Introduction
Prostate cancer is the second most prevalent cancer
among males (1) and accounts for a
notable proportion of cancer-related fatalities, ranking fifth
worldwide (2) in this regard.
Approximately 8% of patients present with de novo metastatic
disease (3), while one-third of
localized prostate cancer cases progress to metastatic disease
during the clinical course (4). The
definition of disease volume has continued to evolve among patients
with metastatic disease, since disease volume plays a crucial role
in determining prognosis. Prior to the findings of the CHAARTED
(5) and STAMPEDE (6) trials in 2015 and 2016 respectively,
conventional treatment for metastatic hormone-sensitive prostate
cancer relied primarily on androgen deprivation therapy (ADT) with
median progression-free survival (PFS) time typically ranging from
24 to 36 months (7), resulting in
an early transition to metastatic castrate-resistant prostate
cancer.
Historically, the role of chemotherapy was confined
to cases of castrate-resistant metastatic prostate cancer (8,9). Yet,
the emergence of key data (5,6)
indicated that the integration of upfront chemotherapy demonstrates
prolonged PFS and overall survival (OS) compared with ADT alone
(5,6). The CHAARTED and STAMPEDE trials
demonstrated that the combination of upfront chemotherapy and ADT
leads to improvements in OS time of 1.1 and 1.8 years,
respectively, for hormone-sensitive metastatic prostate cancer
(3,4). These results led to the rapid
incorporation of combination therapy with ADT and docetaxel into
international guidelines, including those from the National
Comprehensive Cancer Network (10)
and European Society for Medical Oncology (11) in 2015, establishing the new standard
of care for metastatic hormone-sensitive prostate cancer.
Consequently, Shaukat Khanum Memorial Cancer Hospital and Research
Centre (SKMCH and RC), based in Lahore and Peshawar, Pakistan,
aligned its practices with this paradigm shift, and adopted the use
of upfront chemotherapy with docetaxel alongside ADT in the
treatment of patients presenting with hormone-sensitive metastatic
prostate cancer.
Subsequently, the treatment landscape underwent
further transformation with the advent of the role of novel
antiandrogens in hormone-sensitive metastatic prostate cancer,
which improved survival rates and enhanced the quality of life for
patients with metastatic prostate cancer (5,6,12).
Data from STAMPEDE trial suggested superior efficacy outcomes and a
more favourable toxicity profile with upfront novel antiandrogens,
particularly for those with low-volume disease (13,14).
However, despite these advancements, cost constraints have hindered
the widespread adoption of antiandrogen therapy, especially in
resource-limited settings such as in the present study (15). Currently, limited data exists on the
efficacy of combination therapy for metastatic castration-sensitive
prostate cancer in Pakistan. Notable trials (5,6,12)
establishing chemohormonal therapy as the standard of care
predominantly involved Caucasian populations, with minimal
representation from South Asian groups. There is a lack of trial
and real-world data specific to the present patient population,
which may have poorer performance status and factors not typically
accounted for in clinical trials, such as elderly patients, and
patients from ethnic minorities and lower socioeconomic groups. In
addition, standard guidelines (16,17)
for treating low-volume disease with antiandrogens are also based
on data from settings where access to these medications is not as
limited. Therefore, it was crucial to evaluate the impact of
chemohormonal therapy in low-volume disease, while considering the
broader effects of financial limitations on tailoring therapy in
resource-limited settings. Therefore, the present retrospective
study was conducted with a focus on patients with hormone-sensitive
metastatic prostate cancer, irrespective of disease volume, who
underwent upfront chemohormonal therapy within the Pakistani
patient population, offering insights into its efficacy and
relevance in the present setting. The objective was to assess the
efficacy and tolerability of this approach and explore the impact
of disease volume on these outcomes. Furthermore, analysis was
conducted to assess the impact of subsequent lines of treatment on
overall disease outcomes. The aim of present study was to
investigate the efficacy and toxicity of the upfront docetaxel in
patients with hormone-sensitive metastatic prostate cancer and
assess the impact in patients with low- and high-volume
disease.
Materials and methods
Study design and population
The present analysis was a retrospective,
longitudinal, observational and single-centre study conducted on
patients with hormone-sensitive metastatic prostate cancer who were
treated with upfront chemotherapy. The patients registered at SKMCH
and RC in Lahore and Peshawar between January 2016 and December
2019 were included in the present study. The inclusion criteria
were as follows: Patients with newly diagnosed de novo
hormone-sensitive metastatic prostate cancer with a confirmed
diagnosis of prostate cancer on histopathology, who were treated
with upfront chemotherapy with docetaxel within 6 months of
diagnosis were included in the present study. The exclusion
criteria were as follows: i) Patients with prostate cancer without
a histopathological diagnosis; ii) patients with localized disease
only; iii) patients with castrate resistant metastatic prostate
cancer; iv) patients with hormone-sensitive metastatic prostate
cancer who were not started on chemotherapy within 6 months of
diagnosis; and v) patients treated with upfront antiandrogens (for
example abiraterone and enzalutamide) were excluded from the
present study.
