Introduction
Although cancer treatment has made great progress,
it is a major cause of morbidity and mortality worldwide (1,2).
According to data from the Global Cancer Report, ~19.3 million new
cancer cases and ~10 million cancer death cases occurred worldwide
in 2020, including ~4.57 million new cancer cases and ~3 million
cancer deaths in China alone (3).
With improvements in medical treatments, traditional
cancer therapies, such as surgery, chemotherapy and radiotherapy,
have evolved. Although these treatments can inhibit the growth of
tumor cells, there are certain harmful effects on normal cells
(4–7). Due to the heterogeneity of cancer,
gene mutations of different cancer cells in patients with the same
type of tumor are not necessarily the same, resulting in increased
difficulties in cancer treatment (8). Drugs that improve cancer treatment
effects and reverse drug resistance have become the focal point in
the current research on drug-resistant tumors (9–14).
However, due to the interactions between different drugs, the
efficacy of combination therapy remains suboptimal in clinical
practice (15) and there are still
adverse reactions caused by chemotherapy (16). Therefore, it is necessary to perform
further research to develop safe and effective cancer
treatments.
The tumor microenvironment (TME) refers to the
surrounding microenvironment in which tumor cells exist, including
surrounding blood vessels, immune cells, cancer-associated
fibroblasts, myeloid-derived suppressor cells, signaling molecules
and the extracellular matrix (Fig.
1) (17). The interaction
between tumor cells and the microenvironment can be a molecular
target for tumor therapy. The cellular and molecular composition
corresponding to the TME and the chemical and physical factors
involved in tumor growth have attracted increased research
interest. Multiple studies have confirmed that the TME's cellular
and molecular composition affects tumor cell growth, invasion and
metastasis (18–21).
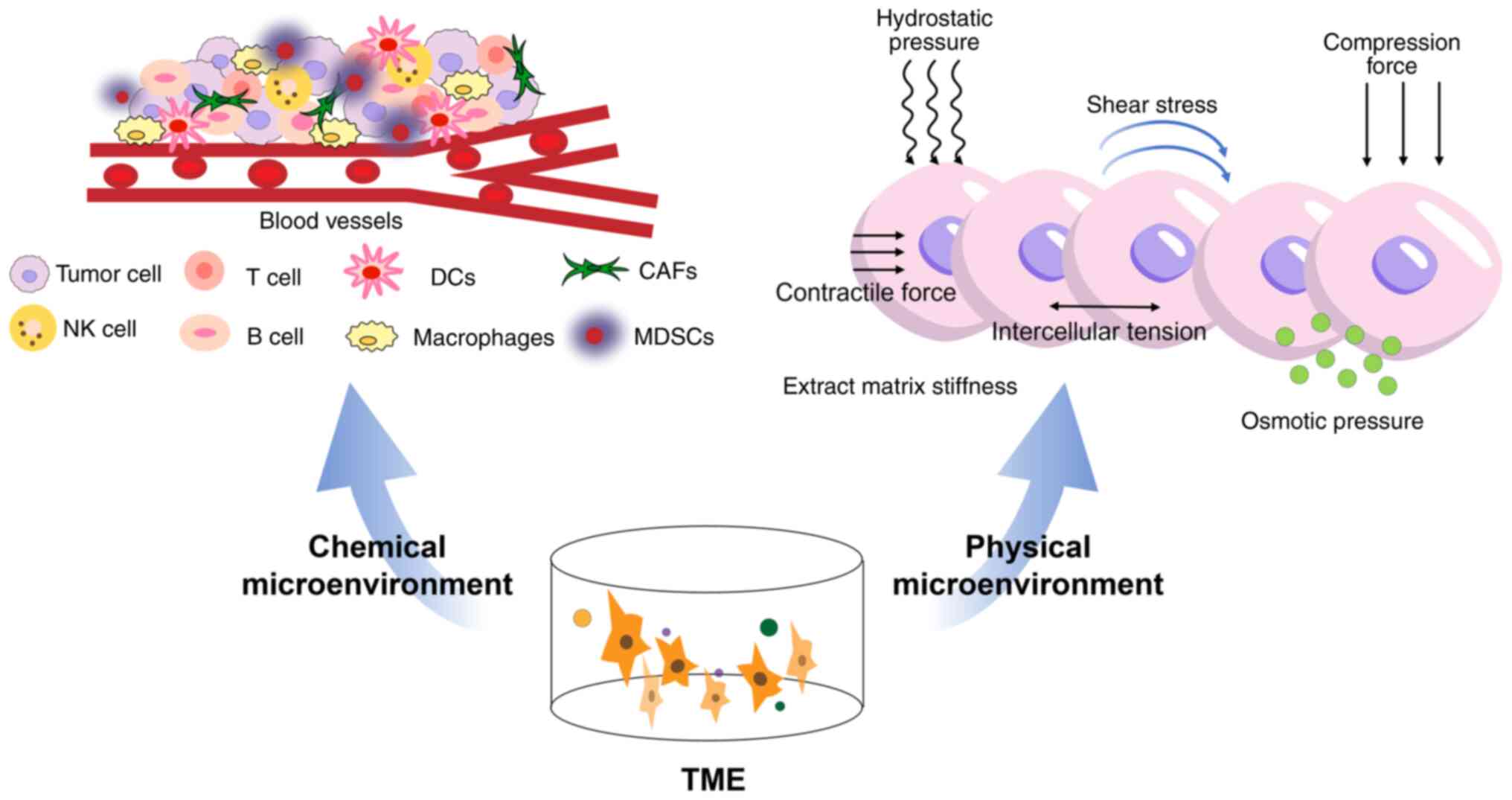 | Figure 1.Composition of the TME. The TME is
composed of the chemical microenvironment and the physical
microenvironment. The chemical microenvironment is populated by
numerous diverse cell types, including blood vessels, and immune
cells, e.g., T and B cells, DCs, NK cells, MDSCs, CAFs and
macrophages. The physical microenvironment includes osmotic
pressure, intercellular tension, contractile force, shear stress,
hydrostatic pressure, compression force and extract matrix
stiffness. TME, tumor microenvironment; DCs, dendritic cells; NK,
natural killer; MDSC, myeloid-derived suppressor cells; CAF,
cancer-associated fibroblasts. |
Immunotherapy is a novel cancer therapy method
(22). Immune cells in the immune
system, such as natural killer (NK) cells, serve an important role
in tumor immunotherapy and interact with the TME to inhibit the
growth of tumor cells (Fig. 1)
(23). A number of studies have
focused on genetic and biochemical factors as the causes of
malignant tumors (20,21). However, physical factors in the TME
have not been widely studied (24).
Tumor cells are typically confined to specific microenvironments
and changes in physical factors in the TME will also affect the
behavior of tumor cells, such as solid stresses generated by tumor
growth (25). Therefore, the
changing characteristics of physical factors in the
microenvironment also serve a key role in tumor development.
Physical therapy for tumors, by changing the living
environment of tumor cells through physical means, may become a new
and effective treatment strategy that can be used to treat most
types of tumor diseases. The present review aimed to summarize the
mechanisms of application of physical stimuli such as pressure,
temperature, light, sound and other therapies, in tumor treatment,
to provide a basis for new treatments and research on tumors.
Pressure
Over the past decades, studies have reported the
critical role of mechanics in the TME (26,27).
Cells in tissues such as the heart, lung and skeleton encounter
nanoscale to macroscale forces that are integral to their function,
such as the shear stress induced by blood flow on a vessel wall
(Fig. 1) (28). Cancer cells have been reported to be
fibrosis of normal cells; the cellular architecture in tumors is
severely altered and is typically characterized by a stiffened
extracellular matrix (29). Cells
can sense exogenous forces through mechanical transduction and
transform them into biological signals (30). The TME affects the growth of tumor
cells through physical and chemical stimulation (31) and responds to changes the tumor
cells in the TME. Mechanical factors include contractile force,
shear stress, hydrostatic pressure, intercellular tension and
extracellular matrix stiffness. Changes in mechanical factors in
the TME can affect tumor transformation, invasion and metastasis
(32). Therefore, the study of
tumor mechanics may be a future direction for developing
tumor-targeted drugs. When analyzing the force on cells from a
microscopic perspective, the cells are affected by two sets of
balancing forces: Gravity and supporting force; the swelling
pushing force of osmotic pressure on the cell membrane and the
pulling force of the cytoskeleton pulling the cell membrane inward
(Fig. 1). Gravity and support are
weak and negligible. The balance between the swelling force of
osmotic pressure and the pulling force of the cytoskeleton is a
basis for cell survival. If this mechanical balance is destroyed,
changes in the cellular microenvironment occur, affecting the
biological function of the cell, including tumor cells. Pressure
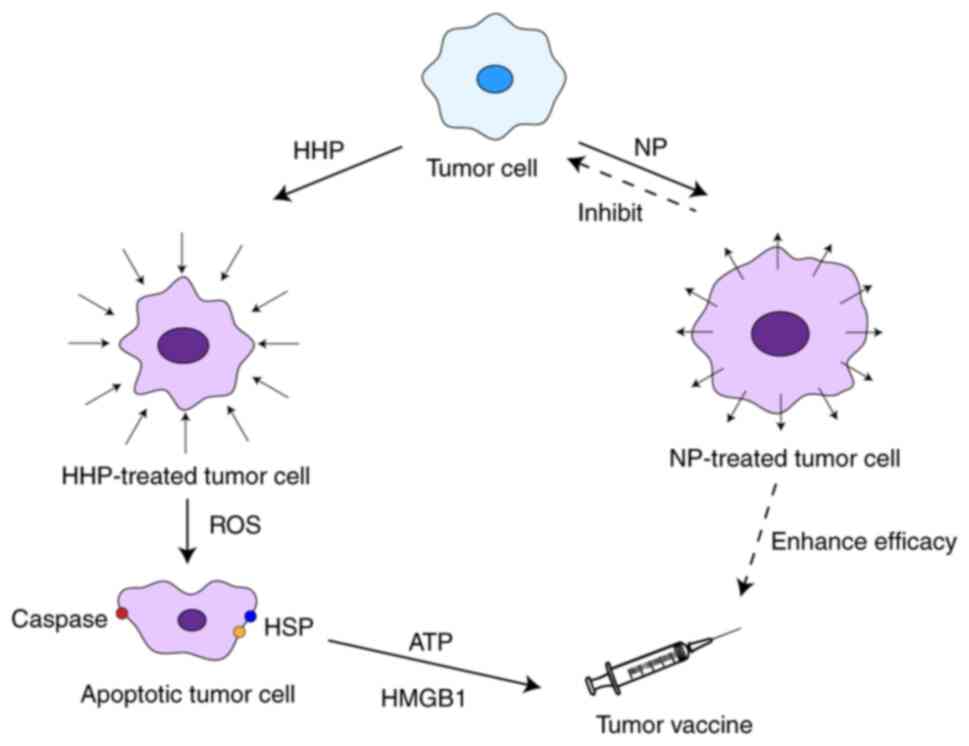
therapy can be divided into high-pressure therapy (HHP) and
negative-pressure therapy (NP). HHP induces immunogenic cell death
(ICD) in tumor cells and shows broad prospects in developing tumor
vaccines and enhancing tumor cell sensitivity. NP uses the defects
in the osmotic pressure regulation ability of tumor cells to
rupture the cell membrane by reducing the external pressure
environment, thereby selectively killing tumor cells without
damaging normal cells.
HHP
HHP was first used to inactivate microorganisms in
milk (33). It can damage microbial
cell membranes and cell walls, change the cell morphology, affect
intracellular enzyme activity and transport intracellular nutrients
and waste, thereby killing spoilage and pathogenic bacteria in food
(34). HHP technology is widely
used in numerous industries, including food preservation and
sterilization and also biotechnology fields, such as
pharmaceuticals.
