70%), desmin− |
|
|
|
|
|
|
|
|
| and
HMB45− |
|
|
|
|
| 60 | Vaginal
bleeding | 5.8 | ≤3 per single | S-100+
(focal), vimentin+, | No | NA |
|
|
|
|
|
| HPF | CD34+
(50%), desmin− and |
|
|
|
|
|
|
|
|
|
HMB45− |
|
|
|
|
| 25 | Vaginal
bleeding | 8 | ≤4 per single | S-100+
(focal), vimentin+, | No | NA |
|
|
|
|
|
| HPF | CD34+
(<10%), desmin− |
|
|
|
|
|
|
|
|
| and
HMB45− |
|
|
|
| Akhavan et
al, 2012 | 53 | Purulent,
malodor | 7 | Scattered
mitotic | S-100+,
vimentin+, desmin+ | No | G12P11 | (14) |
|
|
| vaginal
discharge |
| figures | (focal),
CK− and HMB45− |
|
|
|
| Dong et al,
2014 | 45 | Vaginal
bleeding | 3.7×2.6 | Scattered
mitotic |
S-100+ | NA | NA | (15) |
|
|
|
|
| figures |
|
|
|
|
| Sangiorgio et
al, 2018 | 45 | Vaginal
bleeding | 4 | ≤40 mitoses
per | S-100+,
CD34+ (10%), | No | G4P2 | (2) |
|
|
|
|
| 10 HPF | CD10−,
desmin−, SMA− and |
|
|
|
|
|
|
|
|
|
HMB45− |
|
|
|
| Zhang et al,
2022 | 46 | Vaginal
bleeding | NA | NA | S-100+,
vimentin+, CK− and | No | G?P2 | (16) |
|
|
|
|
|
|
CD34− |
|
|
|
| Current case | 35 | Vaginal
bleeding | 4×3.5×2 | ≤30 mitoses
per | S-100+,
vimentin+, | No | G1P1 | - |
|
|
|
|
| 10 HPF | desmin−,
CK−, HMB45− and |
|
|
|
|
|
|
|
|
|
Melan-A− |
|
|
|
The reported age of cervical patients with MPNST
ranges from 21–73 years, with a median age of 47 years and a mean
age of 45.3 years. The patient in the present report was 36 years
old. The clinical manifestation in these patients is abnormal
vaginal bleeding. Furthermore, it has been reported that 40–50% of
patients with MPNST have NF-1, which has been associated with a
more aggressive disease (3,17,18).
The first symptom of the patient in the present case was vaginal
bleeding and the patient had no history of NF-1. MPNSTs can easily
metastasize to the lungs, but regional lymph node metastasis is
rare (17–19). As a result of early detection, lymph
node metastasis and distal organ metastasis did not occur in the
patient in the present study.
Cervical MPNST is similar to neoplasms of the same
type occurring in other soft tissues. The neoplasms measure 3–4 cm
and are generally polypoid or cervical canal masses that may invade
the cervical interstitium but do not destroy the endocervical
glands (7). The tumor is composed
of tightly packed atypical spindle cells with high mitotic
activity, and its growth pattern is similar to that of
fibrosarcoma, arranged in herringbone, storiform or nodular
patterns (5–8). The tumor cells exhibit diffuse growth
and can form alternating distributions of rich and sparse areas of
cells. Dense tumor cells are commonly found around blood vessels in
mucoid areas, which is called the plexiform pattern. At high
magnification, the tumor cells show the morphological
characteristics of Schwann cells, with deeply stained and irregular
nuclei, round or tapered nuclei, comma or bullet nuclei,
easy-to-see mitotic images and mostly elongated wavy cells in the
sparse area. In addition, large cells with obvious pleomorphism and
multinucleated giant cells were commonly seen in one-third of MPNST
cases. Geographic necrosis was also observed in two-thirds of MPNST
cases. The tumors are rich in thick-walled blood vessels and focal
multidirectional differentiation, such as chondro-osteoid
differentiation, rhabdomyoblastic differentiation or
angiosarcomatoid differentiation, is found in 10–15% of cases
(17,19).
The morphological characteristics of MPNSTs can aid
in diagnosing the tumors as malignant, but they need to be
differentiated from other smooth muscle, neurogenic and mesenchymal
malignancies. Therefore, immunohistochemistry is important, as the
best known marker, S-100, is expressed in 50–90% of MPNSTs
(20), but it is usually expressed
locally (19). The higher the
degree of malignancy of the tumor, the more primitive the cell
differentiation is and the lower the expression rate of S-100
(21,22). Tumor cells also tend to express
Vimentin, SOX-10 and p53, and can express CD56, CD57, PGP9.5 and
other neuropathic markers, with a Ki67 index of 5–65%. The
morphological and immunohistochemical phenotypes in the present
case were consistent with the diagnosis of MPNST. Dense and
intermingled spindle cells were accompanied by numerous mitotic and
multinuclear giant cells. Bleeding and necrosis were observed in
certain areas. The cells were positive for S-100, SOX-10 and CD99,
and neural markers, such as CD56 and PGP9.5, were also positively
expressed, with a Ki67 index of 20–50%. Both smooth muscle and
melanin markers were negative, further confirming the diagnosis of
MPNST in the patient.
