Introduction
Lung cancer remains the leading cause of
cancer-related morbidity and mortality globally; it accounts for ~2
million new cases and 1.76 million deaths per year, with rates
continuing to increase (1).
Non-small-cell lung cancer (NSCLC) accounts for >85% of all lung
cancer cases (2). A notable
proportion of patients are diagnosed at advanced stages of the
disease, and the treatment for these patients typically involves
radiation therapy, chemotherapy, targeted therapy and immunotherapy
(3,4). However, despite these comprehensive
approaches, outcomes for patients with advanced lung cancer are
often poor, with such cases classified as advanced refractory lung
cancer (5). Currently, there is no
established optimal clinical treatment for advanced refractory
NSCLC, with most patients receiving primarily palliative and
symptomatic supportive care (6).
This highlights the urgent need for developing more effective and
targeted therapeutic strategies to improve the prognosis of these
patients.
Advancements in precision medicine have highlighted
the clinical potential of localized therapies, such as bronchial
artery chemoembolization (BACE) and iodine-125 seed implantation,
which are increasingly recognized as important therapeutic options.
Iodine-125 seed implantation, a type of brachytherapy, involves
placing iodine-125 seeds directly into tumor tissues. These seeds
emit low-dose γ-rays continuously, selectively targeting and
destroying cancer cells whilst minimizing radiation exposure to
surrounding healthy tissues and reducing collateral damage
(7). BACE delivers chemotherapeutic
agents and embolic materials directly into tumor-feeding arteries
using a catheter, ensuring high localized drug concentrations. This
approach not only enhances the effects of the drugs but also
decreases the blood supply to the tumor, inducing ischemia, which
ultimately result in necrosis (8).
Compared with systemic chemotherapy, BACE markedly improves the
local efficacy of chemotherapeutic drugs whilst reducing systemic
side effects (9). However,
conventional embolization materials, such as gelatin sponge and
polyvinyl alcohol particles, have notable limitations, including
incomplete embolization and a higher risk of complications
(10).
8Spheres microspheres, a novel embolic material
comprising a polyvinyl alcohol skeleton with covalent bonding and
cross-linking agents, exhibit excellent elasticity and compliance.
These characteristics enable more uniform embolization of
tumor-feeding arteries, thereby improving therapeutic outcomes, and
their potential as a localized therapy has attracted considerable
attention (11).
Building on these advancements, the present study
aimed to evaluate the safety and efficacy of combining 8Spheres
microsphere embolization with iodine-125 seed implantation for
treating advanced refractory NSCLC. Using retrospective clinical
data, the study compared outcomes between the combined treatment
