Introduction
Pancreatic ductal adenocarcinoma (PDA) is the fourth
leading cause of cancer-related deaths in Japan as of 2021
(1). Although surgical resection is
necessary for curative treatment, approximately 80% of patients
with PDA present with an unresectable disease. Postoperative
chemotherapy is performed with TS-1, gemcitabine, and other agents;
however, tumor recurrence or metastasis typically occurs within 1
year of treatment (2). PDA of the
head (hPDA) is more frequently diagnosed than PDA of the body/tail
(btPDA) owing to the higher incidence of clinical biliary
obstruction symptoms such as jaundice. Consequently, hPDA is
identified at an earlier, more treatable stage than btPDA, leading
to a more favorable prognosis (3).
Birnbaum et al (4) reported
differences in gene expression between the PDA types. Specifically,
the expression of genes associated with epithelial cell development
and differentiation, smooth muscle cells, and transforming growth
factor β (TGFβ) is higher in patients with btPDA than in those with
hPDA (4,5). Moffitt et al (6) classified PDA into classical and basal
cell types based on gene expression analysis findings and reported
that the basal cell types were more likely to respond to
chemotherapy. Additionally, Moll et al (7) reported that the Moffitt classification
can be based on the expression levels of GATA6, a transcriptional
regulator required for normal pancreatic development, and keratin
5, expressed in basal cells (8).
Therefore, we evaluated the levels of not only GATA6 and
cytokeratin 5/6 (CK5/6) but also hepatocyte nuclear factor-1β
(HNF1β, expressed in the intestinal tract), S-100A4 (expressed in
cholangiocarcinoma), keratin 81 (KRT81, a marker of basal cells),
pancreatic and duodenal homeobox 1 (PDX1), NK6 homeobox 1 (NKX6.1,
responsible for pancreatic development), TGFβ, and SMAD family
member 4 (SMAD4, associated with the development of PDA) using
immunostaining (9–13).
Regarding the histological and morphological
classification of PDA, the large duct type (PDA-L) exhibits a more
favorable overall prognosis than the small duct type (PDA-S), even
if the tumor diameter is larger in the former type (14). Patients with colon cancer exhibiting
more severe fibrosis reportedly exhibit a poorer prognosis
(15). Existing literature
indicates a stark contrast in gene expression between hPDA and
btPDA. However, the comprehensive evaluation of clinical data and
protein expression to elucidate differences between these PDA types
remains limited. In this study, we aimed to investigate the
difference in tumor location (hPDA/btPDA) or prognosis based on
protein expression levels or histological features (PDA-L/PDA-S and
fibrotic focus [FF]).
Materials and methods
Case selection
We retrospectively collected data on 60 patients
with PDA who underwent resection at Oita University Hospital,
between November 2014 and February 2021. Surgery was performed in
all operable cases. The patients who underwent neochemoradiotherapy
were omitted. Cases were selected sequentially, starting with the
newest case, retrospectively to reduce bias. Based on clinical,
imaging, and macroscopic findings, the patients were stratified
into two groups by tumor origin within the pancreas (hPDA and
btPDA). We obtained clinical data from patient records, including
sex, age, tumor location, tumor size (20 mm or not; pT1 or not),
lymph node metastasis status (positive or negative), tumor stage (1
or over), overall survival (OS) (dead or alive), and histological
diagnosis.
Histological staining
Hematoxylin and eosin (HE) staining and
immunohistochemical (IHC) staining were performed manually.
Formalin-fixed paraffin-embedded tissue blocks showing
representative histology were selected for each case from our
internal tissue archive and sliced into 4-µm-thick sections.
Briefly, the sections were deparaffinized in xylene and rehydrated
in graded alcohol. Endogenous peroxidase activity was quenched by
incubating the sections with 3% hydrogen peroxide for 20 min at
25°C. The subsequent process was determined according to the
antibodies used (Table I) (9–13).
Immunoreaction was visualized using the Histofine Simple Stain
(MULTI) (Nichirei Biosciences, Tokyo, Japan). The nuclei were
counterstained with Mayer's hematoxylin.
 | Table I.List of antibodies used. |
Table I.
