Introduction
Acute myeloid leukemia (AML) is a highly
heterogeneous hematological malignancy that poses significant
treatment challenges. Despite considerable advancements in
induction and consolidation therapies for AML in recent years, the
risk of relapse after achieving complete remission (CR) remains
high (1). Specifically, among
patients aged <60 years, >50% experienced relapse, whereas
relapse rates reached 90% in those aged ≥60 years. This high
disease recurrence rate contributes to an aggregated 5-year overall
survival (OS) of 30.5% across all cases (2). This underscores the urgent need for
effective and safe post-remission treatment strategies to enhance
disease-free survival and ultimately OS survival. While
consolidation therapy has been established to improve survival
outcomes in low-risk patients with AML, maintenance therapy, a
standard treatment component for certain hematological
malignancies, such as acute lymphoblastic leukemia and acute
promyelocytic leukemia (3,4), remains a contentious issue in AML. For
patients with AML harboring high-risk factors for recurrence and
who have undergone allogeneic hematopoietic stem cell
transplantation, maintenance therapy is deemed necessary to
mitigate the risk of recurrence (5,6).
However, for those with intermediate-to-low-risk disease,
maintenance therapy is not yet part of the standard treatment
regimen due to insufficient evidence of its benefits. Thus, the
exploration of maintenance therapy for patients with intermediate
and low-risk AML is of importance.
The combination of azacitidine (AZA) and venetoclax
(VEN) has demonstrated synergistic effects, enhancing the
anti-leukemic efficacy while potentially reducing drug dosages and
associated toxicities (7,8). This VEN and hypomethylating agent
regimen has emerged as the standard treatment for elderly or unfit
patients with AML who are not suitable for intensive chemotherapy,
where unfitness is defined as meeting ≥1 of the following: Eastern
Cooperative Oncology Group performance status ≥2; Hematopoietic
Cell Transplantation Comorbidity Index ≥3; organ dysfunction; or
active infection requiring intravenous antimicrobial therapy
(9–11). Although studies have explored the
use of AZA or VEN individually for maintenance therapy in AML
(12), there is a paucity of
research on the application of the combined VEN plus AZA (VEN-AZA)
regimen for maintenance therapy in patients with
low-to-intermediate-risk AML.
In the present study, a retrospective analysis of 43
patients newly diagnosed with intermediate-to-low-risk AML who
underwent maintenance therapy at Beijing Luhe Hospital Affiliated
to Capital Medical University (Beijing, China) was conducted to
evaluate the efficacy and safety of the combination regimen of
VEN-AZA in maintenance therapy for patients with this disease.
Materials and methods
Patient selection and data
collection
A retrospective analysis of patients with primary
intermediate-to-low-risk AML admitted to Beijing Luhe Hospital
Affiliated to Capital Medical University between March 2018 and
June 2024 was conducted. Prognostic risk stratification was
conducted according to the 2022 European Leukemia Net AML criteria
(13). The inclusion criteria were
as follows: i) Patients who remained in CR following induction and
consolidation therapy, with blasts of <5% and no evidence of
extramedullary tumor infiltration and received either VEN-AZA
treatment regimen or no treatment; ii) patients who were not
eligible for or unwilling to undergo allogeneic or autologous
hematopoietic stem cell transplantation; iii) patients who had not
received other targeted drugs; and iv) patients with complete
clinical data. The study variables including the patient's age,
sex, prognosis stratification, white blood cell (WBC) count,
platelet count, lactate dehydrogenase levels, bone marrow blast
percentage, chromosomal analysis, fusion genes, gene mutations and
minimal residual disease (MRD) monitoring prior to maintenance
therapy were retrospectively extracted from electronic medical
records.
Diagnostic data collection Bone marrow
morphological examination was performed using Wright's staining,
with 200 nucleated cells counted under an oil-immersion lens. All
molecular analyses, including reverse transcription-quantitative
PCR (RT-qPCR) for AML1-ETO, CBFβ-MYH11 and HOX11 expression
quantification, as well as next-generation sequencing (NGS)-based
mutation profiling of 248 genes, were performed by Hightrust
Diagnostics Medical Laboratory (Beijing, China). The RT-qPCR primer
sequence information for various genes is shown in Table I. For NGS, libraries were
constructed using the Hemaseq™ Myeloid 248 Gene Mutation Detection
Kit (Healthy Biotechnology Co., Ltd; cat. no. S0918-S), a
hybridization capture-based panel targeting exons and flanking ±20
bp intronic regions of 248 genes, including core driver genes [such
as fms related receptor tyrosine kinase 3 (FLT3), nucleophosmin 1
(NPM1) and CCAAT enhancer binding protein α (CEBPA)], epigenetic
regulators [(such as DNA methyltransferase 3α (DNMT3A), tet
methylcytosine dioxygenase 2 (TET2), isocitrate dehydrogenase
[NADP(+)] 1/2 (IDH1/2)], signaling pathway genes (such as Janus
kinase 2 and calreticulin) and spliceosome complex genes (such as
splicing factor 3b subunit 1 and serine and arginine rich splicing
factor 2).
 | Table I.PCR primer sequence information. |
Table I.