group and the iodine-125 seed implantation group. Furthermore, the
analysis assessed the potential of the combined regimen to enhance
therapeutic effectiveness, extend patient survival, minimize
treatment-related side effects and provide a scientific foundation
for the broader adoption of this innovative therapeutic
approach.
Materials and methods
Patient population
The present study retrospectively reviewed 45
patients with advanced refractory NSCLC who were treated at The
First Affiliated Hospital of Chongqing Medical University
(Chongqing, China) between January 2020 and December 2022. The
study received approval from the Ethics Committee of the First
Affiliated Hospital of Chongqing Medical University. As a
retrospective analysis, the need for patient consent was waived,
and all data were anonymized. The inclusion criteria were as
follows: i) Histopathologically-confirmed diagnosis of NSCLC; ii)
tumor-node-metastasis (TNM) stage (12) IIIB or IVA with manageable distant
metastatic tumors; iii) prior treatment failure, including
radiotherapy, chemotherapy, targeted therapy and immunotherapy; iv)
at least one measurable tumor focus as defined by Response
Evaluation Criteria in Solid Tumors (RECIST) (13); v) expected survival of >6 months
based on clinical assessment at the start of treatment; and vi) an
Eastern Cooperative Oncology Group (ECOG) performance status score
(14) of 0–2. The exclusion
criteria included the following: i) History of iodine allergy or
allergy to the chemotherapeutic drugs used; ii) comorbid severe
cardiovascular disease (New York Heart Association class III–IV)
(15), severe psychiatric disorder
or other severe physical illness; iii) immune dysfunction or
coagulation dysfunction; and iv) cognitive dysfunction.
The patients were categorized into a test group and
a control group (Table I). The test
group comprised 23 patients who received 8Spheres microsphere
embolization combined with bronchial artery perfusion chemotherapy
and iodine-125 seed implantation. This group included 13 men and 10
women, with 8 patients aged ≤60 years and 15 aged >60 years. A
total of 14 patients had adenocarcinoma, and nine had squamous
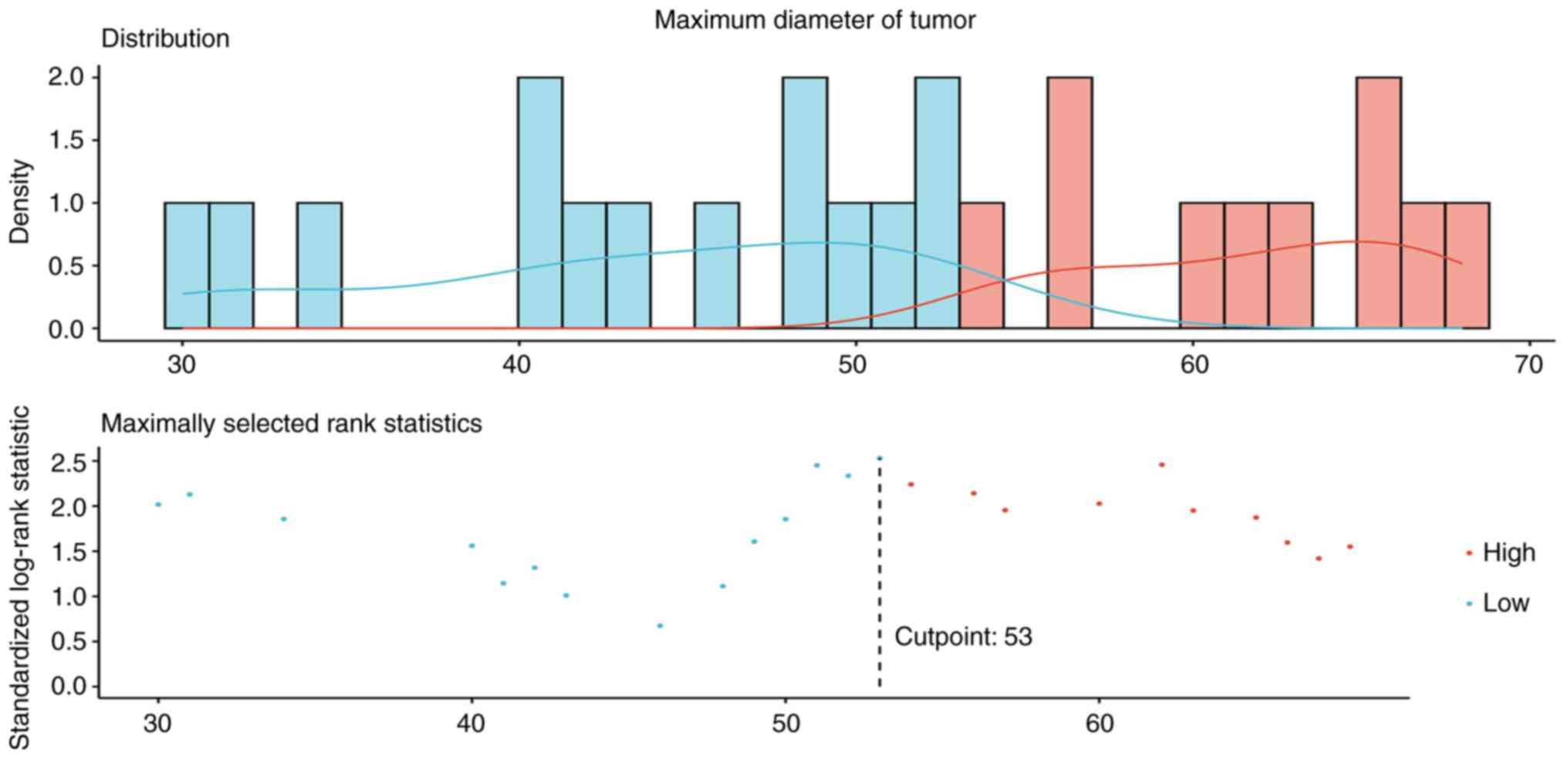
carcinoma. Tumor diameters were ≤53 mm in 12 cases and >53 mm in
11 cases. TNM staging indicated stage IIIB in 13 patients and stage
IVA in 10 patients. The control group consisted of 22 patients
treated with iodine-125 seed implantation alone. This group
included 12 men and 10 women, with 4 patients aged ≤60 years and 18
aged >60 years. Adenocarcinoma was observed in 13 cases, and
squamous carcinoma in 9 cases. Tumor diameters were ≤53 mm in 13
cases and >53 mm in 9 cases (Fig.
1). TNM staging indicated stage IIIB in 10 patients and stage
IVA in 12 patients. All patients were last followed up in June
2024.
 | Table I.Clinical characteristics of the
patients in each treatment group. |
Table I.
Clinical characteristics of the
