Introduction
Nasopharyngeal cancer (NPC) is a rare epithelial
carcinoma with an extremely uneven geographical distribution. As
represented by GLOBOCAN data for 2020, NPC contributes to only 0.7%
of the total number of new cancer cases worldwide, and 70% of these
cases are diagnosed in Eastern and Southeastern Asia (1). According to the 4th edition of the
World Health Organization classification, these carcinomas are
divided into three types: non-keratinizing (subdivided into
differentiated and undifferentiated), keratinizing and basaloid
squamous cell types (2).
Non-keratinizing NPC (NK-NPC) has distinct features such as ethnic
disparities, radio- and chemosensitivity, metastatic potential and
well-proven role of Epstein Barr virus in its pathogenesis
(3). Due to its sensitivity to
anti-neoplastic treatment, a large proportion of patients, even in
advanced stages of disease, could be cured or achieve long-term
remission (3).
Late presentation of NK-NPC requires multimodality
treatment. Radiotherapy (RT) is a mainstay of the curative-intent
approach, with high doses (70 Gy) needed for the eradication of the
gross tumor and doses of 50–60 Gy required for regions of
microscopic infiltration risk (4).
Chemotherapy, in different schemes of application (induction,
concomitant and adjuvant) is added. Cisplatin-based concomitant
chemotherapy has become a treatment standard when considering data
from randomized clinical trials and meta-analyses, which has
indicated that combined treatment improves overall survival, local
recurrence-free survival and distant metastasis-free survival rates
(5–7).
This intensive combined therapeutic approach
inevitably leads to numerous toxicities, both acute and late. In
contrast to acute toxicities, late sequelae are in general
unrecognized, underestimated and often lacking adequate therapy.
Nevertheless, their paramount importance comes from a tendency to
progress over time, affecting patients' quality of life (QoL),
increasing morbidity and even causing mortality. Published
literature reporting late morbidity in NK-NPC survivors is scarce,
often inconsistent and, following uneven geographical distribution,
predominantly driven from the Asian population of patients with
endemic NK-NPC (8–11). Therefore, the present study aimed to
analyze the occurrence and severity of late toxicities following
CRT in patients with strictly non-endemic NK-NPC.
Materials and methods
Study design
The present clinical retrospective study was
conducted at the Institute for Oncology and Radiology of Serbia
(Belgrade, Serbia) between January 2015 and December 2020. The
Multidisciplinary Tumor Board made treatment decisions for every
patient based on current recommendations. Written informed consent
was obtained from all subjects, who were fully aware of the planned
combined treatment and its potential acute and late toxicities.
Information on data publication was included in the consent.
Confidentiality was maintained throughout the study with the
protection of subjects' personal information. Anonymity was
carefully maintained and no disclosure of any personal identifiers
was allowed.
Patient characteristics
A total of 36 adult patients were included from our
non-endemic region (state of Serbia), all with histologically
confirmed, non-metastatic, NK-NPC. Since the Institute for Oncology
and Radiology of Serbia is a referral center for Serbia, patients
not only from Belgrade but from other regions were also included.
Patients treated with palliative intent or previously treated for
another malignancy, those with refractory disease or patients with
Eastern Cooperative Oncology Group performance score (ECOG PS) ≥2
were excluded (12). Based on local
findings upon an ear, nose and throat examination with
fiber-endoscopy, a full blood count with biochemical analysis and
radiological imaging [multi-slice computed tomography (CT)/magnetic
resonance imaging of splanchnocranium/head and neck, lung X-rays
and ultrasound of the abdomen], patients were staged according to
the seventh edition of the American Joint Committee on Cancer/Union
for International Cancer Control (AJCC/UICC) staging system up till
2018 and according to the eight edition of the AJCC/UICC staging
system onwards (13,14).
RT
Radical RT was planned for every patient with a
tumor dose of 70 Gy in 35 fractions, with a standardized
fractionation regimen (2 Gy per fraction) and conformal technique.
After patient immobilization in a supine position with a
thermoplastic immobilization mask, a CT simulation of the head and
neck from the vertex to the fifth thoracic vertebra was performed
obtaining 2.5-mm thick slices with intravenous iodine contrast.
According to the institutional treatment protocol
for the Institute of Oncology and Radiology of Serbia and the
International Commission on Radiation Units and Measurements
Reports 50, 62 and 83, target volumes were delineated on each slide
of the CT simulation (15–17). The gross tumor volume (GTV) was
defined by the primary tumor and macroscopic metastatic lymph
nodes.
The high-risk clinical target volume (CTV) included
the GTV with 5-mm margins, and a tumor dose (TD) of 70 Gy in 35
fractions was prescribed to that volume. Intermediate-risk CTV
covered the entire nasopharynx, parapharyngeal space, clivus, base
of the skull, pterygoid fossa, posterior half of ethmoidal sinus,
inferior sphenoid sinus or the whole sphenoid sinus if the tumor
invaded its inferior parts, the posterior edge of the nasal cavity
and the maxillary sinuses. A TD of 60–64 Gy in 30–32 fractions was
prescribed to this volume. Low-risk CTV encompassed
intermediate-risk CTV with prophylactic neck node level I–V and
VIIa bilaterally, with a TD of 50 Gy in 25 fractions prescribed.
This standardized dose configuration was planned for every patient,
respecting the dose constraints on the organs at risk.
To account for the patient motion and set-up error,
each CTV was expanded by 3–5 mm, thus obtaining the planning tumor
volume. More narrow margins (1–3 mm) were used in situations when
the primary tumor was adjacent to a critical neurological
structure.
On each slice, organs at risk, including the brain
stem, spinal cord, temporal lobes, brain optic nerves, optic
chiasm, lens, parotid glands, mandible and temporomandibular
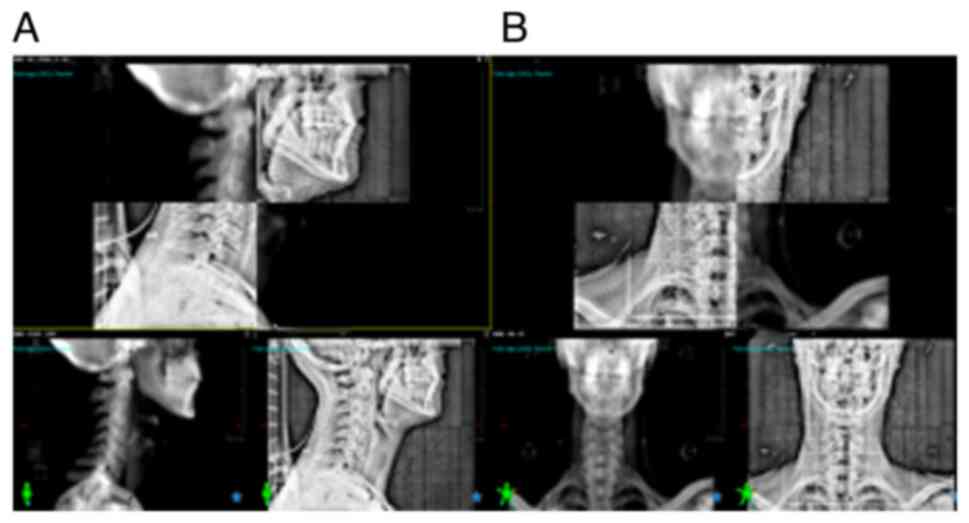
joints, were also outlined (Fig.
