Introduction
Breast cancer is globally the most common cancer
among women (1), and existing
treatments mainly include surgery, chemotherapy, endocrine therapy,
targeted therapy and radiotherapy. Radiotherapy is typically
administered to patients who have undergone breast conserving
surgery, those with lymph node metastasis and those who possess
large (≥5 cm) masses (2).
Radiotherapy induces double-stranded DNA breaks through ionizing
radiation, and as healthy cells have a stronger DNA repair ability,
radiotherapy typically targets and kills tumor cells (3). Although radiotherapy significantly
improves the prognosis of patients with malignant tumors, exposure
to radiation damages the skin. Radiation-induced skin injury (RSI)
is divided into two types: i) Acute RSI with skin desquamation,
necrosis, ulceration and hemorrhage; and ii) chronic RSI with
chronic ulceration, radiation-induced keratosis pilaris, capillary
dilatation, fibrosis and skin cancer (4). Radiation-induced non-melanoma skin
cancer (NMSC) occurs more frequently in patients undergoing
radiotherapy on the face, neck and head, while skin cancer induced
by postoperative radiotherapy for related breast cancers is rare
(5). In addition, RSI is described
as latent, progressive and persistent, and usually causes
irreversible damage to the microvessels and vascular endothelial
cells in the skin. Specifically, radiation-associated fibrosis
damages the lymphatic vessels and blood vessels in the irradiated
area, which can lead to skin ulcers and difficulties in wound
healing. It has been reported that 30% of patients who receive
radiotherapy to the breast or chest wall will develop severe skin
fibrosis (6). Patients with breast
cancer who undergo breast conserving surgery typically require
postoperative radiotherapy, which often results in side effects
that also affect the postoperative aesthetics of the patient. The
present case report describes the case of a patient with squamous
cell carcinoma (SCC) of the skin induced after postoperative
radiotherapy for breast cancer (Fig.
1), and discusses the causes and solutions to the postoperative
wound healing difficulties for skin cancer.
Case report
Patient
A 43-year-old woman noticed a palpable nodule at the
9 o'clock position on the right breast in January 2020. In March
2020, the patient sought medical attention at the Affiliated
Hospital of Guangdong Medical University (Zhanjiang, Guangdong,
China), where a breast core needle biopsy was performed, revealing
ductal carcinoma in situ (DCIS) of the right breast (the
pathological findings revealed cells with irregular nuclear size
and shape, increased chromatin, and the presence of cancer cells
within the mammary ducts; Fig. 2A),
with focal invasion suspected. After 5 days, the patient underwent
simultaneous breast-conserving surgery for right-sided breast
cancer and a right axillary sentinel lymph node biopsy.
The results of the postoperative pathology led to a
diagnosis of invasive carcinoma of the right breast with ductal
carcinoma in situ, with the diameter of the invasive lesion
being 0.5 cm and no metastasis present in the sentinel lymph nodes
(0/6). The immunohistochemistry (IHC) results were as follows:
Estrogen receptor (ER) (no expression in the invasive carcinoma and
carcinoma in situ), progesterone receptor (PR) (no
expression in the invasive carcinoma and carcinoma in situ),
human epidermal growth factor receptor 2 (HER2) (3+) and Ki-67 (50%
in the carcinoma in situ and 60% in the invasive carcinoma).
The patient was diagnosed with right breast invasive carcinoma with
ductal carcinoma in situ [pT1N0M0 stage IA with HER2
overexpression, according to the Eighth Edition of the AJCC Cancer
Staging Manual: Breast Cancer (7)]
at 6 days post-surgery. Based on the postoperative pathology, the
patient had indications for both chemotherapy and targeted therapy.
After surgery, the patient received chemotherapy and targeted
therapy, including 4 cycles of 110 mg docetaxel and 900 mg
cyclophosphamide, plus trastuzumab (initially 8 mg/kg, then 6 mg/kg
from the second cycle for the follow-up) every 21 days for 1
year.
According to the Chinese Society of Clinical
Oncology Breast Cancer diagnosis and treatment guidelines (2), patients who undergo breast-conserving
surgery require postoperative radiotherapy. The radiotherapy
modality for this patient was volumetric modulated arc therapy. The
patient received radiotherapy in July 2020 according to the
following scheme: Planning target volume (PTV), 5,000 cGy/25
fractions/5 weeks; PTVboost, 6,000 cGy/25 fractions/5 weeks
(subsequent skin cancer appeared in the PTVboost area). The patient
presented with erythema, dry flaky skin and a small amount of wet
flaking skin [grade 2; in accordance with the grading system
developed by The Radiation Therapy Oncology Group and The European
Organization for Research and Treatment of Cancer (8)] after radiotherapy, and was instructed
to keep their skin dry, protect it from light and prevent friction.
At 3 months post-radiotherapy, the skin erythema had resolved, and
the skin was only slightly dry, with no dry or wet flaking
observed. All treatments for the patient were completed at the
Affiliated Hospital of Guangdong Medical University. After the
completion of treatment, the patient did not require any additional
special treatment and only needed regular follow-up examinations.
Follow-ups were conducted every 3 months in the first and second
years, and every 6 months from the third year onward. No
abnormalities were detected during the follow-up period from March
2020 to July 2023.
