Introduction
Globally, pancreatic cancer is ranked seventh in
terms of causing cancer-related fatalities, resulting in 432,242
deaths in 2018 (1). The majority of
patients with pancreatic cancer are diagnosed at an advanced stage
or found to have an unresectable tumor (2,3), and
this is the primary contributor to a decrease in survival rate of
<10% (4,5). Although survival rates have improved
only slightly since advances in medical treatment, the morbidity of
pancreatic cancer continues to increase (2).
Tumor-associated pain is a highly prevalent symptom
in individuals diagnosed with pancreatic cancer that manifests in
>80% of cases (6). Despite the
utilization of various palliative chemotherapy regimens and
standard palliative therapies, nearly 75% of patients with locally
advanced disease experience significant cancer-related abdominal or
back pain, significantly diminishing their quality of life. Given
the unfavorable prognosis of these patients and as a significant
number of patients are unwilling or unable to tolerate
chemotherapy, the primary objective of therapy for pancreatic
cancer in advanced stages is to optimize the quality of life and
enhance overall survival (OS) (7).
Several minimally invasive local treatments have
been used to reduce pain and improve survival rate in patients
diagnosed with unresectable pancreatic cancer. High-intensity
focused ultrasound (HIFU) is a non-invasive, repeatable thermal
ablation technique (8). HIFU aims
to deliver the energy required to raise and maintain the focal
region above 60°C, in order to cause coagulative necrosis and
immediate cell death without affecting surrounding organs through
heat effect, cavitation or potential immunological effects
(9–11). In addition to achieving local tumor
control and alleviating tumor-associated pain, repeated HIFU has
the potential to positively influence both progression-free
survival and OS in patients (7,12,13).
Radioactive iodine-125 (125I) seed implantation is a
commonly employed treatment option for malignant tumors due to its
effectiveness, minimal invasiveness and low risk of complications
(14,15). Compared with external radiotherapy,
it has the advantages of a short treatment time; continuous
radiotherapy, which can irradiate the tumor cells of different
division cycles continuously, improving the radiosensitivity and
producing a high radiobiological effect; and repeatability, as well
as a low incidence of radiation-related adverse reactions (14). In a previous study where patients
underwent systemic chemotherapy, thermal therapy, molecular target
therapy, immune treatment, gene treatment, Chinese medicine
treatment, radiation therapy including iodine-125 seed interstitial
brachytherapy, nutrition support or symptomatic treatment, the
clinical benefit rate of the seed implantation group was 92.3%,
which was significantly greater than that of the control group
(41.7%; P<0.01) in patients with unresectable pancreatic cancer.
The clinical evaluation indicators included pain relief,
consumption of analgesic drugs, physical condition [Karnofsky
Performance Scale (KPS) score] (16) and body weight in this study
(17).
Previous reports have confirmed the efficacy of HIFU
combined with external radiotherapy in treating pancreatic cancer
(18,19). A decrease in blood flow caused by
HIFU ablation may impede heat dissipation, resulting in damage to
tumor cells due to hypoxia and enhancing the efficacy of
radiotherapy (19). Radiotherapy
has been proven to be effective at targeting oxygen-rich cells,
while hyperthermia has also been shown to be effective at treating
hypoxic cells (20,21). Therefore, we hypothesized that the
combination of repeated HIFU treatment and radioactive
125I seed implantation may further improve the clinical
outcome, as the curative effect of the existing treatments is
generally poor for treating advanced pancreatic cancer. A
retrospective case series analysis was conducted to assess the
safety and feasibility of the combination of these two minimally
invasive treatments for treating patients with advanced pancreatic
cancer.
Patients and methods
Research type
This research was conducted as a single-center,
non-controlled and non-blinded retrospective study.
Patients
A total of 52 patients diagnosed with advanced
pancreatic cancer between March 31, 2015, and March 31, 2021, were
included in the present study. The date of last follow-up was
August 31, 2021. Patients were enrolled according to the following
inclusion criteria: i) An age of ≥18 years; ii) histologically or
cytologically diagnosed pancreatic carcinoma [Union for
International Cancer Control (UICC stages III and IV] (22); iii) KPS score ≥50, and an expected
survival time of >3 months; iv) sufficiently visible tumor on
ultrasound (diameter ≥2 and ≤8 cm); v) platelet count
≥75×109/l (normal reference range,
125–350×109/l) and prothrombin time (PT) that is normal
or prolonged by <3 seconds (normal reference range, 9.4–12.5
sec) (23) vi) ineligible for or
refused to undergo surgery and unwilling or unable to tolerate
chemotherapy; and vii) receipt of more than one HIFU treatment
combined with radioactive 125I seed implantation in the
pancreatic lesions. The exclusion criteria were as follows: i)
Underwent surgery, chemotherapy or external radiation; ii) there
was no suitable path for percutaneous pancreatic puncture; iii)
serious cardiac and cerebrovascular events; iv) unable to cooperate
with efficacy evaluation and treatment; and v) there were severe
safety problems, obvious effects or lack of effects that required
termination of treatment. Study approval was obtained from The
Ethics Committee of Huadong Hospital Affiliated to Fudan University
(approval no. 2021K040; Shanghai, China), and oral consent for the
publication of clinical data was obtained from all patients.