In recent years, research on the effect of HHP on
tumor cells has been increasing. HHP can induce the ICD of tumor
cells. Immunogenic cell death is a type of regulatory cell death
that can activate the host to generate adaptive immune responses.
The ability to reshape the TME through multiple mechanisms may
increase the success of immunotherapy (35). HHP-induced immunogenic cell death is
driven by excessive reactive oxygen species (ROS) production, which
triggers a rapid integrated stress response, protein kinase R-like
endoplasmic reticulum kinase-mediated eukaryotic translation
initiation factor 2 subunit-α (eIF2α) phosphorylation and the
sequential activation of caspases-2, −8 and −3 (36). Fucikova et al (37) reported that HHP rapidly induced the
surface expression of heat shock protein (HSP)70, HSP90 and
calreticulin, while also promoting the release of high-mobility
group box 1 and ATP. In addition, HHP treatment activated key
features of the endoplasmic reticulum stress-mediated apoptotic
pathway, including ROS production, eIF2α phosphorylation and
caspase-8 activation. HHP has also been reported as a production
technology for tumor vaccines. HHP-treated cells can be
cryopreserved and retain their immunogenicity. In addition,
clinical trials have reported that HHP-treated tumor vaccines
induce specific immune responses to tumor cells (Fig. 2) (38).
Although HHP has shown potential in cancer
treatment, it faces several limitations. HHP is mainly used in
vitro, while precise in vivo methods are yet to be
developed. HHP remains experimental, lacking established treatment
guidelines. Further research is needed to optimize pressure
control, improve application techniques and explore combination
therapies to enhance safety and effectiveness.
NP
The cell membrane is a semi-permeable lipid bilayer,
and the cytoskeleton and sodium and potassium pump of tumor cells
are abnormal, which may lead to a decrease in the osmotic pressure
regulation ability of tumor cells. Atmospheric pressure is one of
the environmental factors necessary for the survival of life. The
different types of tissues and cells in the body have different
levels of adaptability to the living environment. Compared with
normal cells, the cytoskeleton and sodium and potassium pump of
malignant tumor cells are abnormal. Malignant tumor cells have a
decreased ability to control their intracellular osmotic pressure
compared with normal cells and are less tolerant of hypotonic
stress. As atmospheric pressure and intracellular osmotic pressure
exert opposite effects, the osmotic pressure regulation function of
tumor cells is impaired. If the cells are in an environment with
low atmospheric pressure, tumor cells rupture and die before normal
cells (39). It has been reported
that NP inhibits the proliferation and metastasis of pancreatic
cancer cells (40). Furthermore,
applying NP after injecting synthetic severe acute respiratory
syndrome coronavirus 2 DNA vaccine in rats improved the immune
effect of the vaccine (Fig. 2)
(41). Although there is currently
a scarcity of research on the effects of NP on tumor cells, the
hypothesis that NP kill tumor cells without affecting normal cells
appears feasible. This may potentially be a new research direction
for tumor treatment.
Temperature
Environmental temperature is a critical factor that
influences the viability of organisms. When the environmental
temperature of tumor cells reaches ~50°C, cells experience thermal
damage. When the temperature reaches 55°C, collagen denatures,
whereas when the temperature is >60°C, cellular structures such
as mitochondria change and tumor cells begin to undergo necrosis.
On the contrary, when the temperature drops from −4 to −21°C, ice
crystals form outside the tumor cells and the cells begin to
dehydrate. When the temperature drops to −40°C, homogeneous ice
crystals form inside the cells, which is critical for cell death.
Therefore, temperature changes have an impact on the viability of
tumor cells. According to the different modes of temperature
action, temperature therapy is divided into hyperthermia and
cryotherapy. Hyperthermia changes tumor cell membrane permeability,
protein denaturation and DNA synthesis, thereby inducing cell
apoptosis or necrosis. Cryotherapy results in mechanical damage to
the cell membrane, thereby inducing tumor cell necrosis and
apoptosis. The subsequent review sections will introduce the
mechanism of action, research progress and clinical application of
thermotherapy and cryotherapy to provide a theoretical and
experimental basis for further exploring the potential of
temperature therapy in tumor treatment.
Hyperthermia
Hyperthermia, a treatment that increases the
temperature to 39–45°C to induce cell death through apoptosis or
necrosis (42), is a low-toxic
tumor treatment, which is currently used clinically. According to
different heating techniques and heating locations, hyperthermia
techniques are divided into whole-body hyperthermia (43), local hyperthermia and local-regional
hyperthermia (44). Whole-body
hyperthermia is a widely explored approach in oncology, where the
body temperature is elevated to 38.5–41.5°C and maintained for a
certain period. This is typically achieved using non-invasive
surface heating methods, such as infrared electromagnetic waves,
which penetrate the subcutaneous layer and directly heat the blood
in the capillaries, thereby raising the overall body temperature.
Various techniques have been developed for local hyperthermia to
target tumor tissues more precisely while minimizing harm to normal
tissues. These include infrared water window heating, intracavitary
hyperthermia and high-intensity focused ultrasound (45).
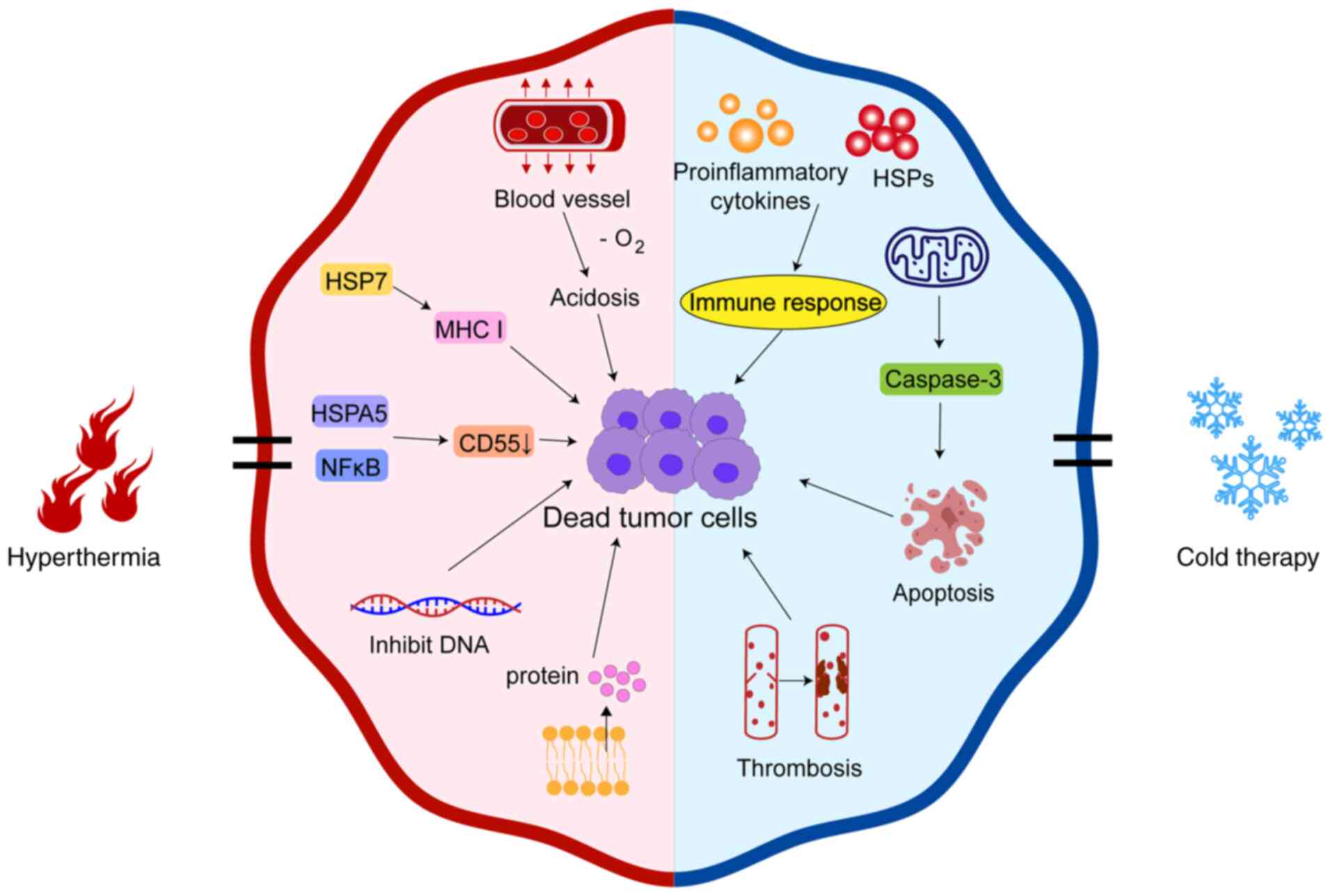
Hyperthermia indirectly kills tumor cells. Heating
causes reactive expansion of blood vessels in normal tissue around
the tumor, which causes further reduction of blood flow in the
tumor tissue, leading to hypoxia in the tumor tissue. This induces
a state of hypoxia, hyponutrition and low blood flow for an
extended period of time, causing the accumulation of lactic acid
and inducing TME changes. The pH drops, inducing tumor acidosis
(46). Hyperthermia can also
upregulate the expression levels of HSP70, thereby increasing the
immune antigenicity of tumor cells, inhibiting their proliferation
and migration and inducing tumor cell death (47). In mouse models of nasopharyngeal
carcinoma, hyperthermia can upregulate the expression levels of
HSPA5, downregulate CD55 expression levels through HSPA5/NF-κB
signal transduction and activation of the complement cascade and
inhibit tumor development (Fig. 3)
(48).
Hyperthermia can also directly kill tumor cells, as
it can inhibit the synthesis of RNA, DNA and protein, preventing
the proliferation of tumor cells (46). When the temperature of the body is
maintained at >40°C for a certain period of time, the
cytoskeleton of tumor cells is destroyed, causing their protein to
denature and cell complex enzymes to be affected, thus inhibiting
the DNA synthesis process until the cells die. It has also been
shown that hyperthermia can induce apoptosis of tumor cells by
activating the caspase family of proteins (Fig. 3) (49).
Hyperthermia increases the efficacy of chemotherapy
and radiotherapy. Hyperthermia and radiotherapy have different
sites of action in the cell proliferation cycle. Hyperthermia
mainly affects S-phase cells (46),
whereas radiotherapy mainly affects G1 and
G2/M phase cells (50).
The combination of the two has synergistic effects on the cell
cycle. A study on breast cancer cells showed that radiofrequency
heating at 13.56 MHz and 43°C for 20 min significantly reduced the
S-phase cell population, while at the same time significantly
increasing the G2/M-phase cell population, enhancing the
efficacy of radiotherapy (51). Of
all types of DNA damage, DNA double-strand breaks are considered
the most harmful (52). Heating can
cause double-stranded DNA breaks or reduce DNA damage repair
(53). The ionization effect of
radiation in organisms exerts its killing effect on tumor cells by
directly or indirectly causing cell DNA strand breaks. DNA is the
main target of ionizing radiation and ionizing radiation has a
cytotoxic effect, causing single- and double-strand DNA breaks
(54). Although radiation causes a
certain degree of direct killing effect on tumor vascular
endothelial cells, it also induces the expression of vascular
endothelial growth factor (VEGF), making tumor vessels more
resistant to radiation. Hyperthermia can inhibit the expression of
tumor-derived VEGF and its products, thereby hindering the
proliferation of tumor vascular endothelium and reshaping of
extracellular matrix, inhibiting tumor growth and metastasis. Liang
et al (55) reported that
hyperthermia can inhibit VEGF gene expression and protein synthesis
levels, indicating that heating can inhibit the proliferation of
endothelial cells and the formation of tumor neovascularization,
thereby enhancing the radiation effect on tumors. Heating changes
the permeability of cell membranes, making it easier for drugs to
enter tumor cells and improving the penetration and absorption of
chemotherapeutic drugs. Therefore, hyperthermia and
chemotherapeutic drugs serve a synergistic role in increasing the
cytotoxicity of chemotherapeutic drugs and exerting antitumor
effects. In addition, hypoxia in tumor cells is an important factor
leading to drug resistance. Therefore, adding hyperthermia
treatment alongside chemotherapy can also eliminate chemotherapy
resistance and improve the efficacy of drugs (Fig. 3) (56).
