Introduction
In recent decades, there has been a notable increase
in the global incidence of differentiated thyroid cancer (DTC).
According to GLOBOCAN 2020 data on cancer incidence and mortality,
thyroid cancer ranks ninth overall and fifth among female
malignancies for incidence (1).
Follicular thyroid carcinoma (FTC) is a relatively uncommon subtype
of DTC that originates from thyroid follicular cells. Its incidence
is markedly lower than that of papillary thyroid carcinoma (PTC),
accounting for 5–15% of all thyroid malignancies. However, FTC
exhibits higher malignancy compared with PTC, demonstrating a
tendency for hematogenous spread and a higher risk of distant
metastasis. The most frequent sites of FTC metastasis are the bones
and lungs (2–4). The prognosis of FTC primarily depends
on the clinical stage at diagnosis, with early diagnosis and
treatment generally associated with more favorable outcomes. By
contrast, advanced or recurrent cases tend to have a less favorable
prognosis. In addition, FTC is more susceptible to
dedifferentiation and resistance to radioactive iodine (RAI)
therapy (5), which complicates
clinical management by limiting therapeutic options and increasing
the risk of disease progression and recurrence.
Decorin (DCN) is a small leucine-rich proteoglycan
(PG) predominantly present in the extracellular matrix of mammalian
connective tissues. This protein exhibits diverse biological
functions, including inhibition of collagen fiber formation and
regulation of cell proliferation, migration and adhesion (6). While the role of DCN in breast, liver
and colorectal cancers is well documented (7–12), its
involvement in FTC remains poorly characterized. An early study by
Arnaldi et al (13)
demonstrated the downregulation of DCN mRNA levels in FTC tissues,
a finding later corroborated at the protein level by our previous
study in which FTC and adjacent noncancerous tissues were compared
(14). However, the functional
relevance of DCN downregulation to the pathogenesis of FTC,
particularly its impact on tumor cell behavior, has not yet been
elucidated. Therefore, the present study sought to clarify the role
of DCN in the progression of FTC. By comprehensively examining the
association between DCN expression levels and the proliferation,
migration and invasion capabilities of FTC cells, the study aimed
to provide evidence supporting the potential of DCN as a
therapeutic target for this malignancy.
Materials and methods
Main materials
The Nthy-ori3-1 human noncancerous thyroid cell line
and FTC-133 human FTC cell line were acquired from iCell Bioscience
Inc. Another human FTC cell line, FTC-238, was obtained from
Shanghai WheLab Bioscience Ltd. Dulbecco's Modified Eagle's Medium
(DMEM)/F12 and RPMI-1640 cell culture media were sourced from iCell
Bioscience Inc., while Gibco fetal bovine serum (FBS) was purchased
from Thermo Fisher Scientific, Inc. Penicillin-streptomycin and
trypsin solutions were obtained from Beyotime Institute of
Biotechnology. The bicinchoninic acid (BCA) protein quantitative
kit was also procured from Beyotime Institute of Biotechnology.
Primary antibodies against DCN and β-actin, as well as Cell
Counting Kit-8 (CCK-8) reagents, were acquired from Wuhan Sanying
Biotechnology. The Transwell chamber was obtained from Corning,
Inc. The quantitative polymerase chain reaction (qPCR) kit and
total RNA extraction kit (Vazyme FastPure Cell/Tissue Total RNA
Isolation Kit V2; cat. no. RC112-01) were sourced from Vazyme
Biotech Co., Ltd., while the reverse transcription (RT) kit was
procured from Takara Bio, Inc. Lentiviruses
(pcSLenti-EF1-EGFP-P2A-Puro-CMV-DCN-3×FLAG-WPRE; 3rd generation
lentiviral system) for the overexpression of DCN and for use as a
transfection control were designed and packaged by OBiO Technology
(Shanghai) Corp., Ltd.
Cell culture
Nthy-ori3-1 and FTC-133 cells were maintained in
RPMI-1640 and the FTC-238 cells were cultured in DMEM/F12; both
media were supplemented with 1% penicillin-streptomycin solution
and 10% FBS. All cells were incubated at 37°C with 5%
CO2, with the cell culture medium refreshed every 24–48
h based on cell confluency. Upon reaching 90% confluency, adherent
cells were detached using trypsin solution and passaged for
subsequent experiments up to the sixth or seventh generation. All
cell culture procedures were conducted under sterile conditions,
and all experiments utilized cells in the logarithmic growth
phase.
RT-qPCR
Total RNA was extracted from cells in each group
during the logarithmic growth phase using a Vazyme FastPure Kit
(cat. no. RC112-01). An ultra-microspectrophotometer was utilized
to determine the total RNA concentration of the extract. Following
this, cDNA synthesis was performed using the Takara PrimeScript™ RT
reagent Kit with gDNA Eraser (Perfect Real Time; cat. no. RR047A).