However, several malignant tumors composed of
spindle cells can occur in the cervix, creating the need for a
differential diagnosis (2,13). These malignant tumors include the
following: i) Spindle cell leiomyosarcoma (LMS) is composed of
intersecting long bundles of spindle cells with eosinophilic
cytoplasm and rod-shaped, cigar-shaped nuclei or nucleolar
vacuoles. This tumor has moderate-to-significant nuclear atypia and
high mitotic activity. The diagnosis of LMS requires the
immunoreactivity of tumor cells to smooth muscle markers, such as
desmin, caldesmon or SMA. ii) Low-grade endometrial stromal
sarcomas have infiltrating edges and extensive invasion of the
muscular layer. The tumor cells are relatively uniform in size and
shape, with round or ovoid nuclei, obscure nucleoli and unclear
cell borders. Certain cases may be dominated by spindle cells. This
tumor expresses ER, PR, CD10 and vimentin. iii) Adenosarcoma is a
low-grade malignant tumor consisting of benign glands and malignant
stroma, with a characteristic lobular tumor structure. Tumor cells
form a cuff-like structure around the gland. The sarcomatous
component can overgrow benign glands, known as ‘sarcomatous
overgrowth’. It can be distinguished from other cervical sarcomas
by its morphological features after extensive sampling. iv)
Malignant melanomas have several histological forms, the most
common of which are spindle cell type (desmoplastic type) and
epithelioid cell type. Pigment is only found in ~50% of the tumors.
The tumor cells usually have different degrees of pleomorphism,
very active nuclear division and common nuclear and cytoplasmic
inclusion bodies, and a few cases can also be seen as
multinucleated giant cells. HMB-45, Melan-A and S-100 are commonly
expressed in malignant melanomas, but certain cases are negative
for melanocytic markers (2,13).
The treatment for cervical MPNST is an abdominal
hysterectomy with bilateral salpingo-oopherectomy (23). As lymph node metastasis is rare, a
pelvic lymph node dissection may or may not be performed. If there
is a need for fertility in young, nulliparous patients, a radical
vaginal trachelectomy may be attempted (11). High-dose radiotherapy is recommended
for tumors ≥5 cm with microscopically positive incisal margins
(24). Chemotherapy is beneficial
for survival and for the prevention of metastasis in patients with
MPNST (25). Kroep et al
(26) reported that doxorubicin-IFO
regimens were superior to other regimens for MPNSTs. However, there
is no specific clinical treatment plan. The chemotherapy plus
radiotherapy plan adopted for the patient in the present case was a
trial treatment, with IFO, VP16 and DDP being common chemotherapy
drugs for the treatment of malignant tumors. Adjuvant radiotherapy
was added to prevent recurrence, as the tumor deeply infiltrated
the cervical stroma.
In conclusion, cervical MPNST is rare, and the
morphological and immunohistochemical phenotypes overlap with
several types of cervical tumor. During diagnosis, attention should
be given to adequate sampling, careful observation of the
morphology under the microscope and adequate immunohistochemistry
for a potential differential diagnosis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data used and/or analyzed during this study are
included in this published article. Due to patient privacy concerns
and the data protection, the NGS data has not been submitted to a
public repository.
Authors' contributions
XL was responsible for collecting the clinical and
pathological data of the patient, for study conception and for
writing the manuscript. LL contributed to the analysis of the case
data and revision of the manuscript. XL and LL confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and its
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singh D, Vignat J, Lorenzoni V, Eslahi M,
Ginsburg O, Lauby-Secretan B, Arbyn M, Basu P, Bray F and
Vaccarella S: Global estimates of incidence and mortality of
cervical cancer in 2020: A baseline analysis of the WHO global
cervical cancer elimination initiative. Lancet Glob Health.
11:e197–e206. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sangiorgio V, Zanagnolo V, Aletti G,
Bocciolone L, Bruni S, Landoni F, Colombo N, Maggioni A and
Ricciardi E: Fibroblastic malignant peripheral nerve sheath tumour
of the uterine cervix: Report of a case and literature review with
emphasis on possible differential diagnosis. Int J Gynecol Pathol.
37:497–503. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sordillo PP, Helson L, Hajdu SI, Magill
GB, Kosloff C, Golbey RB and Beattie EJ: Malignant
schwannoma-clinical characteristics, survival, and response to
therapy. Cancer. 47:2503–2509. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fadare O: Uncommon sarcomas of the uterine
cervix: A review of selected entities. Diagn Pathol. 1:302006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sloan D: Diagnosis of a tumor with an
unusual presentation in the pelvis. Am J Obstet Gynecol.
