Introduction
Chronic myeloid leukaemia (CML) is a neoplastic
disease of hematopoietic stem cells (1). Reports from European CML registries
show incidence rates ranging from 0,7-1/100.000 persons per year.
The incidence rises with age, and men are more likely to develop
CML than women. Both sexes have an incidence peak of between 57–60
years of age (2). However, notably,
the disease burden of CML has decreased globally in the past 3
decades in countries with high social-demographic indices.
Programmes to control modifiable risk factors such as smoking and
high body mass index may be helpful in further diminishing the
mortality of this disease (3).
The majority of patients with CML harbour the
pathognomonic BCR-ABL gene. This specific cytogenic aberration is
distinguished by a reciprocal translocation of chromosomes 9 and
22. The BCR-ABL gene is formed on chromosome 22, when a subunit of
chromosome 9 attaches, and this newly formed chromosome 22 is
called the ‘Philadelphia Chromosome’ (4).
CML is a triphasic disease comprising a chronic
phase, an accelerated phase and, finally, the terminal blast
crisis. Most patients are diagnosed and start their treatment in
the chronic phase (5).
Imatinib is a tyrosine kinase inhibitor (TKI), which
was approved by the United States Food and Drug Administration in
2001 for the first-line treatment of BCR-ABL-positive CML, and it
is still one of the preferred choices of treatment agents. However,
if a patient does not respond to imatinib, second- and
third-generation TKIs can be applied. A curative treatment approach
is allogeneic stem cell transplantation (6). Moreover, the goal of treatment in CML
is to achieve a complete remission under TKI application. The
endpoints for treatment efficacy are either the normalization of
blood cell count, loss of the Philadelphia Chromosome, or a
specific decrease in BCR-ABL gene expression, which are classified
as complete hematologic response, complete cytogenetic response and
complete molecular response (CMR), respectively (7).
Imatinib is the first approved TKI in this
indication and still represents one of the standard first-line
therapies, regardless of age (6).
Older patients are often underrepresented in clinical studies due
to stringent inclusion and exclusion criteria of co-morbidities;
however, they comprise a high percentage of haemato-oncological
patients in real-world settings. Therefore, the present study aimed
to determine to what extent older patients with CML differ from
younger patients in terms of response and tolerability of
imatinib.
Patients and methods
Study design and population
The present study had a single-centre retrospective
design. Patients diagnosed with BCR-ABL positive CML and treated
with imatinib between January 2011 and December 2021 at the
University Hospital Krems (Krems, Austria) were included in the
analysis. Patients with a first-line therapy other than imatinib,
were aged <18 years of age or were diagnosed other cancer types
were excluded.
Included patients were divided into 2 groups:
Patients aged ≥60 years and patients aged <60 years at the onset
of treatment with imatinib. The United Nations define ‘older’
people as those aged ≥60 years
(emergency.unhcr.org/protection/persons-risk/older-persons;
accessed April 24, 2025); therefore, this age limit was chosen.
The present study was approved by the Institutional
Review Board and the Ethics Committee of the Lower Austria federal
state (approval no. GS4-EK-4/825-2022), which is the legal entity
of the ‘Niederösterreichische Landesgesundheitsagentur’ that
operates all hospitals in Lower Austria, including the University
Hospital Krems. Furthermore, the present was performed according to
the Declaration of Helsinki. Due to the retrospective nature of the
study, informed consent was waived by the Commission for Scientific
Integrity and Ethics at the Karl Landsteiner University of Health
Sciences.
Data collection
Patients were identified via the International
Classification of Diseases-10 Code C92.1. Data were taken from the
electronic records of the routine medical visits of patients.
Information was stored either at the data management system
‘Medical Process Administrator’ implemented at the University
Hospital Krems or the ‘Oncology Information System’ of Lower
Austria. The information on remission rates was obtained from case
records. The CMR was used to obtain information about remission
rate, duration and depth.
Data on remission depth was acquired by reviewing
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) tests detecting the BCR-ABL1 gene. These tests were
performed by the commercial provider Labdia Labordiagnostik GmbH.
The specific forward and reverse primer sequences, including that
of the normalization control, used for RT-qPCR were not disclosed
by the company. CMR was defined as CMR 3, CMR 4 or CMR 4,5: CMR 3,
also known as major molecular response, was defined as ≤0.1%
BCR-ABL1 international scale (IS) or ≥3.0 log reduction; CMR 4 was
defined as ≤0.01% BCR-ABL1 IS or ≥4.0 log reduction; and CMR 4,5
was defined as ≤0.0032% BCR-ABL1 IS or ≥4.5 log reduction. The
limit for a detectable BCR-ABL1 gene level was set to 0.000319.
Variables under consideration were stage at
diagnosis, remission rate, remission duration, remission depth,
relapse, duration of medication, side effects and survival.
