Introduction
Pulmonary large cell neuroendocrine carcinoma
(LCNEC) is a rare disease, accounting for ~3% of all lung cancer
cases (1,2). The cancer is highly invasive, with
rapid development and hidden symptoms, and is difficult to diagnose
early (3). In total, ~40% of
patients with LCNEC are initially diagnosed at stage IV, with no
opportunity for surgery. Hence, the overall prognosis is poor, with
a median overall survival (mOS) time of only 8–12 months (4). In 2015, the World Health Organization
defined LCNEC as a unique subtype of lung neuroendocrine neoplasms
and one of the subtypes of non-small cell lung cancer (NSCLC)
(5). However, its biological and
clinical characteristics, and prognostic factors, are similar to
those of SCLC (6). Next-generation
sequencing has revealed molecular heterogeneity among patients with
LCNEC that could be roughly divided into two subtypes: The
SCLC-like subtype that mainly co-occurs with TP53 and
RB1 mutations, and the NSCLC-like subtype that is mainly
associated with KRAS, STK11/KEAP1 and/or TP53
mutations (7,8). Studies have shown that the NSCLC-like
and SCLC-like subsets have no differences in terms of mOS and
median progression-free survival (mPFS) (7). However, Zhuo et al (9) demonstrated that genomic subtype
analysis plays a role in the prognostic and therapeutic decisions
for patients with LCNEC.
The clinical management of LCNEC is controversial
due of its low incidence rate and unusual pathological features.
Radical surgical resection is primarily recommended for early stage
LCNEC (10), with reported OS
benefits for patients with resectable LCNEC (11–13).
Postoperative platinum-based single-agent or multi-agent adjuvant
chemotherapy can prolong survival time (14,15);
however, utilizing preoperative neoadjuvant chemotherapy remains
controversial due to uncertain efficacy (3). Currently, no standardized treatment
strategy exists for the multi-disciplinary and comprehensive
treatment of advanced LCNEC, with most regimens relying on
chemotherapy. Existing evidence supports the efficacy of drugs
commonly used in the treatment of SCLC, such as platinum-etoposide
(SCLC-like regimen), and advanced LCNEC (16–18).
The selection of a first-line treatment for metastatic LCNEC poses
challenges due to the predominantly small scale and retrospective
nature of available studies, with some yielding conflicting
results, as some choose SCLC-like regimens, while other select
NSCLC-like regimens (4).
Immune checkpoint inhibitors (ICIs) targeting
programmed death protein 1 (PD-1) and programmed death ligand 1
(PD-L1) have transformed treatment planning for patients with
driver mutation-negative advanced NSCLC (19,20).
Due to the lack of prospective evidence, the efficacy of
immunotherapy in LCNEC remains unestablished. Data from existing
case reports and small retrospective studies are promising,
indicating that the mPFS time of patients with advanced LCNEC using
ICIs was 4.2–14.2 months, while the mOS time was 11.8 months
(21–24). However, the efficacy and safety of
ICIs in patients with LCNEC remain controversial. Therefore,
further assessment is necessary to validate the effect of LCNEC on
ICI efficacy in a more homogeneous patient subgroup, especially
given that the treatment regimen may considerably affect outcomes.
The present study aimed to evaluate the effect of ICIs on treatment
response and survival outcomes in patients with advanced LCNEC.
Materials and methods
Patient selection and clinical
data
The present retrospective study included patients
with pathologically confirmed advanced LCNEC (stage IV; poorly
differentiated, large cell, abundant cytoplasm and multiple
necrosis) who visited The First Affiliated Hospital of Zhengzhou
University (Zhengzhou, China) between January 1, 2019, and
September 9, 2021. The main inclusion criteria were as follows: i)
Pathologically confirmed LCNEC, mixed LCNEC and SCLC, and mixed
LCNEC and NSCLC with a predominant LCNEC component; and ii) the
presence of at least one observable or measurable lesion. The
exclusion criteria were as follows: i) The presence of non-primary
lung cancer; ii) previous or current malignant tumors of other
types; and iii) uncontrolled dysfunction of any major organ. After
obtaining approval from the Institutional Ethical Review Board of
the First Affiliated Hospital of Zhengzhou University (approval no.
2022-KY-0592-002), baseline demographic [including age, sex,
Eastern Cooperative Oncology Group performance status (ECOG PS
score) (25), and smoking history],
clinical, pathological and treatment characteristics, were
retrospectively collected, in addition to toxicity and outcome
data.
Study design and treatment
methods
Patients were divided into group A (patients who
received ICIs as any treatment line; n=73) and group B (patients
who did not receive ICIs; n=75). Group A was further divided into
group C (patients who received chemotherapy combined with ICIs;
n=34) and group D (patients who received anti-angiogenic agents
combined with ICIs; n=32) for analysis. Additionally, patients were
divided into pure LCNEC and mixed LCNEC (squamous cell carcinoma,
adenocarcinoma and small-cell carcinoma) based on the pathological
type.
Chemotherapy consisted of etoposide (80–100
mg/m2, administered on days 1–3 of each 21-day cycle),
pemetrexed (500 mg/m2), docetaxel (75 mg/m2)
or paclitaxel (175 mg/m2) (administered on day 1 of each
cycle), with or without carboplatin (area under the curve, 4–6),
cisplatin (75–80 mg/m2) or nedaplatin (80–100
mg/m2) (administered on day 1 of each 21-day cycle),
based on the investigator's judgment. Patients in groups A and C
received 4–6 cycles of chemotherapy plus ICIs, followed by
maintenance ICIs every 3 weeks. Anti-angiogenic therapy consisted
of anlotinib (8–12 mg, taken orally once a day for 2 weeks with a
1-week break), apatinib (250 mg, taken continuously) and
bevacizumab (15 mg/kg, administered on day 1 of each 21-day cycle).
ICIs consisted of pembrolizumab (200 mg), camrelizumab (200 mg),
sintilimab (200 mg), toripalimab (240 mg), tislelizumab (200 mg),
atezolizumab (1,200 mg) or durvalumab (1,500 mg, administered on
day 1 of each 21-day cycle). Patients continued treatment until
disease progression based on investigator assessment, unacceptable
toxicity or other discontinuation criteria. Continuation of the
study's treatment regimen following disease progression was
permitted if clinical benefit was proven. Group C was comprised of
34 patients as follows: Patients with NSCLC who received
pemetrexed-platinum (n=1), paclitaxel-platinum (n=5),
docetaxel-platinum (n=2) and gemcitabine-platinum (n=2), and
patients with SCLC who received etoposide-platinum (n=20) (4
patients received thoracic radiotherapy and 1 patient received
palliative radiotherapy for brain metastasis) and
irinotecan-platinum (n=4). In total, 32 patients were included in
group D, and the following anti-angiogenesis drugs were used:
Anlotinib, apatinib and bevacizumab. A single patient with brain
metastasis received palliative radiotherapy.
Evaluation of efficacy
The revised Response Evaluation Criteria in Solid
Tumors, version 1.1 (26), was used
to evaluate the treatment efficacy, including complete response,
partial response (PR), stable disease (SD) and progressive disease
(PD) statuses. PFS was defined as the time from the start of ICI
treatment to the date of disease progression or death due to any
cause. Overall survival (OS) was defined as the time from advanced
disease diagnosis until death or censored at the last follow-up
visit. Immune-related adverse events (irAEs) were graded using the
National Cancer Institute's Common Terminology Criteria for Adverse
Events, version 5.0 (27). Duration
of follow-up was calculated from the time of advanced disease
diagnosis until the last follow-up visit or censored at death. The
cut-off date for data collection was June 14, 2022.
Statistical analysis
Categorical variables are presented as numbers and
percentiles. Medians and ranges are reported for continuous
variables. Fisher's exact and χ2 tests were used to
compare the baseline demographic, clinical and pathological
characteristics (except for age, tumor size and Ki-67 values, which
are continuous variables that were tested using an independent
sample t-test). The Kaplan-Meier method was used to estimate
survival rates and the log-rank test was used to analyze
between-group survival differences. Case characteristics were
regarded as independent prognostic factors if they showed a
significant association (P<0.05) in the multivariable regression
with a Cox proportional hazards model. Statistical analyses were
conducted using SPSS 26.0 (IBM Corp). P<0.05 was used to
indicate a statistically significant difference.
