Introduction
Melanoma is characterized by the malignant
transformation of melanocytes. This skin cancer is highly
aggressive and drug-resistant, with a survival rate of only 29.8%
after 5 years, and causes ~55,500 deaths annually (1,2). At
present, the primary therapeutic strategy for melanoma involves
surgical excision of the primary tumor followed by adjuvant
treatment to prevent recurrence of the malignancy (3). First-line adjuvant treatments for
melanoma include interferon-α and dabrafenib. However, these
treatments have not been successful in markedly improving overall
survival and often result in severe side effects (4–7).
Therefore, the development of new adjuvants with high efficacy and
low toxicity is required for the treatment of melanoma.
In recent years, Traditional Chinese Medicine (TCM)
has gained increasing attention for its potential in the
development of novel drugs for postoperative adjuvant treatment,
due to its high efficacy and low toxicity (8–10).
Atractylodes macrocephala, known as ‘Baizhu’ in Chinese, is
a perennial herb with a history spanning thousands of years in
treating various disorders, including spleen hypofunction, diarrhea
and cancer (11,12). Both in vitro and in
vivo experimental studies have demonstrated the antitumor
activity of A. macrocephala rhizome extract (13–15).
Atractylenolide I (AT) is a sesquiterpenoid lactone extracted from
the A. macrocephala rhizome. AT induces apoptosis and
suppresses glycolysis in colorectal cancer cells (16). The suppressive role of AT in
tumorigenesis has also been reported in breast cancer (15). AT induces apoptosis and cell cycle
arrest in melanoma cells via the extracellular regulated protein
kinase/glycogen synthase kinase 3β axis (17). Additionally, Xu et al
(18) discovered that AT enhances
the responsiveness to immune checkpoint blockade therapy by
activating tumor antigen presentation in colorectal cancer cell
implanted C57BL/6 mice and human patient-derived colorectal cancer
organoid models. Therefore, the promising therapeutic potential of
AT in melanoma is supported by various studies. However, its
precise role and underlying mechanisms remain largely unknown.
Furthermore, the multitude of targets and complex interactions
between AT and melanoma pose a major challenge in understanding the
antitumor mechanism of action of AT.
Network pharmacology has recently emerged as a
robust method for systematically revealing the biological
mechanisms underlying complex diseases and natural ingredients in
TCM (19,20). Unlike the traditional ‘one
symptom-one target-one drug’ dogma, network pharmacology prefers to
establish a ‘compound-protein/gene-disease’ synergistic network,
sharing a similar holistic philosophy as that of TCM (21). This approach effectively provides an
understanding of the mechanisms underlying complex interactions
between diseases and TCM preparations, making it valuable for new
drug discovery and, in particular, the modern analysis of TCM
treatments. For example, Ganoderma lucidum, known as ‘Ling
Zhi’ in China, is a typical TCM with anticancer properties, whose
mechanism of action was analyzed using network pharmacology and
experimental data (22).
Additionally, Li et al (23)
utilized network pharmacology to predict the immunoregulatory
mechanisms of ginseng leaves in lung cancer. These findings
indicate the potential value of network pharmacology as a strategy
for enhancing the understanding of the underlying mechanisms of TCM
in disease treatment.
Considering the complex interactions between AT and
melanoma cells, to the best of our knowledge, the present study was
the first to utilize network pharmacology and integrate
experimental verification to uncover the underlying therapeutic
mechanisms of AT in treating melanoma.
Materials and methods
Cell culture
B16 cells were obtained from Procell Life Science
& Technology Co., Ltd. and A875 cells were acquired from the
American Type Culture Collection. Both cell types were cultured in
RPMI 1640 medium (MilliporeSigma), supplemented with
penicillin/streptomycin (Corning, Inc.) and 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), at 37°C in a humidified
atmosphere with 5% CO2.
Cell viability assay
B16 and A875 cells in the logarithmic phase were
trypsinized, counted and seeded in 96-well plates at a density of
5×103 cells/well in RPMI 1640 medium containing 10%
fetal bovine serum. To investigate the cytotoxicity of AT (purity
>98%; Chengdu Push Bio-Technology Co., Ltd.) in B16 cells and
A875 cells, cells were cultured in media only (control), AT at 25
µM (AT-low group; AT-L), 50 µM (AT-medium group; AT-M) and 100 µM
(AT-high group; AT-H) were added to the experimental wells for 24 h
at 37°C. After treatment, the original medium was replaced with 100
µl fresh medium containing 10 µl Cell Counting Kit-8 (CCK-8)
reagent (cat. no. C0043; Beyotime Institute of Biotechnology).
Optical density was measured at 450 nm using a microplate reader
(Bio-Rad Laboratories, Inc.) to calculate the cell viability after
a 2 h incubation with CCK-8 in a humidified incubator with 5%
CO2 at 37°C. The assay was repeated thrice.
Colony formation assay
B16 and A875 cells were seeded in 6-well plates at a
density of 2,000 cells/well (Corning, Inc.) and incubated for 10
days. The cells were then fixed with 4% paraformaldehyde for 30 min
at room temperature, stained with crystal violet (Beijing Solarbio
Science & Technology Co., Ltd.) at 25°C for 30 min and washed
thrice with 1X phosphate buffered saline. A cell population
containing >50 cells (24) was
considered a single colony. The number of colonies was counted
using a light microscope (Leica Microsystems GmbH) and evaluated by
ImageJ software (version 1.52m; National Institutes of Health,)
using the ColonyArea plugin (https://imagej.net/plugins/colonyarea). The assay was
performed in parallel using three biological replicates.
Sphere formation assay
To generate spheres, 4×103 B16 and A875
cells, with or without AT treatment, were seeded in 24-well plates
coated with 0.5 mg/ml poly-2-hydroxyethyl methacrylate ethanol
solution (MilliporeSigma) to prevent cell attachment. To further
promote the formation of sphere, cells were cultured in 1 ml RPMI
1640 supplemented with 20 ng/ml epidermal growth factor (Stemcell
Technologies, Inc.), a 1:50 dilution of B27 supplement (Gibco;
Thermo Fisher Scientific, Inc.) and 20 ng/ml recombinant human
basic fibroblast growth factor (Promo Kine; PromoCell GmbH) and
incubated in a humidified 5% CO2 incubator at 37°C for 7
days. Cell density was maintained at 4 cells/µl to prevent cell
aggregation. Spheres (containing >50 cells) were counted through
inverted light microscopy and data were evaluated by ImageJ and the
ImageJ Bio-Formats Plugin (https://www.openmicroscopy.org/bio-formats/). The
experiment was performed thrice to facilitate the statistical
analysis of the data.
Wound healing assay
B16 and A875 cells were seeded into 6-well plates.
Once the cells were at 90% confluency, the cell layer was disturbed
using a sterile 200 µl pipette tip to generate a linear scratch
wound on the cell surface. Subsequently, the cells were cultured in
serum-free RPMI 1640 medium, with images collected at 0 and 24 h.
Wound closure was assessed through a light microscopy and
quantified using ImageJ software. The wound closure was calculated
as the wound healing rate using the following formula: Wound
healing rate (%)= (wound width at 0 h-wound width at 24 h)/wound
width at 0 h ×100%. The assay was performed independently three
times in parallel.
AT target prediction
The Similarity Ensemble Approach (https://sea.bkslab.org/) and Swiss TargetPrediction
(http://www.swisstargetprediction.ch/) databases were
used to predict protein target for screening, with ‘Homo
sapiens’ specified as the restriction condition.