Statistical analysis
The following patient characteristics were analysed
descriptively: Age; sex; stage at initial diagnosis; lactate
dehydrogenase (LDH) level at diagnosis; LDH at the start of
imatinib therapy; spleen size at the start of imatinib therapy;
number of leukocytes at diagnosis; and number of leukocytes at the
start of imatinib therapy. The parameter spleen size was divided
into 2 groups: Normal spleen, <12 cm in length; and enlarged
spleen, ≥12 cm in length.
The normality of the two persistent variables were
assessed using the Shapiro-Wilk test, and for group comparisons,
the Mann-Whitney test was used. Categorical variables were assessed
using the χ2 test or Fisher's exact test. Overall
survival (OS) and progression-free survival (PFS) were assessed
using Kaplan-Meier curves and evaluated using the log-rank test. An
OS event was defined by death, whereas PFS was defined as
progression of disease or death from any cause.
The data were analysed according to an intent to
treat approach: Each patient that received first-line therapy with
an imatinib regimen was eligible.
All statistical analyses were performed using SPSS
v26 (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patients' characteristics
The present retrospective single-centre analysis
included patients with BCR-ABL-positive CML who were treated with
imatinib between January 2011 and December 2021 at the University
Hospital Krems. A total of 22 patients were included, of whom 9
were aged ≥60 years (‘older’ group) and 13 were aged <60 years
(‘younger’ group). Moreover, 5/22 patients were female. All
patients were diagnosed in a chronic state of CML. The median age
was 44 years in the younger group and 73 years in the older group.
The median LDH level at diagnosis was 457 U/l in the younger group
and 413 U/l in the older group (normal range, 20–250 U/l). The
median LDH level at the start of imatinib therapy was lower, with a
median value of 428 U/l in the younger group and 385 U/l in the
older group. The LDH levels ranged from 204–1,027 U/l at diagnosis
and 168–934 U/l at the start of imatinib therapy. The median white
blood cell (WBC) level at diagnosis was 61.45 G/l in the younger
group and 38.51 G/l in the older group (normal laboratory reference
value, 3.90–10.20 G/l). By contrast, the median WBC level at the
start of imatinib therapy was 51.69 G/l in the younger group and
30.25 G/l in the older group. Overall, the WBC level at diagnosis
ranged from 12.73–421.94 G/l and 11.08–156.55 G/l at the start of
imatinib therapy (Table I).
 | Table I.Patient characteristics (n=22). |
Table I.
Patient characteristics (n=22).
|
| Age group |
|---|
|
|
|
|---|
| Parameter | <60 years | ≥60 years |
|---|
| Sex |
|
|
| Male | 9 (69) | 8 (89) |
|
Female | 4 (31) | 1 (11) |
| Age, years | 44 (23, 57) | 73 (60, 86) |
| Diagnosis in chronic
stage | 13 (100) | 9 (100) |
| LDH, U/l |
|
|
| At
diagnosis | 457 (237, 1,027) | 413 (204, 878) |
| At start
of imatinib therapy | 428 (243, 934) | 385 (168, 642) |
| WBC, G/l |
|
|
| At
diagnosis | 61.45 (14.77,
421.94) | 38.51 (12.73,
195.47) |
| At start
of imatinib therapy | 51.69 (14.77,
156.55) | 30.25 (11.08,
56.89) |
| Spleen size |
|
|
|
Normal | 2 (15.4) | 7 (55.6) |
|
Enlarged | 10 (76.9) | 2 (22.2) |
| Missing
data | 1 (7.7) | 1 (11.1) |
| Splenectomy performed
prior to CML diagnosis | 0 (0) | 1 (11.1) |
Spleen size was categorized into 2 groups: Normal
and enlarged. In the younger group, 10 patients had enlarged
spleens, two had normal-sized spleens and one patient had no record
of spleen size. No splenectomies were reported in this group. A
total of 5 patients had normal-sized spleens in the older group,
two had enlarged spleens and one patient had no record of spleen
size. In this group, one patient underwent a splenectomy prior to
CML diagnosis (Table I).
Treatment discontinuation due to
progression or side effects
Overall, 5/22 patients (22.7%) had to end imatinib
therapy due to disease progression. Moreover, 4/22 patients (18,2%)
ended imatinib therapy due to side effects during treatment,
comprising severe nausea, joint pain, bloating, lack of
concentration, fatigue, exanthema, oedema, pruritus, muscle
cramping and wound healing disorder.
In the younger group, 3/13 patients (23.1%) had to
end imatinib therapy due to progression and 1/13 (7.7%) ended the
therapy due to side effects. In the older group, 2/9 patients
(22.2%) had to end imatinib therapy due to progression and 3/9
(33.3%) due to side effects. Furthermore, 1/9 (11.1%) patients in
the older group died; however, this was not associated with
CML.