patients in each treatment group.
| Characteristic | Test group
(n=23) | Control group
(n=22) | χ2 | P-value |
|---|
| Age |
|
| 1.585 | 0.208 |
| ≤60
years | 8 (34.8) | 4 (18.2) |
|
|
| >60
years | 15 (65.2) | 18 (81.8) |
|
|
| Sex |
|
| 0.018 | 0.894 |
|
Male | 13 (56.5) | 12 (54.5) |
|
|
|
Female | 10 (43.5) | 10 (45.5) |
|
|
| ECOG
PSa |
|
| N/A | 0.999 |
| 0 | 1 (4.4) | 1 (4.6) |
|
|
| 1 | 15 (65.2) | 14 (63.6) |
|
|
| 2 | 7 (30.4) | 7 (31.8) |
|
|
| Pathological
type |
|
| 0.015 | 0.903 |
|
Adenocarcinoma | 14 (60.9) | 13 (59.1) |
|
|
|
Squamous cell carcinoma | 9 (39.1) | 9 (40.9) |
|
|
| TNM stage |
|
| 0.551 | 0.458 |
|
IIIB | 13 (56.5) | 10 (45.5) |
|
|
|
IVA | 10 (43.5) | 12 (54.5) |
|
|
| Maximum tumor
diameter |
|
| 0.218 | 0.641 |
| ≤53
mm | 12 (52.2) | 13 (59.1) |
|
|
| >53
mm | 11 (47.8) | 9 (40.9) |
|
|
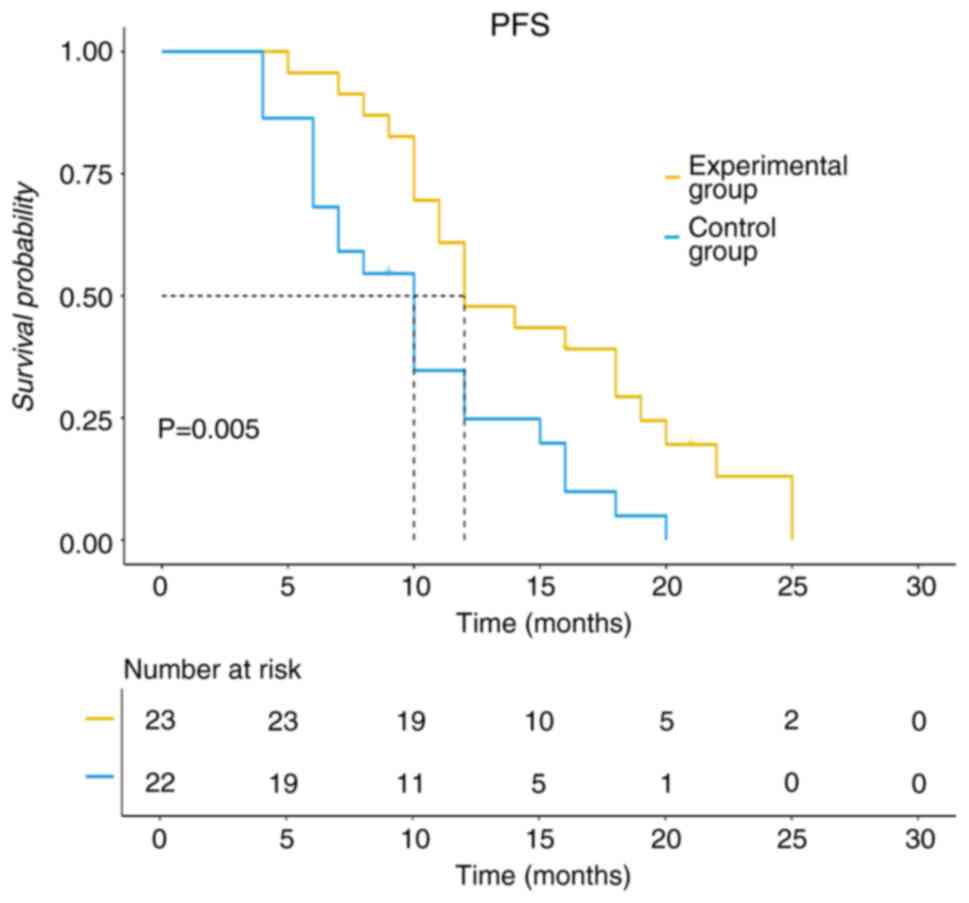
| PFS | 12 (10–19) | 10 (6–12) | 2.769 | 0.006 |
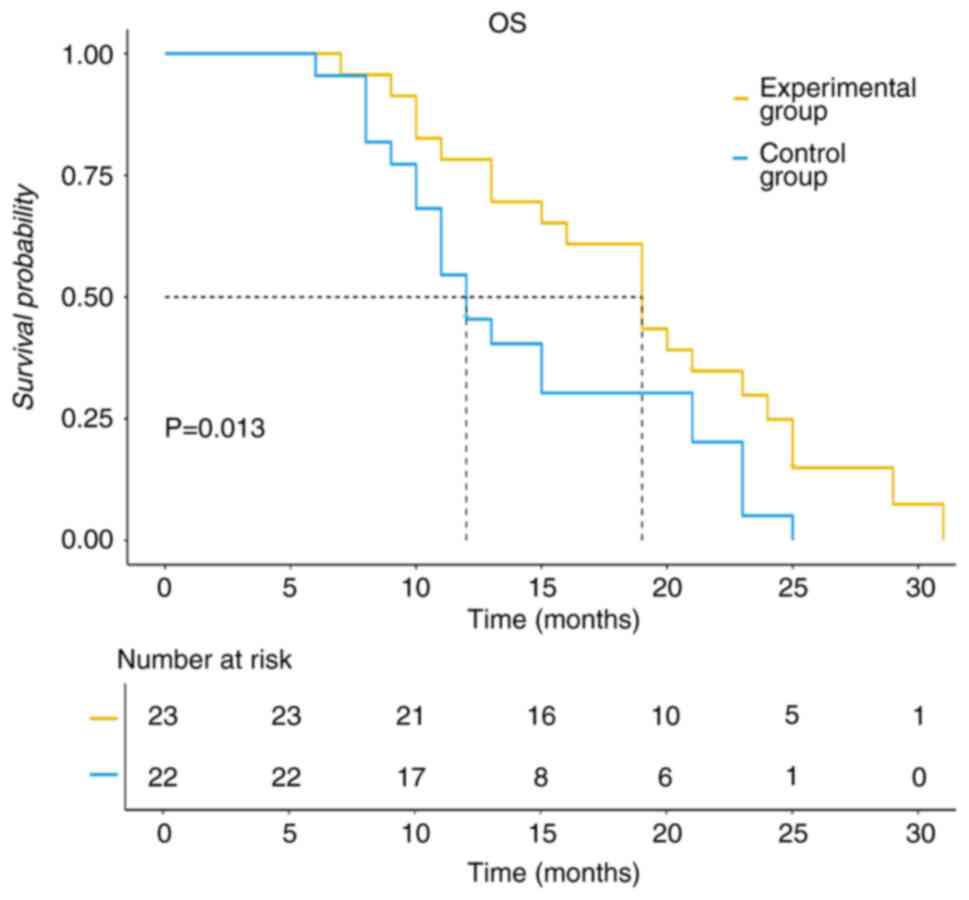
| OS | 19 (13–24) | 12 (10–21) | 2.140 | 0.032 |
Treatment methods
Standard preoperative evaluations were performed on
all patients, which included screenings for infectious disorders
(human immunodeficiency virus/acquired immunodeficiency syndrome,
syphilis and hepatitis), coagulation function evaluations, liver
and kidney function testing and comprehensive blood and urine
tests. Additional evaluations included electrocardiograms, cardiac
enzyme tests and contrast-enhanced chest CT scans. Informed consent
was obtained from all patients for iodine-125 seed implantation,
bronchial artery chemoembolization and other related
procedures.
Patients in the control group received only
iodine-125 seed implantation. Treatment plans were created using
the Therapy Planning System (Beijing Astro Technology Co., Ltd.),
which outlined the needle insertion route and angle, the number of
needles to be implanted and the arrangement of the particles. The
iodine-125 seeds, purchased from Beijing Atom High-Tech Co., Ltd.,
had a diameter of 0.8 mm and a length of 4.5 mm, with a titanium
shell, an activity of 0.6–0.8 mCi, a half-life of 59.6 days, and a
prescribed dose of 120–140 Gy. The procedure was performed under
local anesthesia using a Revolution™ HD CT scanner (GE
Healthcare). CT-guided implantation was performed using a layer
thickness of 5 mm, following the planned protocol.
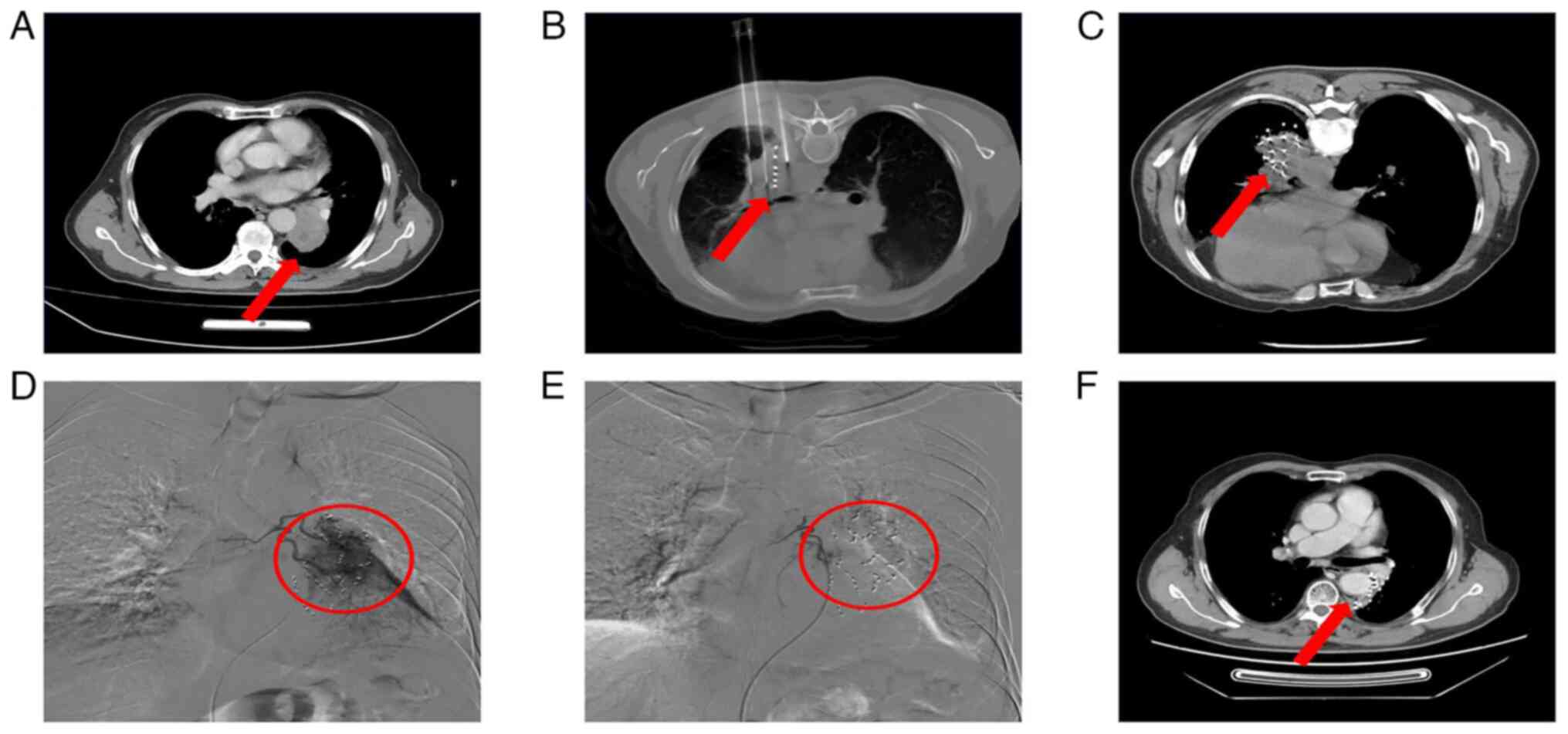
Patients in the test group underwent BACE 4–6 days
after iodine-125 seed implantation (using the same protocol as in
the control group). The procedure involved the following steps: The
right femoral artery was punctured and a 5F RLG catheter (Terumo
Corporation) was selectively inserted into the bronchial artery for
angiography. Other arteries, such as the phrenic and internal
thoracic arteries, were also assessed to identify the blood supply
of the tumor if needed. A 2.7F microcatheter (APT Medical Inc.) was
then super-selectively inserted into the target tumor vessel.