Results
Demographic and clinical
characteristics
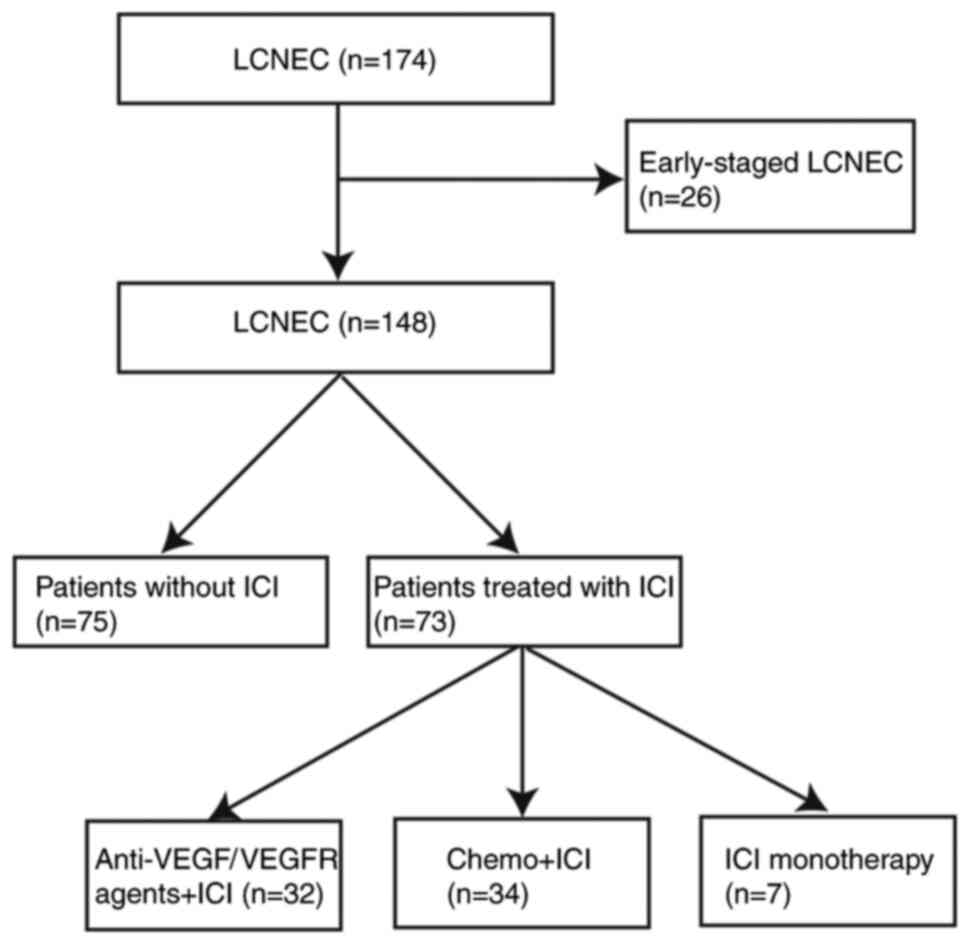
In total, 174 patients with histologically confirmed
LCNEC were diagnosed at The First Affiliated Hospital of Zhengzhou
University between January 1, 2019, and September 9, 2021. A total
of 26 patients with early stage disease were excluded. Thus, 148
patients were clinically evaluated (Fig. 1). The baseline demographic, clinical
and pathological characteristics of the 148 patients are summarized
in Table I, and the detailed
treatment regimens and outcomes for all patients are presented in
Table II. All patients in the
clinical analysis underwent follow-up from the time of pathological
diagnosis to June 14, 2022, or until death.
 | Table I.Baseline clinical, pathological and
treatment characteristics of patients with advanced large cell
neuroendocrine carcinoma of divided according to exposure to
ICI. |
Table I.
Baseline clinical, pathological and
treatment characteristics of patients with advanced large cell
neuroendocrine carcinoma of divided according to exposure to
ICI.
|
Characteristics | Patients treated
with ICI (group A; n=73) | Patients not
treated with ICI (group B; n=75) | P-value | All patients
(n=148) |
|---|
| Median age (range),
years | 67 (32–84) | 66 (34–86) | 0.892 | 67 (32–86) |
| Sex, n (%) |
|
| 0.405 |
|
|
Male | 63 (86.3) | 68 (90.7) |
| 131 (88.5) |
|
Female | 10 (13.7) | 7 (9.3) |
| 17 (11.5) |
| Smoking history, n
(%) |
|
| 0.716 |
|
|
Current/former smoker | 44 (60.3) | 43 (57.3) |
| 87 (58.8) |
| Never
smoker | 29 (39.7) | 32 (42.7) |
| 61 (41.2) |
| ECOG PS at
diagnosis, n (%) |
|
| 0.934 |
|
|
0-1 | 58 (79.5) | 60 (80.0) |
| 118 (79.7) |
| ≥2 | 15 (20.5) | 15 (20.0) |
| 30 (20.3) |
| Primary tumor
location, n (%) |
|
| 0.509 |
|
|
Left | 39 (53.4) | 36 (48.0) |
| 75 (50.7) |
|
Right | 34 (46.6) | 39 (52.0) |
| 73 (49.3) |
| Median primary
tumor size (IQR), mm | 38.5
(27.5–57.0) | 42.0
(27.4–59.0) | 0.324 | 40.9
(27.5–58.0) |
| Median Ki-67
(IQR) | 80 (67.3–80.0) | 80.0
(60.0–80.0) | 0.680 | 80.0
(66.2–80.0) |
| Histological
subtype, n (%) |
|
| 0.782 |
|
|
LCNEC | 50 (68.5) | 54 (72.0) |
| 104 (70.3) |
| Mixed
LCNEC + NSCLC | 5 (6.8) | 6 (8.0) |
| 11 (7.4) |
| Mixed
LCNEC + SCLC | 18 (24.7) | 15 (20.0) |
| 33 (22.3) |
| Lymph node
metastases, n (%) |
|
| 0.370 |
|
|
Yes | 65 (89.0) | 63 (84.0) |
| 128 (86.5) |
| No | 8 (11.0) | 12 (16.0) |
| 20 (13.5) |
| N, n (%) |
|
| 0.483 |
|
| N0 | 8 (11.0) | 12 (16.0) |
| 20 (12.5) |
| N1 | 6 (8.2) | 4 (5.3) |
| 10 (6.8) |
| N2 | 33 (45.2) | 39 (52.0) |
| 72 (38.6) |
| N3 | 26 (35.6) | 20 (26.7) |
| 46 (31.1) |
| M, n (%) |
|
| 0.420 |
|
| M0 | 30 (41.1) | 26 (34.7) |
| 56 (37.8) |
| M1 | 43 (58.9) | 49 (65.3) |
| 92 (62.2) |
| Number of
metastatic organs, n (%) |
|
| 0.959 |
|
| 0 | 39 (53.4) | 41 (54.7) |
| 80 (54.1) |
| 1 | 21 (28.8) | 20 (26.7) |
| 41 (27.7) |
| ≥2 | 13 (17.8) | 14 (18.7) |
| 27 (18.2) |
| Brain metastases, n
(%) |
|
| 0.368 |
|
|
Yes | 17 (23.3) | 13 (17.3) |
| 30 (20.3) |
| No | 56 (76.7) | 62 (82.7) |
| 118 (79.7) |
| Liver metastases, n
(%) |
|
| 0.948 |
|
|
Yes | 10 (13.7) | 10 (13.3) |
| 20 (13.5) |
| No | 63 (86.3) | 65 (86.7) |
| 128 (86.5) |
| Bone metastases, n
(%) |
|
| 0.188 |
|
|
Yes | 8 (11.0) | 14 (18.7) |
| 22 (14.9) |
| No | 65 (89.0) | 61 (81.3) |
| 126 (85.1) |
| NLR, n (%) |
|
| 0.722 |
|
|
<5 | 61 (83.6) | 61 (81.3) |
| 122 (82.4) |
| ≥5 | 12 (16.4) | 14 (18.7) |
| 26 (17.6) |
| CEA, n (%) |
|
| 0.233 |
|
| <5
ng/ml | 46 (63.0) | 40 (53.3) |
| 86 (58.1) |
| ≥5
ng/ml | 27 (37.0) | 35 (46.7) |
| 62 (41.9) |
| NSE, n (%) |
|
| 0.020a |
|
|
<16.3 ng/ml | 35 (47.9) | 22 (29.3) |
| 57 (38.5) |
| ≥16.3
ng/ml | 38 (52.1) | 53 (70.7) |
| 91 (61.5) |
| CA125, n (%) |
|
| 0.033a |
|
| <35
U/ml | 63 (86.3) | 54 (72.0) |
| 117 (79.1) |
| ≥35
U/ml | 10 (13.7) | 21 (28.0) |
| 31 (20.9) |
| CYFRA21-1, n
(%) |
|
| 0.325 |
|
| <3.3
ng/ml | 28 (38.4) | 23 (30.7) |
| 51 (34.5) |
| ≥3.3
ng/ml | 45 (61.6) | 52 (69.3) |
| 97 (65.5) |
| LDH, n (%) |
|
| 0.355 |
|
| <240
U/l | 22 (30.1) | 28 (37.3) |
| 50 (33.8) |
| ≥240
U/l | 51 (69.9) | 47 (62.7) |
| 98 (66.2) |
| Cholesterol, n
(%) |
|
| 0.063 |
|
| <5.2
mg/dl | 67 (91.8) | 61 (81.3) |
| 128 (86.5) |
| ≥5.2
mg/dl | 6 (8.2) | 14 (18.7) |
| 20 (13.5) |
| Triglycerides, n
(%) |
|
| 0.951 |
|
| <1.7
mg/dl | 64 (87.7) | 66 (88.0) |
| 130 (87.8) |
| ≥1.7
mg/dl | 9 (12.3) | 9 (12.0) |
| 18 (12.2) |
| Treatment details,
n (%) |
|
|
|
|
| ICI
alone | 7 (9.6) |
|
|
|
|
Chemotherapy + ICI | 34 (46.6) |
|
|
|
|
Anti-angiogenesis + ICI | 32 (43.8) |
|
|
|
| Name of ICI, n
(%) |
|
|
|
|
|
Durvalumab | 6 (9.1) |
|
|
|
|
Toripalimab | 3 (4.5) |
|
|
|
|
Camrelizumab | 45 (62.1) |
|
|
|
|
Sintilimab | 11 (15.2) |
|
|
|
|
Atezolizumab | 1 (1.5) |
|
|
|
|
Tislelizumab | 5 (6.1) |
|
|
|
|
Pembrolizumab | 2 (1.5) |
|
|
|
 | Table II.Details of treatment in 73 patients
who underwent different treatment regimens. |
Table II.