At a total of 3 years post-surgery, the patient
complained of a skin nodule at the right breast scar, which was
noticed while taking a shower in July 2023, with no obvious
triggers. This condition persisted for 2 months. The surface of the
skin nodule was initially ulcerated and then gradually crusted
over. During this period, the patient did not visit the hospital
for medical care, nor was any treatment administered. In September
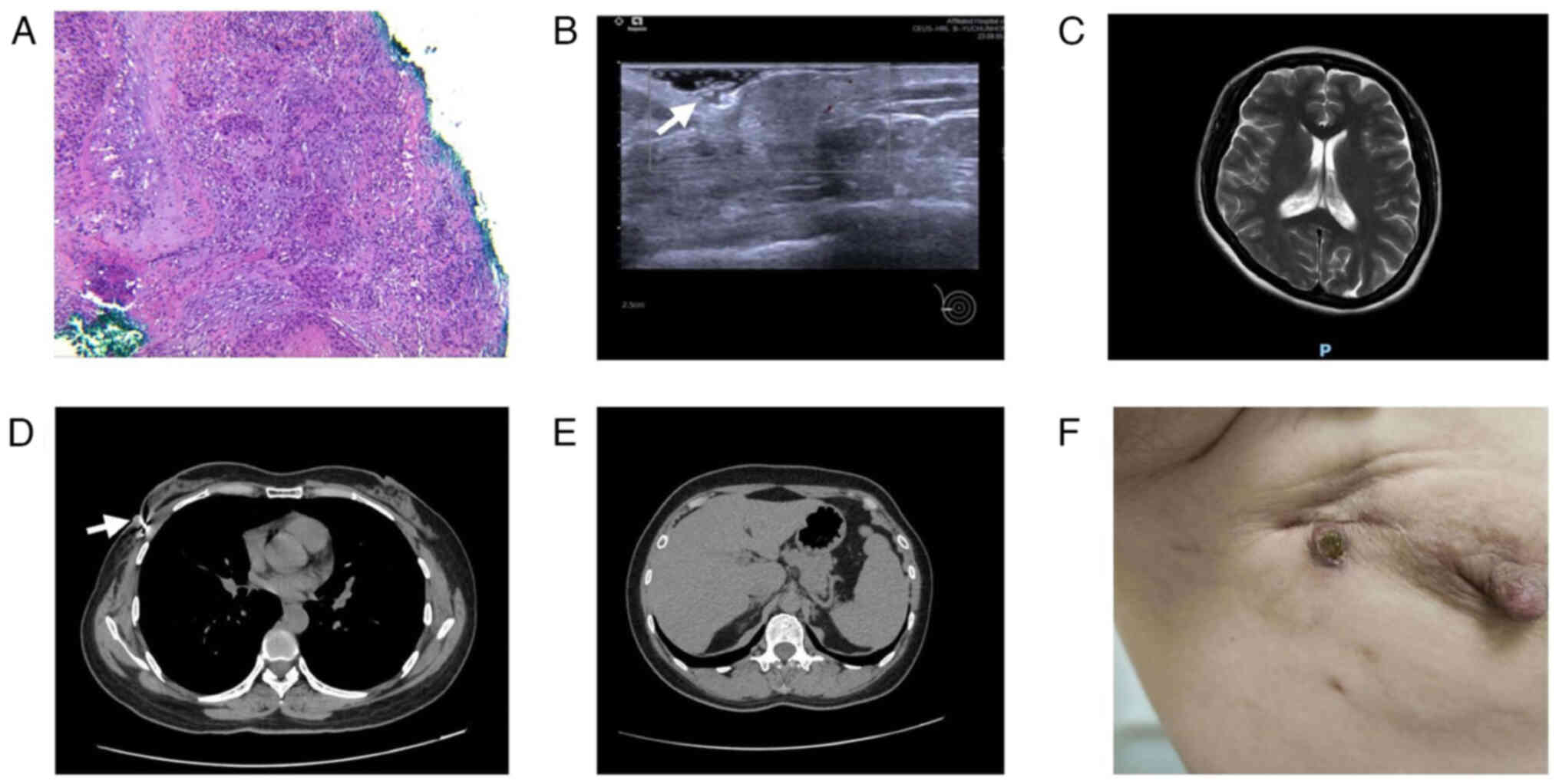
2023, breast ultrasonography at the Affiliated Hospital of
Guangdong Medical University showed an isoechoic nodule in the
right breast at the 9 o'clock direction of the surgical scar,
measuring ~1.1×0.9 cm in size [breast imaging-reporting and data
system class 4B, according to the second edition of the American
College of Radiology BI-RADS US (9); Fig.
2B]. There were no signs of local or distant metastases in the
head magnetic resonance imaging scans (Fig. 2C), or the chest (Fig. 2D) and upper abdomen (Fig. 2E) enhanced computed tomography
scans.
Physical examination results
A round raised nodule was observed at the scar in
the right breast, measuring ~1.1×0.9 cm in size with a hard texture
and unclear boundary. The nodule presented as a central black scab
with a dark red periphery, with no breakage or bleeding. There was
a hard texture to the surrounding skin and soft tissues, poor
mobility and no sense of tenderness. No obvious enlarged lymph
nodes were detected in the right axilla and the upper and lower
fossae of the clavicle (Fig.
2F).
Course of treatment and postoperative
pathology
In September 2023, the patient underwent excision
and biopsy of the right breast skin nodule due to the possibility
of breast cancer recurrence, which could not be ruled out. During
the operation, the nodule and surrounding 1 cm of skin tissue were
removed. The intraoperative frozen-section examination showed that
the right breast skin had focal ulcer formation, squamous
epithelial hyperplasia and inflammatory cell infiltration. The
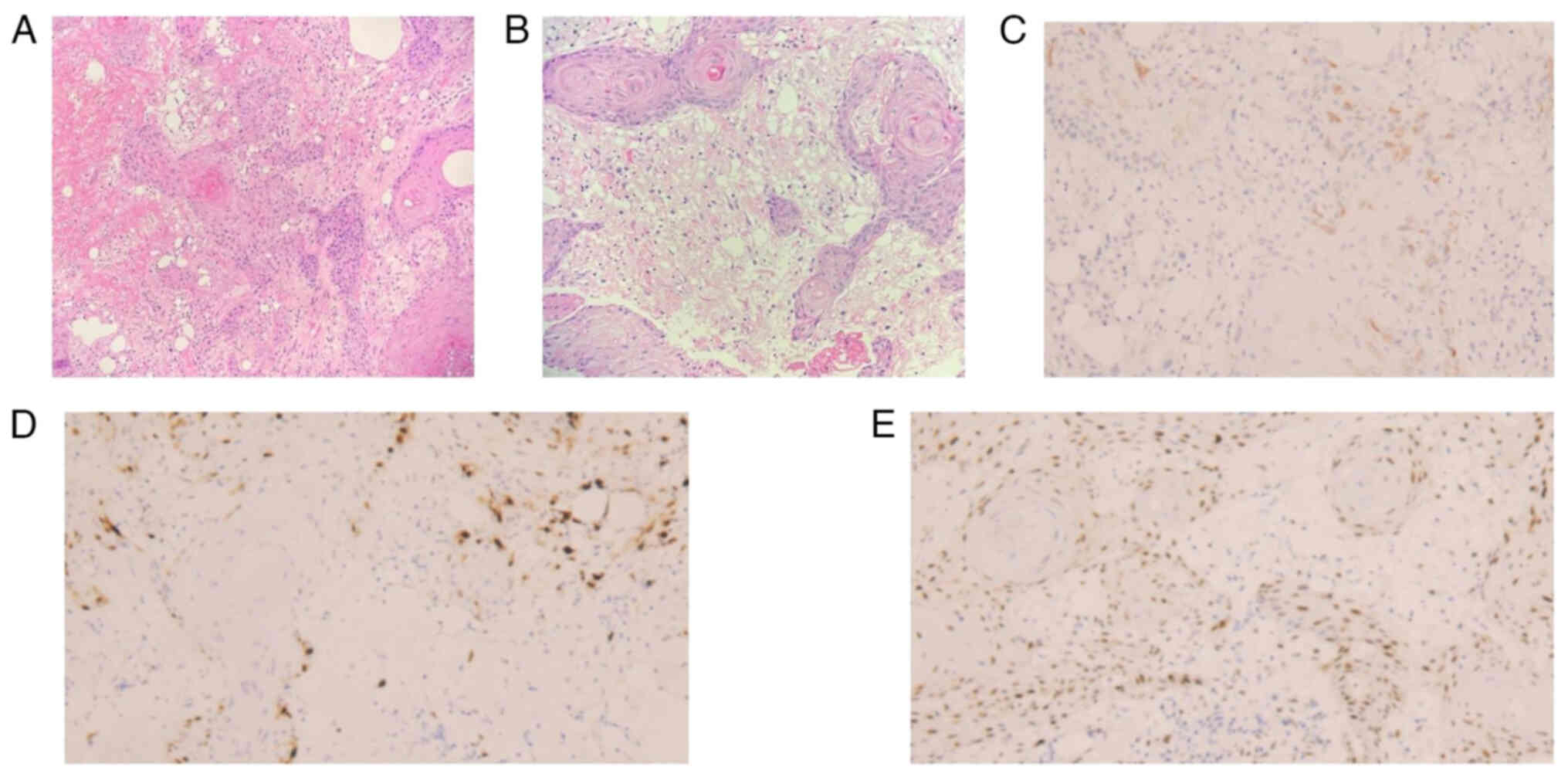
results of the postoperative routine pathology were as follows
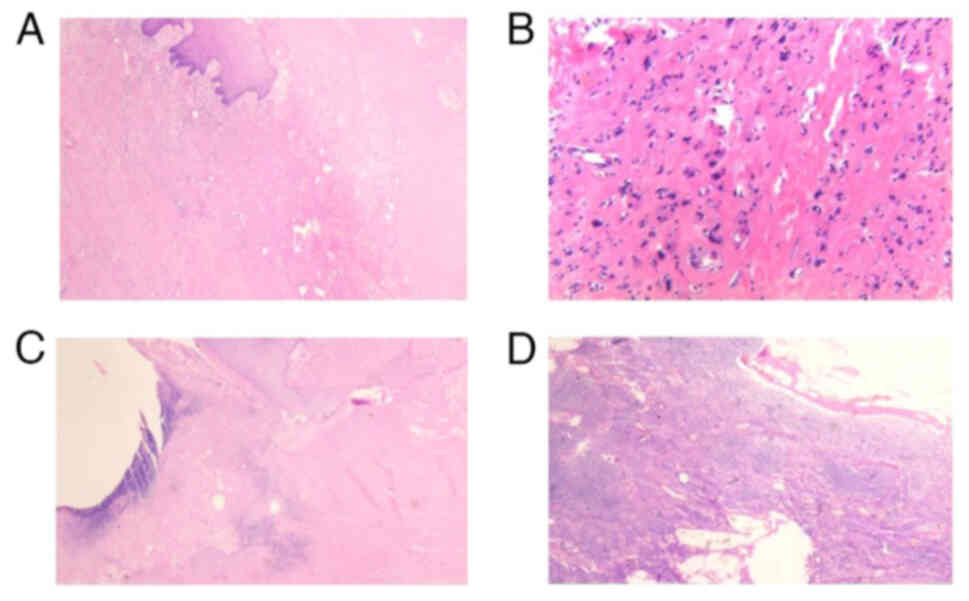
(Fig. 3A and B): The right breast