159:826–827. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Junge J, Horn T and Bock J: Primary
malignant schwannoma of the uterine cervix. Case report. Br J
Obstet Gynaecol. 96:111–116. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keel SB, Clement PB, Prat J and Young RH:
Malignant schwannoma of the uterine cervix: A study of three cases.
Int J Gynecol Pathol. 17:223–230. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lallas TA, Mehaffey PC, Lager DJ, Van
Voorhis BJ and Sorosky JI: Malignant cervical schwannoma: An
unusual pelvic tumor. Gynecol Oncol. 72:238–242. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bernstein HB, Broman JH, Apicelli A and
Kredentser DC: Primary malignant schwannoma of the uterine cervix:
A case report and literature review. Gynecol Oncol. 74:288–292.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Di Giovannantonio L, Bellocci R,
Zappacosta R, Zappacosta B, Castrataro A, Liberatore M, Liberati M
and Angelucci D: Primary malignant schwannoma of the uterine
cervix: A malignant tumor with unusual behaviour. A case report.
Pathologica. 97:7–9. 2005.(In Italian). PubMed/NCBI
|
|
11
|
Rodriguez AO, Truskinovsky AM, Kasrazadeh
M and Leiserowitz GS: Case report: Malignant peripheral nerve
sheath tumor of the uterine cervix treated with radical vaginal
trachelectomy. Gynecol Oncol. 100:201–204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim NR, Chung DH, Park CY and Ha SY:
Malignant peripheral nerve sheath tumor of the uterine cervix
expressing both S-100 protein and HMB-45. J Obstet Gynaecol Res.
35:1136–1141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mills AM, Karamchandani JR, Vogel H and
Longacre TA: Endocervical fibroblastic malignant peripheral nerve
sheath tumor (neurofibrosarcoma): Report of a novel entity possibly
related to endocervical CD34 fibrocytes. Am J Surg Pathol.
35:404–412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akhavan A, Moghimi M, Karimi-Zarchi M and
Navabii H: Malignant peripheral nerve sheet tumour of cervix. BMJ
Case Rep. 2012:bcr02201258642012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong A, Zuo C, Wang Y, Zhai Z and Xu M:
Enhanced CT and FDG PET/CT in primary malignant peripheral nerve
sheath tumor of the uterine cervix. Clin Nucl Med. 39:825–827.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang T, Wang Z, Wang R, Wu X, Zhao M and
Su F: Malignant peripheral nerve sheath tumor of the cervix. J Coll
Physicians Surg Pak. 32:S24–S27. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ducatman BS, Scheithauer BW, Piepgras DG,
Reiman HM and Ilstrup DM: Malignant peripheral nerve sheath tumors.
A clinicopathologic study of 120 cases. Cancer. 57:2006–2021. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong WW, Hirose T, Scheithauer BW, Schild
SE and Gunderson LL: Malignant peripheral nerve sheath tumor:
Analysis of treatment outcome. Int J Radiat Oncol Biol Phys.
42:351–360. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hruban RH, Shiu MH, Senie RT and Woodruff
JM: Malignant peripheral nerve sheath tumors of the buttock and
lower extremity. A study of 43 cases. Cancer. 66:1253–1265. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stasik CJ and Tawfik O: Malignant
peripheral nerve sheath tumor with rhabdomyosarcomatous
differentiation (malignant triton tumor). Arch Pathol Lab Med.
130:1878–1881. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weiss SW, Langloss JM and Enzinger FM:
Value of S-100 protein in the diagnosis of soft tissue tumors with
particular reference to benign and malignant Schwann cell tumors.
Lab Invest. 49:299–308. 1983.PubMed/NCBI
|
|
22
|
Wick MR, Swanson PE, Scheithauer BW and
Manivel JC: Malignant peripheral nerve sheath tumor. An
immunohistochemical study of 62 cases. Am J Clin Pathol.
87:425–433. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wright JD, Rosenblum K, Huettner PC, Mutch
DG, Rader JS, Powell MA and Gibb RK: Cervical sarcomas: An analysis
of incidence and outcome. Gynecol Oncol. 99:348–351. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leachman BK and Galloway TJ: The role for
radiation therapy in the management of sarcoma. Surg Clin North Am.
96:1127–1139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moretti VM, Crawford EA, Staddon AP,
Lackman RD and Ogilvie CM: Early outcomes for malignant peripheral
nerve sheath tumor treated with chemotherapy. Am J Clin Oncol.
34:417–421. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kroep JR, Ouali M, Gelderblom H, Le Cesne
A, Dekker TJA, Van Glabbeke M, Hogendoorn PCW and Hohenberger P:
First-line chemotherapy for malignant peripheral nerve sheath tumor
(MPNST) versus other histological soft tissue sarcoma subtypes and
as a prognostic factor for MPNST: An EORTC soft tissue and bone
sarcoma group study. Ann Oncol. 22:207–214. 2011. View Article : Google Scholar : PubMed/NCBI
|