1). Irradiation was delivered on linear accelerators with
integrated multi-leaf collimators and high-energy photons. Adequate
patient positioning on the RT machine underwent regular everyday
verifications with kV or MV portals and/or cone beam CT.
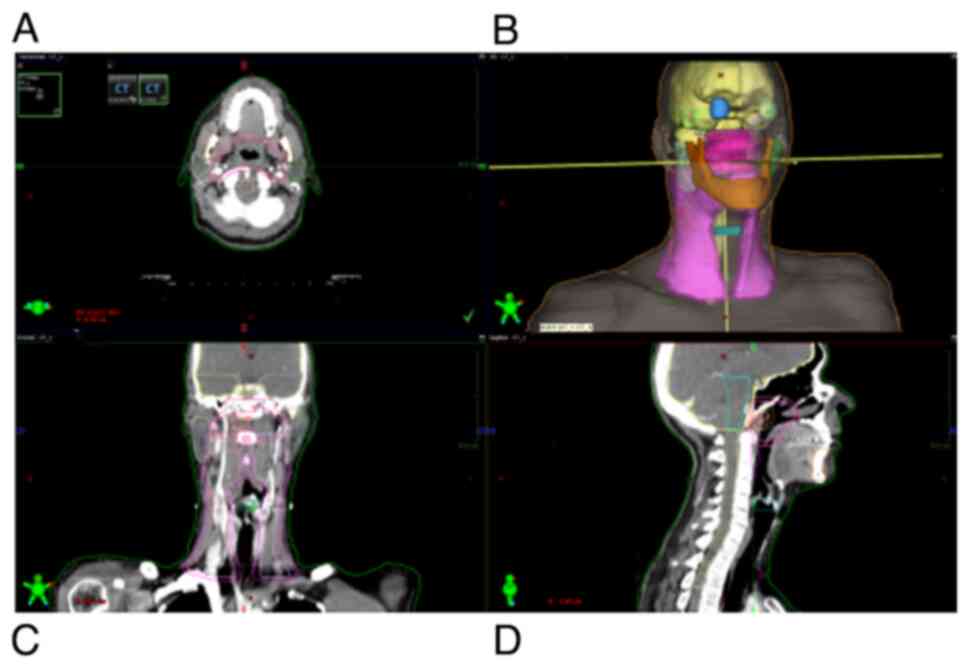 | Figure 1.Organs at risk and tumor volume
delineation in three planes and in three-dimensional reconstruction
on treatment planning CT simulation taken from the vertex to the
fifth thoracic vertebra. (A) Axial plane, (B) three-dimensional
reconstruction, (C) coronal plane and (D) sagittal plane. Colors
representing OAR: Brain, yellow; brainstem, cyan; optic chiasm,
light orange; spinal cord, light yellow; temporal lobe left,
purple; temporal lobe right, light green; eye globe right, blue;
eye globe left, translucent green; mandible, dark orange; parotid
gland left, dark green; parotid gland right, mint; glottis, dark
cyan. Colors representing tumor volumes: GTVprimary, red; GTVnodal,
dark red; CTV50, magenta; PTV50, pink; CTV60, dark magenta; PTV60,
dark pink; CTV70, light pink; PTV70, light orange. GTV, gross tumor
volume; CTV, clinical target volume; PTV, planning target
volume. |
Everyday patient's positioning on the RT machine
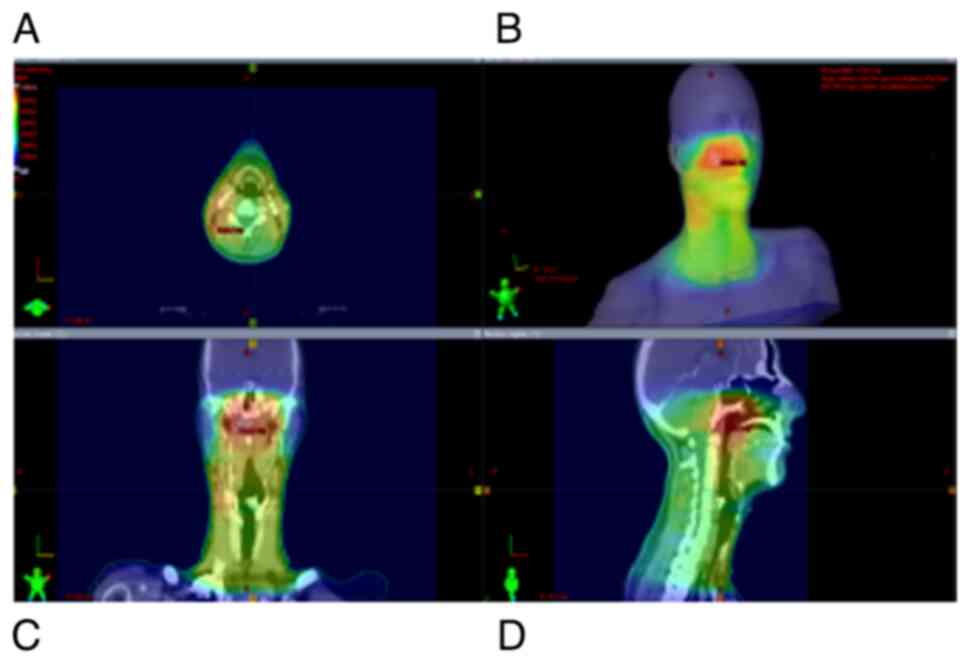
demanded a high level of precision, as shown in Fig. 2. Over the study period, RT for
treating NPC patients was delivered with the 3D conformal technique
up until 2018, and with the intensity-modulated radiation therapy
(IMRT) technique afterward, following its implementation in the
Institute of Oncology and Radiology of Serbia (Fig. 3).
Faces shown in the top right panel of Figs. 1 and 3 are digital 3D reconstructions obtained
from three planes (axial, coronal and sagittal) of RT treatment
planning CT, which represents a virtual patient, i.e. a 3D model of
a particular patient which is the concept of 3D-conformal RT.