nodule was formed of skin tissue and the formation of a necrotic
ulcer was observed locally; the peripheral squamous epithelium had
significant hyperplasia with keratinization, and some of the
epithelium was scattered within the stroma. The results of the IHC
were as follows (Figs. 3 and
4): Insulin-like growth factor 2
mRNA-binding protein 3 IMP3(partial+), Ki-67(~30%), tumor protein
p53 (wild-type expression), tumor protein p63(+), cytokeratin
(CK)(+), CK5/6(+) and HER2(−). The combination of these results
were consistent with highly differentiated SCC, the surgical
horizontal resection margins and basal margins are negative. The
postoperative diagnosis was a follows: i) Skin SCC of the right
breast (pT1N0M0 stage I); and ii) after breast conserving surgery
for right breast cancer (pT1N0M0 stage IA HER2 overexpression
type).
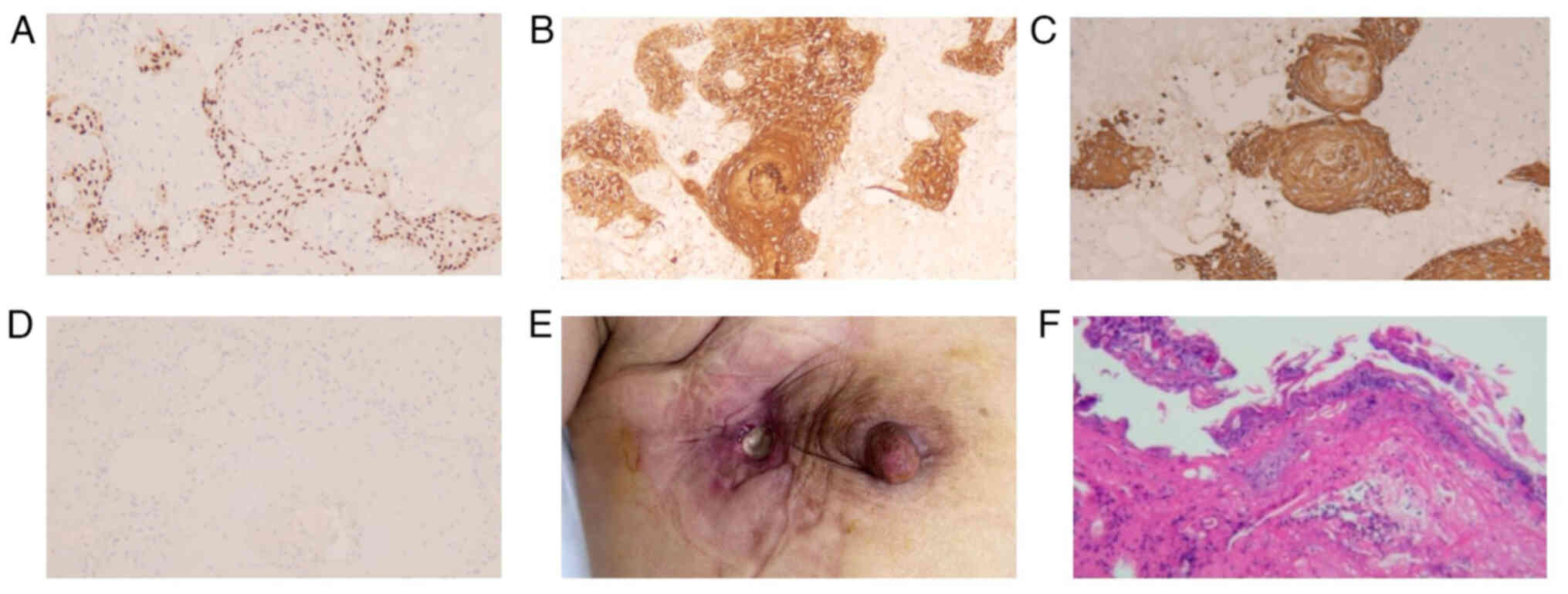
In November 2023, the patient identified an ulcer
located at the scar after the excision of the right breast skin
nodule (Fig. 4E), measuring ~2×1 cm
in size, that secreted a foul-smelling purulent fluid, with red,
swollen and painful surrounding skin. A skin biopsy (H&E
staining) indicated that the surface epithelium of the submitted
skin tissue was hyperplastic with hyperkeratosis, parakeratosis,
and local inflammatory exudation and necrosis (Fig. 4F). Considering that conservative
treatment of this ulcer is not effective, a partial mastectomy of
the right breast, an excision of the descending branch of the
thoracodorsal vasculature myocutaneous flap, a right mammaplasty
and a right nipple areola flap repair were performed 6 days after
the patient was admitted. The postoperative pathology demonstrated
that: i) The right breast skin ulcer had formed accompanied by
local tissue necrosis of the skin, hyaline degeneration and
inflammatory cell infiltration of the surrounding stroma, and that
large hyperchromatic deformed cells were observed in local areas
(Fig. 5A and B); ii) no tumor was
found in the upper, lower, inner, basal or incisional margin
tissues of the right breast submitted for examination during the
operation (Fig. 5C); and iii)
reactive hyperplasia was observed in one lymph node that was sent
for examination (Fig. 5D).