As a supplement to existing tumor treatment plans,
thermotherapy has a promising prospect in the clinical treatment of
advanced relapsed tumors. A meta-analysis analyzed the efficacy of
thermal radiation therapy and showed that the overall complete
response rate in the thermal radiation therapy group was 54.9%,
which was significantly higher compared with that of the single
radiation therapy group, at 39.8% (57). Although hyperthermia is a promising
cancer treatment, it has several limitations and side effects, such
as maintaining an optimal therapeutic temperature while avoiding
damage to surrounding tissues, burns, pain and thermal damage to
healthy tissues. Further studies are required to determine the
future of thermal therapy, such as expanding the application of
thermal therapy in cancer treatment, combining thermal therapy with
other treatment methods to obtain more accurate data and provide a
basis for clinical treatment.
Cold therapy
Cryoablation technology is increasingly being
utilized in the minimally invasive treatment of tumors. At present,
cryoablation technology mainly includes liquid or gas as a medium
to quickly drop the local temperature of the tumor to <-140°C to
generate ice crystals. This causes the cell membrane to rupture and
occlude the microvessels in the ablation area, resulting in
ischemia and necrosis of tumor cells. Cryoablation induces tumor
cell death through multiple mechanisms. Cryoablation can induce
cell membrane damage to tumor cells, activate the immune response,
induce apoptosis and cause vascular embolisms.
After cryoablation forms ice crystals, the cell
membrane undergoes mechanical damage and activates a series of
antitumor immune reactions, causing tumor necrosis (58). Cells that are not directly killed by
cryoablation may induce apoptosis through a pathway mediated by
mitochondria that activates the caspases family (59). Cryoablation can also cause vascular
embolism, leading to diminished blood flow to the tumor and
amplifying its cytotoxic effect on the tumor. When the temperature
drops, the tissue's extracellular fluid freezes and the development
of extracellular ice elevates the osmotic pressure external to the
cell, causing the fluid to transfer from the inside to the outside
of the cell. In this hypertonic environment, alterations in the
intracellular pH and cellular constituents may result in cellular
injury (60). Cryoablation can also
cause changes in the immune function of tumors. Cells that die due
to osmotic shock or mechanical damage from ice crystals release
their intracellular contents into the extracellular space. Numerous
types of content are immunostimulatory, such as pro-inflammatory
cytokines, HSPs and other related factors. Their receptors can
activate the immune response (61).
Studies have shown that after cryoablation, tumor antigens are
released and the activation of lysosome-related pathways leads to
the overexpression of the synaptosome-associated protein 23 and
syntaxin binding protein 2, thereby promoting the activation of
immune effector cells and suppressing the release of
immunosuppressive factors, ultimately leading to enhanced antitumor
immunity (62). In addition, Seki
et al (63) reported that
the glucose catabolic ability of brown adipose tissue is enhanced
under low-temperature environments, resulting in decreased blood
sugar and insulin tolerance. This reduces the metabolism of the
body, thereby decreasing the catabolic ability of glucose in the
tumor, inhibiting tumor growth and providing a potential new
treatment method for cancer therapy (Fig. 3).
There are numerous reports on the application of
cryoablation in numerous organs, but the effect of cryoablation
treatment using cryoablation is not identical and is closely
associated with the heterogeneity of the tumor. Guo et al
(64) summarized the clinical
application status of cryoablation, indicating that cryoablation
technology has advantages such as minimal invasiveness and fewer
complications. The cooling rate, target temperature, time at target
temperature, thaw rate and number of cycles are the main parameters
that directly affect the therapeutic effect of cryoablation
technology. In the future, auxiliary treatment of cryoablation may
rely on the optimization of these parameters to maximize the
surgical efficacy and minimize surgical complications, reducing the
tumor burden.
Phototherapy
Phototherapy is a method of exposing a patient to
light to treat disease. Phototherapy has been reported to be a safe
tumor treatment in a number of previous studies, mainly focusing on
photothermal therapy (PTT) and photodynamic therapy (PDT) (65). PTT irradiates the photothermal agent
(PTA) with light at a specific wavelength, which heats the PTA and
kills tumor cells, whereas PDT produces a large number of ROS under
the specific wavelength to kill tumor cells (66). Photosensitizers are key to PDT and
PTT can improve treatment efficiency and efficacy without the
requirement of any external photothermal contrast agents (66). During clinical treatment, PTA and
photosensitizers are typically administered through intravenous or
local applications. Through active or passive targeted delivery,
they accumulate in tumor tissue and light of a specific wavelength
is used to irradiate the tumor tissue locally. The following
sections in this review will introduce the mechanism of action,
research progress and clinical application of PTT and PDT,
providing a theoretical basis and practical reference for the
further development of phototherapy in tumor treatment.
PTT
PTT uses photothermal conversion nanomaterials
targeted to tumor sites to absorb and convert light energy into
heat under near-infrared irradiation, thereby inducing tumor cell
death (67). It has become a highly
efficient and minimally invasive method for the treatment of
primary tumors (68) and has few
side effects, a high specificity and can be repeated. In PTT, PTA
enhances the heating of local cells and tissues. Through the local
use of photosensitizers and minimally invasive near-infrared
radiation, PTT-induced hyperthermia can be controlled to minimize
damage to non-targeted tissues.
As aforementioned, hyperthermia can induce tumor
cells to release antigens, pro-inflammatory cytokines and
immunogenic intracellular substrates, promoting immune activation
and causing apoptosis. PTT treatment causes effects akin to those
of hyperthermia. Ali et al (69) irradiated MCF-7 human breast cancer
cells with PTT and showed that subsequent to 2 min of laser
irradiation, the cells primarily underwent apoptosis. Further
research on biomarkers of the apoptosis pathway showed that the
mitochondrial pathway mediates PTT-induced apoptosis through
activation of Bid (70). HSPs are a
category of proteins synthesized by cells in response to stress
sources and are involved in the mechanism of PTT-induced cell
death. Wang et al (71)
synthesized indocyanine green-loaded vanadium oxide nanoparticles
(VO2NPs) for pH-activated near-infrared luminescent
imaging-guided enhanced photothermal tumor ablation. In the acidic
TME, VO2NPs decompose and release VO2+, which
inhibits the function of HSP60. PTT can also induce the activation
of immune function. Deng et al (72) loaded SNX-2112, an HSP90 inhibitor,
on a graphene oxide carrier for near-infrared (NIR) irradiation.
The results showed that NIR can induce downregulation of the
expression levels of HSP90 and programmed cell death ligand 1 in
tumor cells, whilst upregulating the expression of CD69, an
activation marker of T cells. In addition, the increase in tumor
blood flow caused by PTT also leads to an increase in microvascular
permeability and improves drug accumulation in tumors (Fig. 4) (73).
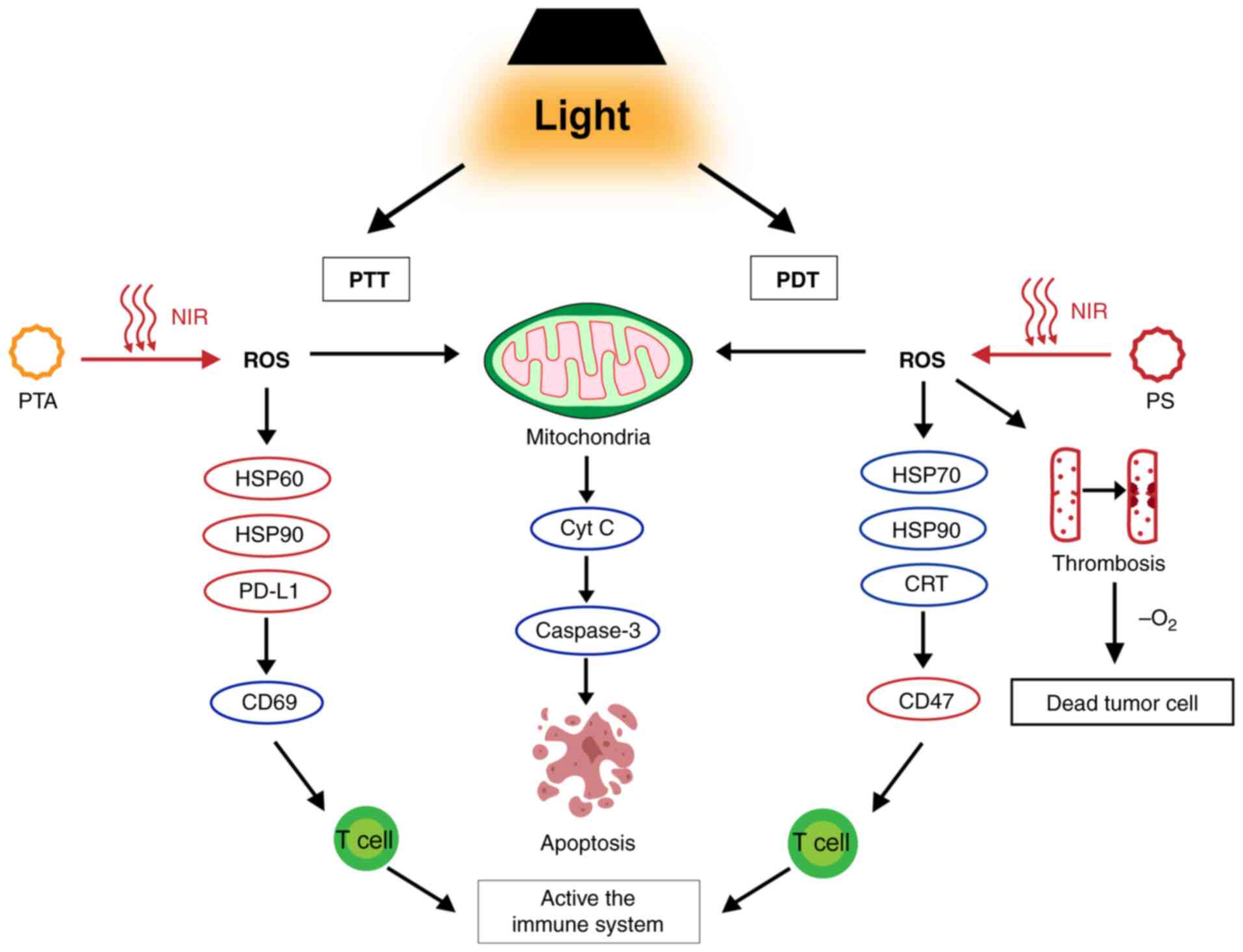 | Figure 4.Mechanism of phototherapy.
Phototherapy is categorized into PTT and PDT. PTT involves NIR
irradiation of the PTA, increases the temperature, activates
caspase-3 via the mitochondrial pathway, inhibits HSP60 and induces
cell death. It inhibits HSP90 and PD-L1, upregulates CD69 and
activates immune function. PDT involves NIR irradiation of the PS
and produces ROS, which kills cells by inducing apoptosis and
necrosis. It can form a thrombus and eventually lead to cell death.