Chemotherapy drugs were administered first, following oncological
guidelines (National Comprehensive Cancer Network Guidelines for
Non-Small-Cell Lung Cancer) (16).
For patients with adenocarcinoma, 500 mg/m2 pemetrexed
disodium + 75 mg/m2cisplatin was administered, whilst
for patients with squamous carcinoma, 135 mg/m2
paclitaxel + area under the curve 5 carboplatin was administered.
After chemotherapy, embolization was performed using 8Spheres
microspheres (Suzhou Hengrui Medical Devices Co., Ltd.) with a
diameter of 300–500 µm. The microspheres were injected into the
target vessel at a rate of 1 ml/min. The embolization effect was
evaluated using digital subtraction angiography (DSA). If needed,
additional embolization was performed based on DSA findings.
Efficacy evaluation
Efficacy was evaluated using the RECIST 1.1
guidelines (13). Efficacy
categories included complete remission (CR), defined as the total
disappearance of all target lesions; partial remission (PR),
defined as a ≥30% reduction in the maximum diameter of target
lesions; stable disease (SD), defined as insufficient change in
lesion size to meet the criteria for PR or progressive disease
(PD); and PD, defined as a ≥20% increase in the maximum diameter of
target lesions. The efficacy metrics were calculated using the
following formulae: Objective response rate (ORR)=CR + PR; and
disease control rate (DCR)=CR + PR + SD. Patients were evaluated
for efficacy at 2, 4, and 6 months after treatment.
Adverse reactions and
complications
All adverse reactions and complications occurring
during and after treatment were recorded, including pneumothorax,
chest pain, fever, gastrointestinal symptoms and hemoptysis. The
Common Terminology Criteria for Adverse Events was used to
categorize adverse reactions (17)
and promptly addressed to ensure patient safety.
Follow-up
All patients were regularly followed up after
treatment until June 2024, death or loss to follow-up. Follow-up
visits were scheduled every 2 months during the first 6 months and
every 3 months thereafter. These visits included physical
examinations, assessments of lesion progression, blood tests,
evaluations of liver and kidney function, and chest imaging.
Follow-ups focused on evaluating progression-free survival (PFS)
and overall survival (OS).
Statistical analysis
Data were analyzed using SPSS 29.0 (IBM Corp.) and R
(version 4.3.1; The R Foundation). The normality of continuous
variables was assessed using the Shapiro-Wilk test. Based on the
distribution, continuous data are presented as mean ± standard
deviation if normally distributed, or as median (interquartile
range) if not. Baseline characteristics between the groups were
compared using either a two-sample t-test or a Wilcoxon rank-sum
test, depending on the data distribution. For categorical data
analysis, the χ2 test was applied for variables with
expected frequencies ≥5 in all cells, whereas the Fisher's exact
test was used to analyze variables with small sample sizes or
expected frequencies <5. Survival analyses included all patients
and were performed using the Kaplan-Meier method, with group
comparisons made using the log-rank test. The optimal cutoff value
for the maximum tumor diameter, as a treatment-related continuous
variable, was calculated using the surv_cutpoint function in the R
package ‘survminer’ (version 0.5.0) (18). The effect sizes of several factors
were assessed using Cox proportional hazards analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
Analysis of treatment response
At 2 months, the ORR for patients in the test and
control groups was 82.6 and 40.9%, respectively
(χ2=8.318; P<0.05), whilst the DCR was 100% for both
groups. At 4 months, the ORR was 87.0 and 59.1%, respectively
(χ2=4.465; P<0.05), and the DCR was 95.7 and 86.4%,
respectively (χ2=0.325; P>0.05). At 6 months, the ORR
was 82.6 and 50.0%, respectively (χ2=5.380; P<0.05),
whilst the DCR was 91.3 and 77.3%, respectively
(χ2=0.786; P>0.05). These findings are summarized in
Table II. A comparison between the
imaging results for patients in the experimental group before and
after treatment is presented in Fig.
2.
 | Table II.Comparison of outcomes in patients
with different treatments. |
Table II.
Comparison of outcomes in patients
with different treatments.