Remission rates
Overall, 13/22 patients (59.1%) went into remission,
of which eight were aged <60 years (61.5%) and five were aged
≥60 years old (55.6%) (Table II).
No significant difference in the remission rates between the
different age groups was demonstrated.
 | Table II.Remission rate. |
Table II.
Remission rate.
|
| Remission (%) |
|
|---|
|
|
|
|
|---|
| Group | No | Yes | Total |
|---|
| <60 years | 5 (38.5) | 8 (61.5) | 13 |
| ≥60 years | 4 (44.4) | 5 (55.6) | 9 |
| Total | 9 (41.0) | 13 (59.0) | 22 |
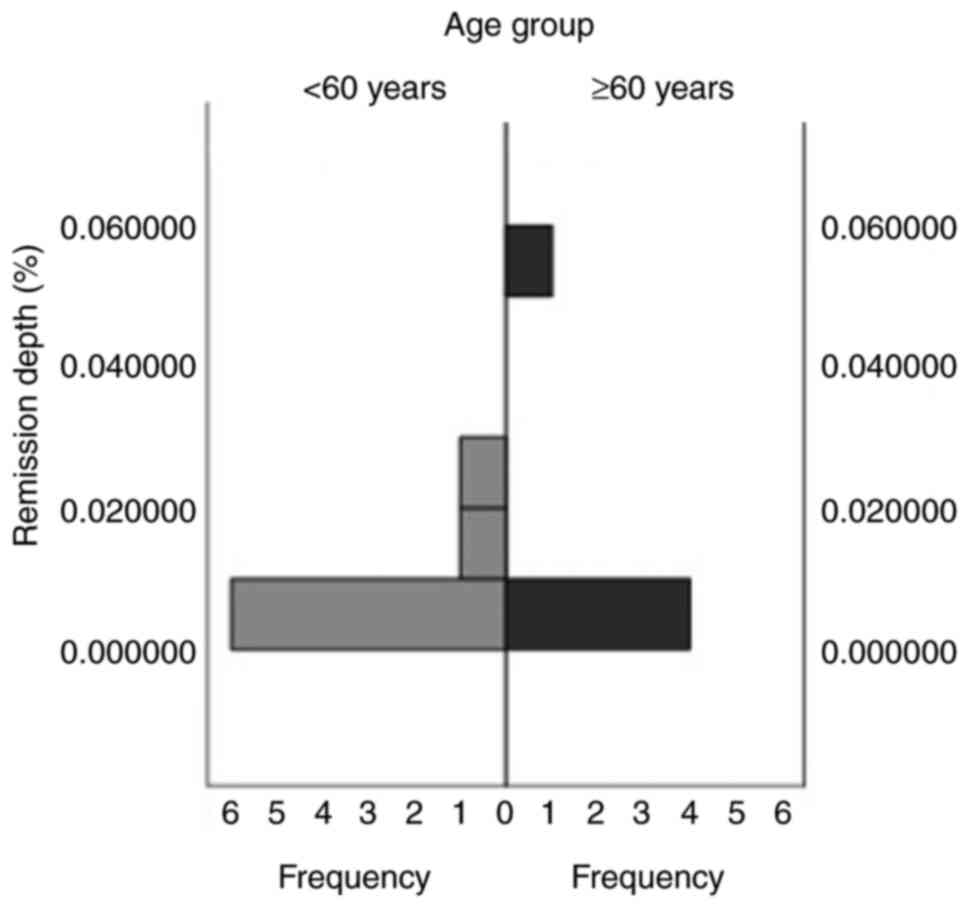
Remission depth
A total of 61.5% of patients went into remission in
the younger group, compared with 55.6% in the older group. A total
of 6 patients in the younger group (46.2%) and 3 in the older group
(33.3%) achieved CMR 4,5 (Fig. 1).
In both groups, one patient achieved CMR 4 (younger group, 7.7%;
older group, 11.1%). Moreover, one patient achieved CMR 3 in both
groups. No significant difference was demonstrated between the two
age groups with regard to remission depth.