Melanoma target collection
Melanoma-related targets were sourced from various
online medical databases, including GeneCards (https://www.genecards.org/) (25), DrugBank (https://go.drugbank.com/) (26), Online Mendelian Inheritance in Man
(https://www.omim.org/) (27) and Therapeutic Target Database
(https://db.idrblab.net/ttd/) (28). After obtaining melanoma-related
disease targets, duplicates were removed and a database of
disease-target information was created. Subsequently, melanoma
disease targets were compared with AT therapeutic targets to
identify overlapping targets. The significance threshold was set at
-log(p) ≥5.
Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) analyses
The data were obtained using the following
restriction conditions: ‘OFFICIAL-GENE-SYMBOL’, ‘P<0.05’ and
‘Homo sapiens’. GO and KEGG pathway enrichment analyses were
conducted using the DAVID database (29,30).
The results were ranked in descending order based on the degree of
target enrichment. Subsequently, the top 10 processes and pathways
were selected and visualized.
Construction of the target-pathway
network
Intersecting targets between AT and melanoma as well
as notable pathways predicted using the KEGG database (https://www.kegg.jp/) were imported into Cytoscape
3.7.0 (https://cytoscape.org/) to construct the
component-target-pathway network of AT. The importance of AT and
the targets was determined according to Degree ≥5 (31), which indicates the total number of
routes related to a node by other nodes. Increasingly higher degree
values indicate increasing importance.
RNA interference and
overexpression
To knock down phosphatidylinositol 3-kinase (PI3K)
expression, B16 cells were transfected with PI3K-specific small
interfering RNAs (siRNAs; Qiagen, Inc.) using Lipofectamine RNAiMAX
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. AllStars negative control (NC) siRNA
(Qiagen, Inc.) was used as the experimental control. The sequences
of the specific genes used in the present study are as follows:
PI3K (#1) sense strand, 5′-AGAAAACCGCCUUAUGGAGUC-3′ and antisense
strand, 5′-CUCCAUAAGGCGGUUUUCUAU-3′; PI3K (#2) sense strand,
5′-AUAGAAAACCGCCUUAUGGAG-3′ and antisense strand,
5′-CCAUAAGGCGGUUUUCUAUGU-3′; siNC 5′-AATTCTCCGAACGTGTCACGT-3′ and
antisense strand, 5′-UUAAGAGGCTTGCACAGTGCA-3′. For PI3K
overexpression in A875 cells, the cDNA encoding PI3K (NCBI
reference sequence, NM_006218.4) was amplified via PCR and
subcloned into the pcDNA3.1 vector (Invitrogen; Thermo Fisher
Scientific, Inc.) to construct the PI3K overexpression (oe) vector,
with an empty vector serving as a NC. B16 and A875 cells seeded in
6-well plates at a density of 1×106 cells/well were
transfected with the aforementioned siRNAs or vectors
(PI3K-specific siRNAs and siNC, 50 nM; PI3K oe vector and empty
vector, 2 µg) at 37°C. The expression levels of PI3K in transfected
cells were quantified using reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blotting 48 h
post-transfection.
RT-qPCR
Total RNA was isolated from B16 cells and A875 using
TRIzol reagent according to the manufacturer's instructions
(Invitrogen; Thermo Fisher Scientific, Inc.). Reverse Transcription
was performed with the GoScript™ Reverse Transcription kit (Promega
Corporation) according to the manufacturer's instructions. Briefly,
500 ng of total RNA was reverse transcribed in a 20 µl reaction
volume using oligo(dT) primers and M-MLV reverse transcriptase. The
reaction was incubated at 42°C for 60 min, followed by enzyme
inactivation at 70°C for 15 min. qPCR was subsequently performed on
the LightCycler System 2.0 (Roche Diagnostics GmbH) with the
following thermocycling conditions: Initial pre-denaturation for 2
min at 90°C, followed by 40 cycles at 93°C for 10 sec, 60°C for 15
sec and 72°C for 15 sec. Evaluation of the solubility curve was
performed at 95°C for 5 sec and 60°C for 1 min, followed by cooling
at 42°C for 30 sec. The primer sequences for PI3K and β-actin are
listed in Table SI. The expression
level of each gene was analyzed using a SYBR Green kit (Promega
Corporation). Relative quantitation analysis was conducted based on
the 2−ΔΔCq method (32),
with β-actin serving as the internal control. The RT-qPCR assays
were conducted in triplicate.
Western blotting
Briefly, RIPA buffer (Beyotime Institute of
Biotechnology) containing a protease inhibitor cocktail (Roche
Diagnostics GmbH) was used to extract proteins from the cells.
Protein concentrations were determined using a BCA kit (Beyotime
Institute of Biotechnology). Next, all protein samples (20 µg per
lane) were separated using 8% sodium dodecyl-sulfate polyacrylamide
gel electrophoresis and transferred to polyvinylidene difluoride
membranes. Different membranes were used for probing phosphorylated
and non-phosphorylated proteins to ensure optimal blocking and
detection conditions. The membranes were blocked for 2 h at 25°C
using 8% skim milk for β-actin as well as non-phosphorylated
proteins, and 5% bovine serum albumin (Gibco; Thermo Fisher
Scientific, Inc.) for the phosphorylated proteins. The membranes
were then incubated with the corresponding primary antibodies at
4°C overnight, followed by incubation with secondary antibodies for
1 h at 25°C. Finally, the ECL Kit (cat. no. ab65623; Abcam) was
used to visualize the protein bands through an ECL imaging system
(Tanon Science and Technology Co., Ltd.). Band intensity was
semi-quantified using ImageJ. The following antibodies were
provided by Beyotime Institute of Biotechnology: PI3K antibody
(cat. no. AF7749; 1:1,000), Phosphorylated (p)-PI3K (cat. no.
AF5905; 1:1,000), protein kinase B (AKT; cat. no. AA326; 1:1,000),
p-AKT (Ser473; cat. no. AA329; 1:1,000), p-AKT
(Thr308; cat. no. AA331; 1:1,000), mammalian target of
rapamycin (mTOR; cat. no. AF1648; 1:1,000), p-mTOR
(Ser2448; cat. no. AF5869; 1:1,000) and β-actin (cat.
no. AF0003; 1:2,000). p-mTOR antibody (Ser2481; cat. no.
abs130934; 1:1,000) was purchased from Absin Bioscience, Inc. The
HRP-conjugated secondary antibodies were purchased from Abcam (cat.
nos. ab6721 and ab6728; 1:2,000). All western blotting experiments
were repeated thrice.
Statistical analysis
GraphPad Prism (version 8.0.2; Dotmatics) was used
for the data analyses. All data are presented as the mean ±
standard error of the mean. The normality of the data was tested
using the Shapiro-Wilk test, with P<0.05 considered to indicate
normally distributed data. Homogeneity of variance was tested using
Levene's test, with P>0.05 considered to indicate homogenous
data. For data that met normal distribution assumptions and equal
variances, statistical analyses were performed through one-way
analysis of variance and Bonferroni post hoc test for multiple
comparisons. Two-way ANOVA followed by Bonferroni post hoc test was
used for experiments involving multiple independent variables (such
as time and dose-dependent comparisons of the cytotoxicity of AT).
P<0.05 was considered to indicate a statistically significant
difference.