HIFU instrument and therapy
HIFU was conducted using a HIFUNIT-9000 system
(Shanghai A&S Science Technology Development Co., Ltd.). This
system is an ultrasound-guided device equipped with an overhead
treatment probe (24). No
anesthesia was required for HIFU treatment. The main parameters of
the HIFU equipment used were as follows: Sound intensity, 5–10
kW/cm2; power, 60–100%; therapy depth, 2–15 cm; and
focal spot, 3×3×8 mm. The number of transducers was 3 to 6, which
is consistent with number of acoustic irradiations of a single
focal spot (8–16 times). The ratio of the unit launch time to the
intermission time was 1:2. The position of the pancreatic lesions
was initially determined using ultrasound, computed tomography
(CT), magnetic resonance imaging and/or positron emission
tomography/CT. Real-time ultrasound was utilized to precisely
identify and target the pancreatic tumors via the use of an
integrated probe. The focus of the ablation energy was carefully
controlled to move sequentially along the X, Y and Z axes until the
target lesion was fully encompassed from point to surface and
ultimately throughout the entire body. Each session lasted for
40–50 min and occurred once per day for a total of five sessions in
a course. If a tumor with a larger volume could not be completely
scanned within the initial five sessions, additional treatment were
administered until the predetermined target area was fully covered.
Patients were instructed to avoid milk or other aerogenic food and
to fast for 6–8 h before HIFU therapy. The interval between the two
treatment courses was at least 4 weeks.
Ultrasound-guided seed
implantation
CT scans were obtained 1–2 weeks prior to seed
implantation to assess the exact location and volume of the tumors.
The dose distribution was subsequently calculated via tumor
brachytherapy via the Seeds Implanted & 3D Planning System
(TPS; Beijing Feitian Zhaoye Technology Co., Ltd.). The
125I seeds, manufactured by Shanghai Xinke
Pharmaceutical Co., Ltd., were made from silver rods that absorbed
125I. These seeds were then enclosed in a titanium
capsule, which was welded using laser technology. Each seed had a
diameter of 0.8 mm and a length of 4.5 mm. The wall of the titanium
capsule had a thickness of 0.05 mm. The 125I seeds
produce γ rays with two energy levels: 5% at 35 keV and 95% at 28
keV. These materials have a half-life of 59.6 days, a half-value
thickness of 0.025 mm of lead, a penetration depth of 17 mm, an
incipient rate of 7 cGy/h and activities ranging from 0.4 to 0.8
mCi. The procedure was performed percutaneously under the guidance
of ultrasound and local infiltration anesthesia. Under real-time
ultrasound guidance, 18-gauge needles were inserted into the tumor
mass at intervals of 1.0 cm in a parallel array. The needles
extended at least 0.5–1.0 cm beyond the margins of the pancreatic
lesions. Using a specialized applicator (Syncor Pharmaceutical,
Ltd.), 125I seeds were carefully implanted after needle
insertion, with a spacing of 1 cm between seeds within the same
needle (25). Given the impact of
fibrosis resulting from hyperthermia on radiation effects, HIFU
should be performed either after or concurrently with radiotherapy
(26). However, patients who suffer
from pain often prioritize treatments involving less trauma in the
real world. Therefore, HIFU treatment preceded seed implantation
therapy in the patients in the present study. Patients were
instructed to avoid milk or other aerogenic food, and to fast for
6–8 h. Oral laxatives were additionally taken for bowel preparation
before radioactive particle implantation. The interval between HIFU
treatment and 125I seed implantation was 1–4 weeks.
Patient follow-up and evaluation
OS was defined as the period spanning from the date
of the pathologically confirmed diagnosis until either the date of
the last follow-up or the date of death. Survival was selected as
the primary endpoint, and physical status and pain as the secondary
endpoints. Patient follow-up occurred at 1 and 2 months after
125I seed implantation, and then follow-up was
subsequently conducted every 3 months to obtain survival and
related adverse reaction data. Censoring took place if patients
were still alive at the last follow-up or if they died due to other
causes. The patients' medical history, physical examinations,
laboratory examinations, pain responses, performance status scores
and tumor imaging prior to treatment were collected, as well as the
results at the 1- and 2-month intervals after 125I seed
implantation. KPS scores ranging from 0 to 100 were utilized. The
pain response was assessed using a numeric rating scale (NRS)
(27) ranging from 0 to 10, where 0
represented no pain and 10 represented unbearable pain, with scores
of 1–3 indicating mild pain, scores of 4–6 indicating moderate pain
and scores of 7–10 indicating severe pain. The laboratory tests
included a complete urinalysis, blood analysis, amylase and CA19-9
levels, serum chemistry, electrocardiogram and chest X-ray. Tumor
imaging involved the use of B-mode ultrasound and CT.