| A, 2 months |
|---|
|
|---|
| Outcome | Test group
(n=23) | Control group
(n=22) |
|---|
| CR | 2 | 0 |
| PR | 17 | 9 |
| SD | 4 | 13 |
| PD | 0 | 0 |
| ORR, % | 82.6 | 40.9 |
| DCR, % | 100.0 | 100.0 |
|
| B, 4
months |
|
| Outcome | Test group
(n=23) | Control group
(n=22) |
|
| CR | 5 | 3 |
| PR | 15 | 10 |
| SD | 2 | 6 |
| PD | 1 | 3 |
| ORR, % | 87.0 | 59.1 |
| DCR, % | 95.7 | 86.4 |
|
| C, 6
months |
|
| Outcome | Test group
(n=23) | Control group
(n=22) |
|
| CR | 7 | 3 |
| PR | 12 | 8 |
| SD | 2 | 6 |
| PD | 2 | 5 |
| ORR, % | 82.6 | 50.0 |
| DCR, % | 91.3 | 77.3 |
Analysis of survival probability
The baseline characteristics of patients selecting
different treatment regimens were comparable between the two groups
(P>0.05); however, statistically significant differences were
demonstrated for the median PFS and OS between the groups (Table I). Among the patients in the test
group, 12/23 (52.2%) had previously received chemotherapy with the
aforementioned regimens, whilst 11/23 (47.8%) had not. In the
control group, 10/22 (45.5%) had previously received chemotherapy
with the aforementioned regimens, and 12/22 (54.5%) had not. The
results revealed no significant difference in ORR (P=0.213) or DCR
(P=0.456) between subgroups, suggesting that prior chemotherapeutic
agents use did not significantly influence the observed treatment
efficacy (Table SI). The median
PFS for the experimental group was 12 months, whilst for the
control group, it was 10 months (P<0.05; Fig. 3). The median OS for the experimental
group was 19 months, compared with 12 months for the control group
(P<0.05; Fig. 4). The cutoff
value for the maximum tumor diameter was determined to be 53 mm
using the surv_cutpoint function in R. Univariate analysis of OS
and PFS demonstrated that both the treatment regimen and maximum
tumor diameter had a significant impact on patient outcomes.
According to the results of the univariate Cox regression analysis,
the factors influencing OS included the treatment regimen and the
maximum tumor diameter (Table
III). Furthermore, multivariable Cox regression analysis with
forward stepwise selection (Forward LR) indicated that, after
controlling for tumor size, patients receiving the control regimen
had a 2.291-fold higher risk of death than those in the test group
[95% confidence interval (CI), 1.194–4.396]. Similarly, patients
with tumors of >53 mm had a 2.723-fold higher risk of death than
those with tumors of ≤53 mm (95% CI, 1.416–5.238) (Table IV). Moreover, univariate Cox
regression analysis revealed that both the treatment regimen and
tumor size were significant factors influencing PFS (Table V). Multivariable analysis confirmed
these findings, demonstrating that after adjusting for tumor size,
patients in the control group had a 2.567-fold higher recurrence
risk compared with those in the test group (95% CI, 1.332–4.949).
Additionally, patients with tumors of >53 mm had a 2.440-fold
higher recurrence risk than those with smaller tumors (95% CI,
1.276–4.665) (Table VI). In
summary, the combined univariate and multivariable analyses
indicate that the experimental therapy achieved superior PFS and OS
outcomes compared with the control regimen, and it was more
effective in delaying disease recurrence [HR (PFS)=2.567 and HR
(OS)=2.291].
 | Table III.Univariate Cox analysis of different
characteristics based on overall survival. |
Table III.
Univariate Cox analysis of different
characteristics based on overall survival.
|
|
|
|
|
|
| 95.0% CI for
HR |
|---|
|
|
|
|
|
|
|
|
|---|
| Variable | B | SE | Wald | P-value | HR | Lower | Upper |
|---|
| Age (>60 vs. ≤60
years) | 0.298 | 0.358 | 0.692 | 0.405 | 1.347 | 0.668 | 2.716 |
| Sex (Female vs.
male) | 0.191 | 0.316 | 0.364 | 0.546 | 1.210 | 0.651 | 2.249 |
| Group (Control vs.
test) | 0.654 | 0.324 | 4.081 | 0.043 | 1.924 | 1.020 | 3.631 |
| ECOG
PSa |
|
| 0.375 | 0.829 |
|
|
|
| 0 | Ref. |
|
|
|
|
|
|
| 1 | 0.294 | 0.736 | 0.159 | 0.690 | 1.341 | 0.317 | 5.672 |
| 2 | 0.110 | 0.774 | 0.020 | 0.887 | 1.117 | 0.245 | 5.089 |
| Pathological type
(SCC vs. AC) | 0.255 | 0.322 | 0.629 | 0.428 | 1.291 | 0.687 | 2.427 |
| TNM stage (IVA vs.
IIIB) | 0.161 | 0.314 | 0.263 | 0.608 | 1.175 | 0.635 | 2.175 |
| Maximum tumor
diameter |
|
|
|
|
|
|
|
| (>53 vs. ≤53
mm) | 0.849 | 0.324 | 6.853 | 0.009 | 2.336 | 1.238 | 4.410 |
 | Table IV.Multivariate Cox analysis of
different characteristics based on overall survival. |
Table IV.
Multivariate Cox analysis of
different characteristics based on overall survival.
|
|
|
|
|
|
| 95.0% CI for
HR |
|---|
|
|
|
|
|
|
|
|
|---|
| Variable | B | SE | Wald | P-value | HR | Lower | Upper |
|---|
| Group (Control vs.
test) | 0.829 | 0.333 | 6.212 | 0.013 | 2.291 | 1.194 | 4.396 |
| Maximum tumor
diameter | 1.002 | 0.334 | 9.011 | 0.003 | 2.723 | 1.416 | 5.238 |
| (>53 vs. ≤53
mm) |
|
|
|
|
|
|
|
 | Table V.Univariate Cox analysis of different
characteristics based on progression-free survival. |
Table V.
Univariate Cox analysis of different
characteristics based on progression-free survival.
|
|
|
|
|
|
| 95.0% CI for
HR |
|---|
|
|
|
|
|
|
|
|
|---|
| Variable | B | SE | Wald | P-value | HR | Lower | Upper |
|---|
| Age (>60 vs. ≤60
years) | 0.228 | 0.355 | 0.410 | 0.522 | 1.256 | 0.626 | 2.519 |
| Sex (Female vs.
male) | 0.276 | 0.314 | 0.775 | 0.379 | 1.318 | 0.712 | 2.439 |
| Group (Control vs.
test) | 0.819 | 0.329 | 6.206 | 0.013 | 2.269 | 1.191 | 4.322 |
| ECOG
PSa |
|
| 0.129 | 0.938 |
|
|
|
| 0 | Ref. |
|
|
|
|
|
|
| 1 | 0.247 | 0.736 | 0.113 | 0.737 | 1.281 | 0.303 | 5.420 |
| 2 | 0.188 | 0.770 | 0.060 | 0.807 | 1.207 | 0.267 | 5.455 |
| Pathological type
(SCC vs. AC) | 0.403 | 0.323 | 1.562 | 0.211 | 1.497 | 0.795 | 2.818 |
| TNM stage (IVA vs.
IIIB) | 0.222 | 0.312 | 0.508 | 0.476 | 1.249 | 0.678 | 2.302 |
| Maximum tumor
diameter | 0.763 | 0.325 | 5.511 | 0.019 | 2.146 | 1.134 | 4.059 |
| (>53 vs. ≤53
mm) |
|
|
|
|
|
|
|
 | Table VI.Multivariate Cox analysis of
different characteristics based on progression-free survival. |
Table VI.