Surgical methods
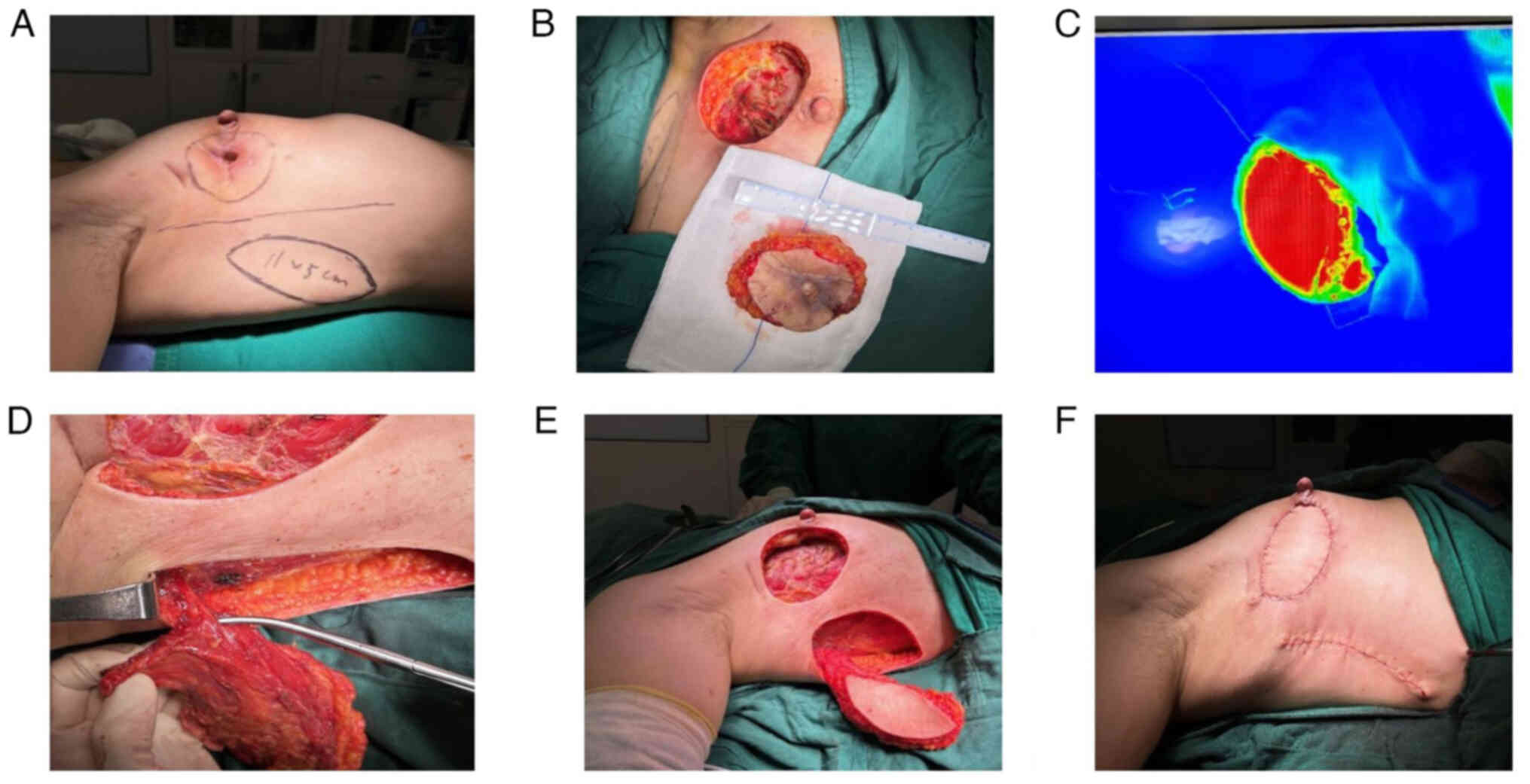
Prior to the operation, the size of the skin ulcer
surface at the 9 o'clock position on the right chest wall, the scar
tissue area around it and the fibrotic tissue after radiotherapy
were measured. A color Doppler ultrasound was used to locate the
position of the thoracodorsal vessels, and the location of the
descending branch flap of the thoracodorsal vessels was used as a
horizontal axis. A section ~10 cm below the axillary fold on the
right chest wall area was used as a fusiform flap with a length of
~11 cm and a width of ~5 cm (Fig.
6A). After demarcation, attention was paid to the tension of
the flaps on both sides to avoid the occurrence of skin flap
necrosis due to excessive tension.
Before the operation, gentian violet was used to
demarcate the incision range on the skin, that is, an oval incision
centered on the ulceration. The extent of the excision included
breast tissue and scar tissue in the outer quadrant of the breast,
including part of the chest muscle, measuring 5×8 cm. After the
incision, the remaining necrotic tissue and deep surface underwent
debridement, and the bleeding tissue was exposed without
abnormality (Fig. 6B).
Subsequently, the thoracodorsal vascular muscle flap
was separated as follows: i) The skin and subcutaneous tissues were
incised sequentially along the preoperative right lateral thoracic
wall to the surface of the latissimus dorsi muscle. ii) the main
trunk of the thoracodorsal vessels was exposed after exploration,
and then the descending branches of the thoracodorsal vessels were
found along its course. The surrounding tissues and small branch
vessels were separated, paying attention to the position of the
thoracodorsal nerves during the process to avoid injury. iii) The
flap of the descending branch of the thoracodorsal vessels and a
small portion of the latissimus dorsi muscle were separated
together, and after separation, indocyanine green was injected
intravenously to clarify the flap blood flow (Indocyanine Green
Fluorescence Imaging System; Fig. 6C
and D). A suitable subcutaneous tunnel was made between the
breast remnant cavity and the lateral descending thoracodorsal
vascular muscle flap. The size of this tunnel was large enough to
avoid vascular distortion and compression (Fig. 6E). The free descending thoracodorsal
vascular flap was transferred to the breast remnant cavity and then
the flap was plasticized and cut according to the trauma. The flap
was fixed on the right side of the breast defect by absorptive
suture and the retained flap was sutured with the skin to reshape
the breast (Fig. 6F).