Procedure
After selecting the patients appropriate to the
aforementioned criteria, data was retrieved from the hospital
information system (HIS) database of SKMCH and RC. Patients had
been treated with docetaxel at a dose of 75 mg/m2 every
3 weeks and doses were reduced in the event of toxicity to 60
mg/m2. For patients that developed febrile neutropenia,
granulocyte colony-stimulating factor was used as a secondary
prophylaxis. Patients with osseous metastasis were treated with
zoledronic acid to prevent fractures. HIS records were reviewed to
collect data for baseline characteristics including age, Gleason
score, mode of ADT (surgical or pharmacological) and disease burden
(low-volume vs. high-volume). The records were reviewed for the
number of cycles of chemotherapy, and the type and grade of
toxicity. Toxicity grading was performed according to the Common
Toxicity Criteria for Adverse Events (version 5) guidelines
(18). The data-cut off for the
present study was 20 February 2024.
Study definitions
Low-volume disease was defined as: Metastatic
hormone-sensitive prostate cancer with ≤5 sites of metastasis in
bones or lymph nodes with no visceral metastasis. High-volume
disease (19) was defined as:
Metastatic hormone-sensitive prostate cancer with >5 sites of
metastasis in bones or lymph nodes with or without visceral
metastasis. OS was defined as the duration between diagnosis and
death. PFS was defined as the duration between the start of
chemotherapy to biochemical or radiological progression.
The specific primary objectives investigated were as
follows: OS and PFS. The secondary objectives were as follows:
Toxicity, impact of disease burden on the OS and PFS, and impact of
further lines of treatment on PFS and OS.
Statistical analysis
IBM SPSS Statistics 29.0 was used to conduct the
analysis. Counts and percentages were computed for the categorical
variables and descriptive statistics were used for age. The
survival analysis was conducted using the Kaplan-Meier method and
the log-rank test was applied to evaluate the survival
distributions. OS and PFS were estimated and each were subsequently
stratified by the disease burden (low-volume vs. high-volume).
Death was the outcome used for OS and for PFS, both disease
progression and death were used as endpoints. The log-rank test in
survival analysis reported a χ2 value and a P-value was
computed. The analysis of variance (ANOVA) test was used to compare
the difference in the mean survival time between the three groups
following the second-line of treatment (no treatment, antiandrogens
and rechallenge therapy). The interval computed for the patients on
second-line therapy began at progression and finished at death or
last encounter. An a-level of 0.05 or P≤0.05 was considered to
indicate a statistically significant difference.
Results
The present study included 167 male patients with
hormone-sensitive metastatic prostate cancer. The baseline
characteristics of included patients are shown in Table I. The median age was 66 years with
an age range of 45–80 years. Among the patients, 63 (37.7%) were
aged 45 to 64 years, while 104 (62.3%) were in the 65 to 80-year
age group. In the present study, 2 patients (1.2%) had a Gleason
score of 6, 30 patients (18.0%) scored 7, 28 patients (16.8%)
scored 8, 86 patients (51.1%) scored 9 and 9 patients (5.4%) scored
10. Overall, 123 patients (73.7%) had a Gleason score between 8 and
10. The Gleason score was unknown for 12 patients (7.2%).
Prostate-specific antigen was low (≤10) in 16 (9.6%), while it was
high (>10) in 151 (90.4%) patients. All patients underwent
castration, predominantly through surgical means (n=136; 81.4%). In
remaining patients pharmacological castration with leuprolide was
performed (castration with bicalutamide/flutamide only was not
done). Additionally, 66 patients (39.5%) had low-volume disease,
while 101 patients (60.5%) presented with high-volume disease
(Table I).
 | Table I.Baseline characteristics. |
Table I.
Baseline characteristics.