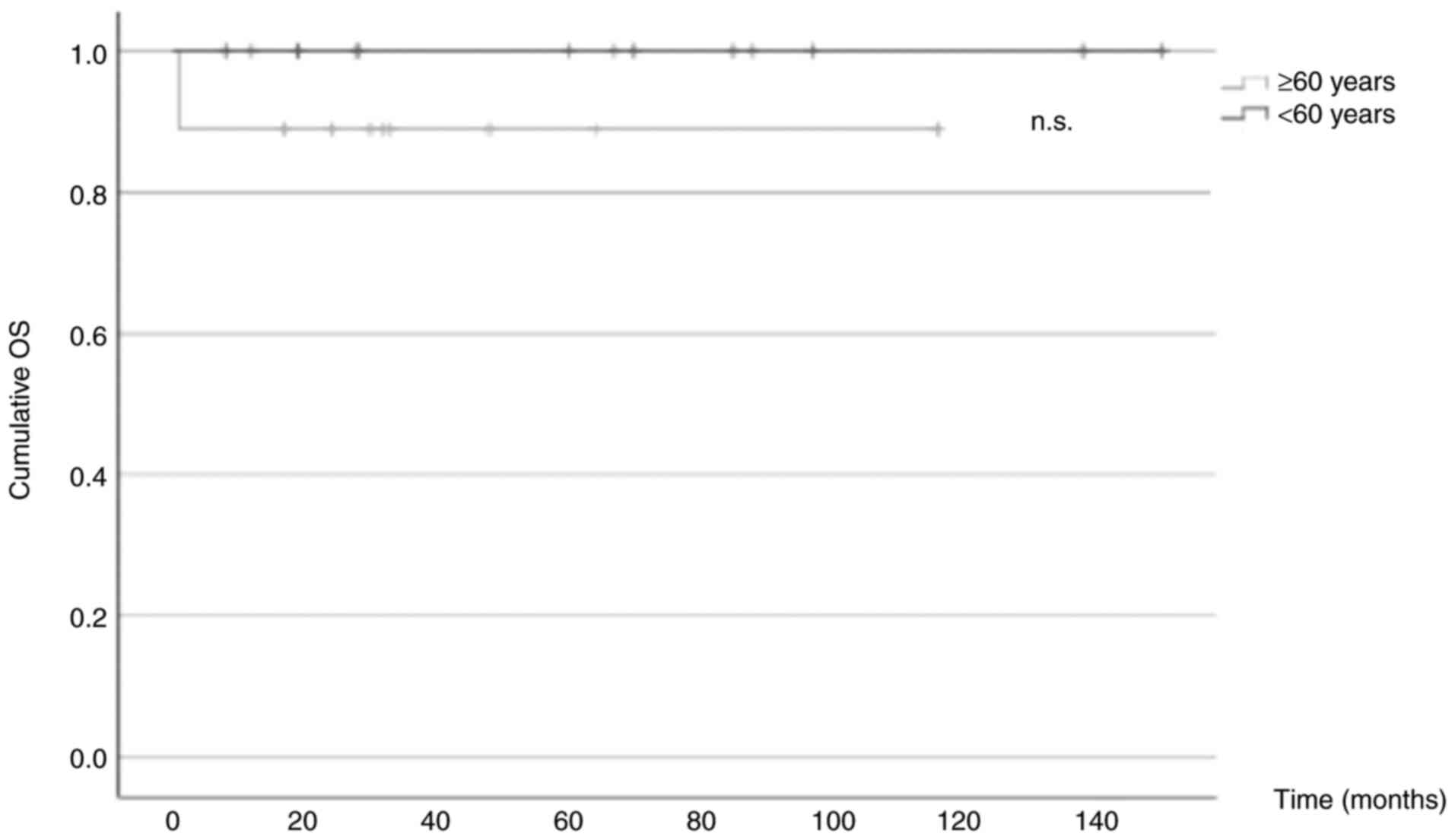
OS
Only 1 patient included in the present study died
(4.5%), who belonged to the older group (11.1%). Thus, a 10-year
survival rate of 100% in the younger group and 90% in the older
group was observed. Kaplan-Meier curves were plotted, and a
log-rank test was performed to assess any significant differences
between the 2 groups. The results revealed a nonsignificant P-value
of 0.229 (Fig. 2).