Multivariate Cox analysis of
different characteristics based on progression-free survival.
|
|
|
|
|
|
| 95.0% CI for
HR |
|---|
|
|
|
|
|
|
|
|
|---|
| Variable | B | SE | Wald | P-value | HR | Lower | Upper |
|---|
| Group (Control vs.
test) | 0.943 | 0.335 | 7.929 | 0.005 | 2.567 | 1.332 | 4.949 |
| Maximum tumor
diameter | 0.892 | 0.331 | 7.273 | 0.007 | 2.440 | 1.276 | 4.665 |
| (>53 vs. ≤53
mm) |
|
|
|
|
|
|
|
Adverse events
No severe adverse events, such as treatment-related
death, unintended embolism or grade ≥3 hematologic toxicity,
occurred in either group. Common adverse events, including
pneumothorax, hemoptysis, fever, chest pain and nausea, all
resolved with symptomatic treatment. Furthermore, the incidence of
adverse events did not significantly differ between the two groups
(Table VII).
 | Table VII.Adverse events. |
Table VII.
Adverse events.
| Adverse event | Test group
(n=23) | Control group
(n=22) | P-value |
|---|
| Pneumothorax | 2 (8.7) | 3 (13.6) | 0.647 |
| Hemoptysis | 3 (13.0) | 3 (13.7) | 1.000 |
| Chest pain | 6 (26.1) | 5 (22.7) | 0.756 |
| Fever | 5 (21.7) | 3 (13.6) | 0.594 |
| Nausea | 2 (8.7) | 2 (9.1) | 1.000 |
Discussion
Treating advanced refractory NSCLC is challenging.
Local therapies such as BACE and iodine-125 seed implantation have
received considerable attention and are increasingly applied in
clinical practice and research (19). Therefore, the present study aimed to
evaluate the efficacy and safety of 8Spheres microsphere
embolization combined with iodine-125 seed implantation for
advanced refractory lung cancer, and compare it with iodine-125
seed implantation alone. The results indicate that the combination
therapy outperformed iodine-125 seed implantation alone in terms of
local tumor control, OS and PFS, suggesting the potential benefits
of this combined approach for patients with advanced refractory
lung cancer.
In the present study, the ORR, DCR, median PFS and
median OS were significantly higher in the test group compared with
that in the control group (P<0.05). Furthermore, the test group
demonstrated improved efficacy in delaying disease recurrence [HR
(PFS)=2.567 and HR (OS)=2.291].
Whilst certain patients experienced mild adverse
effects, such as chest pain and fever, these were managed
effectively with symptomatic treatment, and no serious
complications occurred. This highlights the therapeutic advantage
of 8Spheres microspheres, which are considered to be effective due
to their role as a vascular embolic agent. The optimal cutoff value
for the maximum tumor diameter, determined using the surv_cutpoint
function in R, was revealed to be 53 mm. Patients with tumors
exceeding this size had a worse survival prognosis than those with
tumors less than this size. Therefore, this cutoff value may aid in
stratifying prognostic risk and guide treatment planning in
clinical practice. In summary, tumor size in advanced refractory
lung cancer appears to significantly influence patient
prognosis.
The efficacy of iodine-125 seed therapy has been
well-established in prostate cancer and spinal metastases (20,21).
In a study by Cheng et al (22), six patients with NSCLC received
iodine-125 seed implantation. A total of five patients achieved CR,
and one achieved PR 1 month after implantation, with an ORR of
100%, a median OS of 26 months, and a median PFS of 12 months. In
another study by Sui et al (23), three patients with advanced NSCLC
treated with iodine-125 seed brachytherapy and anti-programmed cell
death protein-1 antibodies achieved CR or PR. Moreover, compared
with conventional whole-body radiotherapy, iodine-125 seed
implantation reduces radiation exposure to surrounding tissues,
minimizes treatment-related side effects, and is suitable for
inoperable patients or those unable to tolerate systemic
chemotherapy (24). However,
iodine-125 seed therapy has limitations, particularly in addressing
the complex tumor-blood supply system.
BACE has also been assessed for treating advanced
lung cancer (25). In a study by
Zhu et al (26), BACE
combined with apatinib improved outcomes in patients with lung
cancer. Among the 47 patients who underwent the BACE procedure, the
observation group had a median PFS of 322 days, compared with 209
days in the control group (P<0.05), with 1-year survival rates
of 76.19 and 46.15%, respectively (P<0.05). However, traditional
embolization materials such as polyvinyl alcohol (PVA) particles
and gelatin sponge particles have limitations. PVA particles may
cause incomplete embolization due to uneven size distribution,
whilst gelatin sponge particles, being short-acting embolic agents,
may result in tumor recurrence or complications (27,28).
8Sphere microspheres are a novel type of embolic
material made from a macromolecular cross-linked polymer, with PVA
as the main chain. These microspheres have a regular spherical
shape and the ability to compress, which allows them to travel
further within blood vessels. These properties enable precise
distribution in the blood supply artery of a tumor during
embolization, resulting in terminal embolization with a stable and
predictable effect (11). In a
study by Zhang et al (29),
15 patients with uterine fibroids underwent uterine artery
embolization using 8Spheres microspheres, with no serious adverse
effects. At 6 months post-surgery, the volumes of the uterus and
dominant smooth muscle tumors notably decreased from 340.0±35.8 and
100.6±24.3 cm3, respectively (baseline) to 266.6±30.9
and 56.1±17.3 cm3. These results indicate the efficacy
and safety of 8Spheres microspheres in solid tumor embolization.
Furthermore, 8Spheres microsphere embolization is also associated
with minimal adverse effects. In a study by Zhou et al
(30), partial splenic artery
embolization using 8Spheres microspheres in patients with
hepatocellular carcinoma and hypersplenism not only achieved
permanent vascular embolization but also markedly reduced
inflammatory responses, resulting in a lower incidence of fever and
pain. The enhanced therapeutic efficacy of the combined treatment
is likely due to multiple biological mechanisms. Vascular
embolization with 8Spheres microspheres induces tumor hypoxia and
the simultaneous inhibition of tumor cell proliferation and
invasion (29). In a study by Chao
et al (31), TACE was
reported to be associated with the modulation of serum angiogenic,
inflammatory and cell growth cytokines in patients with
hepatocellular carcinoma (HCC). Additionally, the ischemic and
hypoxic environment created by embolization may alter the immune
microenvironment of the tumor, potentially enhancing the immune
response against the tumor (32).