| Clinicopathological
variables | No. of patients
(%) |
|---|
| Age, years |
|
|
Median | 66 |
|
Range | 45-80 |
| Gleason score |
|
| 6 | 2 (1.2) |
| 7 | 30 (18.0) |
| 8 | 28 (16.8) |
| 9 | 86 (51.5) |
| 10 | 9 (5.4) |
|
Unknown | 12 (7.2) |
| Prostate-specific
antigen |
|
| Normal
(≤10) | 16 (9.6) |
| High
(>10) | 151 (90.4) |
| Castration |
|
|
Pharmacological | 31 (18.6) |
|
Surgical | 136 (81.4) |
| Disease volume |
|
|
Low-volume | 66 (39.5) |
|
High-volume | 101 (60.5) |
There were 100 (59.9%) patients who had disease
progression (Table II). The median
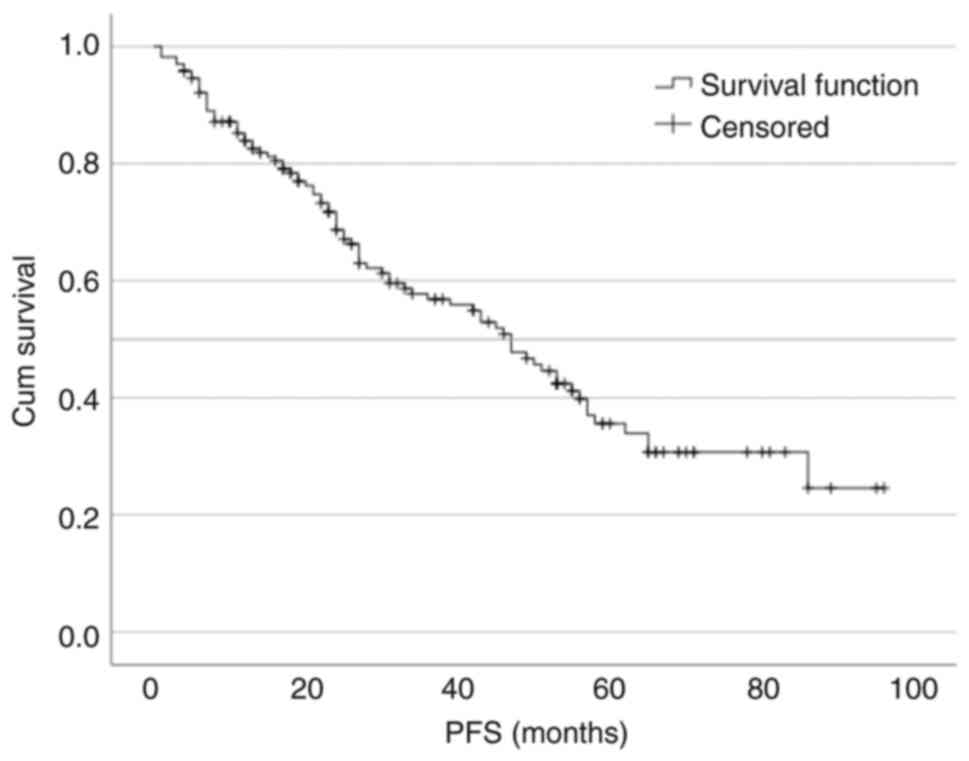
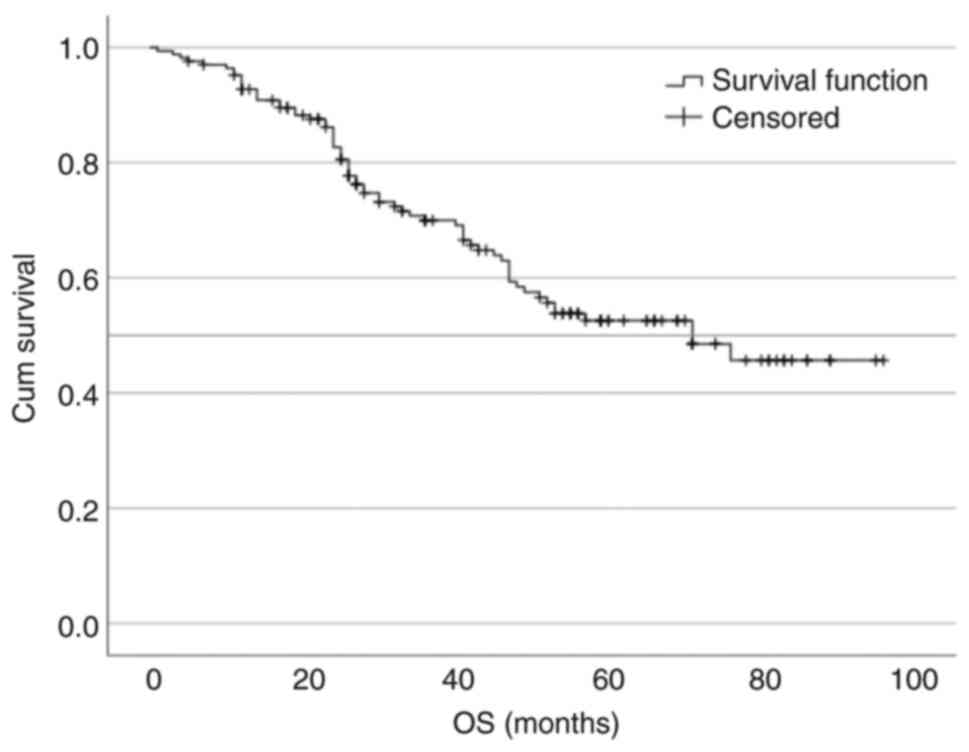
PFS was 47 months (95% CI, 37.503–56.497; Fig. 1). There were 101 (60.5%) patients
still alive at the time of data cut off (Table II), with a median OS of 71 months
(Fig. 2). Out of 66 deaths, 62
(93.9%) were related to cancer, predominantly disease progression,
while 4 (6%) were related to comorbidities. The cause of death in
these 4 patients was either a cardiovascular event or ischemic
heart disease.
 | Table II.Clinical outcomes. |
Table II.