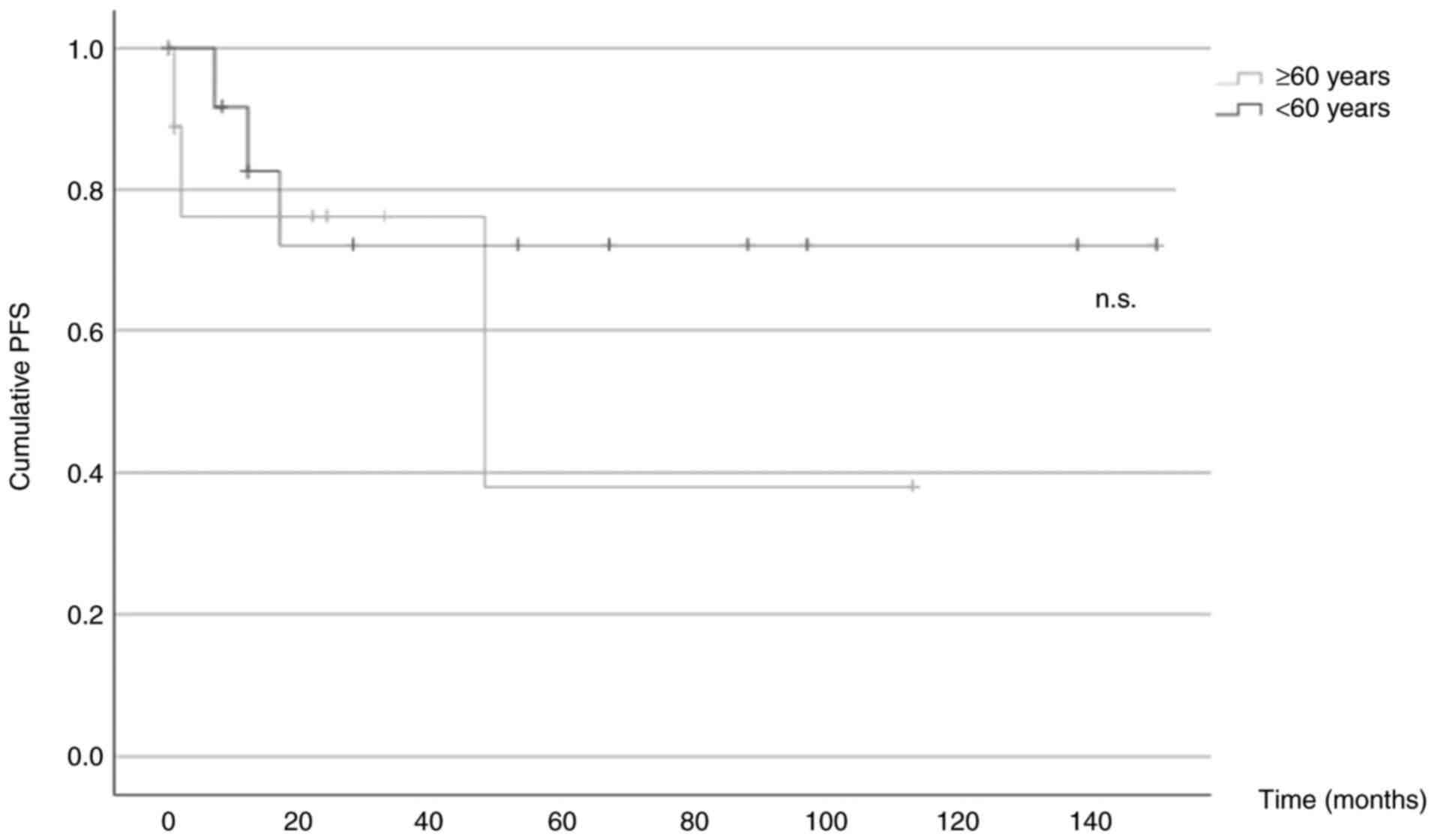
PFS
A total of 5/22 patients (22.7%) experienced disease
progression, with 2/9 patients (22.2%) in the older group and 3/13
patients (23.1%) in the younger group. Kaplan-Meier curves were
plotted, and a log-rank test was performed to assess any
significant differences between the 2 groups (95% confidence
interval, 36.1–115.5 in the older cohort vs. 74.6–148.9 in the
younger cohort). The results revealed a nonsignificant P-value of
0.485 (Fig. 3).
Discussion
In total, 22 patients were included in the present
analysis, of whom five were female. An overlap of men was expected,
as there is a higher prevalence of CML in men than women (8), however not to such a high extent. In
the present study, the relatively small sample size likely caused
this shift. Moreover, all the patients in the present study were
diagnosed in the chronic phase. As a CML diagnosis is typically
made in the chronic phase (8), no
difference in the two groups was to be expected in this regard.
The median LDH level at diagnosis was 457 U/l in the
younger group and 413 U/l in the older group; however, the median
LDH level at the start of imatinib therapy was lower compared with
their respective median LDH at diagnosis, with a median level of
428 U/l in the younger group and 385 U/l in the older group.
Moreover, when comparing the median WBC level at diagnosis with
that at the start of imatinib therapy, both groups demonstrated a
lower median WBC level at the start of imatinib therapy. The median
WBC at diagnosis was 61.45 G/l in the younger group and 38.51 G/l
in the older group. In comparison, the median WBC at the start of
imatinib therapy was 51.69 G/l in the younger group and 30.25 G/l
in the older group. Overall, the WBC at diagnosis ranged from
12.73–421.94 G/l and the WBC at the start of therapy start ranged
from 11.08–156.55 G/l. These findings were expected as most
patients with newly diagnosed CML receive hydroxyurea before the
start of TKI therapy which leads to a reduction in the levels of
WBC, reticulocytes and LDH (9).
Hydroxyurea is a chemical agent which suppresses bone marrow
activity, initiates megaloblastosis and produces antitumour effects
(9). It can be useful in the
primary management of CML until the BCR-ABL1 gene is confirmed. The
medication is usually started if patients suffer from symptoms
and/or when the leucocyte count is very high. To reduce the risk of
hyperviscosity syndrome, hydroxyurea is administered until the TKI
is started (9).