List of antibodies used.
| Antigen | Cat. no. | Dilution | Antigen
retrieval | Temperature,°C | Reaction time | Other | Source |
|---|
| S-100A4 | ab133554 | 1:500 | pH 9,
autoclave | 4 | 16 h | - | Abcam |
| GATA6 | AF1700 | 1:40 | pH 6,
autoclave | 25 | 30 min | Simple stain | R&D Systems,
Inc. |
| HNF1β | 12533-1-AP | 1:500 | pH 6,
autoclave | 25 | 30 min | Simple stain | Proteintech Group,
Inc. |
| PDX1 | ab47383 | 1:1,000 | pH 6,
autoclave | 4 | 16 h | - | Abcam |
| NKX6.1 | ab221549 | 1:500 | pH 6,
autoclave | 4 | 16 h | - | Abcam |
| CK5/6 | D5/16 B4 | 1:50 | pH 6,
autoclave | 4 | 16 h | Simple stain | Dako; Agilent |
|
|
|
|
|
|
|
| Technologies,
Inc. |
| KRT81 | sc-100929 | 1:50 | pH 6,
autoclave | 4 | 16 h | Simple stain | Santa Cruz |
|
|
|
|
|
|
|
| Biotechnology,
Inc. |
| SMAD4 | sc-7966 | 1:50 | - | 4 | 16 h | Simple stain | Santa Cruz |
|
|
|
|
|
|
|
| Biotechnology,
Inc. |
| TGFβ | 21898-1-AP | 1:50 | - | 25 | 30 min | - | Proteintech Group,
Inc. |
Histopathological evaluation
We evaluated the histological patterns and FF using
HE staining. The histological patterns of PDA-L and PDA-S were
evaluated following a previously reported method as a binary
variable (14). Tumor glands
>0.5 mm and occupying >50% of the total tumor area were
defined as PDA-L, and those not meeting these criteria were defined
as PDA-S (Fig. 1A and B) in the
representative largest cut surface (14). FF was evaluated using a four-grade
system (0, 1, 2, and 3) (Fig. 1C-E)
(16). Grade 0 (G0) specimens
primarily comprised infiltrating carcinoma without FF, and grade 1
(G1) specimens exhibited abundant fibroblasts arranged in a
storiform pattern. Grade 2 (G2) specimens comprised intermediate
fibroblasts mixed with collagen fibers and grade 3 (G3) specimens
primarily comprised hyalinized collagen fibers. Tumor cells were
rarely observed in FF. Based on assessment results, G1 and G2
specimens were categorized as having low fibrosis and G3 specimens
were categorized as exhibiting high fibrosis (17).
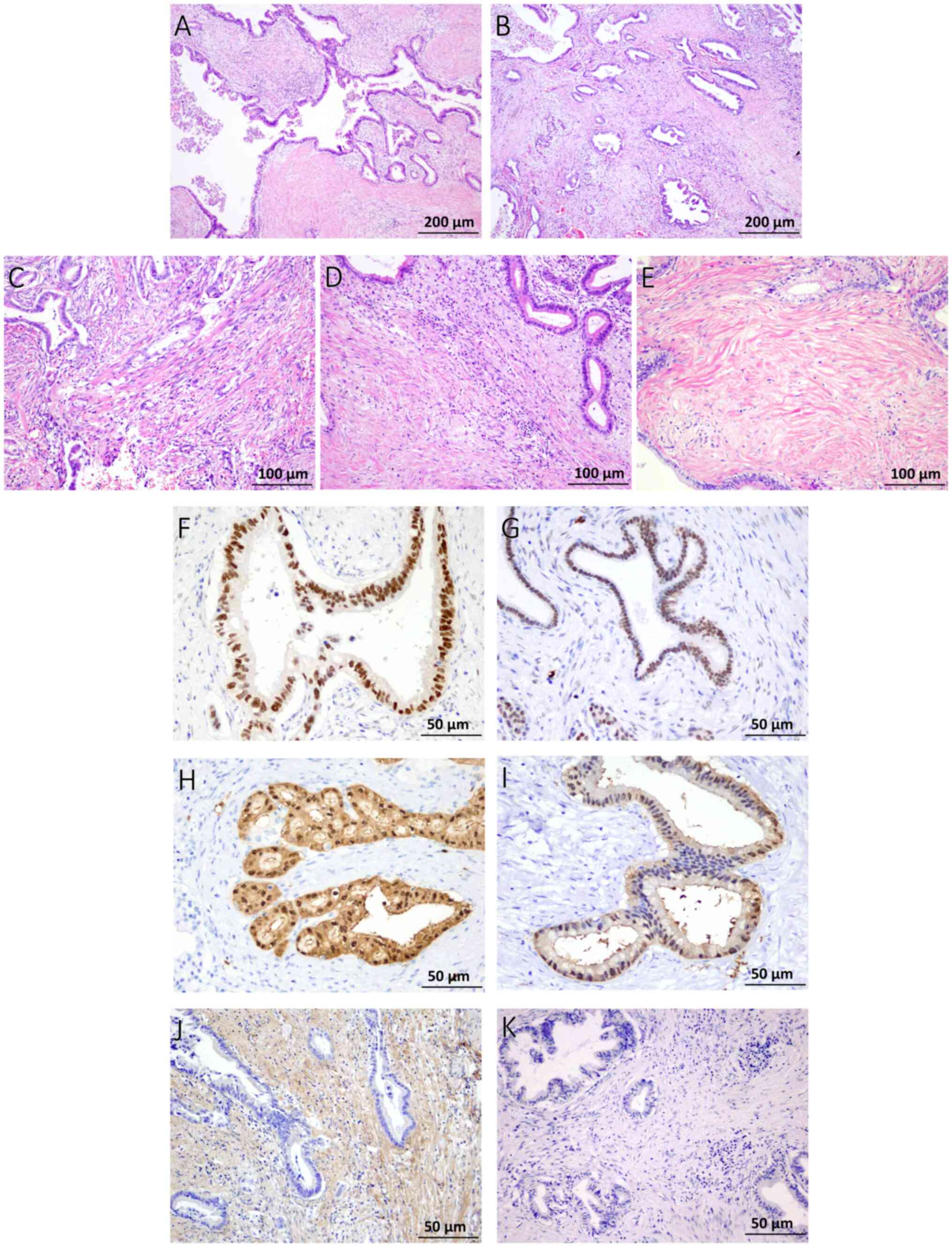 | Figure 1.Evaluation of the histological
patterns in PDA-L and PDA-S. (A) Tumor glands >0.5 mm and
occupying >50% of the total tumor area were defined as PDA-L,
and (B) the others were defined as PDA-S. Assessment of FF. (C)
Samples rated grade 1 had abundant fibroblasts arranged in a
storiform pattern, (D) those rated grade 2 consisted of
intermediate fibroblasts mixed with collagen fibers and (E) those
rated grade 3 consisted mainly of hyalinized collagen fibers. (E)