Results
AT inhibits the proliferation,
stemness and migration of melanoma cells
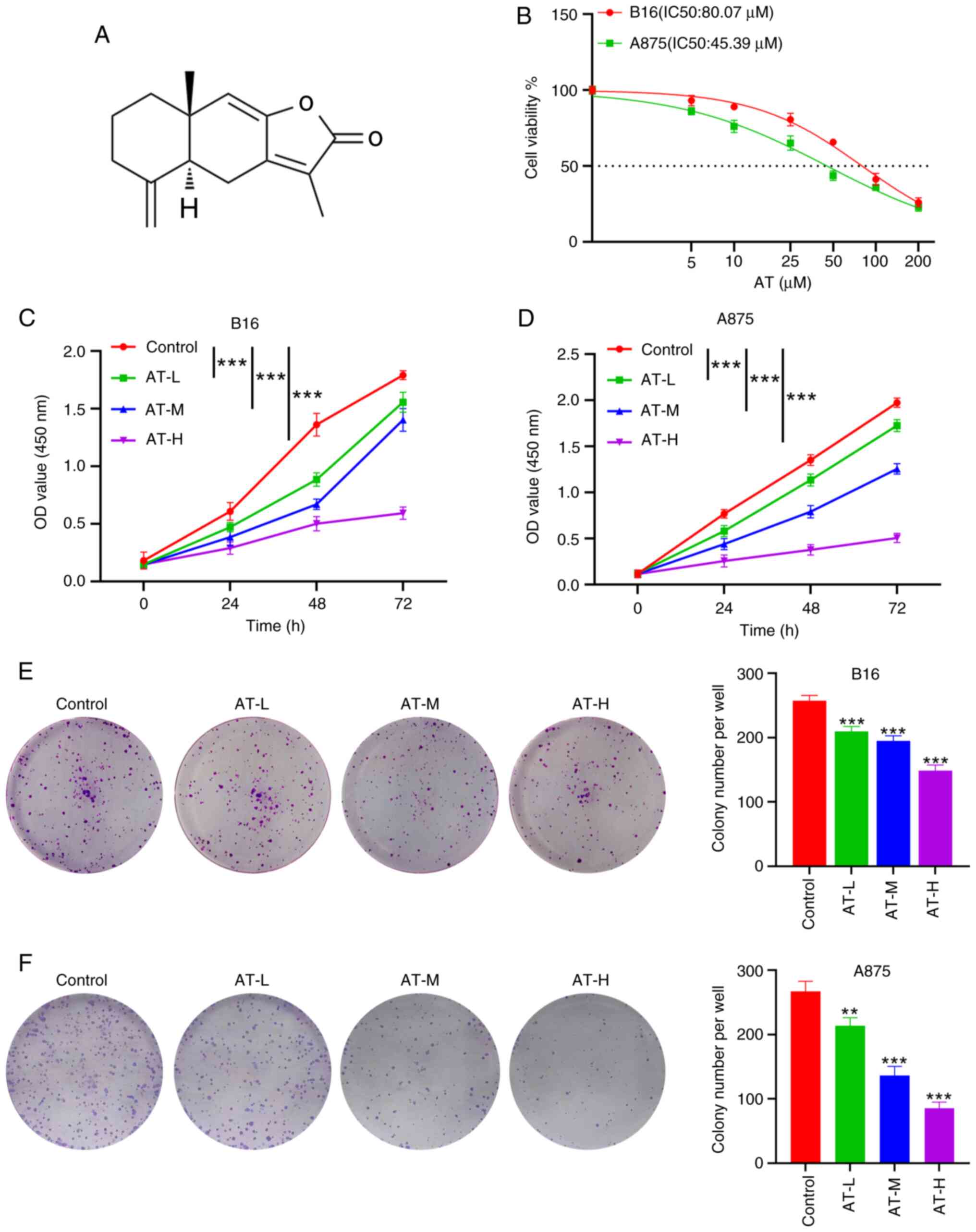
The chemical structure of AT is shown in Fig. 1A. To evaluate the antitumor activity
of AT, B16 and A875 melanoma cells were initially co-cultured with
a series of concentrations of AT (5, 10, 25, 50, 100 and 200 µM)
for 24 h to calculate the half-maximal inhibitory concentration
(IC50) of AT. The AT IC50 values were 80.07
µM for B16 cells and 45.39 µM for A875 cells (Fig. 1B). Next, doses of 25 µM (AT-L), 50
µM (AT-M) and 100 µM (AT-H) were chosen to explore the cytotoxicity
of AT in melanoma cells. After 24, 48 and 72 h of treatment, the
viability of B16 cells was significantly reduced by all three AT
doses in both a time-dependent (P<0.001) and dose-dependent
(P<0.001) manner, as revealed by the CCK-8 assay (Fig. 1C). Similar findings were observed in
A875 cells (both P<0.001; Fig.
1D), supporting the antitumor effect of AT on melanoma cells.
Additionally, a colony formation assay was performed to evaluate
the antiproliferative effect of AT on melanoma cells. Compared with
the colony numbers in the control group, the number of melanoma
cell colonies in the AT-L, AT-M and AT-H groups were significantly
reduced (Fig. 1E and F).
Collectively, these results indicate a suppressive role for AT in
melanoma proliferation.
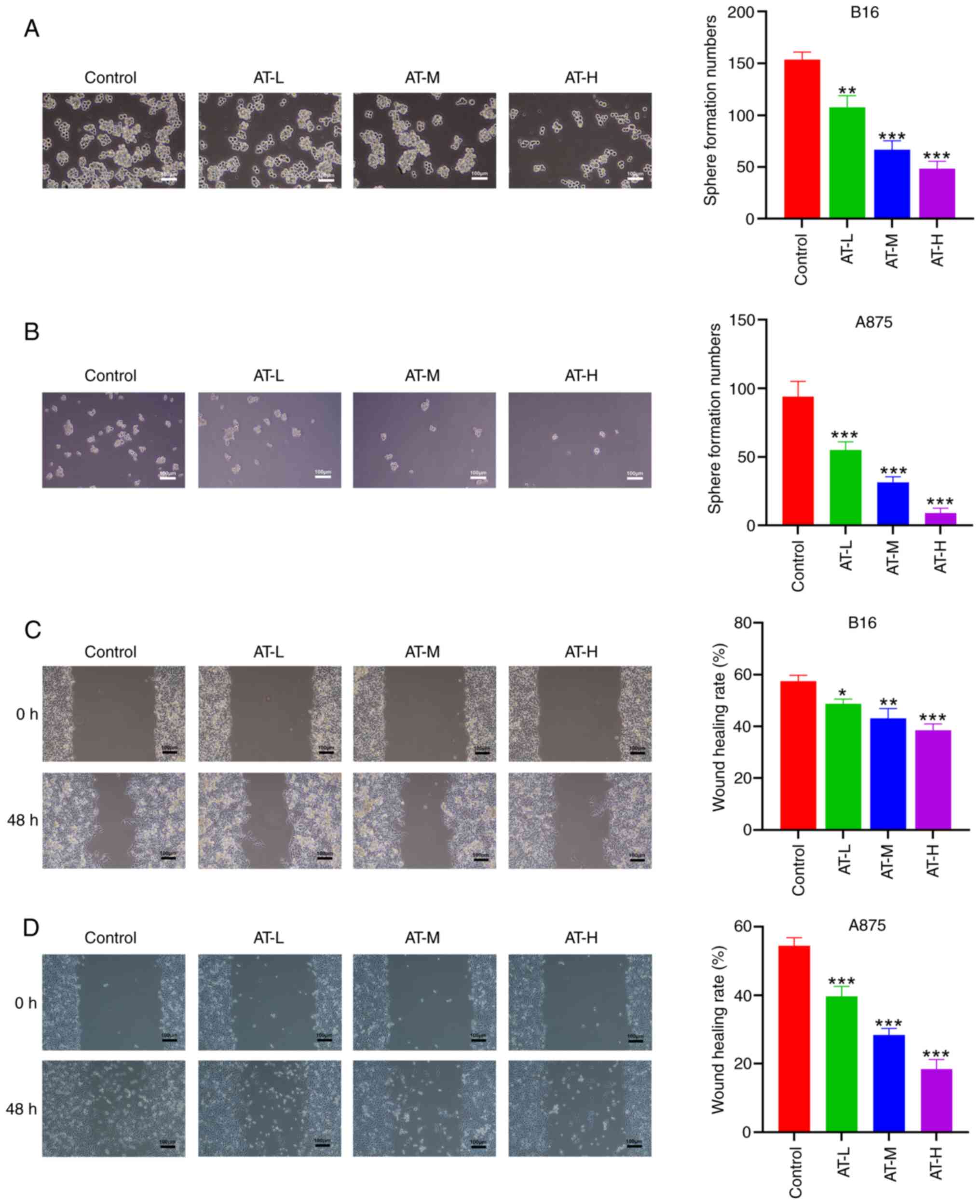
Since cancer stemness plays a crucial role in the
recurrence and metastasis of malignancy (33), the suppressive effect of AT on the
stemness of melanoma cells was examined using a sphere formation
assay. The results indicated a significant decrease in the number
of spheres formed by the B16 and A875 cells after AT treatment
(Fig. 2A and B). The collective
results suggest that AT could inhibit the stemness of melanoma
cells. Additionally, as shown in Fig.