Briefly, genomic DNA was removed by incubation at 42°C for 2 min,
and reverse transcription was conducted at 37°C for 15 min using
the kit's RT Primer Mix. The reaction was terminated by heating at
85°C for 5 sec to inactivate the enzyme, as per the manufacturer's
guidelines. Then, qPCR was carried out using a SYBR Green-based
master mix on the Analytik Jena qTOWER3 Real-Time PCR System
(Model: qTOWER3G; Analytik Jena GmbH). The thermal cycling protocol
included an initial denaturation step at 95°C for 3 min, followed
by 40 cycles of amplification consisting of denaturation at 95°C
for 5 sec and a combined annealing/extension step at 60°C for 5
sec, with fluorescence signal acquisition at the end of the
annealing/extension phase. To validate amplification specificity, a
melting curve analysis was subsequently performed by gradually
heating the samples from 65 to 95°C at a rate of 0.5°C per
increment. All reactions were conducted in triplicate to ensure
technical reproducibility. DCN RNA expression levels were
normalized to those of the internal reference β-actin, and the
relative mRNA expression level of DCN was calculated using the
2−ΔΔCq method (15).
Table I lists the sequences of the
primers used.
 | Table I.Primer sequences utilized for
quantitative polymerase chain reaction. |
Table I.
Primer sequences utilized for
quantitative polymerase chain reaction.
| ID | Sequence (5′-
3′) |
|---|
| β-actin-F |
CCTGGCACCCAGCACAAT |
| β-actin-R |
GGGCCGGACTCGTCATAC |
| DCN-F |
GGCTGGACCGTTTCAACAGAGAG |
| DCN-R |
AAGATGGCATTGACAGCGGAAGG |
Western blotting (WB)
The cells in each group were lysed on ice using RIPA
lysis buffer [50 mM Tris (VETEC), 150 mM NaCl [Tianjin DaMao
Chemical Reagent Partnership (Limited Partnership)], 1% Triton
X-100 (Jianglai Bio; Shanghai Future Industrial Co., Ltd.), 0.1%
SDS (Vetec), 1% sodium deoxycholate (Zeye Biological), 1 mM EDTA
(Amresco, LLC), supplemented with 5 mM NaF (Shanghai Macklin
Biochemical Co., Ltd.), 2 mM sodium pyrophosphate (Shanghai
Qincheng Biotechnology Co., Ltd.), 2 mM β-glycerophosphate (Qimin
Bio, www.shkambio.com), 1 mM Na3VO4 (Shanghai
Baishun Biotechnology Co., Ltd.), 1 mM PMSF (Beyotime Institute of
Biotechnology) and protease inhibitor cocktail (Beijing Biolab
Technology Co., Ltd.); all stored at 4°C protected from light]. The
protein concentration of the lysate was determined using a BCA
protein assay kit. Then, equal amounts of proteins (20 µg per lane)
were separated via 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis, transferred to a PVDF membrane, and blocked with
1X Tris-buffered saline with 0.1% Tween-20 (TBST) containing 5%
skimmed milk for 1 h at 25°C. The membranes were then incubated
with primary antibodies against DCN (cat. no. 14667; 1:1,000;
Proteintech Group, Inc.) and β-actin (cat. no. 66009-1-Ig; 1:5,000;
Proteintech Group, Inc.) at room temperature (25°C) for 1 h. After
washing five times with 1X TBST, HRP-conjugated goat anti-rabbit
secondary antibodies (cat. no. GNI9310-R) diluted 1:5,000 in
blocking buffer were added, and the membranes were incubated at
room temperature for an additional 1 h. Following five more washes
with 1X TBST, the target protein bands were visualized utilizing an
enhanced chemiluminescence reagent (Ultrasensitive ECL; cat. no.
1810202; Shanghai Qinxiang Scientific Instrument Co., Ltd.), with
the images generated and captured by a gel imaging system. Protein
band intensities were analyzed using ImageJ software (version
1.54g; National Institutes of Health), supported by Java 1.8.0_345
(64-bit). Finally, the relative expression of DCN was evaluated,
using β-actin as an internal reference.
Lentiviral infection
FTC-133 and FTC-238 cells were divided into a
negative control (NC) group and a DCN overexpression (OE-DCN)
group, which were respectively infected with control lentiviral
particles and recombinant lentiviral particles carrying the DCN
gene. Lentiviral infection was performed using optimized
multiplicities of infection of 80 for FTC-133 cells and 10 for
FTC-238 cells. Recombinant lentiviral particles carrying the DCN
gene [titer, 2.41×109 transducing units (TU)/ml] and
control particles (titer, 1.69×109 TU/ml) were used.
After 12 h, the medium was replaced with fresh complete medium.