Figs. 1 and 3 are presented to depict the extreme
proximity of tumor volumes and healthy structures (organs at risk)
in patients' radiation plans, as well as localization and volume of
skin and subcutaneous tissues included in the irradiated
volume.
Chemotherapy
All patients received concurrent cisplatin weekly,
with a dose of 40 mg/m2. The aim was to administer at
least 5 or 6 cycles of mono cisplatin a 40 mg/m2
concurrent with radiation. Neoadjuvant chemotherapy was
administered in high-risk patients with locoregional extended
disease. In a subgroup of patients who did not start treatment with
neoadjuvant chemotherapy, yet were assessed as high risk for local
recurrence or distant dissemination upon the completion of CRT, an
adjuvant chemotherapy was applied. Neoadjuvant, as well as an
adjuvant chemotherapy consisted of cisplatin (80 mg/m2)
with 5-fluorouracil (800 mg/m2) continuous infusion for
5 days, recycled every 3 weeks for three cycles.
Follow-up and statistical
analysis
After combined CRT treatment, patients were followed
up every 3 months during the first 2 years, every 6 months from
year 2 to year 5, and once a year thereafter. The late toxicities
were clinician-reported and graded according to the the Radiation
Therapy Oncology Group/European Organization for Research and
Treatment of Cancer ‘Late Radiation Morbidity Scoring Schema’
(18).
Measurements of descriptive statistics are used for
interpretation of the parameters of importance. Categorical
variables are described using frequencies (percentages), while
mean, median, standard deviation and range are used for numeric
variables. For testing normal data distribution, the
Kolmogorov-Smirnov and Shapiro-Wilk tests were used. P<0.05 was
considered to indicate a statistically significant difference. To
compare disease characteristics, treatment details and toxicities,
statistical tests were selected based on data type and
distribution: The Wilcoxon rank-sum test for continuous variables,
and Fisher's exact test for categorical variables. The statistical
analysis was performed with the program R [version 4.3.1
(2023-06-16 ucrt) - ‘Beagle Scouts’; Copyright© 2023 The
R Foundation for Statistical Computing; Platform:
×86_64-w64-mingw32/x64 (64-bit)] (www.r-project.org; downloaded: August 21, 2023).
Results
All patients were >18 years old, with a median
age of 49.5 years (range, 18–71 years) and with good performance
status (ECOG PS≤1). Of the 36 patients included, 27 were men and 9
were women, with a male/female ratio of 3:1. The majority of
patients presented in the advanced stage of disease. Specifically,
47.2% (17 patients) were in clinical stage III and 27.8% (10
patients) were in stage IVA. A total of 25% of the cohort (9
patients) was diagnosed in stage II (Table I).
 | Table I.Patients and tumor
characteristics. |
Table I.
Patients and tumor
characteristics.
| Characteristic | n (%) |
|---|
| Age, years |
|
|
Median | 49.5 |
|
Range | 18-71 |
| Sex |
|
|
Men | 27 (75.0) |
|
Women | 9 (25.0) |
| ECOG PS |
|
| 0 | 26 (72.2) |
| 1 | 10 (27.8) |
| Comorbidity |
|
|
HTA | 6 (16.7) |
| Heart
disease | 2 (5.6) |
|
Diabetes mellitus | 3 (8.3) |
|
Lung | 1 (2.8) |
|
Depression | 1 (2.8) |
|
Hepatitis | 1 (2.8) |
| Stage |
|
| II | 9 (25.0) |
|
III | 17 (47.2) |
|
IVA | 10 (27.8) |
| T stage |
|
| T1 | 6 (16.7) |
| T2 | 10 (27.8) |
| T3 | 12 (33.33) |
| T4 | 8 (22.2) |
| N stage |
|
| N0 | 4 (11.1) |
| N1 | 12 (33.3) |
| N2 | 17 (47.2) |
| N3 | 3 (8.3) |
In 29 patients (80.6%), treatment commenced with
induction chemotherapy, and all patients completed three cycles. In
the adjuvant setting, 6 patients (16.7%) received chemotherapy,
with a median of two cycles. Whether chemotherapy was administered
in the induction or adjuvant approach, all patients were scheduled
for CRT according to the Multidisciplinary Tumor Board decision
based on current treatment protocols.
Over the study period, RT was delivered with IMRT in
just over one-third of patients (14 patients; 38.9%). The rest of
the patients were treated with 3D conformal RT. The median TD
reached was 68.64 Gy, with a range of 44–70 Gy. Out of the 36
patients, 32 (88.9%) received a TD of 70 Gy in 35 fractions. Only 4
patients did not reach the prescribed dose. In 2 of these patients,
therapy was terminated earlier due to Covid infection (one after a
TD of 62 Gy and the other after a TD of 64 Gy), while 1 patient
refused to continue with CRT after manifestation of an acute
toxicity of severe grade [mucositis grade III according to CTCAE
vs. 4.3 (19)] and reached a TD of
40 Gy. In the fourth patient, the reason for failure to receive the
planned TD was the development of neurological symptomatology
unrelated to primary disease after a TD of 60 Gy.
In the absence of contraindications, from the start
of RT, chemopotentiation was applied with mono cisplatin in the
weekly regimen, with a dose of 40 mg/m2. Six cycles were
realized in 3 patients (8.3%). Most of the patients (11 patients;
30.6%) received five cycles. Four cycles of weekly cisplatin were
applied in 7 patients (19.4%) and three cycles in another 7
patients (19.4%). Five patients (13.9%) received two cycles of
concurrent chemotherapy. The predominant reasons for chemotherapy
withdrawal were hematological and non-hematological acute
toxicities, namely mucositis grade III, dermatitis grade III,
febrile neutropenia, neutropenia grade III and thrombopenia grade
III.
The median number of concomitant cycles was four. In
total, 3 patients (8.3%) received only one cycle. Of these, 1
patient withdrew from further therapy after one cycle of cisplatin
and a TD of 40 Gy due to severe acute toxicity, namely mucositis
grade III, as aforementioned. In another patient, also previously
mentioned, CRT was interrupted after one cycle of mono cisplatin
and a TD of 60 Gy due to central nervous symptomatology
development. In the third patient, further application of
concomitant CT was interrupted due to HCV infection activation, but
RT was completed to a TD of 70 Gy/35 fractions.
After the completion of CRT, during regular
follow-up, late sequelae of any sort were registered in 30 out of
the 36 patients (83.3%). No late sequelae of any type were found in
6 patients (16.7%) (Table II).
 | Table II.Distribution of the late
toxicities. |
Table II.