It also upregulates HSP70, HSP90 and CRT, activating the immune
system. PTT, photothermal therapy; PDT, photodynamic therapy; NIR,
near-infrared; PTA, photothermal agent; HSP, heat shock protein;
PS, photosensitizer; ROS, reactive oxygen species; PD-L1,
programmed cell death ligand 1; Cyt C, cytochrome C; CRT,
calreticulin. |
To solve the limitations of PTT monotherapy, a
series of PTT-based combination therapies have been studied. Nam
et al (74) reported that
PTT in combination with chemotherapy can trigger effective
antitumor immunity against disseminated tumors. A
polydopamine-coated spiked gold nanoparticle, SGNP@PDA, combined
with the chemotherapy drug doxorubicin, has been developed for the
chemotherapy combined with PTT. This combination therapy enhances
the antitumor effect of the drug and eliminates local and untreated
distant tumors, achieving survival rates >85% in a bilateral
mouse tumor model of CT26 colon cancer.
In clinical trials, PTT combined with immunotherapy
can produce synergistic effects, reduce primary tumors, control
untreated metastasis and prolong patient survival. It can be used
to treat various types of tumor, such as lung, breast, esophageal,
colon and bladder cancers (75).
PTT shows promise for antitumor therapy but faces certain
difficulties in the clinical application of the treatment. For
instance, in clinical practice, it is difficult to master the light
conditions required for the conversion of photosensitizers.
However, with the continuous development of nanotechnology, the
clinical progress of PTT for tumor treatments will likely improve
over time.
PDT
PDT is a relatively new minimally invasive treatment
method that relies on the selective accumulation of
photosensitizers in cancer cells to generate cytotoxic ROS when
excited by light of certain wavelengths to ultimately kill cancer
cells (76). PDT is based on three
main components: Light, photosensitizer and oxygen. There are three
mechanisms of action by which PDT destroys tumor cells: i) ROS
produced by the photochemical reaction of PDT can directly kill
cells by inducing apoptosis and necrosis; ii) PDT damages
tumor-related blood vessels, resulting in interruption of the
oxygen and nutrient supply, which leads to indirect cell death
caused by hypoxia; and iii) PDT can induce an inflammatory response
and activate the immune response against tumor cells (77).
Light activates the photosensitizer from the ground
state to the excited state and energy transfers to molecular oxygen
to form ROS. In addition to causing oxidative damage to
macromolecules in tumor cells (78), ROS can also directly kill tumor
cells by inducing necrosis or apoptosis. During PDT, most
photosensitizers destroy mitochondrial function, cause cells to
release cytochrome c and activate the caspase pathway to
induce apoptosis of tumor cells. Liu et al (79) used photosensitizer aloe-emodin
(AE)-mediated PDT to inhibit human oral squamous cell carcinoma
both in vitro and in vivo. AE-PDT can kill tumor
cells and inhibit their migration. In the PDT-treated group, the
protein expression levels of Bcl-2 and Bcl-2/Bax were significantly
decreased, while the protein expression levels of Bax and Caspase-3
were significantly increased. De Miguel et al (80) studied the effect of PDT on skull and
spinal osteosarcoma in Balb/c nude mice and reported that the
volume of bone matrix increased after PDT treatment and the size of
the tumor decreased, which may be related to increased necrosis
(Fig. 4).
During PDT, when activated by light, the endothelial
cells of the tumor vascular system produce ROS, destroying the
tumor vascular endothelial cells and causing thrombosis. This
results in a series of corresponding physiological reactions, such
as the release of vasoactive molecules and increased vascular
permeability (81), leading to the
interruption of the supply of oxygen and nutrients to the tumor and
death of tumor cells. Dolmans et al (82) used the photosensitizer MV640, which
targets blood vessels, to study the mechanism of PDT damage of
tumor blood vessels. The tumor tissue showed dose-dependent effects
of this treatment, such as blood stasis, ischemia, internal tumor
hemorrhage, disappearance of blood vessels and thrombosis, and
inhibited tumor growth due to ischemia and hypoxia. Other studies
have shown that PDT can reduce the expression levels of NF-κB
through a ROS-mediated mechanism and activation of NF-κB serves an
important role in the tumor mechanism induced by blood vessel
photosensitivity (Fig. 4) (83,84).
In addition, PDT can also disrupt immune homeostasis
within tumors, activate corresponding immune and inflammatory
reactions and produce anti-tumor effects. Zheng et al
(85) performed a study of PDT in
the treatment of Lewis lung cancer in mice and showed that a large
number of damage-related proteins were released after the tumor
cells were damaged, which in turn mediated the body's antitumor
immune response (Fig. 4).
PDT has been reported to achieve clinical efficacy
in the treatment of skin, esophageal and bladder cancers. A
preliminary study demonstrated the effectiveness and safety of PDT
in patients with bladder cancer who had complete transurethral
resection and were confirmed without serious adverse reactions
(86). PDT is an effective and safe
alternative to traditional treatments with a low incidence rate of
tumors, but its widespread use is currently limited due to the high
cost of photosensitizers and light source equipment required by
PDT. Photosensitizers may also cause skin light allergies and the
tissue penetration of light is limited; therefore, PDT is
restricted in its application for certain types of deep tumor such
as glioma. With the development of new photosensitizers, light
source technologies and combined treatment strategies, PDT may have
broad future application prospects (87).
Sonodynamic therapy (SDT)
As a new non-invasive therapy derived from PDT, SDT
has been previously researched and explored. The principle of SDT
is similar to that of PDT. SDT uses low-intensity ultrasound to
stimulate disease sites, activate sonosensitizers and generate ROS,
inducing tumor cell death. Different types of sonosensitizers,
including organic molecules and inorganic nanomaterials, such as
piezoelectric materials, semiconductors and metallic materials,
have been explored over the past few years. Due to the rapid
development of nanotechnology, a variety of nanomaterials have been
prepared into sonosensitizers or nanocarriers for sonosensitizers,
markedly improving the targeting, stability and biological safety
of sonosensitizers. Chen et al (88) used stanene-based nanosheets as
nano-sound sensitizers to enhance the antitumor efficacy of SDT.
The antitumor mechanism of SDT occurs under low-intensity
ultrasound irradiation as the caspase pathway is activated and
apoptosis of tumor cells is induced. Li et al (89) reported that sinoporphyrin
sodium-mediated SDT significantly increased intracellular ROS. The
elevated production of ROS increases the expression levels of p53
and Bax while suppressing Bcl-2 expression, resulting in the
activation of caspase-3 and ultimately triggering cellular
apoptosis. Yang et al (90)
reported that SDT mediates and induces apoptosis, which is related
to the formation of cell membrane pores caused by the destruction
and cavitation effect of microbubbles (Fig. 5).
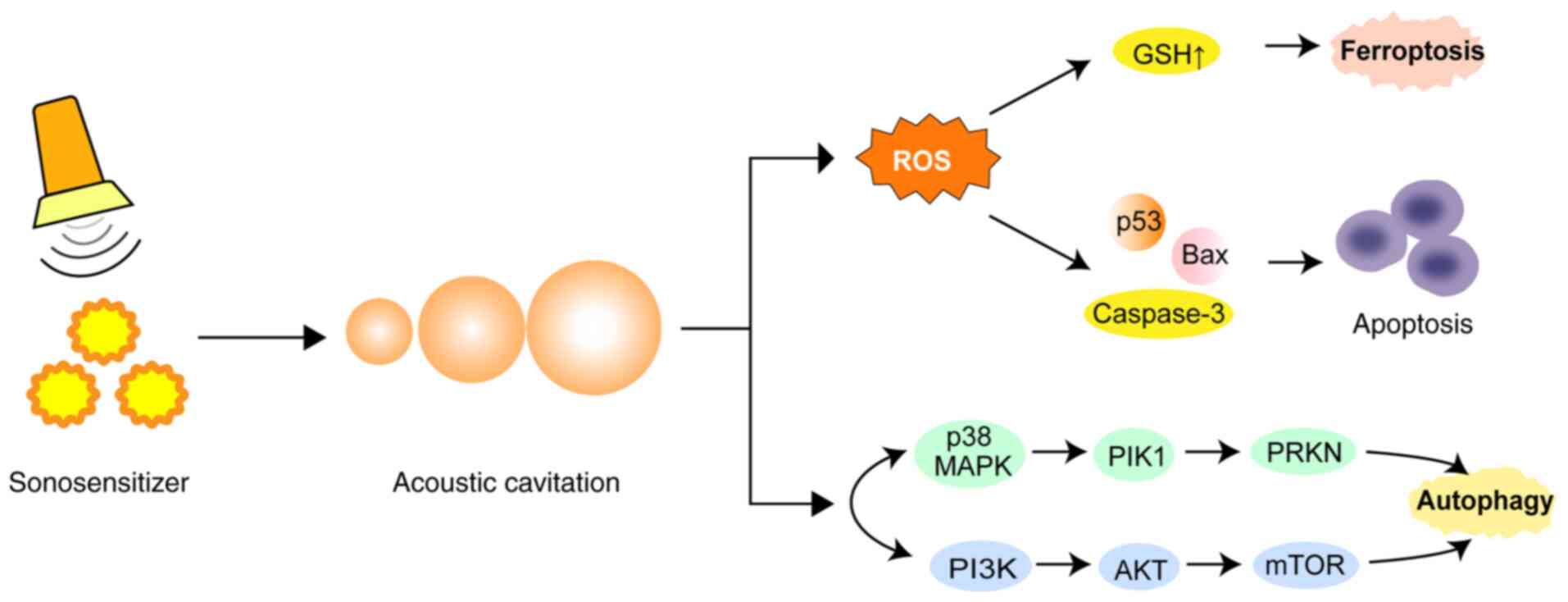 | Figure 5.Mechanism of sonosensitizers. SDT can
mediate the antitumor response, activates sonosensitizers and
generates ROS through apoptosis, ferroptosis and autophagy, thereby
inducing tumor cell death. SDT, sonodynamic therapy; ROS, reactive
oxygen species; ROS, reactive oxygen species; GSH, glutathione;
p53, tumor protein p53; Bax, bcl-2-associated × protein; p38, tumor
protein p38; MAPK, mitogen-activated protein kinase; PIK1,
PTEN-induced kinase 1; PRKN, parkin RBR E3 ubiquitin protein
ligase; PI3K, phosphatidylinositol-3-kinase; AKT, protein kinase B;
mTOR, mammalian target of rapamycin. |
Autophagy is the process of degradation of proteins
or organelles within cells and is an important defensive and
protective mechanism of cells (91). Autophagy starts from the endoplasmic
reticulum and regulates the formation of autophagy bodies under the
collaborative action of the UNC51-like kinase-1 kinase, PI3K and
autophagy-related protein 9 complexes (92,93).
It has been shown that SDT can induce autophagy through the
MAPK/p38-PTEN-induced putative kinase 1-parkin RBR E3 ubiquitin
protein ligase mitochondrial autophagy and PI3K/Akt/mTOR pathways
(94). Ferroptosis is a
non-apoptotic regulated cell death caused by iron accumulation and
subsequent lipid peroxidation (95). Sun et al (96) prepared a metal-organic framework
biomimetic nanosystem, mFeP@si, as a
sonosensitizer, mediating the rupture of lysosomal membranes to
achieve lysosomal escape, accelerating ROS production and
glutathione depletion and further triggering iron death (Fig. 5).
As an emerging technology, SDT is still in the
exploration stage. Research on SDT in brain tumors, such as
glioblastoma, is underway and preliminary results show that SDT can
penetrate the blood-brain barrier and has potential therapeutic
value (97). Despite facing
challenges, such as sound sensitizer development and ultrasound
parameter optimization, SDT may potentially become an important
supplementary therapy for the treatment of tumors as research
progresses. In the future, the scale of clinical trials needs to be
further expanded to verify their safety and efficacy.
Other new therapies
With continuous tumor treatment research, other
types of new physical therapies have also attracted attention.
