Introduction
Corded and hyalinized endometrioid carcinoma (CHEC)
is a rare histological variant of endometrioid carcinoma (EC). The
presence of corded components within the hyalinized stroma,
combined with histological changes typical of EC, contribute to its
diagnosis. This distinctive morphology was initially described by
Clement and Young (1) in a review
in 2002. In 2005, Murray et al (2) analyzed the clinicopathological
features of 31 similar cases and coined the term CHEC. To date,
>60 cases with clinicopathological features of CHEC have been
reported (2–7). The majority of these cases are
classified as low-grade [International Federation of Gynecology and
Obstetrics (FIGO) G1-G2] (8), with
only seven cases exhibiting the high-grade component (FIGO G3)
(5–7). Ladwig et al (5) revealed that low-grade CHEC shares
similarity with classic EC, exhibiting β-catenin (CTNNB1; 7/7),
PIK3CA(Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic
Subunit Alpha, 6/7) and PTEN (6/7) mutations, with only one case of
high-grade CHEC presenting with overexpression of p53 (1/7).
Additionally, CHEC with high-grade components exhibits
overexpression of p53 (2/5) and mismatch repair deficiency (MMRd;
3/5), while a unique case of CHEC with focal bizarre cells
exhibited no specific molecular alterations (7). CHEC with high-grade components can be
confused with carcinosarcoma, both in histological morphology and
molecular phenotype, which poses a challenge for pathological
diagnosis. The present study describes the case of a patient with
high-grade CHEC and also provides a review of the relevant
literature (5–7) to summarize the clinicopathological and
molecular phenotypical features to prevent misdiagnoses.
Case report
A 38-year-old female patient was admitted to the
Department of Gynecology at Shandong Provincial Hospital Affiliated
to Shandong First Medical University in Jinan, China, in November
2023, with symptoms of intermittent vaginal bleeding and abdominal
pain persisting for >3 months. She had irregular menstruation
since menarche at the age of 18 years and had been receiving a
combination of traditional Chinese and Western medicines since the
age of 27 years. At the age of 31 years, the patient underwent
endometrial curettage, which indicated atypical endometrial
hyperplasia. Preoperative endometrial biopsy confirmed the
diagnosis of carcinosarcoma. There was no reported family history
of tumors. A gynecological B-mode ultrasound (transabdominal probe
frequency, 3.5 MHz; depth 18 cm, focal zone set at the uterine
corpus with medium gain adjustment) revealed the deep invasion of
endometrial cancer into the myometrial layer and cervix, and MRI
scans (3.0T, T2-weighted turbo spin-echo sequence: Repetition/Echo
Time 4,000/100 msec, slice thickness 5 mm, Field of View 30 cm;
diffusion-weighted imaging with b=1,000 s/mm2) confirmed
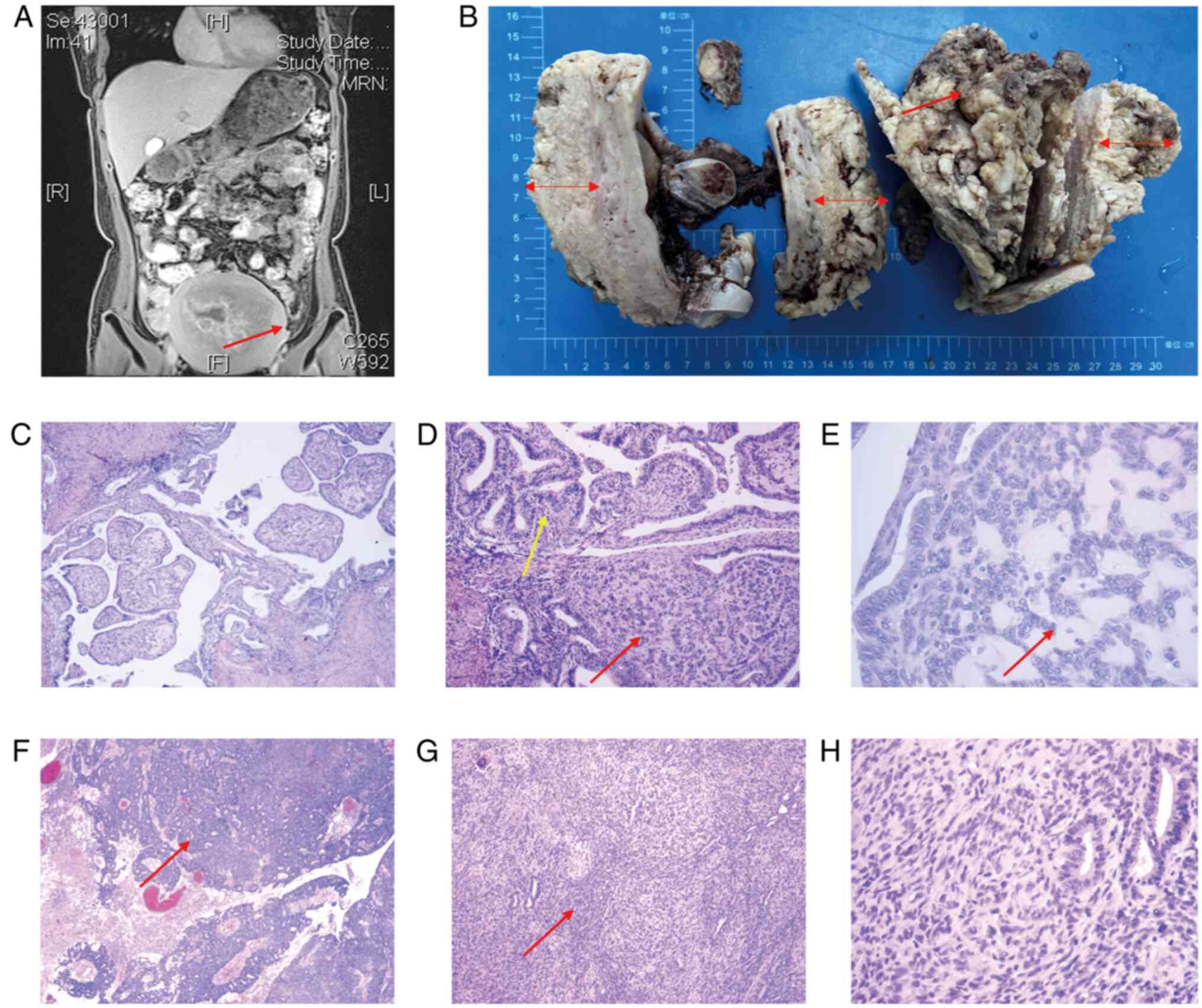
the marked irregular thickening of the endometrium with invasion
into the myometrium and cervix (Fig.