Clinical outcomes.
| Disease
progression | No. of patients
(%) |
|---|
| No | 66 (39.5) |
|
Yes | 101 (60.5) |
| Patient status |
|
|
Alive | 101 (60.5) |
|
Dead | 66 (39.5) |
| Cause of death |
|
|
Cancer-related | 62 (93.9) |
|
Comorbidities-related | 4 (6.0) |
There was no statistically significant difference in
the OS or PFS distributions by age group (45–64 vs. 65–80 years;
P>0.05; data not shown). Gleason score (≤6, low-risk; 7,
intermediate-risk; and ≥8, high risk) had no impact on the PFS;
however, was notably associated with OS. The median OS for patients
with high-risk Gleason score was 71 months vs. not reached for
intermediate-risk group (P=0.046; data not shown). In the low-risk
group there were two cases and both were censored; therefore, no
statistics were computed.
The median PFS for low-volume vs. high-volume
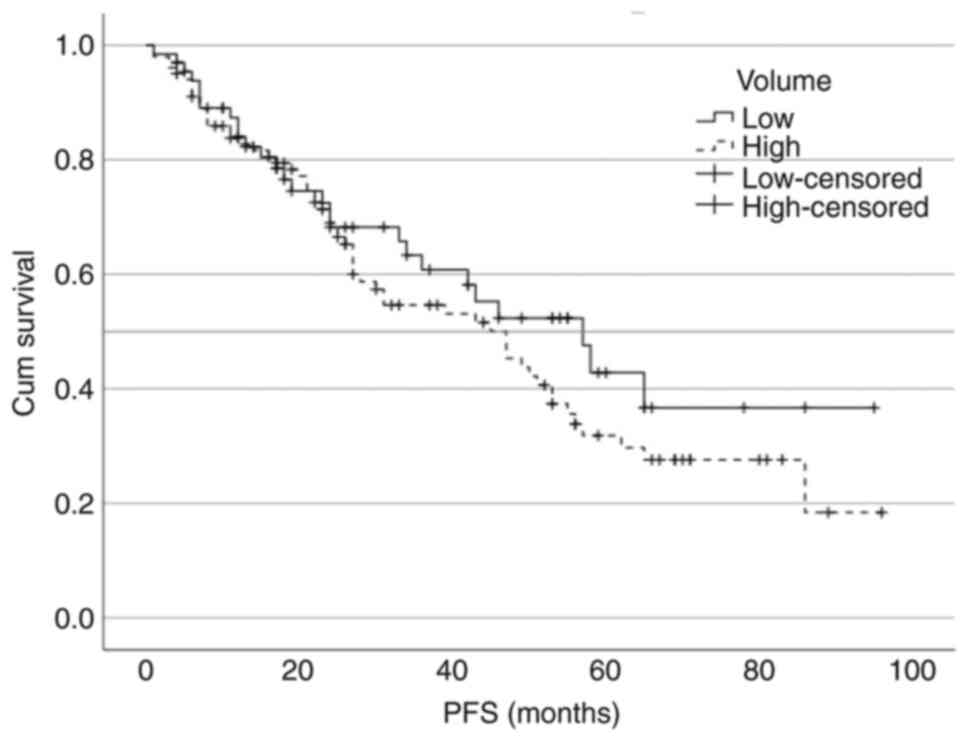
disease was 57 vs. 47 months, respectively (P=0.276; Fig. 3). The median OS for low- and
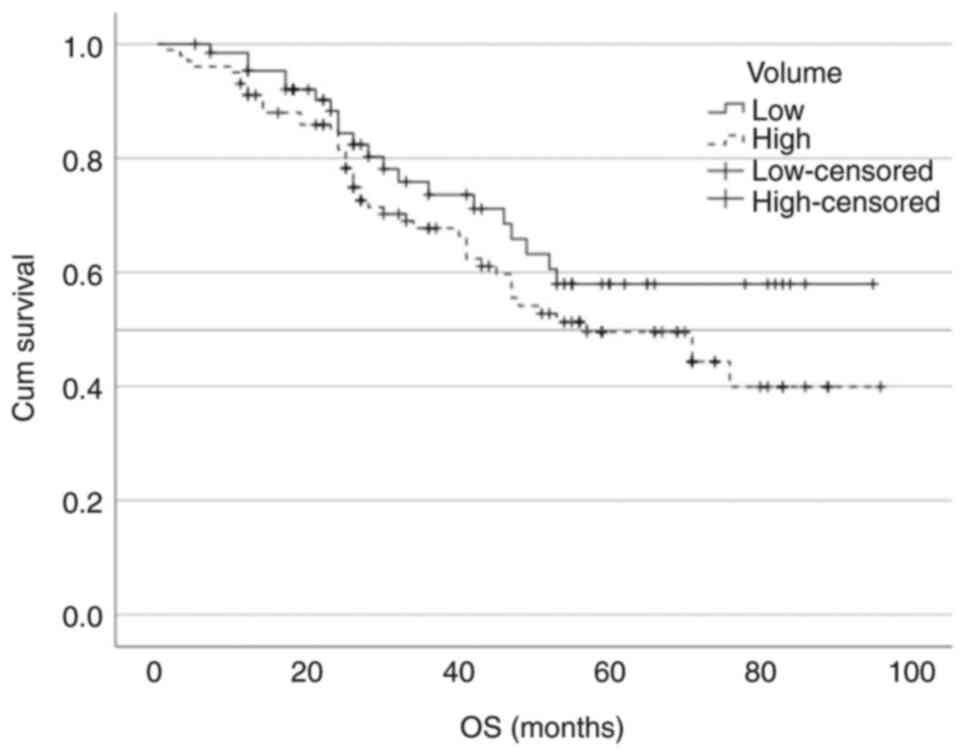
high-volume disease was not reached vs. 57 months, respectively
(P=0.192; Fig. 4). The comparison
of the survival curves by disease volume using the log-rank test
was not statistically significant either for the PFS
[χ2=1.18; degrees of freedom (df)=1; P=0.276] or the OS
(χ2=1.70; df=1; P=0.192).