These include magnetic therapy, acupuncture and local
interventional therapy, which impact the growth, invasion and
metastasis of tumor cells through different biophysical mechanisms.
The following sections will introduce the mechanisms of action of
these novel therapies and their potential applications in tumor
therapy.
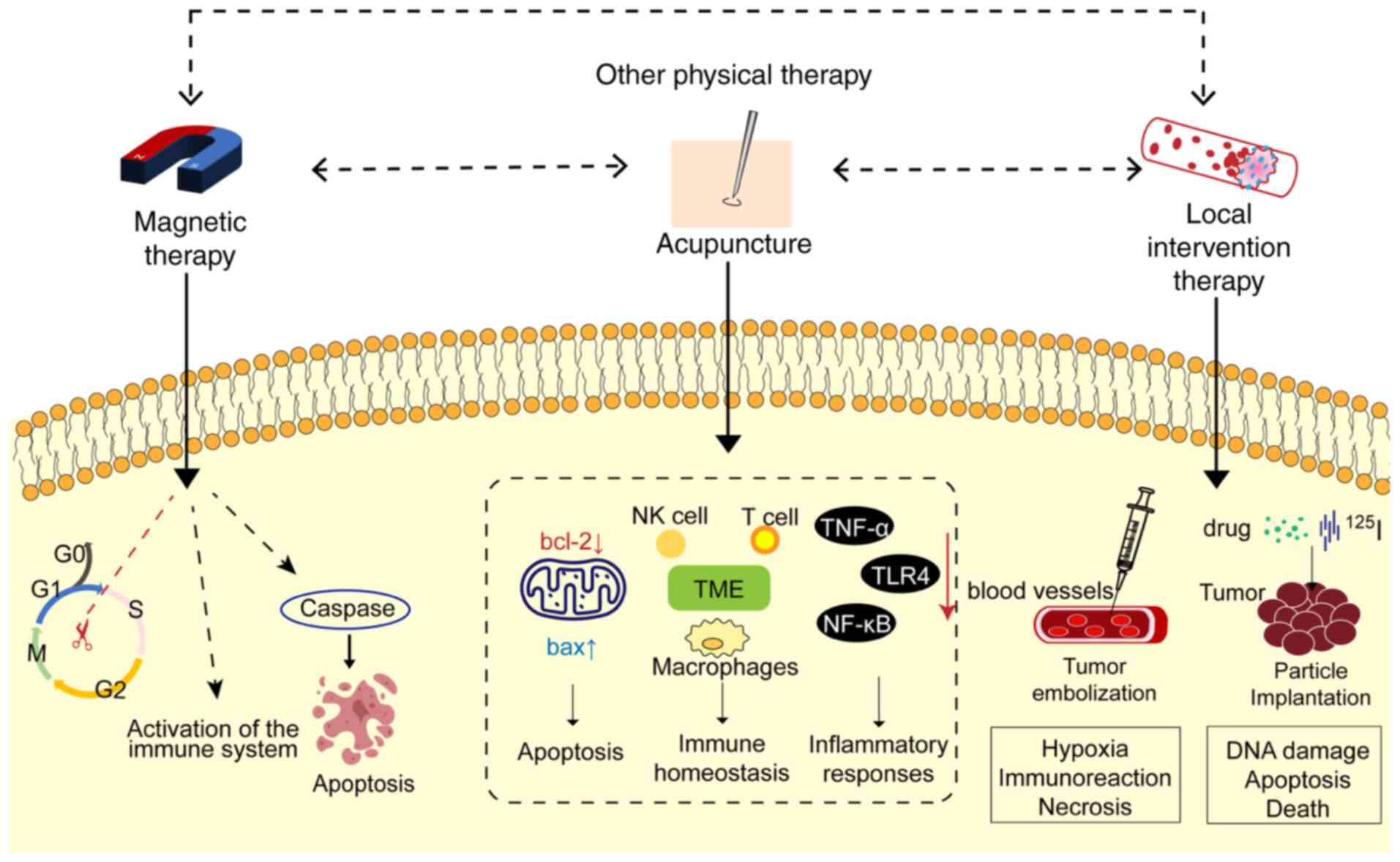
Magnetic therapy
Magnetic therapy involves an external physical
stimulus and produces complex biological effects. It has been shown
that appropriate magnetic field stimulation can prevent
inflammation and oxidative stress and alleviate or repair
neurological damage, and magnetic fields may have protective
effects in various diseases. Magnetic therapy for cancer includes
pulsed electric field (PEF) and pulsed electromagnetic field
(PEMF).
The PEF is generated by applying a voltage to two
electrodes. Under the action of PEF, the permeability of the cell
membrane increases, causing mitochondrial vacuoles to produce and
release cytochrome c. When acting on the nucleus, DNA double
strand breakage may occur. PEF can block the transfer of cells from
G0/G1 phase to S+G2/M phase.
Kulbacka et al (98)
reported that nanosecond PEF reverses drug resistance in gastric
cancer cells by inducing oxidative stress and apoptosis. PEF can
also regulate immune function and induce antitumor effects. Pastori
et al (99) showed that PEF
has an immunomodulatory effect on a breast cancer model of mice,
inhibiting tumor growth and improving the survival rate of mice
(Fig. 6).
PEMF is used to treat various diseases, including
osteoarthritis (100) and
Parkinson's disease (101). Both
in vitro and in vivo research on PEMF-related tumors
is becoming more widespread. PEMF can inhibit cell proliferation,
block tumor angiogenesis, alter the cell cycle, directly kill or
induce tumor cell apoptosis and exert antitumor effects. Filipovic
et al (102) studied the
effect of a 50-Hz magnetic field on different cancer cell systems,
indicating that PEMF therapy has an antiproliferative effect, which
inhibits cell division by destroying the mitotic spindle structure,
leading to chromosome separation errors and inducing apoptosis. Guo
et al (103) reported that
the cell cycle of cancer cells is affected under low-frequency
magnetic fields. PEMF also increases doxorubicin-induced DNA damage
by inhibiting DNA topoisomerase II alpha. In another study, 8 Hz
PEMF was used to irradiate MCF-7 and MDA-MB-231 breast cancer
epithelial cells and FF95 normal fibroblasts. It was found that the
proliferation rate and activity of breast cancer cells were
reduced, whilst the death and aging of breast cancer cells were
induced (Fig. 6) (104).
Currently, the clinical application of PEMF is
limited and Barbault et al (105) published the first report using
PEMF therapy and magnetic fields of specific frequencies to show
the therapeutic effect of PEMF in local tumor treatment. Clinical
research has also revealed that PEMF relieves pain associated with
cancer (106). Although the
biological effects of PEMF have been verified in certain studies,
its specific mechanism still needs to be explored in depth. PEMF
therapy provides a safe and well-tolerated approach for tumor
treatment.
Acupuncture
Acupuncture involves the stimulation of certain
acupoints, activating peripheral nerves, sending sensory
information from the spinal cord to the brain, engaging peripheral
autonomic nerve pathways and modulating the body's physiological
state (107). According to the
concepts of Traditional Chinese Medicine, acupuncture has the
functions of strengthening the body, dredging the meridians,
promoting the movement of qi and blood and harmonizing the visceral
organs. Clinical studies on the treatment of tumor complications
with acupuncture have shown that acupuncture can effectively
improve the symptoms of tumor complications and delay the growth of
tumors to a certain extent, acting as a safe and efficient physical
therapy method (108). Acupuncture
primarily exerts an antitumor effect by enhancing and optimizing
the tumor immune microenvironment, improving the local angiogenesis
of the tumor and promoting tumor cell apoptosis. In the immune
microenvironment, NK cells, CD8+ T cells,
CD4+ T cells, macrophages and related cytokines are
associated with the treatment of tumors by acupuncture and
moxibustion (109). A study using
Lewis lung cancer model mice showed that acupuncture and
moxibustion can promote the production of NK cells by regulating
adrenergic signals, restoring the immune homeostasis of the body
and improving its antitumor capabilities (110). Electroacupuncture at ‘Zusanli’ can
alleviate renal injury in mice with colorectal cancer after
5-fluorouracil chemotherapy, and its mechanism of action may be
related to regulating renal oxidative stress, reducing the
inflammatory response and inhibiting apoptosis (111). Acupuncture may also reduce the
overexpression of NF-κB and the excessive release of inflammatory
factors by inhibiting the overexpression of TNF-α and Toll-like
receptor 4, thereby reducing the occurrence of liver inflammation
in cis-diaminodichloroplatinum (DDP) mice, reducing the side
effects of DDP on the liver and serving an effective role in liver
protection (112). Acupuncture can
significantly reduce the protein expression levels of Bcl-2 in
tumor cells of tumor-bearing mice, increase the protein expression
levels of Bax, reduce the ratio of Bcl-2/Bax and promote tumor cell
apoptosis (113). Previous
clinical studies have reported that acupuncture and moxibustion
have an effect on the symptoms associated with tumors, including
gastrointestinal adverse reactions (114), cancerous pain (115), insomnia (116) and anxiety (117). Therefore, the use of acupuncture
and moxibustion to treat tumors may provide new ideas for clinical
practice, providing a basis for further study (Fig. 6).
Local intervention therapy
As a type of tumor physical therapy, local
interventional therapy relies on imaging guidance to accurately
deliver the therapeutic device to the tumor site, achieving direct
effects on the lesions, including tumor embolization and granule
implantation.
Tumor embolization is an imaging-guided local
interventional treatment method. This involves injecting
embolization materials or drugs directly into the tumor blood
supply artery, blocking the tumor blood supply and inducing hypoxic
necrosis. The tumor blood supply artery is selectively blocked by
embolizers and forms a thrombus to induce tumor hypoxia and
nutritional deficiency (118).
Cancer cells in a stressed state can affect cell migration,
angiogenesis and metastasis by activating the antitumor immune
response. It has been reported that in a rat model of liver cancer,
hepatocellular carcinoma cells develop hypoxia stress due to
embolisms (119). Avritscher et
al (120) reported that after
embolization treatment, tumor-infiltrating lymphocytes and IL-17
expression levels in type 17 T-helper cells increased, activating
the antitumor immune response and reducing cell migration. Tumor
embolization has important clinical value in a variety of solid
tumors. Maclean et al (121) used a novel absorbable gel
microsphere to embolize 23 patients undergoing uterine fibroid
embolization. These results showed that tumor necrosis occurred in
83% of the patients (Fig. 6).
Particle implantation treatment achieves precise
local treatment by implanting radioisotope or antitumor drug
carrier microparticles directly into the tumor. Granule
implantation treatment depends on the role of radioactive or drug
particles in the tumor. The implanted particles act directly on
tumor cells, causing DNA damage and further inducing apoptosis.
Zhang et al (122) showed that implanting
125I radioactive particles in a mouse model of liver
cancer upregulated the expression levels of proinflammatory
cytokines in cancer cells and enhanced cytokine-induced killer
cell-mediated apoptosis by activating caspase-3. In a retrospective
study, 27 patients with bone metastatic tumors were treated with
125I particles to treat pain relief after surgery
without any serious complications (Fig.
6). Local interventional treatment has few complications.
Through precise positioning, it provides minimally invasive and
individualized treatment plans for patients with solid tumors, but
it also has limitations (123). In
the future, through strategies such as the combination of drugs,
immunotherapy and improving drug delivery, this treatment may
improve the efficacy of tumors, reduce adverse reactions and
provide more accurate and efficient individualized treatment plans
for patients with refractory tumors.
Conclusion and perspectives
Tumors cause a multi-factor, long-term type of
disease. In certain cases, it is impossible to completely kill all
tumor cells, which can lead to excessive medical treatments
administered to patients. Compared with other diseases, the
currently existing measures such as surgery, radiotherapy,
chemotherapy and biotherapy do not have high efficacy rates.