Tumor cells were seldom observed in FF. (A-E) Hematoxylin and eosin
stain. (F and G) Immunohistochemistry results for GATA6 and HNF1β.
(F) GATA6 positivity is shown, displaying an intensity score of 2+
and a population score of 3+ (positive). (G) HNF1β positivity is
also shown (intensity score of 2+; population score of 3+).
Immunohistochemistry results for S-100A4. (H) Sections showing
complete staining across the entire gland duct epithelium were
designated as S-100A4high, whereas (I) sections showing
partial staining of the gland duct epithelium were designated as
S-100A4low. Immunohistochemistry results for TGFβ. The
stroma exhibited (J) positive and (K) negative staining for TGFβ.
FF, fibrotic focus; GATA6, GATA binding protein 6; HNF1β,
hepatocyte nuclear factor-1β; PDA-L, large duct pattern; PDA-S,
small duct pattern; S-100A4, S100 calcium binding protein A4. |
During immunohistochemistry, reactivity was scored
using three grades of colorimetric intensity (0, 1+, and 2+) and
four grades of positive cell population (0, 1+, 2+, and 3+)
(Figs. 1F and G, and S1). According to intensity, specimens
were scored as follows: strong staining, 2+; weak staining, 1+; and
no staining, 0. For the positive cell population grading, a
staining grade of 3+ was detected in 50%, 2+ in 49–10%, and 1+ in
10–1% of the cells. Furthermore, specimens exhibiting negative
staining were assigned a score of 0 (18). If positive intensity score was 2+
and 1+, we classified by the population grade (S-100A4, GATA6,
HNF1β, PDX1, NKX6.1, KRT81, SMAD4, and TGFβ). For S-100A4, cases in
which the gland duct epithelium was partially stained were defined
as S-100A4low, whereas those in which the entire
epithelium was stained were defined as S-100A4high
(Fig. 1H and I). Additionally, the
stromal staining was evaluated separately for the presence of TGFβ
expression (Fig. 1J and K).
Statistical analysis
The χ2 test, Welch's t-test and Fisher's
exact test were performed using R (version 4.4.2), with the
significance level set at 95%. If the calculated P-value was
smaller than the one-sided P-value, it indicated a significant
difference and warranted evaluation. The association between
proteins was assessed using Phi coefficient, with a >0.2
indicating significance. The Mann-Whitney U test was performed to
determine the association between PDA-L/PDA-S and S-100A4
expression, OS and lymph node metastasis, and OS and tumor stage.
The Wilcoxon Signed Rank test was used to determine the association
between GATA6 and HNF1β expression.