Safety assessment
Adverse events commonly associated with HIFU and
125I seed implantation were recorded, and the severity
of adverse events was graded according to the Common Terminology
Criteria for Adverse Events, version 4 (28). The occurrence of adverse events
associated with HIFU, such as burns, fever, pancreatitis, abdominal
pain, hemorrhage, jaundice, intestinal necrosis and
gastrointestinal perforation, as documented in previous studies
(12,18), was monitored throughout the entire
follow-up period. Additionally, after the implantation of
125I seeds, the occurrence of fever, upper
gastrointestinal bleeding or perforation, pancreatitis, pancreatic
fistula, radiation enteritis and cholangiolitis was monitored.
Statistical analysis
All the statistical analyses were conducted using
SPSS (version 24; IBM Corp.). The median, mean, range, standard
deviation (SD) and exact 95% confidence intervals (CIs) were
calculated. Non-normally distributed data are expressed as medians
(ranges). Normally distributed data are presented as the mean ±
standard deviation. Categorical variables were compared using
χ2 analysis or Fisher's exact test, and exact 95% CIs
were computed. A variance test confirmed that the overall variance
of KPS and NRS scores at the three time points was homogeneous,
with P-values of 0.578 and 0.637, respectively. ANOVA and Dunnett's
test were used to make a multiple comparisons of KPS and NRS scores
before and after HIFU combined with 125I seed
implantation therapy. Survival time was evaluated using the
Kaplan-Meier method. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
A retrospective evaluation was conducted on 52
patients, for whom the median age was 66 years. The tumors were
mainly located in the head of the pancreas (44.2%). Among the 52
patients, the mean diameter was 4.3±1.0 cm. The pathological type
was confirmed in all patients via percutaneous pancreatic puncture
or endoscopic ultrasonography-guided fine-needle aspiration.
Clinical stage IV pancreatic cancer accounted for 65.4% of the
cases according to the UICC. The number of HIFU treatment courses
patients received ranged from 2–6 times with a median course number
of 3.2 times. All patients successfully underwent 125I
seed implantation. The detailed characteristics of the patients are
outlined in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Value |
|---|
| Sex, n (%) |
|
|
Male | 34 (65.4) |
|
Female | 18 (34.6) |
| Mean age (range),
years | 66.0±10.1
(46–87) |
| Tumor location, n
(%) |
|
|
Head | 23 (44.2) |
|
Neck | 4 (7.7) |
|
Body | 5 (9.6) |
|
Body-tail | 16 (30.8) |
|
Tail | 4 (7.7) |
| UICC stage, n
(%) |
|
|
III | 18 (34.6) |
| IV | 34 (65.4) |
| Mean tumor size
(range) |
|
|
Diameter, cm | 4.3±1.0
(2.1–7.1) |
| Volume,
cm3 | 51.2±43.8
(6.8–266) |
| Pathological type,
n (%) |
|
| Ductal
adenocarcinoma | 38 (73.1) |
|
Adenocarcinoma | 10 (19.2) |
|
Adenosquamous carcinoma | 4 (7.7) |
| Mean no. of HIFU
courses (range) | 3.2±1.6 (2–6) |
| Iodine-125
seeds |
|
|
Intensity (range), mCi | 0.55±0.06
(0.4–0.7) |
| Mean
number (range) | 36.2±11.1
(13–70) |
| Mean
dose (range), mCi | 21.1±7.6
(4.8–42) |
Clinical response evaluation
ANOVA test results of KPS and NRS scores both
revealed significant differences among the three time points
(Tables II and III). The mean KPS score of the patients
was 62.7±6.3 (95% CI, 61.3–70.6) at baseline, 73.7±7.9 (95% CI,
68.8–80.2; P<0.001) at 1 month and 68.8±6.5 (95% CI, 64.7–72.5;
P<0.001) at 2 months after combined treatment. KPS score
significantly increased following combined therapy (P<0.001), as
evidenced by improvements in sleep time, nutritional status and
functional level. Thus, the overall quality of life improved. A
total of 3 patients had a KPS score of 90 at 1 month after combined
therapy.
 | Table II.Comparison of KPS score before and
after HIFU combined with 125I seed implantation
therapy. |
Table II.