PCR primer sequence information.
| Gene name | Forward primer
sequence (5′-3′) | Reverse primer
sequence (5′-3′) |
|---|
| AML1-ETO |
CACCTACCACAGAGCCATCAAA |
ATCCACAGGTGAGTCTGGCATT |
| CBFβ-MYH11 |
CATTAGCACAACAGGCCTTTGA |
AGGGCCCGCTTGGACTT |
| HOX11 |
TGGATGGAGAGTAACCGCAGAT |
GGGCGTCCGGTTCTGATA |
For bone marrow MRD detection, a Canto II flow
cytometer (Becton, Dickinson and Company) with Diva software was
used to acquire and analyze the data. Antibody reagents from
Becton, Dickinson and Company and Beckman Coulter, Inc. were used
to label CD34, CD117, CD38, HLA-DR, CD33, CD13, CD15, CD14, CD4,
CD7, CD19 and CD56. After collecting 500,000 CD45+ cells, abnormal
cell populations were identified by combining leukemia-associated
immunophenotype and ‘different from normal’ approaches. The
threshold was set at 0.1% as per the European LeukemiaNet
recommendations to distinguish negative and positive results
(14). Additionally, RT-qPCR
targeting AML1-ETO and CBFβ-MYH11 fusion-gene transcripts was used
for MRD detection.
Maintenance therapy and efficacy
endpoint
Patients in the maintenance therapy group received
VEN-AZA maintenance therapy 1 month after consolidation therapy.
The specific VEN-AZA dosing regimen was as follows: Subcutaneous
injection of 75 mg/m2 AZA for 7 consecutive days and the
dose of VEN was reduced from the standard 400 mg daily to 200 mg on
days 1–21. If grade 4 myelosuppression (based on the Common
Terminology Criteria for Adverse Events version 5.0) occurred
during treatment (15), the VEN
treatment was paused and supportive treatments such as leukocyte
elevation, anti-infection measures or component blood transfusions
were provided. One treatment cycle lasted 2–3 months. During the
maintenance treatment period, an assessment was conducted per
treatment cycle. The methods for detecting MRD included qPCR for
detecting fusion genes and flow cytometry to identify
leukemia-associated abnormal phenotypes in the bone marrow cells of
patients. The primary efficacy endpoint was progression-free
survival (PFS), defined as the time from the onset of the disease
to recurrence, death or end of follow-up.
Statistical analysis
Continuous variables are presented as the median and
range (minimum to maximum). Intergroup comparisons were conducted
using the Kruskal-Wallis rank-sum test. For categorical variables,
proportional differences were analyzed via Fisher's exact test when
any expected cell count was <10; otherwise, the χ2
test was applied (Table II). The
impact of maintenance therapy on PFS was evaluated using
Kaplan-Meier survival curves and log-rank tests. Differences in PFS
were assessed using univariate and multivariate Cox proportional
hazards regression analysis. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using Empower(R) (www.empowerstats.com; X&Y solutions, Inc.) and R
version 3.6.1 (http://www.R-project.org).
 | Table II.Clinical and biological
characteristics of patients with AML at diagnosis. |
Table II.
Clinical and biological
characteristics of patients with AML at diagnosis.