1A). Intraoperative frozen section analysis initially
misdiagnosed the lesion as carcinosarcoma. The patient underwent a
hysterectomy with bilateral salpingo-oophorectomy.
Upon a gross examination of radical hysterectomy
specimens following surgery, the uterus was enlarged, presenting as
a uniformly exophytic mass within the uterine cavity. The tumor was
firm, with gray-white tissue diffusely invading the myometrium and
the cervical stroma with a size of ~15×10×4 cm3
(Fig. 1B). The specimens were fixed
in 10% neutral buffered formalin at 25°C for 24 h, sectioned at 4
µm thickness, stained with hematoxylin (5 min) and eosin (2 min at
25°C), and examined using an optical microscope. Under microscopic
examination, two distinct morphological forms were evident. The
majority of the tumor consisted of typical low-grade EC, while the
corded components were embedded within the hyalinized stroma
(Fig. 1E). The corded components
were arranged in strips, small cell clusters and spindle cells,
intermingling with the adenocarcinoma component. Locally, there was
a transition between the two components (Fig. 1C and D). Additionally, areas of
squamous/morular differentiation were observed. At a higher
magnification, the nuclei of both the classic EC and corded
components exhibited relatively consistent and mild to moderate
dysplasia, with a limited number of mitotic figures (Fig. 1E). Notably, ~20% of the tumor
displayed an increased density of spindle-shaped cells (Fig. 1F), and in some areas, a small amount
of hyalinized stroma was visible. Moreover, a few glands and
spindle cells fused with each other (Fig. 1G). In some regions, a uniformly pale
or eosinophilic appearance is observed, often with well-demarcated
borders, corresponding to ground pattern necrosis. Under a
high-powered microscope, severe cell dysplasia was observed, and
mitoses were visible (~50 cells/10 high-power fields; Fig. 1H). The histological classification
revealed that 80% of the tumor was categorized as low-grade (FIGO
G1)(8) and 20% as high-grade (FIGO
G3). The tumor cells infiltrated more than half of the uterine wall
and extended into the cervical stroma, without evident vascular
invasion or metastasis into the pelvic lymph nodes.
Tissue sections were baked at 65°C for 1 h, followed
by dewaxing in 100% xylene and rehydrated through graded ethanol
series (100, 95, 80, 70%). After rinsing in PBS, antigen retrieval
was performed using a pressure cooker with preheated retrieval
buffer: sections were heated at full pressure for 3 min after
boiling, cooled naturally, and washed in PBS. Endogenous peroxidase
activity was blocked with 3% hydrogen peroxide (10 min; room
temperature), followed by PBS rinsing. Primary antibodies: AE1/AE3,
PAX-8, ER, PR, EMA and E-cadherin, β-catenin, CR, MLH1, MSH2, MSH6,
PMS2, Ki-67, p53 (Zhongshan Golden Bridge Biotechnology) were
applied and incubated overnight at 4°C in a humidified chamber.
After PBS washing, universal secondary antibodies (rabbit/mouse
IgG; 1:200 dilution, PV-9000, Zhongshan Golden Bridge
Biotechnology) conjugated with horseradish peroxidase were
incubated at 37°C for 30 min. DAB was applied for 4–6 min (room
temperature), and reactions were stopped by rinsing under running
water. Sections were counterstained with hematoxylin (1 min),
differentiated in 1% acid alcohol (3 sec), blued in ammonia water
(30 sec), dehydrated through graded alcohols (70–100 %), and
mounted with neutral resin. Staining results were analyzed using
Nikon Eclipse E600 light microscope (Nikon Corporation, Japan).
