Introduction
Metastatic tumors to the pancreas represent a
minority of all pancreatic malignancies, typically ranging from 2
to 5% based on reported data (1).
Melanoma, renal cell and lung carcinoma are the most common tumors
to metastasize to the pancreas, while metastasis of tumors from the
esophagus is rare comprising <5% of all pancreatic metastases
(2,3). At present, three commonly used methods
exist for detecting metastatic tumors to the pancreas: Endoscopic
ultrasound-guided fine-needle aspiration (EUS-FNA), surgical
intervention and autopsy (4–6).
Metastasis to the pancreas is frequently misidentified as a primary
tumor, which leads to potentially inappropriate treatment (7). There is a lack of conclusive evidence
regarding the effectiveness of surgical resection in prolonging
survival of patients with metastatic tumors to the pancreas
(8). Accurate identification of the
primary site, precise pathological diagnosis and thorough
preoperative staging are key factors for achieving optimal patient
prognosis; this approach ensures patients receive effective
treatment. According to the most recent esophageal cancer diagnosis
and treatment guidelines from the National Comprehensive Cancer
Network of America (9), patients
with metastatic esophageal cancer are, in most cases, not
considered candidates for surgical resections. Therefore,
misdiagnosing metastatic tumors as dual origin primary tumors for
radical resection may lead to unnecessary harm to patients.
EUS-FNA is the primary diagnostic method for
obtaining pathological diagnosis of abdominal tumors, particularly
pancreatic tumors, due to high accuracy and low rate of
complications. EUS-FNA has been shown to have a diagnostic accuracy
of >90% in detecting pancreatic tumors (10,11).
Therefore, in patients with malignant synchronous or metachronous
pancreatic tumors, it is recommended to consider EUS-FNA to
determine if the pancreatic tumor is primary or secondary. Cortez
et al (8) demonstrated that
EUS-FNA can assist patients with single pancreatic metastasis by
avoiding unnecessary pancreatic tumor resection.
While there are some reported cases (2–6,12,13)
of esophageal tumor metastasis to the pancreas, they remain rare
occurrences. The present case study aimed to provide insights for
diagnosis and treatment of metastatic tumors to the pancreas.
Case report
A 53-year-old female patient presented to Guangyuan
Central Hospital (Sichuan, China) in March 2024 with dysphagia and
epigastric pain that had lasted for 3 months, with no other
accompanying symptoms. The patient underwent upper gastrointestinal
