Introduction
Steatohepatitic hepatocellular carcinoma (SH-HCC) is
a histological subtype of HCC which was first described by Salomao
et al (1,2) and that accounts for 11–19% HCC cases
(3–5). The association between metabolic
disorders, such as diabetes mellitus (DM) and hyperlipidemia has
been repeatedly reported in SH-HCC (1–5).
Histologically, it is characterized by steatosis, peritumoral
fibrosis, inflammatory infiltrates, balloon-like swelling of tumor
cells, and Mallory-Denk body-like cytoplasmic inclusions. The name
‘steatohepatitic’ HCC comes from the histopathologic similarity to
steatohepatitis, although the molecular mechanism causing these
morphological changes in tumor cells is still unknown.
The Hedgehog (Hh) signaling pathway is involved in
normal embryonic development and wound healing, and its
dysregulation plays an important role in tumor progression
(6,7). The aberrant activation of Hh signaling
pathway and overexpression of related molecules such as sonic
hedgehog (SHh), patched, smoothened, or glioma-associated oncogene
(GLI) are associated with poor prognosis in various cancers such as
triple-negative breast cancer, ovarian cancer, small cell lung
carcinoma, colon cancer, and liver cancer, including HCC (8–15).
Several Hh signaling inhibitors have been used to treat advanced
basal cell carcinoma in clinical practice (16). The Hh pathway is also activated
during non-neoplastic liver injury (17). Rangwala et al (18) reported that the SHh protein is
overexpressed in ballooned hepatocytes, which is the histological
hallmark of steatohepatitis. Studies have shown that
immunohistochemistry of the SHh protein in patients with
non-alcoholic fatty liver disease facilitates fibrosis stage
prediction and ballooned hepatocyte detection (19,20).
We hypothesized that SHh molecule expression in
SH-HCC is substantially higher than that in conventional HCC
(C-HCC). We investigated SHh mRNA and protein expression in
SH-HCC and compared them with those in C-HCC, which is not
classified as a special HCC subtype. We also examined the clinical
significance of SHh overexpression in patients with HCC.
Materials and methods
Subjects
We reviewed 1,360 patients with HCC cases resected
at Kurume University Hospital between April 2003 and March 2020.
SH-HCC diagnosis was confirmed if the HCC fulfilled all four of the
following criteria: Intratumoral steatosis (>5% tumor cells),
peritumoral fibrosis, intratumoral inflammatory infiltrates, and
tumor cell ballooning, and the tumors with these findings are
predominant (≥50%). The histological evaluation was performed using
routine H&E staining, as well as Azan or Masson's trichrome
staining to assess fibrosis. Archived tissue samples were used for
gene expression analysis and immunohistochemistry.
Gene expression analysis by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Frozen materials from 22 SH-HCC and 24 C-HCC
(control) tissue samples were subjected to RT-qPCR to quantify the
mRNA expression levels. Total RNA was isolated using the RNeasy
Protect Mini Kit (Qiagen, Hilden, Germany) and quantitatively
analyzed using a NanoDrop ND-1000 (NanoDrop Technologies,
Wilmington, DE, USA). Total RNA was reverse transcribed using the
Reverse Transcription System (Promega, Madison, WI, USA). RT-qPCR
was performed in an ABI7500 Real-Time PCR System (Applied
Biosystems, Waltham, MA, USA) using TaqMan PCR assay probe/primers
for the housekeeping gene β-actin (Hs99999903_m1) and
SHh (HS00179843_m1). Each PCR was performed in duplicate.
The average cycle quantification (Cq) values per duplicate were
calculated for SHh and the housekeeping gene, yielding ∆Cq.
To determine the SHh gene expression levels relative to the
housekeeping gene, we calculated the 2−∆Cq values
(21).