Details of treatment in 73 patients
who underwent different treatment regimens.
| Case no. | Systemic treatment
lines | Treatment
details | Radiotherapy | Efficacy |
|---|
| 1 | 1st-line | Etoposide +
carboplatin + durvalumab | Whole-brain
radiotherapy | SD |
| 2 | 1st-line | Etoposide +
cisplatin + durvalumab |
| PR |
| 3 | 1st-line | Etoposide +
lobaplatin + camrelizumab |
| PR |
| 4 | 1st-line | Etoposide +
carboplatin + camrelizumab | Thoracic
radiotherapy | PR |
| 5 | 1st-line | Etoposide +
carboplatin + camrelizumab | Thoracic
radiotherapy | PR |
| 6 | 1st-line | Irinotecan +
nedaplatin + durvalumab |
| PR |
| 7 | 1st-line | Etoposide +
carboplatin + camrelizumab |
| PR |
| 8 | 1st-line | Etoposide +
cisplatin + durvalumab |
| SD |
| 9 | 1st-line | Paclitaxel +
tislelizumab |
| SD |
| 10 | 1st-line | Etoposide +
carboplatin + camrelizumab |
| SD |
| 11 | 1st-line | Etoposide +
cisplatin + durvalumab |
| SD |
| 12 | 1st-line | Etoposide +
nedaplatin + tislelizumab |
| PR |
| 13 | 1st-line | Etoposide +
cisplatin + camrelizumab | Thoracic
radiotherapy | SD |
| 14 | 1st-line | Paclitaxel +
nedaplatin + sintilimab |
| SD |
| 15 | 1st-line | Etoposide +
carboplatin + camrelizumab |
| PR |
| 16 | 1st-line | Etoposide +
cisplatin + camrelizumab |
| PD |
| 17 | 1st-line | Etoposide +
nedaplatin + atezolizumab | Thoracic
radiotherapy | PR |
| 18 | 1st-line | Etoposide +
nedaplatin + sintilimab |
| PR |
| 19 | 1st-line | Etoposide +
lobaplatin + camrelizumab |
| PR |
| 20 | 2nd-line | Etoposide +
nedaplatin + camrelizumab |
| PR |
| 21 | 2nd-line | Docetaxel +
camrelizumab |
| PR |
| 22 | 2nd-line | Paclitaxel +
carboplatin + camrelizumab |
| SD |
| 23 | 2nd-line | Gemcitabine +
cisplatin + camrelizumab |
| SD |
| 24 | 2nd-line | Irinotecan +
toripalimab |
| PD |
| 25 | 2nd-line | Etoposide +
nedaplatin + camrelizumab |
| SD |
| 26 | 2nd-line | Etoposide +
camrelizumab |
| SD |
| 27 | 2nd-line | Docetaxel +
sintilimab |
| SD |
| 28 | 2nd-line | Irinotecan +
lobaplatin + camrelizumab |
| SD |
| 29 | 2nd-line | Irinotecan +
sintilimab |
| PD |
| 30 | 2nd-line | Paclitaxel +
carboplatin + camrelizumab |
| PR |
| 31 | 3rd-line | Gemcitabine +
carboplatin + tislelizumab |
| PD |
| 32 | 3rd-line | Paclitaxel +
carboplatin + camrelizumab |
| SD |
| 33 | 3rd-line | Pemetrexed +
carboplatin + camrelizumab |
| SD |
| 34 | 3rd-line | Etoposide +
lobaplatin + camrelizumab |
| SD |
| 35 | 1st-line | Sintilimab +
anlotinib |
| PR |
| 36 | 1st-line | Camrelizumab +
apatinib |
| PR |
| 37 | 2nd-line | Camrelizumab +
anlotinib |
| SD |
| 38 | 2nd-line | Sintilimab +
anlotinib |
| SD |
| 39 | 2nd-line | Camrelizumab +
apatinib |
| PR |
| 40 | 2nd-line | Camrelizumab +
anlotinib |
| SD |
| 41 | 2nd-line | Camrelizumab +
anlotinib |
| SD |
| 42 | 2nd-line | Durvalumab +
anlotinib |
| SD |
| 43 | 2nd-line | Camrelizumab +
anlotinib |
| PR |
| 44 | 2nd-line | Camrelizumab +
anlotinib |
| SD |
| 45 | 2nd-line | Camrelizumab +
anlotinib |
| PD |
| 46 | 2nd-line | Camrelizumab +
bevacizumab |
| SD |
| 47 | 2nd-line | Sintilimab +
anlotinib |
| PR |
| 48 | 2nd-line | Camrelizumab +
apatinib |
| SD |
| 49 | 2nd-line | Sintilimab +
anlotinib |
| SD |
| 50 | 2nd-line | Camrelizumab +
anlotinib |
| SD |
| 51 | 2nd-line | Camrelizumab +
apatinib |
| PR |
| 52 | 2nd-line | Camrelizumab +
anlotinib |
| PD |
| 53 | 2nd-line | Camrelizumab +
anlotinib |
| SD |
| 54 | 2nd-line | Toripalimab +
anlotinib |
| PR |
| 55 | 3rd-line | Tislelizumab +
anlotinib |
| SD |
| 56 | 3rd-line | Camrelizumab +
apatinib |
| SD |
| 57 | 3rd-line | Toripalimab +
anlotinib |
| SD |
| 58 | 3rd-line | Pembrolizumab +
anlotinib | Whole-brain
radiotherapy | SD |
| 59 | 3rd-line | Camrelizumab +
anlotinib |
| PD |
| 60 | 3rd-line | Camrelizumab +
anlotinib |
| PD |
| 61 | 3rd-line | Sintilimab +
anlotinib |
| PR |
| 62 | 4th-line | Sintilimab +
anlotinib |
| PD |
| 63 | 4th-line | Camrelizumab +
anlotinib |
| SD |
| 64 | 5th-line | Camrelizumab +
anlotinib |
| SD |
| 65 | 5th-line | Camrelizumab +
anlotinib |
| SD |
| 66 | 6th-line | Camrelizumab +
bevacizumab |
| SD |
| 67 | 2nd-line | Camrelizumab |
| SD |
| 68 | 2nd-line | Camrelizumab |
| PD |
| 69 | 2nd-line | Camrelizumab |
| PD |
| 70 | 2nd-line | Pembrolizumab |
| SD |
| 71 | 3rd-line | Tislelizumab |
| SD |
| 72 | 3rd-line | Camrelizumab |
| PR |
| 73 | 3rd-line | Sintilimab |
| SD |
The patients had a median age of 67 years (age
range, 32–86 years), were mostly men (88.5%) and had a history of
smoking (58.8%). Patients with an ECOG PS score ≥2 at diagnosis
accounted for 20.3% of the population. LCNEC occurred in the lung
lobe, and no significant differences were observed in the
distribution of LCNEC between the left and right lungs. All the
patients had stage IV LCNEC at diagnosis, while 20.3% had brain
metastases, 13.5% had liver metastases and 14.9% had bone
metastases. Moreover, 104 patients (70.3%) had pure LCNEC and 44
had combined LCNEC (mixed LCNEC and SCLC, 22.3%; mixed LCNEC and
NSCLC, 7.4%). The positive rate of serum tumor marker cytokeratin
19 fragment antigen 21-1 (CYFRA21-1) was the highest (65.5%),
followed by neuron-specific enolase (NSE) (61.5%), and the
positivity rates for carcinoembryonic antigen (CEA) and cancer
antigen (CA)-125 were low. The levels of peripheral blood markers
such as the neutrophil/lymphocyte ratio (NLR), cholesterol and
triglyceride were low, while the lactate dehydrogenase (LDH) level
was higher than normal in ~66.2% of patients. The median Ki-67
level was 80.0% [inter-quartile range (IQR), 66.2–80.0%]. Group B
had more patients with increased NSE and CA125 levels (P=0.033 and
P=0.020, respectively) than group A. All patients received
chemotherapy, anti-angiogenic therapy or immunotherapy according to
their condition, and some patients received radiotherapy. Of these,
73 patients received ICIs (group A) and 75 patients did not receive
ICIs (group B). In group A, 7 patients (9.6%) received
immunotherapy alone, 34 patients (46.6%) received chemotherapy
combined with immunotherapy and 32 patients (43.8%) received
anti-angiogenic combined immunotherapy (Tables I, II, and Fig.
2).