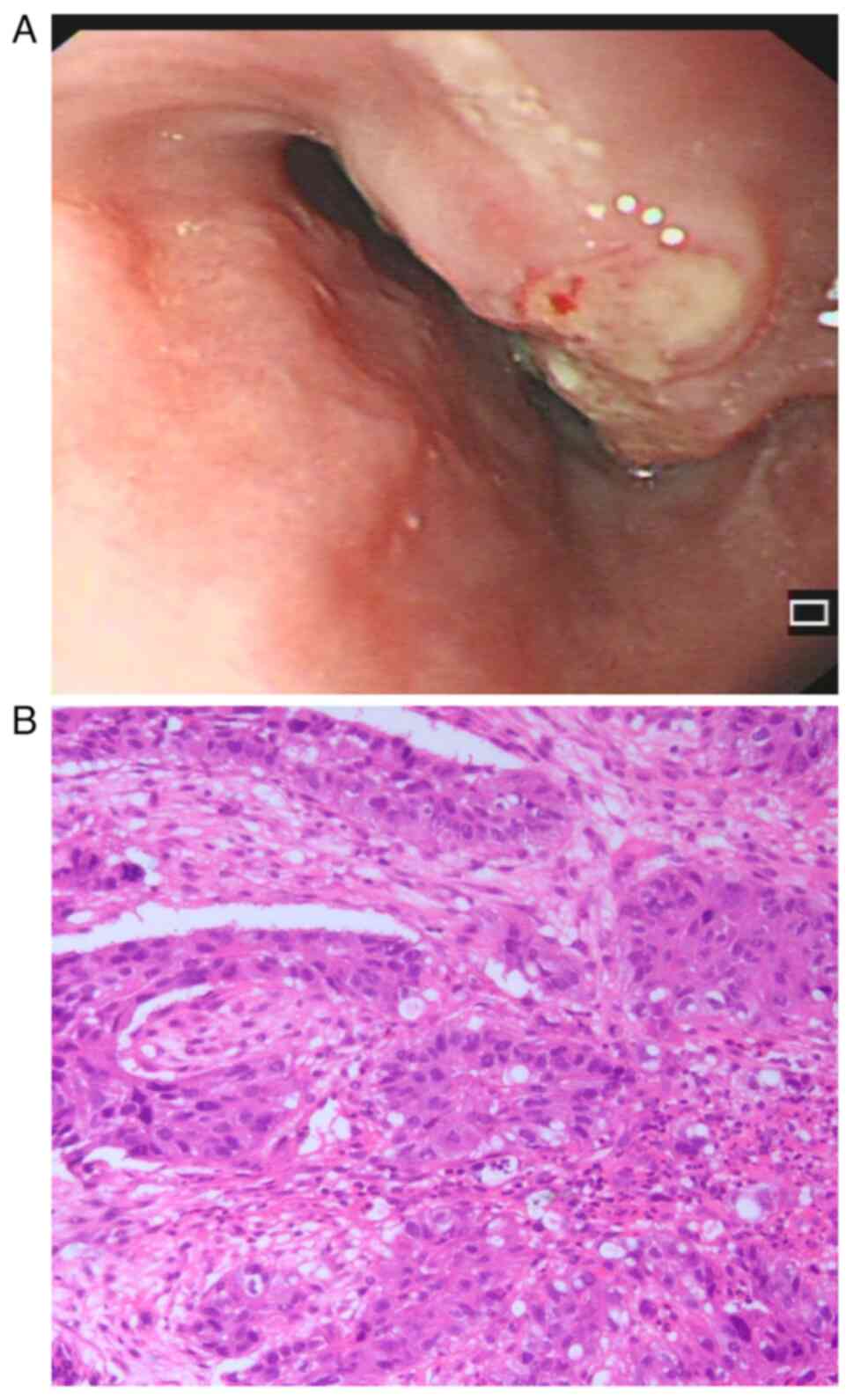
endoscopy and pathological biopsy and was diagnosed with squamous
cell carcinoma of the lower esophagus (Fig. 1A and B). The patient did not exhibit
any other symptoms such as malnutrition, anemia or jaundice. The
levels of carbohydrate antigen 19-9 and 125 in the blood test were
10.06 U/ml (normal range <37.00 U/ml) and 10.33 U/ml (normal
range <35.00 U/ml), respectively. From a computed tomography
(CT) scan (256-row GE Revolution CT, Revolution APEX; GE
Healthcare; slice thickness was 1 mm) prior to surgery on March
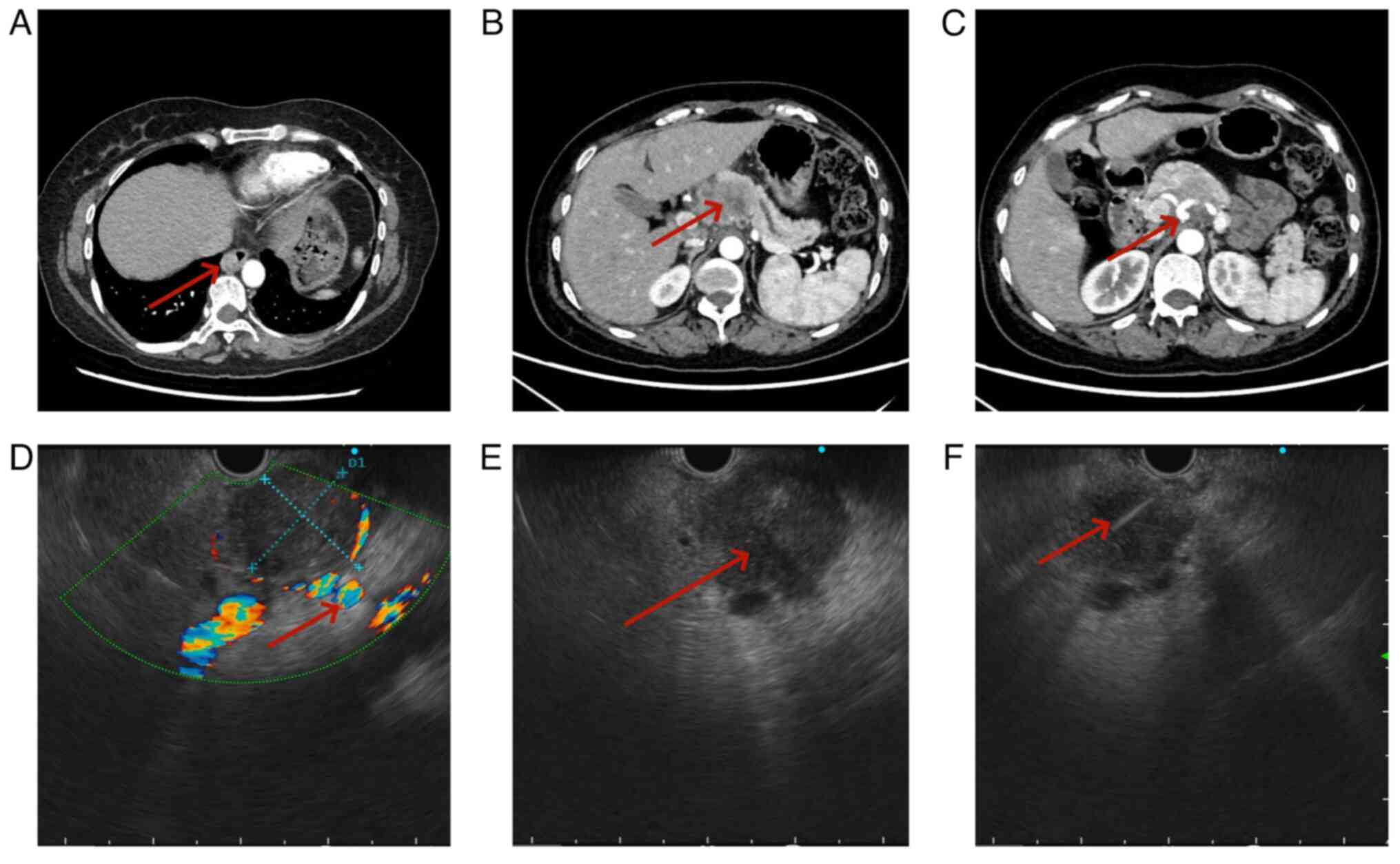
2024, the thoracic surgeon identified a pancreatic tumor (Fig. 2A-C). Due to the potential of the
tumor to infiltrate the celiac trunk vessels and multiple
retroperitoneal lymph nodes, the multidisciplinary treatment (MDT)
team opted to verify the pathological diagnosis of the tumor before
proceeding with extensive multi-organ surgery. Subsequently, the
patient underwent EUS scanning in April 2024, demonstrating a tumor
measuring ~34.4×30.2 mm in the body of the pancreas. The
echogenicity characteristics indicated that, in comparison with
normal pancreatic tissue, there was a lower echo intensity with
uniform punctate medium-high echoes present, which were slightly