Immunohistochemistry
Specimens (4 µm) were cut from formalin-fixed,
paraffn-embedded blocks. Immunostaining for SHh (clone EP1190Y,
ab53281, dilution 1:4,000; Abcam, Cambridge, MA, USA) was performed
using the Ventana Benchmark (Ventana, Tucson, AZ, USA). Whole
tissue sections from 40 patients with SH-HCC and tissue microarrays
(containing 2×3 and 1×3 mm2 tumor and non-tumor tissue
cores, respectively, per case) from 137 patients with C-HCC were
used for immunohistochemistry. The positive cell percentage in the
tumor was graded as diffuse (>50%), patchy (10–50%), focal
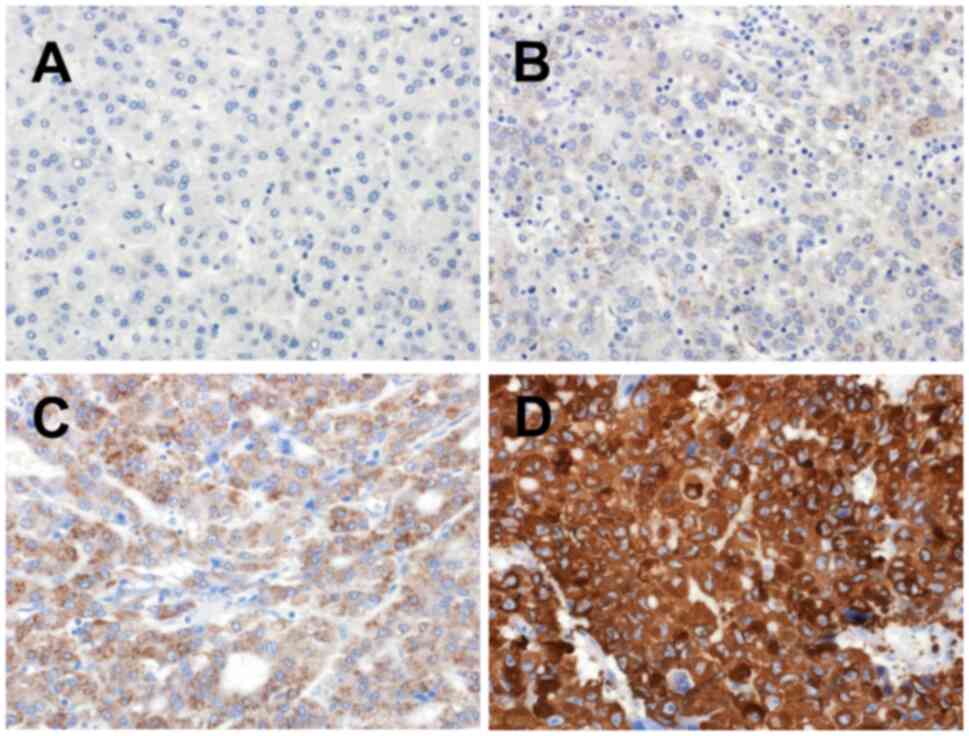
(1–10%), or none (0%). The staining intensity was classified as
strong, moderate, weak, or no. Staining scores were defined as 3
(diffuse strong), 2 (diffuse moderate or patchy strong), 1 (patchy
moderate or focal with any intensity or weakness with any
percentage of tumor cells), and 0 (none). A representative image of
each score is shown in Fig. 1.
Staining scores of 2 and 3 were classified as high expression,
whereas scores of 0 and 1 were classified as low expression.
Survival analysis
The survival analysis included patients with
solitary HCC who underwent curative resection and had no previous
treatment. Overall survival was defined as the time interval
between the date of surgery and death.
Statistics
Data analyses were performed using the JMP pro 15.1
software (SAS Institute, Cary, NC, USA). Categorical variables were
tested using the χ2 or Fisher's exact tests.
Quantitative variables were tested using the Mann-Whitney U test.
The Kaplan-Meier method and log-rank test were used for the
survival analysis. Univariate analysis was conducted to identify
the prognostic factors for overall survival, and multivariate
analysis of the significant factors found in the univariate
analysis was performed using Cox regression analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinicopathological analysis
In this cohort, we identified 40 patients with
SH-HCC, with 3% incidence. The clinicopathological findings are
outlined in Table I. Age, sex,
viral status, serum α-fetoprotein (AFP) and des-γ-carboxy
prothrombin levels, presence or absence of microvascular invasion,
and intrahepatic metastasis were not significantly different
between C-HCC and SH-HCC. The DM frequency was high in patients
with SH-HCC.
 | Table I.Clinicopathological
characteristics. |
Table I.