Comparison of KPS score before and
after HIFU combined with 125I seed implantation
therapy.
|
| KPS score |
|
|
|
|---|
|
|
|
|
P-value(ANOVA)a |
P-value(Dunnett's) |
|---|
| Groups | 90, n (%) | 80, n (%) | 70, n (%) | 60, n (%) | 50, n (%) | Mean ± SD | 95% CI |
|---|
| Pre-therapy | 0 (0.0) | 2 (3.8) | 13 (25.0) | 34 (65.4) | 3 (5.8) | 62.7±6.3 | 61.3–70.6 | P<0.001 | - |
| 1-month
post-therapy | 3 (5.8) | 20 (38.5) | 22 (42.3) | 7 (13.5) | 0 (0.0) | 73.7±7.9 | 68.8–80.2 | - |
P<0.001b |
| 2-months
post-therapy | 0 (0.0) | 8 (15.4) | 30 (57.7) | 14 (26.9) | 0 (0.0) | 68.8±6.5 | 64.7–72.5 | - |
P<0.001c |
 | Table III.Comparison of NRS score before and
after HIFU combined with 125I seed implantation
therapy. |
Table III.
Comparison of NRS score before and
after HIFU combined with 125I seed implantation
therapy.
|
| NRS score |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Groups | No pain scoring 0,
n (%) | Mild pain scoring
1–3, n (%) | Moderate pain
scoring 4–6, n (%) | Severe pain scoring
7–10, n (%) | Mean ± SD | 95% CI | P-value
(ANOVA)a | P-value
(Dunnett's) |
|---|
| Pre-therapy | 0 | 2 (3.8) | 16 (30.8) | 34 (65.4) | 6.7±1.6 | 5.2–6.8 | P<0.001 | - |
| 1-month
post-therapy | 0 | 12 (23.1) | 30 (57.7) | 10 (19.2) | 4.7±1.7 | 3.4–5.5 | - |
P<0.001b |
| 2-months
post-therapy | 0 | 6 (11.5) | 30 (57.7) | 16 (30.8) | 5.4±1.5 | 4.1–5.9 | - |
P<0.001c |
The mean NRS pain assessment score was 6.7±1.6 (95%
CI, 5.2–6.8) at baseline, and 4.7±1.7 (95% CI, 3.4–5.5; P<0.001)
and 5.4±1.5 (95% CI, 4.1–5.9; P<0.001) at 1 and 2 months after
combined treatment, respectively. The number of patients with
severe pain and the recorded NRS score were both significantly
lower at 1 and 2 months after 125I seed implantation
than at baseline (P<0.001). The KPS and NRS scores are shown in
Tables II and III.
Survival
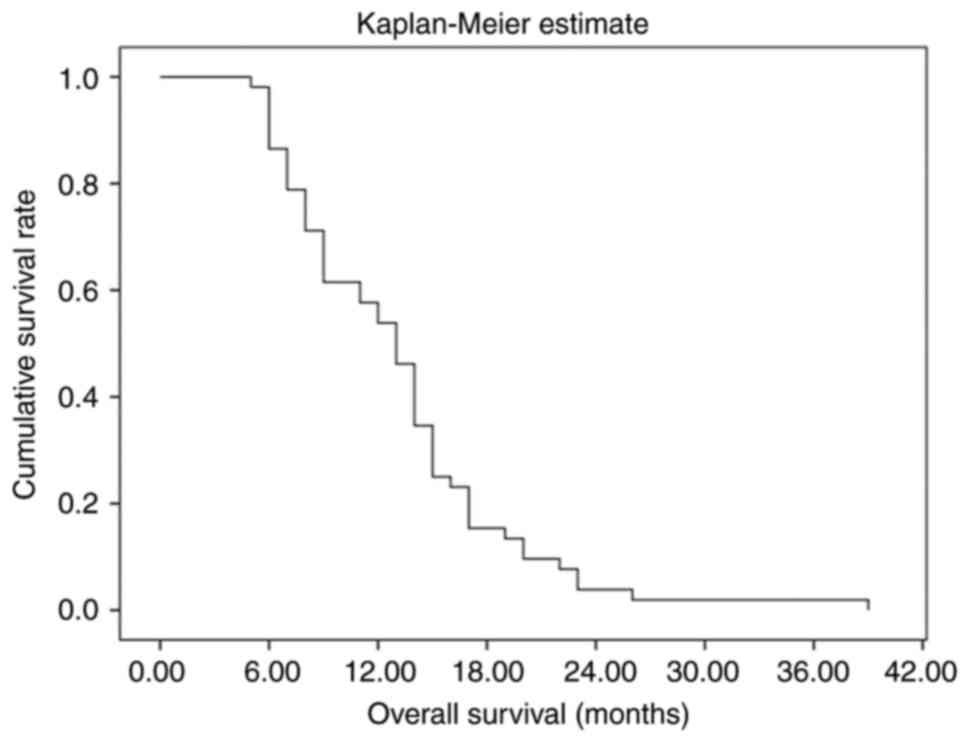
The median OS (mOS) time of patients in the present
study was estimated to be 13.1 months (95% CI, 11.3–14.8). The
longest survival time was 39 months. The 3-, 6-, 9- and 12 month
survival rates were 100.0, 86.5, 61.5 and 53.8%, respectively. In
addition, the 2-year survival rate was 3.8% (Fig. 1).