higher compared with that of the echoes observed in primary
pancreatic cancer. The tumor exhibited clear demarcation from
surrounding pancreatic tissue and invaded compression on the
splenic and common hepatic artery. A few enlarged lymph nodes were
observed in the retroperitoneum. Based on the EUS scan results, the
initial determination of the tumor stage was uT4N1Mx. (According to
the 8th American Joint Committee on Cancer TNM Staging of
Pancreatic Cancer) (14). In April
2024, EUS-FNA was conducted on the tumor in the body of the
pancreas, which resulted in retrieval of a notable number of cells
and tissue (Fig. 2D-F). The
pathologist initially did not consider the present case to be a
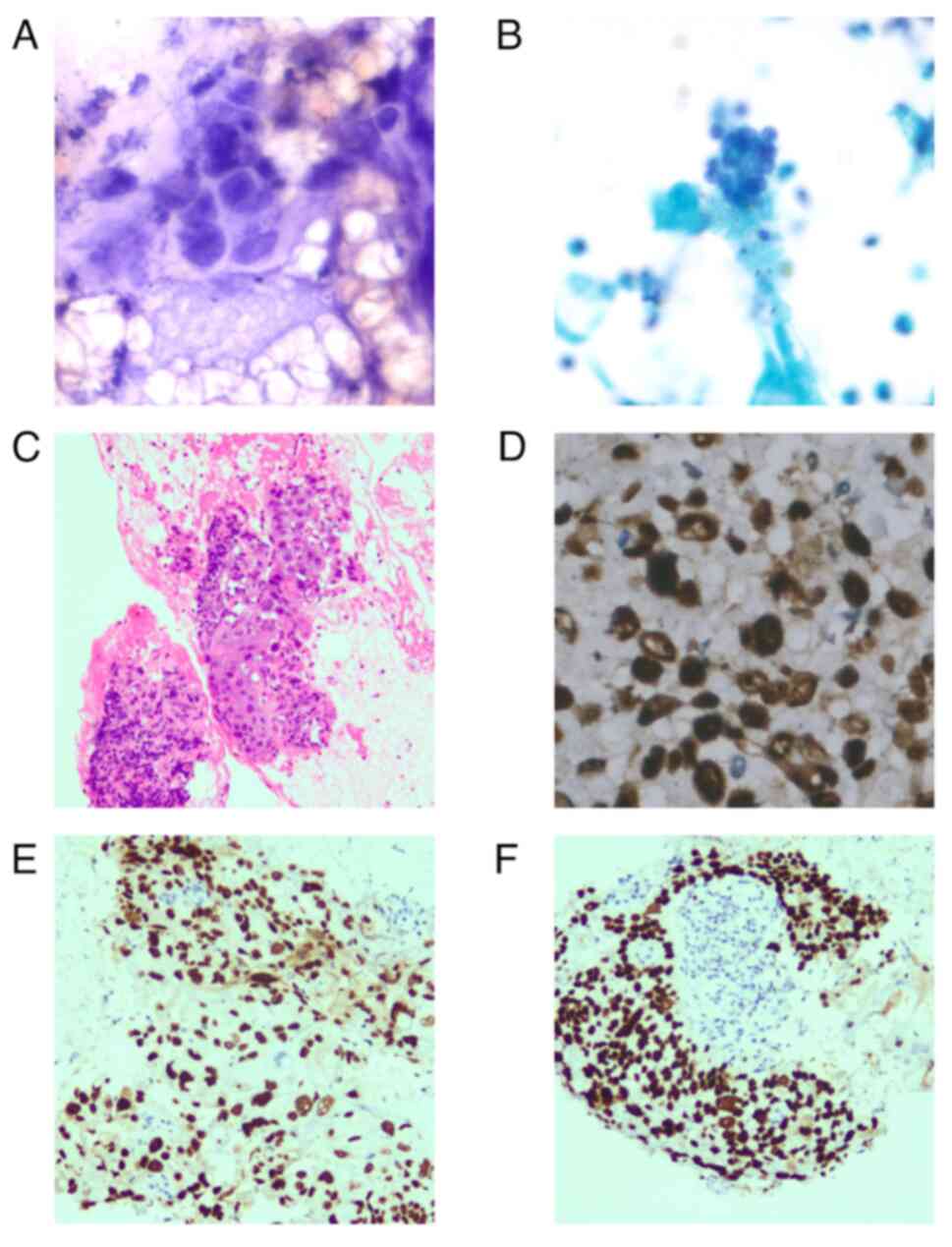
metastatic tumor to the pancreas. However, components indicative of
keratinization and intercellular bridges that were less consistent
with common pancreatic adenocarcinoma were identified in
cytological [hematoxylin and eosin (H&E) and Papanicolaou
stained]and histological (H &E stained)sections. Due to the
patient history of esophageal squamous cell carcinoma,
immunohistochemistry was conducted to assist in diagnosis. P40 and
P63 were positive, with a Ki67 proliferation index of 70% (data not
shown), which confirmed metastasis of esophageal squamous cell
carcinoma to the pancreas (Fig.