Patient outcomes
Efficacy of ICI based on LCNEC
The primary endpoint of this study was OS. The mean
follow-up duration was 18.18 months (IQR, 1.8–56.13 months) in
group A and 12.56 months (IQR, 0.43–40.37 months) in group B. By
the end of follow-up, 34 patients (46.6%) in group A and 50
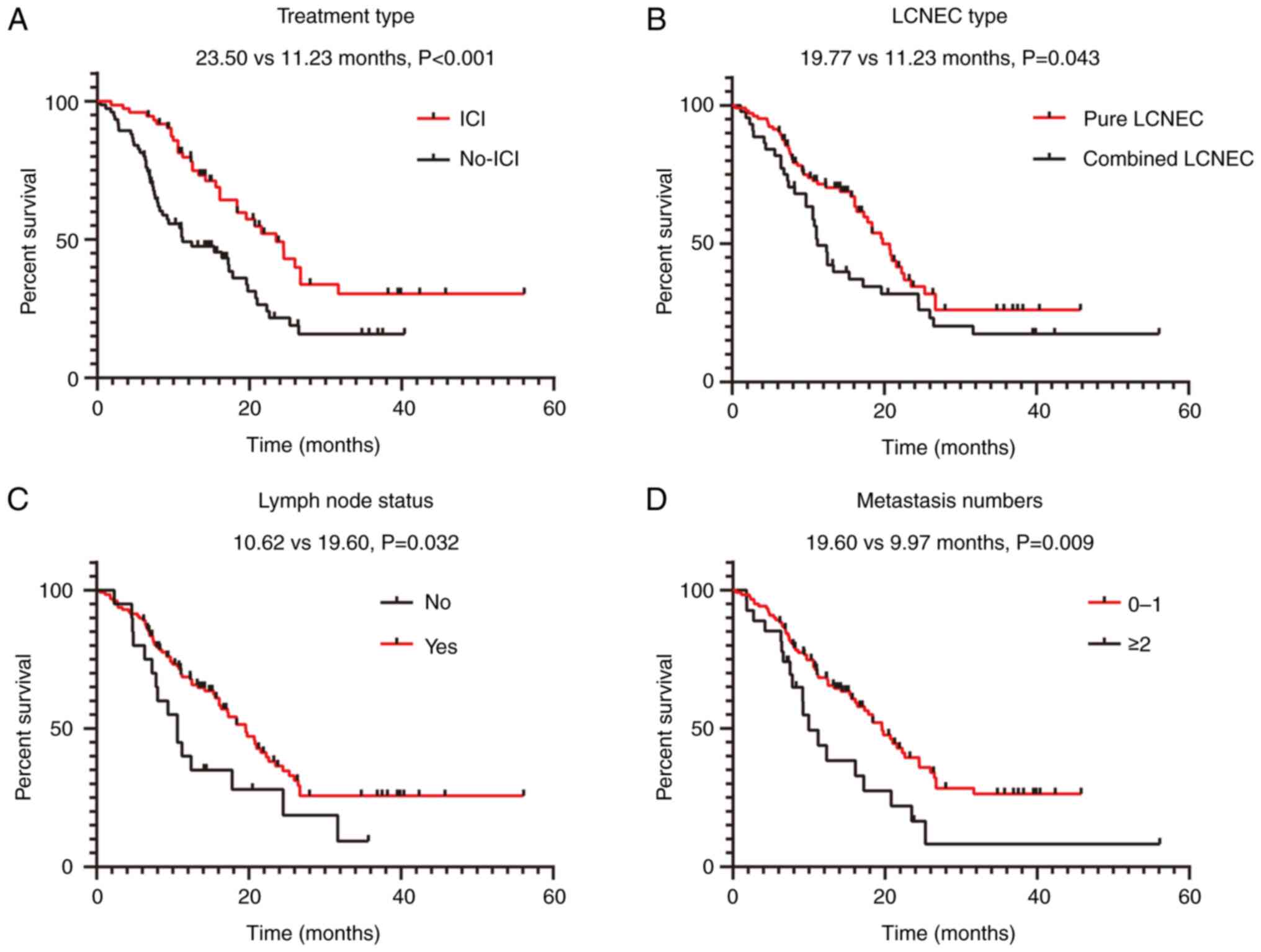
patients (66.7%) in group B had died. The mOS time of group A was
23.500 months [95% confidence interval (CI), 18.524–28.476] and
that of group B was 11.230 months (95% CI, 4.530–18.930). The
survival time of group A was significantly longer than that of
group B (P=0.001) (Fig. 3A). In the
univariate analysis, ICI administration (P<0.001), histological
type (P=0.043), lymph node metastases (P=0.032) and number of
metastatic organs (P=0.009) demonstrated a significant association
with OS (Fig. 3A-D; Table III). However, sex, smoking status,
ECOG PS score, presence of brain, liver and bone metastases, and
levels of NLR, CEA, CA125, CYFRA21-1, LDH, cholesterol and
triglycerides did not demonstrate any significant associations with
OS (all P>0.05) (Table III).
In the multivariate Cox regression analysis model, which
incorporated all factors significantly associated with OS from the
univariate analysis, ICI administration (P<0.001), pathological
type (P=0.005), lymph node metastases (P=0.030) and number of
metastatic organs (P=0.011) were significantly associated with OS
(Table III).
 | Table III.Univariate and multivariate Cox
regression analyses of overall survival since the diagnosis of
advanced disease in patients with advanced large cell
neuroendocrine carcinoma. |
Table III.
Univariate and multivariate Cox
regression analyses of overall survival since the diagnosis of
advanced disease in patients with advanced large cell
neuroendocrine carcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameters | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| ICI (yes vs.
no) | 0.453
(0.292–0.703) |
<0.001a | 0.377
(0.235–0.607) |
<0.001a |
| Sex (male vs.
female) | 0.969
(0.501–1.875) | 0.636 |
|
|
| Smoking (yes vs.
no) | 0.894
(0.579–1.380) | 0.613 |
|
|
| ECOG PS (0–1 vs.
≥2) | 0.920
(0.546–1.551) | 0.754 |
|
|
| Number of
metastatic organs (0–1 vs. ≥2) | 0.429
(0.227–0.812) | 0.009a | 2.595
(1.246–5.403) | 0.011a |
| Lymph node
metastases (yes vs. no) | 0.542
(0.314–0.934) | 0.032a | 0.537
(0.306–0.942) | 0.030a |
| Brain metastases
(yes vs. no) | 1.318
(0.790–2.199) | 0.290 |
|
|
| Liver metastases
(yes vs. no) | 1.091
(0.578–2.058) | 0.789 |
|
|
| Bone metastases
(yes vs. no) | 1.571
(0.867–2.845) | 0.136 |
|
|
| Histological
subtype (pure vs. no) | 1.573
(1.015–2.439) | 0.043a | 1950
(1.222–3.110) | 0.005a |
| NLR (<5 vs.
≥5) | 0.895
(0.516–1.552) | 0.693 |
|
|
| CEA (<5 vs. ≥5
ng/ml) | 1.099
(0.711–1.699) | 0.670 |
|
|
| NSE (<16.3 vs.
≥16.3 ng/ml) | 1.515
(0.946–2.425) | 0.084 |
|
|
| CA125 (<35 vs.
≥35 U/ml) | 1.463
(0.905–2.365) | 0.121 |
|
|
| CYFRA21-1 (<3.3
vs. ≥3.3 ng/ml) | 1.248
(0.793–1.965) | 0.338 |
|
|
| LDH (<240 vs.
≥240 U/l) | 1.315
(0.818–2.114) | 0.258 |
|
|
| Cholesterol
(<5.2 vs. ≥5.2 mg/dl) | 1.711
(0.925–3.165) | 0.087 |
|
|
| Triglycerides
(<1.7 vs. ≥1.7 mg/dl) | 1.157
(0.613–2.186) | 0.653 |
|
|
Efficacy analysis of combination
therapy based on immunotherapy
Comparison of the survival rate
Since different treatment regimens were used in
group A (patients who received ICIs), it was subdivided into group
C (patients who received chemotherapy combined with ICIs; n=34) and
group D (patients who received anti-angiogenic agents combined with
ICIs; n=32). A total of 7 patients who received monotherapy with an
anti-PD-1/PD-L1 agent were not included in the analysis. No
differences were found in baseline characteristics between the two
groups, except for age (P=0.026), and the clinical and pathological
characteristics of the patients are summarized in Table IV.
 | Table IV.Baseline clinical, pathological and
treatment characteristics of patients with advanced large cell
neuroendocrine carcinoma of the lung by treatment regimen. |
Table IV.
Baseline clinical, pathological and
treatment characteristics of patients with advanced large cell
neuroendocrine carcinoma of the lung by treatment regimen.