Stably transduced cells were selected using 1 µg/ml puromycin for
7–14 days until all uninfected cells were eliminated.
Overexpression efficiency was validated by fluorescence microscopy
[≥80% green fluorescent protein (GFP)+ cells], RT-qPCR
and WB.
Cell viability assay
Transfected FTC-133 and FTC-238 cells (OE-DCN and NC
groups for each cell line) in the logarithmic growth phase were
seeded into 96-well plates at a density of 3,000 cells/well.
Following incubation for 0, 24, 48, 72 and 96 h, CCK-8 reagent (10
µl/well) was added and the cells were incubated at 37°C for 1–4 h
to ensure measurements within the linear detection range (optimized
via preliminary tests). The optical density at 450 nm was measured
at multiple time points (1, 2, 3 and 4 h) using a microplate
reader, and the 2-h time point (within the linear phase,
R2>0.98) was selected for final analysis. Cell growth
curves were then generated based on these measurements.
Colony formation assays
Transfected FTC 133 and FTC-238 cells were
trypsinized, counted and seeded into 6-well plates at a density of
700 cells/well. The cells were incubated at 37°C for 14 days. If
any colony reached ≥50 cells prior to the 14-day endpoint, the
culture was immediately terminated and analyzed; otherwise,
incubation proceeded for the full duration. Afterwards, the cells
were gently washed once with PBS and fixed with 1 ml 4%
paraformaldehyde for 45 min at 25°C. Following fixation, the wells
were washed with PBS and stained with 1 ml/well crystal violet dye
for 20 min at 25°C. Finally, the cells were thoroughly rinsed with
PBS and allowed to air-dry. Images were captured against a white
background, and colonies (defined as cell clusters containing ≥50
adherent cells) were quantified using ImageJ software. Cell counts
were performed on the same images to assess viability.
Transwell assay
The invasive ability of the FTC cells was assessed
by Transwell assays, conducted using Matrigel-coated chambers
(pre-coated with 80 µl of BD Matrigel diluted 1:8 in serum-free
medium, followed by polymerization at 37°C for 30 min) in 12-well
plates. The upper and lower chambers were first washed once with
200 and 500 µl serum-free medium, respectively. Cell suspensions
(5×104 cells/ml) from each experimental group were
prepared using serum-free medium. A 200-µl aliquot of cell
suspension was introduced into the upper chamber, while the lower
chamber was filled with 500 µl complete medium supplemented with
20% FBS. Following a 48-h incubation period at 37°C, the chambers
were removed and washed with PBS to eliminate residual media. The
cells were then stained with crystal violet solution for 20 min at
room temperature (25°C). Excess stain was removed with distilled
water, and cells on the upper surface of the membrane were
carefully removed using a cotton swab. Cells that had invaded
through the membrane were visualized and photographed using a
widefield inverted fluorescence microscope (Model: MC-D500U3;
Jiangxi Phoenix Optics Group Co., Ltd.). Three randomly selected
fields from each well were analyzed to count the number of invading
cells.
Wound healing assay
The transfected FTC-133 and FTC-238 cells were
seeded into a 6-well plate with a total of 5×105
cells/well and cultured with 2 ml complete medium containing 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.; cat. no. 10270-106) in
a 5% CO2 incubator at 37°C. When the cell confluency
reached 80–90%, a scratch wound was created in the cells using a
200-µl pipette tip. Immediately after scratching, the medium was
replaced with serum-free medium to minimize the effect on
proliferation. The wound was photographed using an inverted light
microscope (MC-D500U3; Jiangxi Phoenix Optics Group Co., Ltd.) at
×4 magnification, and its width was recorded as the initial wound
size. The 6-well plate was then returned to the 37°C, 5%
CO2 incubator. After 6, 12, 24, 48 and 72 h of
incubation, the wound width was measured at the same location. Cell
motility was calculated using the formula: Cell motility
(%)=[(initial scratch width - scratch width after
migration)/initial scratch width] ×100. The experiments were
performed in triplicate, and the results were averaged for
statistical analysis.
Statistical analysis
Statistical analysis and the visualization of
experimental data were performed using GraphPad Prism 8.0 software
(Dotmatics). Measurement data are presented as the mean ± standard
deviation. For comparisons between two groups, unpaired Student's
t-test was employed. For comparisons among multiple groups, one-way
analysis of variance followed by Tukey's post hoc test was used to
assess pairwise differences. P<0.05 was considered to indicate a
statistically significant difference.