Adverse events
Mild fever occurred in 2 (3.8%) cases and 1 (1.9%)
case after HIFU treatment and 125I seed implantation,
respectively. The patients were subjected to physical cooling and
recovered 1–25 days later. After HIFU treatment and 125I
seed implantation, mild symptoms of gastrointestinal dysfunction,
such as abdominal distension and loss of appetite, were observed in
1 (1.9%) and 2 (3.8%) patients, respectively, and normality was
reached in ~1 week. Abdomen and waist pain occurred in 3 (5.8%)
patients after 125I seed implantation, of which 2 cases
were mild and 1 was moderate, and gradually subsided within 1 week
when they received non-steroidal pain killers. The serum amylase
level increased mildly in 2 (3.8%) patients 1 day after
125I seed implantation, and these patients recovered
within 1 week. No serious complications, such as superficial skin
or subcutaneous tissue injury, upper gastrointestinal tract
bleeding, infection, severe pancreatitis, radiation enteritis,
pancreatic fistula or gastrointestinal tract perforation, were
detected during the follow-up period.
Discussion
Patients with pancreatic cancer are mostly diagnosed
with advanced or unresectable disease, and existing chemotherapy,
radiation therapy, targeted therapy or even immunotherapy are
unsatisfactory and do not significantly improve their quality of
life (1,4). Treatment for patients with advanced
pancreatic cancer should aim to improve quality of life, prolong
survival, avoid toxic or traumatic treatment, and achieve the goal
of tumor control as much as possible (29,30).
HIFU and 125I seed implantation are typical examples of
these methods. The mOS time of patients in the present study was
estimated to be 13.1 months. The 3-, 6-, 9- and 12-month survival
rates were 100.0, 86.5, 61.5 and 53.8%, respectively. These
findings are encouraging, as they demonstrate improved survival
compared with findings of previous studies (31–38) on
HIFU treatment and iodine-125 seed interstitial brachytherapy. In
addition to achieving local tumor control and alleviating
tumor-associated pain, HIFU can significantly impact both
progression-free survival and OS in patients (31). Studies conducted in Asia have
reported a mOS time ranging from 6 to 11 months and a median
progression-free period lasting 5 to 8.4 months in patients with
pancreatic cancer receiving HIFU treatment (32–34).
125I seed implantation has garnered significant
attention due to its potential to increase the radiation dosage
applied to pancreatic tumors while minimizing harm to adjacent
organs (35). For patients with
pancreatic cancer at more than stage III undergoing 125I
seed implantation, the mOS time was 12.8 months, for a total
effective rate of 91%, both of which are superior to traditional
therapy (36). The patients in the
present cohort were mostly diagnosed with stage IV disease, with an
mOS of 13.1 months. A retrospective study indicated that patients
who received a combination of HIFU and gemcitabine experienced the
greatest improvement in survival, with an mOS time of 7.4 months
(37). Li et al (38) conducted a study that demonstrated a
significant increase in the 1-year survival rate for patients who
underwent chemotherapy in combination with 125I seed
implantation than in those who did not undergo chemotherapy (60.7%
vs. 35.9%; P=0.034). The present study observed that the 1-year
survival rate was 53.8% after repeated HIFU treatment combined with
125I seed implantation, which is similar to or even
better than the previously reported data. The improvement in the
survival rate may be related to the thermal effect of HIFU
(18). Radiotherapy has been proven
to be effective at targeting oxygen-rich cells, while hyperthermia
has also been shown to be effective at treating hypoxic cells
(20,21).
Tumor-related pain is a highly prevalent symptom in
patients diagnosed with pancreatic cancer and occurs in >80% of
cases (6). HIFU and 125I
seed implantation can significantly reduce cancer-related pain, and
they may serve as complementary or even alternative approaches to
opioid and plexus neurolysis (39–41).
In one study, the NRS scores of 37 patients diagnosed with
pancreatic cancer who successfully underwent 125I seed
implantation were significantly lower than the preoperative scores
after 1 week, 1 month and 2 months of implantation (P<0.05)
(15). In the present patient
cohort, the number of patients with severe pain and the NRS score
were both significantly lower at 1 and 2 months after
125I seed implantation than at baseline (P<0.001).
The mechanical effect of HIFU appears to induce neuromodulation and
alleviate pain by temporarily blocking nerve activity (42). Tumor cavitation leads to coagulation
necrosis, causing damage or apoptosis of pain fibers innervating
the tumor (43,44). KPS scores improved significantly
after combined therapy, as evidenced by fewer complications and
improvements in nutritional status, sleep duration and functional
level, leading to an overall enhancement in quality of life
(45). The score mainly benefits
from good pain control and tumor-growth control.