Distribution of the late
toxicities.
| Late toxicity | n (%) |
|---|
| Overall
toxicity | 30 (83.3) |
| Neck fibrosis | 25 (69.4) |
| Late
xerostomia | 21 (58.3) |
| Late dysphagia | 2 (5.6) |
| Secondary
hypothyroidism | 4 (11.1) |
| Secondary
neuropathy | 3 (8.3) |
The most common treatment-related late complication
was neck fibrosis, experienced by 25 patients (69.4%) in the form
of skin thickening and induration, muscle atrophy and loss of
subcutaneous fat. Late xerostomia developed in 21 patients (58.3%).
Only xerostomia grade ≤2 with no notable impact on the dietary
habits was recorded as follows: Grade 1 in 41.67% of the cases and
grade 2 in 16.67%. Only 2 patients (5.6%) developed late dysphagia.
As the most serious and potentially life-threatening outcomes of
this sequela, no instance of aspiration pneumonia or the need for a
feeding tube was recorded. Secondary hypothyroidism as a
post-irradiation consequence developed in 4 patients (11.1%) and
was easily corrected with synthetic thyroid hormone. Neuropathy was
noted in 3 patients (8.3%) in the form of facial numbness,
paresthesia and dysesthesia, and palsy of the VII cranial
nerve.
All registered late adverse events were
statistically analyzed. Due to the sporadic occurrence of late
dysphagia, secondary hypothyroidism and secondary neuropathy, no
significant associations to patient, tumor or treatment
characteristics were found, so the late toxicities that are most
likely to affect the majority of patients treated with CRT were
focused upon.
Exploring the associations between patient
characteristics, no statistically significant difference was found
in the frequency of overall late toxicity (P=0.14), neck fibrosis
(P=0.37) and late xerostomia (P>0.999) between men and women. In
the present study, tumor (T) stage, node (N) stage and clinical
stage of disease did not have a significant impact on late toxicity
prevalence (Table III).
 | Table III.Overall toxicity, late neck fibrosis
and late xerostomia associations with tumor and treatment
characteristics analyzed by Fisher's exact test. |
Table III.
Overall toxicity, late neck fibrosis
and late xerostomia associations with tumor and treatment
characteristics analyzed by Fisher's exact test.
| Characteristic | Overall
toxicity | Late neck
fibrosis | Late
xerostomia |
|---|
| Male/female | P=0.14 | P=0.37 | P>0.999 |
| T stage (1–4) | P=0.48 | P=0.90 | P=0.72 |
| N stage (0–3) | P=0.80 | P=0.91 | P=0.43 |
| Clinical stage
(2–4) | P=0.61 | P=0.87 | P=0.51 |
| Neoadj.chemo/no
neoadj.chemo | P=0.58 | P>0.999 | P=0.69 |
| 3D conformal
RT/IMRT | P=0.18 | P>0.999 | P=0.09 |
| Adj.chemo/no
ajd.chemo | P=0.52 | P=0.29 | P=0.65 |
The results imply that the administration of
chemotherapy in different settings, neoadjuvant, concomitant or
adjuvant, has no significant effect on the occurrence of
post-therapy overall toxicity and late neck fibrosis (Table III). However, late xerostomia
occurred significantly more often in patients who received five
cycles compared with that in patients who received less than five
cycles of weekly cisplatin concomitant with RT (P=0.02).
Neoadjuvant and adjuvant chemotherapy applications had no
statistically significant influence on its development.
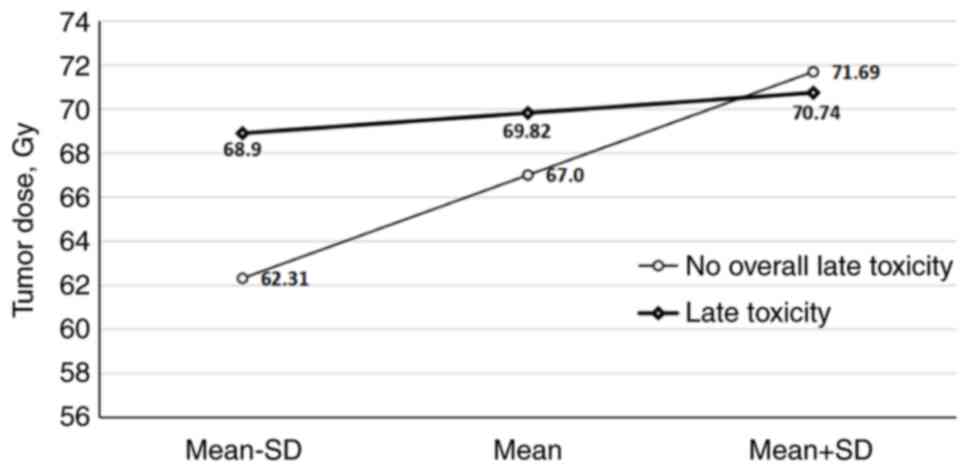
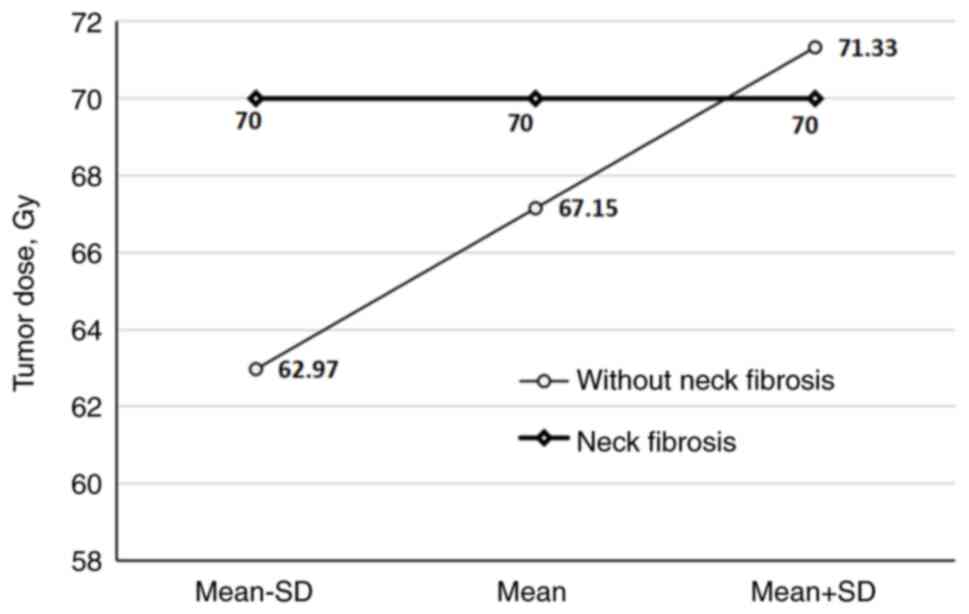
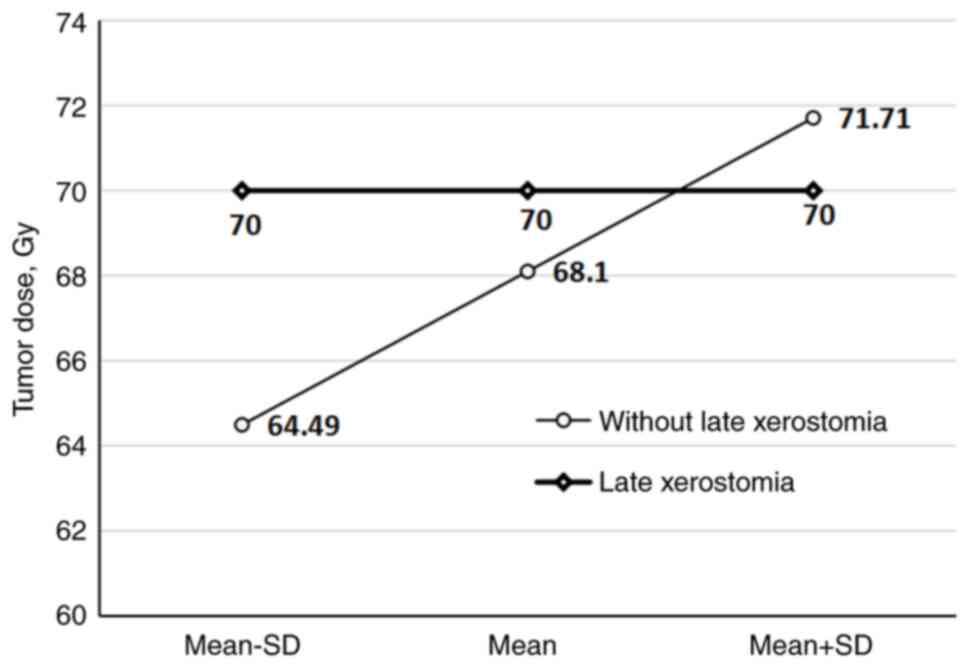
The delivered TD had a substantial impact on the
overall late toxicity, neck fibrosis and late xerostomia frequency
in the patients (P=0.02, P=0.002 and P=0.02, respectively) when a
mean TD of 67.0, 67.15 and 68.1 Gy was reached (Fig. 4, Fig.
5, 6).
Although in the subgroup of 22 patients treated with
3D conformal RT, a type of late post-therapy effect manifested in
66.7% of patients, late fibrosis in 60.0% and secondary xerostomia
in 71.4%, when compared with patients treated with IMRT,
statistical significance with regard to late toxicity incidence was
not reached (P=0.18, P>0.999 and P=0.09, respectively).
Discussion
The last few decades have witnessed immense and
accelerated advances in RT techniques and planning systems, as well
as the appliance of chemotherapy in various sequencing schemes,
along with RT in the management of NPC, thus providing promising
outcomes for treated patients (3,6,20). As
a result of this, survivorship issues emerge. Treatment-induced
late consequences can cause significant morbidity in cancer
survivors, diminish their QoL and even, in rare cases, lead to
death (9). Specific localization of
NK-NPC and notoriously narrow therapeutic margins indicate the