|
Characteristics | Chemo + ICI (group
C; n=34) | Anti-VEGF + ICI
(group D; n=32) | P-value |
|---|
| Age, n (%) |
|
| 0.044a |
| <67
years | 19 (55.9) | 10 (31.3) |
|
| ≥67
years | 15 (44.1) | 22 (68.8) |
|
| Sex, n (%) |
|
| >0.999 |
| Male | 30 (88.2) | 28 (87.5) |
|
| Female | 4 (11.8) | 4 (12.5) |
|
| Smoking history, n
(%) |
|
| 0.05 |
| Current/past
smoker | 22 (64.7) | 13 (40.6) |
|
| Never
smoker | 12 (35.3) | 19 (59.4) |
|
| ECOG PS at
diagnosis, n (%) |
|
| 0.384 |
| 0-1 | 28 (82.4) | 23 (71.9) |
|
| ≥2 | 6 (17.6) | 9 (28.1) |
|
| Histological
subtype, n (%) |
|
| 0.561 |
| LCNEC | 24 (70.6) | 20 (62.5) |
|
| Mixed LCNEC +
NSCLC | 2 (5.9) | 3 (9.4) |
|
| Mixed LCNEC +
SCLC | 8 (23.5) | 9 (28.1) |
|
| N, n (%) |
|
| 0.813 |
| N0 | 3 (8.8) | 3 (9.4) |
|
| N1 | 1 (2.9) | 4 (12.5) |
|
| N2 | 18 (52.9) | 12 (37.5) |
|
| N3 | 12 (35.3) | 13 (40.6) |
|
| M, n (%) |
|
| 0.66 |
| M0 | 12 (35.3) | 13 (40.6) |
|
| M1 | 22 (64.7) | 19 (59.4) |
|
| Brain metastases, n
(%) |
|
| 0.578 |
| Yes | 10 (29.4) | 7 (21.9) |
|
| No | 24 (70.6) | 25 (78.1) |
|
| Liver metastases, n
(%) |
|
| 0.505 |
| Yes | 4 (11.8) | 6 (18.8) |
|
| No | 30 (88.2) | 26 (81.3) |
|
| Bone metastases, n
(%) |
|
| 0.106 |
| Yes | 6 (17.6) | 1 (3.1) |
|
| No | 28 (82.4) | 31 (96.9) |
|
| NLR, n (%) |
|
| 0.104 |
| <5 | 31 (91.2) | 24 (75.0) |
|
| ≥5 | 3 (8.8) | 8 (25.0) |
|
| CEA, n (%) |
|
| >0.999 |
| <5
ng/ml | 21 (61.8) | 20 (62.5) |
|
| ≥5 ng/ml | 13 (38.2) | 12 (37.5) |
|
| NSE, n (%) |
|
| 0.631 |
| <16.3
ng/ml | 17 (50.0) | 14 (43.8) |
|
| ≥16.3
ng/ml | 17 (50.0) | 18 (56.3) |
|
| CA125, n (%) |
|
| 0.734 |
| <35
U/ml | 28 (82.4) | 28 (87.5) |
|
| ≥35 U/ml | 6 (17.6) | 4 (12.5) |
|
| CYFRA21-1, n
(%) |
|
| 0.802 |
| <3.3
ng/ml | 13 (38.2) | 11 (34.4) |
|
| ≥3.3
ng/ml | 21 (61.8) | 21 (65.6) |
|
| LDH, n (%) |
|
| 0.185 |
| <240
U/l | 13 (38.2) | 7 (21.9) |
|
| ≥240 U/l | 21 (61.8) | 25 (78.1) |
|
| Cholesterol, n
(%) |
|
| 0.673 |
| <5.2
mg/dl | 30 (88.2) | 30 (93.8) |
|
| ≥5.2
mg/dl | 4 (11.8) | 2 (6.2) |
|
| Triglycerides, n
(%) |
|
| 0.151 |
| <1.7
mg/dl | 27 (79.4) | 30 (93.8) |
|
| ≥1.7
mg/dl | 7 (20.6) | 2 (6.3) |
|
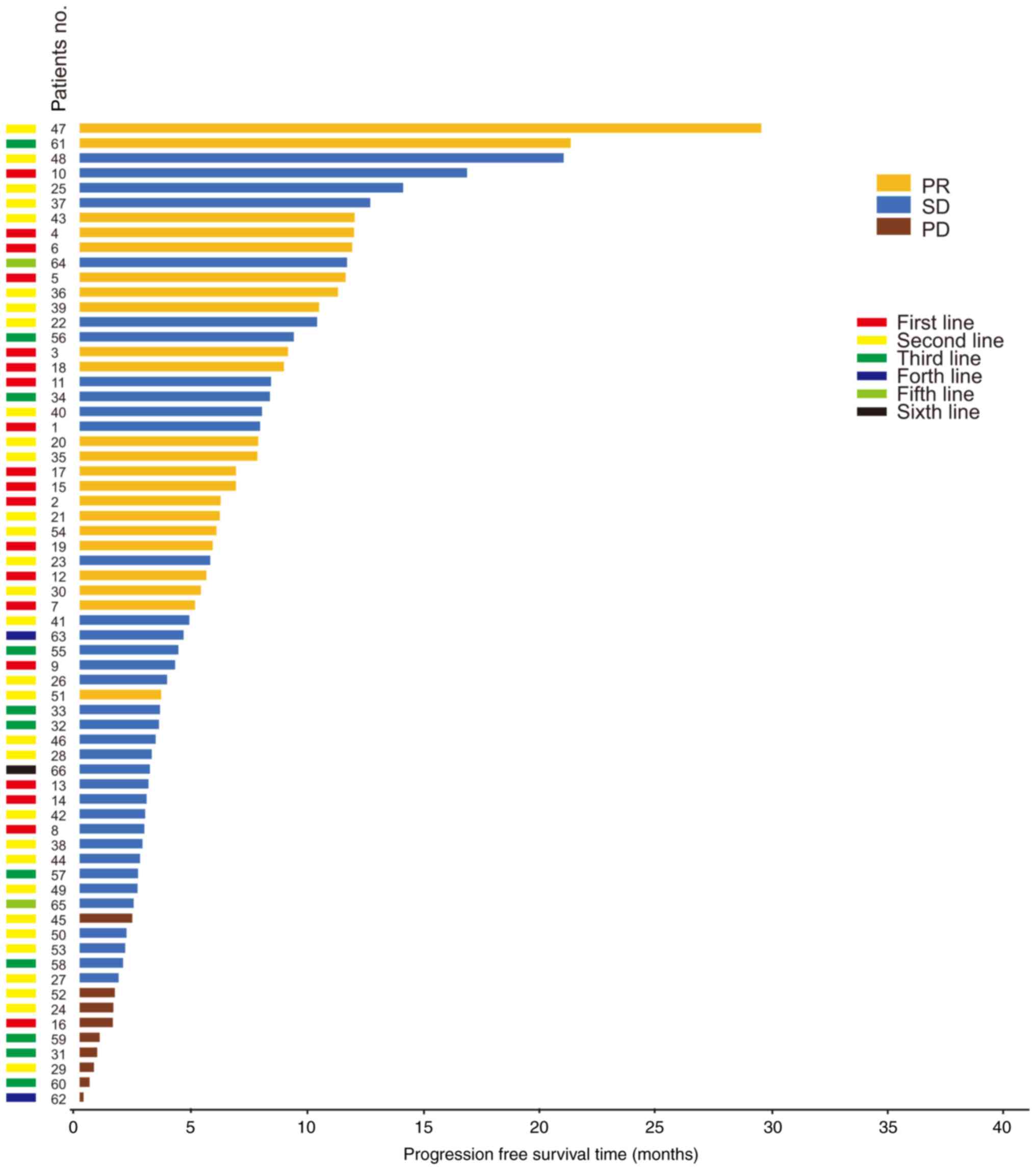
Among the 66 response evaluable patients, 22 (33.3%)
patients achieved a confirmed PR, 35 (53.0%) patients achieved SD
and 9 (13.6%) patients experienced PD. The disease control rate
(DCR) was 86.4% (n=57/66) (Tables
II and V). No significant
differences were found in the DCR between the two groups
(χ2=0.730, P=0.460) (Table V).
 | Table V.Efficacy of different treatment
regimens. |
Table V.
Efficacy of different treatment
regimens.