Results
Clinical characteristics and
morphological findings of hPDA and btPDA
The clinical and pathological characteristics of
patients, clinical relationships, histological and IHC findings,
and clinical and histopathological relationships are shown in
Table II, Table III, Table IV. The study involved 60 patients
with PDA: 30 cases each of hPDA and btPDA. In the hPDA group, the
follow-up duration ranged from 6 to 70 (mean: 28.1) months, and
that in the btPDA group ranged from 4 to 60 (mean: 22.3) months.
Follow-up data regarding prognosis were not available for six
patients with hPDA and one patient with btPDA. Morphologically, 24
and 36 patients were classified as having PDA-L and PDA-S,
respectively. Regarding FF grading, 42 patients had a low FF grade
(G1 and G2), whereas 18 had a high FF grade (G3). No differences
were observed in sex, age, or histological patterns between the
hPDA and btPDA groups (Tables II
and III).
 | Table II.Clinical characteristics of patients
with head pancreatic ductal adenocarcinoma and patients with
body/tail pancreatic ductal adenocarcinoma. |
Table II.
Clinical characteristics of patients
with head pancreatic ductal adenocarcinoma and patients with
body/tail pancreatic ductal adenocarcinoma.
|
| Tumor location |
|
|---|
|
|
|
|
|---|
|
Characteristics | Head (n=30) | Body/tail
(n=30) | P-value |
|---|
| Sex, n
(male/female) | 14/16 | 16/14 | 0.261 |
| Mean age,
years | 72.4 | 70.2 | 0.229 |
| Tumor size, n |
|
| 0.030a |
| <20
mm | 9 | 2 |
|
| ≥20
mm | 21 | 28 |
|
| Lymph node
metastasis, n |
|
| 0.030a |
|
Positive | 21 | 15 |
|
|
Negative | 9 | 15 |
|
| Stage, n |
|
| 0.058a |
| 1 | 9 | 15 |
|
| 2, 3
and 4 | 21 | 15 |
|
| Follow-up,
months |
|
| 0.110 |
|
Range | 6-70 | 4-60 |
|
|
Mean | 28.1 | 22.3 |
|
| Overall survival,
n |
|
| 0.715 |
|
Dead | 18 | 14 |
|
|
Alive | 6 | 15 |
|
 | Table III.Morphological and
immunohistochemistry positivity findings in the head pancreatic
ductal adenocarcinoma and body/tail pancreatic ductal
adenocarcinoma groups. |
Table III.
Morphological and
immunohistochemistry positivity findings in the head pancreatic
ductal adenocarcinoma and body/tail pancreatic ductal
adenocarcinoma groups.
| Morphological and
immunohistochemical findings | Head, n (%) | Body/tail, n
(%) | P-value |
|---|
| Histological
pattern |
|
|
|
| Large
pattern | 13 (43) | 11 (37) | 0.792 |
| Small
pattern | 17 (57) | 19 (63) | 0.347 |
| Fibrotic focus |
|
|
|
| Low
(G1, G2) | 27 (90) | 15 (50) | 0.323 |
| High
(G3) | 3 (10) | 15 (50) | 0.0001 |
| S-100A4
positivity |
|
|
|
|
High | 13 (43) | 20 (67) | 0.125 |
|
Low | 17 (57) | 10 (33) | 0.792 |
| GATA6
positivity | 24 (80) | 29 (97) | 0.999 |
| HNF1β
positivity | 14 (47) | 8 (27) | 0.599 |
| KRT81
positivity | 17 (57) | 22 (73) | 0.425 |
| NKX6.1
positivity | 0 (0) | 0 (0) |
|
| PDX1
positivity | 5 (17) | 8 (27) | 0.759 |
| SMAD4
positivity | 1 (3) | 5 (17) | 0.999 |
| TGFβ (stroma
positivity) | 10 (33) | 20 (67) | 0.667 |
 | Table IV.Relationship between histological
findings and immunohistochemistry results. |
Table IV.
Relationship between histological
findings and immunohistochemistry results.
|
| Histological
pattern |
|
|---|
|
|
|
|
|---|
|
Immunohistochemistry results | Large, n | Small, n | P-value |
|---|
| S-100A4 |
|
| 0.0001a |
|
High | 3 | 29 |
|
|
Low | 21 | 7 |
|
| GATA6 |
|
| 0.0350 |
|
Positive | 24 | 32 |
|
|
Negative | 0 | 4 |
|
| HNF1β |
|
| 0.0010a |
|
Positive | 14 | 8 |
|
|
Negative | 10 | 28 |
|
The hPDA group had smaller tumors than the btPDA
group, and lymph node metastasis was more frequent in the hPDA
group than in the btPDA group (P=0.020 and P=0.0061, respectively).