According to the aim of the study, the score of the
European Organization for Research and Treatment of Cancer Quality
of Life Questionnaire-C30 scale (EORTC QLQ-C30) may be better than
the KPS score for evaluating the quality of life, which was one of
the limitations of the present study. This is because the EORTC
QLQ-C30 scale has higher reliability that assesses 30 factors
including physical function, pain, fatigue, appetite and sleep
disorders.
As HIFU treatment is non-invasive, has few adverse
reactions and is well tolerated by patients, it can be repeated
(8). When the therapeutic effect is
stable disease or progressive disease, the interval between
repeated HIFU treatments for the same site should be >4 weeks,
and repeated HIFU treatment is performed every 1–3 months in
general (13). There is no uniform
standard for the specific time interval. Each patient in the
present cohort was treated with repeated HIFU treatment. The HIFU
treatment was administered to patients 2–6 times for pancreatic
lesions, for a median duration of 3.2 cycles. Ning et al
(46) demonstrated the benefit to
survival time of repeated HIFU operations without a significant
increase in the incidence of side effects. In the study, incomplete
HIFU ablation was common in most patients, and repeated HIFU
operations were conducted for the patients who were able to pass
through HIFU well and were in good economic condition. A recent
study (47) indicated that each
patient underwent a minimum of two cycles of HIFU ablation, with a
1-month interval between each treatment. The response rate to HIFU
ablation was 79.4%. Prolonged survival was associated with an
Eastern Cooperative Oncology Group performance status (48) and subsequent HIFU ablation. No
studies have reported the limitations of the number of HIFU
courses. The current study presented the experience of one center,
but the specific treatment intervals and treatment courses need to
be explored in future large sample and multicenter prospective
studies. As particle implantation therapy is invasive, the damage
from puncture requires time to heal (generally ~1 week), and there
is an interval between the puncture and observation of the combined
therapy; thus, the interval between HIFU and particle implantation
therapy is generally 1–4 weeks. Since combination therapy
comprising repeated HIFU and 125I particle implantation
has rarely been reported, this is only the clinical experience of
the High-Intensity Focused Ultrasound Center of Oncology
Department, Huadong Hospital Affiliated to Fudan University and it
may need to be verified by further studies in the future.
In conclusion, repeated HIFU treatment combined with
125I seed interstitial brachytherapy is effective and
safe. Compared with existing treatment strategies, the benefits for
patients are similar or even better. However, few similar studies
have reported on this combination treatment. The outcomes of this
study are highly encouraging for patients who are unwilling or
unable to tolerate surgery or chemotherapy, despite the small
sample size, lack of controls and potential statistical bias. The
limitations of this study also include the absence of stratified
analysis and prognostic factor analysis. Large, prospective and
multicenter randomized clinical trials are needed to assess the
long-term efficacy of these treatments and determine the
appropriate treatment intervals.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL proposed the study concept, designed the study,
participated in the data collection, interpreted the data and
drafted the manuscript. YZ participated in the data collection. YJ
participated in the data analysis and interpreted the data. JZ
participated in the data analysis. LZ revised the grammatical
problems in the draft, refined the study concept and analyzed
imaging data. ZB was involved in analyzing and interpreting the
data and also revised the manuscript critically for important
intellectual content. HZ refined the study concept and designed the
study, and revised the manuscript critically for important
intellectual content. YL and HZ confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Huadong Hospital Affiliated
to Fudan University (Shanghai, China) approved the present study
(approval no. 2021K040).
Patient consent for publication
Oral consent for publication of clinical data was
obtained from all patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lv W, Yan T, Wang G, Zhao W, Zhang T and
Zhou D: High-intensity focused ultrasound therapy in combination
with gemcitabine for unresectable pancreatic carcinoma. Ther Clin
Risk Manag. 12:687–691. 2016.PubMed/NCBI
|
|
4
|
Khalaf N, El-Serag HB, Abrams HR and
Thrift AP: Burden of pancreatic cancer: From epidemiology to
practice. Clin Gastroenterol Hepatol. 19:876–884. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seufferlein T, Porzner M, Heinemann V,
Tannapfel A, Stuschke M and Uhl W: Ductal pancreatic
adenocarcinoma. Dtsch Arztebl Int. 111:396–402. 2014.PubMed/NCBI
|
|
7
|
Dababou S, Marrocchio C, Rosenberg J,
Bitton R, Pauly KB, Napoli A, Hwang JH and Ghanouni P: A
meta-analysis of palliative treatment of pancreatic cancer with
high intensity focused ultrasound. J Ther Ultrasound. 5:92017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sofuni A, Asai Y, Mukai S, Yamamoto K and
Itoi T: High-intensity focused ultrasound therapy for pancreatic
cancer. J Med Ultrason (2001). May 12–2022.(Epub ahead of print).