Similar to the present research, a study by
Castagnetti et al (10)
assessed the differences in patients with CML between those classed
as ‘young adults’ (18–29 years old), ‘adults’ (30–59 years old) and
‘elderly’ (>60 years old) by promoting several multicentric
prospective clinical studies over a time span of 40 years. The
authors collected data on the hematologic characteristic of
patients before the start of any treatment and the study reported
that the young adults had a median WBC level at diagnosis of 61 G/l
(range, 15–880 G/l), the adults had a median WBC level at diagnosis
of 57 G/l (range, 13–780 G/l) and the elderly had a median WBC
level at diagnosis of 59 G/l (12–544 G/l). These results indicate
that the maximum WBC levels decline with age, a finding that was
also demonstrated in the present study, where patients in the
younger group had WBC levels at diagnosis ranging from 14.77–421.94
G/l and older patients had WBC levels at diagnosis ranging from
12.73–195,42 G/l.
Spleen size was categorized into 2 groups: Normal
and enlarged. In the younger group, 76.9% of spleens were enlarged
(n=10), whereas only 15.4% (n=2) were of normal size. Furthermore,
one patient had no record of spleen size (7.7%) and there were no
splenectomies performed in this group. By contrast, 55.6% of the
spleens in the older group were of normal size (n=5) and only 22.2%
(n=2) were enlarged. Additionally, one patient had no record of
spleen size (11.1%) and one patient underwent a splenectomy (11.1%)
prior to CML diagnosis. The aforementioned study by Castagnetti
et al (10) also assessed
the differences in spleen size among young adults (18–29 years
old), adults (30–59 years old) and the elderly (>60 years old).
The study reported that 71% of the young adults had enlarged
spleens, followed by 63% of the adults and 55% of the elderly.
These results support the findings of the present study of an
increased number of enlarged spleens in the younger patient cohort
(<60 years).
In the present study, 59.01% of all patients went
into remission. A total of 8 (61.5%) were in the younger group,
compared with 5 (55.6%) in the older group; however, this
difference was not statistically significant. In-line with this
finding, a study by Saussele et al (11) reported that age was not a relevant
marker for remission rate in the TKI era. Another study by Cortes
et al (12) evaluated the
effects of age on prognosis in patients with CML and divided their
study group into patients aged ≥60 and <60 years. The study
reported that there was no significant difference in remission
rates between the two age groups. However, a marked difference
between the study by Cortes et al and the present study is
that Cortes et al treated their patients with imatinib after
failed interferon-α therapy. The authors suggested that the
association between CML therapy and older age in regard to
prognosis notably diminished, whereas allogeneic transplantation or
interferon-α therapy are associated with numerous side effects in
elder patients. Therefore, the generally well-tolerated modern
treatment with imatinib and higher-generation TKIs could be the
reason for the lack of differences in remission rates between
different age groups.
Furthermore, of the 13/22 (59.1%) patients that went
into remission in the present study, most achieved CMR 4.5 (younger
group, 46.2%; older group, 33.3%). Moreover, one patient in both
groups achieved CMR 4 (younger group, 7.7%; older group, 11.1%).
Additionally, in both groups there was 1 patient who achieved CMR
3. In the aforementioned study by Cortes et al (12), no difference in remission rates was
reported between the two age groups (<60 and ≥60 years old);
however, there was a difference in remission depth. A total of 44%
of the older patient group had attained complete cytogenetic
remission in comparison with the younger group (56%). However, a
10-year observation of patients in the randomized CML-study IV, who
were distributed into four different age groups (16–29, 30–44,
45–59 and ≥60 years), reported that there were no significant
differences in CMR 4 (13).
In the present study, 27.3% of patients had to end
the therapy due to side effects, such as severe nausea, joint pain,
bloating, lack of concentration, fatigue, exanthema, oedema,
pruritus, muscle cramping and wound healing disorder (younger
group, 23.1%; older group, 33.3%). Only 1/9 (11.1%) patients in the
older group died, and none in the younger group. Adattini et
al (14) assessed the efficacy
and safety of first-line imatinib treatment in patients with CML
with a mean age of 55 years. The study reported side effects
comprising anaemia, superficial oedema, leukopenia, neutropenia,
thrombocytopenia, fatigue, muscle cramps and infection as the most
frequent. In the study, all 89 included patients experienced at
least one adverse effect associated with imatinib treatment.
Additionally, in the present study, 22.7% of
patients had to end imatinib therapy due to disease progression
(younger group, 23.1%; older group, 11.1%). There was also no
significant difference between the PFS of the two age groups.
Progression of disease can occur due to several reasons, such as
mutations of the BCR-ABL gene, development of resistances or a low
cytogenetic response (15). In the
study by Adattini et al (14), 31% of all patients had to
discontinue their treatment with imatinib due to poor response.