2C and D, the migration of both B16 and A875 cells was also
significantly hindered by AT treatment. The collective data
indicate that AT effectively inhibits the proliferation, stemness
and migration of melanoma cells in a dose-dependent manner.
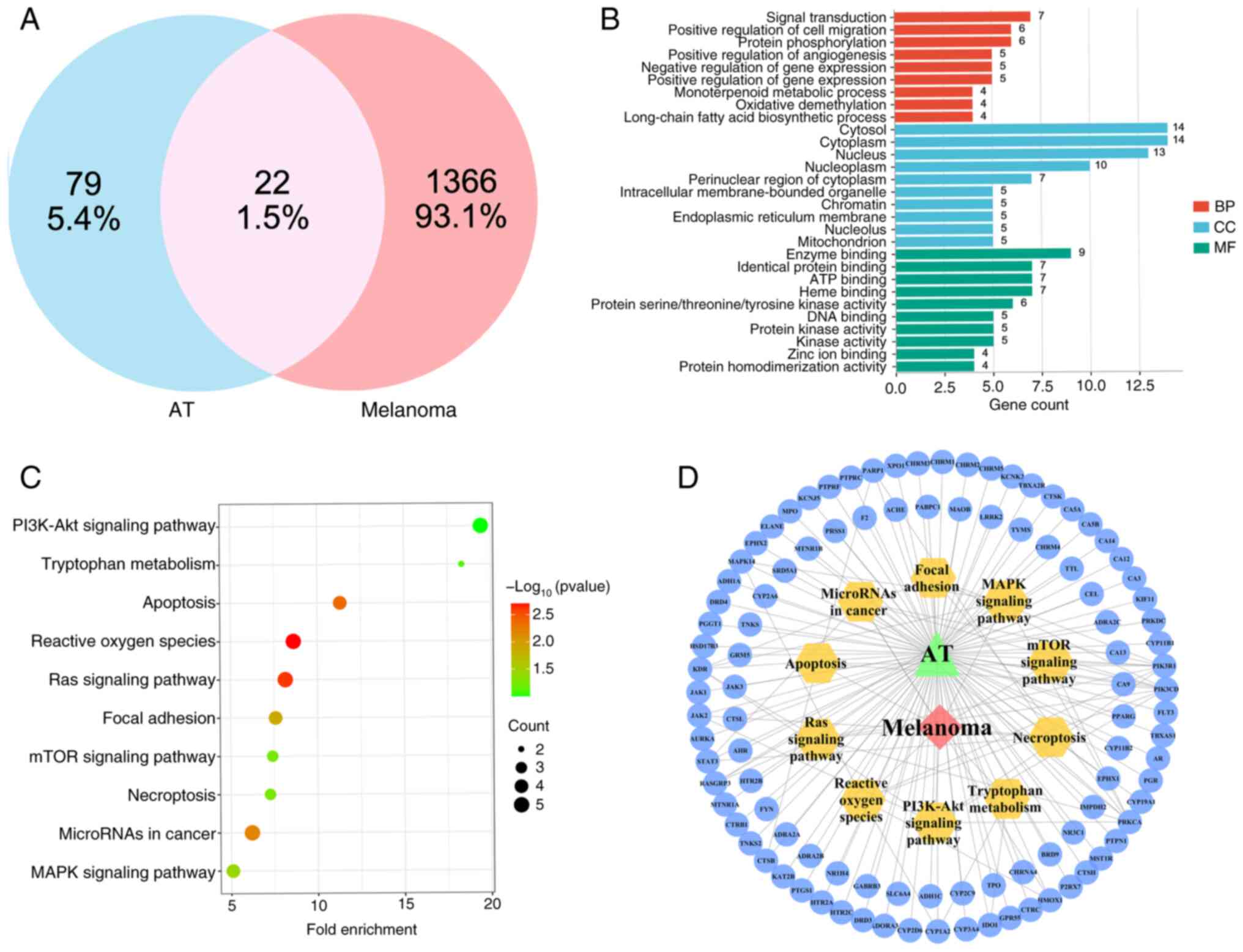
Prediction of the mechanism of action
of AT via network pharmacology
After confirming the antitumor effects of AT, its
mechanism of action was the next focus. However, the therapeutic
targets and interactions between AT and melanoma are complex,
making it difficult to determine the most likely therapeutic
pathway. Network pharmacology was used to predict the main axis and
reveal the potential therapeutic mechanisms of AT in melanoma. A
total of 22 overlapping target genes were identified from the 79 AT
and 1,366 melanoma target genes (Fig.
3A). Subsequently, all intersecting target genes were subjected
to GO and KEGG enrichment analyses using the DAVID database. The
results revealed that 105 biological processes, 16 related cellular
components and 78 molecular functions were involved in the
interactions between AT and melanoma. The pathways associated with
the top 10 intersecting targets were considered for the
construction of a GO enrichment analysis pathway map (Fig. 3B).
KEGG enrichment analysis was used to determine the
signaling pathways associated with the anti-melanoma effects of AT.
KEGG enrichment analysis yielded 62 statistically significant
pathways, and the top ten KEGG enrichment analyses are shown in
Fig. 3C. Among these, the ‘PI3K-Akt
signaling pathway’ stood out as a potential axis in AT treatment,
with a high -log10(P-value) of 9.99×10−2 and
a gene count of 5. Since the mTOR signaling pathway is a well-known
downstream pathway of the PI3K/AKT signaling pathway, the
PI3K/AKT/mTOR axis was chosen for further exploration of the
therapeutic mechanism of AT. Additionally, the PI3K/AKT and mTOR
signaling pathways were selected as crucial pathways due to their
high degree values (8 for the PI3K/AKT axis and 5 for the mTOR
axis) in the target-pathway network (Fig. 3D). Therefore, based on the network
pharmacology results, it is reasonable to consider the
PI3K/AKT/mTOR axis as the mechanism underlying the therapeutic
effects of AT in melanoma.