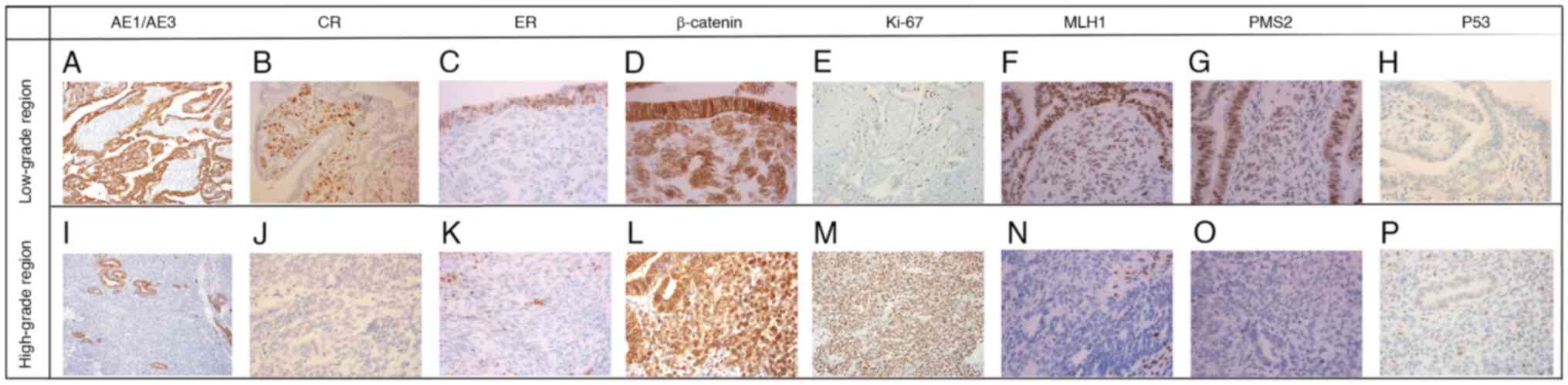
Immunohistochemistry revealed that AE1/AE3, PAX-8, ER, PR, EMA and
E-cadherin were positive in the low-grade EC, and β-catenin
demonstrated membranous positivity (Fig. 2D; Table
I). CR and α-inhibin were negative in the low-grade EC region,
and the Ki-67 index was ~10% (Fig.
2E). In the low-grade corded components, PAX-8, CR and
α-inhibin were positive, β-catenin displayed cytoplasmic and
nuclear positivity (Fig. 2L) and
AE1/AE3, EMA and E-cadherin were negative. The Ki-67 index of the
low-grade corded region was 1% (Fig.
2E). MMR protein and wild-type p53 protein were found both in
the low-grade EC and corded components. In the high-grade area,
PAX-8 was weakly positive, β-catenin demonstrated nuclear
positivity and the Ki-67 index was ~70%. AE1/AE3, EMA, E-cadherin,
CR, α-inhibin, ER and PR were negative. The tumor cells exhibit
wild-type p53 status, characterized by weak or absent nuclear
staining, comparable with the pattern observed in normal cells.
This aligns with our molecular testing results, which confirm the
absence of pathogenic mutations in the TP53 gene. In addition, MMRd
was detected in the high-grade tumor cells.
 | Table I.Immunohistochemistry of the present
case. |
Table I.
Immunohistochemistry of the present
case.
|
| Low-grade
region |
|
|---|
|
|
|
|
|---|
| Protein | Epithelial
region | Corded region | High-grade
region |
|---|
| PAX-8 | + | + | ± |
| AE1/AE3 | + | − | − |
| EMA | + | − | − |
| E-cadherin | + | − | − |
| CR | − | + | − |
| α-inhibin | − | + | − |
| β-catenin | M+ | C+/N+ | N+ |
| ER | + | − | − |
| PR | + | − | − |
| Ki-67 | ~10% | ~1% | ~70% |
| P53 staining | WT | WT | WT |
The sequencing analysis utilized the NEBNext Ultra
II DNA Library Prep Kit (Catalogue No. E7645L; New England Biolabs,
USA) for library preparation. Sample quality was verified using an
Agilent 4200 TapeStation System with High Sensitivity D1000
ScreenTape (Agilent Technologies) to ensure DNA integrity (DIN
≥7.0) and tumor cell content (≥20%). Sequencing was performed on
Illumina platforms (NovaSeq 6000/NextSeq 500/MiSeq/iSeq 100) with
paired-end 150-bp reads, employing the NovaSeq 6000 S4 Reagent Kit
(Catalogue No. 20028312; Illumina, USA). The final library was
quantified to 1.8 nM using a Qubit 4.0 Fluorometer (Thermo Fisher
Scientific) and KAPA Library Quantification Kit (Roche). Data
analysis utilized BWA-MEM v0.7.17 for alignment, GATK v4.2.6.1 for
variant calling, ANNOVAR (2023-05 -01) for annotation, and IGV
v2.12.3 for visualization, ensuring stringent quality metrics (Q30:
93.95%, average depth: 824×). Next-generation sequencing was
performed to assess microsatellite instability (MSI) and a tumor
panel (typically covering 30–50 genes) was used, including DNA
polymerase ε, catalytic subunit (POLE), TP53, PIK3CA, CTNNB1 and
serine/threonine kinase 11 (STK11), for detection of mutations in