Additionally, the hPDA group had a more advanced tumor stage than
the btPDA group (P=0.030). OS was analyzed according to tumor
location and histological patterns; the OS of patients was not
significantly different between the groups (Table II). In contrast, the degree of
fibrosis differed between the hPDA and btPDA groups, and the btPDA
group had a higher FF grade than the hPDA group (P=0.0001).
IHC results of the hPDA and btPDA
groups
The immunostaining results are shown in Table III, and Figs. 1 and S1. S-100A4 positivity was observed in all
patients; furthermore, S-100A4high was observed in 33
and S-100A4low in 27 of the 60 patients.
S-100A4high was observed in 13 of the 30 patients with
hPDA and 20 of the 30 patients with btPDA. All were negative for
PDX1, NKX6.1 and SMAD4, but some cells were positive for CK5/6 and
KRT81 (Fig. S1). No differences
were observed in the expression levels of all proteins between the
hPDA and btPDA groups.
IHC results of the PDA-L and PDA-S
groups
Variations in protein expression levels were
observed between the PDA-L and PDA-S groups (Table IV). In the PDA-L group, S-100A4
high was observed in 3 and S-100A4low was
observed in 21 of the 24 patients. In the PDA-S group,
S-100A4high was observed in 29 and S-100A4low
was observed in 7 of the 36 patients. S-100A4high was
significantly associated with PDA-S (Table IV). S-100A4high showed a
PDA-S pattern and S-100A4low showed a PDA-L pattern.
GATA6 positivity was observed in 56 and GATA6 negativity was
observed in 4 of the 60 patients. Of the 56 GATA6-positive
patients, 24 patients were categorized under PDA-L and 32 under
PDA-S. HNF-1β expression was positive in 22 of the 60 patients and
negative in 38 of the 60 patients. In the PDA-L group, 14 of the 24
patients showed HNF-1β positivity, whereas in the PDA-S group, 8 of
the 36 patients showed HNF-1β positivity. Patients with PDA-L
showed GATA6 and HNF1β positivity (P=0.0350 and P=0.0010,
respectively). Of the 53 patients who showed GATA6 positivity, 22
showed HNF1β positivity, 14 of whom had PDA-L. A significant
association was observed between histological pattern and GATA6
expression (P=0.0350), and between histological pattern and HNF-1β
expression (P=0.0010) (Table IV).
All 27 patients with S-100A4low showed GATA6 positivity;
of the 33 patients with S-100A4high, 27 showed GATA6
positivity. GATA6 expression was associated with HNF-1β expression
(Table SI).
Relationship among clinical
information, histological findings, and IHC results
With regard to OS, 9 of the 20 patients with PDA-L
died of the disease; in contrast, 23 of the 33 patients with PDA-S
died of the disease (P=0.136) (Table
SII). However, OS was significantly related to GATA6 positivity
(P=0.0001) (Table SII). Tumor size
had no relation with HNF1β or GATA6 positivity (P=0.267 and
P>0.999, respectively) (Table
SIII). Furthermore, no positive association was found between
FF and TGF-β expression in the stroma (P>0.999) (Table SIV).
Association between factors
We investigated the association between various
factors, with a particular focus on OS (Table SII). There was a significant
association between poor OS and positive lymph node metastasis
(P=0.016) and advanced stage (P=0.016) (Table SII). Furthermore, there were no
significant associations between tumor location and histological
subtypes (PDA-L and PDA-S), nor between tumor location and protein
expression (Table III).
Discussion
hPDA was significantly associated with small tumors,
increased lymph node metastasis, advanced stages, and low FF grade
tendency in this study. Patients with hPDA exhibited advanced
stages owing to increased lymph node metastasis, even though a
small tumor can trigger the onset of clinical symptoms and early
detection. This observation may be attributed to the presence of
immature fibrosis (low FF grade), facilitated by early detection.
However, there was no relationship between hPDA and OS; instead, it
was associated with increased lymph node metastasis and advanced
tumor stage. This finding may be owing to the limited number of
cases. A high FF grade tended to be also associated with poor OS,
as reported by a previous study on colon cancer; nonetheless, in
our study, a low FF grade was not associated with poor OS (15). The study findings did not establish
a definitive relationship between each clinical factor and poor OS.
However, other studies have suggested that HNF1β is an indicator of
poor prognosis in pancreatic cancer, and our results are consistent
with this finding (19). These
findings suggest that protein expression is related to the
morphology rather than tumor location, elucidating the notable
association between HNF1β expression and OS. Therefore, the
expressed protein may affect the choice of drug to be used. For
example, high HNF1β expression may be associated with drug
resistance in colorectal adenocarcinoma, and HNF1β expression may
affect cisplatin nephrotoxicity (20,21).