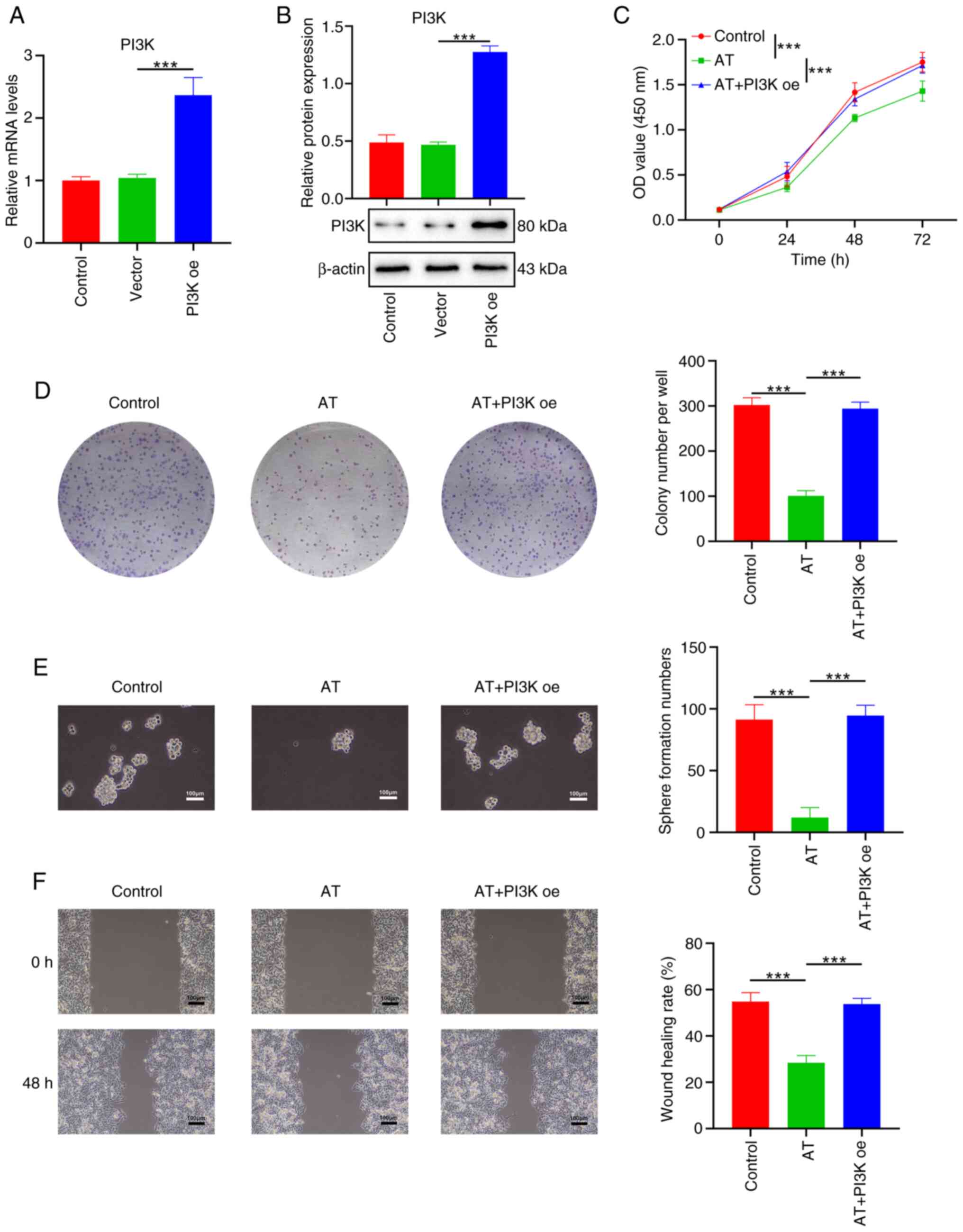
Overexpression of PI3K reverses the
suppressive effects of AT in melanoma cells
The role of PI3K in the suppressive effects of AT on
melanoma cells was explored. Transfection of the PI3K oe vector
successfully overexpressed PI3K in A875 cells, as demonstrated by
RT-qPCR (Fig. 4A) and western
blotting (Fig. 4B). Consistently,
AT treatment significantly suppressed the cell viability,
proliferation, stemness as well as migration of B16 cells (Fig. 4C-F), while the effects of AT were
reversed by overexpressing PI3K (Fig.
4C-F).
To confirm the contribution of the PI3K/AKT/mTOR
pathway to the effects AT of observed, the effects after PI3K
knockdown were studied. The efficiency of PI3K knockdown was
measured at both the RNA and protein levels, and siPI3K#2 was
selected for subsequent experiments based on the results (Fig. S1A and B). As expected, the cell
viability, proliferation, stemness and migration of B16 cells were
significantly reduced after knocking down PI3K expression, which
indicated that PI3K contributes to the malignant phenotypes of B16
cells. Additionally, in the absence of PI3K, AT did not
significantly affect the cell viability, proliferation, stemness
and migration of B16 cells, supporting the hypothesis that the
inhibitory effect of AT on melanoma cells relies on its regulation
of PI3K (Fig. S1C-F). In summary,
PI3K signaling was demonstrated to be a major axis through which AT
inhibits the viability, proliferation, stemness and migration of
melanoma cells.
AT inhibits the PI3K/AKT/mTOR pathway
in melanoma cells
After determining the role of PI3K signaling in AT
activity, the effect of AT on the expression of the PI3K/AKT/mTOR
axis in B16 cells was investigated. As shown in Fig. 5A, AT treatment significantly
decreased the phosphorylation of PI3K and AKT. As a downstream
target of the PI3K/AKT axis, mTOR signaling has been widely
reported to promote proliferation in various cancer types (34). In the present study, the
phosphorylation of mTOR was significantly suppressed by AT
treatment, as shown in Fig. 5B.
These results further imply that the inhibition of the
PI3K/AKT/mTOR axis may be an underlying mechanism of action of AT
in melanoma treatment.