possibility of morphological, functional and esthetic damage.
Unlike acute toxicities, late sequelae are globally more likely to
be unrecognized, underestimated and left without adequate
treatment, even though the majority of patients can suffer from one
or several toxicities. Published data on this topic tend to be
scarce and inconsistent, but also follow the extremely unequal
geographical and ethnic distributions of NK-NPC, dominantly
obtained from the Asian population of patients (8–11). In
such circumstances, results gained from the present retrospective
study of patients with non-endemic NK-NPC have to be discussed and
compared mainly with results registered in Asian-based studies.
In the present study, a male predominance was found
regarding sex distribution, with 75% of patients being men. This is
in strong concordance with data from a meta-analysis by Blanchard
et al (3), which reported
74% of patients being male, and with GLOBOCAN data (1), which imply a male to female ratio of
2–3:1. The median age of 49.5 years is in line with the median age
of <50 years reported in some previous studies (3,21). The
majority of patients in the current study presented in the
locoregional advanced stage of disease, with 72% in stages III and
IVA, due to the aggressive biological behavior of NPC-NK, as shown
across the literature (3,8,9,21,22).
Given their immense potential to negatively
influence the QoL of NPC survivors, late sequelae are gaining
importance within the contemporary scientific community. Much of
our knowledge of this relationship is based on clinician-reported
records. As it has been accepted that clinicians may inadvertently
underestimate symptoms and their severity, oncological interest is
shifting to patient-reported outcomes (PROs) (23,24).
However, the paucity of data from NPC survivors also affects this
aspect of investigation, and only a limited number of clinical
trials have included PROs when performing QoL assessments (22). One of the more recent and important
investigations was performed by McDowell et al (22) in a non-endemic medical center. The
study showed that the five highest-scoring items of PROs were
problems with dry mouth, mucus, swallowing or chewing, memory, and
teeth or gums. Moderate associations between physician-reported
adverse events and PROs were seen for dysphagia, xerostomia,
dysarthria, voice/speech and aspiration. To overcome short-comings
of reporting late post-treatment events, trials dealing with QoL
are suggested to incorporated both PROs and clinician-reported
adverse events.
In the present clinician-reported study, more than
three-quarters of patients developed some form of late toxicity
after completion of combined therapy. To the best of our knowledge,
there is a paucity of data regarding overall post-treatment
toxicities in this particular type of cancer after CRT. Several
reasons can be provided in the explanation of this such as
heterogeneity of baseline patients characteristics, different study
designs, heterogeneity of therapy approaches, rarity of tumor and
insufficient reporting. In a study conducted by Lee et al
(8), in 422 patients treated with
3D conformal RT with a conventional or accelerated fractionation
regimen, with or without chemotherapy, the overall toxicity rate
was 27%, with a markedly greater rate of 37% in the
chemotherapy-based group (16). In
another study performed by Zeng et al (9), higher overall incidence of one or more
late toxicities was registered in 55.8% of patients. Also, the
study emphasized that a notably greater frequency of late sequelae
was found in patients treated with CRT compared to those treated
with RT alone (63.2 vs. 42.0%). Although it is hard to make a
comparison among heterogeneous groups of patients and those with
different treatments, it could be argued that, among the other
aforementioned reasons, differences in ethnicity may play a role in
the greater incidence of overall toxicities in the present study
compared with the incidence of general toxicities in these studies
on the endemic population. No significant impacts of patient and
tumor characteristics on overall post-therapy consequences were
found in the study, in contrast to findings from the study by Zeng
et al (9), which found that
T and N stage categories were significant factors for late
toxicities in general. Previously, Lee et al (8) determined that only patient age was a
strong significant factor in overall late toxicities, but not T and
N tumor stage.