| Characteristic | Off maintenance
therapy, n=21 | On maintenance
therapy, n=22 | P-value |
|---|
| Median age at
diagnosis, years (range) | 59.00
(24.00–81.00) | 49.00
(21.00–75.00) | 0.165 |
| Median white blood
cells, ×109/l (range) | 8.59
(0.84–362.06) | 7.46
(0.88–76.20) | 0.078 |
| Median hemoglobin,
g/l (range) | 85.00
(51.00–150.00) | 86.50
(34.00–127.00) | 0.560 |
| Median platelets,
×109/l (range) | 43.00
(8.00–265.00) | 51.50
(2.00–333.00) | 0.806 |
| Median bone marrow
blast count, % (range) | 62.00
(1.00–91.00) | 44.75
(13.00–78.00) | 0.521 |
| Sex, n (%) |
|
| 0.432 |
|
Female | 8 (38.10) | 11 (50.00) |
|
| Male | 13 (61.90) | 11 (50.00) |
|
| Gene fusion, n
(%) |
|
| 0.956 |
|
Negative | 9 (42.86) | 11 (50.00) |
|
|
AML1-ETO | 7 (33.33) | 7 (31.82) |
|
|
HOX11 | 4 (19.05) | 3 (13.64) |
|
|
CBFβ-MYH11 | 1 (4.76) | 1 (4.55) |
|
| NPM1 status n
(%) |
|
| 0.280 |
| Wild
type | 13 (61.90) | 10 (45.45) |
|
|
Mutant | 8 (38.10) | 12 (54.55) |
|
| CEBPA status, n
(%) |
|
| 0.021 |
| Wild
type | 14 (66.67) | 21 (95.45) |
|
|
Mutant | 7 (33.33) | 1 (4.55) |
|
| FLT3 status, n
(%) |
|
| 0.252 |
| Wild
type | 15 (71.43) | 12 (54.55) |
|
|
Mutant | 6 (28.57) | 10 (45.45) |
|
| DNMT3A status, n
(%) |
|
| 0.069 |
| Wild
type | 14 (66.67) | 20 (90.91) |
|
|
Mutant | 7 (33.33) | 2 (9.09) |
|
| KIT status, n
(%) |
|
| 0.475 |
| Wild
type | 16 (76.19) | 19 (86.36) |
|
|
Mutant | 5 (23.81) | 3 (13.64) |
|
| ASXL1 status, n
(%) |
|
| >0.999 |
| Wild
type | 20 (95.24) | 20 (90.91) |
|
|
Mutant | 1 (4.76) | 2 (9.09) |
|
| ASXL2 status, n
(%) |
|
| 0.607 |
| Wild
type | 19 (90.48) | 21 (95.45) |
|
|
Mutant | 2 (9.52) | 1 (4.55) |
|
| NRAS status, n
(%) |
|
| 0.664 |
| Wild
type | 19 (90.48) | 18 (81.82) |
|
|
Mutant | 2 (9.52) | 4 (18.18) |
|
| IDH2 status, n
(%) |
|
| 0.664 |
| Wild
type | 19 (90.48) | 18 (81.82) |
|
|
Mutant | 2 (9.52) | 4 (18.18) |
|
| IDH1 status, n
(%) |
|
| >0.999 |
| Wild
type | 20 (95.24) | 20 (90.91) |
|
|
Mutant | 1 (4.76) | 2 (9.09) |
|
| TET2 status, n
(%) |
|
| 0.412 |
| Wild
type | 17 (80.95) | 20 (90.91) |
|
|
Mutant | 4 (19.05) | 2 (9.09) |
|
| Karyotype, n
(%) |
|
| >0.999 |
|
Normal | 12 (57.14) | 12 (54.55) |
|
|
Favorable | 2 (9.52) | 3 (13.64) |
|
|
Other | 7 (33.33) | 7 (31.82) |
|
| Prognostic
stratification, n (%) |
|
| 0.048 |
|
Favorable group | 13 (61.90) | 7 (31.82) |
|
|
Intermediate group | 8 (38.10) | 15 (68.18) |
|
| Minimal residual
disease, n (%) |
|
| 0.607 |
|
Negative | 14 (66.67) | 13 (59.09) |
|
|
Positive | 7 (33.33) | 9 (40.91) |
|
Results
Characteristics of the cohort
The present study retrospectively analyzed 43 cases
of newly diagnosed patients with intermediate-to-low-risk AML who
remained in CR following the completion of induction and
consolidation therapy Among them, there were 10 patients in the
maintenance therapy group who received intensive treatment during
the induction phase and 11 patients who received low-intensity
treatment [based on hypomethylating agents (HMAs) or low-dose
cytarabine]. Thus, there were 22 patients who received maintenance
therapy. The median number of maintenance treatment cycles was 8
(range, 2–18). Among these patients, 59.09% (n=13) achieved
MRD-negative (MRDneg) status following induction and consolidation
therapy. There were 21 patients who did not receive maintenance
therapy, with 66.67% (n=14) achieving MRDneg status following the
induction and consolidation therapy. There was no significant
difference in the MRDneg status between the off-maintenance therapy
group and the maintenance therapy group at enrollment. The gene
mutations with the highest frequency in the 43 patients were NPM1,
CEBPA, FLT3, DNMT3A, KIT, ASXL transcriptional regulator 1 (ASXL1),
ASXL2, NRAS, IDH1, IDH2 and TET2. The comparison of basic clinical
characteristics between the two groups is shown in Table II. The proportion of patients with
CEBPA mutations was higher in the off-maintenance therapy group
(33.33% vs. 4.55%; P=0.021), and the proportion of patients in the
favorable group was also higher in the off-maintenance therapy
group (61.90 vs. 31.82%; P=0.048). During the study period, the
most common grade 3–4 hematological adverse events in the
maintenance treatment group were neutropenia (n=2) and
thrombocytopenia (n=4), and the most common non-hematological
adverse event was respiratory infection (Table III). Grade 3–4 adverse events were
experienced by 9 patients during cycles 1–2 of treatment. No deaths
occurred during the maintenance treatment period.