There were 100 patients who had disease progression.
However, only 20 patients received second-line therapy (Table III). The impact of second-line
therapy was not statistically significant (P=0.496). The mean
survival time following the second line of treatment for the
antiandrogens group (n=7) was 15.14±8.84 months and that of the
rechallenge therapy group (n=13) was 12.46±9.51 months (t=0.616;
df=20; two-sided P=0.546), with a mean difference of 2.68 months
(95% CI, −6.47–11.83) between the groups. The difference in median
OS in the second-line therapy groups was 9.88 months for patients
without treatment, 15.14 months for those treated with
antiandrogens and 12.46 months for those with rechallenge
chemotherapy.
 | Table III.Impact of second-line therapy on
OS. |
Table III.
Impact of second-line therapy on
OS.
| Second-line therapy
in patients with progression | OS, months | P-value | No. of patients
(%) |
|---|
| Total | 100 (100) |
| 0.496 |
| None | 80 (80.0) | 9.88 |
|
| Antiandrogens
(abiraterone or enzalutamide) | 7 (7.0) | 15.14 |
|
| Chemotherapy
rechallenge with docetaxel | 13 (13.0) | 12.46 |
|
In the present study, 131 patients (78.4%) completed
6 cycles of chemotherapy. There were 8 patients (7.8%) who
completed 8 cycles of chemotherapy. Grade 3–4 treatment-related
toxicities were observed in ~37.8% of patients. Moreover, one death
was associated with neutropenic sepsis and colitis; a 69-year-old
patient who was diagnosed with metastatic and locally advanced
prostate cancer, and presented with low BMI (BMI, 19), bowel
obstruction and right obstructive uropathy. The patient was managed
with a right percutaneous nephrostomy and loop colostomy.
Postoperatively, the patient experienced high stoma output. After
receiving the first cycle of docetaxel with a 20% dose reduction,
the patient developed neutropenic sepsis and colitis. The patient
succumbed to sepsis despite all supportive measures. The most
common toxicities were mucositis with 53 (31.7%) patients, febrile
neutropenia with 44 (26.3%) patients and sepsis with 29 (17.4%)
patients. Overall, 116 (69.4%) patients required dose reduction
mainly due to neutropenia, sepsis, mucositis and performance status
(Table IV).
 | Table IV.Tolerance and toxicities. |
Table IV.
Tolerance and toxicities.
| Parameter | No. of patients
(%) |
|---|
| No. of cycles of
docetaxel |
|
| 1 | 1 (0.6) |
| 2 | 0 (0.0) |
| 3 | 5 (3.0) |
| 4 | 6 (3.6) |
| 5 | 5 (3.0) |
| 6 | 131 (78.4) |
| 7 | 1 (0.6) |
| 8 | 13 (7.8) |
| 9 | 2 (1.2) |
| 10 | 2 (1.2) |
| 11 | 0 (0.0) |
| 12 | 1 (0.6) |
| Toxicity,
grade |
|
| 1 | 16 (9.6) |
| 2 | 85 (50.9) |
| 3 | 35 (21.0) |
| 4 | 28 (16.8) |
| 5 | 1 (0.6) |
| Type of
toxicity |
|
|
Diarrhoea and vomiting | 2 (1.2) |
|
Peripheral neuropathy | 8 (4.8) |
|
Mucositis | 53 (31.7) |
| Febrile
neutropenia | 44 (26.3) |
|
COVID-19 | 3 (1.8) |
|
Mixed | 28 (16.8) |
|
Sepsis | 29 (17.4) |
| Dose reduction | 116 (69.4) |
Discussion
The findings of the present study provided insight
on the characteristics of metastatic prostate cancer within the
Pakistani population and treatment efficacy and outcomes,
particularly in the context of limited economic resources and
access to advanced therapies. The health economics of cancer
treatments cannot be underestimated. Within the constraints of
limited resources, in a low-income country with the majority of
patients having a poor socioeconomic status, free access to
docetaxel due to its low cost makes this treatment relatively
affordable.