Post-operative follow-up
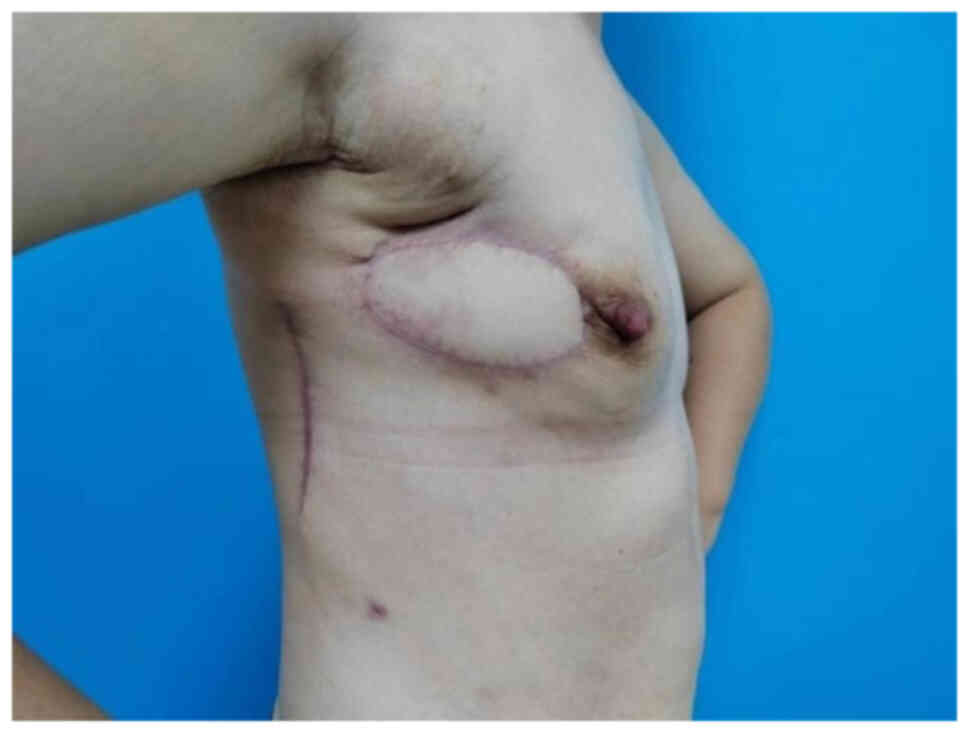
At the 6-month postoperative follow-up in May 2024,
the patient exhibited successful wound healing of the flap and did
not complain of discomfort. The flap showed no signs of
hyperpigmentation or edema (Fig.
7). The patient's last follow-up was in December 2024, and no
new tumors were found during the examination.
H&E staining and
immunohistochemistry (IHC)
Samples were fixed in 10% neutral buffered formalin
at room temperature for 12 h. After tissue dehydration and
clearing, the samples were immersed in melted paraffin at 60°C
twice, each for 1 h. Sections were cut to 5 µm, dewaxed and
subjected to H&E staining. H&E staining were performed at
room temperature for 5 min, and the slides were observed under an
optical microscope.
For IHC, the following primary antibodies were
applied: Anti-ER (ready-to-use antibody; catalog no.: 790-4324;
incubation for 32 min; Roche Diagnostics GmbH), anti-PR
(ready-to-use antibody; catalog no. 790-2223; incubation for 24
min; Roche Diagnostics GmbH), anti-HER2 (ready-to-use antibody;
catalog no. 790-2991; incubation for 8 min; Roche Diagnostics
GmbH), anti-IMP3 (dilution, 1:100; catalog no. MAB-0725; incubation
for 40 min; Fuzhou Maixin Biotechnology Development Co., Ltd.),
anti-Ki-67 (ready-to-use antibody; catalog no. 790-4286; incubation
for 16 min; Roche Diagnostics GmbH), anti-p53 (ready-to-use
antibody; catalog no. 790-2912; incubation for 32 min; Roche
Diagnostics GmbH), anti-p63 (dilution, 1:150; catalog no. MAB-0674;
incubation for 32 min; Fuzhou Maixin Biotechnology Development Co.,
Ltd.), CK (dilution, 1:100; catalog no. A1901; incubation for 30
min; Guangzhou LBP Medicine Science & Technology Co.,Ltd.) and
CK5/6 (dilution, 1:150; catalog no. MAB-0692; incubation for 36
min; Fuzhou Maixin Biotechnology Development Co., Ltd.). The
incubations were performed on the Roche Ventana automated IHC
platform at 36°C. Subsequently, the secondary antibody from the
UltraView Universal DAB Detection Kit (ready-to-use; catalog no.
760-500; Roche Diagnostics GmbH) was automatically incubated using
the Ventana platform for 8 min. Streptavidin-HRP (ready-to-use;
catalog no. 760-500; Roche Diagnostics GmbH) was added and
automatically incubated on the Ventana platform for 10 min.
3,3′-Diaminobenzidine (ready-to-use; catalog no. 760-700; Roche
Diagnostics GmbH) was used as the chromogenic substrate and
automatically developed on the Ventana platform for 8 min. Finally,
the nuclei were counterstained with hematoxylin for 30 sec, and the
slides were observed under an optical microscope (Olympus
Corporation).
Discussion
The pathogenesis of cutaneous SCC (cSCC) mainly
includes ultraviolet radiation, human papilloma infection, TP53
gene mutation, chronic scarring, transplantation-related immunity,
ionizing radiation and low-dose arsenic exposure. Among them,
prolonged ultraviolet radiation is the most common pathogenesis of
cSCC, which is mainly found in exposed areas such as the scalp,
face and backs of the hands (10).
In the present case, the diagnosis of the patient
needed to be differentiated from other diseases. Firstly, the
patient did not have a history of long-term ultraviolet (UV)
radiation exposure on the chest wall, an area that is less exposed
to UV radiation compared with other parts of the body. Therefore,
the likelihood of primary cSCC occurring at this location was low.
Additionally, the patient had a clear history of radiotherapy and
presented with post-radiation skin fibrosis, which further
supported the exclusion of primary cSCC. Secondly, the patient had
a previous history of breast cancer. When the skin nodule was first
detected, the possibility of breast cancer recurrence or metastasis
could not initially be ruled out. However, based on the
postoperative pathological findings, the tumor cells were arranged
in nests or sheets with the presence of keratin pearls. IHC showed
positive expression of CK5/6 and p63, indicating a squamous cell
origin, which is inconsistent with the pathological features of
breast cancer. Additionally, breast cancer recurrence or metastasis
is often accompanied by other metastatic lesions, but no other
abnormalities were found in the patient's examination results.
Therefore, the possibility of breast cancer recurrence or
metastasis was ruled out. Thirdly, the patient developed SCC of the
skin in the irradiated area 3 years after radiotherapy, and it is
noteworthy that this nodule was located in a scar. Cases of
malignant transformation of scar tissue have been previously
reported (11) and this condition
is termed a Marjolin ulcer (MU) (12). In patients with MU, the primary
cause of skin SCC is due to recurrent ulceration and persistent
irritation of a non-healing wound. MU therefore often occurs in
post-burn scarring and is often more aggressive, with research
results showing that 20–36% of patients have lymph node metastasis
at the time of initial diagnosis (13). The clinical presentation of the
present study patient was similar to that of MU. However, the
patient did not present with ulceration or poor healing of the scar
after postoperative radiotherapy. The patient's skin cancer was a
well-differentiated cSCC without regional lymph node metastasis,
which differs from the pathological characteristics of MU. In
addition, the skin nodule initially found in this case was only
ulcerated for a short period of time and gradually scabbed over.