Results
FTC cells express low levels of
DCN
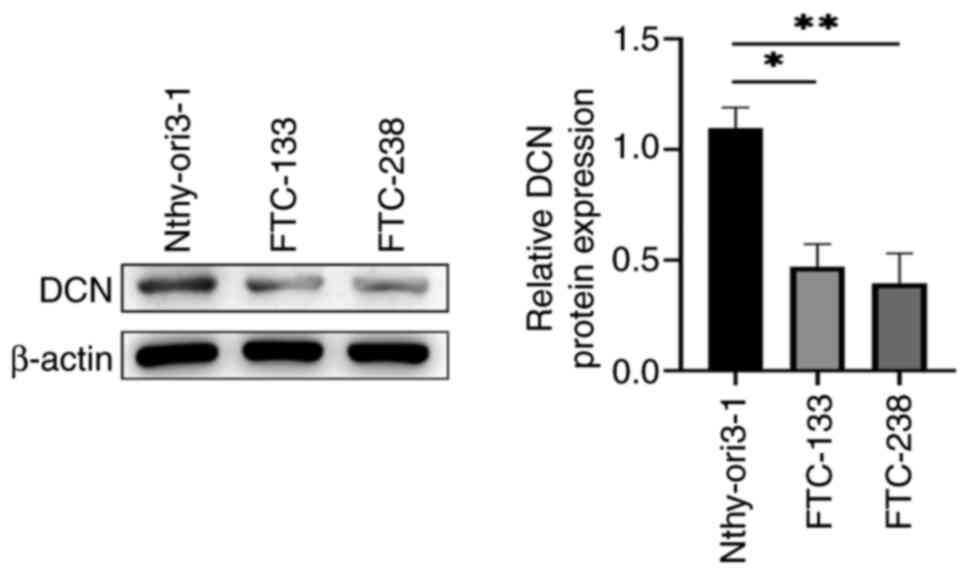
The expression levels of DCN in FTC and noncancerous
thyroid cell lines were compared. WB revealed that the expression
levels of DCN in the FTC cell lines were significantly lower
compared with those in the Nthy-ori3-1 noncancerous thyroid cell
line (P<0.05; Fig. 1).
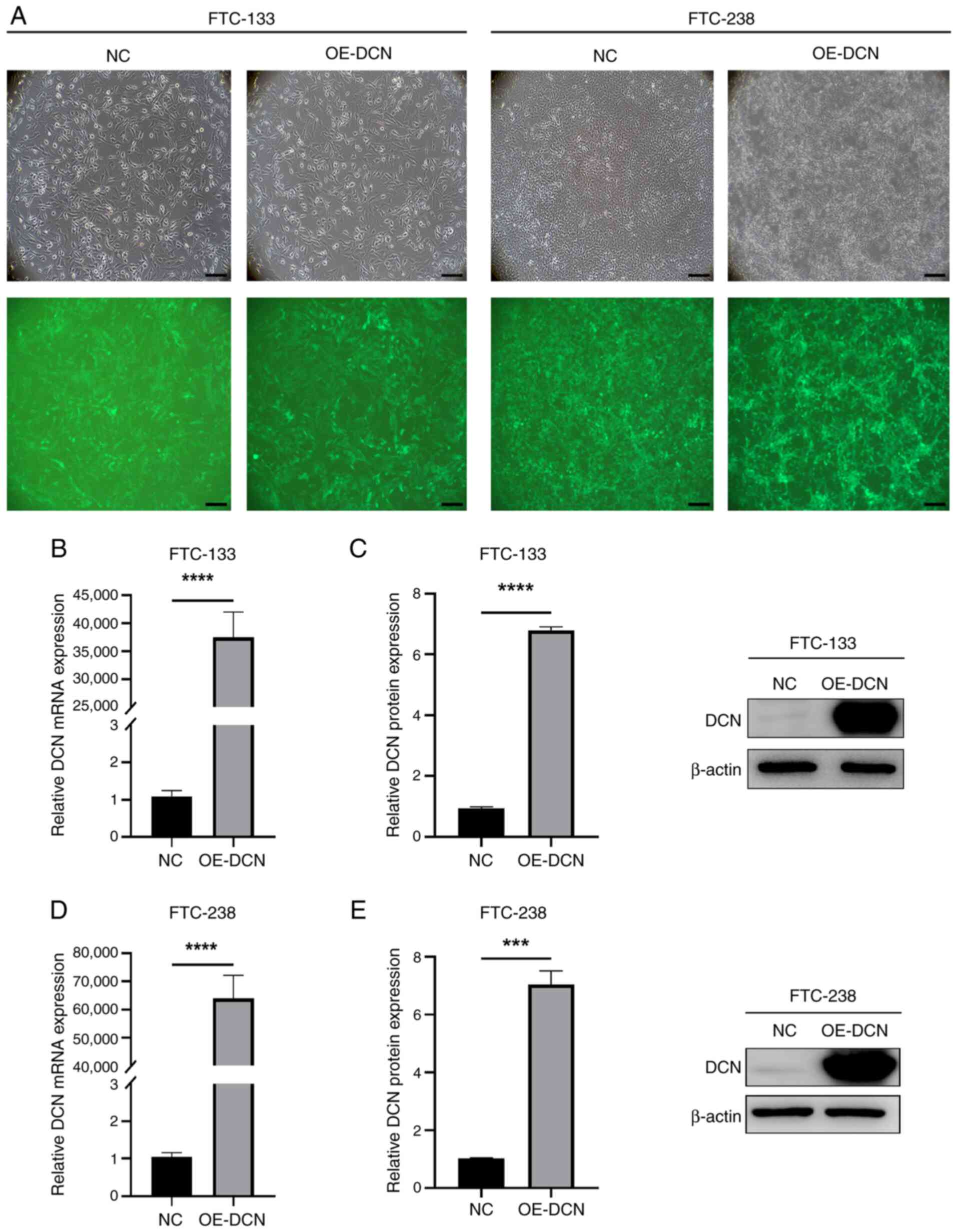
Establishment of DCN-overexpressing
FTC cell lines
To investigate the impact of DCN overexpression on
FTC cells, FTC-133 and FTC-238 cell lines were infected with
lentiviral particles overexpressing both DCN and enhanced green
fluorescent protein as a reporter gene to establish two cellular
models of DCN overexpression. As shown by the fluorescence
microscopy images in Fig. 2A, the
infection rates for the FTC-133 and FTC-238 cell lines were ≥90%.