 | Table III.Adverse events of maintenance
treatment in patients with acute myeloid leukemia. |
Table III.
Adverse events of maintenance
treatment in patients with acute myeloid leukemia.
| Adverse event | Grade 3–4, n
(%) |
|---|
| Neutropenia | 2 (9) |
| Anemia | 0 (0) |
|
Thrombocytopenia | 4 (19) |
| Digestive tract
symptom | 1 (4) |
| Fatigue | 0 (0) |
| Respiratory
infection | 2 (9) |
| Pain | 0 (0) |
| Heart disease | 1 (4) |
| Liver damage | 0 (0) |
Prognostic value
Clinical and pathological parameters predictive of
PFS were further investigated through univariate and multivariate
Cox regression models. In the univariate analysis, maintenance
therapy selection, WBC and age were significantly associated with
PFS (Table IV). Patients with AML
who did not accept maintenance therapy showed a significantly
unfavorable PFS. With a median follow-up of 29.6 months (7–74), the
median PFS was not reached in patients with AML receiving
maintenance therapy, while the median PFS time for those not
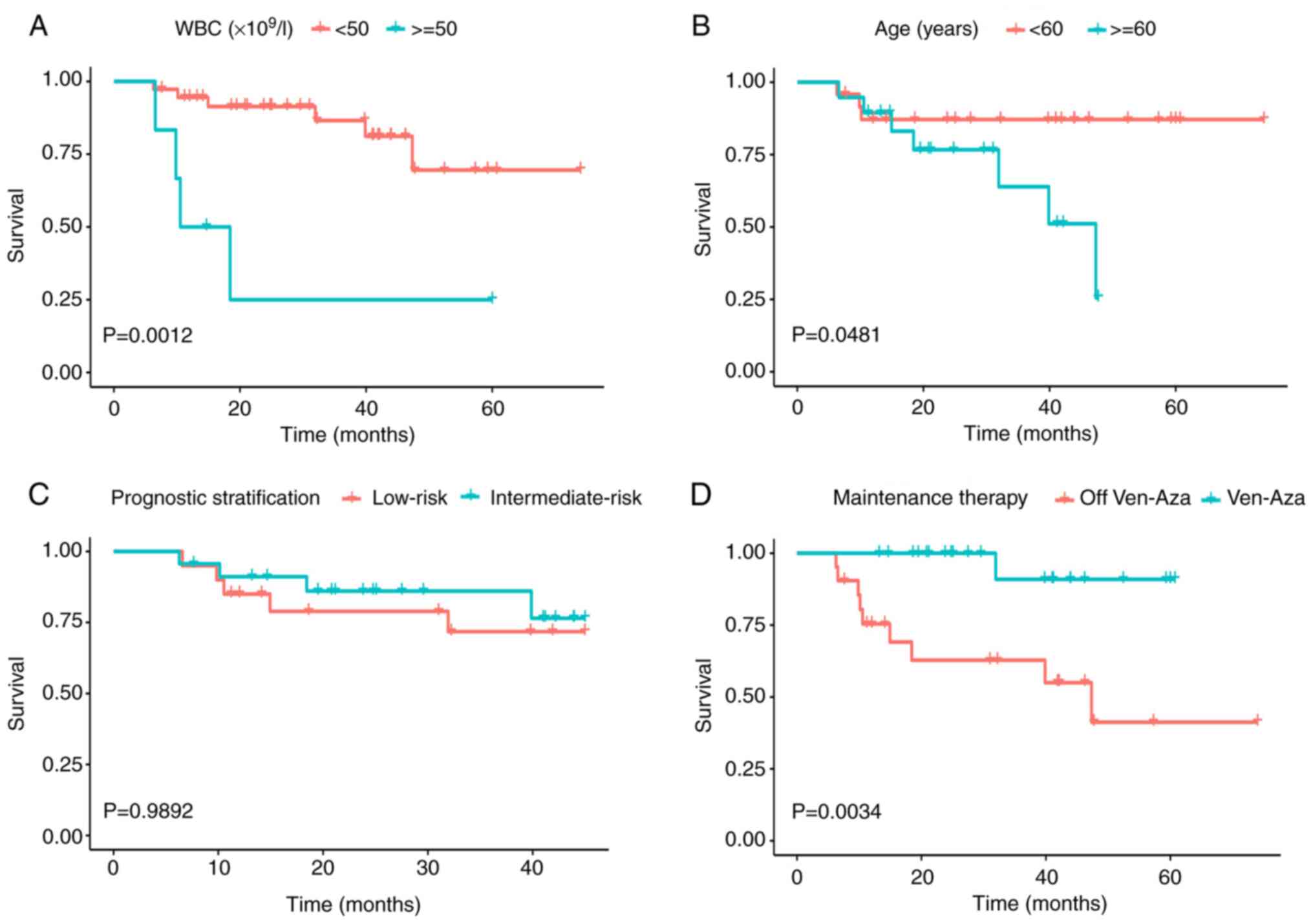
receiving maintenance therapy was 47.3 months (P=0.0034; Fig. 1D). The PFS time decreased in
patients with WBC ≥50×109/l and those aged ≥60 (P=0.0012
and P=0.0481, respectively; Fig. 1A and
B). No difference in PFS was observed between the
intermediate-risk and low-risk patients (P=0.9892; Fig. 1C).
 | Table IV.Univariate analysis of
progression-free survival. |
Table IV.
Univariate analysis of
progression-free survival.
|
| Progression-free
survival |
|---|
|
|
|
|---|
| Characteristic | HR (95% CI) | P-value |
|---|
| Sex |
|
|
|
Male | 1.0 |
|
|
Female | 1.6 (0.4–6.2) | 0.495 |
| Age | 1.1 (1.0–1.1) | 0.027 |
| White blood cells
count | 1.0 (1.0–1.0) | <0.001 |
| Hemoglobin
count | 1.0 (1.0–1.0) | 0.487 |