| Response | Chemo + ICI | Anti-VEGF +
ICI | P-value | ICI alone |
|---|
| Complete response,
n (%) | 0 (0.0) | 0 (0.0) |
| 0 (0.0) |
| Partial response, n
(%) | 14 (41.2) | 8 (25.0) |
| 1 (14.3) |
| Stable disease, n
(%) | 16 (47.0) | 19 (59.4) |
| 4 (57.1) |
| Progressive
disease, n (%) | 4 (11.8) | 5 (15.6) |
| 2 (28.6) |
| DCR, % | 88.2 | 84.4 | 0.460 |
|
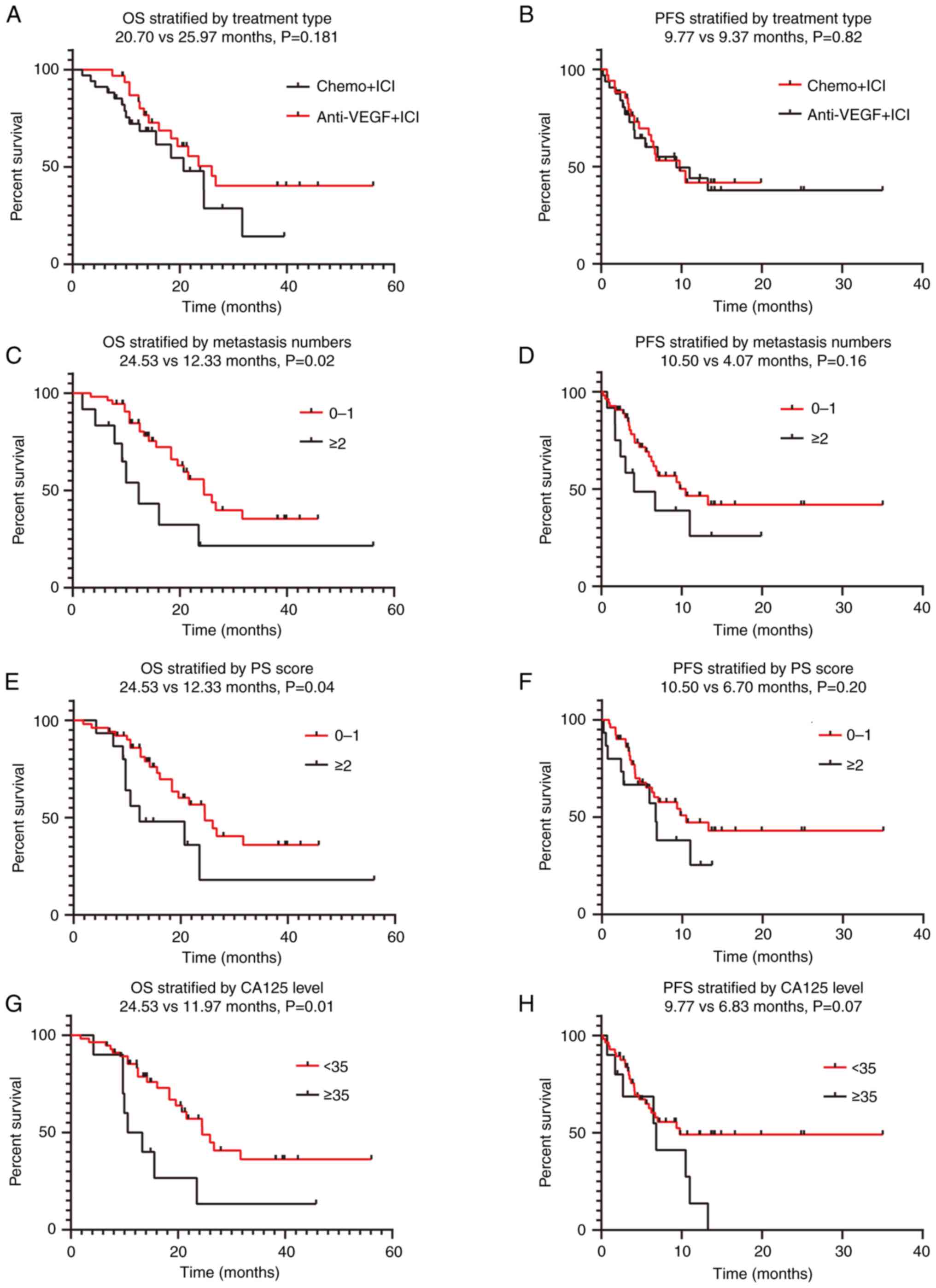
The median follow-up period was 13.7 months in group
C (IQR, 9.25–18.95 months) and 18.97 months in group D (IQR,
12.38–35.33 months). At the end of the study period, 16 patients
(47.1%) in group C and 15 patients (46.9%) in group D had died. The
median OS times were 20.70 months (95% CI, 12.065–29.335) and 25.97
months (95% CI, 19.095–32.839) in groups C and D, respectively
(P=0.181; Fig. 4). The PFS times
were 9.77 months (95% CI, 4.991–14.542) and 9.37 months (95% CI,
2.015–16.718) in groups C and D, respectively (P=0.82; Fig. 4). Immuno-based combination therapy
(group C vs. group D) exhibited no significant association with
regard to mPFS and mOS. Univariate analysis showed that the ECOG PS
score (P=0.045), number of metastatic organs (P=0.02), and CA125
level (P=0.01) exhibited a significant association with regard to
mOS but not mPFS (Fig. 4; Table VI). In addition, age, sex, smoking
status, pathological type (pure LCNEC vs. mixed LCNEC), presence of
brain, liver and bone metastases, and levels of NLR, CEA,
CYFRA21-1, LDH, cholesterol and triglycerides were also not
associated with OS (all P>0.05). However, in the multivariate
Cox regression analysis, no statistical significance was observed
between the CA125 level and OS (P=0.070) (Table VI).
 | Table VI.Univariate and multivariate Cox
regression analyses of overall survival in patients with advanced
LCNEC by treatment regimen. |
Table VI.
Univariate and multivariate Cox
regression analyses of overall survival in patients with advanced
LCNEC by treatment regimen.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameters | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Treatments (chemo +
ICI vs. anti-VEGF) | 0.617
(0.302–1.263) | 0.187 |
|
|
| Age (<67 vs. ≥67
years) | 0.949
(0.468–1.924) | 0.884 |
|
|
| Sex (male vs.
female) | 1.255
(0.437–3.606) | 0.673 |
|
|
| Smoking (yes vs.
no) | 0.738
(0.363–1.500) | 0.401 |
|
|
| ECOG PS (0–1 vs.
≥2) | 2.238
(1.019–4.915) | 0.045a | 1.356
(0.511–3.599) | 0.541 |
| Number of
metastatic organs (0–1 vs. ≥2) | 2.531
(1.125–5.697) | 0.025a | 1.856
(0.692–4.982) | 0.220 |
| Lymph node
metastases (yes vs. no) | 0.558
(0.214–1.456) | 0.233 |
|
|
| Brain metastases
(yes vs. no) | 1.165
(0.520–2.609) | 0.710 |
|
|
| Liver metastases
(yes vs. no) | 0.976
(0.373–2.555) | 0.961 |
|
|
| Bone metastases
(yes vs. no) | 1.411
(0.426–4.671) | 0.573 |
|
|
| Histological
subtype (pure vs. no) | 1.212
(0.584–2.515) | 0.607 |
|
|
| NLR (<5 vs.
≥5) | 1.029
(0.438–2.415) | 0.948 |
|
|
| CEA (<5 vs. ≥5
ng/ml) | 0.845
(0.396–1.801) | 0.662 |
|
|
| NSE (<16.3 vs.
≥16.3 ng/ml) | 1.049
(0.507–2.167) | 0.898 |
|
|
| CA125 (<35 vs.
≥35 U/ml) | 2.699
(1.199–6.075) | 0.016a | 2.207
(0.938–5.191) | 0.070 |
| CYFRA21-1 (<3.3
vs. ≥3.3 ng/ml) | 1.078
(0.518–2.245) | 0.840 |
|
|
| LDH (<240 vs.
≥240 U/l) | 1.214
(0.542–2.718) | 0.637 |
|
|
| Cholesterol
(<5.2 vs. ≥5.2 mg/dl) | 2.744
(0.807–9.328) | 0.106 |
|
|
| Triglycerides
(<1.7 vs. ≥1.7 mg/dl) | 1.396
(0.479–4.067) | 0.541 |
|
|
irAEs
No statistically significant differences were
observed between the development of irAEs and the use of combined
immunotherapy regimen (group C vs. group D, P=0.48). The frequency
of corticosteroid use was significantly higher in group C than that
in group D (P=0.001). The most common irAEs were hypothyroidism
(n=5) and immune-associated pneumonitis (n=3). No statistically
significant differences were observed with regard to irAE grade
(P=0.49) and permanent treatment discontinuation due to irAEs
(P=0.65) between the two groups (Table VII).
 | Table VII.Safety analysis of the total study
population based on advanced large cell neuroendocrine carcinoma of
the lung. |
Table VII.
Safety analysis of the total study
population based on advanced large cell neuroendocrine carcinoma of
the lung.
| Parameter | Chemo + ICI
(n=34) | Anti-VEGF + ICI
(n=32) | P-value | ICI alone
(n=7) |
|---|
| Any irAEs, n
(%) |
|
| 0.48 |
|
| Yes
(n=20) | 7 (35.0) | 9 (45.0) |
| 4 (20.0) |
| No
(n=53) | 27 (50.9) | 23 (43.4) |
| 3 (5.7) |
| irAEs Grade, n
(%) |
|
| 0.49 |
|
| 2
(n=7) | 3 (42.9) | 4 (57.1) |
| 0 (0.0) |
| 3
(n=11) | 3 (27.3) | 5 (45.5) |
| 3 (27.3) |
| 4-5
(n=2) | 1 (50.0) | 0 (0.0) |
| 1 (50.0) |
| Treatment permanent
discontinuation, n (%) |
|
| 0.65 |
|
| Yes
(n=10) | 4 (40.0) | 5 (50.0) |
| 1 (10.0) |
| No
(n=63) | 30 (47.6) | 27 (42.9) |
| 6 (11.1) |
| Systemic
corticosteroid use, n (%) |
|
| 0.001 |
|
| Yes
(n=35) | 24 (68.6) | 9 (25.7) |
| 2 (5.7) |
| No
(n=38) | 10 (26.3) | 23 (60.5) |
| 5 (13.2) |
| irAE type, n
(%) |
|
|
|
|
|
Dermatitis (n=2) | 0 (0.0) | 1 (11.1) |
| 1 (25.0) |
|
Reactive cutaneous capillary
endothelial proliferation (n=2) | 1 (14.3) | 1 (11.1) |
| 0 (0.0) |
|
Hypothyroidism (n=5) | 2 (28.5) | 2 (22.3) |
| 1 (25.0) |
|
Hyperthyroidism (n=1) | 0 (0.0) | 1 (11.1) |
| 0 (0.0) |
|
Autoimmune diabetes mellitus
(n=1) | 1 (14.3) | 0 (0.0) |
| 0 (0.0) |
|
Adrenocortical dysfunction
(n=1) | 0 (0.0) | 1 (11.1) |
| 0 (0.0) |
|
Hypophysitis (n=2) | 1 (14.3) | 1 (11.1) |
| 0 (0.0) |
|
Pneumonitis (n=3) | 1 (14.3) | 1 (11.1) |
| 1 (25.0) |
| Immune
myocarditis (n=2) | 0 (0.0) | 1 (11.1) |
| 1 (25.0) |
|
Anaphylactic shock (n=1) | 1 (14.3) | 0 (0.0) |
| 0 (0.0) |
Discussion
The prognosis of patients with LCNEC is generally
poor, and the 5-year survival rate of those with advanced stage
disease is as low as 8% (28).