Therefore, it was hypothesized that the cSCC of the patient was not
as a result of malignant transformation of the scar tissue.
There have been multiple previous reports on
radiation-induced skin cancer. In 1995, Landthaler et al
(14) retrospectively analyzed 612
radiation sites from 522 patients and observed 12 cases of basal
cell carcinoma (2.0%) and 9 cases of SCC (1.5%). Subsequently, a
prospective analysis of the Childhood Cancer Survivor Study cohort,
which included 13,132 childhood cancer survivors, identified NMSC
in 213 patients. Among these, 90% had received radiotherapy, and
90% of the tumors developed within the irradiated field (15). Basal cell carcinoma and SCC are the
two main types of post-radiotherapy cutaneous malignancy, with a
long latency period reported and being described as a late
complication of ionizing radiation. Radiation-induced skin cancers
can develop at any time from 2–65 years after radiation exposure
(16). Although the probability of
malignancy induced by radiation therapy is low, a study found a
positive correlation between radiotherapy for breast cancer and
cutaneous melanoma (17). A
subsequent analysis by Rezaei et al (18), that included 875,880 patients with
breast cancer (50.3% of whom received radiotherapy), found that
patients who were treated with radiotherapy were more likely to
develop skin cancer than those who did not receive radiotherapy (8%
increase). Although this study did not include SCC or basal cell
carcinoma of the skin, statistical analysis revealed that melanoma
and angiosarcoma were the most common types of non-keratinocytic
skin cancer among patients with breast cancer following
radiotherapy or other treatments. In addition, a review of relevant
literature also identified case reports of NMSC induced by
postoperative radiotherapy for breast cancer, with pathological
results consistently showing SCC (19–21).
In the case reported by Loveland-Jones et al (19), the patient had undergone lumpectomy
and whole breast radiation therapy. At 9 years after surgery, a
non-healing ulcer of the right nipple was detected. The patient
underwent a wide excision of the nipple-areolar complex, and
pathological examination revealed a moderately to poorly
differentiated invasive SCC. IHC showed positive expression results
for CK5/6, p63 and CK, and negative expression results for ER, PR
and HER2. The surgical approach in this case was consistent with
that of the present patient, and the SCC also developed within the
radiation field. Similarly, IHC in this case showed positive
expression of CK5/6 and p63, which are associated with SCC, and
lack of expression of HER-2, which is associated with breast
cancer. Therefore, based on the aforementioned reasons, we consider
that the cSCC in the present patient was induced by radiation
therapy.
With regard to the mechanism of radiation-induced
skin cancer, firstly, radiation causes skin cancer mainly due to
direct and indirect DNA damage, reactive oxygen species (ROS)
production and local immune dysregulation. Cellular damage due to
excessive ROS production is the result of interference with cell
membranes, proteins and DNA, which alters the overall biological
activity. As a result of these effects, oxidative products are
formed that have mutagenic properties, which initiates the
oncogenic process within the epidermal cells (4). Secondly, chronic stimulation is also a
recognized theory, as research has found that inflammation is
involved in the development of skin cancer (22). For example, chronic inflammatory
stimulation due to radiation dermatitis and radioactive ulcers
after radiotherapy is both a carcinogenic and a cancer-promoting
factor. van Vloten et al (23) followed up 360 patients who had
received radiotherapy for benign diseases of the head and neck, and
found skin tumors in 21 of them. In 8 out of the 21 patients, 10
skin carcinomas were detected at recall. The study also concluded
that the severity of radiation dermatitis was associated with a
higher incidence of skin cancer. Furthermore, the dry contracture
of the skin in the radiation area and the small amount of
pigmentation observed in this area, as described in the present
case report 3 years after radiotherapy, is in line with the
clinical manifestations of chronic radiation dermatitis. Therefore,
it was concluded that the patient described in the present case
report developed SCC of the skin after radiotherapy, which may also
be related to long-term stimulation of chronic radiation
dermatitis. The diagnostic criteria for radiotherapy-induced skin
cancer and malignant tumors induced by radiotherapy converge can be
summarized as follows: i) A definite history of radiation exposure;
ii) tumors occurring at the irradiated site; iii) a long latent
period; and iv) pathological and histological confirmation or
histological types different from the primary tumor (24).
Surgery is the primary treatment for skin SCC. For
patients with early stage skin cancer, the radical cure rate of
surgical resection can reach up to 95%; this consists of standard
excision, Mohs micrographic surgery (MMS) and curettage and
electrocautery, with standard excision being the most commonly used
(25). For low- and high-risk
cSCCs, radial resection margins of 4 and 6 mm, respectively,
demonstrated 95% oncological clearance (25). For low-risk cSCC, National
Comprehensive Cancer Network guidelines recommend that excision
margins are 4–6 mm of the normal skin and that the margins are
pathologically negative (10). For
high-risk cSCC with multiple risk factors, there is insufficient
clinical data and no definitive criteria for peripheral margins,
but relevant studies have found that MMS is more effective than
standard excision for high-risk cSCC (26). The main non-surgical treatment
modalities for cSCC are radiotherapy, chemotherapy, photodynamic
therapy (PDT) and cryotherapy (27). Among them, cryotherapy or PDT are
only suitable for low-risk skin cancers without a clear
histological diagnosis and proof of tumor clearance, while
radiotherapy and chemotherapy are mostly used for patients with
advanced cancers.
In the present case report, the skin nodule of the
patient appeared on the right side of the postoperative scar from
breast cancer surgery. The possibility of recurrence of breast
cancer could not be excluded; however, combined with the wishes of
the patient and the circumstances, surgical excision and
intraoperative frozen section pathology were performed. In
addition, the results of intraoperative frozen pathology only
described squamous epithelial hyperplasia, and did not diagnose SCC
of the skin clearly. Therefore, considering the limitations of the
intraoperative frozen pathology and the fact that the patient had a
history of radiotherapy, a standard excision of skin cancer was
performed, and ~1 cm of normal skin around the skin nodule was
excised. This was confirmed to be cSCC by postoperative routine
pathology, and there was no tumor residue on the margins of the
incision.
A total of 20 days after the excision of the skin
SCC, the patient developed a wound ulcer. Therefore, the
possibility of tumor remnants could not be excluded, and a biopsy
of the skin tissue was performed. The results of the biopsy
indicated that only skin ulcer formation had occurred, and it was
hypothesized that the main cause of the ulcers was RSI after
radiotherapy.