Furthermore, the study analysed the probability of having to switch
to a second- or third-generation TKI, resulting in a 15% chance of
switching therapy within 12 months, 27% within 2 years and 46%
within 5 years upon initiation of imatinib therapy. In the study by
Castagnetti et al (10),
which compared young adults (18–29 years old), adults (30–59 years
old) and the elderly (>60 years old), the probability of disease
progression at 8 years was 16% among the young adults, 5% among the
adults and 7% among the elderly. Another study by Gugliotta et
al (15) assessed age
differences in patients treated with imatinib and divided 559
included patients into two groups (<65 and ≥65 years old). The
PFS for the patient group of <65 years old was 90%, whereas
patients aged ≥65 years had an PFS of 75%. However, after excluding
the deaths unrelated to CML progression, the PFS was 93 and 91% for
patients aged <65 and ≥65 years, respectively. The results of
the present study revealed no significant difference between the
younger and older patients regarding PFS, which is in-line with the
literature.
In the present study cohort, 1/22 (4.5%) patients
died at the age of 83, and this was not due to CML. These findings
lead to a 10-year overall survival rate of 100% in the younger
group and 90% in the older group. This difference in OS was not
statistically significant. Cortes et al (12) also reported that age had no impact
on either achieving response or survival. Moreover, the study by
Castagnetti et al (10)
reported an 8-year survival rate of 93% in the young adult group,
95% in the adult group and 89% in the elderly cohort. These
findings suggest that there is no significant difference in OS
between older and younger patients, when investigating CML-related
deaths. Furthermore, imatinib is still among the preferred
first-line therapy options for CML treatment, as in comparison with
higher-generation TKIs such as nilotinib and dasatinib, no
significant differences in OS have been reported (16,17).
The present study has certain limitations. As it was
retrospective, there was a risk of selection bias and the influence
of confounding factors, meaning that the study population could be
biased toward a particular subgroup. Therefore, the results may not
reflect the true population, which makes it difficult to generalize
the findings to broader groups. Another limitation is the small
sample size, as it was performed at a single centre. Variations in
entry dates and differences in follow-up durations may have also
affected the results. Additionally, the statistical approach of the
study was not designed to determine the strength of associations.
Notably, both a small sample size and selection bias exacerbate the
impact of each other: A small sample size increases the risk that
any biases in the selection of participants will distort the
findings, and the limited sample may be disproportionately affected
by these biases, reducing the robustness of the conclusions of the
study. For future research, larger studies are needed to enable
subgroup analyses and account for confounding factors such as
co-existing health conditions and other clinical characteristics.
Moreover, real-world data from multiple centres should be collected
and further investigated in meta-analyses, which help to gain more
representative and reliable conclusions.
In conclusion, the present study demonstrated that,
in a real-world setting of imatinib treatment in patients with
BCR-ABL-positive CML, age had no significant impact on remission
rates, remission depth, PFS and OS. With a 10-year-survival rate of
100% for patients aged <60 years and 90% for patients aged ≥60
years, the treatment outcomes of patients with CML in the study
cohort were good. Furthermore, the results revealed that, in-line
with previous studies, the impact of age as a factor has diminished
in the modern era of TKI treatment, due to their high efficacy and
good tolerability. However, further studies with larger patient
groups and the inclusion of newer TKIs are needed to gain further
comprehensive insights.
Acknowledgements
The authors would like to acknowledge the
contribution of NÖ Landesgesundheitsagentur, legal entity of
University Hospitals in Lower Austria, for providing the
organizational framework to conduct this research. The present work
was based on the masters' thesis of RG, submitted at the Karl
Landsteiner University of Health Sciences to acquire the academic
title of Medical Doctor.
Funding
This study was supported by the Open Access Publishing Fund of
Karl Landsteiner University of Health Sciences, Krems, Austria.
This research did not receive any specific grant from funding
agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Conceptualization was performed by RG, GK, MP and
JS; methodology by RG, GK, MP and JS; validation by RG and JS;
formal analysis by RG and JS; investigation by RG and JS; resources
were provided by MP and JS; data curation by RG and JS;
writing-original draft preparation by RG and JS; writing-review and
editing by RG, GK, MP and JS; visualization by RG and JS;
supervision by GK, MP and JS; project administration by RG and JS
and funding acquisition by MP and JS. RG and JS confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board and the Ethics Committee of Lower Austria (approval
no. GS4-EK-4/825-2022) and was performed according to the
Declaration of Helsinki. Due to the retrospective nature of the
study, the Commission for Scientific Integrity and Ethics at the
Karl Landsteiner University of Health Sciences waived the
requirement for informed consent.
Patient consent for publication
Not applicable.