| Platelets
count | 1.0 (1.0–1.0) | 0.575 |
| Prognostic
stratification |
| 0.989 |
|
Favorable group | 1.0 |
|
|
Intermediate group | 1.0 (0.3–3.5) |
|
| Maintenance
therapy |
| 0.021 |
| Off
VEN-AZA | 1.0 |
|
|
VEN-AZA | 0.1 (0.0–0.7) |
|
| Minimal residual
disease |
| 0.107 |
|
Negative | 1.0 |
|
|
Positive | 2.8 (0.8–10.1) |
|
Study end points
The multivariate Cox regression model was employed
to examine the association between VEN-AZA maintenance therapy and
PFS, with adjustments made for potential confounding factors
(Table V). In both the unadjusted
and adjusted models, it was observed that patients with AML who
underwent VEN-AZA maintenance treatment exhibited a decreased risk
of the primary endpoint event, compared with those not receiving
maintenance treatment. In the unadjusted model, the hazard ratio
(HR) was determined to be 0.09, with a 95% confidence interval (CI)
of 0.01–0.69. In the Adjusted I model, the adjustment factors
included the age of the patients at the time of diagnosis, which
was categorized as either ≥60 years old or <60 years old, and
the WBC count was specified as ≥50×109/l or
<50×109/l. The corresponding HR was found to be 0.1,
with a 95% CI of 0.01–0.85. In the Adjusted II model, the
adjustment factors comprised the age of the patients at diagnosis
(≥60 years old or <60 years old), the WBC count
(≥50×109/l or <50×109/l), prognostic
stratification and mutations in the DNMT3A, NRAS, IDH2, IDH1, TET2,
ASXL1 and ASXL2 genes, as well as MRD prior to maintenance
treatment. The HR was calculated to be 0.06, with a 95% CI of
0.00–0.77.
 | Table V.Association between VEN-AZA
maintenance therapy and the endpoint of acute myeloid leukemia
recurrence in multivariate Cox proportional-hazards analysis. |
Table V.