Currently, the first-line treatment of metastatic LCNEC is still
controversial, and the chemotherapy regimen for advanced LCNEC
tends to be the same regimen as that for SCLC. Based on the results
of the present study and those of current small-scale and
retrospective studies, the prognosis remains poor, with an mOS time
of 8–12 months (17,29). The combination of immunotherapy with
etoposide-platinum chemotherapy has been identified as the standard
first-line treatment for advanced SCLC (30,31).
However, due to the rarity of LNCEC and the lack of prospective
evidence, the efficacy of immunotherapy in LCNEC has not been
determined. According to the present findings, the mOS of the ICI
group was 23.500 months, which was double that of the non-ICI group
(11.230 months). The results of univariate and multivariate
analyses further supported the positive effect of ICI on the OS
time of patients with advanced LCNEC. The present results are in
line with the previously reported data on LCNEC (21), suggesting that immunotherapy is a
superior treatment and providing valuable insight regarding
possible therapeutic options for advanced LCNEC. For instance, a
recent retrospective study (24)
assessed the efficacy and safety of ICIs in 37 patients with
advanced LCNEC and concluded that patients receiving
mono-immunotherapy or a combination of different ICIs exhibited a
favorable prognosis with an objective response rate of 33%, an mPFS
time of 4.2 months and a mOS time of 11.8 months. In addition, a
real-world study (21) showed that
ICIs had a positive impact on OS, with mOS time of 12.4 months in
patients who received ICIs and 6.0 months in patients who did not.
Agar et al (22) reported
that the mOS time of patients administered nivolumab treatment was
12.1 months (95% CI, 7.10–14.20), indicating that nivolumab as a
second-line treatment or beyond showed a high level of tumor
response and prolonged OS time in patients with advanced LCNEC.
This result is consistent with the present finding that ICI
administration is associated with prolonged OS time in patients
with LCNEC.
In population-based studies, age, sex, primary tumor
size, lymph node metastasis and tumor stage have been identified as
prognostic factors for lung cancer survival (32,33).
Previous studies have shown that LCNEC is highly prevalent in
elderly men with a history of smoking (3). In the present study, the median age of
patients was 67 years, with a predominance of men. Among them,
58.8% had a history of smoking and 100% had stage IV LCNEC at the
time of diagnosis, with distant metastases mainly in the brain,
liver and bones. Serum tumor markers such as CEA, NSE, CA125 and
CYFRA21-1 are important indicators of an auxiliary diagnosis of
lung cancer (34). In the present
study, CYFRA21-1 had the highest positivity rate, followed by NSE,
while the positivity rates of CEA and CA125 were low. Studies have
shown that peripheral blood markers such as NLR and LDH are
high-risk factors for a poor prognosis in patients with NSCLC
(35). In the present study, 61.5
and 66.2% of the patients had elevated NLR and LDH levels,
respectively, but univariate and multivariate analyses showed no
statistical association between these markers and OS. Considering
the presence of differences in baseline and treatment
characteristics favoring patients treated with ICIs in the present
cohort, further analysis after elimination of confounding factors
is required. A recent retrospective study of 251 patients with
LCNEC after surgical resection (36) showed that only lymphatic
infiltration was an independent prognostic factor. The present
study showed a significant association between OS and lymph node
metastasis and the number of metastatic organs. Patients with lymph
node metastasis and >2 metastatic organs had a poor prognosis.
Thus, lymphatic invasion affects surgical efficacy in the early
stage and functions as an independent risk factor of poor prognosis
in patients with advanced LCNEC. In addition, distant metastasis is
an indicator of prognosis.
In clinical practice, the incidence of LCNEC with
other lung cancer subtypes (e.g., SCLC, adenocarcinoma and squamous
cell carcinoma) is ~10% (37,38).
However, the differences in clinical characteristics, prognosis and
treatment between pure LCNEC and mixed LCNEC remain unclear. Zhang
et al (16) showed that the
mOS time of patients with pure LCNEC was significantly greater than
that of patients with combined LCNEC (P=0.083). The present study
explored the difference in prognosis between pure and combined
LCNEC. The results of the univariate and multivariate analyses
showed that the histological type of LCNEC was significantly
associated with the mOS time, and the difference in the mOS time
between patients with pure LCNEC and combined LCNEC was
statistically significant. Therefore, the prognosis of mixed LCNEC
was worse than that of pure LCNEC. However, the retrospective
nature and limited sample size of the present study may impact the
ability to draw a definite conclusion. Nevertheless, the data
indicate that the heterogeneity of pathological components may be
related to prognosis.
Anti-angiogenic agents can regulate the tumor immune
microenvironment and exert synergistic action when combined with
ICIs (38,39), highlighting the potential of the
combined application of antitumor therapy as a new treatment
strategy for advanced LCNEC. In a multicenter, open-label,
single-arm, phase II clinical study of surufatinib in combination
with toripalimab in patients with advanced neuroendocrine carcinoma
(NCT04169672) (40), 21 patients
received combination therapy. Among 20 tumor evaluable patients,
the objective response rate (4 patients achieved confirmed PR and
10 patients achieved SD) and DCR were 20 and 70%, respectively. The
median PFS time was 3.94 months (95% CI, 1.31 to unknown). Based on
these findings, the present study further analyzed the efficacy and
influencing factors of combined chemotherapy and anti-angiogenesis
therapy on the basis of immunotherapy. In the present study, the
difference in the DCR between the two groups was not statistically
significant. Moreover, immuno-based combination therapy
(chemotherapy vs. anti-vascular endothelial growth factor therapy)
was not significantly associated with the mPFS or mOS time. Further
analysis showed that the ECOG PS score, number of metastatic organs
and CA125 level were significantly associated with a poor
prognosis, but not with PFS time. In the multivariate Cox
regression analysis, CA125 level was still significantly associated
with the mOS time. No statistically significant differences were
found with regard to the effects of ICI combination therapy on mOS
time among patients with elevated levels of NLR, CEA, CYFRA21-1,
LDH, cholesterol and triglycerides. Furthermore, patients with
advanced LCNEC in the enrolled cohort with a better ECOG PS score,
fewer metastatic organs and lower CA125 levels had better outcomes
after ICI-based systemic therapy than their counterparts.
Considering that 34 and 32 patients were included in groups C and
D, respectively, in this retrospective study, the influence of the
small sample size and selection bias, and other related factors,
could not be excluded. Therefore, expanding the sample size to
conduct a multi-cohort study is necessary to verify the
findings.
This single-center retrospective study had several
limitations. First, no centralized histological review of the
enrolled patients was present. LCNEC may be difficult to diagnose,
and a small biopsy is usually insufficient; thus, a surgical lung
biopsy is usually required (3,4). In
the present study, the diagnosis of patients depended on findings
from histological examinations, and only some patients underwent
next-generation sequencing to determine the pathological type.
Second, comprehensive molecular tumor characteristic data that
could be applied to most patients were lacking, and the PD-L1
status of some patients was unknown. Therefore, the study did not
analyze the prognostic correlation between PD-L1 expression levels
and ICI treatment, which needs to be analyzed in future studies.
Lastly, other limitations to the present study included its
retrospective design, lack of a central pathology assessment and a
small sample size of patients receiving ICIs. Overall, the results
of the present study suggest that ICIs are a reasonable choice for
the treatment of advanced LCNEC in the absence of other treatment
options, but prospective clinical studies are needed to confirm the
value of ICIs in the treatment of LCNEC.
Acknowledgements
Not applicable.
Funding
This study was funded by the Joint Construction Project of Henan
Province and Ministry (grant no. LHGJ20190013).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NY, SG, SS and XL conceived and designed the study.
Administrative support was provided by NY and XL. SG, ZZ and SS
provided study materials or patients. Collection and assembly of
data was performed by NY and SG. Data analysis and interpretation
was performed by NY, SG, ZZ and SS. NY, SG, SS and XL confirm the
authenticity of all the raw data. All authors helped to write the
manuscript. All authors have read and approved the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the First Affiliated
Hospital of Zhengzhou University approved this study (approval no.
2022-KY-0592-002). The requirement for informed consent was waived
by the Ethics Committee of the First Affiliated Hospital of
Zhengzhou University due to the retrospective design and use of
anonymized patient information. All procedures have been performed
in accordance with the Declaration of Helsinki.
Patient consent for publication
The requirement for informed consent was waived by
the Ethics Committee of the First Affiliated Hospital of Zhengzhou
University (approval no. 2022-KY-0592-002).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Takei H, Asamura H, Maeshima A, Suzuki K,
Kondo H, Niki T, Yamada T, Tsuchiya R and Matsuno Y: Large cell
neuroendocrine carcinoma of the lung: A clinicopathologic study of
eighty-seven cases. J Thorac Cardiovasc Surg. 124:285–292. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iyoda A, Hiroshima K, Toyozaki T, Haga Y,
Fujisawa T and Ohwada H: Clinical characterization of pulmonary
large cell neuroendocrine carcinoma and large cell carcinoma with
neuroendocrine morphology. Cancer. 91:1992–2000. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Corbett V, Arnold S, Anthony L and Chauhan
A: Management of large cell neuroendocrine carcinoma. Front Oncol.
11:6531622021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andrini E, Marchese PV, De Biase D,
Mosconi C, Siepe G, Panzuto F, Ardizzoni A, Campana D and Lamberti
G: Large cell neuroendocrine carcinoma of the lung: Current
understanding and challenges. J Clin Med. 11:14612022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 World Health Organization
classification of lung tumors: Impact of genetic, clinical and
radiologic advances since the 2004 classification. J Thorac Oncol.