3A-F). Tissue was immersed in 10% neutral formalin at room
temperature for >20 h, after which it was embedded in paraffin
blocks. The paraffin blocks were sectioned to a thickness of 4 µm
and baked at 60°C for 30-60 min. Paraffin sections were immersed in
fresh xylene and descending ethanol solutions, respectively. The
tissue was then rinsed with tap water and PBS and heated to 100°C.
Following treatment with 3% H2O2 for 10 mins
at room temperature, 10% normal goat serum (Scientific Phygene) was
added at 37°C for 30 min to block nonspecific binding. Primary
antibodies against Ki-67 (cat. no. SP6, 1:200, MXB
biotechnologies), p63(MX013, 1:200, MXB biotechnologies), and p40
(MX048, 1:200, MXB biotechnologies) were added and incubated at
37°C for 2 h. The samples were then washed 3 times with PBS (2
mins). Subsequently, added horseradish peroxidase (HRP)-labeled
goat anti-rabbit IgG as the secondary antibody (Everything
Biotechnology Co, Hefei, China, BL003A) for P40 and Ki67, and
HRP-labeled goat anti-mouse IgG as the secondary antibody
(Everything Biotechnology Co, Hefei, China, BL001A; all 1:200 and
incubated at 37°C for 30 mins. The samples were washed again 3
times with PBS with each wash lasting 2 mins. A volume of 10 µl
freshly prepared DAB solution was added to the samples at room
temperature for 5-8 min, samples were rinsed with tap water and
counterstained with hematoxylin for 30-60 sec at room temperature,
followed by rinsing with tap water to restore the blue color. The
samples were dehydrated by using ethanol gradients. Sections were
treated with xylene 3 times for 2 mins each to achieve
transparency. Finally, the sections were sealed with neutral gum
and observed under a light microscope (Olympus, Tokyo, Japan). The
Papanicolaou staining was conducted as follows: Slides were
immersed in 95% ethanol solution for a minimum of 15 mins at room
temperature. Following washing with clean water 2 to 3 times, they
were stained with hematoxylin dye. Once the coloration was clearly
visible, the slides were rinsed with water. Subsequently, the
slides were immersed in a lithium carbonate solution for 1 to 2
mins. After reverting to blue, the slides were rinsed with water
and then transferred to a 95% ethanol solution for dehydration,
allowing them to sit for 1 min to eliminate any excess water and
immersed in orange G6 stain for 3 mins. Following this, they were
immersed in 80, 95, and 95% ethanol solutions for 10 sec each. The
samples were placed in EA36 solution followed by thorough rinsing
with water. Finally, the samples were dehydrated using 95% ethanol
3 times and sealed with gum.
Following discussions with the MDT team, the patient
elected to forgo surgery and instead underwent a combination of
radiotherapy and chemotherapy concurrently. The patient received
cisplatin-based chemotherapy regimen in conjunction with
fluorouracil, (Cisplatin, 80 mg/m2, iv, d1 and
Capecitabine, 1,000 mg/m2 oral, twice daily, d1-14)
alongside concurrent radiotherapy for esophageal and pancreatic
tumors (60 Gy in 30 fractions). In May 2025, the patient finished
the initial cycle of treatment. Ongoing monitoring will be
conducted to evaluate the efficacy and long-term outcomes of this
treatment approach.
Discussion
While most pancreatic tumors are primarily of
pancreatic origin, metastatic tumors may occasionally arise.