Clinicopathological
characteristics.
| Characteristic | C-HCC (n=1,320) | SH-HCC (n=40) | P-value |
|---|
| Mean± SD age,
years | 68.9±9.6 | 70.8±8.1 | 0.2576 |
| Male/female, n
(%) | 974 (74%)/346
(26%) | 31 (78%)/9 (22%) | 0.5984 |
| HBsAg -/+, n (%) | 1,095 (83%)/225
(17%) | 34 (85%)/6 (15%) | 0.7343 |
| HCV Ab -/+, n
(%) | 577 (44%)/743
(56%) | 16 (40%)/24
(60%) | 0.6409 |
| HBsAg + or HCVAb
+/others, n (%) | 950 (72%)/370
(28%) | 28 (70%)/12
(30%) | 0.7848 |
| DM -/+, n (%) | 862 (65%)/458
(35%) | 16 (40%)/24
(60%) | 0.001 |
| Median AFP,
ng/ml | 11.3 | 11.1 | 0.9962 |
| Median DCP,
mAU/ml | 63 | 53 | 0.2522 |
| Mean ± SD maximum
tumor diameter, mm | 32.8±24.3 | 24.9±9.8 | 0.1469 |
| Microvascular
invasion -/+, n (%) | 652 (49%)/668
(51%) | 16 (40%)/24
(60%) | 0.2417 |
| Intrahepatic
metastasis -/+, n (%) | 1140 (86%)/180
(14%) | 33 (83%)/7
(17%) | 0.4845 |
SHh gene and protein expression
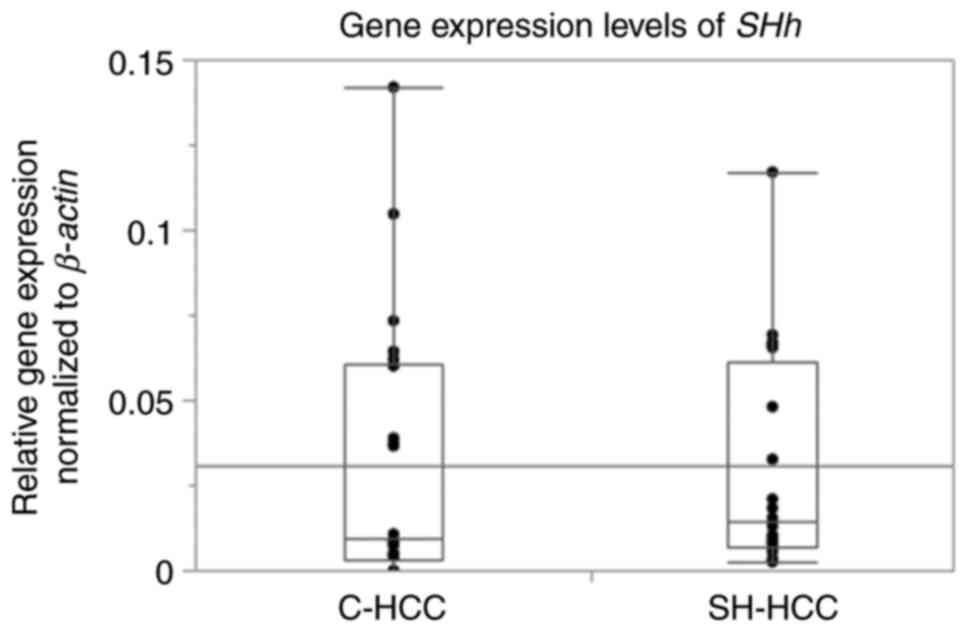
SHh mRNA expression was detected in 20
patients with SH-HCC but not in five patients with C-HCC.
SHh gene expression levels were not significantly different
between SH-HCC and C-HCC (Fig. 2).