the low- and high-grade regions. Both the low- and high-grade
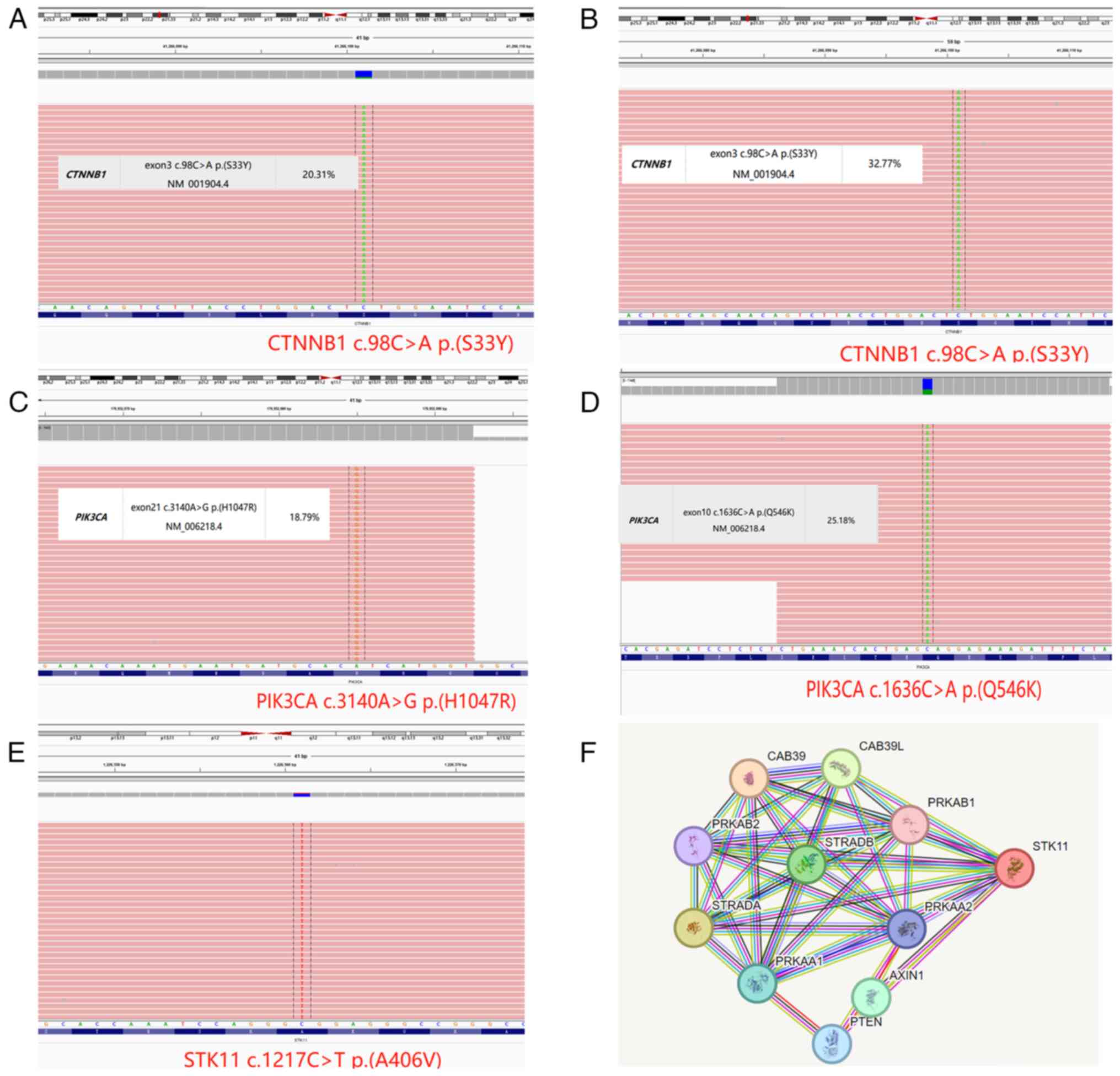
components exhibited mutations in CTNNB1 (exon 3; Fig. 3A and B) and PIK3CA (exon 21;
Fig. 3C and D; Table II). POLE mutations were not
identified in either low- or high-grade CHEC. Furthermore, only the
high-grade region components had high MSI (MSI-H), whereas the
low-grade components exhibited no special molecular profile (NSMP).
STK11 mutation was identified exclusively in the high-grade region,
located in exon 9, involving a change from C to T at base 1,217 of
the encoded protein (Fig. 3E).
Conversely, the STK11 mutation was not detected in the low-grade
region. Protein-protein interaction (PPI; dataset obtained from the
STRING database (string-db.org/) analysis of STK11 revealed a
potential downstream network (Fig.
3F).
 | Table II.Molecular features of the present
case. |
Table II.
Molecular features of the present
case.
| Gene | Low-grade
region | High-grade
region |
|---|
| TP53 | WT | WT |
| MLH1, PMS2 | No D | D |
| MSH2, MSH6 | No D | No D |
| CTNNB1 | MT | MT |
| PIK3CA | MT | MT |
| MSI | Low | High |
| STK11 | WT | MT |
Pathological diagnosis of CHEC was confirmed, with
20% of the region classified as high-grade (FIGO G3). The FIGO
stage was classified as IIA. Following the surgical procedure, the
patient underwent three cycles of chemotherapy with lomustine at
240 mg/cycle and carboplatin at 600 mg/cycle, administered every 4
weeks. The patient underwent pelvic CT scans every 6 months, with
tumor marker assessment (including CA-125) performed at the
12-month mark. If elevated, CA-125 levels were monitored every 3
months. No recurrence was detected during the 16-month follow-up
period.
Discussion
To date, 62 cases of CHEC have been reported
(2–7); patients with CHEC are typically
younger compared with those with conventional EC with a median age
at diagnosis of ~49.8 years compared to 61 years for conventional
EC (9). CHEC exhibits
characteristic structural features, consisting of a combination of
classical EC structures and corded structures with hyalinized
degeneration, which are merged together. The corded component
accounts <5->90% of the tumor, with the majority being
low-grade (FIGO G1 or G2) and FIGO stage I (68%). Notably, the
majority of the patients with CHEC survived and remained
disease-free at the last follow-up (70%). Therefore, CHEC is a
subtype of low-grade EC exhibiting a favorable prognosis, and the
prognostic outcome of CHEC is comparable to conventional low-grade
EC (10). However, seven cases of
CHEC with high-grade components have been reported, which exhibit
MMR deficiency, p53 abnormalities, and/or poor prognosis (5–7), in
which the EC and/or corded components exhibit marked heterogeneity
and moderate to severe atypia. These features suggest a high degree
of tumor complexity, as observed in the present case.