The histological PDA-L pattern was related to
S-100A4low, GATA6 positivity, and HNF1β positivity.
GATA6 is an important transcriptional regulator during pancreatic
development, particularly in the formation of exocrine glands in
the pancreas (22–24). It is required for gland development
and is considered an oncogene in pancreatic cancer pathogenesis
(22,24). GATA6 is reportedly highly expressed
in classical type pancreatic cancer based on Moffitt's
classification (6). Compared with
low GATA6 expression in the basal cell type, high GATA6 expression
has been observed in the classical type, which suppresses the basal
cell-like changes, resulting in a better prognosis (6,14,25,26).
This observation may be related to factors of
epithelial-mesenchymal transition (EMT), such as decreased
expression of E-cadherin and increased expression of vimentin that
occur with decreased GATA6 expression. Tumors with high GATA6
expression have ductal structures (26). In the present study, high GATA6
expression was associated with PDA-L, which may be a crucial factor
for the formation of ductal structures. Increased GATA6 expression
reportedly increases HNF1β expression during pancreatic development
(26). Although genetic alterations
were not discussed in this study, PDA-L was regarded as the
classical type and PDA-S as the basal cell type in the Moffitt's
classification (6,14,15).
Future studies should examine the relationship among tumor
location, morphological features, and genetic alterations.
Our findings revealed that GATA6 and HNF1β
expression was high, whereas S-100A4 expression was low in PDA-L.
S-100A4 is expressed in various cancer types other than PDA and
acts by inhibiting p53 release and increasing epidermal growth
factor receptor expression (27,28).
S-100A4 is associated with EMT, and the prognosis of
S-100A4-positive PDA is poorer than that of S-100A4-negative PDA
(27,28). Furthermore, S-100A4 promotes cell
invasion and metastasis by degrading the extracellular matrix,
which is involved in the upregulation of matrix metalloproteinases
(MMPs), especially MMP-9, MMP-13, and MMP-2. They play important
roles in tumor metastasis (28).
Additionally, increased MMP9 expression decreases the expression of
the long noncoding RNA GATA6-AS, a cancer suppressor gene,
resulting in repressive feedback in endometrial cancer (29). Based on these findings, the
characteristics of patients with GATA6 positivity, HNF-1β
positivity, and S-100A4low are consistent with those of
patients with PDA-L. High GATA6 positivity in treatment-naive PDA
favors good survival and can manifest a potent anti-tumor immune
microenvironment (30). These
protein expressions are linked to drug choice, and histological
analysis becomes important. Furthermore, btPDA was associated with
a high FF grade tendency and S-100A4high in this study.
This finding is consistent with the results of a previous study,
which suggested that fibrosis is more pronounced in btPDA and that
S-100A4 expression plays an important role in PDA fibrosis
(4).
This study has some limitations. First, it included
a small cohort of 60 Japanese patients with pancreatic cancer,
employing a retrospective study design. Although the cohort size
was small, this study represents a promising preliminary
investigation. Most patients were recruited before 2019, and a few
who underwent neochemoradiotherapy were omitted. Additionally,
variations in the disease course resulted in incomplete follow-up
for some patients. Moreover, we focused on histopathological
aspects, using limited clinical data and without taking into
account potential differences in treatment response among the
groups. Future investigations should focus on larger cohorts and
comprehensive clinical data.
Despite the limitations, our findings suggest that
hPDA was significantly associated with small tumors, more frequent
lymph node metastasis, more advanced stages, and low FF grade
trend. The tumor origin could not influence tumor prognosis, but
GATA6 was related to poor OS. In terms of histological findings,
the PDA-L pattern was related to S-100A4low, GATA6
positivity, and HNF1β positivity. We conclude that protein
expression shows a stronger association with tumor morphology than
with its location. The combination of morphology and protein
expression could serve as an indicator for PDA prognosis depending
on the choice of certain drugs used in treatment. In addition,
histological classification may be useful in PDA treatment.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are not
publicly available as they could compromise the privacy of research
participants but may be requested from the corresponding
author.
Authors' contributions
HN conceived and designed the experiments. HN and KA
performed the experiments. HN, KA, RK, KK and TD analyzed the data.
HN and KA wrote the manuscript. HN, KA and TD confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
tenets of The Declaration of Helsinki (2013). The study protocol
was reviewed and approved by the Institutional Ethics Committee and
Review Board of Oita University (approval no. 2641; Yufu, Japan).