In a previous study, hepatic artery embolization (HAE) was reported
to enhance intratumoral and peritumoral programmed death-ligand 1
(PD-L1) expression in a rat HCC model. The hypoxia-inducible
factor-1α pathway is a possible mechanism underlying increased
intratumoral PD-L1 expression after HAE (32). Moreover, in a study by Kang et
al (33), the iodine-125 seed
promoted the apoptosis of cholangiocarcinoma cells and induced the
activation of the ROS/p53 pathway in a dose-dependent manner. The
combination of 8Spheres microsphere vascular embolization of the
tumor blood supply and induction of tumor hypoxia, and iodine-125
seed implantation for radiation damage to the tumor, enhanced the
therapeutic effect on the tumor. However, further studies are
required to elucidate these mechanisms in more detail. In a
single-arm pilot study by Chen et al (34), embosphere microsphere embolization
combined with iodine-125 seeds was used to treat patients with
locally advanced stage III NSCLC after radiotherapy failure. Among
the 28 patients, the 6-month ORR and DCR were 71.42 and 92.86%,
respectively, with no serious complications observed during
follow-up. The median PFS was 8 months (95% CI, 7.3–8.8 months). In
the present study, the ORR, DCR and median PFS in the combination
therapy group were higher than those reported by Chen et al.
We hypothesize that the improved treatment efficacy in the present
study was associated with the superior size distribution, enhanced
spherical stability and improved retention characteristics of the
8Spheres microspheres.
Compared with standard-of-care treatments, such as
immunotherapy-based regimens and radiation therapy, the combined
treatment of 8Spheres microsphere embolization and iodine-125 seed
implantation offers several advantages. The minimally invasive
nature of the procedure and the enhanced therapeutic effect due to
the synergistic action of the two modalities make it a promising
option for patients with advanced refractory NSCLC. However, the
combined treatment may not be suitable for all patients, and
further studies are needed to determine the optimal indications and
patient selection criteria.
The present study has several limitations. First, it
was a retrospective analysis with a small sample size and a
relatively short follow-up period, which could introduce selection
bias. To more comprehensively evaluate the long-term efficacy and
safety of the treatment regimen, larger-scale prospective
randomized controlled trials are warranted. In future prospective
studies, it is recommended that propensity score matching be
incorporated. This approach can help adjust for potential
confounders and further validate the findings obtained in the
current study, thereby providing more robust and reliable evidence
for clinical practice. Moreover, despite all patients receiving the
same treatment, individual variations in response could affect
efficacy and safety. Whilst the present study reports follow-up
data up to June 2024, long-term survival outcomes (such as 3–5
years follow-up) are crucial for evaluating the true efficacy of
the treatment. Future studies should aim to provide long-term data
on survival trends, late-onset adverse events and patterns of
disease recurrence. This will further validate the clinical
benefits of the combined treatment. In addition, future research
should focus on more precise patient stratification and
individualized treatment regimens so as to offer improved options
for patients with refractory NSCLC. The current study used the
sequence of seed implantation followed by embolization; however,
future studies should evaluate the clinical outcomes of pre-seed
implantation embolization. This modification may enhance tumor
hypoxia and improve the efficacy of iodine-125 radiotherapy.
Furthermore, genetic alterations (such as EGFR, ALK and KRAS
mutations) were not systematically recorded in patient medical
records, limiting the ability to stratify outcomes by molecular
profiles. Future prospective studies should integrate comprehensive
genomic profiling to refine prognostic models and identify
biomarkers predictive of treatment response. Finally, although
histological subtypes (adenocarcinoma vs. squamous carcinoma) and
baseline ECOG performance status were balanced between groups,
subgroup analyses stratified by these factors were not performed
due to the small sample size. More extensive studies should explore
whether treatment efficacy differs across histological subtypes or
patient functional status.
In conclusion, the combination of 8Spheres
microsphere embolization and iodine-125 seed implantation presents
a promising treatment option for patients with advanced refractory
NSCLC. The procedure is straightforward, minimally invasive and
safe, making it a promising option for clinical application.
Furthermore, this approach significantly enhances therapeutic
efficacy, extends survival and is minimally invasive, meaning that
it well-suited for clinical use. However, further research is
required to evaluate its long-term effects and explore optimized
treatment strategies.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LR and HP conceived and designed this study. LR, LJ
and YL participated in data collection and data curation
(organizing and maintaining data). LJ, YL and HP analyzed the data.
LR, LJ and HP drafted the manuscript and all authors reviewed it.
YL and HP confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethics Committee of the First Affiliated Hospital of Chongqing
Medical University (approval no. K2023-470). The requirement for
informed consent for participation was waived due to the
retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CR
|
complete remission
|
|
DCR
|
disease control rate
|
|
DSA
|
digital subtraction angiography
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
NSCLC
|
non-small cell lung cancer
|
|
ORR
|
objective response rate
|
|
OS
|
overall survival
|
|
PD
|
progressive disease
|
|
PFS
|
progression-free survival
|
|
PR
|
partial remission
|
|
RECIST
|
Response Evaluation Criteria in Solid
Tumors
|
|
SD
|
stable disease
|
References
|
1
|
Thai AA, Solomon BJ, Sequist LV, Gainor JF
and Heist RS: Lung cancer. Lancet. 398:535–554. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller M and Hanna N: Advances in systemic
therapy for non-small cell lung cancer. BMJ. 9:n23632021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meyer ML, Fitzgerald BG, Paz-Ares L,
Cappuzzo F, Jänne PA, Peters S and Hirsch FR: New promises and
challenges in the treatment of advanced non-small-cell lung cancer.
Lancet. 404:803–822. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Remon J, Soria JC and Peters S; ESMO
Guidelines Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Early and locally advanced non-small-cell lung cancer: An update of
the ESMO clinical practice guidelines focusing on diagnosis,
staging, systemic and local therapy. Ann Oncol. 32:1637–1642. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao YW, Liu S, Qin H, Sun JB, Su M, Yu
GJ, Zhou J, Gao F, Wang RY, Zhao T and Zhao GS: Efficacy and safety
of CalliSpheres drug-eluting beads for bronchial arterial
chemoembolization for refractory non-small-cell lung cancer and its
impact on quality of life: A multicenter prospective study. Front
Oncol. 13:11109172023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zha B, Zhang Y, Yang R and Kamili M:
Efficacy and safety of anlotinib as a third-line treatment of
advanced non-small cell lung cancer: A meta-analysis of randomized
controlled trials. Oncol Lett. 24:2292022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kou F, Gao S, Liu S, Wang X, Chen H, Zhu
X, Guo J, Zhang X, Feng A and Liu B: Preliminary clinical efficacy
of iodine-125 seed implantation for the treatment of advanced
malignant lung tumors. J Cancer Res Ther. 15:1567–1573. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He G, Yang K, Zhang X, Pan J, Han A, Gao
Z, Li Y and Wang W: Bronchial artery chemoembolization with
drug-eluting beads versus bronchial artery infusion followed by
polyvinyl alcohol particles embolization for advanced squamous cell
lung cancer: A retrospective study. Eur J Radiol. 161:1107472023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du L and Morgensztern D: Chemotherapy for
advanced-stage non-small cell lung cancer. Cancer J. 21:366–370.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Komada T, Suzuki K, Mizuno T, Ebata T,
Matsushima M, Naganawa S and Nagino M: Efficacy of percutaneous
transhepatic portal vein embolization using gelatin sponge
particles and metal coils. Acta Radiol Open.