The scores for SHh immunostaining are presented in Table II, with 63 (46%) and two (5%)
scoring 0, 30 (22%) and eight (20%) scoring 1, 21 (15%) and 12
(30%) scoring 2, and 23 (17%) and 18 (45%) scoring 3 in C-HCC and
SH-HCC, respectively. Thus, the SHh staining scores were
significantly different between SH-HCC and C-HCC (P=0.000003).
Representative histological images of the tumor and non-tumor
regions of SH-HCC, along with the immunohistochemical staining
images of SHh, are presented in Fig.
S1.
 | Table II.SHh immunoexpression in tumor
cells. |
Table II.
SHh immunoexpression in tumor
cells.
| Group | - | + | ++ | +++ |
|---|
| Conventional
HCC | 63 | 30 | 21 | 23 |
| Steatohepatitic
HCC | 2 | 8 | 12 | 18 |
Prognosis
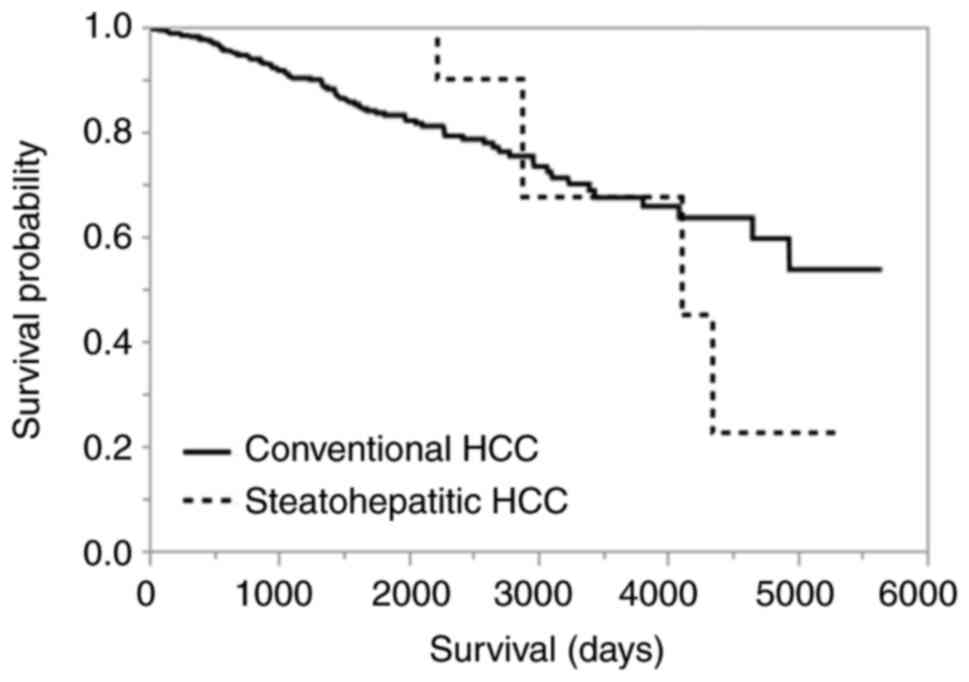
The overall survival was not significantly different
between SH-HCC and C-HCC (P=0.8146; Fig. 3). Among all HCCs, including C-HCC
and SH-HCC, the presence of vascular invasion, intrahepatic
metastasis, high serum AFP (>200 ng/ml), and high SHh expression
in immunohistochemistry were poor prognostic factors in univariate
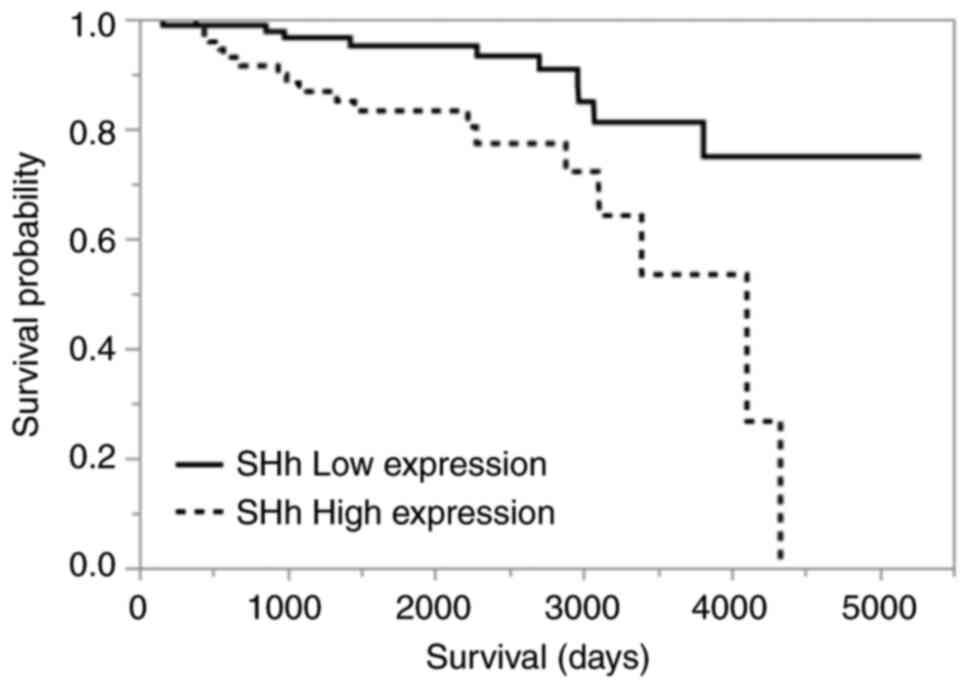
analysis. In multivariate analysis, only high SHh expression was
identified as an independent poor prognostic factor (Tables III and IV). Kaplan-Meier curves comparing the
high- and low-SHh expression groups are shown in Fig. 4 (P=0.0022).
 | Table III.Prognostic factors in univariate
analysis for overall survival. |
Table III.
Prognostic factors in univariate
analysis for overall survival.
| Factor | P-value | HR (95% CI) |
|---|
| Steatohepatitic
HCC | 0.8114 | 0.8867628
(0.2704958–2.1310151) |
| Microvascular
invasion | 0.0111 | 1.7962606
(1.1475853–2.8828483) |
| Intrahepatic