In addition, RT-qPCR analyses revealed that DCN mRNA expression
levels in the OE-DCN groups were significantly increased compared
with those in the corresponding NC groups (P<0.0001; Fig. 2B and D). WB analyses corroborated
the RT-qPCR results, with significantly increased DCN protein
expression levels in the OE-DCN groups compared with those in the
corresponding NC groups (P<0.001; Fig. 2C and E).
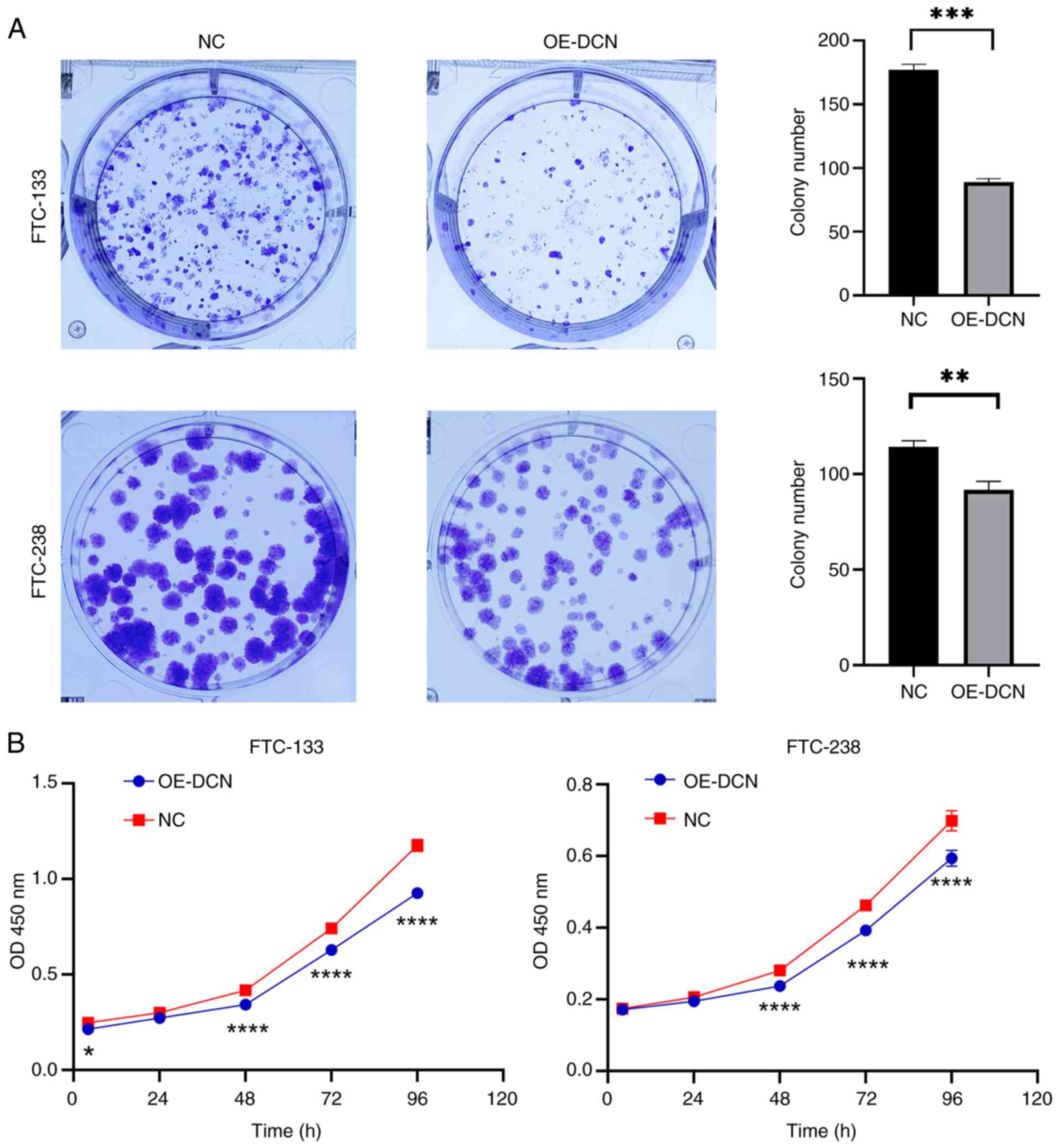
DCN overexpression suppresses the
proliferation of FTC cells
The effect of DCN overexpression on the
proliferation of FTC cells was investigated. In the colony
formation assay, the clonogenic capacity of the OE-DCN groups of
FTC-133 and FTC-238 cells was significantly decreased compared with
that of the corresponding NC groups (P<0.01; Fig. 3A). In addition, CCK-8 assays
demonstrated that cell viability in the OE-DCN groups was
significantly lower compared with that in the respective NC groups
(P<0.05; Fig. 3B). These
findings indicate that DCN overexpression inhibits the
proliferation of FTC cells.