Therefore, finding new types of effective treatments is of high
priority. With the advancement of the social economy, science and
technology, modern mechanized manufacturing provides material
guarantee and infinite possibilities for physiotherapy. Instruments
and equipment can be used repeatedly and their applications in
healthcare are more promising compared with chemotherapy.
Physiotherapies may potentially be used in palliative therapy for
advanced tumors, due to their decreased side effects and the low
cost of treatment (124).
Physical therapy, as an emerging cancer treatment
method, has become a research hotspot. Compared with application
bottlenecks associated with treatments such as chemotherapy and
biotherapy, physical therapy can achieve precise localized
treatment with minimal systemic toxicity and side effects. The
present review summarized the antitumor mechanisms of physical
therapy methods such as pressure, temperature, light, sound and
magnetism, demonstrating the broad research potential and
applicability of physical therapy for tumors. Although physical
therapy has made significant progress in experimental research,
there are relatively few clinical trials for these treatment types.
First, clinical trials are limited to the current level of
understanding and technology. Second, within a certain range, the
degree of influence of physical factors is insufficient. Since
physical therapy often relies on instruments and materials that
alter the internal physical factors of tumors, further basic and
clinical research is required to determine the mechanism of action
of physical therapy in tumor treatment and its potential for
clinical applications. Future research on the physical therapy of
tumors may provide a strong scientific basis for the use of these
therapeutic methods in the treatment of tumors.
Acknowledgements
Not applicable.
Funding
This study was supported by the Key Project of Technology
Department of Zhejiang Province (grant no. 2021C03087), Project of
Medical and Health Research Program of Zhejiang Province (grant no.
2022PY084), Project of Science and Technology on Traditional
Chinese Medicine of Zhejiang Province (grant no. 2021ZB185) and the
National Training Program of Innovation and Entrepreneurship for
Undergraduates (grant no. 202310346078X).
Availability of data and materials
Not applicable.
Authors' contributions
JX, CJ and GC were involved in the conception and
design of the study, drafting and revision of the manuscript and
preparation of the figures. HS, YW, CS, ShaL, TY, YS, YL and YF
drafted and revised the manuscript. QZ, ShuL and TX revised the
manuscript. Data authentication is not applicable. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhai B, Chen P, Wang W, Liu S, Feng J,
Duan T, Xiang Y, Zhang R, Zhang M, Han X, et al: An
ATF24 peptide-functionalized β-elemene-nanostructured
lipid carrier combined with cisplatin for bladder cancer treatment.
Cancer Biol Med. 17:676–692. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou J, Kang Y, Gao Y, Ye XY, Zhang H and
Xie T: β-Elemene inhibits epithelial-mesenchymal transformation in
non-small cell lung cancer by targeting ALDH3B2/RPSA axis. Biochem
Pharmacol. 232:1167092025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zraik IM and Heß-Busch Y: Management of
chemotherapy side effects and their long-term sequelae. Urologe A.
60:862–871. 2021.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chaudary N, Hill RP and Milosevic M:
Targeting the CXCL12/CXCR4 pathway to reduce radiation treatment
side effects. Radiother Oncol. 194:1101942024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen P, Wu Q, Feng J, Yan L, Sun Y, Liu S,
Xiang Y, Zhang M, Pan T, Chen X, et al: Erianin, a novel dibenzyl
compound in Dendrobium extract, inhibits lung cancer cell growth
and migration via calcium/calmodulin-dependent ferroptosis. Signal
Transduct Target Ther. 5:512020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bai R, Zhu J, Bai Z, Mao Q, Zhang Y, Hui
Z, Luo X, Ye XY and Xie T: Second generation β-elemene nitric oxide
derivatives with reasonable linkers: Potential hybrids against
malignant brain glioma. J Enzyme Inhib Med Chem. 37:379–385. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu SX, Liang ZM, Wu QB, Shou L, Huang XX,
Zhu QR, Xie H, Mei RY, Zhang RN, Zhai XY, et al: A novel diagnostic
and therapeutic strategy for cancer patients by integrating Chinese
medicine syndrome differentiation and precision medicine. Chin J
Integr Med. 28:867–871. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan T, Feng Y, Wang W, Wang R, Yin L, Zeng
Y, Zeng Z and Xie T: Cabazitaxel-loaded human serum albumin
nanoparticles combined with TGFβ-1 siRNA lipid nanoparticles for
the treatment of paclitaxel-resistant non-small cell lung cancer.
Cancer Nanotechnol. 14:702023. View Article : Google Scholar
|
|
10
|
Xu YX, Chen YM, Zhang MJ, Ren YY, Wu P,
Chen L, Zhang HM, Zhou JL and Xie T: Screening of anti-cancer
compounds from Vaccariae Semen by lung cancer A549 cell fishing and
UHPLC-LTQ Orbitrap MS. J Chromatogr B Analyt Technol Biomed Life
Sci. 1228:1238512023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sui X, Zhang M, Han X, Zhang R, Chen L,
Liu Y, Xiang Y and Xie T: Combination of traditional Chinese
medicine and epidermal growth factor receptor tyrosine kinase
inhibitors in the treatment of non-small cell lung cancer: A
systematic review and meta-analysis. Medicine (Baltimore).
99:e206832020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Zeng H, You Y, Wang R, Tan T, Wang
W, Yin L, Zeng Z, Zeng Y and Xie T: Active targeting of orthotopic
glioma using biomimetic liposomes co-loaded elemene and cabazitaxel
modified by transferritin. J Nanobiotechnology. 19:2892021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu S, Li Q, Li G, Zhang Q, Zhuo L, Han X,
Zhang M, Chen X, Pan T, Yan L, et al: The mechanism of
m6A methyltransferase METTL3-mediated autophagy in
reversing gefitinib resistance in NSCLC cells by β-elemene. Cell
Death Dis. 11:9692020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen P, Li X, Zhang R, Liu S, Xiang Y,
Zhang M, Chen X, Pan T, Yan L, Feng J, et al: Combinative treatment
of β-elemene and cetuximab is sensitive to KRAS mutant colorectal
cancer cells by inducing ferroptosis and inhibiting
epithelial-mesenchymal transformation. Theranostics. 10:5107–5119.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao Q, Feng J, Liu W, Wen C, Wu Y, Liao Q,
Zou L, Sui X, Xie T, Zhang J and Hu Y: Opportunities and challenges
for co-delivery nanomedicines based on combination of
phytochemicals with chemotherapeutic drugs in cancer treatment. Adv
Drug Deliv Rev. 188:1144452022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo X, Wen T and Qu X: Research progress
of adverse events related to PD-1/PD-L1 inhibitors based
combination therapy. Zhongguo Fei Ai Za Zhi. 24:513–518. 2021.(In
Chinese). PubMed/NCBI
|
|
17
|
Sung SY, Hsieh CL, Wu D, Chung LWK and
Johnstone PAS: Tumor microenvironment promotes cancer progression,
metastasis, and therapeutic resistance. Curr Probl Cancer.
31:36–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tung JC, Barnes JM, Desai SR, Sistrunk C,
Conklin MW, Schedin P, Eliceiri KW, Keely PJ, Seewaldt VL and
Weaver VM: Tumor mechanics and metabolic dysfunction. Free Radic
Biol Med. 79:269–280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xian J, Xiao F, Zou J, Luo W, Han S, Liu
Z, Chen Y, Zhu Q, Li M, Yu C, et al: Elemene hydrogel modulates the
tumor immune microenvironment for enhanced treatment of
postoperative cancer recurrence and metastases. J Am Chem Soc.
146:35252–35263. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang N, Gao H, He L, Liu Y, Fan H, Xu Q
and Yang S: Ginsenoside Rb1 is an immune-stimulatory agent with
antiviral activity against enterovirus 71. J Ethnopharmacol.
266:1134012021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W, Jia M, Lian J, Lu S, Zhou J, Fan
Z, Zhu Z, He Y, Huang C, Zhu M, et al: Inhibition of TANK-binding
kinase1 attenuates the astrocyte-mediated neuroinflammatory
response through YAP signaling after spinal cord injury. CNS
Neurosci Ther. 29:2206–2222. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen L, He Y, Zhu J, Zhao S, Qi S, Chen X,
Zhang H, Ni Z, Zhou Y, Chen G, et al: The roles and mechanism of
m6A RNA methylation regulators in cancer immunity.
Biomed Pharmacother. 163:1148392023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu J, Yang Q, Yue Z, Liao B, Cheng H, Li
W, Zhang H, Wang S and Tian Q: Emerging advances in engineered
macrophages for tumor immunotherapy. Cytotherapy. 25:235–244. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Q, Luo Q, Ju Y and Song G: Role of the
mechanical microenvironment in cancer development and progression.
Cancer Biol Med. 17:282–292. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shieh AC: Biomechanical forces shape the
tumor microenvironment. Ann Biomed Eng. 39:1379–1389. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mbeunkui F and Johann DJ Jr: Cancer and
the tumor microenvironment: A review of an essential relationship.
Cancer Chemother Pharmacol. 63:571–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Raudenská M, Navrátil J, Gumulec J and
Masařík M: Mechanobiology of cancerogenesis. Klin Onkol.
34:202–210. 2021.PubMed/NCBI
|
|
28
|
Butcher DT, Alliston T and Weaver VM: A
tense situation: Forcing tumour progression. Nat Rev Cancer.
9:108–122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Egeblad M, Rasch MG and Weaver VM: Dynamic
interplay between the collagen scaffold and tumor evolution. Curr
Opin Cell Biol. 22:697–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hamill OP and Martinac B: Molecular basis
of mechanotransduction in living cells. Physiol Rev. 81:685–740.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghosh D and Dawson MR: Microenvironment
influences cancer cell mechanics from tumor growth to metastasis.
Adv Exp Med Biol. 1092:69–90. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kumar S and Weaver VM: Mechanics,
malignancy, and metastasis: The force journey of a tumor cell.
Cancer Metastasis Rev. 28:113–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Frey B, Janko C, Ebel N, Meister S,
Schlücker E, Meyer-Pittroff R, Fietkau R, Herrmann M and Gaipl US:
Cells under pressure-treatment of eukaryotic cells with high
hydrostatic pressure, from physiologic aspects to pressure induced
cell death. Curr Med Chem. 15:2329–2336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Patterson MF: Microbiology of
pressure-treated foods. J Appl Microbiol. 98:1400–1409. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao LP, Hu JH, Hu D, Wang HJ, Huang CG,
Luo RH, Zhou ZH, Huang XY, Xie T and Lou JS: Hyperprogression, a
challenge of PD-1/PD-L1 inhibitors treatments: potential mechanisms
and coping strategies. Biomed Pharmacother. 150:1129492022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moserova I, Truxova I, Garg AD, Tomala J,
Agostinis P, Cartron PF, Vosahlikova S, Kovar M, Spisek R and
Fucikova J: Caspase-2 and oxidative stress underlie the immunogenic
potential of high hydrostatic pressure-induced cancer cell death.
Oncoimmunology. 6:e12585052017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fucikova J, Moserova I, Truxova I,
Hermanova I, Vancurova I, Partlova S, Fialova A, Sojka L, Cartron
PF, Houska M, et al: High hydrostatic pressure induces immunogenic
cell death in human tumor cells. Int J Cancer. 135:1165–1177. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rozková D, Tiserová H, Fucíková J,
Last'ovicka J, Podrazil M, Ulcová H, Budínský V, Prausová J, Linke
Z, Minárik I, et al: FOCUS on FOCIS: Combined chemo-immunotherapy
for the treatment of hormone-refractory metastatic prostate cancer.