Association between VEN-AZA
maintenance therapy and the endpoint of acute myeloid leukemia
recurrence in multivariate Cox proportional-hazards analysis.
|
| Non-adjusted | Adjust
Ia | Adjust
IIb |
|---|
|
|
|
|
|
|---|
| Therapy | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Off VEN-AZA | 1.0 | 0.0205 | 1.0 | 0.0354 | 1.0 | 0.0309 |
| VEN-AZA | 0.09
(0.01–0.69) |
| 0.10
(0.01–0.85) |
| 0.06
(0.00–0.77) |
|
Discussion
Maintenance therapy for AML plays a pivotal role in
reducing the recurrence risk and enhancing patient survival rates.
The VIALE-A study (16)
demonstrated that combination treatment with VEN-AZA can
significantly improve treatment outcomes. The VEN-AZA regimen is
typically designed as a long-term treatment, which persists until
disease progression or the emergence of unacceptable toxicity
(17). In the present study,
patients with intermediate-to-low-risk AML attained CR following
consolidation therapy. Among those continuing VEN-AZA maintenance
therapy, only 1 case experienced disease progression, with PFS not
reached, showing a significantly longer PFS compared with the
non-maintenance group (median PFS of 47.3 months). Even after
adjusting for age, WBC count, prognostic stratification and
prognosis-related gene mutations, the relapse risk of
maintenance-treated patients remained significantly lower. Pratz
et al (18) demonstrated the
importance of MRD in a cohort of patients with a longer follow-up
(median >20 months). Those who achieved CR without measurable
residual disease also showed a longer duration of remission, OS and
event-free survival compared with patients with MRD positivity. The
VIALE-A study (16) demonstrated
that patients responsive to VEN-AZA who achieved CR with MRD
negativity had a median OS time of ~3 years. The study by Chua
et al (19) confirmed that
patients receiving VEN-based treatment for 12 months and achieving
MRD negativity at the end of treatment had the potential for
durable treatment-free remission (TFR). Deep remission is a key
determinant of long-term survival in patients with AML. During
continuous VEN-AZA treatment in the present study, the MRD of 8
patients turned negative, achieving molecular remission, with no
relapses. By contrast, 7 patients had persistent positive MRD
following consolidation and 6 exhibited relapse. This is consistent
with previously reported results that indicated that late-stage
MRDneg responses were associated with an improved prognosis
(20). This suggested that
intermediate- or low-risk patients may benefit from VEN-AZA
maintenance, likely due to deep remission induced by continuous
treatment, effectively preventing relapse.
The maintenance treatment regimen for AML should
effectively prevent recurrence while avoiding severe
chemotherapy-related adverse reactions. A previous study reported
that the incidence of any-grade infections was 84% in the
combination therapy group and 67% in the single-agent AZA group.
The incidence of severe adverse events was 83% in the combination
therapy group, higher than the 73% in the single-agent AZA group
(21). Of note, in the present
study, no deaths or severe adverse events occurred during the
maintenance treatment of patients. The most common grade 3–4
adverse events were neutropenia, thrombocytopenia and respiratory
tract infections. Most adverse reactions occurred in the first two
cycles of maintenance treatment, and then the frequency gradually
decreased, which may have been associated with the dose reduction
of VEN or the adjustment of the administration time. As
demonstrated in the retrospective analysis by Xin et al
(22), in patients who responded to
the combination therapy of HMAs and VEN, the incidence of
infectious complications was relatively high, and the
infection-related mortality was also at a high level. The
infection-related mortality has an adverse impact on the OS of this
vulnerable patient population with AML. Elderly patients with AML
or those unfit for intensive chemotherapy are more likely to be
affected by treatment-related toxicities during long-term treatment
(23). Therefore, for patients with
intermediate- or low-risk AML who achieve deep (MRD ≤0.01%) and
sustained CR, attempting to discontinue treatment and obtaining the
benefits of treatment-free survival (TFS) could be a feasible
strategy.
However, whether discontinuing treatment is the best
strategy for achieving an improvement in quality of life and TFR
remains controversial (24). In the
present study, the median number of maintenance treatment cycles
was 8 (range, 2–18), yet we were unable to determine the minimum
number of VEN-AZA treatment cycles associated with a longer TFR. As
reported in the study by Garciaz et al (25), patients who had received >10
previous cycles of treatment had the longest TFS, and a minimum of
5 cycles were associated with an acceptable TFR. Therefore, further
exploration to determine the optimal number of cycles and dosage
for maintenance therapy is needed.