The need for informed consent was waived due to the retrospective
nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
btPDA
|
body/tail pancreatic ductal
adenocarcinoma
|
|
CK5/6
|
cytokeratin 5/6
|
|
EMT
|
epithelial-mesenchymal transition
|
|
FF
|
fibrotic focus
|
|
G0
|
grade 0
|
|
G1
|
grade 1
|
|
G2
|
grade 2
|
|
G3
|
grade 3
|
|
hPDA
|
head pancreatic ductal
adenocarcinoma
|
|
HNF1β
|
hepatocyte nuclear factor-1β
|
|
IHC
|
immunohistochemical
|
|
KRT81
|
keratin 81
|
|
PDA-L
|
large duct pattern
|
|
NKX6.1
|
NK6 homeobox 1
|
|
PDX1
|
pancreatic and duodenal homeobox 1
|
|
PDA
|
pancreatic ductal adenocarcinoma
|
|
PDA-S
|
small duct pattern
|
References
|
1
|
Ushio J, Kanno A, Ikeda E, Ando K, Nagai
H, Miwata T, Kawasaki Y, Tada Y, Yokoyama K, Numao N, et al:
Pancreatic Ductal Adenocarcinoma: Epidemiology and Risk Factors.
Diagnostics (Basel). 11:5622021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neoptolemos JP, Palmer DH, Ghaneh P,
Psarelli EE, Valle JW, Halloran CM, Faluyi O, O'Reilly DA,
Cunningham D, Wadsley J, et al: Comparison of adjuvant gemcitabine
and capecitabine with gemcitabine monotherapy in patients with
resected pancreatic cancer (ESPAC-4): A multicentre, open-label,
randomised, phase 3 trial. Lancet. 389:1011–1024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee M, Kwon W, Kim H, Byun Y, Han Y, Kang
JS, Choi YJ and Jang JY: The role of location of tumor in the
prognosis of the pancreatic cancer. Cancers (Basel). 12:20362020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Birnbaum DJ, Bertucci F, Finetti P,
Birnbaum D and Mamessier E: Head and body/tail pancreatic
carcinomas are not the same tumors. Cancers (Basel). 11:4972019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ling Q, Xu X, Zheng SS and Kalthoff H: The
diversity between pancreatic head and body/tail cancers: Clinical
parameters and in vitro models. Hepatobiliary Pancreat Dis Int.
12:480–487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moffitt RA, Marayati R, Flate EL, Volmar
KE, Loeza SG, Hoadley KA, Rashid NU, Williams LA, Eaton SC, Chung
AH, et al: Virtual microdissection identifies distinct tumor- and
stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat
Genet. 47:1168–1178. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moll R, Franke WW, Schiller DL, Geiger B
and Krepler R: The catalog of human cytokeratins: Patterns of
expression in normal epithelia, tumors and cultured cells. Cell.
31:11–24. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Kane GM, Grünwald BT, Jang GH, Masoomian
M, Picardo S, Grant RC, Denroche RE, Zhang A, Wang Y, Miller JK, et
al: Correction: GATA6 expression distinguishes classical and
basal-like subtypes in advanced pancreatic cancer. Clin Cancer Res.
28:27152022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bártů M, Dundr P, Němejcová K, Tichá I,
Hojný H and Hájková N: The role of HNF1B in tumorigenesis of solid
tumours: A review of current knowledge. Folia Biol (Praha).
64:71–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen H, Xu C, Jin Q and Liu Z: S100
protein family in human cancer. Am J Cancer Res. 4:89–115.
2014.PubMed/NCBI
|
|
11
|
Zhou X, Hu K, Bailey P, Springfeld C, Roth
S, Kurilov R, Brors B, Gress T, Buchholz M, An J, et al:
Corrigendum: clinical impact of molecular subtyping of pancreatic
cancer. Front Cell Dev Biol. 11:11795592023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao M, Mishra L and Deng CX: The role of
TGF-β/SMAD4 signaling in cancer. Int J Biol Sci. 14:111–123. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roy N, Takeuchi KK, Ruggeri JM, Bailey P,
Chang D, Li J, Leonhardt L, Puri S, Hoffman MT, Gao S, et al: PDX1
dynamically regulates pancreatic ductal adenocarcinoma initiation
and maintenance. Genes Dev. 30:2669–2683. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim SJ, Choi SJ, Yang J, Kim D, Kim DW,
Byun JH and Hong SM: Pancreatic ductal adenocarcinoma with a
predominant large duct pattern has better recurrence-free survival
than conventional pancreatic ductal adenocarcinoma: A comprehensive
histopathological, immunohistochemical, and mutational study. Hum
Pathol. 127:39–49. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ueno H, Ishiguro M, Nakatani E, Ishikawa
T, Uetake H, Murotani K, Matsui S, Teramukai S, Sugai T, Ajioka Y,
et al: Prognostic value of desmoplastic reaction characterisation
in stage II colon cancer: Prospective validation in a Phase 3 study
(SACURA Trial). Br J Cancer. 124:1088–1097. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fujitani Y: Transcriptional regulation of
pancreas development and β-cell function (Review). Endocr J.