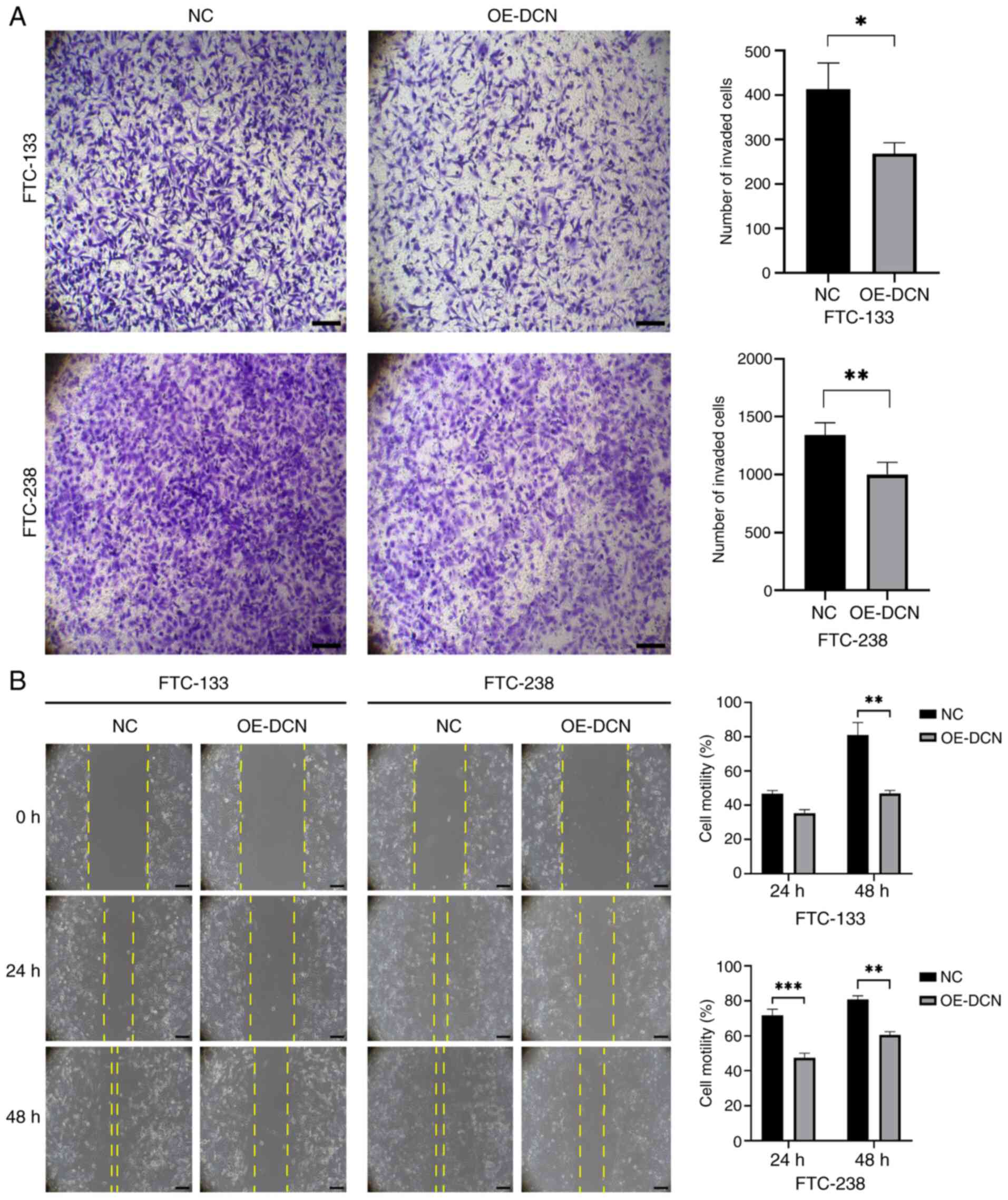
DCN overexpression suppresses the
invasion and migration of FTC cells
Finally, the effects of DCN overexpression on FTC
cell invasion and migration were evaluated. Transwell assay results
indicated that DCN overexpression significantly reduced the
invasive ability of FTC cells compared with that of the NC group
(P<0.05; Fig. 4A). In addition,
wound healing assay results demonstrated that DCN overexpression
significantly impaired the edge-to-center wound healing of FTC
cells relative to that of the respective NC group (P<0.01;
Fig. 4B). These findings
collectively indicate that DCN overexpression suppresses FTC cell
invasion and migration.