It was demonstrated that the median age of
presentation for upfront metastatic prostate cancer in the present
study population aligned closely with international (5,6,12) and
national (20–22) data, underscoring the consistency of
disease characteristics across different demographics and
corroborates findings from aforementioned studies, emphasizing the
universality of prostate cancer demographics.
In terms of treatment modalities for metastatic
prostate cancer, while pharmacological castration is predominantly
used in developed countries (6),
the present study highlighted the prevalence of surgical castration
in the present population due to socioeconomic factors and
challenges with treatment compliance. This underscores the
significance of tailored approaches to therapy based on resource
availability and patient demographics. Moreover, the present study
demonstrated a high proportion of patients with high Gleason
scores, mirroring findings from published data (6,23).
This underscores the aggressive nature of the disease in the
present population, necessitating robust treatment strategies.
Regarding chemotherapy protocols, the approach of
the present study, which offered 6–10 cycles of docetaxel, aligns
with how the guidelines (16,17) on
the management of prostate cancer have evolved. In the initial
trials >6 cycles of chemotherapy were given (12), Therefore, initially, the present
centre adopted the approach of 6–10 cycles; however, as further
data emerged (5,6) the approach was switched to 6 cycles of
chemotherapy.
Recent advancements (16,24)
have delineated distinct standards of care based on disease volume,
with novel antiandrogens established as the preferred option for
low-volume disease, whereas chemotherapy remains a key treatment
strategy for high-volume disease. The present retrospective
analysis provided insights on the treatment patterns and outcomes
observed in a cohort of patients with prostate cancer, some of whom
were managed prior to the crystallization of explicit guidelines
(25,26) regarding treatment stratification by
disease volume (27). Notably, the
present patient cohort received upfront chemohormonal therapy,
irrespective of disease volume, reflecting the prevailing
guidelines and economic considerations at the time. This approach,
though divergent from contemporary standards (28,29),
yielded a median OS of 71 months, comparable to outcomes reported
in landmark trials such as STAMPEDE (6), where docetaxel and ADT conferred a
median OS of 81 months. By contrast, the median OS observed in the
GETUG-AFU-15 trial (12), where
treatment modalities may not have mirrored current standards, was
notably lower at 58.9 months. Despite limitations such as the lack
of access to subsequent lines of therapy upon disease progression,
the present findings underscore the reasonable survival outcomes
achieved with the adopted treatment strategy. The present study
suggested that the efficacy of this approach could transcend the
availability of advanced therapies, as evidenced by outcomes
superior to those reported in previous studies such as the
GETUG-AFU-15 trial, which was conducted during a period when novel
antiandrogens were less accessible. Notably, despite constraints on
access to novel agents, these data reflect improved PFS and OS
rates, suggesting the efficacy of the upfront chemotherapy approach
within resource limitations. The present data provided evidence
that could support the use of this treatment within the constraints
within low-medium income countries where access to certain cancer
treatments is dependent on affordability.
The finding that there were no significant
differences in PFS or OS between low- and high-volume disease
demonstrates the efficacy of the upfront chemotherapy approach
across disease burdens; the cost-effectiveness of the present
approach potentially makes it a valuable and equitable option,
particularly in resource-limited settings.
Limited use of second-line therapy post-progression
was observed, primarily due to cost constraints and patient
frailty, which highlighted the challenges in accessing advanced
treatments. Despite this, patients showed comparable survival
outcomes to international data (6,12),
indicating the clinical impact of the present approach. However,
the small sample size of the present study limits the ability to
control for confounding factors such as age, comorbidities,
performance status, disease burden or severity which could
independently affect outcomes. Further multicentre studies are
warranted to enhance statistical power and provide a more
representative patient population.
In terms of treatment tolerability, the present
study demonstrated comparable completion rates of chemotherapy
cycles to international benchmarks (5,6,12),
albeit with higher incidences of toxicities, particularly
neutropenic sepsis. However, despite these challenges, overall
treatment-related mortality was low, highlighting the importance of
diligent monitoring and supportive care in resource-limited
settings. Additionally, 19 patients received >6 cycles of
chemotherapy. When docetaxel was first introduced for metastatic
hormone-sensitive prostate cancer, guidelines regarding the optimal
number of cycles were not well defined (12). As a result, a number of patients in
the present study who initiated treatment before precise guidelines
were established continued therapy >6 cycles at the discretion
of their oncologists if good tolerance was demonstrated.