Radiotherapy-induced dermatofibrosis is a chronic
and irreversible disease that occurs weeks to years after radiation
exposure and is characterized by changes in skin appearance, loss
of skin elasticity and contractures, and hardness of the skin that
is difficult to pinch and crease, accompanied by capillary
dilatation, pain and itching. Radiation-associated dermatofibrosis
is also accompanied by damage to the vascular system, which is
characterized by a decrease in the density of the microvascular
network and vascular morphological changes (28). Therefore, it was considered that
there were two main reasons for the poor wound healing in the
patient described in the present case report. Firstly, the
radiation damage destroyed the blood vessels and thus led to poor
blood flow in the flap in this area, thus making it difficult to
heal. Secondly, the radiation-related fibrosis led to high tension
at the flap on both sides after the tumor resection, which
ultimately led to the poor healing outcome. A previous study showed
that early aggressive management of chronic non-healing wounds and
peripheral flap grafting after excision of unstable scar tissue can
improve the prognosis of patients with cSCC (29). Therefore, in the present study, part
of the right breast where the ulcer had formed was excised and a
free flap repair was performed.
The common flaps for this type of chest wall
reconstruction are the latissimus dorsi muscle flap, localized
arbitrary flap and rectus abdominis muscle flap (30). However, in the present study, due
the condition of the patient and the extent of the right
mastectomy, the descending thoracodorsal vascular flap was selected
for the repair of the chest wall. The thoracodorsal vascular flap
is also often used for reconstruction after breast-conserving
surgery for breast cancer (31),
and it must be noted that its use in the repair in a variety of
soft-tissue defects has improved results (32). Furthermore, as the patient in the
present study had undergone breast-conserving surgery in the past,
and the amount of subcutaneous fat was insufficient, the descending
branches of the thoracodorsal vascular musculocutaneous flap were
selected to repair the defect. The advantages of this type of
musculocutaneous flap were firstly that the location of the
incision, namely the lateral chest wall, was relatively hidden, and
there was no need to change the position of the patient during the
operation. Secondly, most of the latissimus dorsi muscle was
preserved in our case, which reduced the likelihood of hematoma in
the back and shoulder joint dysfunction, and there were fewer
complications in the donor area. It must be noted that the
thoracodorsal vascular flap has limitations, including the
possibility of mutation of the branches emanating from the
thoracodorsal vascular, the limited width of the flap and the
time-consuming intraoperative fine separation (33,34),
However, this type of repair is simpler than other flaps, has fewer
postoperative complications, and can be used in other patients with
chest wall skin cancer or localized chest wall ulcers after
radiotherapy. In the present case report, the breast shape of the
patient was improved while the patient underwent flap repair using
the descending branches of the thoracodorsal vascular
musculocutaneous flap. Radiotherapy has become a key part of
postoperative treatment for breast cancer, and although SCC of the
skin is rare after radiotherapy for breast cancer, physicians
should remain vigilant. The area receiving the radiotherapy will
have a postoperative scar and the side effects of radiotherapy can
induce skin cancer. Therefore, a biopsy should be performed as
early as possible to clarify the pathology and any follow-up
treatment. In addition, in view of the poor wound healing described
in the present case, radiotherapy may lead to the occurrence of
skin fibrosis. As there is no effective prevention and treatment
method for radiation fibrosis, immediate flap transfer repair
should be considered at the time of surgery, especially for those
patients who need surgical excision for skin cancer after
breast-conserving surgery, in order to reduce the risk of adverse
events and to improve the appearance of the breasts while obtaining
improved therapeutic effects.
Finally, RSI has a long latency period and therefore
should be prevented early. With the development of radiotherapy
technology, intensity modulated radiation therapy, volumetric
modulated arc therapy, helical tomotherapy, fixed field tomotherapy
and other treatment modalities in clinical practice, research has
found that the correct choice of radiotherapy modality can
effectively reduce the side effects associated with radiotherapy
(35,36). As radiotherapy-related skin damage
can lead to skin cancer, and despite certain treatments delaying or
reducing the risk of radiation-induced fibrosis (RIF), the key to
preventing RIF is to reduce the radiation dose to exposed healthy
skin areas. Future advancements in radiotherapy should focus on
improving targeting precision and minimizing radiation exposure to
healthy tissues, thereby enhancing patient quality of life.
Radiotherapy is a primary cancer treatment; however,
relevant measures must be taken to prevent RSI and reduce the
incidence of adverse reactions. Drugs that can aid this must be
further researched and applied clinically. In addition, rational
selection of radiotherapy techniques, individualized design, and
early use of relevant drugs to treat and prevent chronic
radioactive skin injury must be implemented, regardless of the
severity of the RSI. Finally, patients should be followed up for a
long period of time after radiotherapy. If skin nodules, ulcers or
other chronic suspected wounds develop, biopsies should be
performed early to exclude the possibility of malignant lesions.
Any required follow-up treatment should be carried out as soon as
possible, and the feasibility of surgical excision of the patient
should be considered comprehensively based on the condition of the
skin condition. In the future, in addition to the more commonly
used surgical interventions, improved treatment options should be
further explored.
In conclusion, radiotherapy can lead to the
development of skin cancer, and its related skin injury may bring
difficulties to the treatment of skin cancer induced after
radiotherapy. Therefore, during radiotherapy, more attention should
be paid to the treatment of RSI. In addition, following
radiotherapy, more attention should be paid to the skin condition
of the area where radiotherapy was received, in addition to the
postoperative recurrence and wound recovery. If skin cancer has
developed after radiotherapy, a comprehensive evaluation should be
performed, and if surgical resection of the tumor is feasible, a
simple flap repair or reconstruction surgery should be considered
immediately after the skin cancer is resected, as described in the
present study, to avoid the occurrence of adverse events.
Acknowledgements
Not applicable.
Funding
Funding was provided by the Guangdong General Colleges and
Universities Characteristic and Innovative Projects (grant no.
2021KTSCX037).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YQZ contributed to conception and design of the case
report. JWL and PQ contributed to data collection and analysis. SCH
and ZMY advised on patient treatment and performed the surgery. ZZL
and MY obtained medical images (e.g. MRI, CT and ultrasound), YYT,
CYC and ZZL contributed to data analysis and interpretation. YYT,
SCH, PQ, ZMY, CYC, MY, ZZL, JWL and YQZ contributed to manuscript
writing and final approval of the manuscript. All authors have read
and approved the final version of the manuscript. SCH and YQZ
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Affiliated Hospital of Guangdong Medical University (Zhanjiang,
China; (approval no. PJKT2024-116).
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the present case report and any
accompanying identifiable images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Hao C, Wang K, Zhang J, Chen J, Liu
Y, Nie J, Yan M, Liu Q, Geng C, et al: Chinese society of clinical
oncology (CSCO) breast cancer guidelines 2024. Transl Breast Cancer
Res. 5:182024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramseier JY, Ferreira MN and Leventhal JS:
Dermatologic toxicities associated with radiation therapy in women
with breast cancer. Int J Womens Dermatol. 6:349–356. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu C, Wei J, Wang X, Zhao Q, Lv J, Tan Z,
Xin Y and Jiang X: Radiation-induced skin reactions: Oxidative
damage mechanism and antioxidant protection. Front Cell Dev Biol.
12:14805712024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cuperus E, Leguit R, Albregts M and
Toonstra J: Post radiation skin tumors: Basal cell carcinomas,
squamous cell carcinomas and angiosarcomas. A review of this late
effect of radiotherapy. Eur J Dermatol. 23:749–757. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borrelli MR, Shen AH, Lee GK, Momeni A,
Longaker MT and Wan DC: Radiation-induced skin fibrosis:
Pathogenesis, current treatment options, and emerging therapeutics.