64:477–486. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding JH, Xiao Y, Zhao S, Xu Y, Xiao YL,
Shao ZM, Jiang YZ and Di GH: Integrated analysis reveals the
molecular features of fibrosis in triple-negative breast cancer.
Mol Ther Oncolytics. 24:624–635. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Allison KH, Hammond MEH, Dowsett M,
McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR,
Chavez-MacGregor M, Perlmutter J, et al: Estrogen and progesterone
receptor testing in breast cancer: ASCO/CAP guideline update. J
Clin Oncol. 38:1346–1366. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu DD, Guo SW, Jing YY, Dong YL and Wei
LX: A review on hepatocyte nuclear factor-1beta and tumor. Cell
Biosci. 5:582015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao F, Gong W, He H, Zhang Z, Yang H, Shao
F, Gao Y and He J: Integrated multi-omics analysis reveals clinical
significance of hepatocyte nuclear factor-1β in tumor immune
microenvironment, immunotherapy and prognostic prediction for colon
adenocarcinoma. Cancer Immunol Immunother. 74:382024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Hao J, Du Z, Li P, Hu J, Ruan M,
Li S, Ma Y and Lou Q: Inhibition of hepatocyte nuclear factor 1β
contributes to cisplatin nephrotoxicity via regulation of nf-κb
pathway. J Cell Mol Med. 25:2861–2871. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bailey P, Chang DK, Nones K, Johns AL,
Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC,
et al: Genomic analyses identify molecular subtypes of pancreatic
cancer. Nature. 531:47–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kloesch B, Ionasz V, Paliwal S, Hruschka
N, Martinez de Villarreal J, Öllinger R, Mueller S, Dienes HP,
Schindl M, Gruber ES, et al: A GATA6-centred gene regulatory
network involving HNFs and ΔNp63 controls plasticity and immune
escape in pancreatic cancer. Gut. 71:766–777. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martinelli P, Carrillo-de Santa Pau E, Cox
T, Sainz B Jr, Dusetti N, Greenhalf W, Rinaldi L, Costello E,
Ghaneh P, Malats N, et al: GATA6 regulates EMT and tumour
dissemination, and is a marker of response to adjuvant chemotherapy
in pancreatic cancer. Gut. 66:1665–1676. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang MX, Coates RF, Ambaye A, Gardner JA,
Zubarick R, Gao Y, Skelly J, Liu JG and Mino-Kenudson M:
Investigation of HNF-1B as a diagnostic biomarker for pancreatic
ductal adenocarcinoma. Biomark Res. 6:252018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Che P, Yang Y, Han X, Hu M, Sellers JC,
Londono-Joshi AI, Cai GQ, Buchsbaum DJ, Christein JD, Tang Q, et
al: S100A4 promotes pancreatic cancer progression through a dual
signaling pathway mediated by Src and focal adhesion kinase. Sci
Rep. 5:84532015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martinelli P, Cañamero M, del Pozo N,
Madriles F, Zapata A and Real FX: Gata6 is required for complete
acinar differentiation and maintenance of the exocrine pancreas in
adult mice. Gut. 62:1481–1488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Andrés MP, Jackson RJ, Felipe I,
Zagorac S, Pilarsky C, Schlitter AM, Martinez de Villareal J, Jang
GH, Costello E, Gallinger S, et al: GATA4 and GATA6
loss-of-expression is associated with extinction of the classical
programme and poor outcome in pancreatic ductal adenocarcinoma.
Gut. 72:535–548. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao Y, Zou X, Wang G, Liu Y, Zhang C, Lu
W and Li Q: Effects of GATA6-AS/MMP9 on malignant progression of
endometrial carcinoma. J BUON. 26:1789–1795. 2021.PubMed/NCBI
|
|
30
|
van Eijck CWF, Real FX, Malats N, Vadgama
D, van den Bosch TPP, Doukas M, van Eijck CHJ and Mustafa DAM;
Dutch Pancreatic Cancer Group (DPCG), : GATA6 identifies an
immune-enriched phenotype linked to favorable outcomes in patients
with pancreatic cancer undergoing upfront surgery. Cell Rep Med.
5:1015572024. View Article : Google Scholar : PubMed/NCBI
|