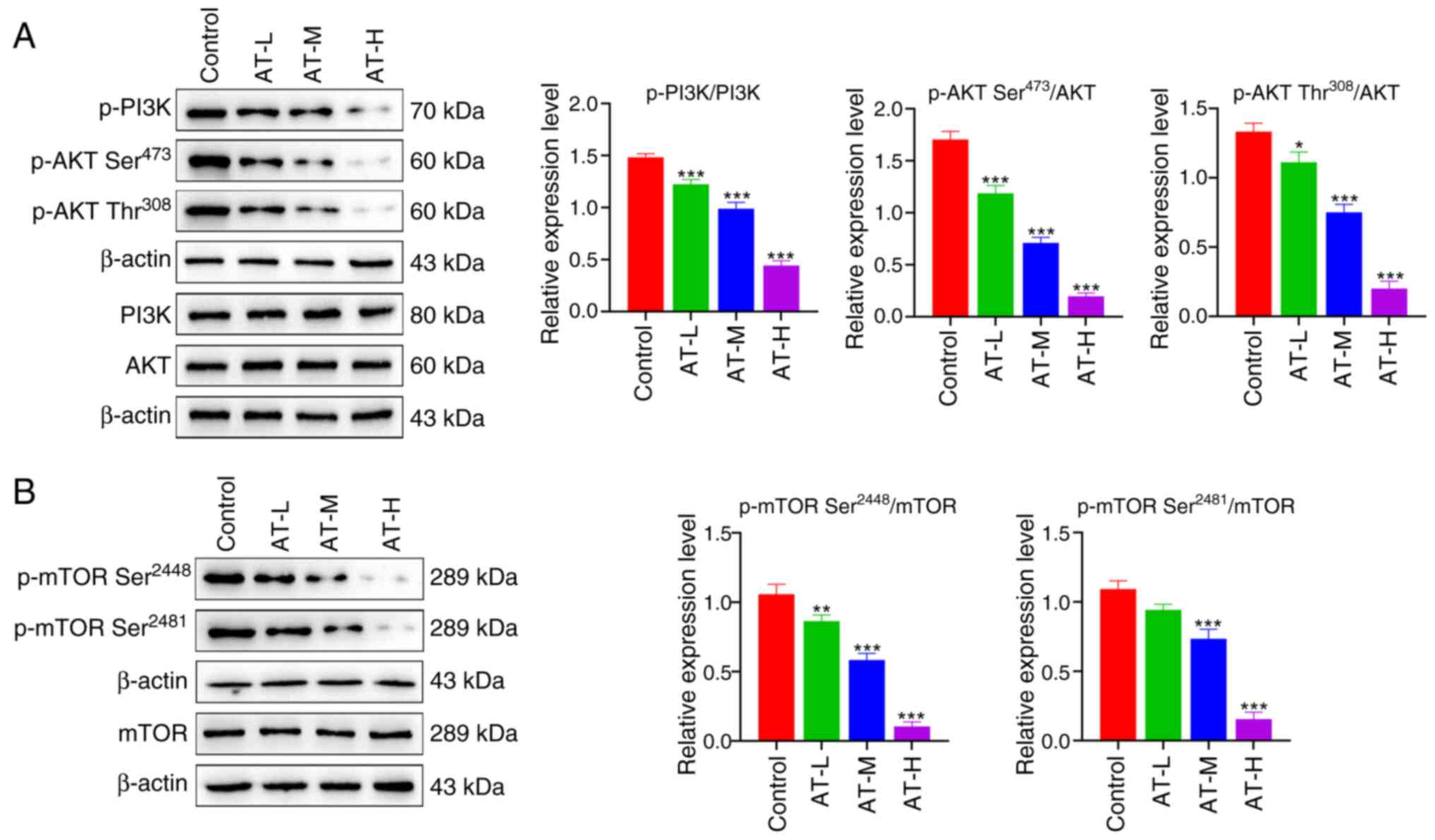 | Figure 5.AT inhibits the PI3K/AKT/mTOR pathway
in melanoma cells. (A) Expression levels of p-PI3K, PI3K, p-AKT
(Ser473), p-AKT (Thr308) and AKT were
measured via western blotting. (B) Expression levels of p-mTOR
(Ser2448), p-mTOR (Ser2481) and mTOR were
measured via western blotting. *P<0.05, **P<0.01,
***P<0.001 vs. the control group. AT, atractylenolide I; AT-L,
AT-low; AT-M, AT-medium; AT-H, AT-high; p-, phosphorylated; PI3K,
phosphatidylinositol 3-kinase; AKT, protein kinase B; mTOR,
mammalian target of rapamycin. |
Discussion
AT has been used to treat splenic and stomach
ailments in Asia for centuries (35,36).
Previous pharmacological studies have reported various activities
of AT, such as antioxidant, anti-inflammatory and anticancer
activities (37–39). Melanoma is one of the most common
skin carcinomas worldwide and affects millions of individuals
annually (40). The results of the
present study demonstrated that AT effectively inhibited the growth
and proliferation of melanoma cells, underscoring its potential for
melanoma treatment.
Since malignant metastasis always occurs in patients
with advanced melanoma and poses a major challenge to clinical
therapy (41), the suppressive
effect of AT on the migration of melanoma cells was investigated in
the present study. AT treatment significantly inhibited cell
migration, suggesting the potential of AT as an adjuvant treatment
to prevent melanoma metastasis. Similar to these results, Fu et
al (42) demonstrated that AT
inhibits the migration of melanoma cells and suggested that the
role of AT may be mediated by the Janus kinase 2 (JAK2)/signal
transducer and activator of transcription 3 (STAT3) pathway.
However, the bioinformatics analysis performed in the present study
suggested the potential role of the PI3K/AKT signaling pathway in
AT activity during melanoma treatment and the JAK2/STAT3 pathway
was not revealed in the analysis. Hence, the JAK2/STAT3 pathway was
not explored further in the present study. Metastatic progression
is affected by various factors, including post-transcriptional
regulation, cancer stemness and epithelial-mesenchymal transition
(EMT). Among these, stemness has been shown to play a role in AT
treatment of melanoma metastasis (43). Cancer stemness is often
characterized by the ability of specific cancer cells to
self-renew, differentiate and regenerate. The induction of stemness
in cancer cells is closely related to the proliferation, migration
and drug resistance of melanoma cells (44,45).
In the present study, AT treatment significantly inhibited the
stemness of B16 cells, which may be a key factor in the therapeutic
effect of AT on melanoma metastasis. EMT is a process characterized
by the loss of epithelial cell markers and upregulation of
mesenchymal cell markers. This process is another critical factor
in cancer cell metastasis (46). Li
et al (47) showed that
fibronectin 1 promotes melanoma proliferation and metastasis
through apoptosis and EMT. Additionally, evidence suggests a
positive crosstalk between stemness and EMT in cancer cells
(48), with EMT transcription
factors playing a role in regulating tumor cell stemness (49). Notably, a previous study
demonstrated the suppression of EMT by AT in prostate cancer cells
(50). Considering the marked
inhibitory effect of AT on the stemness of melanoma cells, EMT may
also be suppressed by AT treatment.
Although the inhibitory effect of AT on cancer
progression has been demonstrated, its specific mechanisms of
action remain unknown. In the present study, the mechanism
underlying the anti-melanoma effects of AT was investigated using
network pharmacology. Based on these data, the PI3K/AKT signaling
pathway was considered the key pathway for AT activity, whereas
mTOR was identified as a notable downstream effector of the PI3K
axis. The crucial role of PI3K/AKT signaling in melanoma
pathophysiology has been emphasized by network pharmacology and
extensive preliminary research (51,52).
Inhibiting the PI3K/AKT/mTOR axis sensitizes melanoma cells to
cisplatin and temozolomide (53).
Previous studies on other diseases have demonstrated a relationship
between AT and the PI3K/AKT/mTOR pathway. For example, AT
effectively inhibits colorectal tumor progression both in
vitro and in vivo, mainly by regulating the AKT/mTOR
signaling pathway (14). In
addition, the antitumor effect of AT in bladder cancer cells
reportedly relies on the inhibition of the PI3K/Akt/mTOR signaling
pathway (54). Moreover, Wang et
al (55) recently demonstrated
that AT suppresses the osteogenic differentiation of human valve
interstitial cells by regulating the PI3K/AKT pathway. Similarly,
the present study confirmed that this axis is essential for AT
activity in melanoma cells, as revealed by PI3K knockdown and
overexpression experiments.
PI3K is an intracellular phosphatidylinositol kinase
that regulates cell survival, growth, proliferation, angiogenesis
and metabolism in human cancer via the PI3K/AKT/mTOR pathway
(56,57). In the present study, the
phosphorylation of PI3K and AKT in B16 cells was significantly
inhibited by AT treatment, suggesting that AT inhibits melanoma
cells through the PI3K signaling pathway. AT has been shown to
effectively inhibit the migration of B16 cells and the
phosphorylation of AKT (58), which
is consistent with the results of the present study. Following
activation, AKT promotes the phosphorylation of tuberous sclerosis
complex 2, which then activates the mammalian target of rapamycin
complex 1 (mTORC1). AKT directly activates mTORC1 by
phosphorylating Ser2448 (59). In
the present study, mTOR phosphorylation at different sites (Ser
2448 and Ser2481) was significantly downregulated by AT
treatment.
Taken together, the results of the present study
confirm the anti-melanoma effects of AT. Additionally, the
PI3K/AKT/mTOR axis was identified as an underlying mechanism of
action of AT, as revealed by network pharmacology predictions and
experimental validation. The significant suppressive effect of AT
on melanoma suggests its potential as a novel drug for melanoma
treatment. AT has several advantages that may facilitate its future
use. First, clinical evidence suggests that AT is safe and exhibits
low toxicity without serious adverse reactions (60). Second, a previous study reported
that AT sensitizes human ovarian cancer cells to paclitaxel,
indicating that the combination of AT with current chemotherapies
may overcome drug resistance (61).