Nearly 70% of patients in the present group
developed subcutaneous neck fibrosis as the most frequent late
toxicity after CRT treatment. In the study conducted by Huang et
al (10), secondary neck
fibrosis was registered in 71.1% of cases in the group of patients
with NPC irradiated using a non-IMRT planning technique; however,
in the group of patients irradiated with IMRT, the frequency of
this late sequela was considerably lower at 34% (18). It should be underlined no
chemotherapy was administered in the study by Huang et al
(10). Zeng et al (9) recorded a neck fibrosis incidence rate
of 34.3% in patients treated with IMRT, and a considerably higher
rate of 45.1% in the group of patients who underwent combined CRT.
In one of the most comprehensive studies coming from a center
outside an endemic region, the study conducted by McDowell et
al (22) reported subcutaneous
neck fibrosis in 55% of patients. Again, RT doses were delivered
with IMRT in all patients. Although this study informs on
physician-reported late toxicities and PROs in patients with NPC
treated with IMRT in a non-endemic center, it should be highlighted
that the majority of the investigated cohort was comprised of
patients born in endemic regions. In addition, 7% of patients in
this study did not receive chemopotentiation. More recently, in
2019, Zhao et al published 10-year results of a phase 2
prospective study where subcutaneous neck fibrosis was present in
91% patients, predominantly of a lesser grade (11). As can be seen from these data, a
wide range of cumulative incidences of this late side effect span
across different clinical studies, even though they were all
conducted on patients from endemic regions, which brings us back to
the pressing issue of inhomogeneity of study designs. It seems that
the present group of non-endemic patients may be more prone to the
neck fibrosis, when keeping in mind that IMRT was applied in just
over one-third of patients and that all of them received one or
more cycles of cisplatin.
According to the previously published studies on
head and neck tumors of various subsites treated with RT, the
frequency of late xerostomia ranged from 64 to 95% depending on
tumor localization, RT planning technique and delivered TD, but
also depending on patient-orientated factors (10,25).
Several studies have strongly supported IMRT in terms of decreasing
permanent xerostomia (20,26,27).
In the present study, late xerostomia was recorded in almost
two-thirds of patients (58.3%), with grade 2 found in 16.7% of
patients. This result aligns closely with that from a study
performed by Lee et al (8),
where only 14% of patients reported grade 2 ×erostomia and 35% of
the patients did not develop xerostomia. In a study by Zeng et
al (9) conducted in 2014 on NPC
survivors irradiated with IMRT with or without chemotherapy,
xerostomia was observed in 78.1% of patients. However, a notably
higher incidence of this long-term consequence was registered in
the study by McDowell et al (22) performed in a non-endemic RT center.
Overall, 95% of the patients treated with IMRT suffered from
low-grade late xerostomia according to physician-reported outcomes
(22). Several reasons for this
discrepancy compared with the present results could be offered,
from the different study designs (cross-sectional cohort study vs.
retrospective study), the longer time to event (subjects enrolled
were disease-free ≥4 years after treatment), the influence of
sample size and differences in adverse events reporting, to the
impact of ethnicity on toxicity manifestation (majority of patients
were of Asian descent). In the study by Huang et al
(10), female survivors were
observed to have a 2.6-fold higher probability of secondary
xerostomia, but a significant association could not be confirmed
between patient and tumor demographics and the later development of
xerostomia (10). While it is well
established that the development and degree of xerostomia is
largely dependent on the dose and volume of the salivary gland in
the radiation field, the role of chemotherapy is less clear and
literature data are somewhat contrasting. Zeng et al
(9) concluded that chemotherapy was
not a relevant factor affecting xerostomia, which agrees with
studies by Miah et al (28)
and Chao et al (29), where
the research was performed on patients with head and neck cancer.
By contrast, the study by Ou et al (30) demonstrated that concurrent
chemotherapy significantly increased xerostomia compared to RT
alone (46.4 vs. 36.3%), as well as total cisplatin dose increasing
overall late toxicities. That being said, the present result is
comparable with the findings from previously mentioned studies
regarding the cumulative prevalence rate of xerostomia even though
the patients were irradiated both with non-IMRT and IMRT planning
techniques. Also, in alignment with the results by Ou et al
(30), the present study
demonstrated a statically significant impact of cisplatin
chemopotentiation on late xerostomia prevalence, with a median of 5
cycles (P=0.02).
The only common significant factor for overall late
sequela and the most prevalent ones (subcutaneous neck fibrosis and
late xerostomia) that could be identified in the present study was
the delivered TD (P=0.02, P=0.002 and P=0.02, respectively). The
difference became statistically significant when the mean TDs of
67.0, 67.15 and 68.1 Gy were reached. The cumulative incidences of
these three chronic toxicities were numerically notably lower in
the subgroup of patients irradiated with IMRT in comparison to
patients irradiated with 3D conformal RT, but the differences did
not reach statistical significance, possibly due to the small
sample size.
On the matter of cranial neuropathy, which presented
with numbness, faint pain and cranial nerve palsy in the current
patients, the record of 8.3% in this population of patients is
comparable with published data. For example, in a study performed
by Huang et al (10), in the
group of patients irradiated with non-IMRT, neuropathy was found in
19.1% of cases, while notably less, only 5.0% of cases, exhibited
neuropathy in the group of patients irradiated with IMRT. Also,
Zeng et al (9) reported a
frequency of 2.2% for cranial neuropathy in patients treated with
CRT, and Zhao et al (11)
reported a frequency of 6.5% in patients irradiated with the IMRT
technique only. These numbers are in close alignment with the
results of the present study. Notably, during the study period, no
case of temporal lobe necrosis, as the most severe scenario of
neuropathy, was registered in the present patient cohort.
One of the most troublesome late sequela is late
dysphagia due to its potential to cause aspiration and a decrease
in body mass index. Across the literature, data are scarce and
heterogeneous. Huang et al (10) found 53.5% of cases with late
dysphagia in a non-IMRT group of patients and 22% of cases in the
IMRT group. By contrast, only 1.3% of this sequela was reported in
the study by Ou et al (30).
The present finding of 5.6% late dysphagia of lesser grade is
closer to that of the latter study.
Secondary hypothyroidism was registered in 11.1% of
cases in the present study, which is slightly higher than the rate
in the study by Lee et al (8), where 6.6% patients developed this
hormonal dysfunction.
As already mentioned, NK-NPC is a malignant disease
with prominent ethnic and geographical disparities, so the vast
majority of studies considered regarding this topic are conducted
in the endemic population, with practically no studies performed in
non-endemic regions (8–11,29,30).