metastasis | 0.0028 | 2.5861964
(1.4155532–4.4294630) |
| Preoperative serum
AFP >200 ng/ml | 0.0220 | 1.7185646
(1.0569048–2.7110127) |
| SHh high
expression | 0.0027 | 3.2124137
(1.4932731–7.3222227) |
 | Table IV.Prognostic factors in the
multivariate analysis of overall survival. |
Table IV.
Prognostic factors in the
multivariate analysis of overall survival.
| Factor | P-value | HR (95% CI) |
|---|
| Microvascular
invasion | 0.3217 | 1.5765628
(0.6545625–4.0347770) |
| Intrahepatic
metastasis | 0.9352 | 0.9537622
(0.2631614–2.7704849) |
| Preoperative serum
AFP >200 ng/ml | 0.3124 | 1.6533953
(0.5939074–3.9806890) |
| SHh high
expression | 0.0069 | 3.0577992
(1.3542579–7.3759824) |
Discussion
We demonstrated a high SHh protein expression in
SH-HCC and its prognostic significance in HCCs. In steatohepatitis,
ballooned hepatocytes express the SHh protein, whereas
non-ballooned hepatocytes do not. Therefore, SHh
immunohistochemistry facilitates pathological diagnosis of
steatohepatitis (18,20). Several cases in our study showed
diffuse SHh expression in tumors. We confirmed consistent SHh
protein expression in ballooned tumor cells and various degrees of
expression in non-ballooned tumor cells, suggesting that SHh is
involved in the ballooning of HCC tumor cells and ballooned
hepatocytes in steatohepatitis, and can be expressed on HCC tumor
cells without ballooning because of its association with cell
proliferation signaling. Therefore, SHh immunohistochemistry is not
always useful in discriminating SH-HCC cases from C-HCC cases.
Despite many studies on Hh signaling activation in HCCs, limited
reports have focused on a specific SH-HCC subgroup; thus, our study
is significant in this regard (13–15).