Although the present study demonstrated the
advantages of VEN-AZA maintenance therapy in reducing the
recurrence risk and prolonging survival in patients with
intermediate- or low-risk AML, it should be noted that the study
has limitations. The small sample size increases the likelihood of
failing to detect true treatment effects. As a retrospective study,
a number of confounding factors, including prior consolidation
chemotherapy regimens and prophylactic antibiotic choices, were not
included, which may have inflated the observed treatment benefits.
Additionally, the limited follow-up duration might have led to an
underestimation of late toxicities. Future research should focus on
conducting large-scale, long-term and strictly controlled clinical
trials to accurately determine the minimum number of VEN-AZA
maintenance treatment cycles required to achieve durable TFR
without compromising treatment efficacy. Additionally, given that
existing studies have shown that molecular changes, such as NPM1
and IDH mutations, are associated with a higher benefit from
VEN-AZA treatment (26),
identifying predictive biomarkers to accurately screen out patients
who are most likely to benefit from VEN-AZA maintenance therapy
will lay the foundation for developing truly individualized
treatment strategies.
Acknowledgements
Not applicable.
Funding
The present study was funded by Youth Support Project of Luhe
Hospital (grant no. LHYH2024-LC07).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DZ and XD conceptualized the study, designed the
methodology and interpreted data. DZ and HZ conducted the
statistical analyses and validated the analytical models. XD
drafted the manuscript and coordinated revisions. YZ, XC and JQ
completed the acquisition and preliminary interpretation of
clinical data. TC participated in the study design. HZ adjudicated
clinical endpoints, resolved discrepancies in adverse event
attribution and critically revised the manuscript for scientific
accuracy. HZ and XC confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Affiliated Beijing Luhe Hospital of Capital Medical University
(Beijing, China; approval no. 2024-KY-108). Given the retrospective
nature of the study and the use of de-identified patient data, the
requirement for informed consent was waived by the Institutional
Review Board. The study was conducted in accordance with the
ethical standards of the Declaration of Helsinki and its later
amendments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yuan XL, Lai XY, Wu YB, Yang L, Shi J, Liu
L, Zhao Y, Yu J, Huang H and Luo Y: Risk factors and prediction
model of early relapse in acute myeloid leukemia after allogeneic
hematopoietic cell transplantation. Blood. 140:12915. 2022.
View Article : Google Scholar
|
|
2
|
DiNardo CD, Erba HP, Freeman SD and Wei
AH: Acute myeloid leukaemia. Lancet. 401:2073–2086. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toksvang LN, Lee SHR, Yang JJ and
Schmiegelow K: Maintenance therapy for acute lymphoblastic
leukemia: Basic science and clinical translations. Leukemia.
36:1749–1758. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanz MA, Fenaux P, Tallman MS, Estey EH,
Löwenberg B, Naoe T, Lengfelder E, Döhner H, Burnett AK, Chen SJ,
et al: Management of acute promyelocytic leukemia: Updated
recommendations from an expert panel of the European LeukemiaNet.
Blood. 133:1630–1643. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kinsella FAM, Maroto MAL, Loke J and
Craddock C: Strategies to reduce relapse risk in patients
undergoing allogeneic stem cell transplantation for acute myeloid
leukaemia. Br J Haematol. 204:2173–2183. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Molica M, Breccia M, Foa R, Jabbour E and
Kadia TM: Maintenance therapy in AML: The past, the present and the
future. Am J Hematol. 94:1254–1265. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsao T, Shi Y, Kornblau S, Lu H, Konoplev
S, Antony A, Ruvolo V, Qiu YH, Zhang N, Coombes KR, et al:
Concomitant inhibition of DNA methyltransferase and BCL-2 protein
function synergistically induce mitochondrial apoptosis in acute
myelogenous leukemia cells. Ann Hematol. 91:1861–1870. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garciaz S, Hospital MA, Alary AS, Saillard
C, Hicheri Y, Mohty B, Rey J, D'Incan E, Charbonnier A, Villetard
F, et al: Azacitidine plus venetoclax for the treatment of relapsed
and newly diagnosed acute myeloid leukemia patients. Cancers.
14:20252022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
DiNardo CD, Pratz K, Pullarkat V, Jonas
BA, Arellano M, Becker PS, Frankfurt O, Konopleva M, Wei AH,
Kantarjian HM, et al: Venetoclax combined with decitabine or
azacitidine in treatment-naive, elderly patients with acute myeloid
leukemia. Blood. 133:7–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jonas BA and Pollyea DA: How we use
venetoclax with hypomethylating agents for the treatment of newly
diagnosed patients with acute myeloid leukemia. Leukemia.