The present study reviewed eight cases (including
the present case) of CHEC with high-grade components (Table III). The median age of the
patients in these cases was 59 years (range, 34–71 years), similar
to that of conventional EC but older than that of low-grade CHEC
patients. Notably, 5/8 cases were classified as FIGO stage I, 1/8
as FIGO stage II and 2/8 as FIGO stage III. All eight cases had
traditional EC components, 3/8 cases exhibited low-grade EC, 2/8
exhibited high-grade EC and 3/8 displayed both high- and low-grade
EC (Table III). Furthermore, 7/8
cases exhibited squamous/morular differentiation and there was
marked variation in the proportion of corded and hyalinized
components with a median of 27.5% (range, 15.0–90%). In the
previously reported cases (5–7), the
corded component was consistently characterized by moderate to
severe dysplasia. Similarly, in the present case, the corded and
hyalinized degeneration components displayed both low- and
high-grade elements. Within the low-grade region, the cells
exhibited mild characteristics, with minimal mitoses and a Ki-67
index of 1%. High-grade corded components displayed marked
heterogeneity, characterized by pronounced cellular pleomorphism,
visible mitotic figures and necrosis. Intravascular tumor thrombi
were found in only 1/8 cases. Notably, despite the presence of a
high-grade component, all patients eligible for a follow-up
examination (6/8) experienced disease-free survival.
 | Table III.Clinical, pathological and molecular
findings of high-grade corded and hyalinized EC. |
Table III.
Clinical, pathological and molecular
findings of high-grade corded and hyalinized EC.
|
|
|
| Corded region | Traditional EC
region |
|
|
|
|
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Case number | Age, years | FIGO stage | % | Nuclear atypia | G1/G2 | G3 | LVSI | Squamous
differentiation | FU, months | β-catenin | CTNNB1 | POLE | p53 | MMR | Molecular
subtypes | (Refs.) |
|---|
| 1 | 53 | IA | 20 | Moderate/severe,
focal anaplasia | Yes | No | None | Yes | NED (27) | N | WT | WT | WT | Loss | MMRd | (7) |
| 2 | 60 | IIIA | 25 |
Moderate/severe | Yes | Yes | None | Yes | Lost FU | M | WT | WT | WT | Loss | MMRd | (7) |
| 3 | 71 | IA | 15 |
Moderate/severe | Yes | Yes | None | Yes | NED (10) | N | WT | WT | O | Retained | p53abn | (7) |
| 4 | 58 | IA | 40 |
Moderate/severe | No | Yes | None | Yes | NED (2) | M | WT | WT | O | Retained | p53abn | (7) |
| 5 | 74 | IB | 15 |
Moderate/severe | No | Yes | None | Yes | NED (30) | M | WT | WT | WT | Loss | MMRd | (7) |
| 6 | 34 | IIIC | 75 |
Moderate/severe | Yes | No | Yes | Yes | None | N | None | WT | WT | Retained | NSMP | (6) |
| 7 | 64 | IA | >90 |
Moderate/severe | Yes | No | None | Not described | NED (13) | N | Mut | WT | O | Retained | p53abn | (5) |
| 8 | 38 | IIA | 30 | Mild/severe | Yes | Yes | None | Yes | NED (4) | N | Mutt | WT | WT | Loss | MMRd | Present study |
High-grade CHEC poses a challenge for differential
diagnoses of carcinosarcoma and dedifferentiated carcinoma.
Carcinosarcoma was diagnosed at both the biopsy and in the analysis
of the frozen sections in the present case study. Following
surgery, morphological analysis revealed classic low-grade CHEC,
with high-grade epithelioid cells arranged in cords with a
hyalinized stroma. The high-grade corded component was
superficially located, merged with the endometrioid component and
accompanied by prominent squamous differentiation with nuclear
expression of β-catenin with a CTNNB1 mutation. However, no p53
upregulation was observed, which could have aided with differential
diagnosis.
Common gene mutations in endometrial cancer include
PTEN (82%), PIK3CA (54%), AT-rich interaction domain 1A (54%),
PIK3R1 (36%) and CTNNB1 (34%). (11). In the present case, PIK3CA and
CTNNB1 mutations were found in both high- and low-grade CHEC.
Notably, MSI-H and the STK11 mutation were found only in high-grade
CHEC. Previously, there have been increasing reports of STK11 gene
mutations occurring in EC (12–14),
which may be associated with the malignant progression of EC. Based
on the present case, STK11 may be associated with the high-grade
transformation of CHEC.
According to The Cancer Genome Atlas, EC is
classified into four molecular subtypes: ‘POLE-mut’ in the presence
of POLE mutation, ‘MMRd’ in the absence of POLE mutations but with
MMRd, ‘p53abn’ with an aberrant p53 expression in the absence of
POLE mutations and MMRd and ‘NSMP’ as POLE-wild-type,
MMR-proficient and p53-wild-type (15). In the pooled high-grade CHEC cases,
the molecular subtypes observed were p53abn (3/8), MMRd (4/8) and
NSMP (1/8), with no POLE-mut cases. The association of p53abn with
a worse prognosis of patients with EC further supports the
hypothesis that high-grade CHEC is a more malignant tumor.