Recently, Van Treeck et al reported that the Hh pathway was
upregulated in SH-HCC by comparing paired non-neoplastic liver
tissues using pathway analysis (22). We confirmed SHh protein
overexpression in SH-HCC than that in C-HCC. However, High
SHh gene expression in SH-HCC was not significant in this
study, and the correlation between gene and protein expression
levels in the paired samples was unclear owing to the limited
frozen tissue samples for RT-qPCR, which did not adequately
represent SHh protein expression on glass slides when behaviors
were heterogeneous.
High SHh protein expression in tumor cells is a
significant independent poor prognostic factor. Previous studies
have also reported that Hh signaling activation is significantly
associated with poor prognosis in various cancers (8–12). The
expression of other Hh pathway-related molecules, such as GLI-1 and
GLI-2, correlate with poor prognosis in patients with HCC (13,14).
Hh signaling pathway activation is associated with tumor
invasiveness and progression as well as epithelial-mesenchymal
transition (7). Recent reports have
also suggested that Hh signaling pathway activation induces tumor
immunosuppression and resists immune checkpoint inhibitors
(23,24). In contrast, the presence of
tumor-infiltrating lymphocytes, a definitive feature of SH-HCC,
predicts the benefits of immune checkpoint inhibitors. Although we
found no patients treated with immune checkpoint inhibitors after
initial resection in this study, we believe that studying how these
two conflicting events affect the therapeutic effect of immune
checkpoint inhibitors will aid in the development of personalized
treatment.
The histological presentation of
steatohepatitis-like features in HCCs is not associated with
patient prognosis. This outcome is consistent with that of previous
studies (2,4). Our diagnostic criteria for SH-HCC were
derived from the original criteria developed by Salomao et
al (1) with certain
modifications. Salomao et al (1) proposed five histological
characteristics for SH-HCC diagnosis: intratumoral steatosis,
peritumoral fibrosis, intratumoral inflammatory infiltrates, tumor
cell ballooning, and Mallory-Denk body-like intracytoplasmic
material in the tumor cells. We excluded Mallory-Denk body-like
intracytoplasmic material from the diagnostic criteria. In cases
where we identified this particular feature, the other four
criteria were also present. Additionally, Mallory-Denk bodies are
frequently observed in steatohepatitis but are not a mandatory
criterion for steatohepatitis diagnosis (25,26).
Hence, we do not consider the Mallory-Denk body-like
intracytoplasmic material to be a crucial factor. Salomao et
al (1,2) reported that 27% of SH-HCC cases had
minimal or no fibrosis in their initial report, whereas 90% had
fibrosis in subsequent reports. In this study, we found that all
HCCs with the three fundamental features required for SH-HCC,
showed intratumoral fibrosis, even at a very low proportion. The
presence of these three fundamental findings is necessary and
sufficient for SH-HCC diagnosis, although peritumoral fibrosis is a
highly distinctive and definitive feature of SH-HCC compared with
that of C-HCC, which is medullary and lacks fibrosis.
SH-HCC prevalence in our cohort was 3%, which is
lower than the 13–19% observed in previous studies involving
various etiologies (2–5) due to several potential reasons,
including disparities in the cut-off used to quantify the
steatohepatitic area within the tumor. Some researchers have
utilized 5% cut-off, whereas we used 50% cut-off. Moreover, the
viral status and frequency of metabolic diseases may have been
different in each cohort. Furthermore, steatohepatitis finding
interpretation by pathologists may vary. Inter-observer variability
is a persistent issue in steatohepatitis diagnosis. Specifically,
the assessment of ‘ballooning’ largely depends on individual
subjectivity. Similar challenges may arise in the assessment of the
presence of ‘tumor cell ballooning,’ leading to differences in
SH-HCC prevalence. Although histological HCC subtyping does not
currently affect treatment strategies, it could pose a challenge
for realizing individualized therapies in the future.
Among the patient characteristics, DM frequency was
the most significantly different between the C-HCC and SH-HCC
groups. Individuals with SH-HCC have a higher prevalence of DM than
those with C-HCC, although viral status was not significantly
different. This association has been corroborated by multiple
studies, highlighting the potential relationship between SH-HCC and
underlying metabolic disorders that cause steatohepatitis (1–5).