Incorporating existing treatment guidelines into
low-income countries presents challenges, particularly when
tailoring therapies to financial constraints. The present findings
offer supporting evidence to physicians in such settings regarding
the efficacy of this approach. While abiraterone is preferred for
low-volume disease due to its quality-of-life benefits (16,24),
the present study highlighted the feasibility of docetaxel with
acceptable toxicity, thereby reducing financial burden.
Moreover, the present study addresses a key research
gap by including South Asian patients, who are often
underrepresented in notable clinical trials. This inclusion
provides key insights into disease behaviour within this
population, supporting the need for more diverse representation in
future studies. Limitations of present study are that it was a
retrospective, single institution study with only a small
proportion of patients who went on to receive second line therapy
and head-to-head comparison with novel antiandrogens was not
possible.
In conclusion, the present study underscored the
importance of tailored management strategies in the context of
limited resources and access to advanced therapies. While
challenges exist, it was demonstrated that effective treatment
outcomes may be achieved through utilization of available resources
and adaptation of international guidelines to local contexts.
Further research is warranted to optimize treatment protocols and
improve outcomes for patients with prostate cancer in similar
resource-constrained settings. Future work includes the continued
collection of prospective data, as reporting outcomes such as
toxicity and efficacy in the real-world setting remains key in the
changing landscape of cancer management. To combat the cost
constraints at the policy-making level, the use of biosimilars and
access programs for novel therapies is warranted.
Acknowledgements
The authors would like to thank Mr. M. Ikram Jamil
(Department of Management Information Systems, Shaukat Khanum
Memorial Cancer Hospital and Research Centre, Peshawar, Pakistan)
for help with the formatting of the figures.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MQ, YI and SAH contributed to the conception and
study design. Data collection was conducted by SR and AA.
Statistical analysis was performed by FB. YI and MQ completed the
manuscript writing, with manuscript review provided by SAH. MQ and
SAH confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Based on its retrospective nature, with information
in existence being recorded without subject identification and
without contact with the study subjects, the present study was
granted waiver by the Institutional Review Board of Shaukat Khanum
Memorial Trust (Lahore, Pakistan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript, and
subsequently, the authors revised and edited the content produced
by the AI tools as necessary, taking full responsibility for the
ultimate content of the present manuscript.
References
|
1
|
World Health Organization, . Global cancer
burden growing, amidst mounting need for services. https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-servicesJune
8–2024
|
|
2
|
Wang L, Lu B, He M, Wang Y, Wang Z and Du
L: Prostate cancer incidence and mortality: Global status and
temporal trends in 89 countries from 2000 to 2019. Front Public
Health. 10:8110442022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
CDC. United States Cancer Statistics, .
Prostate Cancer Incidence by Stage at Diagnosis. https://www.cdc.gov/united-states-cancer-statistics/publications/prostate-cancer.htmlMarch
22–2025
|
|
4
|
Shore ND, Moul JW, Pienta KJ, Czernin J,
King MT and Freedland SJ: Biochemical recurrence in patients with
prostate cancer after primary definitive therapy: Treatment based
on risk stratification. Prostate Cancer Prostatic Dis. 27:192–201.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sweeney CJ, Chen YH, Carducci M, Liu G,
Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, et
al: Chemohormonal therapy in metastatic hormone-sensitive prostate
cancer. N Engl J Med. 373:737–746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
James ND, Sydes MR, Clarke NW, Mason MD,
Dearnaley DP, Spears MR, Ritchie AW, Parker CC, Russell JM, Attard
G, et al: Addition of docetaxel, zoledronic acid, or both to
first-line long-term hormone therapy in prostate cancer (STAMPEDE):
Survival results from an adaptive, multiarm, multistage, platform
randomised controlled trial. Lancet. 387:1163–1177. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
James ND, Spears MR, Clarke NW, Dearnaley
DP, De Bono JS, Gale J, Hetherington J, Hoskin PJ, Jones RJ, Laing
R, et al: Survival with newly diagnosed metastatic prostate cancer
in the ‘docetaxel era’: Data from 917 patients in the control arm
of the STAMPEDE trial (MRC PR08, CRUK/06/019). Eur Urol.