Clin Immunol. 131:1–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang F and Jiang RM: Negative pressure
may become a new method for treatment of malignancies. J Air Force
Med Univ. 43:120–122. 2022.(In Chinese).
|
|
40
|
Yang X, Sun B, Zhu H and Jiang Z:
Suppression effects of negative pressure on the proliferation and
metastasis in human pancreatic cancer cells. J Cancer Res Ther.
11:195–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lallow EO, Jhumur NC, Ahmed I, Kudchodkar
SB, Roberts CC, Jeong M, Melnik JM, Park SH, Muthumani K, Shan JW,
et al: Novel suction-based in vivo cutaneous DNA transfection
platform. Sci Adv. 7:eabj06112021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Szelényi Z and Komoly S: Thermoregulation:
From basic neuroscience to clinical neurology, part 2. Temperature
(Austin). 6:7–10. 2018. View Article : Google Scholar
|
|
43
|
Vertree RA, Leeth A, Girouard M, Roach JD
and Zwischenberger JB: Whole-body hyperthermia: A review of theory,
design and application. Perfusion. 17:279–290. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chia BSH, Ho SZ, Tan HQ, Chua MLK and Tuan
JKL: A review of the current clinical evidence for loco-regional
moderate hyperthermia in the adjunct management of cancers. Cancers
(Basel). 15:3462023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kok HP, Cressman ENK, Ceelen W, Brace CL,
Ivkov R, Grüll H, Ter Haar G, Wust P and Crezee J: Heating
technology for malignant tumors: A review. Int J Hyperthermia.
37:711–741. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hildebrandt B, Wust P, Ahlers O, Dieing A,
Sreenivasa G, Kerner T, Felix R and Riess H: The cellular and
molecular basis of hyperthermia. Crit Rev Oncol Hematol. 43:33–56.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ito A, Shinkai M, Honda H, Wakabayashi T,
Yoshida J and Kobayashi T: Augmentation of MHC class I antigen
presentation via heat shock protein expression by hyperthermia.
Cancer Immunol Immunother. 50:515–522. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen C, Ren A, Yi Q, Cai J, Khan M, Lin Y,
Huang Z, Lin J, Zhang J, Liu W, et al: Therapeutic hyperthermia
regulates complement C3 activation and suppresses tumor development
through HSPA5/NFκB/CD55 pathway in nasopharyngeal carcinoma. Clin
Exp Immunol. 213:221–234. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Paulson JR, Luedtke RL, Suydam S, Obi D
and Xie L: ABT-263, an inhibitor of Bcl-2 family antiapoptotic
proteins, sensitizes prometaphase-arrested HeLa cells to apoptosis
induced by mild hyperthermia. Int J Biochem Res Rev. 31:23–34.
2022. View Article : Google Scholar
|
|
50
|
Dillon MT, Good JS and Harrington KJ:
Selective targeting of the G2/M cell cycle checkpoint to improve
the therapeutic index of radiotherapy. Clin Oncol (R Coll Radiol).
26:257–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hatamian M, Hashemi B, Mahdavi SR,
Soleimani M and Khalafi L: Effect of 13.56 MHz radiofrequency
hyperthermia on mitotic cell cycle arrest in MCF7 breast cancer
cell line and suggest a time interval for radiotherapy. J Cancer
Res Ther. 19:447–451. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hu S, Hui Z, Lirussi F, Garrido C, Ye XY
and Xie T: Small molecule DNA-PK inhibitors as potential cancer
therapy: A patent review (2010-present). Expert Opin Ther Pat.
31:435–452. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
van Oorschot B, Granata G, Di Franco S,
Ten Cate R, Rodermond HM, Todaro M, Medema JP and Franken NA:
Targeting DNA double strand break repair with hyperthermia and
DNA-PKcs inhibition to enhance the effect of radiation treatment.
Oncotarget. 7:65504–65513. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Goldstein M and Kastan MB: The DNA damage
response: Implications for tumor responses to radiation and
chemotherapy. Annu Rev Med. 66:129–143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liang X, Zhou H, Liu X, He Y, Tang Y, Zhu
G, Zheng M and Yang J: Effect of local hyperthermia on
lymphangiogenic factors VEGF-C and -D in a nude mouse xenograft
model of tongue squamous cell carcinoma. Oral Oncol. 46:111–115.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Razavi R and Harrison LE: Thermal
sensitization using induced oxidative stress decreases tumor growth
in an in vivo model of hyperthermic intraperitoneal perfusion. Ann
Surg Oncol. 17:304–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Datta NR, Ordóñez SG, Gaipl US, Paulides
MM, Crezee H, Gellermann J, Marder D, Puric E and Bodis S: Local
hyperthermia combined with radiotherapy and-/or chemotherapy:
Recent advances and promises for the future. Cancer Treat Rev.
41:742–753. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Baust JG, Snyder KK, Santucci KL,
Robilotto AT, Van Buskirk RG and Baust JM: Cryoablation: Physical
and molecular basis with putative immunological consequences. Int J
Hyperthermia. 36 (Suppl 1):S10–S16. 2019. View Article : Google Scholar
|
|
59
|
Wen J, Duan Y, Zou Y, Nie Z, Feng H,
Lugnani F and Baust JG: Cryoablation induces necrosis and apoptosis
in lung adenocarcinoma in mice. Technol Cancer Res Treat.
6:635–640. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen Z, Meng L, Zhang J and Zhang X:
Progress in the cryoablation and cryoimmunotherapy for tumor. Front
Immunol. 14:10940092023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shao Q, O'Flanagan S, Lam T, Roy P, Pelaez
F, Burbach BJ, Azarin SM, Shimizu Y and Bischof JC: Engineering T
cell response to cancer antigens by choice of focal therapeutic
conditions. Int J Hyperthermia. 36:130–138. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wu Y, Cao F, Zhou D, Chen S, Qi H, Huang
T, Tan H, Shen L and Fan W: Cryoablation reshapes the immune
microenvironment in the distal tumor and enhances the anti-tumor
immunity. Front Immunol. 13:9304612022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Seki T, Yang Y, Sun X, Lim S, Xie S, Guo
Z, Xiong W, Kuroda M, Sakaue H, Hosaka K, et al: Brown-fat-mediated
tumour suppression by cold-altered global metabolism. Nature.
608:421–428. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Guo RQ, Guo XX, Li YM, Bie ZX, Li B and Li
XG: Cryoablation, high-intensity focused ultrasound, irreversible
electroporation, and vascular-targeted photodynamic therapy for
prostate cancer: A systemic review and meta-analysis. Int J Clin
Oncol. 26:461–484. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kurz B, Berneburg M, Bäumler W and Karrer
S: Phototherapy: Theory and practice. J Dtsch Dermatol Ges.
21:882–897. 2023. View Article : Google Scholar
|
|
66
|
Li X, Lovell JF, Yoon J and Chen X:
Clinical development and potential of photothermal and photodynamic
therapies for cancer. Nat Rev Clin Oncol. 17:657–674. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yang X, Zhang H, Wu Z, Chen Q, Zheng W,
Shen Q, Wei Q, Shen JW and Guo Y: Tumor therapy utilizing
dual-responsive nanoparticles: A multifaceted approach integrating
calcium-overload and PTT/CDT/chemotherapy. J Control Release.
376:646–658. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Vankayala R and Hwang KC:
Near-infrared-light-activatable nanomaterial-mediated
phototheranostic nanomedicines: An emerging paradigm for cancer
treatment. Adv Mater. 30:e17063202018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ali MRK, Ibrahim IM, Ali HR, Selim SA and
El-Sayed MA: Treatment of natural mammary gland tumors in canines
and felines using gold nanorods-assisted plasmonic photothermal
therapy to induce tumor apoptosis. Int J Nanomedicine.
11:4849–4863. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Pérez-Hernández M, Del Pino P, Mitchell
SG, Moros M, Stepien G, Pelaz B, Parak WJ, Gálvez EM, Pardo J and
de la Fuente JM: Dissecting the molecular mechanism of apoptosis
during photothermal therapy using gold nanoprisms. ACS Nano.
9:52–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang S, Li L, Ning X, Xue P and Liu Y:
pH-activated heat shock protein inhibition and radical generation
enhanced NIR luminescence imaging-guided photothermal tumour
ablation. Int J Pharm. 566:40–45. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Deng X, Guan W, Qing X, Yang W, Que Y, Tan
L, Liang H, Zhang Z, Wang B, Liu X, et al: Ultrafast
low-temperature photothermal therapy activates autophagy and
recovers immunity for efficient antitumor treatment. ACS Appl Mater
Interfaces. 12:4265–4275. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhao P, Zheng M, Yue C, Luo Z, Gong P, Gao
G, Sheng Z, Zheng C and Cai L: Improving drug accumulation and
photothermal efficacy in tumor depending on size of ICG loaded
lipid-polymer nanoparticles. Biomaterials. 35:6037–6046. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Nam J, Son S, Ochyl LJ, Kuai R,
Schwendeman A and Moon JJ: Chemo-photothermal therapy combination
elicits anti-tumor immunity against advanced metastatic cancer. Nat
Commun. 9:10742018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li T, Ashrafizadeh M, Shang Y, Nuri Ertas
Y and Orive G: Chitosan-functionalized bioplatforms and hydrogels
in breast cancer: Immunotherapy, phototherapy and clinical
perspectives. Drug Discov Today. 29:1038512024. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, et al: Photodynamic therapy of cancer: An update. CA Cancer J
Clin. 61:250–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Castano AP, Mroz P and Hamblin MR:
Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer.
6:535–545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tian R, Sun W, Li M, Long S, Li M, Fan J,
Guo L and Peng X: Development of a novel anti-tumor theranostic
platform: A near-infrared molecular upconversion sensitizer for
deep-seated cancer photodynamic therapy. Chem Sci. 10:10106–10112.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu YQ, Meng PS, Zhang HC, Liu X, Wang MX,
Cao WW, Hu Z and Zhang ZG: Inhibitory effect of aloe emodin
mediated photodynamic therapy on human oral mucosa carcinoma in
vitro and in vivo. Biomed Pharmacother. 97:697–707. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
de Miguel GC, Abrantes AM, Laranjo M,
Grizotto AYK, Camporeze B, Pereira JA, Brites G, Serra A, Pineiro
M, Rocha-Gonsalves A, et al: A new therapeutic proposal for
inoperable osteosarcoma: Photodynamic therapy. Photodiagnosis
Photodyn Ther. 21:79–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Krupka M, Bartusik-Aebisher D, Strzelczyk
N, Latos M, Sieroń A, Cieślar G, Aebisher D, Czarnecka M,
Kawczyk-Krupka A and Latos W: The role of autofluorescence,
photodynamic diagnosis and Photodynamic therapy in malignant tumors
of the duodenum. Photodiagnosis Photodyn Ther. 32:1019812020.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Dolmans DEGJ, Kadambi A, Hill JS, Waters
CA, Robinson BC, Walker JP, Fukumura D and Jain RK: Vascular
accumulation of a novel photosensitizer, MV6401, causes selective
thrombosis in tumor vessels after photodynamic therapy. Cancer Res.
62:2151–2156. 2002.PubMed/NCBI
|
|
83
|
Zhao Y, Zhao Y, Ma Q, Zhang H, Liu Y, Hong
J, Ding Z, Liu M and Han J: Novel carrier-free nanoparticles
composed of 7-ethyl-10-hydroxycamptothecin and chlorin e6:
Self-assembly mechanism investigation and in vitro/in vivo
evaluation. Colloids Surf B Biointerfaces. 188:1107222020.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu C, Sun S, Feng Q, Wu G, Wu Y, Kong N,
Yu Z, Yao J, Zhang X, Chen W, et al: Arsenene nanodots with
selective killing effects and their low-dose combination with
ß-elemene for cancer therapy. Adv Mater. 33:e21020542021.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zheng Y, Yin G, Le V, Zhang A, Chen S,
Liang X and Liu J: Photodynamic-therapy activates immune response
by disrupting immunity homeostasis of tumor cells, which generates
vaccine for cancer therapy. Int J Biol Sci. 12:120–132. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lee JY, Diaz RR, Cho KS, Lim MS, Chung JS,
Kim WT, Ham WS and Choi YD: Efficacy and safety of photodynamic
therapy for recurrent, high grade nonmuscle invasive bladder cancer
refractory or intolerant to bacille Calmette-Guérin immunotherapy.