Discussion
The increasing prevalence of thyroid carcinoma,
particularly DTC, has attracted considerable clinical attention.
While early-stage cases often respond favorably to surgical
intervention, advanced or recurrent FTC presents substantial
therapeutic challenges (16). The
American Thyroid Association guidelines emphasize that FTC has a
tendency for dedifferentiation and resistance to RAI therapy, with
20–30% of patients with distant metastases, such as pulmonary or
skeletal metastases, becoming RAI-refractory (RAI-R) (5). Cases with distant metastases
demonstrate a marked reduction in 10-year survival rate (<20%
for metastatic disease vs. >90% for localized disease) (17), highlighting the importance of
understanding FTC pathogenesis and identifying novel therapeutic
targets.
DCN, a small leucine-rich PG, has been recognized as
a potent tumor suppressor across various malignancies. For example,
in inflammatory breast cancer, DCN suppresses invasion and tumor
growth by destabilizing E-cadherin and inhibiting EGFR/ERK
signaling (7). DCN also inhibits
breast cancer metastasis through its anti-lymphangiogenic functions
(8). In addition, in hepatocellular
carcinoma, DCN deficiency has been associated with increased
tumorigenesis via mechanisms involving cell cycle arrest, caspase-3
activation and the suppression of proliferation (9,10).
Similarly, studies using colorectal cancer models have demonstrated
that the downregulation of DCN expression promotes hepatic
metastasis by dysregulating receptor tyrosine kinases (11,12).
Arnaldi et al (13) extended
these observations to FTC, reporting reduced DCN mRNA levels in
follicular thyroid tumors. Furthermore, our previous study
corroborated these findings at the histological and cellular
levels, revealing significant downregulation of DCN in FTC tissues
and cell lines (14). This
convergence of evidence indicates that DCN is a molecular regulator
of tumor suppression across diverse malignancies, including
FTC.
Expanding upon our previous histological and
bioinformatics study (14), which
identified DCN as a differentially expressed gene in FTC and
verified its diminished protein abundance in tumor tissues, the
present study aimed to elucidate its functional significance. Two
principal advancements were achieved: Firstly, comparative analyses
revealed that DCN expression in FTC cell lines was significantly
downregulated compared with that in normal thyroid follicular
cells, extending tissue-level observations to in vitro
models. Secondly, lentivirus-mediated DCN overexpression in FTC-133
and FTC-238 cells significantly suppressed their malignant
phenotypes, as evidenced by reduced viability and decreased
migration and invasion capabilities. These findings establish a
direct association between increased DCN expression and suppressed
FTC aggressiveness, providing functional validation of the
mRNA-level observations reported by Arnaldi et al (13).
While the present study offers valuable insights,
several limitations require consideration. Firstly, the mechanistic
foundations of the antitumor effects of DCN remain unexplored; its
downstream signaling pathways merit investigation in future
studies. Secondly, the findings are derived only from two FTC cell
lines; validation across additional models, such as primary tumor
cells or patient-derived xenografts, would enhance the
generalizability of the conclusions. Thirdly, the study lacks in
vivo evidence; animal models are necessary to confirm the
therapeutic potential of DCN. Lastly, clinical associations between
DCN expression levels and patient outcomes, including survival and
RAI resistance, remain unaddressed, necessitating prospective
cohort studies.