10:1243–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrara MG, Stefani A, Simbolo M, Pilotto
S, Martini M, Lococo F, Vita E, Chiappetta M, Cancellieri A,
D'Argento E, et al: Large cell Neuro-Endocrine carcinoma of the
lung: Current treatment options and potential future opportunities.
Front Oncol. 11:6502932021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rekhtman N, Pietanza MC, Hellmann MD,
Naidoo J, Arora A, Won H, Halpenny DF, Wang H, Tian SK, Litvak AM,
et al: Next-Generation sequencing of pulmonary large cell
neuroendocrine carcinoma reveals small cell carcinoma-like and
non-small cell carcinoma-like subsets. Clin Cancer Res.
22:3618–3629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
George J, Walter V, Peifer M, Alexandrov
LB, Seidel D, Leenders F, Maas L, Müller C, Dahmen I, Delhomme TM,
et al: Integrative genomic profiling of large-cell neuroendocrine
carcinomas reveals distinct subtypes of high-grade neuroendocrine
lung tumors. Nat Commun. 9:10482018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhuo M, Guan Y, Yang X, Hong L, Wang Y, Li
Z, Chen R, Abbas HA, Chang L, Gong Y, et al: The prognostic and
therapeutic role of genomic subtyping by sequencing tumor or
cell-free DNA in pulmonary large-cell neuroendocrine carcinoma.
Clin Cancer Res. 26:892–901. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Postmus PE, Kerr KM, Oudkerk M, Senan S,
Waller DA, Vansteenkiste J, Escriu C and Peters S; ESMO Guidelines
Committee, : Early and locally advanced non-small-cell lung cancer
(NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 28 (Suppl_4):iv1–iv21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Welter S, Aigner C and Roesel C: The role
of surgery in high grade neuroendocrine tumours of the lung. J
Thorac Dis. 9 (Suppl 15):S1474–S1483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moon JY, Choi SH, Kim TH, Lee J, Pyo JH,
Kim YT, Lee SJ, Yoon HI, Cho J and Lee CG: Clinical features and
treatment outcomes of resected large cell neuroendocrine carcinoma
of the lung. Radiat Oncol J. 39:288–296. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu J, Gong D, Wang Y, Chi B, Zhang J, Hu S
and Min L: The demographic and treatment options for patients with
large cell neuroendocrine carcinoma of the lung. Cancer Med.
8:2979–2993. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wakeam E, Adibfar A, Stokes S, Leighl NB,
Giuliani ME, Varghese TK Jr and Darling GE: Defining the role of
adjuvant therapy for early-stage large cell neuroendocrine
carcinoma. J Thorac Cardiovasc Surg. 159:2043–2054.e9. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iyoda A, Hiroshima K, Moriya Y, Takiguchi
Y, Sekine Y, Shibuya K, Iizasa T, Kimura H, Nakatani Y and Fujisawa
T: Prospective study of adjuvant chemotherapy for pulmonary large
cell neuroendocrine carcinoma. Ann Thorac Surg. 82:1802–1807. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang JT, Li Y, Yan LX, Zhu ZF, Dong XR,
Chu Q, Wu L, Zhang HM, Xu CW, Lin G, et al: Disparity in clinical
outcomes between pure and combined pulmonary large-cell
neuroendocrine carcinoma: A multi-center retrospective study. Lung
Cancer. 139:118–123. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Le Treut J, Sault MC, Lena H, Souquet PJ,
Vergnenegre A, Le Caer H, Berard H, Boffa S, Monnet I, Damotte D
and Chouaid C: Multicentre phase II study of Cisplatin-Etoposide
chemotherapy for advanced large-cell neuroendocrine lung carcinoma:
the GFPC 0302 study. Ann Oncol. 24:1548–1552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rossi G, Cavazza A, Marchioni A, Longo L,
Migaldi M, Sartori G, Bigiani N, Schirosi L, Casali C, Morandi U,
et al: Role of chemotherapy and the receptor tyrosine kinases KIT,
PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine
carcinoma of the lung. J Clin Oncol. 23:8774–8785. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanna NH, Robinson AG, Temin S, Baker S,
Brahmer JR, Ellis PM, Gaspar LE, Haddad RY, Hesketh PJ, Jain D, et
al: Therapy for Stage IV non-small-cell lung cancer with driver
alterations: ASCO and OH (CCO) joint guideline update. J Clin
Oncol. 39:1040–1091. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dudnik E, Kareff S, Moskovitz M, Kim C,
Liu SV, Lobachov A, Gottfried T, Urban D, Zer A, Rotem O, et al:
Real-world survival outcomes with immune checkpoint inhibitors in
large-cell neuroendocrine tumors of lung. J Immunother Cancer.
9:e0019992021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Agar C, Geier M, Léveiller G, Lamy R,
Bizec JL, Tiercin M, Bernier C, Robinet G, Léna H, Ricordel C and
Corre R: Brief report on the efficacy of nivolumab in patients with
previously treated advanced large-cell neuroendocrine cancer of the
lung. JTO Clin Res Rep. 2:1001292021.PubMed/NCBI
|
|
23
|
Chauhan A, Arnold SM, Kolesar J, Thomas
HE, Evers M and Anthony L: Immune checkpoint inhibitors in large
cell neuroendocrine carcinoma: Current status. Oncotarget.
9:14738–14740. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sherman S, Rotem O, Shochat T, Zer A,
Moore A and Dudnik E: Efficacy of immune check-point inhibitors
(ICPi) in large cell neuroendocrine tumors of lung (LCNEC). Lung
Cancer. 143:40–46. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yates JW, Chalmer B and McKegney FP:
Evaluation of patients with advanced cancer using the Karnofsky
performance status. Cancer. 45:2220–2224. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Common Terminology Criteria for Adverse
Events (CTCAE) v5.0, . 2017.https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11
|
|
28
|
Lowczak A, Kolasinska-Cwikla A, Osowiecka
K, Glinka L, Palucki J, Rzepko R, Doboszynska A and Cwikla JB:
Outcomes of patients with pulmonary large cell neuroendocrine
carcinoma in I–IV stage. Medicina (Kaunas). 57:1182021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fujiwara Y, Sekine I, Tsuta K, Ohe Y,
Kunitoh H, Yamamoto N, Nokihara H, Yamada K and Tamura T: Effect of
platinum combined with irinotecan or paclitaxel against large cell
neuroendocrine carcinoma of the lung. Jpn J Clin Oncol. 37:482–486.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Horn L, Mansfield AS, Szczesna A, Havel L,
Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio
M, et al: First-line atezolizumab plus chemotherapy in
extensive-stage small-cell lung cancer. N Engl J Med.
379:2220–2229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goldman JW, Dvorkin M, Chen Y, Reinmuth N,
Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH,
et al: Durvalumab, with or without tremelimumab, plus
platinum-etoposide versus platinum-etoposide alone in first-line
treatment of extensive-stage small-cell lung cancer (CASPIAN):
Updated results from a randomised, controlled, open-label, phase 3
trial. Lancet Oncol. 22:51–65. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Q, Xu Z, Chen X, Zheng L, Yu Y, Zhao
X, Chen M, Luo B, Wang J and Sun J: Clinicopathological
characteristics and prognostic factors of pulmonary large cell
neuroendocrine carcinoma: A large population-based analysis. Thorac
Cancer. 10:751–760. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He Y, Liu H, Wang S and Chen Y: Prognostic
nomogram predicts overall survival in pulmonary large cell
neuroendocrine carcinoma. PLoS One. 14:e02232752019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen ZQ, Huang LS and Zhu B: Assessment of
seven clinical tumor markers in diagnosis of non-small-cell lung
cancer. Dis Markers. 2018:98451232018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peng L, Wang Y, Liu F, Qiu X, Zhang X,
Fang C, Qian X and Li Y: Peripheral blood markers predictive of
outcome and immune-related adverse events in advanced non-small
cell lung cancer treated with PD-1 inhibitors. Cancer Immunol
Immunother. 69:1813–1822. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roesel C, Welter S, Kambartel KO,
Weinreich G, Krbek T, Serke M, Ibrahim M, Alnajdawi Y, Plönes T and
Aigner C: Prognostic markers in resected large cell neuroendocrine
carcinoma: A multicentre retrospective analysis. J Thorac Dis.
12:466–476. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyoshi T, Umemura S, Matsumura Y, Mimaki
S, Tada S, Makinoshima H, Ishii G, Udagawa H, Matsumoto S, Yoh K,
et al: Genomic profiling of large-cell neuroendocrine carcinoma of
the lung. Clin Cancer Res. 23:757–765. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liang H and Wang M: Prospect of
immunotherapy combined with anti-angiogenic agents in patients with
advanced non-small cell lung cancer. Cancer Manag Res.
11:7707–7719. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yi M, Jiao D, Qin S, Chu Q, Wu K and Li A:
Synergistic effect of immune checkpoint blockade and
anti-angiogenesis in cancer treatment. Mol Cancer. 18:602019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shen L, Yu X, Lu M, Zhang X, Cheng Y,
Zhang Y, Li Z, Xu J, Weng D, Wu C, et al: Surufatinib in
combination with toripalimab in patients with advanced
neuroendocrine carcinoma: Results from a multicenter, open-label,
single-arm, phase II trial. J Clin Oncol. 39:e161992021. View Article : Google Scholar
|