Thus, AT is a valuable natural component that may play a role in
the treatment of melanoma, either individually or in
combination with other chemotherapeutic agents.
However, the present study has some limitations.
Since all experiments were conducted in vitro, robust animal
models are needed to verify the anti-melanoma properties and
underlying mechanisms of action of AT. The PI3K family consists of
three classes of phosphatidylinositol kinases (I/II/III);
therefore, the regulatory role of AT should be thoroughly explored
by further investigating which phosphatidylinositol kinases AT
interacts with. Although the suppressive effect of AT on PI3K
expression has been confirmed, further investigation is required to
understand the interaction between AT and PI3K. Further detailed
research on the regulation of the PI3K/AKT/mTOR axis by AT is
required.
In conclusion, the present study demonstrated the
anti-melanoma activity of AT and identified the PI3K/AKT/mTOR axis
as a key mechanism underlying its therapeutic action, as confirmed
through network pharmacology and experimental validation.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XCX and PK designed the experiments. PK and HC
performed the experiments and analyzed the results. PK and HC
confirm the authenticity of all the raw data. XCX wrote and revised
the manuscript. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huang AC and Zappasodi R: A decade of
checkpoint blockade immunotherapy in melanoma: Understanding the
molecular basis for immune sensitivity and resistance. Nat Immunol.
23:660–670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schadendorf D, van Akkooi ACJ, Berking C,
Griewank KG, Gutzmer R, Hauschild A, Stang A, Roesch A and Ugurel
S: Melanoma. Lancet. 392:971–984. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eddy K and Chen S: Overcoming immune
evasion in melanoma. Int J Mol Sci. 21:89842020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rubin KM, Vona K, Madden K, McGettigan S
and Braun IM: Side effects in melanoma patients receiving adjuvant
interferon alfa-2b therapy: A nurse's perspective. Support Care
Cancer. 20:1601–1611. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hauschild A, Gogas H, Tarhini A, Middleton
MR, Testori A, Dréno B and Kirkwood JM: Practical guidelines for
the management of interferon-alpha-2b side effects in patients
receiving adjuvant treatment for melanoma: Expert opinion. Cancer.
112:982–994. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hersey P, Tiffen JC and Gallagher SJ:
Shedding light on dabrafenib-induced fevers in patients with
melanoma. Lancet Oncol. 20:1637–1638. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sloot S, Fedorenko IV, Smalley KS and
Gibney GT: Long-term effects of BRAF inhibitors in melanoma
treatment: Friend or foe? Expert Opin Pharmacother. 15:589–592.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu X, Li M, Wang X, Dang Z, Yu L, Wang X,
Jiang Y and Yang Z: Effects of adjuvant traditional Chinese
medicine therapy on long-term survival in patients with
hepatocellular carcinoma. Phytomedicine. 62:1529302019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang R, Sun Q, Wang F, Liu Y, Li X, Chen
T, Wu X, Tang H, Zhou M, Zhang S, et al: Efficacy and safety of
Chinese herbal medicine on ovarian cancer after reduction surgery
and adjuvant chemotherapy: A systematic review and meta-analysis.
Front Oncol. 9:7302019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Y, Wang M, Wang Z, Qiu J, Wang Y, Li
J, Dong F, Huang X, Zhao J and Xu T: Polysaccharides from hawthorn
fruit alleviate high-fat diet-induced NAFLD in mice by improving
gut microbiota dysbiosis and hepatic metabolic disorder.
Phytomedicine. 139:1564582025. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoang LS, Tran MH, Lee JS, Ngo QM, Woo MH
and Min BS: Inflammatory inhibitory activity of sesquiterpenoids
from atractylodes macrocephala Rhizomes. Chem Pharm Bull (Tokyo).
64:507–511. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shu YT, Kao KT and Weng CS: In vitro
antibacterial and cytotoxic activities of plasma-modified
polyethylene terephthalate nonwoven dressing with aqueous extract
of Rhizome Atractylodes macrocephala. Mater Sci Eng C Mater Biol
Appl. 77:606–612. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kou N, Cho H, Kim HE, Sun Q, Ahn K, Ji H,
Choi H and Kim O: Anti-cancer effect of Atractylodes macrocephala
extract by double induction of apoptotic and autophagic cell death
in head and neck cancer cells. Bangladesh J Pharmacol. 12:140–146.
2017. View Article : Google Scholar
|
|
14
|
Wang K, Huang W, Sang X, Wu X, Shan Q,
Tang D, Xu X and Cao G: Atractylenolide I inhibits colorectal
cancer cell proliferation by affecting metabolism and stemness via
AKT/mTOR signaling. Phytomedicine. 68:1531912020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Long F, Lin H, Zhang X, Zhang J, Xiao H
and Wang T: Atractylenolide-I suppresses tumorigenesis of breast
cancer by inhibiting toll-like receptor 4-mediated nuclear
factor-κB signaling pathway. Front Pharmacol. 11:5989392020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Wang Y, Liu Z, Guo X, Miao Z and Ma
S: Atractylenolide I induces apoptosis and suppresses glycolysis by
blocking the JAK2/STAT3 signaling pathway in colorectal cancer
cells. Front Pharmacol. 11:2732020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye Y, Chao XJ, Wu JF, Cheng BC, Su T, Fu
XQ, Li T, Guo H, Tse AK, Kwan HY, et al: ERK/GSK3β signaling is
involved in atractylenolide I-induced apoptosis and cell cycle
arrest in melanoma cells. Oncol Rep. 34:1543–1548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu H, Van der Jeught K, Zhou Z, Zhang L,
Yu T, Sun Y, Li Y, Wan C, So KM, Liu D, et al: Atractylenolide I
enhances responsiveness to immune checkpoint blockade therapy by
activating tumor antigen presentation. J Clin Invest.
131:e1468322021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khanal P and Patil BM: Integration of
network and experimental pharmacology to decipher the antidiabetic
action of Duranta repens L. J Integr Med. 19:66–77. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng P, Su HF, Ye CY, Qiu SW, Shi A, Wang
JZ, Zhou XW and Tian Q: A tau pathogenesis-based network
pharmacology approach for exploring the protections of Chuanxiong
Rhizoma in Alzheimer's disease. Front Pharmacol. 13:8778062022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nogales C, Mamdouh ZM, List M, Kiel C,
Casas AI and Schmidt H: Network pharmacology: Curing causal
mechanisms instead of treating symptoms. Trends Pharmacol Sci.
43:136–150. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao RL and He YM: Network pharmacology
analysis of the anti-cancer pharmacological mechanisms of Ganoderma
lucidum extract with experimental support using Hepa1-6-bearing C57
BL/6 mice. J Ethnopharmacol. 210:287–295. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li ZH, Yu D, Huang NN, Wu JK, Du XW and
Wang XJ: Immunoregulatory mechanism studies of ginseng leaves on
lung cancer based on network pharmacology and molecular docking.
Sci Rep. 11:182012021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen B, Liu C, Long H, Bai G, Zhu Y and Xu
H: N6-methyladenosine-induced long non-coding RNA PVT1
regulates the miR-27b-3p/BLM axis to promote prostate cancer
progression. Int J Oncol. 62:162023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Safran M, Rosen N, Twik M, BarShir R,
Stein TI, Dahary D, Fishilevich S and Lancet D: The GeneCards
suite. Practical Guide to Life Science Databases. Abugessaisa I and
Kasukawa T: Springer Nature Singapore; Singapore: pp. 27–56. 2021,
View Article : Google Scholar
|
|
26
|
Wishart DS, Feunang YD, Guo AC, Lo EJ,
Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, et al:
DrugBank 5.0: A major update to the DrugBank database for 2018.