67:1028–1038. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et
al: Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petrylak DP, Tangen CM, Hussain MHA, Lara
PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M,
et al: Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mohler JL, Armstrong AJ, Bahnson RR,
D'Amico AV, Davis BJ, Eastham JA, Enke CA, Farrington TA, Higano
CS, Horwitz EM, et al: Prostate cancer, version 1.2016. J Natl
Compr Canc Netw. 14:19–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parker C, Gillessen S, Heidenreich A and
Horwich A; ESMO Guidelines Committee, : Cancer of the prostate:
ESMO clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 26 (Suppl 5):v69–v77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gravis G, Fizazi K, Joly F, Oudard S,
Priou F, Esterni B, Latorzeff I, Delva R, Krakowski I, Laguerre B,
et al: Androgen-deprivation therapy alone or with docetaxel in
non-castrate metastatic prostate cancer (GETUG-AFU 15): A
randomised, open-label, phase 3 trial. Lancet Oncol. 14:149–158.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
James ND, Clarke NW, Cook A, Ali A, Hoyle
AP, Attard G, Brawley CD, Chowdhury S, Cross WR, Dearnaley DP, et
al: Abiraterone acetate plus prednisolone for metastatic patients
starting hormone therapy: 5-Year follow-up results from the
STAMPEDE randomised trial (NCT00268476). Int J Cancer. 151:422–434.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hoyle AP, Ali A, James ND, Cook A, Parker
CC, de Bono JS, Attard G, Chowdhury S, Cross WR, Dearnaley DP, et
al: Abiraterone in ‘high-’ and ‘low-risk’ metastatic
hormone-sensitive prostate cancer. Eur Urol. 76:719–728. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Menges D, Yebyo HG, Sivec-Muniz S, Haile
SR, Barbier MC, Tomonaga Y, Schwenkglenks M and Puhan MA:
Treatments for metastatic hormone-sensitive prostate cancer:
Systematic review, network meta-analysis, and benefit-harm
assessment. Eur Urol Oncol. 5:605–616. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology (NCCN
Guidelines®) Prostate Cancer, V1. 2025.https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdfMarch
23–2025
|
|
17
|
Parker C, Castro E, Fizazi K, Heidenreich
A, Ost P, Procopio G, Tombal B and Gillessen S; ESMO Guidelines
Committee, : Prostate cancer: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 31:1119–1134. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
U.S. Department of Health and Human
Services, . Common Terminology Criteria for Adverse Events (CTCAE)
version 5.0. March 23–2025
|
|
19
|
Ferriero M, Prata F, Anceschi U, Astore S,
Bove AM, Brassetti A, Calabrò F, Chiellino S, De Nunzio C, Facchini
G, et al: Oncological outcomes of patients with high-volume mCRPC:
Results from a longitudinal real-life multicenter cohort. Cancers
(Basel). 15:48092023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Badar F and Mahmood S: Cancer in Lahore,
Pakistan, 2010–2019: An incidence study. BMJ Open. 11:e0470492021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akhtar S, Hassan F, Ahmad S, El-Affendi MA
and Khan MI: The prevalence of prostate cancer in Pakistan: A
systematic review and meta-analysis. Heliyon. 9:e203502023.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu Y, Mo M, Wei Y, Wu J, Pan J, Freedland
SJ, Zheng Y and Ye D: Epidemiology and genomics of prostate cancer
in Asian men. Nat Rev Urol. 18:282–301. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shabbir A, Khan UT, Nisar D, Rath PK,
Kumari C, Zehra T and Hasan SM: Frequency and trends of prostatic
diseases in a subset of Karachi population: A retrospective study.
Pak J Med Dent. 10:17–23. 2021.
|
|
24
|
Rush HL, Murphy L, Morgans AK, Clarke NW,
Cook AD, Attard G, Macnair A, Dearnaley DP, Parker CC, Russell JM,
et al: Quality of life in men with prostate cancer randomly
allocated to receive docetaxel or abiraterone in the STAMPEDE
trial. J Clin Oncol. 40:825–836. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chi KN, Agarwal N, Bjartell A, Chung BH,
Pereira de Santana Gomes AJ, Given R, Juárez Soto Á, Merseburger
AS, Özgüroğlu M, Uemura H, et al: Apalutamide for metastatic,
castration-sensitive prostate cancer. N Engl J Med. 381:13–24.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Davis ID, Martin AJ, Stockler MR, Begbie
S, Chi KN, Chowdhury S, Coskinas X, Frydenberg M, Hague WE, Horvath
LG, et al: Enzalutamide with standard first-line therapy in
metastatic prostate cancer. N Engl J Med. 381:121–131. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vale CL, Fisher DJ, Godolphin PJ,
Rydzewska LH, Boher JM, Burdett S, Chen YH, Clarke NW, Fizazi K,
Gravis G, et al: Which patients with metastatic hormone-sensitive
prostate cancer benefit from docetaxel: A systematic review and
meta-analysis of individual participant data from randomised
trials. Lancet Oncol. 24:783–797. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fizazi K, Foulon S, Carles J, Roubaud G,
McDermott R, Fléchon A, Tombal B, Supiot S, Berthold D, Ronchin P,
et al: Abiraterone plus prednisone added to androgen deprivation
therapy and docetaxel in de novo metastatic castration-sensitive
prostate cancer (PEACE-1): A multicentre, open-label, randomised,
phase 3 study with a 2×2 factorial design. Lancet. 399:1695–1707.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smith MR, Hussain M, Saad F, Fizazi K,
Sternberg CN, Crawford ED, Kopyltsov E, Park CH, Alekseev B,
Montesa-Pino Á, et al: Darolutamide and survival in metastatic,
hormone-sensitive prostate cancer. N Engl J Med. 386:1132–1142.
2022. View Article : Google Scholar : PubMed/NCBI
|