Esophageal cancer represents a rare source of metastatic tumors to
the pancreas. As esophageal cancer is the ninth most common
malignant tumor and the seventh leading cause of cancer-related
mortality from GLOBOCAN 2018 (15),
reports of pancreatic metastasis originating from esophageal cancer
may increase in the future, as detection methods and awareness
improve. The predominant histological type of esophageal cancer is
squamous cell carcinoma. The incidence of esophageal cancer is
highest in Asia, particularly in China, with a higher prevalence in
males compared with females (13.6 per 100,000 vs. 4.3 in women
(16,17). Lymph node metastasis is considered
to be a key factor impacting the survival of patients with
esophageal cancer. Typical areas of lymph node metastasis in
esophageal cancer vary depending on location of the tumor. For
lower esophageal cancer, the incidence of lymph node metastasis in
the perigastric region is higher compared with the lower and upper
mediastinum regions (18). Middle
and lower esophageal cancers are more likely to metastasize to
celiac lymph nodes, which can lead to involvement of the pancreas
through metastasis to the paraaorta, celiac artery, perigastric
region, posterior surface of the pancreatic head, and station 16a1
or16a2 of the common hepatic artery (19). Depending on involvement of multiple
lymph nodes, pancreatic metastasis of esophageal tumors may occur
(20,21). Esophageal squamous cell carcinoma
with pancreatic metastasis is a rare occurrence, representing
<5% of all pancreatic tumors (2). The available literature on this topic
consists of sporadic case reports with limited information,
resulting in a lack of unified treatment guidelines. The present
studies analyzed existing 11 cases of pancreatic metastasis of
esophageal cancer to inform future treatment strategies (Table I) (1–4,13,15,16).
The age of patients with pancreatic metastasis of esophageal cancer
was >50 years old. Patients often presented with non-specific
symptoms that resemble those of solitary esophageal or pancreatic
cancer, including abdominal pain, jaundice, and dysphagia. Tumors
in the lower esophagus exhibited a higher incidence of metastasis
to the pancreas, particularly to the body and tail of the organ,
compared with upper esophageal cancer. As there is currently no
standardized diagnosis and treatment plan, this may have resulted
in the generally short survival time (< two years) (22).
 | Table I.Cases of pancreatic metastasis in
esophageal cancer. |
Table I.
Cases of pancreatic metastasis in
esophageal cancer.
| No. of cases | Age, years | Sex | Symptoms | Site of primary
esophageal tumor | Site of the primary
tumor metastasis to the pancreas | Method of
diagnosis | Treatment | Survival time,
months | (Refs.) |
|---|
| 2 | NA | NA | NA | NA | NA | EUS-FNA | NA | NA | (2) |
| 1 | 54 | Male | Chest discomfort | Lower | Tail | Surgery | Surgery and
chemotherapy | 9 | (4) |
| 4 | NA | NA | NA | NA | NA | Autopsy | NA | NA | (5) |
| 1 | 70 | Female | NA | NA | Tail | Surgery | Surgery | 24 | (6) |
| 1 | 68 | Male | NA | Lower | Body | Surgery | Surgery and
chemotherapy | 9 | (3) |
| 1 | 67 | Male | Abdomen pain | Upper, middle and
lower | Tail and body | EUS-FNA | Adjuvant therapy | 1 | (12) |
| 1 | 64 | NA | Weight loss | NA | Head | EUS-FNA | Radiotherapy | 10 | (13) |
The prognosis for distant metastatic esophageal
cancer is generally poor compared with that of localized esophageal
cancer (23). There is debate on
whether simultaneous surgical resection of metastases and primary
lesions can enhance patient prognosis (24,25).
Nonetheless, unnecessary surgical interventions may result in
increased surgical trauma and financial burden for patients. The
results of the present study and previous clinical experience
indicate the importance of obtaining a precise pathological
diagnosis as it serves as a key foundation for determining
treatment strategy. It is advisable for patients with pancreatic or
malignant tumors in other locations to utilize pathological
diagnosis to differentiate between primary and metastatic
pancreatic tumors. Therefore, EUS-FNA could be recommended as the
primary method for obtaining pathological tissue from pancreatic
solid tumors (26). EUS-FNA should
be considered in suspected cases of metastatic tumors to the
pancreas. In the present case, distinct ultrasound characteristics
of pancreatic metastasis of esophageal squamous cell carcinoma were
identified. Metastatic tumors to the pancreas exhibit higher
echogenicity, uniformity and well-defined boundaries in contrast to
primary tumors: Previous studies have seldom summarized the
endoscopic ultrasound morphological image characteristics of
metastatic tumors to the pancreas for diagnosis (22,27).