Nucleic Acids Res. 46:D1074–D1082. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hamosh A, Amberger JS, Bocchini C, Scott
AF and Rasmussen SA: Online mendelian inheritance in man
(OMIM®): Victor McKusick's magnum opus. Am J Med Genet
A. 185:3259–3265. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou Y, Zhang Y, Zhao D, Yu X, Shen X,
Zhou Y, Wang S, Qiu Y, Chen Y and Zhu F: TTD: Therapeutic target
database describing target druggability information. Nucleic Acids
Res. 52:D1465–D1477. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang DW, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang DW, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tianyu Z and Liying G: Identifying the
molecular targets and mechanisms of xuebijing injection for the
treatment of COVID-19 via network parmacology and molecular
docking. Bioengineered. 12:2274–2287. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen P, Hsu WH, Han J, Xia Y and DePinho
RA: Cancer stemness meets immunity: From mechanism to therapy. Cell
Rep. 34:1085972021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun SY: mTOR-targeted cancer therapy:
Great target but disappointing clinical outcomes, why? Front Med.
15:221–231. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song HP, Li RL, Zhou C, Cai X and Huang
HY: Atractylodes macrocephala Koidz stimulates intestinal
epithelial cell migration through a polyamine dependent mechanism.
J Ethnopharmacol. 159:23–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song HP, Hou XQ, Li RY, Yu R, Li X, Zhou
SN, Huang HY, Cai X and Zhou C: Atractylenolide I stimulates
intestinal epithelial repair through polyamine-mediated Ca(2+)
signaling pathway. Phytomedicine. 28:27–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu M, Wang RB, Xing JH and Tang YX:
Atractylenolide inhibits apoptosis and oxidative stress of
HTR-8/SVneo cells by activating MAPK/ERK signalling in
preeclampsia. Phytomedicine. 93:1537732021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Du Z, Ma Z, Lai S, Ding Q, Hu Z, Yang W,
Qian Q, Zhu L, Dou X and Li S: Atractylenolide I ameliorates
acetaminophen-induced acute liver injury via the TLR4/MAPKs/NF-κB
signaling pathways. Front Pharmacol. 13:7974992022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fan M, Gu X, Zhang W, Shen Q, Zhang R,
Fang Q, Wang Y, Guo X, Zhang X and Liu X: Atractylenolide I
ameliorates cancer cachexia through inhibiting biogenesis of IL-6
and tumour-derived extracellular vesicles. J Cachexia Sarcopenia
Muscle. 13:2724–2739. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Waseh S and Lee JB: Advances in melanoma:
Epidemiology, diagnosis, and prognosis. Front Med (Lausanne).
10:12684792023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kibbi N, Kluger H and Choi JN: Melanoma:
Clinical presentations. Cancer Treat Res. 167:107–129. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fu XQ, Chou JY, Li T, Zhu PL, Li JK, Yin
CL, Su T, Guo H, Lee KW, Hossen MJ, et al: The JAK2/STAT3 pathway
is involved in the anti-melanoma effects of atractylenolide I. Exp
Dermatol. 27:201–204. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Castaneda M, den Hollander P, Kuburich NA,
Rosen JM and Mani SA: Mechanisms of cancer metastasis. Semin Cancer
Biol. 87:17–31. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wei CY, Zhu MX, Yang YW, Zhang PF, Yang X,
Peng R, Gao C, Lu JC, Wang L, Deng XY, et al: Downregulation of
RNF128 activates Wnt/βMani-catenin signaling to induce cellular EMT
and stemness via CD44 and CTTN ubiquitination in melanoma. J
Hematol Oncol. 12:212019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gong Q, Yu H, Ding G, Ma J, Wang Y and
Cheng X: Suppression of stemness and enhancement of
chemosensibility in the resistant melanoma were induced by
Astragalus polysaccharide through PD-L1 downregulation. Eur J
Pharmacol. 916:1747262022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li B, Shen W, Peng H, Li Y, Chen F, Zheng
L, Xu J and Jia L: Fibronectin 1 promotes melanoma proliferation
and metastasis by inhibiting apoptosis and regulating EMT. Onco
Targets Ther. 12:3207–3221. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pan G, Liu Y, Shang L, Zhou F and Yang S:
EMT-associated microRNAs and their roles in cancer stemness and
drug resistance. Cancer Commun (Lond). 41:199–217. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Battula VL, Evans KW, Hollier BG, Shi Y,
Marini FC, Ayyanan A, Wang RY, Brisken C, Guerra R and Andreeff M
SA: Epithelial-mesenchymal transition-derived cells exhibit
multilineage differentiation potential similar to mesenchymal stem
cells. Stem Cells. 28:1435–1445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Qiao P and Tian Z: Atractylenolide I
inhibits EMT and enhances the antitumor effect of cabozantinib in
prostate cancer via targeting Hsp27. Front Oncol. 12:10848842022.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Smalley KS: Understanding melanoma
signaling networks as the basis for molecular targeted therapy. J
Invest Dermatol. 130:28–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang Y, Chen SJ, Ma T, Long Q, Chen L, Xu
KX and Cao Y: Promotion of apoptosis in melanoma cells by taxifolin
through the PI3K/AKT signaling pathway: Screening of natural
products using WGCNA and CMAP platforms. Int Immunopharmacol.
138:1125172024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sinnberg T, Lasithiotakis K, Niessner H,
Schittek B, Flaherty KT, Kulms D, Maczey E, Campos M, Gogel J,
Garbe C and Meier F: Inhibition of PI3K-AKT-mTOR signaling
sensitizes melanoma cells to cisplatin and temozolomide. J Invest
Dermatol. 129:1500–1515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yu R, Yu BX, Chen JF, Lv XY, Yan ZJ, Cheng
Y and Ma Q: Anti-tumor effects of Atractylenolide I on bladder
cancer cells. J Exp Clin Cancer Res. 35:402016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang J, Zhang P, Zhang J, Ma Z, Tian X,
Liu Y, Lv G and Qu L: Atractylenolide-1 targets FLT3 to regulate
PI3K/AKT/HIF1-α pathway to inhibit osteogenic differentiation of
human valve interstitial cells. Front Pharmacol. 13:8997752022.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Akinleye A, Avvaru P, Furqan M, Song Y and
Liu D: Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer
therapeutics. J Hematol Oncol. 6:882013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Koyasu S: The role of PI3K in immune
cells. Nat Immunol. 4:313–319. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yan Y, Chou GX, Hui W, Chu JH, Fong WF and
Yu ZL: Effects of sesquiterpenes isolated from largehead
atractylodes rhizome on growth, migration, and differentiation of
B16 melanoma cells. Integr Cancer Ther. 10:92–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu Y, Jia Z, Dong L, Wang R and Qiu G: A
randomized pilot study of atractylenolide I on gastric cancer
cachexia patients. Evid Based Complement Alternat Med. 5:337–344.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Huang JM, Zhang GN, Shi Y, Zha X, Zhu Y,
Wang MM, Lin Q, Wang W, Lu HY, Ma SQ, et al: Atractylenolide-I
sensitizes human ovarian cancer cells to paclitaxel by blocking
activation of TLR4/MyD88-dependent pathway. Sci Rep. 4:38402014.
View Article : Google Scholar : PubMed/NCBI
|