High-grade CHEC with p53abn typically exhibits more aggressive
biological behaviors. Additionally, p53abn tumors are associated
with genomic instability, leading to enhanced resistance to
standard chemotherapy, a higher risk of recurrence and reduced
progression-free and overall survival (16,17).
Notably, all three cases with p53 overexpression were FIGO stage I,
potentially due to the early stage of the cancer and possibly other
favorable factors in those individual cases. This does not
necessarily contradict the overall trend, and the available
follow-up information did not indicate a worse prognosis compared
with the non-p53abn type. However, all three p53abn cases were FIGO
stage 1 and had relatively short follow-up periods, necessitating
larger case series and longer follow-up for further investigation.
MMRd was present in 4/8 cases, which suggests the need for germline
and MSH1 promoter hypermethylation testing in combination with
family history, to differentiate between Lynch syndrome
associated-EC vs. sporadic EC (18). Immunotherapy is generally beneficial
for MMRd tumors (19). The present
case exhibited MMRd based on molecular detection and MLH1 and PMS2
protein deficiency based on immunohistochemistry only in the
high-grade region. Further genetic testing confirmed that the
low-grade region belonged to the NSMP subtype. While it has been
hypothesized that high-grade CHEC should be treated as an
aggressive tumor (20), the limited
number of high-grade CHEC cases necessitates the collection of more
cases to investigate prognosis and the efficacy of
immunotherapy.
In the present study, the molecular analysis
detected mutations in STK11 in the high-grade component of CHEC.
STK11 encodes the liver kinase B1 (LKB1) protein, which serves a
key role in transmitting signal events by phosphorylating
downstream molecules, such as STE20-Related Adaptor (STRAD), PTEN
and p21/CDK inhibitor 1A (15,21).
The STK11 gene is involved in essential cellular functions,
including regulation of the cell cycle, angiogenesis and the DNA
damage response (22,23). STK11 mutations are commonly
associated with Peutz-Jeghers syndrome, characterized by pigmented
skin and oral lesions, as well as gastrointestinal polyps (24,25).
Individuals with this syndrome are at a 10–18-fold greater risk of
developing tumors, particularly gastrointestinal tumors, and may
also develop tumors in other sites, such as the breast, uterus,
ovary, testes and pancreas (26,27).
The present patient lacked a history of mucosal melanosis and
gastrointestinal polyps but had a prolonged medication history.
Alterations in the STK11 gene may co-occur with high-grade CHEC,
possibly contributing to the aggressive nature of these tumors
(12).
Using the STRING database, the hub genes of STK11
were identified in the PPI network (Fig. 3D). LKB1 forms heterotrimers with
STRAD and calcium binding protein 39 (CAB39) (28), which enhances the phosphorylation
activity of LKB1 and regulates signaling pathways in vivo,
such as the AMP-activated protein kinase (AMPK) pathway (29). CAB39-like protein, an analogue of
CAB39, interacts with the LKB1-STRAD complex to activate and
phosphorylate AMPKα/β (30). PTEN
and STK11 serve different functions; however, PTEN can interact
with LKB1 and is affected by the phosphorylation of LKB1 (31). Axin 1 (AXIN1) is associated with the
AMPK signaling pathway (32,33),
although AXIN1 and STK11 do not directly interact, they
collaboratively regulate cellular processes through integrated
networks involving metabolic regulation (AMPK/mTOR), proliferative
signaling (Wnt/β-catenin), and stress response pathways to suppress
abnormal cell proliferation. Loss of function in the protein can
disrupt the balance of these pathways, leading to dysregulation
that drives tumor growth (34).
LKB1 is a primary upstream regulator of AMPK. It directly
phosphorylates the α-subunit at a critical site (Thr172),
activating AMPK in response to low energy states (35). By contrast, p21 is activated by LKB1
(36), which prevents cells from
entering the S-phase by inhibiting CDK activity, thereby inhibiting
cell proliferation (37). In CHEC,
STK11 mutations disrupt LKB1 tumor-suppressive roles in metabolism,
proliferation, and genomic stability. These aberrations synergize
with CTNNB1/PIK3CA mutations to drive malignant progression
(38). Further investigations are
needed to explore this association.
Immunohistochemical and molecular analyses have
demonstrated that almost all low-grade CHEC cases exhibit
widespread nuclear β-catenin expression (10). However, a noticeable decrease in
nuclear β-catenin positivity was demonstrated in high-grade CHEC
cases (5/8 cases exhibited nuclear positivity) and only 2/8 cases
of high-grade CHEC exhibited CTNNB1 mutations. The present study
solely assessed hotspot mutations within exon 3. Hence, the
potential occurrence of other hotspot mutations or alterations in
the Wnt/β-catenin pathway cannot be ruled out (7). Thus, a greater number of cases and
further molecular tests are required.
In conclusion, the present study described a patient
with CHEC with a high-grade component, molecularly classified as
MSI-H and accompanied by mutations in PIK3CA, CTNNB1 and STK11. The
selection of different differentiation regions of the tumor for
immunohistochemistry and molecular detection may yield different
results, which will impact the treatment of patients. Therefore, it
is important to consider the selection of tumor regions for
molecular detection. Due to the rarity of high-grade CHEC and
potential for diagnostic confusion, additional comprehensive
studies involving larger cohorts should be performed in the
future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82201293).
Availability of data and materials
The data generated in the present study may be found
in the Mendeley Data database under accession number
10.17632/fjhp6pwjwp.1 or at the following URL: https://data.mendeley.com/datasets/fjhp6pwjwp/1.