33:2795–2804. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klepin HD: Definition of unfit for
standard acute myeloid leukemia therapy. Curr Hematol Malig Rep.
11:537–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Senapati J, Kadia TM and Ravandi F:
Maintenance therapy in acute myeloid leukemia: Advances and
controversies. Haematologica. 108:2289–2304. 2023.PubMed/NCBI
|
|
13
|
Döhner H, Wei AH, Appelbaum FR, Craddock
C, DiNardo CD, Dombret H, Ebert BL, Fenaux P, Godley LA, Hasserjian
RP, et al: Diagnosis and management of AML in adults: 2022
recommendations from an international expert panel on behalf of the
ELN. Blood. 140:1345–1377. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schuurhuis GJ, Heuser M, Freeman S, Béné
MC, Buccisano F, Cloos J, Grimwade D, Haferlach T, Hills RK,
Hourigan CS, et al: Minimal/measurable residual disease in AML: A
consensus document from the European LeukemiaNet MRD Working Party.
Blood. 131:1275–1291. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Freites-Martinez A, Santana N,
Arias-Santiago S and Viera A: Using the common terminology criteria
for adverse events (CTCAE-Version 5.0) to evaluate the severity of
adverse events of anticancer therapies. Actas Dermosifiliogr (Engl
Ed). 112:90–92. 2021.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
DiNardo CD, Jonas BA, Pullarkat V, Thirman
MJ, Garcia JS, Wei AH, Konopleva M, Döhner H, Letai A, Fenaux P, et
al: Azacitidine and venetoclax in previously untreated acute
myeloid leukemia. N Engl J Med. 383:617–629. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei AH, Montesinos P, Ivanov V, DiNardo
CD, Novak J, Laribi K, Kim I, Stevens DA, Fiedler W, Pagoni M, et
al: Venetoclax plus LDAC for newly diagnosed AML ineligible for
intensive chemotherapy: A phase 3 randomized placebo-controlled
trial. Blood. 135:2137–2145. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pratz KW, Jonas BA, Pullarkat V, Recher C,
Schuh AC, Thirman MJ, Garcia JS, DiNardo CD, Vorobyev V,
Fracchiolla NS, et al: Measurable residual disease response and
prognosis in Treatment-Naïve acute myeloid leukemia with venetoclax
and azacitidine. J Clin Oncol. 40:855–865. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chua CC, Hammond D, Kent A, Tiong IS,
Konopleva MY, Pollyea DA, DiNardo CD and Wei AH: Treatment-free
remission after ceasing venetoclax-based therapy in patients with
acute myeloid leukemia. Blood Adv. 6:3879–3883. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bernardi M, Ferrara F, Carrabba MG,
Mastaglio S, Lorentino F, Vago L and Ciceri F: MRD in
Venetoclax-based treatment for AML: Does it really matter? Front
Oncol. 12:8908712022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alsouqi A, Geramita E and Im A: Treatment
of acute myeloid leukemia in older adults. Cancers (Basel).
5:54092023. View Article : Google Scholar
|
|
22
|
Xin F, Yu YH, Shen XL and Zhang GX: The
efficacy of the combination of venetoclax and hypomethylating
agents versus HAG agents in patients with acute myeloid leukemia: A
retrospective study. Hematology. 29:23503192024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rosko AE, Cordoba R, Abel G, Artz A, Loh
KP and Klepin HD: Advances in management for older adults with
hematologic malignancies. J Clin Oncol. 39:2102–2114. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patel KK, Zeidan AM, Shallis RM, Prebet T,
Podoltsev N and Huntington SF: Cost-effectiveness of azacitidine
and venetoclax in unfit patients with previously untreated acute
myeloid leukemia. Blood Adv. 5:994–1002. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garciaz S, Dumas PY, Bertoli S, Sallman
DA, Decroocq J, Belhabri A, Orvain C, Aspas Requena G, Simand C,
Laribi K, et al: Outcomes of acute myeloid leukemia patients who
responded to venetoclax and azacitidine and stopped treatment. Am J
Hematol. 99:1870–1876. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pollyea DA, DiNardo CD, Arellano ML,
Pigneux A, Fiedler W, Konopleva M, Rizzieri DA, Smith BD, Shinagawa
A, Lemoli RM, et al: Impact of venetoclax and azacitidine in
Treatment-Naïve patients with acute myeloid leukemia and IDH1/2
mutations. Clin Cancer Res. 28:2753–2761. 2022. View Article : Google Scholar : PubMed/NCBI
|