Nonetheless, viral etiologies without metabolic syndrome can also
cause SH-HCC. Concurrent fatty liver disease and steatohepatitis
can easily be overlooked in patients with chronic viral hepatitis
(20). Thus, a thorough evaluation
of the non-neoplastic background of the liver is increasingly
important in clinical practice.
In conclusion, this study demonstrated significant
upregulation of SHh protein expression in SH-HCC than that in
C-HCC, which is an independent predictor of an unfavorable
prognosis in HCC. Our analysis confirmed no correlation between
steatohepatitis histology and patient outcome, and a strong
association between steatohepatitis histology and DM incidence,
which is consistent with the results of prior research. Thus, SHh
could potentially serve as a promising therapeutic target for
patients with HCC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HK and RK conducted a reassessment of past
pathological samples, evaluated new SHh immunostaining slides, and
confirmed the authenticity of all the raw data. HK, YN, JA, ON and
HY designed the research study. HK, SO, MiO and MaO collected the
data. HK, RK, SO, MiO, MaO, SM, YM, YK, YY and MN analyzed the
data. HK wrote the paper. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Kurume University School of Medicine (approval no.
459; Kurume, Japan) on December 24, 2020. Informed consent was not
required for this retrospective study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Salomao M, Yu WM, Brown RS Jr, Emond JC
and Lefkowitch JH: Steatohepatitic hepatocellular carcinoma
(SH-HCC): A distinctive histological variant of HCC in hepatitis C
virus-related cirrhosis with associated NAFLD/NASH. Am J Surg
Pathol. 34:1630–1636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Salomao M, Remotti H, Vaughan R, Siegel
AB, Lefkowitch JH and Moreira RK: The steatohepatitic variant of
hepatocellular carcinoma and its association with underlying
steatohepatitis. Hum Pathol. 43:737–746. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jain D, Nayak NC, Kumaran V and Saigal S:
Steatohepatitic hepatocellular carcinoma, a morphologic indicator
of associated metabolic risk factors: A study from India. Arch
Pathol Lab Med. 137:961–966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shibahara J, Ando S, Sakamoto Y, Kokudo N
and Fukayama M: Hepatocellular carcinoma with steatohepatitic
features: A clinicopathological study of Japanese patients.
Histopathology. 64:951–962. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamaoka K, Saitoh S, Kinowaki K, Fujiyama
S, Kawamura Y, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Suzuki F,
et al: Clinicopathological assessment of steatohepatitic
hepatocellular carcinoma. Clin Res Hepatol Gastroenterol.
46:1017992022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carballo GB, Honorato JR, de Lopes GPF and
Spohr TCLSE: A highlight on Sonic hedgehog pathway. Cell Commun
Signal. 16:112018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Skoda AM, Simovic D, Karin V, Kardum V,
Vranic S and Serman L: The role of the Hedgehog signaling pathway
in cancer: A comprehensive review. Bosn J Basic Med Sci. 18:8–20.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Noman AS, Uddin M, Rahman MZ, Nayeem MJ,
Alam SS, Khatun Z, Wahiduzzaman M, Sultana A, Rahman ML, Ali MY, et
al: Overexpression of sonic hedgehog in the triple negative breast
cancer: Clinicopathological characteristics of high burden breast
cancer patients from Bangladesh. Sci Rep. 6:188302016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liao X, Siu MK, Au CW, Wong ES, Chan HY,
Ip PP, Ngan HY and Cheung AN: Aberrant activation of hedgehog
signaling pathway in ovarian cancers: Effect on prognosis, cell
invasion and differentiation. Carcinogenesis. 30:131–140. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lim S, Lim SM, Kim MJ, Park SY and Kim JH:
Sonic hedgehog pathway as the prognostic marker in patients with
extensive stage small cell lung cancer. Yonsei Med J. 60:898–904.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu M, Li X, Liu T, Leng A and Zhang G:
Prognostic value of hedgehog signaling pathway in patients with
colon cancer. Med Oncol. 29:1010–1016. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang L, Tan YX, Jiang BG, Pan YF, Li SX,
Yang GZ, Wang M, Wang Q, Zhang J, Zhou WP, et al: The prognostic
significance and therapeutic potential of hedgehog signaling in
intrahepatic cholangiocellular carcinoma. Clin Cancer Res.