View Article : Google Scholar
|
|
9
|
Sapareto SA and Dewey WC: Thermal dose
determination in cancer therapy. Int J Radiat Oncol Biol Phys.
10:787–800. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dewhirst MW, Viglianti BL, Lora-Michiels
M, Hanson M and Hoopes PJ: Basic principles of thermal dosimetry
and thermal thresholds for tissue damage from hyperthermia. Int J
Hyperthermia. 19:267–294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu F, Hu Z, Qiu L, Hui C, Li C, Zhong P
and Zhang J: Boosting high-intensity focused ultrasound-induced
anti-tumor immunity using a sparse-scan strategy that can more
effectively promote dendritic cell maturation. J Transl Med.
8:72010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang K, Zhu H, Meng Z, Chen Z, Lin J, Shen
Y and Gao H: Safety evaluation of high-intensity focused ultrasound
in patients with pancreatic cancer. Onkologie. 36:88–92.
2013.PubMed/NCBI
|
|
13
|
Sofuni A, Asai Y, Tsuchiya T, Ishii K,
Tanaka R, Tonozuka R, Honjo M, Mukai S, Nagai K, Yamamoto K, et al:
Novel therapeutic method for unresectable pancreatic cancer-the
impact of the long-term research in therapeutic effect of
high-intensity focused ultrasound (HIFU) therapy. Curr Oncol.
28:4845–4861. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duan F, Su XL, Wei ZX, Kong DW, Huang TY
and Wang S: Efficacy of computed tomography-guided implantation of
125I seeds in the treatment of refractory malignant tumors
accompanied with cancer pain and its influence on tumor markers in
the serum. Eur Rev Med Pharmacol Sci. 22:1595–1601. 2018.PubMed/NCBI
|
|
15
|
Fan T and Zhou JY: Computed
tomography-guided 125I radioactive seed implantation therapy for
pancreatic cancer pain. J Coll Physicians Surg Pak. 30:364–368.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mor V, Laliberte L, Morris JN and Wiemann
M: The karnofsky performance status scale. An examination of its
reliability and validity in a research setting. Cancer.
53:2002–2007. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niu H, Zhang X, Wang B, Zhou Z, Wang J and
Xu Z: The clinical utility of image-guided iodine-125 seed in
patients with unresectable pancreatic cancer. Tumour Biol.
37:2219–2223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Borasi G, Russo G, Alongi F, Nahum A,
Candiano GC, Stefano A, Gilardi MC and Messa C: High-intensity
focused ultrasound plus concomitant radiotherapy: A new weapon in
oncology? J Ther Ultrasound. 1:62013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li YJ, Huang GL, Sun XL, Zhao XC and Li
ZG: The combination therapy of high-intensity focused ultrasound
with radiotherapy in locally advanced pancreatic carcinoma. World J
Surg Oncol. 14:602016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Minniti G, Goldsmith C and Brada M:
Radiotherapy. Handb Clin Neurol. 104:215–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
DeNardo GL and DeNardo SJ: Update: Turning
the heat on cancer. Cancer Biother Radiopharm. 23:671–680.
2008.PubMed/NCBI
|
|
22
|
Kakar S, Pawlik TM, Allen PJ and Vauthey
JN: Exocrine pancreas. Pancreatic adenocarcinoma. AJCC cancer
staging manual. 8th ed. New York: Springer-Verlag; 2016
|
|
23
|
Marinova M, Rauch M, Mücke M, Rolke R,
Gonzalez-Carmona MA, Henseler J, Cuhls H, Radbruch L, Strassburg
CP, Zhang L, et al: High-intensity focused ultrasound (HIFU) for
pancreatic carcinoma: Evaluation of feasibility, reduction of
tumour volume and pain intensity. Eur Radiol. 26:4047–4056. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao H, Yang G, Wang D, Yu X, Zhang Y, Zhu
J, Ji Y, Zhong B, Zhao W, Yang Z and Aziz F: Concurrent gemcitabine
and high-intensity focused ultrasound therapy in patients with
locally advanced pancreatic cancer. Anticancer Drugs. 21:447–452.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Jiang Y, Li J, Tian S, Ran W and
Xiu D: Intraoperative ultrasound-guided iodine-125 seed
implantation for unresectable pancreatic carcinoma. J Exp Clin
Cancer Res. 28:882009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Y: High-intensity focused ultrasound
treatment for advanced pancreatic cancer. Gastroenterol Res Pract.