However, there is limited research on ultrasonic features of
pancreatic metastasis of esophageal squamous cell carcinoma, and
the occurrence of this type of tumor is rare. Further studies with
larger sample size are needed to draw further conclusions.
In conclusion, metastatic tumors to the pancreas are
rare and typically present as a pancreatic mass accompanied by
extra-pancreatic malignant tumors. It is important to consider the
potential for metastasis, rather than solely focusing on
dual-source tumors. EUS-FNA may serve as an effective pathological
complement for this patient population. The present study
demonstrates the feasibility of obtaining pancreatic tissue via
EUS-FNA to confirm metastatic pancreatic cancer, thereby
facilitating the development of treatment strategies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JT and WZ collected data and wrote the manuscript.
JT and ZW confirm the authenticity of all the raw data. JT, XX and
WZ performed imaging and histopathological analysis. PZ was
involved in drafting the manuscript, revising it critically for
important intellectual content, patient data analysis and gave
final approval of the version to be published. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of any potentially identifiable images or
data contained in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ballarin R, Spaggiari M, Cautero N, De
Ruvo N, Montalti R, Longo C, Pecchi A, Giacobazzi P, De Marco G,
D'Amico G, et al: Pancreatic metastases from renal cell carcinoma:
The state of the art. World J Gastroenterol. 17:4747–4756. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Novotny A, Sell E and Mehrotra S:
Metastatic tumors to the pancreas, a 12-year single institution
review. Diagn Cytopathol. 49:1233–1236. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okamoto H, Hara Y, Chin M, Hagiwara M,
Onodera Y, Horii S, Shirahata Y, Kamei T, Hashizume E and Ohuchi N:
An extremely rare case of pancreatic metastasis of esophageal
squamous cell carcinoma. World J Gastroenterol. 20:593–597. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Denda Y, Matsuo Y, Nonoyama K, Murase H,
Kato T, Hayashi Y, Imafuji H, Saito K, Morimoto M, Kato H, et al:
Simultaneous presentation and resection of esophageal cancer and
metastasis to the pancreas: Α case report and literature review.
Mol Clin Oncol. 20:22023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adsay NV, Andea A, Basturk O, Kilinc N,
Nassar H and Cheng JD: Secondary tumors of the pancreas: An
analysis of a surgical and autopsy database and review of the
literature. Virchows Arch. 444:527–535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koizumi W, Kitago M, Shinoda M, Yagi H,
Abe Y, Oshima G, Hori S, Inomata K, Kawakubo H, Kawaida M and
Kitagawa Y: Successful resection of pancreatic metastasis from
oesophageal squamous cell carcinoma: A case report and review of
the literature. BMC Cancer. 19:3202019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin P, Ji X, Ren H, Tang Y and Hao J:
Resection or cryosurgery relates with pancreatic tumor type:
primary pancreatic cancer with previous non-pancreatic cancer or
secondary metastatic cancer within the pancreas. Pancreatology.
14:64–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cortez N, Berzosa M, Mahfouz M, Dvir K,
Galarza Fortuna GM and Ben-David K: Diagnosis and treatment of
metastatic disease to the pancreas. J Laparoendosc Adv Surg Tech A.