Competing interests
JS declares honorarium payments from Abbvie, Amgen,
Angelini, Gilead, Janssen, Kite, Merck, Merck Sharp & Dohme,
Miltenyi, Novartis, Pfizer, Roche and Servier as an invited speaker
or expert consulting not related to CML. The other authors declare
that they have no competing interests.
References
|
1
|
Hehlmann R, Hochhaus A and Baccarani M;
European LeukemiaNet, : Chronic myeloid leukaemia. Lancet.
370:342–350. 20074 View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoglund M, Sandin F and Simonsson B:
Epidemiology of chronic myeloid leukaemia: An update. Ann Hematol.
94 (Suppl 2):S241–S247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ning L, Hu C, Lu P, Que Y, Zhu X and Li D:
Trends in disease burden of chronic myeloid leukemia at the global,
regional, and national levels: A population-based epidemiologic
study. Exp Hematol Oncol. 9:292020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurzrock R, Gutterman JU and Talpaz M: The
molecular genetics of philadelphia chromosome-positive leukemias. N
Engl J Med. 319:990–998. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Le Coutre P, Kreuzer KA, Na IK, Schwarz M,
Lupberger J, Holdhoff M, Baskaynak G, Gschaidmeier H, Platzbecker
U, Ehninger G, et al: Imatinib in philadelphia chromosome-positive
chronic phase cml patients: Molecular and cytogenetic response
rates and prediction of clinical outcome. Am J Hematol. 73:249–255.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shah NP, Bhatia R, Altman JK, Amaya M,
Begna KH, Berman E, Chan O, Clements J, Collins RH, Curtin PT, et
al: Chronic myeloid leukemia, version 2.2024, nccn clinical
practice guidelines in oncology. J Natl Compr Canc Netw. 22:43–69.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mahon FX and Etienne G: Deep molecular
response in chronic myeloid leukemia: The new goal of therapy? Clin
Cancer Res. 20:310–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rinaldi I and Winston K: Chronic myeloid
leukemia, from pathophysiology to treatment-free remission: A
narrative literature review. J Blood Med. 14:261–277. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kennedy BJ: Hydroxyurea therapy in chronic
myelogenous leukemia. Cancer. 29:1052–1056. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Castagnetti F, Gugliotta G, Baccarani M,
Breccia M, Specchia G, Levato L, Abruzzese E, Rossi G, Iurlo A,
Martino B, et al: Differences among young adults, adults and
elderly chronic myeloid leukemia patients. Ann Oncol. 26:185–192.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saussele S, Krauss MP, Hehlmann R,
Lauseker M, Proetel U, Kalmanti L, Hanfstein B, Fabarius A, Kraemer
D, Berdel WE, et al: Impact of comorbidities on overall survival in
patients with chronic myeloid leukemia: Results of the randomized
CML study IV. Blood. 126:42–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cortes J, Talpaz M, O'Brien S, Giles F,
Beth Rios M, Shan J, Faderl S, Garcia-Manero G, Ferrajoli A, Wierda
W and Kantarjian H: Effects of age on prognosis with imatinib
mesylate therapy for patients with philadelphia chromosome-positive
chronic myelogenous leukemia. Cancer. 98:1105–1113. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalmanti L, Saussele S, Lauseker M, Müller
MC, Dietz CT, Heinrich L, Hanfstein B, Proetel U, Fabarius A,
Krause SW, et al: Safety and efficacy of imatinib in cml over a
period of 10 years: Data from the randomized cml-study IV.
Leukemia. 29:1123–1132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adattini JA, Gross AS, Wong Doo N and
McLachlan AJ: Real-world efficacy and safety outcomes of imatinib
treatment in patients with chronic myeloid leukemia: An australian
experience. Pharmacol Res Perspect. 10:e010052022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gugliotta G, Castagnetti F, Palandri F,
Breccia M, Intermesoli T, Capucci A, Martino B, Pregno P, Rupoli S,
Ferrero D, et al: Frontline imatinib treatment of chronic myeloid
leukemia: No impact of age on outcome, a survey by the GIMEMA CML
working party. Blood. 117:5591–5599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cortes JE, Saglio G, Kantarjian HM,
Baccarani M, Mayer J, Boqué C, Shah NP, Chuah C, Casanova L,
Bradley-Garelik B, et al: Final 5-year study results of DASISION:
The dasatinib versus imatinib study in treatment-naive chronic
myeloid leukemia patients trial. J Clin Oncol. 34:2333–2340. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kantarjian HM, Hughes TP, Larson RA, Kim
DW, Issaragrisil S, le Coutre P, Etienne G, Boquimpani C, Pasquini
R, Clark RE, et al: Long-term outcomes with frontline nilotinib
versus imatinib in newly diagnosed chronic myeloid leukemia in
chronic phase: Enestnd 10-year analysis. Leukemia. 35:440–453.
2021. View Article : Google Scholar : PubMed/NCBI
|