2014:2053252014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McCaffery M and Beebe A: Pain: Clinical
manual for nursing practice. Mosby, St Louis, MO: 1989
|
|
28
|
Liu YJ, Zhu GP and Guan XY: Comparison of
the NCI-CTCAE version 4.0 and version 3.0 in assessing
chemoradiation-induced oral mucositis for locally advanced
nasopharyngeal carcinoma. Oral Oncol. 48:554–559. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kolbeinsson HM, Chandana S, Wright GP and
Chung M: Pancreatic cancer: A review of current treatment and novel
therapies. J Invest Surg. 36:21298842023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Torphy RJ, Fujiwara Y and Schulick RD:
Pancreatic cancer treatment: Better, but a long way to go. Surg
Today. 50:1117–1125. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vidal-Jove J, Perich E and Del Castillo
MA: Ultrasound guided high intensity focused ultrasound for
malignant tumors: The Spanish experience of survival advantage in
stage III and IV pancreatic cancer. Ultrason Sonochem. 27:703–706.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li PZ, Zhu SH, He W, Zhu LY, Liu SP, Liu
Y, Wang GH and Ye F: High-intensity focused ultrasound treatment
for patients with unresectable pancreatic cancer. Hepatobiliary
Pancreat Dis Int. 11:655–660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang K, Chen Z, Meng Z, Lin J, Zhou Z,
Wang P, Chen L and Liu L: Analgesic effect of high intensity
focused ultrasound therapy for unresectable pancreatic cancer. Int
J Hyperthermia. 27:101–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao HF, Wang K, Meng ZQ, Chen Z, Lin JH,
Zhou ZH, Wang P, Shi WD and Sheng YH: High intensity focused
ultrasound treatment for patients with local advanced pancreatic
cancer. Hepatogastroenterology. 60:1906–1910. 2013.PubMed/NCBI
|
|
35
|
Peretz T, Nori D, Hilaris B, Manolatos S,
Linares L, Harrison L, Anderson LL, Fuks Z and Brennan MF:
Treatment of primary unresectable carcinoma of the pancreas with
I-125 implantation. Int J Radiat Oncol Biol Phys. 17:931–935. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gai B and Zhang F: Chinese expert
consensus on radioactive 125I seeds interstitial implantation
brachytherapy for pancreatic cancer. J Cancer Res Ther.
14:1455–1462. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ning Z, Xie J, Chen Q, Zhang C, Xu L, Song
L and Meng Z: HIFU is safe, effective, and feasible in pancreatic
cancer patients: A monocentric retrospective study among 523
patients. Onco Targets Ther. 12:1021–1029. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li CG, Zhou ZP, Jia YZ, Tan XL and Song
YY: Radioactive 125I seed implantation for locally advanced
pancreatic cancer: A retrospective analysis of 50 cases. World J
Clin Cases. 8:3743–3750. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang H, Wang J, Jiang Y, Li J, Tian S, Ran
W, Xiu D and Gao Y: The investigation of 125I seed implantation as
a salvage modality for unresectable pancreatic carcinoma. J Exp
Clin Cancer Res. 32:1062013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jin Z, Du Y, Li Z, Jiang Y, Chen J and Liu
Y: Endoscopic ultrasonography-guided interstitial implantation of
iodine 125-seeds combined with chemotherapy in the treatment of
unresectable pancreatic carcinoma: a prospective pilot study.
Endoscopy. 40:314–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhongmin W, Yu L, Fenju L, Kemin C and
Gang H: Clinical efficacy of CT-guided iodine-125 seed implantation
therapy in patients with advanced pancreatic cancer. Eur Radiol.
20:1786–1791. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wrenn SP, Dicker SM, Small EF, Dan NR,
Mleczko M, Schmitz G and Lewin PA: Bursting bubbles and bilayers.
Theranostics. 2:1140–1159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rosenschein U, Furman V, Kerner E, Fabian
I, Bernheim J and Eshel Y: Ultrasound imaging-guided noninvasive
ultrasound thrombolysis: preclinical results. Circulation.
102:238–245. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sofuni A, Moriyasu F, Sano T, Yamada K,
Itokawa F, Tsuchiya T, Tsuji S, Kurihara T, Ishii K and Itoi T: The
current potential of high-intensity focused ultrasound for
pancreatic carcinoma. J Hepatobiliary Pancreat Sci. 18:295–303.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Marinova M, Feradova H, Gonzalez-Carmona
MA, Conrad R, Tonguc T, Thudium M, Becher MU, Kun Z, Gorchev G,
Tomov S, et al: Improving quality of life in pancreatic cancer
patients following high-intensity focused ultrasound (HIFU) in two
European centers. Eur Radiol. 31:5818–5829. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ning ZY, Cheng CS, Xie J, Chen QW, Xu LT,
Zhuang LP, Zhang CY, Song LB, Shi WD, Zhu XY, et al: A
retrospective analysis of survival factors of high intensity
focused ultrasound (HIFU) treatment for unresectable pancreatic
cancer. Discov Med. 21:435–445. 2016.PubMed/NCBI
|
|
47
|
Yang SY, Liu F, Liu Y, Xia FF and Fu YF:
Stent insertion with high-intensity focused ultrasound ablation for
distal biliary obstruction secondary to pancreatic carcinoma.
Medicine (Baltimore). 99:e190992020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|