J Urol. 190:1192–1199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Correia JH, Rodrigues JA, Pimenta S, Dong
T and Yang Z: Photodynamic therapy review: Principles,
photosensitizers, applications, and future directions.
Pharmaceutics. 13:13322021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chen W, Liu C, Ji X, Joseph J, Tang Z,
Ouyang J, Xiao Y, Kong N, Joshi N, Farokhzad OC, et al:
Stanene-based nanosheets for β-elemene delivery and
ultrasound-mediated combination cancer therapy. Angew Chem Int Ed
Engl. 60:7155–7164. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li E, Sun Y, Lv G, Li Y, Zhang Z, Hu Z and
Cao W: Sinoporphyrin sodium based sonodynamic therapy induces
anti-tumor effects in hepatocellular carcinoma and activates
p53/caspase 3 axis. Int J Biochem Cell Biol. 113:104–114. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yang W, Xu H, Liu Q, Liu C, Hu J, Liu P,
Fang T, Bai Y, Zhu J and Xie R: 5-Aminolevulinic acid hydrochloride
loaded microbubbles-mediated sonodynamic therapy in pancreatic
cancer cells. Artif Cells Nanomed Biotechnol. 48:1178–1188. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hong X, Huang S, Jiang H, Ma Q, Qiu J, Luo
Q, Cao C, Xu Y, Chen F, Chen Y, et al: Alcohol-related liver
disease (ALD): Current perspectives on pathogenesis, therapeutic
strategies, and animal models. Front Pharmacol. 15:14324802024.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhang R, Pan T, Xiang Y, Zhang M, Feng J,
Liu S, Duan T, Chen P, Zhai B, Chen X, et al: β-Elemene reverses
the resistance of p53-deficient colorectal cancer cells to
5-fluorouracil by inducing pro-death autophagy and cyclin
D3-dependent cycle arrest. Front Bioeng Biotechnol. 8:3782020.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang R, Zheng Y, Zhu Q, Gu X, Xiang B, Gu
X, Xie T and Sui X: β-Elemene reverses gefitinib resistance in
NSCLC cells by inhibiting lncRNA H19-mediated autophagy.
Pharmaceuticals (Basel). 17:6262024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chen F, Xue Q, He N, Zhang X, Li S and
Zhao C: The association and application of sonodynamic therapy and
autophagy in diseases. Life Sci. 334:1222152023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kong N, Chen X, Feng J, Duan T, Liu S, Sun
X, Chen P, Pan T, Yan L, Jin T, et al: Baicalin induces ferroptosis
in bladder cancer cells by downregulating FTH1. Acta Pharm Sin B.
11:4045–4054. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sun N, Lei Q, Wu M, Gao S, Yang Z, Lv X,
Wei R, Yan F and Cai L: Metal-organic framework-mediated siRNA
delivery and sonodynamic therapy for precisely triggering
ferroptosis and augmenting ICD in osteosarcoma. Mater Today Bio.
26:1010532024. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Inui T, Amitani H, Kubo K, Kuchiike D, Uto
Y, Nishikata T and Mette M: Case report: A non-small cell lung
cancer patient treated with GcMAF, sonodynamic therapy and tumor
treating fields. Anticancer Res. 36:3767–3770. 2016.PubMed/NCBI
|
|
98
|
Kulbacka J, Rembiałkowska N, Szewczyk A,
Rossowska J, Drąg-Zalesińska M, Kulbacki M and Choromańska A:
Nanosecond PEF induces oxidative stress and apoptosis via
proteasomal activity inhibition in gastric adenocarcinoma cells
with drug resistance. Int J Mol Sci. 23:129432022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Pastori C, Nafie EHO, Wagh MS,
Mammarappallil JG and Neal RE II: Pulsed electric field ablation
versus radiofrequency thermal ablation in murine breast cancer
models: Anticancer immune stimulation, tumor response, and abscopal
effects. J Vasc Interv Radiol. 35:442–451.e7. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Iannitti T, Fistetto G, Esposito A,
Rottigni V and Palmieri B: Pulsed electromagnetic field therapy for
management of osteoarthritis-related pain, stiffness and physical
function: Clinical experience in the elderly. Clin Interv Aging.
8:1289–1293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Vadalà M, Vallelunga A, Palmieri L,
Palmieri B, Morales-Medina JC and Iannitti T: Mechanisms and
therapeutic applications of electromagnetic therapy in Parkinson's
disease. Behav Brain Funct. 11:262015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Filipovic N, Djukic T, Radovic M,
Cvetkovic D, Curcic M, Markovic S, Peulic A and Jeremic B:
Electromagnetic field investigation on different cancer cell lines.
Cancer Cell Int. 14:842014. View Article : Google Scholar
|
|
103
|
Guo Y, Yang W, Pu G, Zhu C, Zhu Y, Li J,
Huang Y, Wang B and Chu M: Low frequency vibrating magnetic
field-triggered magnetic microspheres with a nanoflagellum-like
surface for cancer therapy. J Nanobiotechnology. 20:3162022.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Pantelis P, Theocharous G, Veroutis D,
Vagena IA, Polyzou A, Thanos DF, Kyrodimos E, Kotsinas A, Evangelou
K, Lagopati N, et al: Pulsed electromagnetic fields (PEMFs) trigger
cell death and senescence in cancer cells. Int J Mol Sci.
25:24732024. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Barbault A, Costa FP, Bottger B, Munden
RF, Bomholt F, Kuster N and Pasche B: Amplitude-modulated
electromagnetic fields for the treatment of cancer: Discovery of
tumor-specific frequencies and assessment of a novel therapeutic
approach. J Exp Clin Cancer Res. 28:512009. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ross C, Overholt T, Xu R, Badlani G, Evans
RJ, Matthews CA and Walker SJ: Pulsed electromagnetic field (PEMF)
as an adjunct therapy for pain management in interstitial
cystitis/bladder pain syndrome. Int Urogynecol J. 33:487–491. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Longhurst JC: Defining meridians: A modern
basis of understanding. J Acupunct Meridian Stud. 3:67–74. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Gümüş M, Chen CI, Ivanescu C, Kilickap S,
Bondarenko I, Özgüroğlu M, Gogishvili M, Turk HM, Cicin I, Harnett
J, et al: Patient-reported outcomes with cemiplimab monotherapy for
first-line treatment of advanced non-small cell lung cancer with
PD-L1 of ≥50%: The EMPOWER-lung 1 study. Cancer. 129:118–129. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Yu SG, Jing XH, Tang Y, Wu QF, Yin HY, Xie
LS, Zhao N, Lin SR and Chai XN: Acupuncture and moxibustion and
immunity: The actuality and future. Zhen Ci Yan Jiu. 43:747–753.
2018.(In Chinese). PubMed/NCBI
|
|
110
|
Hu D, Shen W, Gong C, Fang C, Yao C, Zhu
X, Wang L, Zhao C and Zhu S: Grain-sized moxibustion promotes NK
cell antitumour immunity by inhibiting adrenergic signalling in
non-small cell lung cancer. J Cell Mol Med. 25:2900–2908. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhang XJ, Lin JM, Chen SI, Lin CJ, Peng J,
Yang XD and Zhao JY: Electroacupuncture at ‘Zusanli(ST36)’
alleviates 5-fluorouracil-induced renal injury in colorectal
cancer-bearing mice by exerting effects on oxidative stress,
inflammatory responses, and cell apoptosis. World J Acupunct-Mox.
33:244–251. 2023. View Article : Google Scholar
|
|
112
|
Wang WZ, Wei XY, Yu DD, Du XY, Zhang C,
Wang JM and Han L: Mechanism of acupuncture and moxibustion
regulating TNF-α/TLR4/NF-κB in improving liver injury in cisplatin
mice. Modernization of Traditional Chinese Medicine and Materia
Medica-World Science and Technology. 25:1055–1060. 2023.(In
Chinese).
|
|
113
|
Yang YZ, Li SS, Guo Y, Huang J, Wang JQ,
Feng YT, Zhao SH, Zhao WJ and Xu ZF: Research progress of
acupuncture and moxibustion for the enhanced synergism of
chemotherapy. Acupuncture Research. 49:634–640. 2024.(In Chinese).
PubMed/NCBI
|
|
114
|
Xing XR and Yin DF: Point injection
combined with acupuncture in patients with advanced malignancies
hiccup. Liaoning J Tradit Chin Med. 43:379–380. 2016.(In
Chinese).
|
|
115
|
Zhang XY: Clinical Study on Treatment of
Cancer Pain by External Application of Anti Cancer Analgesic
Plaster Combined with Acupuncture Three-Step Drug Analgesic Method.
Liaoning J Tradit Chin Med. 49:165–169. 2022.(In Chinese).
|
|
116
|
Wang TT, Cheng J, Wang J, Zou WJ and Song
N: Effect of acupuncture treating cancer-related insomnia: A
systematic review. Liaoning. J Tradit Chin Med. 50:196–201+257.
2023.(In Chinese).
|
|
117
|
Zhao JX and Li J: Action and
characteristic of acupuncture and moxibustion in treatment of
malignant tumor. J Clin Acupunct Moxibustion. 38:99–103. 2022.(In
Chinese).
|
|
118
|
Zhou M, Xie Y, Xu S, Xin J, Wang J, Han T,
Ting R, Zhang J and An F: Hypoxia-activated nanomedicines for
effective cancer therapy. Eur J Med Chem. 195:1122742020.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Cortes AC, Nishiofuku H, Polak U, Minhaj
AA, Lopez MS, Kichikawa K, Qayyum A, Whitley EM and Avritscher R:
Effect of bead size and doxorubicin loading on tumor cellular
injury after transarterial embolization and chemoembolization in a
rat model of hepatocellular carcinoma. Nanomedicine. 39:1024652022.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Avritscher R, Jo N, Polak U, Cortes AC,
Nishiofuku H, Odisio BC, Takaki H, Tam AL, Melancon MP, Yevich S,
et al: Hepatic arterial bland embolization increases Th17 cell
infiltration in a syngeneic rat model of hepatocellular carcinoma.
Cardiovasc Intervent Radiol. 43:311–321. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Maclean D, Vigneswaran G, Bryant T, Modi S
and Hacking N: A retrospective cohort study comparing a novel,
spherical, resorbable particle against five established embolic
agents for uterine fibroid embolisation. Clin Radiol. 76:452–457.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhang J, Wu N, Lian Z, Feng H, Jiang Q,
Chen X, Gong J and Qiao Z: The combined antitumor effects of
125I radioactive particle implantation and
cytokine-induced killer cell therapy on xenograft hepatocellular
carcinoma in a mouse model. Technol Cancer Res Treat. 16:1083–1091.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Kandzari DE, Weber MA, Pathak A, Zidar JP,
Saxena M, David SW, Schmieder RE, Janas AJ, Langer C, Persu A, et
al: Effect of alcohol-mediated renal denervation on blood pressure
in the presence of antihypertensive medications: Primary results
from the TARGET BP I randomized clinical trial. Circulation.
149:1875–1884. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Pyszora A, Budzyński J, Wójcik A, Prokop A
and Krajnik M: Physiotherapy programme reduces fatigue in patients
with advanced cancer receiving palliative care: Randomized
controlled trial. Support Care Cancer. 25:2899–2908. 2017.
View Article : Google Scholar : PubMed/NCBI
|