It is particularly relevant to contrast and compare results driven
from both regions, since treatment protocol is still based on a
‘one size fits all’ approach, and the ultimate goal in modern
oncology tends to be individualization and optimization of
therapy.
Novel promising therapeutical approaches are,
however, changing the landscape of NPC therapy. In the aspect of RT
options, intensity-modulated proton therapy (IMPT) is proven to
have dosimetry advantages over IMRT, thus facilitating the delivery
of markedly reduced radiation doses to surrounding normal tissues.
However, according to the study conducted by Li et al
(31) on 77 patients with
non-metastatic NPC, differences in the incidence of grade 3 or
higher late toxicities between groups of patients treated with IMPT
and IMRT were not significant. The median follow-up in this study
was 2 years and it could be a potential factor impacting such
results.
Current perspectives on immunotherapy and targeted
therapy application in NK-NPC are established in recurrent and
metastatic settings. There are numerous ongoing prospective
clinical trials at phases II and III exploring the role of
treatments in a locoregional advanced stage of NPC (clinical trial
numbers: NCT06019130, NCT04833257, NCT02421640, NCT05628922 and
NCT06781112). Efficacy and safety results are highly
anticipated.
The present study, inevitably, has several
limitations. The study included subjects treated in the Department
of Radiotherapy for Solid Tumors and Hematological Malignancies,
Institute for Oncology and Radiology of Serbia (Belgrade, Serbia)
within a 5-year period, between January 2015 and December 2020. The
study was closed in 2022, so there was a minimum 2-year period of
follow-up, making it enough time for late sequelae to manifest and
to be recorded, as these occur as early as 6 months after
completion of RT. However, this was a shorter follow-up period for
those patients treated in 2020, compared with those treated in
2015, which should be considered as a limitation of the study, as
it could lead to underestimation of late toxicity occurrence in
that subgroup of patients due to the long latency. It has been
proposed by several studies that time-to-event analyses could
address this issue (32,33). The retrospective nature of the study
should be taken as a major limitation factor that could underpower
the conclusions. A relatively small sample number could also be
perceived as a limitation to the results. Further investigations
with pooled data from another non-endemic centers would be of a
tremendous importance.
In conclusion, according to the results of the
present study, the majority of patients with non-endemic NK-NPC who
underwent CRT will experience some form of late toxicities, but
predominantly of lesser grade. The most frequent registered late
toxicity is neck fibrosis, followed by xerostomia. In the constant
attempt to improve treatment, aiming for remission or prolongation
of a patient's lifetime, the importance of late morbidities is
increasingly being recognized and should be justified by further
research, especially in the challenging field of NK-NPC. The impact
of ethnic differences on the manifestation of post-treatment
complications is quite undetermined, and possibly, underestimated,
therefore investigations in the non-endemic population of patients
are warranted.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JJR, TA and MN were responsible for study
conceptualization. JJR, TA and NJK were responsible for the design
of the study methodology. Data acquisition was performed by JJR, VV
and NJK. Statistical analysis was performed by JJR. Data
interpretation was performed by JJR, TA, MN and VV. Original draft
preparation was the responsibility of JJR. TA and MN critically
reviewed and edited the manuscript. Final approval was granted by
TA and MN. JJR, TA and VV confirm the authenticity of all the raw
data. All authors have read and approved the manuscript.
Ethics approval and consent to
participate
The study was conducted according to the guidelines
of the Declaration of Helsinki. Ethical review and approval were
waived in this study, since no experiments or novel treatments were
conducted on humans. The methodology represents a standard
treatment modality that does not need ethical approval. The
decision to treat the patients in the manner described in the study
was made by the Multidisciplinary Tumor Board of the Institute for
Oncology and Radiology of Serbia (Belgrade, Serbia) according to
the current treatment protocols. Written informed consent was
obtained for all subjects involved in the study.
The manuscript is a part of the academic
sub-specialistic thesis of JJR approved by the Faculty of Medicine,
University of Belgrade (Belgrade, Serbia) on May 30, 2022 (protocol
number: 04 BR:20-UON-10). The Institute for Oncology and Radiology
of Serbia serves as a scientific teaching base for the Faculty of
Medicine of University of Belgrade.
Patient consent for publication
Written informed consent was obtained for all
subjects involved in the study. Before obtaining the consent,
patients were thoroughly informed about all aspects of the planned
combined treatment, its effects, possible acute and late
toxicities. All subjects were fully aware that the data would be
published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Naggar AK, Chan JKC, Grandis JR, Takata
T and Slootweg PJ: WHO Classification of Head and Neck Tumours. WHO
Classification of Tumours. 9. 4th edition. IARC Publications; Lyon:
pp. 65–69. 2017
|
|
3
|
Blanchard P, Lee A, Marguet S, Leclercq J,
Ng WT, Ma J, Chan AT, Huang PY, Benhamou E, Zhu G, et al:
Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An
update of the MAC-NPC meta-analysis. Lancet Oncol. 16:645–655.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee AW, Ng WT, Pan JJ, Poh SS, Ahn YC,
AlHussain H, Corry J, Grau C, Grégoire V Harrington KJ, et al:
International guideline for the delineation of the clinical target
volumes (CTV) for nasopharyngeal carcinoma. Radiother Oncol.
126:25–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al-Sarraf M, LeBlanc M, Giri PG, Fu KK,
Cooper J, Vuong T, Forastiere AA, Adams G, Sakr WA, Schuller DE and
Ensley JF: Chemoradiotherapy versus radiotherapy in patients with
advanced nasopharyngeal cancer: Phase III randomized Intergroup
study 0099. J Clin Oncol. 16:1310–1317. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen QY, Wen YF, Guo L, Liu H, Huang PY,
Mo HY, Li NW, Xiang YQ, Luo DH, Qiu F, et al: Concurrent
chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal
carcinoma: Phase III randomized trial. J Natl Cancer Inst.