30:1008–1012. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ajani JA, D'Amico TA, Bentrem DJ, Cooke D,
Corvera C, Das P, Enzinger PC, Enzler T, Farjah F, Gerdes H, et al:
Esophageal and esophagogastric junction cancers, version 2.2023,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 21:393–422. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kitano M, Minaga K, Hatamaru K and Ashida
R: Clinical dilemma of endoscopic ultrasound-guided fine needle
aspiration for resectable pancreatic body and tail cancer. Dig
Endosc. 34:307–316. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lanke G, Stewart JM and Lee JH: Pancreatic
paraganglioma diagnosed by endoscopic ultrasound-guided fine needle
aspiration: A case report and review of literature. World J
Gastroenterol. 27:6322–6331. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Long X, Hu ZN, Wu Y, Song J,
Zhang BX and Chen WX: An extremely atypical presentation of
esophageal squamous cell carcinoma with pancreatic and hepatic
metastases: A case report and overview of the literature. Medicine
(Baltimore). 100:e257852021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fritscher-Ravens A, Sriram PV, Krause C,
Atay Z, Jaeckle S, Thonke F, Brand B, Bohnacker S and Soehendra N:
Detection of pancreatic metastases by EUS-guided fine-needle
aspiration. Gastrointest Endosc. 53:65–70. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang H, Kim SS, Sung MJ, Jo JH, Lee HS,
Chung MJ, Park JY, Park SW, Song SY, Park MS and Bang S: Evaluation
of the 8th edition AJCC staging system for the clinical staging of
pancreatic cancer. Cancers (Basel). 14:46722022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Waters JK and Reznik SI: Update on
management of squamous cell esophageal cancer. Curr Oncol Rep.
24:375–385. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Businello G, Parente P, Mastracci L,
Pennelli G, Traverso G, Milione M, Bellan E, Michelotto M, Kotsafti
A, Grillo F and Fassan M: The pathologic and molecular landscape of
esophageal squamous cell carcinogenesis. Cancers (Basel).
12:21602020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li K, Nie X, Li C, He W, Wang C, Du K, Li
K, Liu K, Li Z, Lu S, et al: Mapping of lymph node metastasis and
efficacy index in thoracic esophageal squamous cell carcinoma: A
large-scale retrospective analysis. Ann Surg Oncol. 30:5856–5865.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen J, Cai W, Lin Y, Chen Y, Zheng Q, Pan
J and Chen C: Patterns and rates of abdominal lymphatic metastasis
following esophageal carcinoma. PLoS One. 12:e01854242017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mogal H, Fields R, Maithel SK and
Votanopoulos K: In patients with localized and resectable gastric
cancer, what is the optimal extent of lymph node dissection-D1
versus D2 versus D3? Ann Surg Oncol. 26:2912–2932. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shaheen O, Ghibour A and Alsaid B:
Esophageal cancer metastases to unexpected sites: A systematic
review. Gastroenterol Res Pract. 2017:16573102017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Betés M, González Vázquez S, Bojórquez A,
Lozano MD, Echeveste JI, García Albarrán L, Muñoz Navas M and
Súbtil JC: Metastatic tumors in the pancreas: The role of
endoscopic ultrasound-guided fine-needle aspiration. Rev Esp Enferm
Dig. 111:345–350. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Zhang Y, Peng L and Zhang L:
Research progress on the predicting factors and coping strategies
for postoperative recurrence of esophageal cancer. Cells.
12:1142022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sugimura K, Tanaka K, Sugase T, Momose K,
Kanemura T, Yamashita K, Makino T, Shiraishi O, Motoori M, Yamasaki
M, et al: Clinical impact of conversion surgery after induction
therapy for esophageal cancer with synchronous distant metastasis:
A multi-institutional retrospective study. Ann Surg Oncol.
31:3437–3447. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu C, Tsai PC, Chien LI, Huang CS, Hsieh
CC, Hsu HS and Hsu PK: Esophagectomy in patients with esophageal
squamous cell carcinoma and distant nodal metastasis. Dis
Esophagus. 37:doae0642024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dumonceau JM, Deprez PH, Jenssen C,
Iglesias-Garcia J, Larghi A, Vanbiervliet G, Aithal GP, Arcidiacono
PG, Bastos P, Carrara S, et al: Indications, results, and clinical
impact of endoscopic ultrasound (EUS)-guided sampling in
gastroenterology: European society of gastrointestinal endoscopy
(ESGE) clinical guideline-updated january 2017. Endoscopy.
49:695–714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Malacara VC, Limón CGL, Quintana OB and
Macías GSG: Metastases to pancreas diagnosed by endoscopic
ultrasound-guided fine-needle aspiration: A case series and review
of imaging and cytologic features. Cytojournal. 20:182023.
View Article : Google Scholar : PubMed/NCBI
|