7:20584601187696872018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu H, Zheng C, Liang B and Xiong B:
Quantitative splenic embolization possible: Application of 8Spheres
conformal microspheres in partial splenic embolization (PSE). BMC
Gastroenterol. 21:4072021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Detterbeck FC, Chansky K, Groome P,
Bolejack V, Crowley J, Shemanski L, Kennedy C, Krasnik M, Peake M,
Rami-Porta R, et al: The IASLC lung cancer staging project:
Methodology and validation used in the development of proposals for
revision of the stage classification of NSCLC in the forthcoming
(eighth) edition of the TNM classification of lung cancer. J Thorac
Oncol. 11:1433–1446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asano R, Kajimoto K, Oka T, Sugiura R,
Okada H, Kamishima K, Hirata T and Sato N; investigators of the
Acute Decompensated Heart Failure Syndromes (ATTEND) registry, :
Association of New York Heart Association functional class IV
symptoms at admission and clinical features with outcomes in
patients hospitalized for acute heart failure syndromes. Int J
Cardiol. 230:585–591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ,
Dobelbower M, et al: Non-small cell lung cancer, version 5.2017,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 15:504–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Atkinson TM, Ryan SJ, Bennett AV, Stover
AM, Saracino RM, Rogak LJ, Jewell ST, Matsoukas K, Li Y and Basch
E: The association between clinician-based common terminology
criteria for adverse events (CTCAE) and patient-reported outcomes
(PRO): A systematic review. Support Care Cancer. 24:3669–3676.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan Z, Yang LC, Chen YQ, Wan WQ, Zhou DH,
Mai HR, Li WL, Yang LH, Lan HK, Chen HQ, et al: Prognostic
significance of MRD and its correlation with arsenic concentration
in pediatric acute promyelocytic leukemia: A retrospective study by
SCCLG-APL group. Ther Adv Hematol. 16:204062072413117742025.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chaft JE, Shyr Y, Sepesi B and Forde PM:
Preoperative and postoperative systemic therapy for operable
non-small-cell lung cancer. J Clin Oncol. 40:546–555. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chin J, Rumble RB, Kollmeier M, Heath E,
Efstathiou J, Dorff T, Berman B, Feifer A, Jacques A and Loblaw DA:
Brachytherapy for patients with prostate cancer: American society
of clinical oncology/cancer care ontario joint guideline update. J
Clin Oncol. 35:1737–1743. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharma R, Sagoo NS, Haider AS, Sharma N,
Haider M, Sharma IK, Igbinigie M, Aya KL, Aoun SG and Vira S:
Iodine-125 radioactive seed brachytherapy as a treatment for spine
and bone metastases: A systematic review and meta-analysis. Surg
Oncol. 38:1016182021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng G, Wang Y, Dong D, Zhang W, Wang L
and Wan Z: To evaluate the efficacy and safety of 125I seed
implantation in SCLC as second line therapy. Medicine (Baltimore).
101:e292512022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sui A, Song H, Yu H, Zhang H, Hu Q, Lei Y,
Zhang L and Wang J: Clinical application of iodine-125 seed
brachytherapy combined with anti-PD-1 antibodies in the treatment
of lung cancer. Clin Ther. 42:1612–1616. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Z, Hu Z, Xiong X and Song X: A review
of the efficacy and safety of iodine-125 seed implantation for lung
cancer treatment. Cancer Treat Res Commun. 41:1008442024.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma X, Zheng D, Zhang J, Dong Y, Li L, Jie
B and Jiang S: Clinical outcomes of vinorelbine loading
CalliSpheres beads in the treatment of previously treated advanced
lung cancer with progressive refractory obstructive atelectasis.
Front Bioeng Biotechnol. 10:10882742022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu J, Xu X, Chen Y, Wang Q, Yue Q, Lei K,
Jia Y, Xiao G and Xu G: Bronchial artery chemoembolization with
apatinib for treatment of central lung squamous cell carcinoma. J
Cancer Res Ther. 18:1432–1435. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luz JHM, Gomes FV, Coimbra E, Costa NV and
Bilhim T: Preoperative portal vein embolization in hepatic surgery:
A review about the embolic materials and their effects on liver
regeneration and outcome. Radiol Res Pract.
2020:92958522020.PubMed/NCBI
|
|
28
|
Liu J, Shi D, Li L, Cao L, Liu J, He J and
Liang Z: Clinical study on the treatment of benign prostatic
hyperplasia by embolization of prostate artery based on embosphere
microspheres and gelatin sponge granules. J Healthc Eng.
2022:14240212022.PubMed/NCBI
|
|
29
|
Zhang Y, Xu Y, Zhang X, Zheng B, Hu W,
Yuan G and Si G: 8Spheres conformal microspheres as embolic agents
for symptomatic uterine leiomyoma therapy in uterine artery
embolization (UAE): A prospective clinical trial. Medicine
(Baltimore). 102:e330992023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou J, Feng Z, Liu S, Li X, Liu Y, Gao F,
Shen J, Zhang YW, Zhao GS and Zhang M: Simultaneous CSM-TACE with
CalliSpheres® and partial splenic embolization using
8spheres® for hepatocellular carcinoma with
hypersplenism: Early prospective multicenter clinical outcome.
Front Oncol. 12:9985002022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chao Y, Wu CY, Kuo CY, Wang JP, Luo JC,
Kao CH, Lee RC, Lee WP and Li CP: Cytokines are associated with
postembolization fever and survival in hepatocellular carcinoma
patients receiving transcatheter arterial chemoembolization.
Hepatol Int. 7:883–892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takaki H, Hirata Y, Ueshima E, Kodama H,
Matsumoto S, Wada R, Suzuki H, Nakasho K and Yamakado K: Hepatic
artery embolization enhances expression of programmed cell death 1
ligand 1 in an orthotopic rat hepatocellular carcinoma model: In
vivo and in vitro experimentation. J Vasc Interv Radiol.
31:1475–1482.e2. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang F, Wu J, Hong L, Zhang P and Song J:
Iodine-125 seed inhibits proliferation and promotes apoptosis of
cholangiocarcinoma cells by inducing the ROS/p53 axis. Funct Integr
Genomics. 24:1142024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen C, Wang W, Yu Z, Tian S, Li Y and
Wang Y: Combination of computed tomography-guided iodine-125
brachytherapy and bronchial arterial chemoembolization for locally
advanced stage III non-small cell lung cancer after failure of
concurrent chemoradiotherapy. Lung Cancer. 146:290–296. 2020.
View Article : Google Scholar : PubMed/NCBI
|