In conclusion, the present study supplements
previous histological and bioinformatics evidence (14) with functional validation,
establishing DCN as a critical tumor suppressor in FTC. DCN
overexpression was found to inhibit the proliferation, migration
and invasion of FTC cells, suggesting that DCN may suppress FTC
progression. These findings highlight the potential of DCN as a
therapeutic target for RAI-R FTC. Future research should focus on
elucidating the underlying mechanisms, including in vivo
validation of the therapeutic effects of DCN using animal models
such as patient-derived xenografts, and clarification of the
downstream signaling pathways through which DCN exerts its
antitumor activity; the EGFR/ERK and MAPK/PI3K-AKT pathways are
suggested as potential candidates. Proteomic profiling and
single-cell RNA sequencing could further uncover DCN-regulated
molecular networks and cellular heterogeneity in FTC
dedifferentiation and RAI resistance. Preclinical validation of
DCN-based strategies, along with clinical studies investigating the
association of DCN expression with metastatic progression and
therapeutic outcomes, will be essential for advancing its
translation into targeted therapies for aggressive FTC.
Acknowledgements
Not applicable.
Funding
This study received financial support from the Young Fund
Project of the Jiading District Health Commission, Shanghai (grant
no. 2021-QN-02), Project of the Science and Technology Commission
of Jiading District, Shanghai, China (grant no. JDKW-2022-0016) and
General Project of the Shanghai Jiading District Health Commission,
China (grant no. 2022-KY-13).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QL was responsible for experimental design, data
analysis and manuscript preparation. YM and XW were responsible for
data acquisition. HG contributed to the experimental design,
supervised the execution of experiments and critically reviewed the
statistical methodology. TY provided conceptual guidance for the
study, interpreted key findings related to clinical relevance and
revised the manuscript for intellectual content. RG participated in
data interpretation, validated the pathological significance of
results and substantially edited the manuscript. HG and RG confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chiapponi C, Hartmann MJM, Schmidt M,
Faust M, Schultheis AM, Bruns CJ and Alakus H: Radioiodine
refractory follicular thyroid cancer and surgery for cervical
relapse. Cancers (Basel). 13:6230–6240. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luvhengo TE, Bombil I, Mokhtari A, Moeng
MS, Demetriou D, Sanders C and Dlamini Z: Multi-omics and
management of follicular carcinoma of the thyroid. Biomedicines.
11:12172023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vuong HG, Le MK, Hassell L, Kondo T and
Kakudo K: The differences in distant metastatic patterns and their
corresponding survival between thyroid cancer subtypes. Head Neck.
44:926–932. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong Y, Zhong J and Dong L: The role of
decorin in autoimmune and inflammatory diseases. J Immunol Res.
2022:12833832022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu X, Villodre ES, Larson R, Rahal OM,
Wang X, Gong Y, Song J, Krishnamurthy S, Ueno NT, Tripathy D, et
al: Decorin-mediated suppression of tumorigenesis, invasion, and
metastasis in inflammatory breast cancer. Commun Biol. 4:722021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mondal DK, Xie C, Pascal GJ, Buraschi S
and Iozzo RV: Decorin suppresses tumor lymphangiogenesis: A
mechanism to curtail cancer progression. Proc Natl Acad Sci USA.
121:e23177601212024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horváth Z, Kovalszky I, Fullár A, Kiss K,
Schaff Z, Iozzo RV and Baghy K: Decorin deficiency promotes hepatic
carcinogenesis. Matrix Biol. 35:194–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reszegi A, Horváth Z, Fehér H, Wichmann B,
Tátrai P, Kovalszky I and Baghy K: Protective role of decorin in
primary hepatocellular carcinoma. Front Oncol. 10:6452020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bi X, Pohl NM, Qian Z, Yang GR, Gou Y,
Guzman G, Kajdacsy-Balla A, Iozzo RV and Yang W: Decorin-mediated
inhibition of colorectal cancer growth and migration is associated
with E-cadherin in vitro and in mice. Carcinogenesis. 33:326–330.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reszegi A, Horváth Z, Karászi K, Regős E,
Postniková V, Tátrai P, Kiss A, Schaff Z, Kovalszky I and Baghy K:
The protective role of decorin in hepatic metastasis of colorectal
carcinoma. Biomolecules. 10:11992020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arnaldi LA, Borra RC, Maciel RM and
Cerutti JM: Gene expression profiles reveal that DCN, DIO1, and
DIO2 are underexpressed in benign and malignant thyroid tumors.
Thyroid. 15:210–221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin Q, Ma Y and Chen P: Identification of
potential biomarkers in follicular thyroid carcinoma:
Bioinformatics and immunohistochemical analyses. Oncologie.
26:311–322. 2024. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Phay JE and Ringel MD: Metastatic
mechanisms in follicular cell-derived thyroid cancer. Endocr Relat
Cancer. 20:R307–R319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karapanou O, Simeakis G, Vlassopoulou B,
Alevizaki M and Saltiki K: Advanced RAI-refractory thyroid cancer:
An update on treatment perspectives. Endocr Relat Cancer.
29:R57–R66. 2022. View Article : Google Scholar : PubMed/NCBI
|