Authors' contributions
ZX and FC wrote the manuscript and performed the
literature review. FC analyzed and interpretated the data. YY and
ZX constructed figures and performed the pathology review. ZC and
HC made substantial contributions to conception and design and
revised the manuscript. All authors have read and approved the
final manuscript. ZX and FC confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Biomedical Research Ethic Committee of the Shandong Provincial
Hospital Affiliated to Shandong First Medical University (approval
no. SWYX2024-572), dated October 2024.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the present case report and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CHEC
|
corded and hyalinized endometrioid
carcinoma
|
|
MSI-H
|
high microsatellite instability
|
|
POLE
|
polymerase ε, catalytic subunit
|
|
MMRd
|
mismatch repair deficiency
|
|
STK11
|
serine/threonine kinase 11
|
|
NSMP
|
no special molecular profile
|
|
LKB1
|
liver kinase B1
|
|
STRAD
|
STE20-Related Adaptor
|
|
CAB39
|
calcium binding protein 39
|
|
AMPK
|
AMP-activated protein kinase
|
|
PI3KCA
|
Phosphatidylinositol-4,5-Bisphosphate
3-Kinase Catalytic Subunit α
|
References
|
1
|
Clement PB and Young RH: Endometrioid
carcinoma of the uterine corpus: A review of its pathology with
emphasis on recent advances and problematic aspects. Adv Anat
Pathol. 9:145–184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murray SK, Clement PB and Young RH:
Endometrioid carcinomas of the uterine corpus with sex cord-like
formations, hyalinization, and other unusual morphologic features:
A report of 31 cases of a neoplasm that may be confused with
carcinosarcoma and other uterine neoplasms. Am J Surg Pathol.
29:157–166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wani Y, Saegusa M and Notohara K: Aberrant
nuclear beta-catenin expression in the spindle or corded cells in
so-called corded and hyalinized endometrioid carcinomas. Another
critical role of the unique morphological feature. Histol
Histopathol. 24:149–155. 2009.PubMed/NCBI
|
|
4
|
Sun YH, Tang SX, Wang L, Yan YB and Zhou
XR: Endometrioid carcinoma with sex cord-like formations and
hyalinization of the uterine corpus: A clinicopathologic analysis
of 5 cases. Zhonghua Bing Li Xue Za Zhi. 45:297–301.
2016.PubMed/NCBI
|
|
5
|
Ladwig NR, Umetsu SE, Zaloudek C, Rabban J
and Garg K: Corded and hyalinized endometrioid adenocarcinoma
(CHEC) of the uterine corpus are characterized by CTNNB1 mutations
and can show adverse clinical outcomes. Int J Gynecol Pathol.
40:103–115. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Safdar NS, Thompson EF, Gilks CB, Isacson
C, Bennett JA, Clarke B, Young RH and Oliva E: Corded and
hyalinized and spindled endometrioid endometrial carcinoma: A
clinicopathologic and molecular analysis of 9 tumors based on the
TCGA classifier. Am J Surg Pathol. 45:1038–1046. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Travaglino A, Arciuolo D, Santoro A,
Raffone A, Pedone Anchora L, Piermattei A, Martinelli M, Mollo A,
Onori ME, Minucci A, et al: Corded and hyalinized endometrioid
endometrial carcinoma with high-grade features: A
clinicopathological and TCGA-based molecular analysis. Virchows
Arch. 482:671–678. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
WHO Classification of Tumours Editorial
Board, . Female Genital Tumours. In: WHO Classiication of Tumours.
Volume 4. 5th. ARC Press; Lyon: 2020
|
|
9
|
Crosbie EJ, Kitson SJ, McAlpine JN,
Mukhopadhyay A, Powell ME and Singh N: Endometrial cancer. Lancet.
399:1412–1428. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Travaglino A, Arciuolo D, Santoro A,
Raffone A, Raimondo D, Casadio P, Seracchioli R, Fulgione C, Guida
M, Mollo A, et al: Corded and hyalinized endometrioid carcinoma:
Summary of clinical, histological, immunohistochemical and
molecular data. Pathol Res Pract. 247:1545152023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leskela S, Pérez-Mies B, Rosa-Rosa JM,
Cristobal E, Biscuola M, Palacios-Berraquero ML, Ong S, Matias-Guiu
Guia X and Palacios J: Molecular basis of tumor heterogeneity in
endometrial carcinosarcoma. Cancers (Basel). 11:9642019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Contreras CM, Gurumurthy S, Haynie JM,
Shirley LJ, Akbay EA, Wingo SN, Schorge JO, Broaddus RR, Wong KK,
Bardeesy N and Castrillon DH: Loss of Lkb1 provokes highly invasive
endometrial adenocarcinomas. Cancer Res. 68:759–766. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xi Q, Kage H, Ogawa M, Matsunaga A,
Nishijima A, Sone K, Kawana K and Oda K: Genomic landscape of
endometrial, ovarian, and cervical cancers in Japan from the
database in the center for cancer genomics and advanced
therapeutics. Cancers (Basel). 16:1362023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanwar PS, Kaneko-Tarui T, Zhang L, Tanaka
Y, Crum CP and Teixeira JM: Stromal liver kinase B1 [STK11]
signaling loss induces oviductal adenomas and endometrial cancer by
activating mammalian target of rapamycin complex 1. PLoS Genet.