Ann Plast Surg. 83 (4S Suppl 1):S59–S64. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giuliano AE, Edge SB and Hortobagyi GN:
Eighth edition of the AJCC cancer staging manual: Breast cancer.
Ann Surg Oncol. 25:1783–1785. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the radiation therapy oncology group (RTOG) and the
European organization for research and treatment of cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spak DA, Plaxco JS, Santiago L, Dryden MJ
and Dogan BE: BI-RADS® fifth edition: A summary of
changes. Diagn Interv Imaging. 98:179–190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Work Group and Invited Reviewers, . Kim
JYS, Kozlow JH, Mittal B, Moyer J, Olenecki T and Rodgers P:
Guidelines of care for the management of cutaneous squamous cell
carcinoma. J Am Acad Dermatol. 78:560–578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiriac AE, Betiu M, Brzezinski P, Di
Martino Ortiz B, Chiriac A, Foia L and Azoicai D: Malignant
degeneration of scars. Cancer Manag Res. 12:10297–10302. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Da Costa JC III: Carcinomatous changes in
an area of chronic ulceration, or Marjolin's ulcer. Ann Surg.
37:496–502. 1903.PubMed/NCBI
|
|
13
|
Saaiq M and Ashraf B: Marjolin's ulcers in
the post-burned lesions and scars. World J Clin Cases. 2:507–514.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Landthaler M, Hagspiel HJ and Braun-Falco
O: Late irradiation damage to the skin caused by soft X-ray
radiation therapy of cutaneous tumors. Arch Dermatol. 131:182–186.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perkins JL, Liu Y, Mitby PA, Neglia JP,
Hammond S, Stovall M, Meadows AT, Hutchinson R, Dreyer ZE, Robison
LL and Mertens AC: Nonmelanoma skin cancer in survivors of
childhood and adolescent cancer: A report from the childhood cancer
survivor study. J Clin Oncol. 23:3733–3741. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meibodi NT, Maleki M, Javidi Z and Nahidi
Y: Clinicopathological evaluation of radiation induced basal cell
carcinoma. Indian J Dermatol. 53:137–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goggins W, Gao W and Tsao H: Association
between female breast cancer and cutaneous melanoma. Int J Cancer.
111:792–794. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rezaei SJ, Eid E, Tang JY, Kurian AW,
Kwong BY and Linos E: Incidence of nonkeratinocyte skin cancer
after breast cancer radiation therapy. JAMA Netw Open.
7:e2416322024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Loveland-Jones CE, Wang F, Bankhead RR,
Huang Y and Reilly KJ: Squamous cell carcinoma of the nipple
following radiation therapy for ductal carcinoma in situ: A case
report. J Med Case Rep. 4:1862010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yokota T, Roppongi T, Kanno K, Tsutsumi H,
Sakamoto I and Fujii T: Radiation-induced squamous cell carcinoma
of the chest wall seven years after adjuvant radiotherapy following
the surgery of breast cancer: A case report. Kyobu Geka.
53:1133–1136. 2000.(In Japanese). PubMed/NCBI
|
|
21
|
Kodama K, Doi O, Higashiyama M, Yokouchi
H, Noguchi S and Koyama H: Chemo-thermotherapy of radiation-induced
squamous cell carcinoma in anterior chest wall. Kyobu Geka.
45:917–920. 1992.(In Japanese). PubMed/NCBI
|
|
22
|
Voiculescu V, Calenic B, Ghita M, Lupu M,
Caruntu A, Moraru L, Voiculescu S, Ion A, Greabu M, Ishkitiev N and
Caruntu C: From normal skin to squamous cell carcinoma: A quest for
novel biomarkers. Dis Markers. 2016:45174922016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Vloten WA, Hermans J and van Daal WA:
Radiation-induced skin cancer and radiodermatitis of the head and
neck. Cancer. 59:411–414. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cahan WG, Woodard HQ, Higinbotham NL,
Stewart FW and Coley BL: Sarcoma arising in irradiated bone: Report
of eleven cases. 1948. Cancer. 82:8–34. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skulsky SL, O'Sullivan B, McArdle O,
Leader M, Roche M, Conlon PJ and O'Neill JP: Review of high-risk
features of cutaneous squamous cell carcinoma and discrepancies
between the American joint committee on cancer and NCCN clinical
practice guidelines in oncology. Head Neck. 39:578–594. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leibovitch I, Huilgol SC, Selva D, Hill D,
Richards S and Paver R: Cutaneous squamous cell carcinoma treated
with Mohs micrographic surgery in Australia I. Experience over 10
years. J Am Acad Dermatol. 53:253–260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lansbury L, Bath-Hextall F, Perkins W,
Stanton W and Leonardi-Bee J: Interventions for non-metastatic
squamous cell carcinoma of the skin: Systematic review and pooled
analysis of observational studies. BMJ. 347:f61532013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang X, Ren H, Guo X, Hu C and Fu J:
Radiation-induced skin injury: Pathogenesis, treatment, and
management. Aging (Albany NY). 12:23379–23393. 2020.PubMed/NCBI
|
|
29
|
Iqbal FM, Sinha Y and Jaffe W: Marjolin's
ulcer: A rare entity with a call for early diagnosis. BMJ Case Rep.
2015:bcr20142081762015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujioka M: Surgical Reconstruction of
radiation injuries. Adv Wound Care (New Rochelle). 3:25–37. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li SQ, Zheng ZF, Li H, Zhang JF, Zheng Y
and Lin LS: Clinical study on the thoracodorsal artery perforator
flap in breast-conserving reconstruction of T2 breast cancer. Surg
Innov. 31:16–25. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sui X, Qing L, Yu F, Wu P and Tang J: The
versatile thoracodorsal artery perforator flap for extremity
reconstruction: From simple to five types of advanced applications
and clinical outcomes. J Orthop Surg Res. 18:9732023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang LC, Wang XC, Bentz ML, Fang BR, Xiong
X, Li XF, Lu Q, Wu Q, Wang RF, Feng W, et al: Clinical application
of the thoracodorsal artery perforator flaps. J Plast Reconstr
Aesthet Surg. 66:193–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Homsy C, Theunissen T and Sadeghi A: The
thoracodorsal artery perforator flap: A powerful tool in breast
reconstruction. Plast Reconstr Surg. 150:755–761. 2022.PubMed/NCBI
|
|
35
|
Chan TY, Tan PW, Tan CW and Tang JI:
Assessing radiation exposure of the left anterior descending
artery, heart and lung in patients with left breast cancer: A
dosimetric comparison between multicatheter accelerated partial
breast irradiation and whole breast external beam radiotherapy.
Radiother Oncol. 117:459–466. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Q, Liu J, Ao N, Yu H, Peng Y, Ou L
and Zhang S: Secondary cancer risk after radiation therapy for
breast cancer with different radiotherapy techniques. Sci Rep.
10:12202020. View Article : Google Scholar : PubMed/NCBI
|