103:1761–1770. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan AT, Leung SF, Ngan RK, Teo PM, Lau
WH, Kwan WH, Hui EP, Yiu HY, Yeo W, Cheung FY, et al: Overall
survival after concurrent cisplatin-radiotherapy compared with
radiotherapy alone in locoregionally advanced nasopharyngeal
carcinoma. J Natl Cancer Inst. 97:536–539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee AW, Ng WT, Hung WM, Choi CW, Tung R,
Ling YH, Cheng PT, Yau TK, Chang AT, Leung SK, et al: Major late
toxicities after conformal radiotherapy for nasopharyngeal
carcinoma-patient- and treatment-related risk factors. Int J Radiat
Oncol Biol Phys. 73:1121–1128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng L, Tian YM, Sun XM, Chen CY, Han F,
Xiao WW, Deng XW and Lu TX: Late toxicities after
intensity-modulated radiotherapy for nasopharyngeal carcinoma:
Patient and treatment-related risk factors. Br J Cancer. 110:49–54.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang TL, Chien CY, Tsai WL, Liao KC, Chou
SY, Lin HC, Dean Luo S, Lee TF, Lee CH and Fang FM: Long-term late
toxicities and quality of life for survivors of nasopharyngeal
carcinoma treated with intensity-modulated radiotherapy versus
non-intensity-modulated radiotherapy. Head Neck. 38 (Suppl
1):E1026–E1032. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao C, Miao JJ, Hua YJ, Wang L, Han F, Lu
LX, Xiao WW, Wu HJ, Zhu MY, Huang SM, et al: Locoregional control
and mild late toxicity after reducing target volumes and radiation
doses in patients with locoregionally advanced nasopharyngeal
carcinoma treated with induction chemotherapy (IC) followed by
concurrent chemoradiotherapy: 10-year results of a phase 2 study.
Int J Radiat Oncol Biol Phys. 104:836–844. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; Paris: 2010, Available from. http://www.springer.com/medicine/surgery/book/978-0-387-88440-0
|
|
14
|
Head and Neck Cancer Study Group (HNCSG),
. Monden N, Asakage T, Kiyota N, Homma A, Matsuura K, Hanai N,
Kodaira T, Zenda S, Fujii H, et al: A review of head and neck
cancer staging system in the TNM classification of malignant tumors
(eight edition). Jpn J Clin Oncol. 49:589–595. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
ICRU Report 50, . Prescribing, Recording,
and Reporting Photon Beam Therapy. International Commission on
Radiation Units and Measurements; Bethesda, MD: 1993
|
|
16
|
ICRU Report 62, . Prescribing, Recording,
and Reporting Photon Beam Therapy (Supplement to ICRU Report 50).
International Commission on Radiation Units and Measurements;
Bethesda, MD: 1999
|
|
17
|
Hodapp N: The ICRU Report 83: Prescribing,
recording, and reporting photon-beam intensity-modulated radiation
therapy (IMRT). Strahlenther Onkol. 188:97–99. 2012.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Late effects consensus conference, .
RTOG/EORTC. Radiother Oncol. 35:5–7. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
U.S. Department of Health and Human
Services, . Common Terminology Criteria for Adverse Events v4.0
(CTCAE). https://www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickreference_5×7.pdfMay
31–2023
|
|
20
|
Lee AW, Ng WT, Chan LL, Hung WM, Chan CC,
Sze HC, Chan OS, Chang AT and Yeung RM: Evolution of treatment for
nasopharyngeal cancer-success and setback in the
intensity-modulated radiotherapy era. Radiother Oncol. 110:377–384.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ozdemir S, Akin M, Coban Y, Yildirim C and
Uzel O: Acute toxicity in nasopharyngeal carcinoma patients treated
with IMRT/VMAT. Asian Pac J Cancer Prev. 16:1897–1900. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McDowell LJ, Rock K, Xu W, Chan B, Waldron
J, Lu L, Ezzat S, Pothier D, Bernstein LJ, So N, et al: Long-term
late toxicity, quality of life, and emotional distress in patients
with nasopharyngeal carcinoma treated with intensity modulated
radiation therapy. Int J Radiat Oncol Biol Phys. 102:340–352. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fromme EK, Eilers KM, Mori M, Hsieh YC and
Beer TM: How accurate is clinician reporting of chemotherapy
adverse effects? A comparison with patient-reported symptoms from
the quality-of-life questionnaire C30. J Clin Oncol. 22:3485–3490.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patrick DL, Ferketich SL, Frame PS, Harris
JJ, Hendricks CB, Levin B, Link MP, Lustig C, McLaughlin J, Ried
LD, et al: National institutes of health state-of-the-science
conference statement: Symptom management in cancer: Pain,
depression, and fatigue, july 15–17, 2002. J Natl Cancer Inst.
95:1110–1117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jasmer KJ, Gilman KE, Muñoz Forti K,
Weisman GA and Limesand KH: Radiation-induced salivary gland
dysfunction: Mechanisms, therapeutics and future directions. J Clin
Med. 9:40952020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alterio D, Gugliandolo SG, Augugliaro M,
Marvaso G, Gandini S, Bellerba F, Russell-Edu SW, De Simone I,
Cinquini M, Starzyńska A, et al: IMRT versus 2D/3D conformal RT in
oropharyngeal cancer: A review of the literature and meta-analysis.
Oral Dis. 27:1644–1653. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nutting CM, Morden JP, Harrington KJ,
Urbano TG, Bhide SA, Clark C, Miles EA, Miah AB, Newbold K, Tanay
M, et al: Parotid-sparing intensity modulated versus conventional
radiotherapy in head and neck cancer (PARSPORT): A phase 3
multicentre randomized controlled trial. Lancet Oncol. 12:127–136.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miah AB, Gulliford SL, Bhide SA, Zaidi SH,
Newbold KL, Harrington KJ and Nutting CM: The effect of concomitant
chemotherapy on parotid gland function following head and neck
IMRT. Radiother Oncol. 106:346–351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chao KS, Deasy JO, Markman J, Haynie J,
Perez CA, Purdy JA and Low DA: A prospective study of salivary
function sparing in patients with head-and-neck cancers receiving
intensity-modulated or three-dimensional radiation therapy: Initial
results. Int J Radiat Oncol Biol Phys. 49:907–916. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ou X, Zhou X, Shi Q, Xing X, Yang Y, Xu T,
Shen C, Wang X, He X, Kong L, et al: Treatment outcomes and late
toxicities of 869 patients with nasopharyngeal carcinoma treated
with definitive intensity modulated radiation therapy: new insight
into the value of total dose of cisplatin and radiation boost.
Oncotarget. 6:38381–38397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, Kitpanit S, Lee A, Mah D, Sine K,
Sherman EJ, Dunn LA, Michel LS, Fetten J, Zakeri K, et al: Toxicity
profiles and survival outcomes among patients with nonmetastatic
nasopharyngeal carcinoma treated with intensity-modulated proton
therapy vs intensity-modulated radiation therapy. JAMA Netw Open.
4:e21132052021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bentzen SM, Dorr W, Anscher MS, Denham JW,
Hauer-Jensen M, Marks LB and Williams J: Normal tissue effects:
Reporting and analysis. Semin Radiat Oncol. 13:189–202. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vittrup AS, Kirchheiner K, Fokdal LU,
Bentze SM, Nout RA, Pötter R and Tanderup K: Reporting of late
morbidity after radiation therapy in large prospective studies: A
descriptive review of the current status. Int J Radiat Oncol Biol
Phys. 105:957–967. 2019. View Article : Google Scholar : PubMed/NCBI
|