8:e10029062012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Launonen V: Mutations in the human
LKB1/STK11 gene. Hum Mutat. 26:291–297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
León-Castillo A, de Boer SM, Powell ME,
Mileshkin LR, Mackay HJ, Leary A, Nijman HW, Singh N, Pollock PM,
Bessette P, et al: Molecular classification of the PORTEC-3 trial
for high-risk endometrial cancer: Impact on prognosis and benefit
from adjuvant therapy. J Clin Oncol. 38:3388–3397. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berek JS, Matias-Guiu X, Creutzberg C,
Fotopoulou C, Gaffney D, Kehoe S, Lindemann K, Mutch D and Concin
N: FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet.
162:383–394. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sinicrope FA: Lynch syndrome-associated
colorectal cancer. N Engl J Med. 379:764–773. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nebot-Bral L, Coutzac C, Kannouche PL and
Chaput N: Why is immunotherapy effective (or not) in patients with
MSI/MMRD tumors? Bull Cancer. 106:105–113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Concin N, Creutzberg CL, Vergote I, Cibula
D, Mirza MR, Marnitz S, Ledermann JA, Bosse T, Chargari C, Fagotti
A, et al: ESGO/ESTRO/ESP guidelines for the management of patients
with endometrial carcinoma. Virchows Arch. 478:153–190. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zyla RE, Hahn E and Hodgson A: Gene of the
month: STK11. J Clin Pathol. 74:681–685. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dzung A, Saltari A, Tiso N, Lyck R, Dummer
R and Levesque MP: STK11 prevents invasion through signal
transducer and activator of transcription 3/5 and FAK repression in
cutaneous melanoma. J Invest Dermatol. 142:1171–1182.e1110. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Azin M and Demehri S: STK11 loss: A novel
mechanism for melanoma metastasis with therapeutic implications. J
Invest Dermatol. 142:1007–1009. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Altamish M, Dahiya R, Singh AK, Mishra A,
Aljabali AAA, Satija S, Mehta M, Dureja H, Prasher P, Negi P, et
al: Role of the serine/threonine kinase 11 (STK11) or liver kinase
B1 (LKB1) gene in Peutz-Jeghers syndrome. Crit Rev Eukaryot Gene
Expr. 30:245–252. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Daniell J, Plazzer JP, Perera A and Macrae
F: An exploration of genotype-phenotype link between Peutz-Jeghers
syndrome and STK11: A review. Fam Cancer. 17:421–427. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bennett JA and Oliva E: The complex and
often confusing history, histology and histogenesis of mesonephric,
STK11 adnexal tumour and mesonephric-like neoplasms of the upper
female genital tract (including broad ligament). Histopathology.
81:280–296. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chung SJ, Nagaraju GP, Nagalingam A,
Muniraj N, Kuppusamy P, Walker A, Woo J, Győrffy B, Gabrielson E,
Saxena NK, et al: ADIPOQ/adiponectin induces cytotoxic autophagy in
breast cancer cells through STK11/LKB1-mediated activation of the
AMPK-ULK1 axis. Autophagy. 13:1386–1403. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeqiraj E, Filippi BM, Deak M, Alessi DR
and van Aalten DM: Structure of the LKB1-STRAD-MO25 complex reveals
an allosteric mechanism of kinase activation. Science.
326:1707–1711. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Meng Q, Sun Q, Xu ZX, Zhou H and
Wang Y: LKB1 deficiency-induced metabolic reprogramming in
tumorigenesis and non-neoplastic diseases. Mol Metab.
44:1011312021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li W, Wong CC, Zhang X, Kang W, Nakatsu G,
Zhao Q, Chen H, Go MYY, Chiu PWY, Wang X, et al: CAB39L elicited an
anti-Warburg effect via a LKB1-AMPK-PGC1α axis to inhibit gastric
tumorigenesis. Oncogene. 37:6383–6398. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jia C, Medina V, Liu C, He L, Qian D,
Taojian T, Okamoto CT and Stiles BL: Crosstalk of LKB1- and
PTEN-regulated signals in liver morphogenesis and tumor
development. Hepatol Commun. 1:153–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yue Y, Zhang C, Zhao X, Liu S, Lv X, Zhang
S, Yang J, Chen L, Duan H, Zhang Y, et al: Tiam1 mediates Rac1
activation and contraction-induced glucose uptake in skeletal
muscle cells. FASEB J. 35:e212102021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ren G, Ding YW, Wang LL and Jiang JD:
Berberine stimulates lysosomal AMPK independent of PEN2 and
maintains cellular AMPK activity through inhibiting the
dephosphorylation regulator UHRF1. Front Pharmacol. 14:11486112023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Z, Li K, Lu C, Feng M, Lin C, Yin G,
Luo D, Liu W, Jin K, Dou Y, et al: Metformin promotes anti-tumor
immunity in STK11 mutant NSCLC through AXIN1-dependent upregulation
of multiple nucleotide metabolites. Oncol Res. 32:1637–1648. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shackelford DB and Shaw RJ: The LKB1-AMPK
pathway: Metabolism and growth control in tumour suppression. Nat
Rev Cancer. 9:563–575. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Deguchi A, Miyoshi H, Kojima Y, Okawa K,
Aoki M and Taketo MM: LKB1 suppresses p21-activated kinase-1 (PAK1)
by phosphorylation of Thr109 in the p21-binding domain. J Biol
Chem. 285:18283–18290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karimian A, Ahmadi Y and Yousefi B:
Multiple functions of p21 in cell cycle, apoptosis and
transcriptional regulation after DNA damage. DNA Repair (Amst).
42:63–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baumgartner C, Yadav AK and Chefetz I:
AMPK-like proteins and their function in female reproduction and
gynecologic cancer. Adv Protein Chem Struct Biol. 134:245–270.
2023. View Article : Google Scholar : PubMed/NCBI
|