19:2014–2024. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Che L, Yuan YH, Jia J and Ren J:
Activation of sonic hedgehog signaling pathway is an independent
potential prognosis predictor in human hepatocellular carcinoma
patients. Chin J Cancer Res. 24:323–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang D, Cao L, Li Y, Lu H, Yang X and Xue
P: Expression of glioma-associated oncogene 2 (Gli 2) is correlated
with poor prognosis in patients with hepatocellular carcinoma
undergoing hepatectomy. World J Surg Oncol. 11:252013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeng KS, Jeng CJ, Jeng WJ, Sheen IS, Li
SY, Leu CM, Tsay YG and Chang CF: Sonic Hedgehog signaling pathway
as a potential target to inhibit the progression of hepatocellular
carcinoma. Oncol Lett. 18:4377–4384. 2019.PubMed/NCBI
|
|
16
|
Nguyen A, Xie P, Litvinov IV and
Lefrançois P: Efficacy and safety of sonic hedgehog inhibitors in
basal cell carcinomas: An updated systematic review and
meta-analysis (2009–2022). Am J Clin Dermatol. 24:359–374. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Machado MV and Diehl AM: Hedgehog
signalling in liver pathophysiology. J Hepatol. 68:550–562. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rangwala F, Guy CD, Lu J, Suzuki A,
Burchette JL, Abdelmalek MF, Chen W and Diehl AM: Increased
production of sonic hedgehog by ballooned hepatocytes. J Pathol.
224:401–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Estep M, Mehta R, Bratthauer G, Alaparthi
L, Monge F, Ali S, Abdelatif D, Younoszai Z, Stepanova M, Goodman
ZD and Younossi ZM: Hepatic sonic hedgehog protein expression
measured by computer assisted morphometry significantly correlates
with features of non-alcoholic steatohepatitis. BMC Gastroenterol.
19:272019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kusano H, Kondo R, Ogasawara S, Omuraya M,
Okudaira M, Mizuochi S, Mihara Y, Kinjo Y, Yano Y, Nakayama M, et
al: Utility of sonic hedgehog and keratin 8/18 immunohistochemistry
for detecting ballooned hepatocytes. Histopathology. 80:974–981.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wong ML and Medrano JF: Real-time PCR for
mRNA quantitation. Biotechniques. 39:75–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Van Treeck BJ, Mounajjed T, Moreira RK,
Orujov M, Allende DS, Bellizzi AM, Lagana SM, Davila JI, Jessen E
and Graham RP: Transcriptomic and proteomic analysis of
steatohepatitic hepatocellular carcinoma reveals novel distinct
biologic features. Am J Clin Pathol. 155:87–96. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Petty AJ, Dai R, Lapalombella R, Baiocchi
RA, Benson DM, Li Z, Huang X and Yang Y: Hedgehog-induced PD-L1 on
tumor-associated macrophages is critical for suppression of
tumor-infiltrating CD8+ T cell function. JCI Insight.
6:e1467072021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang J, Ding Y, Chen Y, Lu J, Chen Y, Wu
G, Xu N, Wang H and Teng L: Pan-cancer analyses reveal that
increased Hedgehog activity correlates with tumor immunosuppression
and resistance to immune checkpoint inhibitors. Cancer Med.
11:847–863. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matteoni CA, Younossi ZM, Gramlich T,
Boparai N, Liu YC and McCullough AJ: Nonalcoholic fatty liver
disease: A spectrum of clinical and pathological severity.
Gastroenterology. 116:1413–1419. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bedossa P, Poitou C, Veyrie N, Bouillot
JL, Basdevant A, Paradis V, Tordjman J and Clement K:
Histopathological algorithm and scoring system for evaluation of
liver lesions in morbidly obese patients. Hepatology. 56:1751–1759.
2012. View Article : Google Scholar : PubMed/NCBI
|